Abstract
Background
Most frequent red cell (RBC) donors and many first-time donors are iron deficient, but meet haemoglobin standards. However, the effects of donation-induced iron deficiency on RBC storage quality are unknown. Thus, we used a mouse model to determine if donor iron deficiency reduced post-transfusion RBC recovery.
Methods
Weanling mice received a control diet or an iron-deficient diet. A third group receiving the iron-deficient diet was also phlebotomised weekly. This provided 3 groups of mice with different iron status: (1) iron replete, (2) mild iron deficiency with iron-deficient erythropoiesis, and (3) iron-deficiency anaemia. At ten weeks of age, blood was collected, leucoreduced, and stored at 4 ºC. After 12 days of storage, 24-hour (h) post-transfusion RBC recovery was quantified in recipients by flow cytometry.
Results
Before blood collection, mean haemoglobin concentrations in the iron-replete, iron-deficient, and iron-deficiency anaemia donor mice were 16.5±0.4, 11.5±0.4, and 7.0±1.4 [g/dL± 1 standard deviation (SD)], respectively (p<0.01 for all comparisons between groups). The 24-h post-transfusion RBC recoveries in recipients receiving transfusions from these three cohorts were 77.1±13.2, 66.5±10.9, and 46.7±15.9 (% ±1 SD), respectively (p<0.05 for all comparisons between groups).
Discussion
In summary, donor iron deficiency significantly reduced 24-h post-transfusion RBC recovery in recipient mice. RBCs from mice with mild iron deficiency and iron-deficient erythropoiesis, with haemoglobin levels similar to those used for human autologous blood donation, had intermediate post-transfusion RBC recovery, as compared to iron-replete donors and those with iron-deficiency anaemia. This suggests that, in addition to the effects of iron deficiency on donor health, frequent blood donation, leading to iron-deficient erythropoiesis, may also have adverse effects for transfusion recipients.
Keywords: red blood cells, iron deficiency, blood donation, mouse model
Introduction
Iron deficiency is common among frequent blood donors, but red blood cell (RBC) storage quality and post-transfusion RBC recovery of RBC units from iron-deficient donors have not been rigorously examined. Evidence from animal and human studies indicates that, when the iron supply for erythropoiesis is inadequate, the RBCs produced have multiple defects that could impair their ability to tolerate refrigerated storage.
Iron deficiency, a decrease in the amount of body iron, is detected clinically by measuring indicators of iron storage (e.g., serum ferritin) and of the adequacy of iron supply to erythropoietic precursors (e.g., RBC zinc protoporphyrin). In the absence of complicating factors, as iron stores decrease, serum ferritin levels decline. In addition, RBC zinc protoporphyrin monitors the supply of iron available for RBC production. In the developing RBC, the insertion of iron into protoporphyrin IX is the final step in producing haeme for incorporation into haemoglobin. If iron is unavailable, divalent zinc is incorporated instead, producing zinc protoporphyrin, which binds to haemoglobin, persists for the life of the RBC, and is a biochemical indicator of a deficient supply of iron for RBC production1,2.
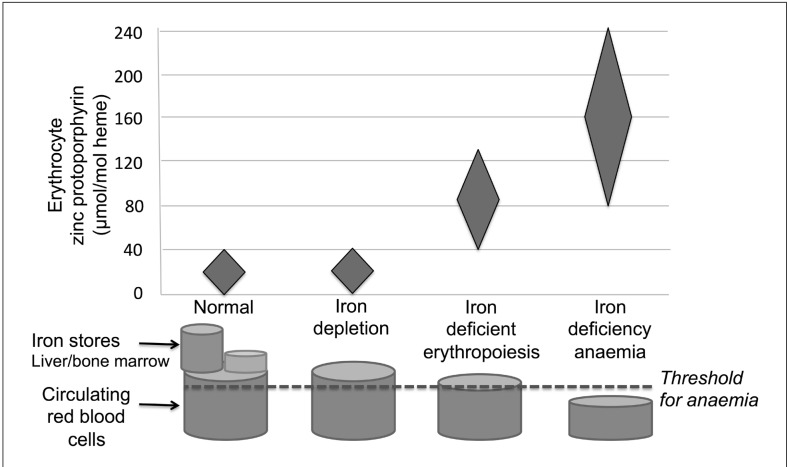
Using these biochemical markers, and other means, four successive stages of iron deficiency can be distinguished (Figure 1):
Figure 1.
Red blood cell (RBC) zinc protoporphyrin progressively increases with increasing severity of iron deficiency.
Iron deficiency progresses from reduced iron stores to iron depletion, in which there is normal erythropoiesis with normal zinc protoporphyrin levels, but decreased ferritin due to absent iron stores. The next stage is iron-deficient erythropoiesis, in which increased RBC levels of zinc protoporphyrin and very low serum ferritin levels reflect both absent iron stores and the lack of sufficient iron for normal haemoglobin production. As iron deficiency progresses further, frank iron deficiency anaemia develops with insufficient iron for maintaining adequate haemoglobin levels. Adapted from Hastka J et al. and Brittenham GM1,2.
Reduced iron stores (data not shown): as iron stores decrease, serum ferritin levels decline proportionally.
Iron depletion: iron stores are absent (e.g., serum ferritin <12 μg/L), but the combination of iron recycled from senescent RBCs and that derived from gastrointestinal iron absorption maintains delivery of iron to the erythroid marrow and other tissues for producing haemoglobin and other functional iron compounds. Because the iron supply for RBC production is maintained, RBC zinc protoporphyrin levels remain in the reference range.
Iron-deficient erythropoiesis: with further reductions in total body iron, the lack of iron limits production of haemoglobin and other iron-requiring compounds, resulting in iron-deficient erythropoiesis. The RBC zinc protoporphyrin concentration in newly formed erythrocytes increases, but any further falls in the nominal serum ferritin levels have no physiological meaning. The effect on the circulating haemoglobin concentration is insufficient to be detected by the standards used to screen blood donors. As recently produced RBCs replace senescent RBCs, the circulating RBC zinc protoporphyrin concentration progressively increases, providing an index reflecting the severity and duration of the inadequate supply of iron for erythropoiesis1,2.
Iron-deficiency anaemia: a further diminution in total body iron produces frank iron-deficiency anaemia with haemoglobin levels falling below the standards used to screen blood donors and leads to their deferral from donation.
Iron deficiency is commonly seen in volunteer blood donors. Although surprisingly prevalent in first-time donors3,4, the prevalence is even higher in the particularly altruistic, frequent, repeat donors, especially among women of childbearing age5,6. For example, in the United States in 2011, of the donors who provided the approximately 15.7 million units of RBCs that were collected, 69% were repeat donors7. In addition, in Canada, approximately 90% of RBC units collected for transfusion are provided by repeat donors8. In the United States, the REDS-II Donor Iron Status Evaluation (RISE) study9 found that up to 49 and 66% of male and female frequent donors, respectively, exhibited either iron depletion (i.e., absent iron stores) or iron-deficient erythropoiesis. Similar frequencies of iron deficiency were reported in Canadian8, Austrian10, Danish11, and Dutch12 populations.
Red blood cells from iron-deficient donors may have impaired tolerance for refrigerated storage and decreased post-transfusion RBC recovery. As examples, RBCs from individuals with iron-deficiency anaemia have decreased levels of endogenous antioxidants13,14, have evidence of oxidative damage15,16, and are more sensitive to oxidative stress14,16 and low pH14; the latter, in particular, decreases progressively during RBC storage17. Furthermore, refrigerated storage induces oxidative stress in donor RBCs and inhibits their oxidative stress defence mechanisms15,18–24. Oxidative damage per se also impairs RBC deformability25, and RBCs from humans, rats, and rabbits with iron-deficiency anaemia have impaired deformability16,26. In addition, the deformability of RBCs from healthy human donors is impaired following refrigerated storage27. Indeed, circulatory RBC lifespan is decreased in humans14,28–30 and in relevant animal models26,31 with iron-deficiency anaemia. In humans, this decreased circulatory lifespan is most likely due to extravascular haemolysis in the spleen28–30 and is corrected by iron repletion14,29. Remarkably, in several older studies14,28,32, RBCs obtained from donors with iron-deficiency anaemia were transfused into healthy recipients, albeit without prior refrigerated storage. In each study, the transfused iron-deficient RBCs had a decreased circulatory lifespan/recovery, most likely due to splenic clearance. In addition, when RBCs obtained from healthy donors were transfused into recipients with iron-deficiency anaemia, the transfused RBCs had a normal lifespan, suggesting that the iron-deficiency-induced defect was intrinsic to the RBC and not due to enhanced clearance mechanisms32,33.
The RBCs transfused in these earlier studies (from the 1940s–1970s) were from donors with iron-deficiency anaemia, not from donors with iron-deficient erythropoiesis. Repeating these studies with modern storage systems and deliberately transfusing healthy human volunteer recipients with allogeneic RBCs obtained from donors with iron-deficiency anaemia are no longer ethically feasible. Nonetheless, if RBCs collected from donors with iron-deficient erythropoiesis do exhibit poor storage quality, this would have particular importance for patients dependent on chronic transfusion therapy, including those with sickle-cell disease and β-thalassaemia. Therefore, we developed a mouse model to determine whether iron-deficient erythropoiesis in donor mice decreased the 24-h post-transfusion recovery of their RBCs in healthy murine recipients.
Materials and methods
Mice
Three murine donor cohorts of different iron status were prepared using wild-type C57BL/6 mice. To this end, weanling male mice were placed on a defined iron control diet of 45 ppm iron (TD.110593, Harlan Laboratories) or an iron-deficient diet of 0–4 ppm iron (TD.110592). A third group was fed the iron-deficient diet and was also phlebotomised weekly (50–100 μL per bleed). This approach generated three groups of mice, respectively, with different iron status: (1) iron replete, (2) mild iron deficiency with iron-deficient erythropoiesis, and (3) iron-deficiency anaemia. At approximately ten weeks of age, blood was collected from each cohort, leucoreduced, packed, and stored at 4 oC in CPDA-1 for 12 days, as described previously34,35.
Transfusion-recipient mice were fed an iron-replete diet and were transgenic for expression of the enhanced Green Fluorescent Protein (eGFP) under the control of the human ubiquitin C promoter and were on the C57BL/6 background. Thus, these mice are syngeneic with the wild-type C57BL/6 RBC donors, except for expression of eGFP, which is expressed in all of their cells, including RBCs.
Laboratory measurements of iron status
Within a week prior to preparing the mouse blood banks, small blood samples were collected from all donor mice by submandibular or saphenous vein bleed and pooled by cage, with 5 mice per cage. Haemoglobin was measured by a modified cyanomethemoglobin assay36. Complete blood counts were performed for the third replicate experiment using an automated haematology analyser (Forcyte Veterinary Hematology Analyzer; Oxford Science Inc., Oxford, CT, USA). To measure zinc protoporphyrin, RBCs were washed with saline and analysed using a haematofluorometer (Model 208; Aviv Biomedical Inc., Lakewood,NJ, USA).
Non-haeme liver iron was determined, as described previously37. This approach was used, rather than determining serum ferritin levels, because measuring the hepatic non-haeme iron concentration is a reference method for evaluating body iron stores. Briefly, the wet weight of each liver obtained at necropsy was quantified, and a weighed, approximately 100 mg portion was dried at 65 °C for 24 h and digested in an acid digestion mixture at 65 °C for 24 h. The iron content of the centrifuged, acidified sample was determined using a bathophenanthroline-based colorometric method. The absorbance at 535 nm of samples and iron standards was measured spectrophotometrically in duplicate and mean values used for calculating total liver iron.
Post-transfusion RBC recovery studies
After 12 days of storage in CPDA-1, 24-h post-transfusion RBC recovery was determined in recipient transgenic mice using flow cytometric detection of transfused RBCs, as described previously34,35. In this approach, the non-fluorescent donor RBCs are detected and quantified against the background of fluorescent, eGFP-expressing recipient RBCs. The experiment was repeated three times in its entirety (n=24 per group total).
Statistical analysis
All statistical analyses were conducted using Prism 6 (Graph Pad Software, La Jolla, CA, USA). All column graphs in figures represent mean and standard deviation. Differences among means were analysed by one-way ANOVA with Tukey's post–test.
Results
Generation of cohorts of blood donor mice with different iron status by dietary manipulation
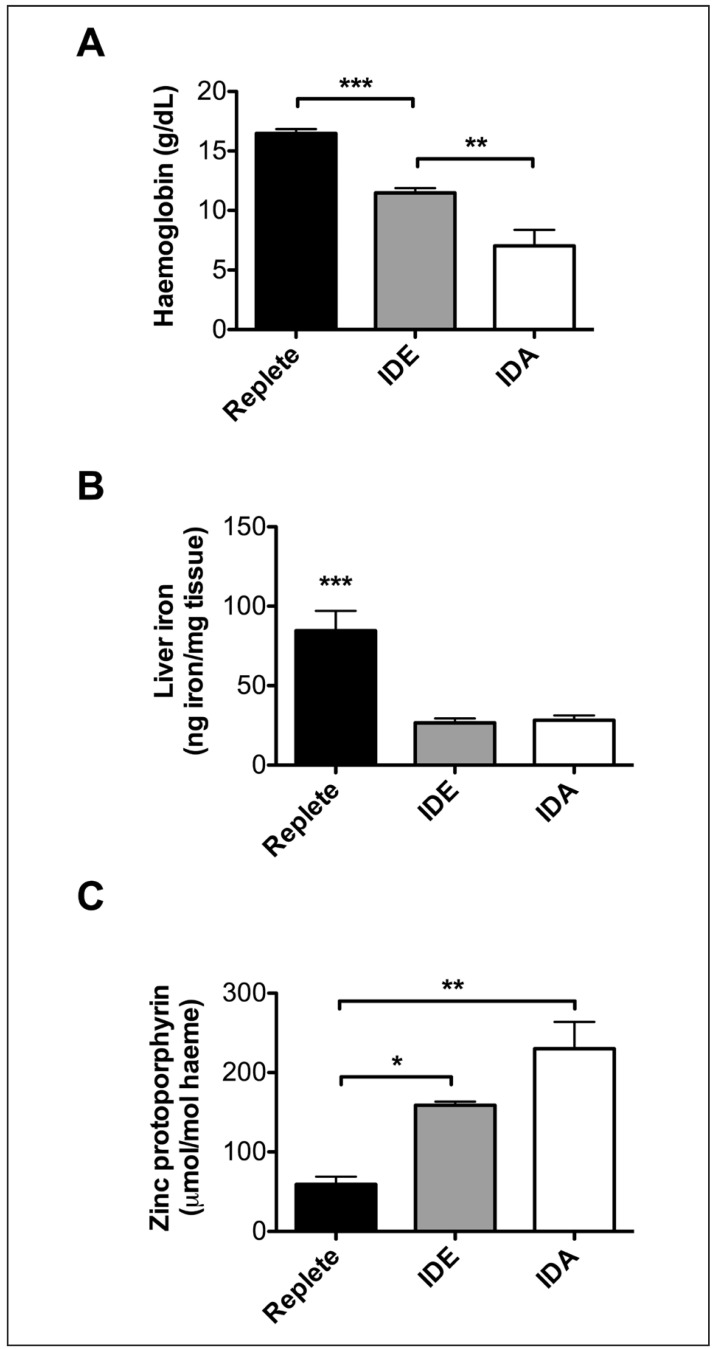
Three groups of weanling mice (n=15–20 per group in each experiment) were fed either an iron-replete or an iron-deficient diet for six weeks. A third group was fed the iron-deficient diet and was phlebotomised weekly to induce severe iron-deficiency anaemia. After six weeks on the diet, the mice were euthanised and blood collected by exsanguination. The average haemoglobin per group (in g/dL ±1 SD) decreased significantly from normal (16.5±0.4) to iron-deficient erythropoiesis (11.5±0.4) to frank iron-deficiency anaemia (7.0±1.4) (Figure 2A). In addition, liver iron measurements (in ng iron/mg tissue ±1 SD) (Figure 2B) demonstrated that iron stores were virtually absent in mice with either iron-deficient erythropoiesis or iron-deficiency anaemia (26.6±2.7 and 28.2±3.0, respectively); in contrast iron-replete mice had normal iron stores (84.6±12.4)38. Finally, zinc protoporphyrin levels (in μmol/mol haeme ±1 SD) were quantified in the pooled blood from each group (Figure 2C); these levels increased in proportion to decreasing iron status, measuring 59.0±9.9, 158.8±4.6, and 230.0±33.9, respectively, in iron-replete mice, mice with iron-deficient erythropoiesis, and mice with severe iron-deficiency anaemia.
Figure 2.
Blood donor mice with varying iron status were generated by dietary manipulation.
Three groups of weanling mice (N=15–20 per group) were fed either an iron-replete or iron-deficient diet (IDE) for 6 weeks. A third group was fed the iron deficient diet and was phlebotomised weekly to induce iron deficiency anaemia (IDA). After 6 weeks on the diet, the mice were euthanised and blood collected by exsanguination. (A) The average haemoglobin per group across 3 replicate experiments. (B) Liver iron, as a measure of total body iron, quantified in 5 representative mice per group from a single experiment. (C) Zinc protoporphyrin levels in the pooled blood from each group across two representative experiments (these measurements were not performed in one of the replicate experiments). *p<0.05, **p<0.01, ***p<0.001 by One-way ANOVA with Tukey's multiple comparison test. ***Without brackets in panel B represents significance compared to both other groups.
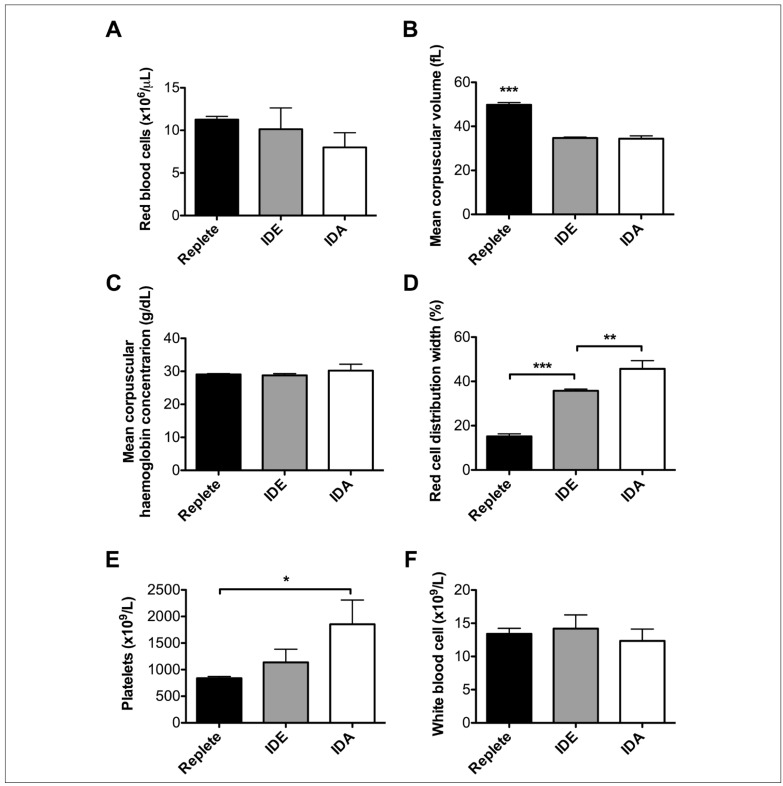
Other aspects of the complete blood count further confirmed the validity of this mouse model of iron deficiency. Thus, the decreasing trend in the number of circulating RBCs with decreasing body iron stores was significant (p<0.05) (Figure 3A). In addition, the mean corpuscular volume was normal in iron-replete mice, but equivalently decreased in mice with mild iron deficiency and iron-deficient erythropoiesis and in those with iron-deficiency anaemia (Figure 3B); as expected, the mean corpuscular haemoglobin concentration was virtually the same across all three groups (Figure 3C). As a marker of dysregulated erythropoiesis, the RBC distribution width increased progressively in proportion to decreasing iron status (Figure 3D). Finally, similar to what has been observed in humans39, murine platelet counts also increased progressively in proportion to decreasing iron status (p<0.01 for linear trend in data) (Figure 3E); nonetheless, as expected, there were no significant changes in the white blood cell counts (WBC) among these three groups of mice (Figure 3F).
Figure 3.
Additional laboratory parameters demonstrating the validity of the mouse model of variable iron status.
Additional parameters were measured on the animals described in Figure 2. Three groups of weanling mice (N=15–20 per group) were fed either an iron-replete or iron-deficient diet (IDE) for 6 weeks. A third group was fed the iron deficient diet and was phlebotomised weekly to induce iron deficiency anaemia (IDA). After 6 weeks on the diet, blood was collected from the saphenous vein and pooled per cage in each group. The measurements are as labeled in the figure panels. *p<0.05, **p<0.01, ***p<0.001 by One-way ANOVA with Tukey's multiple comparison test. ***Without brackets in panel B represents significance compared to both other groups.
Post-transfusion RBC recovery studies
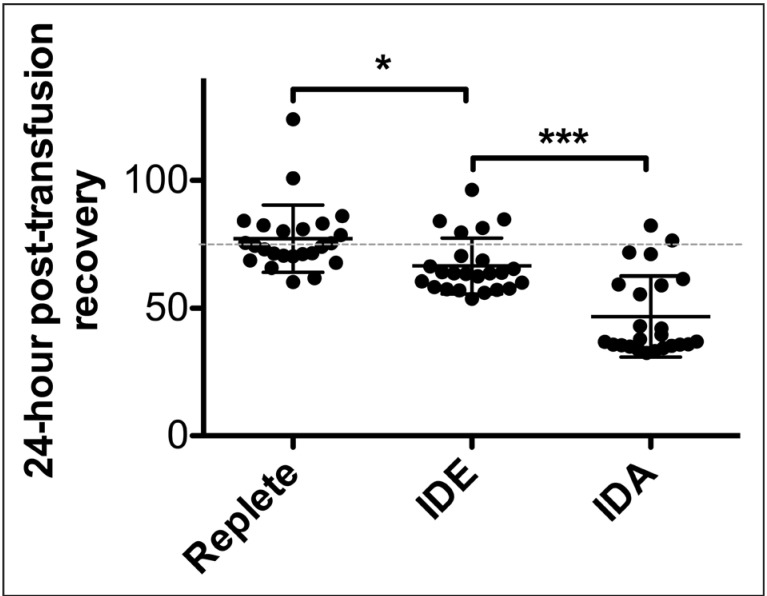
Similar to prior results40, refrigerator-stored, transfused RBCs from iron-replete donors into eGFP congenic, healthy recipients had a mean 24-h post-transfusion recovery of 77.1% ±13.2%, as expected (Figure 4). In addition, as expected from human studies and various animal models14,28,32, the 24-h post-transfusion recovery was decreased using RBCs from donors with severe iron-deficiency anaemia (mean 46.7% ±15.9%; p<0.001) whereas the results using donors with mild iron deficiency and iron-deficient erythropoiesis were intermediate: 66.5% ±10.9% (p<0.05 compared to iron-replete mice).
Figure 4.
Donor RBCs from mice with iron deficiency exhibit decreased 24-hour post-transfusion recovery.
Three groups of adult mice with varying iron status: iron replete, iron-deficient erythropoiesis with mild iron deficiency (IDE), and severe iron deficiency anaemia (IDA) were generated by modifying the iron-content of their diets with or without additional phlebotomy. At ~10 weeks of age, blood was collected from each cohort, pooled, leucoreduced, packed, and refrigerator-stored in CPDA-1 for 12 days. The 24-hour post-transfusion recoveries of the stored RBCs were quantified using flow cytometry in enhanced Green Fluorescent Protein-transgenic recipient mice. The experiment was repeated three times in its entirety (n=8 recipient mice per group per experiment; 24 mice per group total). Shown are the individual 24-hour post-transfusion recovery results for each recipient mouse, combined across all three replicate experiments. *p<0.05, ***p<0.001 by One-way ANOVA with Tukey's multiple comparison test.
Discussion
These results show that murine donor iron deficiency significantly reduced 24-h post-transfusion RBC recovery in healthy recipient mice. RBCs obtained from mice with haemoglobin levels similar to the cut-off used for autologous blood donation in humans, but with mild iron deficiency and iron-deficient erythropoiesis, exhibited intermediate post-transfusion RBC recovery, as compared to iron-replete donors and those with iron-deficiency anaemia. The data in Figures 2 and 3 demonstrate the development of a mouse model of iron deficiency, producing three mouse cohorts with different iron status: (1) iron replete, (2) mild iron deficiency with iron-deficient erythropoiesis, and (3) severe iron-deficiency anaemia. The storage quality of the RBCs obtained from murine donors with mild iron deficiency and iron-deficient erythropoiesis was suboptimal, with a mean 24-h RBC recovery of only 66.5%. These units did not average a 75% recovery and, thus, would not pass the United States Food and Drug Administration regulatory criteria for adequacy. Therefore, these results in a mouse model provide evidence of the need for clinical evaluation of the effects of donor iron deficiency in humans. For patients with urgent transfusion requirements, RBC units with diminished RBC recovery may, or may not, increase the risk of harm41. However, in the chronic transfusion setting (e.g., for transfusion-dependent patients with sickle cell disease, β-thalassaemia major, or other forms of refractory anaemia), transfusing the best quality RBC units, with the highest post-transfusion recoveries, would reduce the number of transfusions required over a lifetime and, thus, reduce the lifetime burden of iron overload42.
One limitation of this study is that the murine cohort with iron-deficient erythropoiesis is slightly more affected than that found in humans who meet typical RBC donation standards. Thus, these mice with mild iron deficiency and iron-deficient erythropoiesis have decreased RBC mean corpuscular volumes and haemoglobin concentrations and a greater elevation in zinc protoporphyrin levels than typically seen in humans with iron-deficient erythropoiesis. Nonetheless, the trend in our results suggests a strong correlation between body iron stores and post-transfusion RBC recovery after refrigerated storage, with decreased recovery expected in donors with iron-deficient erythropoiesis. We acknowledge the truism that "mice aren't human" and, in the current case, that our mouse model of iron-deficient erythropoiesis does not exactly correspond to the human counterpart in every possible way (Figures 2 and 3)43.
Conclusions
In conclusion, it will be important to determine whether or not similar findings are observed in human volunteers. Such a randomised clinical trial is now beginning and will study autologous RBC transfusions in otherwise healthy volunteers with documented iron-deficient erythropoiesis (i.e., the Donor Iron-Deficiency Study. Registered at: clinicaltrial.gov NCT02889133). The results of this human trial will determine whether the adverse effects of iron deficiency are limited to donors or also compromise the quality of RBC units from iron-deficient donors who meet current standards for RBC donation.
Footnotes
Disclaimer
This work was presented, in part, at the 2015 Annual Meeting of the AABB44.
Funding
This work was funded, in part, by NIH R01 HL115557 (SLS) and NIH K08 HL103756 (EAH).
Authorship contributions
EAH and SLS designed the studies. SB performed the experiments with the assistance of EAH. SB, GMB, EAH, and SLS evaluated and interpreted the data. EAH and SLS prepared the first draft of the manuscript. All Authors read, edited, and approved the final manuscript. EAH and SLS contributed equally to this work.
The Authors declare no conflicts of interest.
References
- 1.Brittenham GM. Disorders of iron homeostasis: iron deficiency and overload. In: Hoffman R, Benz EJ, Silberstein LE, et al., editors. Hematology. 6th ed. Philadelphia: Elsevier/Saunders; 2013. pp. 437–49. [Google Scholar]
- 2.Hastka J, Lasserre J-J, Schwarzbeck A, et al. Laboratory tests of iron status: correlation or common sense? Clin Chem. 1996;42:718–24. [PubMed] [Google Scholar]
- 3.Bialkowski W, Bryant BJ, Schlumpf KS, et al. The strategies to reduce iron deficiency in blood donors randomized trial: design, enrolment and early retention. Vox Sang. 2014;108:178–85. doi: 10.1111/vox.12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith GA, Fisher SA, Doree C, Roberts DJ. A systematic review of factors associated with the deferral of donors failing to meet low haemoglobin thresholds. Transfusion Med. 2013;23:309–20. doi: 10.1111/tme.12046. [DOI] [PubMed] [Google Scholar]
- 5.Birgegard G, Schneider K, Ulfberg J. High incidence of iron depletion and restless leg syndrome (RLS) in regular blood donors: intravenous iron sucrose substitution more effective than oral iron. Vox Sang. 2010;99:354–61. doi: 10.1111/j.1423-0410.2010.01368.x. [DOI] [PubMed] [Google Scholar]
- 6.Booth AO, Lim K, Capper H, et al. Iron status and dietary iron intake of female blood donors. Transfusion. 2013;54:770–4. doi: 10.1111/trf.12347. [DOI] [PubMed] [Google Scholar]
- 7.Whitaker BI. The 2011 National Blood Collection and Utilization Survey Report. Washington: Department of Health and Human Services; 2011. pp. 1–98. [Google Scholar]
- 8.Goldman M, Uzicanin S, Scalia V, O'Brien SF. Iron deficiency in Canadian blood donors. Transfusion. 2013;54:775–9. doi: 10.1111/trf.12380. [DOI] [PubMed] [Google Scholar]
- 9.Cable RG, Glynn SA, Kiss JE, et al. Iron deficiency in blood donors: the REDS-II Donor Iron Status Evaluation (RISE) study. Transfusion. 2012;52:702–11. doi: 10.1111/j.1537-2995.2011.03401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Semmelrock MJ, Raggam RB, Amrein K, et al. Reticulocyte hemoglobin content allows early and reliable detection of functional iron deficiency in blood donors. Clin Chim Acta. 2012;413:678–82. doi: 10.1016/j.cca.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 11.Rigas AS, Pedersen OB, Sorensen CJ, et al. No association between iron status and self-reported health-related quality of life in 16,375 Danish blood donors: results from the Danish Blood Donor Study. Transfusion. 2015;55:1752–6. doi: 10.1111/trf.13085. [DOI] [PubMed] [Google Scholar]
- 12.Baart AM, van Noord PAH, Vergouwe Y, et al. High prevalence of subclinical iron deficiency in whole blood donors not deferred for low hemoglobin. Transfusion. 2013;53:1670–7. doi: 10.1111/j.1537-2995.2012.03956.x. [DOI] [PubMed] [Google Scholar]
- 13.Macdougall LG. Red cell metabolism in iron deficiency anemia. III. The relationship between glutathione peroxidase, catalase, serum vitamin E, and susceptibility of iron-deficient red cells to oxidative hemolysis. J Perinatol. 1980;80:775–82. doi: 10.1016/s0022-3476(72)80130-6. [DOI] [PubMed] [Google Scholar]
- 14.Macdougall LG, Judisch JM, Mistry S. Red cell metabolism in iron deficiency anemia. II. The relationship between red cell survival and alterations in red cell metabolism. J Perinatol. 1970;76:660–75. doi: 10.1016/s0022-3476(70)80283-9. [DOI] [PubMed] [Google Scholar]
- 15.Ogunro PS, Ogungbamigbe TO, Muhibi MA. The influence of storage period on the antioxidants level of red blood cells and the plasma before transfusion. African J Med Medical Sci. 2010;39:99–104. [PubMed] [Google Scholar]
- 16.Yip R, Mohandas N, Clark MR, et al. Red cell membrane stiffness in iron deficiency. Blood. 1983;62:99–106. [PubMed] [Google Scholar]
- 17.Bennett-Guerrero E, Veldman TH, Doctor A, et al. Evolution of adverse changes in stored RBCs. PNAS. 2007;104:17063–8. doi: 10.1073/pnas.0708160104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dumaswala UJ. Glutathione loading prevents free radical injury in red blood cells after storage. Free Rad Res. 2000;33:517–29. doi: 10.1080/10715760000301061. [DOI] [PubMed] [Google Scholar]
- 19.Dumaswala UJ, Zhuo L, Mahajan S, et al. Glutathione protects chemokine-scavenging and antioxidative defense functions in human RBCs. Am J Physiol - Cell Physiol. 2001;280:867–73. doi: 10.1152/ajpcell.2001.280.4.C867. [DOI] [PubMed] [Google Scholar]
- 20.Dumont LJ, AuBuchon JP Biomedical Excellence for Safer Transfusion (BEST) Collaborative. Evaluation of proposed FDA criteria for the evaluation of radiolabeled red cell recovery trials. Transfusion. 2008;48:1053–60. doi: 10.1111/j.1537-2995.2008.01642.x. [DOI] [PubMed] [Google Scholar]
- 21.Jozwik M, Jozwik M, Jozwik M, et al. Antioxidant defence of red blood cells and plasma in stored human blood. Clin Chim Acta. 1997;267:129–42. doi: 10.1016/s0009-8981(97)00148-4. [DOI] [PubMed] [Google Scholar]
- 22.Rinalducci S, Marrocco C, Zolla L. Thiol-based regulation of glyceraldehyde-3-phosphate dehydrogenase in blood bank-stored red blood cells: a strategy to counteract oxidative stress. Transfusion. 2015;55:499–506. doi: 10.1111/trf.12855. [DOI] [PubMed] [Google Scholar]
- 23.Reisz JA, Wither MJ, Dzieciatkowska M, et al. Oxidative modifications of glyceraldehyde 3-phosphate dehydrogenase regulate metabolic reprogramming of stored red blood cells. Blood. 2016;128:e32–42. doi: 10.1182/blood-2016-05-714816. [DOI] [PubMed] [Google Scholar]
- 24.Wither M, Dzieciatkowska M, Nemkov T, et al. Hemoglobin oxidation at functional amino acid residues during routine storage of red blood cells. Transfusion. 2016;56:421–6. doi: 10.1111/trf.13363. [DOI] [PubMed] [Google Scholar]
- 25.Mohanty JG, Nagababu E, Rifkind JM. Red blood cell oxidative stress impairs oxygen delivery and induces red blood cell aging. Frontiers Physiol. 2014;5:1–6. doi: 10.3389/fphys.2014.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodvien R, Gillum A, Weintraub LR. Decreased glutathione peroxidase activity secondary to severe iron deficiency: a possible mechanism responsible for the shortened life span of the iron-deficient red cell. Blood. 1974;43:281–9. [PubMed] [Google Scholar]
- 27.Knight JA, Searles DA, Clayton FC. The effect of desferrioxamine on stored erythrocytes: lipid peroxidation, deformability, and morphology. Ann Clin Lab Sci. 2010;26:283–90. [PubMed] [Google Scholar]
- 28.Diez-Ewald M, Layrisse M. Mechanisms of hemolysis in iron deficiency anemia. Further studies. Blood. 1968;32:884–94. [PubMed] [Google Scholar]
- 29.Farid Z, Nichols JH, Schulert AR, Bassily S. Chromium-51 red cell half-life in severe iron deficiency anemia. Am J Trop Med Hyg. 1965;14:605–9. doi: 10.4269/ajtmh.1965.14.375. [DOI] [PubMed] [Google Scholar]
- 30.Layrisse M, Linares J, Roche M. Excess hemolysis in subjects with severe iron deficiency anemia associated and nonassociated with hookworm infection. Blood. 1965;25:73–91. [PubMed] [Google Scholar]
- 31.Kempe DS, Lang PA, Duranton C, et al. Enhanced programmed cell death of iron-deficient erythrocytes. FASEB J. 2005;20:368–70. doi: 10.1096/fj.05-4872fje. [DOI] [PubMed] [Google Scholar]
- 32.Loria A, Sanchez-Medal L, Lisker R, et al. Red cell life span in iron deficiency anaemia. Brit J Haematol. 1967;13:294–302. doi: 10.1111/j.1365-2141.1967.tb08743.x. [DOI] [PubMed] [Google Scholar]
- 33.Brown GM, Hayward OC, Powell EO, Witts LJ. The destruction of transfused erythrocytes in anaemia. J Path Bacteriol. 1944;56:81–94. [Google Scholar]
- 34.Waterman HR, Kapp LM, Howie HL, et al. Analysis of 24-h recovery of transfused stored RBCs in recipient mice of distinct genetic backgrounds. Vox Sang. 2015;109:148–54. doi: 10.1111/vox.12270. [DOI] [PubMed] [Google Scholar]
- 35.Wojczyk BS, Kim N, Bandyopadhyay S, et al. Macrophages clear refrigerator storage-damaged red blood cells and subsequently secrete cytokines in vivo, but not in vitro, in a murine model. Transfusion. 2014;54:3186–97. doi: 10.1111/trf.12755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moore GL, Ledford ME, Merydth A. A micromodification of the Drabkin hemoglobin assay for measuring plasma hemoglobin in the range of 5 to 2000 mg/dl. Biochemical Med. 1981;26:167–73. doi: 10.1016/0006-2944(81)90043-0. [DOI] [PubMed] [Google Scholar]
- 37.Torrance J, Bothwell T. Tissue iron Stores. In: Cook JD, editor. Iron (Methods in Hematology) New York: Churchill Livingstone; 1980. pp. 90–115. [Google Scholar]
- 38.Hubbard AC, Bandyopadhyay S, Wojczyk BS, et al. Effect of dietary iron on fetal growth in pregnant mice. Compar Med. 2013;63:127–35. [PMC free article] [PubMed] [Google Scholar]
- 39.Schloesser LL, Kipp MA, Wenzel FJ. Thrombocytosis in iron-deficiency anemia. J Lab Clin Med. 1965;66:107–14. [Google Scholar]
- 40.Gilson CR, Kraus TS, Hod EA, et al. A novel mouse model of red blood cell storage and posttransfusion in vivo survival. Transfusion. 2009;49:1546–53. doi: 10.1111/j.1537-2995.2009.02173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prudent M, Tissot J-D, Lion N. In vitro assays and clinical trials in red blood cell aging: lost in translation. Transfusion Apher Sci. 2015;52:270–6. doi: 10.1016/j.transci.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 42.Spanos T, Ladis V, Palamidou F, et al. The impact of neocyte transfusion in the management of thalassaemia. Vox Sang. 1996;70:217–23. doi: 10.1111/j.1423-0410.1996.tb01330.x. [DOI] [PubMed] [Google Scholar]
- 43.Zimring JC, Spitalnik SL. On the appropriate use and interpretation of animal models in transfusion medicine research. Transfusion. 2013;53:2334–9. doi: 10.1111/trf.12131. [DOI] [PubMed] [Google Scholar]
- 44.Bandyopadhyay S, Hod EA, Francis RO, et al. Refrigerator-stored red cells from iron-deficient donor mice have reduced 24-hour post-transfusion recovery. Transfusion. 2015;55(Suppl 3):5A. [Abstract P5-030A] [Google Scholar]