Abstract
Bacterial pathogens rely on chemical signaling and environmental cues to regulate disease-causing behavior in complex microenvironments. The human pathogen Streptococcus mutans employs a particularly complex signaling and sensing scheme to regulate genetic competence and other virulence behaviors in the oral biofilms it inhabits. Individual S. mutans cells make the decision to enter the competent state by integrating chemical and physical cues received from their microenvironment along with endogenously produced peptide signals. Studies at the single-cell level, using microfluidics to control the extracellular environment, provide physical insight into how the cells process these inputs to generate complex and often heterogeneous outputs. Fine changes in environmental stimuli can dramatically alter the behavior of the competence circuit. Small shifts in pH can switch the quorum sensing response on or off, while peptide-rich media appear to switch the output from a unimodal to a bimodal behavior. Therefore, depending on environmental cues, the quorum sensing circuitry can either synchronize virulence across the population, or initiate and amplify heterogeneity in that behavior. Much of this complex behavior can be understood within the framework of a quorum sensing system that can operate both as an intercellular signaling mechanism and intracellularly as a noisy bimodal switch.
1. Introduction – Control of virulent behavior in S. mutans
Many pathogenic bacteria employ chemical signaling and sensing schemes to regulate their disease-causing behaviors. These control mechanisms can become particularly complex when the bacteria inhabit biofilms, complex three-dimensional matrices composed of extracellular polysaccharides, proteins and other secretions (Hall-Stoodley et al., 2004). The physical framework of the biofilm allows the bacteria to colonize surfaces and protects them against environmental stresses and host defenses. It also facilitates competitive or cooperative interactions with other bacterial species. The chemically and physically heterogeneous microenvironment within a biofilm provides the bacterial pathogen with a wide range of stimuli and signals generated by the same and different species. The cell integrates and processes these signals in order to control stress responses, coordinate intercellular cooperation or competition, and activate disease-causing behaviors (Li; Tian, 2012).
The human oral pathogen Streptococcus mutans is an important example. This Gram-positive anaerobe forms and inhabits tenacious biofilms on the surface of the tooth. There it can consume sugars from the host’s diet, producing acid fermentation products that erode the tooth enamel. This makes S. mutans a primary causative agent of dental caries, one of the most prevalent infectious diseases in the United States. Dental caries has large economic and social costs through its impact on school and work performance, nutrition, development and overall health (Dye et al., 2007). Understanding how S. mutans causes disease requires understanding how the organism interprets chemical and physical information from its microenvironment in order to activate virulence. Here we examine a number of puzzling aspects of an important virulence pathway – genetic competence – in S. mutans. We describe how those characteristics, when taken together with experiments at the single cell level and stochastic simulations, provide insight into the mechanism by which the organism processes environmental inputs. Our findings strongly suggest the competence pathway can be understood in physical terms as a positive feedback circuit that, unlike a conventional quorum sensing model, can function in two qualitatively different modes – either intercellular or intracellular signaling – in response to fine-tuning from environmental cues.
S. mutans derives virulence in large part from its ability to form and colonize oral biofilms (Koo et al., 2013). However a number of related behaviors allow it to thrive in the acidified, anaerobic biofilm environment and to outcompete commensal species (Senadheera; Cvitkovitch, 2008). It can ferment a diversity of carbohydrates and tolerate acidity as low as pH 3.0 (Matsui; Cvitkovitch, 2010). It can resist oxidative stresses imposed by competing species and it can inhibit the growth of those species by releasing antibacterial peptides known as bacteriocins. Under stress, S. mutans cells can also lyse and release their cellular contents, presumably for benefit of others in the population. Regulation of these behaviors interconnects closely with the regulation of genetic competence. Genetic competence refers to the transient physiologic state during which a bacterium becomes transformable, i.e. able to acquire new traits by taking up and incorporating exogenous DNA.
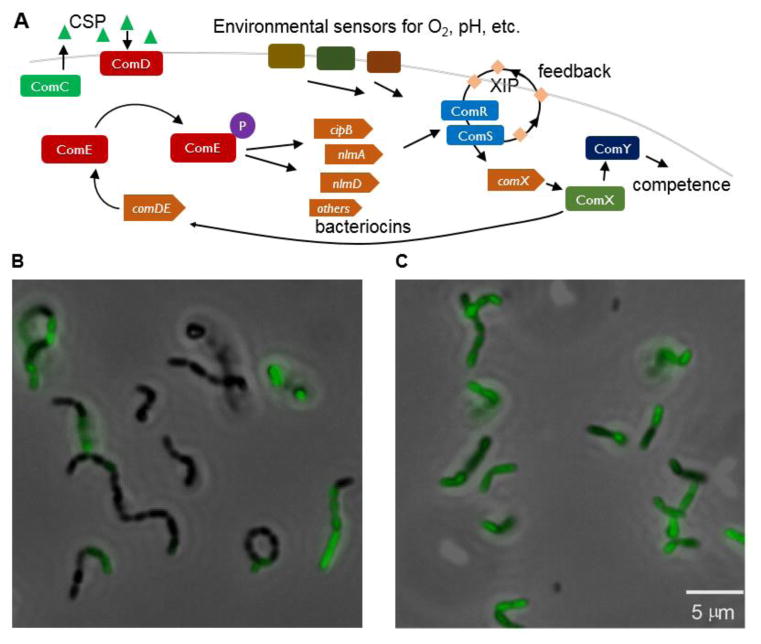
Most of the 80 or so known transformable species of bacteria become transformable only transiently during growth or under certain environmental conditions. Yet while they share similar mechanisms for DNA uptake and processing, the mechanisms that control entry into the competent state differ sharply between species (Johnston et al., 2014). S. mutans exerts tight control over its competent state through a complex transcriptional network outlined in FIGURE 1 (Smith; Spatafora, 2012). Many authors describe this as a quorum sensing network (Waters; Bassler, 2005), whereby the bacteria secrete a diffusible signal into their environment and the accumulation of signal coordinates or synchronizes competence population-wide (Smith; Spatafora, 2012; Fontaine et al., 2014; Johnston et al., 2014). However the quorum sensing description can understate the complexity of the interactions between a bacterium’s microenvironment and its regulatory pathways (Dunn; Stabb, 2007). Certainly in S. mutans the term does not capture the variety of environmental stimuli that interact with the S. mutans network or the fact that competence depends on more than just total bacterial population: Competence regulation in S. mutans employs multiple diffusible signals and shows acute sensitivity to environmental parameters such as pH that vary spatially and temporally in the biofilm (Son et al., 2015b). Moreover, the circuit contains multiple feedback elements that appear to introduce stochasticity and cell-to-cell heterogeneity, raising intriguing questions about how the pathogen integrates multiple environmental cues with endogenously produced chemical signals in order to switch phenotype.
FIGURE 1.
(A) Overview of genetic competence signaling in S. mutans (Smith; Spatafora, 2012). ComC is synthesized, processed and exported as CSP, an extracellular signal peptide. ComD detects CSP and phosphorylates ComE to ComE-P, which acts as a transcriptional activator for several bacteriocin genes. By a mechanism not yet understood, the bacteriocins stimulate the ComRS system. ComR (with XIP or its precursor ComS) forms a transcriptional activator for synthesis of ComX, the master competence regulator. ComX is an alternative sigma factor that stimulates transcription of the late competence genes such as comY that lead to DNA uptake. ComX also drives positive feedback to the upstream comCDE system, as the comE promoter possesses a ComX binding site (Son et al., 2015a). The circuit also integrates a variety of other environmental and physiological inputs such as environmental pH, carbohydrate catabolism, oxidative stress and other mechanisms (Ahn et al., 2007; Ahn et al., 2014; Kaspar et al., 2015).
(B)–(C) Overlaid fluorescence (green) and phase contrast (gray scale) microscopy images of S. mutans responding to exogenous signal peptides (B) CSP (1 μM in complex medium) and (C) XIP (500 nM in defined medium). The green fluorescence from a PcomX-gfp reporter shows that CSP and XIP elicit a bimodal and unimodal response respectively from comX (Son et al., 2012).
Like other streptococci S. mutans uses an alternative sigma factor, denoted ComX or σX, as the master regulator of the competent state (Fontaine et al., 2014). ComX forms a complex with the RNA polymerase and stimulates activation of so-called “late competence genes” for DNA uptake and processing by recognizing a common sequence in their promoters. Activation of the competent state therefore requires activation of comX. The pathway (FIGURE 1) begins with the cell synthesizing (from the 46-amino acid precursor, ComC) the diffusible 21-residue peptide CSP (competence stimulating peptide), which it exports and cleaves from 21 to 18 residues (Hossain; Biswas, 2012). S. mutans detects the accumulated environmental CSP via ComDE, a two-component signal transduction system in which extracellular CSP induces ComD to phosphorylate ComE to ComE-P, a transcriptional activator for several bacteriocin genes.
This CSP-ComDE quorum sensing also exists in other streptococci, such as S. pneumoniae, but their full competence networks are not homologous: While ComE-P directly activates comX in S. pneumoniae, in S. mutans ComE-P does not directly activate comX (Fontaine et al., 2014). Instead, in S. mutans other regulatory steps that are not yet fully understood lie between bacteriocin production and comX activation. These additional steps appear to have an important consequence: while S. pneumoniae competence regulation resembles simple quorum sensing, with environmental CSP triggering the entire population to become competent, only a subpopulation (between 1% and 50%) of S. mutans cells become competent following stimulation with CSP (Aspiras et al., 2004; Lemme et al., 2011; Son et al., 2012). All cells activate the ComDE circuit and bacteriocin production in response to the CSP signal, but only a subset become transformable (Son et al., 2015a).
2. Feedback signals, environmental inputs, and complex behavior of comX
In S. mutans and several other streptococci (but not in S. pneumoniae), the immediate activator of comX is the ComRS system (Mashburn-Warren et al., 2010; Fontaine et al., 2014). ComRS consists of the Rgg- type transcription factor ComR and the 17-residue peptide ComS. ComR detects ComS intracellularly by forming a ComR/ComS complex, the transcriptional activator for both comS and comX. Thus ComS appears to stimulate its own production. In fact, when supplied exogenously to S. mutans, a shorter peptide consisting of residues 11–17 of ComS proved more effective than full length ComS in activating comX. Researchers later detected the short 11–17 peptide, designated XIP (for σX inducing peptide) in the supernatant of mature S. mutans cultures, suggesting that XIP functions as an intercellular signal for competence (Khan et al., 2012; Wenderska et al., 2012; Desai et al., 2012).
Consequently, many authors describe ComRS as a quorum sensing system in which XIP acts as the diffusible signal to drive comX (Federle; Morrison, 2012; Johnston et al., 2014; Fontaine et al., 2014; Wahl et al., 2014; Haustenne et al., 2015; Khan et al., 2016). In this view, S. mutans synthesizes ComS and exports its cleaved form, XIP. Other cells import environmental XIP via the oligopeptide permease Opp, detect it through the intracellular receptor ComR, and the ComR/XIP complex stimulates comS, comX and competence.
This view raises several questions however. The first is why the competence regulatory pathway employs two sequential quorum sensing signals, CSP and XIP. How do the CSP and XIP signals interact to trigger competence? What can the second signal contribute that the first signal cannot? In addition, the view of XIP as an extracellular feedback signal leaves unanswered the question of how CSP induces a bimodal comX response. This bimodality is important because it may generate heterogeneity in other behaviors, such as persistence and lysis, in addition to competence (Leung et al., 2015). A key reason that the origin of bimodality in comX activity remains unresolved is that researchers still lack detailed knowledge of the mechanism by which CSP and comCDE stimulate comX. This lack of clarity has invited a focus on comCDE and the bacteriocins regulated by comCDE as origins of the bimodal behavior of comX. Some authors have proposed that the upstream regulator ComE participates in a noisy feedback loop that allows some cells to stimulate ComE-P (Lemme et al., 2011; Federle; Morrison, 2012). Autofeedback mechanisms operating intracellularly can amplify the effects of noisy gene expression and lead to bimodal expression patterns in a population of cells (Ferrell, 2002; Dubnau; Losick, 2006). Such a mechanism could lead to bimodal stimulation of the bacteriocin pathway and consequently heterogeneous expression of comX and genetic competence in a subpopulation of cells (Federle; Morrison, 2012). Other authors have proposed that heterogeneous production of bacteriocins in response to CSP creates membrane pores that facilitate environmental XIP entry into some, but not all, cells (Reck et al., 2015).
However these models have shortcomings. For example, heterogeneous production of bacteriocin would not explain bimodality – a particular kind of heterogeneity – in competence activation. There are other problems as well. First, the activation of comE in a population of cells is not necessarily bimodal following stimulation by CSP, and the activation of the bacteriocin cipB that is immediately regulated by comCDE is certainly not bimodal (Son et al., 2015a). This tends to undermine comCDE as an origin of bimodality. Second, we have shown (Son et al., 2012) that the presence of an intact comS (encoding the XIP precursor) is absolutely required for bimodal response to CSP; consequently XIP supplied exogenously does not elicit a bimodal response from a comS deletion strain. Interestingly also, we found that the oligopeptide permease gene opp (which admits XIP) is required for comX to respond to exogenous XIP, but it is not required for CSP-induced bimodal response. These findings are difficult to rationalize under either of the scenarios where bimodality originates upstream of the ComRS system. An alternative model that unifies the experimental data is needed.
Single cell studies conducted with microfluidic control of the extracellular environment reveal richer patterns in the bimodality (Son et al., 2012; Son et al., 2015a). These microfluidic studies showed that exogenously-added XIP induces comX population-wide, or unimodally. In fact they showed an unexpected connection between (1) the identity (CSP or XIP) of the exogenous signal provided to the cells, (2) the composition of the growth medium, and (3) heterogeneous behavior of comX (FIGURE 2). When cells grow in a medium that contains assorted small peptides (complex growth medium), exogenous CSP elicits the bimodal response from comX that is described above, while XIP elicits no response from comX. However in a medium that lacks the assorted peptides (chemically defined medium), XIP elicits a unimodal response, with all cells activating comX, while CSP elicits no response. Thus, the composition of the growth medium, via the presence or absence of assorted small peptides, determines the effective signal (CSP or XIP) and the unimodal vs. bimodal behavior of comX.
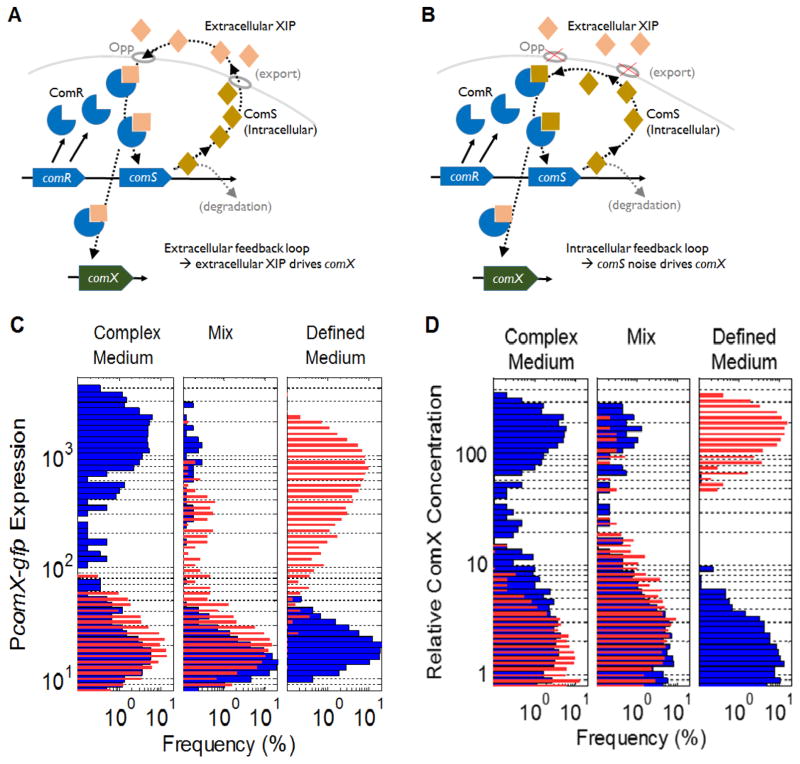
FIGURE 2.
(A)–(B): Model for two modes of ComRS function. (A) In the case of purely extracellular feedback, the cell exports ComS and processes it to XIP which accumulates extracellularly as a quorum sensing signal. XIP enters the cell via the permease Opp and forms (with ComR) an activator complex for comS and comX. Supplying exogenous XIP to a population of S. mutans therefore activates comX in all cells, giving a unimodal distribution of comX activity. (B) In the purely intracellular feedback case the cell does not readily import or export ComS or XIP. However basal levels of endogenously produced ComS can interact with ComR to stimulate further comS activity, forming an intracellular feedback loop. Exogenous XIP then has little effect on comX activity. Stochasticity in basal ComS production leads to heterogeneous (bimodal) comX activity in the population.
(C) Microfluidic data show that growth medium controls signal and response dynamics of the competence circuit. In a medium containing assorted small peptides (complex medium), exogenous CSP (1 μM, blue) induces a bimodal distribution of comX activity (left) in a microfluidic flow chamber. Medium lacking such small peptides (defined medium) quenches comX response to CSP (right). However exogenous XIP (500 nM, red) produces a unimodal response from comX in defined medium (right) and no response in complex medium (left). A hybrid behavior appears in an admixture of 2–5% complex/95–98% defined medium (center), with a weakly bimodal response to either CSP or XIP. These data suggest that ComRS can operate with either extracellular (A) or intracellular (B) feedback, depending on the presence of assorted small peptides in the growth environment.
(D) Results of stochastic simulation of PcomX activity in the bimodal model. Model parameters and equations are given in the Supplemental Information. Histograms show activity of PcomX in an ensemble of cells as calculated in COPASI assuming stimulation with exogenous CSP (300 nM, red) or XIP (1 μM, blue). As described in the Supplemental Information, the change from complex (left) to defined medium (right) is modeled by faster importation of exogenous XIP, but a shorter lifetime for intracellular XIP (or ComS), in the defined medium.
Further, by finely adjusting the composition of the growth medium in a microfluidic flow chamber, the experimenter can tune comX activity from unimodal to bimodal, while also tuning the active (or effective) signal from XIP to CSP (FIGURE 2). Adding just 1–2% complex medium converts a unimodal XIP response to a bimodal response (Son et al., 2012). Interestingly, the cell must have an intact comS in order to exhibit the bimodal response and it must have an intact opp to exhibit the unimodal response (Supplemental FIGURE S1 and (Son et al., 2012)). In short, the presence of small peptides in the medium can tune the response of comX between two qualitatively different modes: a bimodal behavior that requires an endogenous ComS and a unimodal behavior that requires the XIP-importing permease.
3. Environmental tuning of feedback modes
The ComE and bacteriocin models for bimodality, described above, cannot readily explain the origin of these two behaviors. Instead we argue that the data make sense if ComRS can operate in two modes (Figure 2), either as an extracellular or an intracellular feedback loop (Son et al., 2012; Son et al., 2015a). We have constructed a simulation model (FIGURE 2 and Supplemental Information) of the ComRS system in which ComS acts as an intracellular positive feedback signal, stimulating its own production and thereby amplifying stochasticity in basal levels of ComS. In our modeling sufficient feedback strength can trigger ComRS autoactivation and hence comX expression, at least in some cells (bimodality). We hypothesize that the growth medium affects this behavior by modulating the strength of this feedback.
Consider an extracellular mode (FIGURE 2A), where XIP and ComS pass bidirectionally between the cell interior and the environment. Regardless of whether CSP is present to stimulate basal activity in ComRS, any ComS or XIP produced leaves the cell and does not feed autoactivation of the ComRS system or trigger comX. Consequently, CSP does not effectively stimulate comX. However, all cells in a population will import (via Opp) and detect (via ComR) exogenous XIP, leading to stimulation of comS and comX population-wide as long as cells carry opp and comR. Cells do not require a copy of comS to respond to environmental XIP.
Consider also an intracellular mode (FIGURE 2B) where ComS (or XIP) does not cross the cell membrane. Each individual cell retains and accumulates its own ComS (or XIP) and accordingly activates comX independently of other cells. Intracellular positive feedback in ComS production then amplifies cell-to- cell variability in ComS levels, leading to population-wide heterogeneity in the activation of the competence circuit (Dubnau; Losick, 2006). As long as CSP can, even if indirectly, stimulate basal ComS levels, the intracellular feedback model predicts heterogeneity in comX activation in response to CSP.
If small peptides in the growth medium alter the strength of positive feedback, then they will determine whether ComRS functions in the extracellular or intracellular mode. Two plausible mechanisms for such action are that (1) small peptides may interfere with XIP import through Opp, and (2) they may enhance the lifetime of endogenously produced ComS by inhibiting its degradation. The first mechanism could explain why exogenous XIP activates comX in defined medium but not in complex medium. The second mechanism could explain why CSP activates comX in complex medium but not in defined medium. Both mechanisms would shift the balance between extracellular XIP and intracellular CSP as drivers of the comX circuit. As shown in the Supplemental FIGURE S4, neither of these mechanisms alone seems sufficient to explain all the data. Therefore our modeling includes both.
Stochastic simulations of this model in these two modes (FIGURE 2D) closely resemble the experimental data on single cell response in defined and complex growth media (FIGURE 2C). Simulations were performed using COPASI (Hoops et al., 2006) as described in the Supplemental Information.
This hypothesis makes useful predictions. For example, because bistability originates in comS autofeedback, a comS deficient strain should not exhibit bimodal expression of comX, regardless of growth medium or exogenous XIP vs CSP levels: even in a continuous flow chamber that prevents accumulation of environmental XIP via flow, the presence or absence of the comS gene should control comX bimodality. Microfluidic studies confirmed that deletion of comS eliminated bimodal comX activation (Supplemental FIGURE S1). This finding alone seems to rule out models for bimodality (Lemme et al., 2011; Federle; Morrison, 2012; Reck et al., 2015) originating in ComE or bacteriocin expression - upstream of ComRS.
The alternative hypothesis that bimodality originates in comDE implies that the regulatory targets of comE, such as cipB, should be bimodally activated when stimulated by CSP. However we find cipB is activated unimodally in a population stimulated by CSP, indicating that bimodality does not propagate downstream from comDE (Son et al., 2015a). Likewise the hypothesis that cell-to-cell variability in bacteriocin production leads to a variable susceptibility to exogenous XIP cannot explain why cells lacking opp are incapable of responding to exogenous XIP, yet they show a wild-type bimodal response to exogenous CSP: opp is required for the unimodal activation of comX but not for the bimodal activation. Conversely a native comS is required for the bimodal (CSP-driven) activation but not for a unimodal (exogenous XIP-drive) activation (Son et al., 2012). In short, the data link bimodality to an intracellular, rather than extracellular, ComS/XIP signal.
The model of FIGURE 2 also predicts that deletion of opp (encoding the XIP permease) should not prevent exogenous CSP from eliciting a bimodal comX response, although it should prevent exogenous XIP from eliciting any comX response. It also predicts that exogenous CSP should have little effect on cells that are responding to exogenous XIP, and vice versa. Our data confirm both of these predictions (Son et al., 2012). Supplemental FIGURE S2 shows that CSP does not enhance the response to environmental XIP, in contradiction to the bacteriocin model. In addition we previously showed (Figure S5 of (Son et al., 2012)) that comX does not respond at all in a comS deletion strain that is stimulated with CSP+XIP in complex medium, as expected in the model of FIGURE 2.
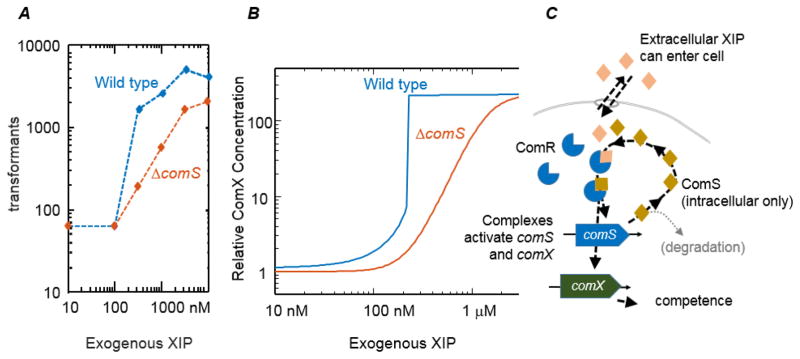
The model also provides insight into the curious finding (FIGURE 3A) that the unimodal response to exogenous XIP is slightly more sensitive when the cell has an intact comS. When provided exogenous XIP, cells with an intact comS gene transformed more efficiently than did cells lacking comS (Mashburn-Warren et al., 2010). Although this result is puzzling if ComS and XIP move freely in and out of the cell, it makes sense if XIP crosses much more readily than does ComS. Supplying exogenous XIP to a wild type cell then creates a hybrid situation in which extracellular XIP can stimulate comS and seed further activation of comS by autofeedback. Cells carrying intact comS are then expected to respond more strongly to exogenous XIP than would a comS deletion mutant. A COPASI simulation of this scenario generated results (FIGURE 3B) closely similar to the experimental data in FIGURE 3A. The Supplemental Information provides further details on the simulation.
FIGURE 3.
(A) Experimental data, adapted from (Mashburn-Warren et al., 2010), show that exogenous XIP (in defined medium) stimulates more transformation in the wild-type S. mutans than in a ΔcomS mutant. Studies by Mashburn-Warren et al. were performed in triplicate, leading to uncertainties (standard deviation) similar to the size of the plotting symbols.
(B) Simulation of a hybrid of the two feedback models in FIGURE 2, where extracellular XIP can cross the cell membrane but ComS cannot. Environmental XIP enters the cell and stimulates the intracellular ComS feedback loop, leading to greater activation of comS and comX in the wildtype (blue curve) than in ΔcomS (red curve).
(C) Diagram of hybrid model. Model details and parameters are given in Supplemental information.
Because it emphasizes an intracellular – rather than extracellular – feedback role for XIP (or ComS), the feedback model raises the question of whether XIP (or ComS) has a significant extracellular, cell-to-cell signaling function in addition to its intracellular function. XIP has been detected in media from S. mutans cultures (Khan et al., 2012; Desai et al., 2012; Wenderska et al., 2012), but its efficacy as a cell-to-cell signal has not been shown conclusively. Interestingly, a study of S. thermophilus found evidence that while that organism may transport its ComS peptide to its cell surface, it does not appear to release it extracellularly (Gardan et al., 2013). Future work is needed to assess the relative importance of intracellular vs extracellular modes of ComS and XIP signaling.
4. Additional sources of heterogeneity
Single-cell studies also show a finely tuned response to microenvironment variables such as pH. A microfluidic study applying fine pH gradients showed that while both CSP and XIP can induce comX activity at neutral pH, comX becomes much less responsive to both signals if the pH falls outside a narrow window, roughly one pH unit wide (Son et al., 2015b). By pH 6 the competence circuit has become almost unresponsive to these signals (FIGURE 4A). This behavior could lead to significant spatial heterogeneity in competence in an S. mutans biofilm, where the local pH can dip below pH 5 and may vary by as much as 1 pH unit over distances of as little as 10 μm (Xiao et al., 2012; Koo et al., 2013).
FIGURE 4.
(A) Response of comX to CSP (blue) and XIP (red) is acutely sensitive to local extracellular pH. In microfluidic studies the median comX activity in a population of S. mutans is maximal near pH 7 and declines sharply away from pH neutral conditions. Dashed lines show spline fit.
(B)–(C) Mechanisms downstream of comX introduce additional heterogeneity. When exogenous XIP is supplied to a dual reporter strain carrying PcomX-gfp and PcomY-rfp, all cells activate comX (as indicated by the green reporter fluorescence) (B), but only a subset also activates comY (indicated by the red fluorescence) (C) and other late competence genes. Thus an additional noisy switch lies between comX and comY.
Even the activation of comX does not ensure transformability (Lemme et al., 2011). FIGURE 4 shows that even among cells that produce ComX in response to CSP, only a subset also activate the downstream competence gene comY. These data imply that an additional stochastic switch acts downstream of ComX, most likely involving MecA/ClpPC, an ATP-dependent proteolytic system common to many bacterial species (Tian et al., 2013). MecA/ClpPC may regulate the levels of ComX and prevent most cells from accumulating sufficient active ComX to induce late competence genes. Such a mechanism appears to operate in S. thermophilus (Wahl et al., 2014). In S. mutans it may give rise to a stochastic and highly nonlinear relationship between the activation of transcription of comY and comX (Buchler; Cross, 2009).
Recent single cell studies have also revealed surprising regulatory feedback from comX to comE (Reck et al., 2015; Son et al., 2015a): comE shows exactly the same XIP and CSP dependence, the same medium- and signal-dependent bimodal/unimodal switching, and the same behaviors with deletion of comS as seen for comX. Therefore while ComE does participate in regulatory autofeedback (Lemme et al., 2011), it is a larger feedback loop that encompasses much of the competence regulon including the ComE-regulated bacteriocins, the ComRS local feedback loop, and ComX. CSP and ComS/XIP play inequivalent roles as competence signals as CSP stimulates the larger loop whereas ComS/XIP operates as a subsidiary feedback system inside the larger loop.
Such nested feedback loops occur in other biological regulatory networks, and may consist of purely negative feedback, mixed positive and negative feedback, or purely positive feedback (Brandman et al., 2005; Tsai et al., 2008; Ray; Igoshin, 2010; Mengel et al., 2012). Interestingly even a simple two-component system may be capable of either positive or negative feedback behavior (Ray; Igoshin, 2010). The presence of negative feedback is often used to drive oscillatory outputs, and the nesting of feedback loops may enhance control over oscillatory period or amplitude (Mengel et al., 2012). In pathways that contain only positive feedback, the nested architecture can provide rapid switching to an output state that is insensitive to noise in the upstream inputs (Brandman et al., 2005). The nested positive feedback in S. mutans may indicate that CSP acts as a quorum sensing stimulus to drive the global ComE-ComX feedback loop and stabilize its output, while XIP (and ComRS) acts intracellularly to generate rapid stochastic switching into either the on or off states of comX activation. Supplemental FIGURE S5 shows stochastic simulations of a competence model containing the nested feedback, and support the interpretation that the larger feedback loop stabilizes the activated state, especially at low or moderate levels of CSP stimulation.
Quorum sensing networks are conventionally interpreted as mechanisms for synchronizing bacterial behavior population-wide. However in the chemically heterogeneous environment of the biofilm the S. mutans regulatory network may potentially amplify, rather than reduce, heterogeneity in competence behavior. The regulation of transformation in S. mutans appears to involve a complex interaction between stochastic switches and interlocked feedback loops, acutely responsive to environmental cues. The system generates stochasticity, but can modulate between bimodal and unimodal behavior depending on microenvironment, and shuts down at extremes of pH. It seems entirely possible that in the biofilm environment this network could generate multiple qualitatively different behaviors in the regulatory systems of different subpopulations. At the same time the presence of global feedback may help to stabilize the multiple output states in the presence of noisy inputs.
Our findings also raise the question of whether ComS (or XIP) has a biological role as an intercellular (quorum) signal, in addition to serving as an intracellular feedback signal. If it is released extracellularly during lysis, for example, it could stimulate competence in nearby S. mutans cells in quorum-sensing fashion, even if the mechanism is unconventional for a quorum sensing.
The stability and precise definition of the growth conditions in a microfluidic flow chamber have little in common with the intricate and variable microenvironment in the biofilm. However, the regulatory circuits of S. mutans show great sensitivity to small changes in their environment. The more finely we control the extracellular inputs, the more complex the cellular behaviors we observe. The patterns of these behaviors give insights into the interpretation of the underlying mechanisms. Microfluidic control, together with single cell analysis, has provided valuable knowledge about the ways that the organism processes environmental cues to activate virulence.
Supplementary Material
Acknowledgments
We thank Dr. Robert A. Burne for many helpful suggestions and Dr. Sang Joon Ahn for providing bacterial reporter strains. We acknowledge funding support from NIDCR 1R01DE023339 and R21DE018826.
References
- Ahn S, Wen ZT, Burne RA. Effects of oxygen on virulence traits of Streptococcus mutans. J Bacteriol. 2007;189:8519–8527. doi: 10.1128/JB.01180-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn S, Kaspar J, Kim JN, Seaton K, Burne RA. Discovery of novel peptides regulating competence development in Streptococcus mutans. J Bacteriol. 2014;196:3735–45. doi: 10.1128/JB.01942-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspiras MB, Ellen RP, Cvitkovitch DG. ComX activity of Streptococcus mutans growing in biofilms. FEMS Microbiol Lett. 2004;238:167–174. doi: 10.1016/j.femsle.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Brandman O, Ferrell JE, Li R, Meyer T. Interlinked fast and slow positive feedback loops drive reliable cell decisions. Science. 2005;310:496–498. doi: 10.1126/science.1113834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchler NE, Cross FR. Protein sequestration generates a flexible ultrasensitive response in a genetic network. Molec Syst Biol. 2009;5:272–272. doi: 10.1038/msb.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai K, Mashburn-Warren L, Federle MJ, Morrison DA. Development of competence for genetic transformation by Streptococcus mutans in a chemically defined medium. J Bacteriol. 2012;194:3774–3780. doi: 10.1128/JB.00337-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau D, Losick R. Bistability in bacteria. Mol Microbiol. 2006;61:564–572. doi: 10.1111/j.1365-2958.2006.05249.x. [DOI] [PubMed] [Google Scholar]
- Dunn AK, Stabb EV. Beyond quorum sensing: The complexities of prokaryotic parliamentary procedures. Anal Bioanal Chem. 2007;387:391–398. doi: 10.1007/s00216-006-0730-9. [DOI] [PubMed] [Google Scholar]
- Dye BA, Tan S, Smith V, Lewis BG, Barker LK, Thornton-Evans G. Trends in oral health status, USA, 1988–1994 and 1999–2004. Vital Health Stat. 2007;11:1–92. [PubMed] [Google Scholar]
- Federle MJ, Morrison DA. One if by land, two if by sea: Signalling to the ranks with CSP and XIP. Mol Microbiol. 2012;86:241–245. doi: 10.1111/mmi.12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrell JE. Self-perpetuating states in signal transduction: Positive feedback, double-negative feedback and bistability. Curr Opin Cell Biol. 2002;14:140–148. doi: 10.1016/s0955-0674(02)00314-9. [DOI] [PubMed] [Google Scholar]
- Fontaine L, Wahl A, Fléchard M, Mignolet J, Hols P. Regulation of competence for natural transformation in streptococci. Infect, Genet, Evol. 2014:343–360. doi: 10.1016/j.meegid.2014.09.010. [DOI] [PubMed] [Google Scholar]
- Gardan R, Besset C, Gitton C, Guillot A, Fontaine L, Hols P, Monnet V. Extracellular life cycle of ComS, the competence-stimulating peptide of Streptococcus thermophilus. J Bacteriol. 2013;195:1845–1855. doi: 10.1128/JB.02196-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: From the natural environment to infectious diseases. Nat Rev Micro. 2004;2:95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- Haustenne L, Bastin G, Hols P, Fontaine L. Modeling of the ComRS signaling pathway reveals the limiting factors controlling competence in Streptococcus thermophilus. Front Microbiol. 2015;6:1413. doi: 10.3389/fmicb.2015.01413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoops S, Sahle S, Gauges R, Lee C, Pahle J, Simus N, Singhal M, Xu L, Mendes P, Kummer U. COPASI—a COmplex PAthway SImulator. Bioinformatics. 2006;22:3067–3074. doi: 10.1093/bioinformatics/btl485. [DOI] [PubMed] [Google Scholar]
- Hossain MS, Biswas I. An extracellular protease, SepM, generates functional competence-stimulating peptide in Streptococcus mutans UA159. J Bacteriol. 2012;194:5886–5896. doi: 10.1128/JB.01381-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston C, Martin B, Fichant G, Polard P, Claverys J. Bacterial transformation: Distribution, shared mechanisms and divergent control. Nat Rev Micro. 2014;12:181–196. doi: 10.1038/nrmicro3199. [DOI] [PubMed] [Google Scholar]
- Kaspar J, Ahn S, Palmer SR, Choi SC, Stanhope MJ, Burne RA. A unique ORF within the comX gene of Streptococcus mutans regulates genetic competence and oxidative stress tolerance. Mol Microbiol. 2015 doi: 10.1111/mmi.12948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan R, Rukke HV, Høvik H, Åmdal HA, Chen T, Morrison DA, Petersen FC. Comprehensive transcriptome profiles of Streptococcus mutans UA159 map core streptococcal competence genes. mSystems. 2016;1:e00038-15. doi: 10.1128/mSystems.00038-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan R, Rukke HV, Ricomini Filho AP, Fimland G, Arntzen MØ, Thiede B, Petersen FC. Extracellular identification of a processed type II ComR/ComS pheromone of Streptococcus mutans. J Bacteriol. 2012;194:3781–3788. doi: 10.1128/JB.00624-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo H, Falsetta ML, Klein MI. The exopolysaccharide matrix: A virulence determinant of cariogenic biofilm. J Dent Res. 2013;92:1065–73. doi: 10.1177/0022034513504218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemme A, Grobe L, Reck M, Tomasch J, Wagner-Dobler I. Subpopulation-specific transcriptome analysis of competence-stimulating-peptide-induced Streptococcus mutans. J Bacteriol. 2011;193:1863–1877. doi: 10.1128/JB.01363-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung V, Dufour D, Levesque CM. Death and survival in Streptococcus mutans: Differing outcomes of a quorum-sensing signaling peptide. Frontiers in Microbiology. 2015;6:1176. doi: 10.3389/fmicb.2015.01176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Tian X. Quorum sensing and bacterial social interactions in biofilms. Sensors. 2012;12:2519–2538. doi: 10.3390/s120302519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashburn-Warren L, Morrison DA, Federle MJ. A novel double-tryptophan peptide pheromone controls competence in Streptococcus spp. via an rgg regulator. Mol Microbiol. 2010;78:589–606. doi: 10.1111/j.1365-2958.2010.07361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui R, Cvitkovitch D. Acid tolerance mechanisms utilized by streptococcus mutans. Future Microbiology. 2010;5:403–417. doi: 10.2217/fmb.09.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengel B, Krishna S, Jensen MH, Trusina A. Nested feedback loops in gene regulation. Physica A: Statistical Mechanics and its Applications. 2012;391:100–106. [Google Scholar]
- Ray JC, Igoshin OA. Adaptable functionality of transcriptional feedback in bacterial two-component systems. PLoS Comput Biol. 2010;6:e1000676. doi: 10.1371/journal.pcbi.1000676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reck M, Tomasch J, Wagner-Döbler I. The alternative sigma factor SigX controls bacteriocin synthesis and competence, the two quorum sensing regulated traits in Streptococcus mutans. PLoS Genet. 2015;11:e1005353. doi: 10.1371/journal.pgen.1005353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senadheera D, Cvitkovitch DG. Quorum sensing and biofilm formation by Streptococcus mutans. In: Utsumi R, editor. Bacterial Signal Transduction: Networks and Drug Targets. Springer; New York: 2008. pp. 178–188. [DOI] [PubMed] [Google Scholar]
- Smith EG, Spatafora GA. Gene regulation in S. mutans. J Dent Res. 2012;91:133–141. doi: 10.1177/0022034511415415. [DOI] [PubMed] [Google Scholar]
- Son M, Shields R, Ahn SJ, Burne RA, Hagen SJ. Bidirectional signaling in the competence regulatory pathway of Streptococcus mutans. FEMS Microbiol Lett. 2015a;362:fnv159. doi: 10.1093/femsle/fnv159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son M, Ghoreshi D, Ahn S, Burne RA, Hagen SJ. Sharply tuned pH response of genetic competence regulation in Streptococcus mutans: A microfluidic study of environmental sensitivity of comX. Appl Environ Microb. 2015b;81:5622–5631. doi: 10.1128/AEM.01421-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son M, Ahn S, Guo Q, Burne RA, Hagen SJ. Microfluidic study of competence regulation in Streptococcus mutans: Environmental inputs modulate bimodal and unimodal expression of comX. Mol Microbiol. 2012;86:258–272. doi: 10.1111/j.1365-2958.2012.08187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X, Dong G, Liu T, Gomez ZA, Wahl A, Hols P, Li Y. MecA protein acts as a negative regulator of genetic competence in Streptococcus mutans. J Bacteriol. 2013;195:5196–5206. doi: 10.1128/JB.00821-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai TY, Choi YS, Ma W, Pomerening JR, Tang C, Ferrell JE. Robust, tunable biological oscillations from interlinked positive and negative feedback loops. Science. 2008;321:126–129. doi: 10.1126/science.1156951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl A, Servais F, Drucbert A, Foulon C, Fontaine L, Hols P. Control of natural transformation in salivarius streptococci through specific degradation of sigma-X by the MecA-ClpCP protease complex. J Bacteriol. 2014;196:2807–2816. doi: 10.1128/JB.01758-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters CM, Bassler BL. Quorum sensing: Cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol. 2005;21:319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- Wenderska IB, Lukenda N, Cordova M, Magarvey N, Cvitkovitch DG, Senadheera DB. A novel function for the competence inducing peptide, XIP, as a cell death effector of Streptococcus mutans. FEMS Microbiol Lett. 2012;336:104–112. doi: 10.1111/j.1574-6968.2012.02660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.