Abstract
Acute ozone exposure induces a classical stress response with elevated circulating stress hormones along with changes in glucose, protein and lipid metabolism in rats, with similar alterations in ozone-exposed humans. These stress-mediated changes over time have been linked to insulin resistance. We hypothesized that acute ozone-induced stress response and metabolic impairment would persist during subchronic episodic exposure and induce peripheral insulin resistance. Male Wistar Kyoto rats were exposed to air or 0.25 ppm or 1.00 ppm ozone, 5 h/day, 3 consecutive days/week (wk) for 13 wks. Pulmonary, metabolic, insulin signaling and stress endpoints were determined immediately after 13 wk or following a 1 wk recovery period (13 wk + 1 wk recovery). We show that episodic ozone exposure is associated with persistent pulmonary injury and inflammation, fasting hyperglycemia, glucose intolerance, as well as, elevated circulating adrenaline and cholesterol when measured at 13 wk, however, these responses were largely reversible following a 1 wk recovery. Moreover, the increases noted acutely after ozone exposure in non-esterified fatty acids and branched chain amino acid levels were not apparent following a subchronic exposure. Neither peripheral or tissue specific insulin resistance nor increased hepatic gluconeogenesis were present after subchronic ozone exposure. Instead, long-term ozone exposure lowered circulating insulin and severely impaired glucose-stimulated beta-cell insulin secretion. Thus, our findings in young-adult rats provide potential insights into epidemiological studies that show a positive association between ozone exposures and type 1 diabetes. Ozone-induced beta-cell dysfunction may secondarily contribute to other tissue-specific metabolic alterations following chronic exposure due to impaired regulation of glucose, lipid, and protein metabolism.
Keywords: Ozone, Stress response, Metabolism, Insulin resistance, Pancreatic beta cells
1. Introduction
In 2011 the International Diabetes Federation reported that the diabetes epidemic affected 366 million people worldwide, with 90% of the reported cases being classified as type 2 diabetes (TIID). Most mitigation efforts of TIID have focused on conventional risk factors such as sedentary lifestyle, obesity, and high-fat/caloric diet. Recently, epidemiological studies have demonstrated a positive association between air pollutants and chronic metabolic conditions (Pearson et al., 2010; Liu et al., 2013; Thiering and Heinrich, 2015). For instance, one study showed an overlap of diabetes cases reported by the Center for Disease Control and Prevention, and high concentrations of particulate matter in the United States in 2004 and 2005 (Pearson et al., 2010). Likewise, several studies have shown a positive association between ozone exposure, insulin resistance, and exacerbation of pre-existing metabolic conditions in the elderly (Stafoggia et al., 2010; Zanobetti and Schwartz, 2011; Kim and Hong, 2012). Another epidemiology study demonstrated that ozone-exposure in children was associated with increased incidence of type 1 diabetes (TID) (Hathout et al., 2006). The causality and thus, the underlying mechanisms for air pollutant-induced metabolic effects are unclear.
Long-term ambient particulate matter exposures are linked to the onset of metabolic risk factors for TIID such as adipose inflammation, mitochondrial dysfunction, hepatic endoplasmic reticulum stress and insulin resistance in mouse models of obesity (Xu et al., 2011; Mendez et al., 2013; Sun et al., 2013). It has been proposed that inhaled air pollutants may cause systemic insulin resistance by: 1) increased circulating proinflammatory cytokines and reactive byproducts, and/or 2) central nervous system (CNS) activation (Rajagopalan and Brook, 2012). While some air pollution studies have demonstrated systemic inflammation (Nurkiewicz et al., 2004; Finnerty et al., 2007; Mutlu et al., 2007; Niwa et al., 2008), many others show no increases in circulating cytokines (Campen et al., 2006; Kooter et al., 2006; Montero et al., 2006; Gottipolu et al., 2009). Therefore, it is likely that air pollutants may act through alternative mechanisms such as the CNS to elicit peripheral metabolic effects.
In some studies exposure to particulate matter has been shown to cause CNS inflammation, oxidative stress, and endoplasmic reticulum stress in the hypothalamus and other regions that regulate the autonomic nervous system (ANS) (Mokoena et al., 2015; Block and Calderon-Garciduenas, 2009; Levesque et al., 2011; Liu et al., 2014). Dysfunction or chronic activation of the sympathetic nervous system (SNS) and the hypothalamic-pituitary-adrenal (HPA)-axis have been implicated in hyperglycemia, obesity, β-cell dysfunction and insulin resistance (Cai, 2013; Nosadini et al., 1983; Surwit and Schneider, 1993; Friedman et al., 1996; Delaunay et al., 1997; Bjorntorp et al., 1999; Rask et al., 2001). Likewise, persistent increases in stress hormones, such as adrenaline (epinephrine) and corticosterone are postulated to induce insulin resistance through increased circulating non-esterified free fatty acids (NEFAs) (Nonogaki, 2000). It is suggested that the continuous supply of NEFAs to the tissues could elevate intracellular ceramide and diacylglycerol levels, which can interfere with insulin signaling through increased IRS-1/2 serine phosphorylation and decreased AKT-mediated glucose uptake (Arner and Langin, 2014; Perry et al., 2014). However, a potential link between the neuronal stress response and insulin resistance has not been examined in air pollution studies.
We have recently reported that acute ozone exposure in rats induces a classical stress response characterized by increases in circulating stress hormones, glucose, NEFAs, and branched chain amino acids (BCAA), suggesting the involvement of the SNS and activation of the HPA axis (Miller et al., 2015). The increases in circulating lipid metabolites and stress hormones were also noted in humans after an acute ozone exposure (Miller et al., 2016a). Interestingly, adrenalectomy prior to ozone exposure resulted in diminution of not only metabolic but also pulmonary effects in rats (Miller et al., 2016b). However, no insulin resistance was observed during an acute ozone exposure in our rat or human studies. This is in contrast to a study conducted by Vella et al. (2015), who demonstrated muscle insulin resistance in rats after 16 h ozone exposure. It is not known if subchronic ozone exposure could lead to long-lasting elevations in circulating stress hormones and lipids or cause peripheral insulin resistance.
The objective of the present study was to examine if weekly episodic ozone exposure would lead to persistent non-reversible increases in stress hormones and metabolic alterations and cause insulin resistance in the liver and muscle tissues in rats. In this study rats were exposed episodically to ozone 5 h/day × 3 consecutive days/week (wk) × 13 wks. Clinical tests for glucose tolerance and insulin resistance as well as secretion were performed intermittently during exposure. Metabolic alterations, hormone levels, lung injury/inflammation, and peripheral insulin signaling were assessed immediately after the last exposure or following a 1wk recovery period.
2. Materials and methods
2.1. Animals
Heathy male Wistar Kyoto (WKY) rats (250–300 g) were obtained from Charles River Laboratories Inc. (Raleigh, NC) at 10wks of age. All rats were housed 2/cage in polycarbonate cages in an isolated animal room maintained at 21 ± 1 °C, 50 ± 5% relative humidity and on a 12 h light/dark cycle. The US EPA NHEERL animal facility is approved by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). All animals received standard (5001) Purina pellet rat chow (Brentwood, MO) and water ad libitum unless otherwise stated. Animals were treated humanely and all efforts were made for alleviation of suffering. The US EPA NHEERL Animal Care and Use Committee approved the research protocol for this study.
2.2. Ozone generation and animal exposures
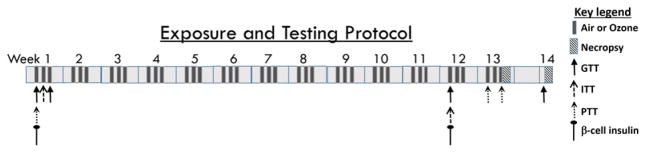
Ozone was generated from oxygen by a silent arc discharge generator (OREC, Phoenix, AZ) and its entry into the Rochester style “Hinners” chambers was controlled by mass flow controllers. The chambers relative mean temperature was 23.3 °C (74 °F) and humidity was 46%. Ozone concentrations were recorded continuously using the photometric API Model 400 ozone analyzer (Teledyne Instruments, San Diego, CA). Animals underwent whole body exposure to 1) filtered air, 2) 0.25 ppm ozone, or 3) 1.0 ppm ozone for 5 h/day, 3 consecutive days/wk for 13 wks. Animals were divided into two cohorts: cohort 1 and cohort 2. For cohort 1, necropsies were performed immediately post final exposure (13 wk). For cohort 2, necropsies were performed after 1 wk recovery in their home cages following the 13 wk exposure (13 wk + 1 wk recovery) to determine if ozone effects were reversible (Fig. 1).
Fig. 1.
Experimental design. Two cohorts of animals were exposed to: 1) filtered air, 2) 0.25 ppm or 3) 1.00 ppm ozone for 5 h/day for 3 consecutive days/wk. for 13 wks (n = 8–10 rats/group). Cohort 1 rats were necropsied immediately after the final exposure. Cohort 2 animals were necropsied after a 1wk recovery period following 13 wks of episodic exposure (13 wk + 1wk recovery). Metabolic testing time points are indicated by arrows for each test: glucose tolerance test (GTT: 1 wk-D1; 1 wk-D3; 12 wk-D1; 13 wk + 1wk recovery; the final GTT conducted six days following last exposure), insulin tolerance test (ITT: 1 wk-D2; 12 wk-D1), pyruvate tolerance test (PTT: 1 wk-D1; 13 wk-D1; 13 wk-D3) and β-cell insulin secretion test (1 wk-D1; 12 wk-D1). All tests were conducted immediately following exposure.
2.3. Intraperitoneal glucose tolerance test (GTT)
Prior to GTT, animals were fasted in air or ozone exposure chambers and during loading/unloading, except for the recovery group where food was withheld for ~6 h. Baseline blood glucose levels were measured by pricking the tip of the tail using a sterile 25 gauge needle and using Bayer Contour Glucometer (Leverkusen, Germany). Animals were then injected intraperitoneally with pharmaceutical grade glucose solution (20% D-glucose; Sigma-Aldrich, St. Louis. MI) at 2 g/10 ml/kg body weight. Glucose levels were measured at 30, 60, 90, and 120 min post glucose injection as reported in our previous studies (Miller et al., 2015, 2016b). GTT was conducted in cohort 1 animals during the 1st wk of exposure immediately following day 1 (1 wk-D1) or day 3 (1 wk-D3) of exposure, and during the 12th wk. immediately following day 1 of exposure (12wk-D1). GTT was also performed in cohort 2 following 1wk recovery (Fig. 1).
2.4. Blood collection for insulin measurement during GTT
During GTT after the first day ozone exposure of week 12 (12 wk-D1), blood samples were collected from the lateral tail vein for insulin measurement before injecting glucose (baseline) and 30 min post glucose injection. Rats were placed in restraint tubes that were mounted on a lab bench device during blood collection. In order to reduce tube stress, rats were acclimatized for 5 min to restrainer tubes for 3 consecutive days prior to the start of the blood collection. The tails were warmed for 1 min using a damp warm cloth and wiped clean. A heparinized 20 gauge sterile needle was inserted into the tail vein, and after discarding the first two drops, ~200 μl of blood sample was collected into serum seperator tubes. Hemostasis was then achieved by pressure using clean sterile gauze. These samples were spun at 3500 ×g for 10 min, serum aliquots were separated, and stored at −80 °C until insulin analysis. For analysis of acute ozone exposure effects on glucose-mediated β-cell insulin secretion, a separate cohort of animals (cohort 3, not shown in Fig. 1) were exposed to air or 1.0 ppm ozone for 4 h. Immediately post exposure, ~50–100 μl blood samples were collected prior to and 30 min post glucose injection from the tail prick. These samples were spun and serum aliquots were stored at −80 °C for insulin measurement as stated above.
2.5. Intraperitoneal insulin tolerance test (ITT)
Cohort 1 underwent ITT at wk 1, day 2 (1 wk-D2), and cohort 2 underwent ITT at wk 12, day 1 (12 wk-D1) (Fig. 1). Immediately following exposure, baseline glucose levels were measured from the tail prick as in the case of GTT. HumulinR (Lilly USA, LLC, Indianapolis, IN) was injected I.P. (1.0 IU diluted in 1 ml saline/kg body weight) and blood glucose was measured at 30, 60, 90 and 120 min post insulin injection in fasted rats (Fig. 1).
2.6. Intraperitoneal pyruvate tolerance test (PTT)
PTT was conducted in cohort 2 at 1 wk-D1, 13 wk-D1 and at 13 wk-D3 (Fig. 1). This procedure indirectly measures hepatic gluconeogenesis in conscious animals. As in the case with GTT and ITT, immediately following exposure, fasting baseline glucose levels were measured using a glucometer through a tail prick at baseline. Pharmaceutical grade sodium pyruvate (Sigma-Aldrich, St. Louis. MI) diluted in saline (1 g/2 ml/kg body weight) was then injected I.P. Blood glucose levels were measured at 30, 60, 90, and 120 min following injection of pyruvate (Fig. 1.).
2.7. Necropsy and sample collection
Cohort 1 animals were necropsied immediately following the 13 wk of exposure (13 wk), while cohort 2 animals were necropsied after a 1 wk recovery period (13 wk + 1 wk recovery) (Fig. 1). Half of the animals (n = 4–5) in each group were injected I.P. with 1.0 IU/kg body weight of HumulinR to accelerate insulin-mediated glucose uptake through AKT phosphorylation 10–15 min prior to necropsy. Rats were euthanized with an I.P. injection of >200 mg/kg sodium pentobarbital (Fatal-Plus diluted 1:1 with saline; Vortech Pharmaceuticals, Ltd., Dearborn, MI). All animals in both groups were fasted 6 h prior to necropsy.
2.8. Blood collection, complete blood count (CBC), and tissue collection
When animals were completely non-responsive to hind paw pinch after Fetal-Plus injection, blood samples were collected through the abdominal aorta followed by exsanguination. CBC was performed from EDTA containing blood tubes using a Beckman-Coulter AcT blood analyzer (Beckman-Coulter Inc., Fullerton, CA). All blood tubes were then centrifuged at 3500 rpm at 4 °C for 10 min. Plasma and serum samples were stored at −80 °C until further analysis. Hepatic and gracilis leg muscle tissues were collected, frozen in liquid nitrogen and stored at −80 °C for later assessment.
2.9. Bronchoalveolar lavage and bronchoalveolar lavage fluid (BALF) processing for pulmonary injury and inflammation assessment
The trachea was cannulated and then a suture was used to tie the left lung. The right lung was lavaged using Ca2+/Mg2+ free phosphate buffered saline (pH 7.4) equal to 28 ml/kg body weight (total lung capacity) × 0.6 (right lung is ~60% of total lung weight). Three in-and-out washes were performed using the same aliquot of buffer and BALF was transfered to ice until processing. Aliquots of BALF were used to determine total cell counts with a Z1 Coulter Counter (Coulter, Inc., Miami, FL) and cell differentials as described previously (Bass et al., 2013). Cell-free BALF aliquots were used to analyze total protein, albumin, γ-glutamyl transpeptidase (GGT) activity, and N-acetyl glucosaminidase (NAG) activity as described previously (Bass et al., 2013).
2.10. Serum and plasma analysis
The catecholamines, adrenaline and noradrenaline, in the EDTA plasma samples were measured using kits from Rocky Mountain Diagnostics (Colorado Springs, CO) per the manufacturer’s protocol. Serum corticosterone concentrations were analyzed employing an immunoassay kit from Arbor Assays (Ann Arbor, MI) per the manufacturer’s protocol. Total cholesterol and triglycerides were measured in serum samples using kits from TECO Diagnostics (Anaheim, CA), while high density lipoprotein (HDL) cholesterol and low density lipoprotein (LDL) cholesterol were measured using kits from Thermo Fisher Scientific, Inc. (Middletown, VA). All lipid kit protocols were modified for use on the Konelab Arena 30 system (Thermo LabSystems, Espoo, Finland). Serum NEFAs were measured by a coupled enzymatic reaction and the resultant hydrogen peroxide detection using a colorimetric probe as per the manufacturer’s protocol (Cell Biolabs, Inc., San Diego, CA). BCAA were measured in serum using an ELISA kit and protocol based on chemiluminescence detection (Abcam, Cambridge, MA). Insulin serum levels were detected using rat-specific chemiluminescence assay kit (Millipore, Billerica, MA) via manufacturer’s instructions. Serum levels of inflammatory cytokines were measured in selected groups of animals (13 wk; air and 1.00 ppm ozone groups) using rat-specific V-Plex Proinflammatory Panel 2 kit based on electrochemiluminescence detection (Meso Scale Discovery, Gaithersburg, MD) via manufacturer’s instructions.
2.11. Insulin signaling assessment in liver and muscle
Liver and muscle tissues from the 13 wk and 13 wk + 1 wk recovery groups were homogenized in lysis buffer containing protease and phosphatase inhibitors using a polytron-type homogenizer. Homogenates were centrifuged at 14,000 ×g at 4 °C for 10 min. The supernatants were analyzed for protein concentrations using Coomassie Plus protein assay kit (Pierce, Rockford, IL) and aliquots were stored at −80 °C for total and phosphoprotein assessment. Phosphorylated AKT (pAKT) and total AKT were analyzed using a rat-specific electrochemiluminescence assay (Meso Scale Discovery, Gaithersburg, MD) via manufacturer’s instructions. The pAKT level for each sample was normalized to total AKT and the relative fold change was calculated based on the air control for non-insulin injected animals at their respective time point.
2.12. General statistics
Graph Pad Prism (6.0) was used for all data analysis. GTT, ITT, and PTT data were analyzed using a two-way repeated measure multivariate analysis of variance (MANOVA). Each of these time-dependent metabolic measures were also analyzed for area under the curve by the trapezoidal method. All other measurements had two independent variables (exposure and time) and therefore these parameters were analyzed by two way analysis of variance (ANOVA) followed by Duncan’s multiple range test. Pairwise comparisons were performed on all data and the level of significance was set at p < 0.05.
3. Results
3.1. Acute and subchronic episodic ozone exposure induces glucose intolerance without impacting insulin tolerance
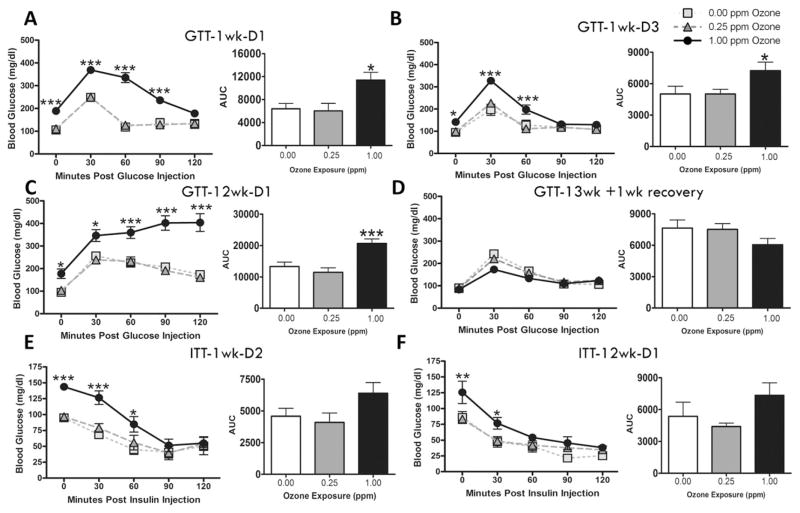
GTT was conducted immediately following exposure at 1 wk-D1, 1 wk-D3, 12 wk-D1 and 13 wk + 1 wk recovery to determine if ozone-induced glucose metabolic impairment would persist during subchronic exposure and if these effects were reversible following a recovery period. Ozone exposure at 1 wk-D1 induced fasting hyperglycemia and glucose intolerance at the 1.00 ppm dose (Fig. 2A). Ozone-induced hyperglycemia and severity of glucose intolerance appeared to be reduced at 1 wk-D3 compared to 1 wk-D1 (Fig. 2B). Ozone exposure led to marked increases in fasting glucose and glucose intolerance at 1.00 ppm at 12 wk-D1 as observed by higher glucose levels at all times after glucose injection and significantly increased area under the curve value (Fig. 2C), which appeared to be more pronounced relative to the 1 wk-D1 time point. A 1 wk recovery resulted in complete reversal of ozone-induced fasting hyperglycemia and glucose intolerance (Fig. 2D).
Fig. 2.
Acute and subchronic ozone exposures induce hyperglycemia and glucose intolerance without impairing insulin tolerance. GTT was conducted in animals at (A) week 1, day 1 (1 wk-D1), (B) week 1, day 3 (1 wk-D3), (C) week 12, day 1 (12 wk-D1), and (D) six days after the 13 wk final exposure (13 wk + 1 wk recovery). ITT was conducted at (E) week 1, day 2 (1 wk-D2) and (F) week 12, day 1 (12 wk-D1). Bar graphs show respective area under the curve (AUC) values for each test. Values indicate mean ± SEM (n = 8–10). *Indicates ozone effect when compared to matching air group (*p < 0.05, *p < 0.01, ***p < 0.001).
ITT was conducted to determine if subchronic ozone-induced glucose intolerance was accompanied with peripheral insulin resistance. Neither the 1 wk-D2 (Fig. 2E) nor 12 wk-D1 (Fig. 2F) assessment indicated impairment in peripheral insulin-mediated glucose clearance. Ozone-induced hyperglycemia was reversed after insulin injection, suggesting effective insulin-mediated glucose uptake.
3.2. Acute ozone increases hepatic gluconeogenesis
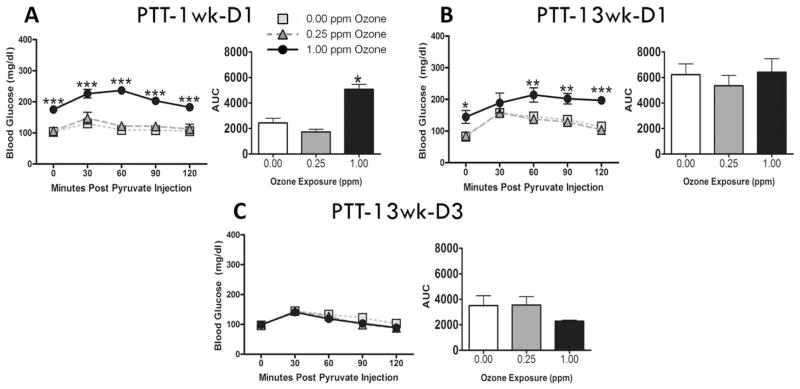
To determine if ozone-induced hyperglycemia resulted from liver gluconeogenesis, PTT was conducted in animals at 1 wk-D1 and 13 wk-D1. During 1 wk-D1, 1.00 ppm ozone-exposed animals were already hyperglycemic and showed a significant increase in blood glucose following pyruvate injection when compared to air or 0.25 ppm groups (Fig. 3A). The AUC further confirmed the stimulation of gluconeogenesis at this time point. In contrast, after a 13wk subchronic ozone exposure no significant increase in gluconeogenesis was observed (Fig. 3B & C).
Fig. 3.
Acute and subchronic ozone exposures induce hepatic gluconeogenesis. PTT was conducted in animals at (A) week 1, day 1 (1 wk-D1), (B) week 13, day 1 (13 wk-D1) and (C) week 13, day 3 (13 wk-D3). Bar graphs show respective area under the curve (AUC) values for each test. Values indicate mean ± SEM (n = 8–10). *Indicates significant ozone effect when compared to matching air group (*p < 0.05, **p < 0.01, ***p < 0.001).
3.3. Acute and subchronic episodic ozone exposure attenuates glucose-stimulated pancreatic β-cell insulin secretion
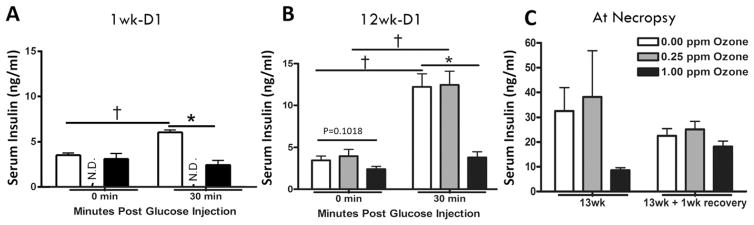
Impairment of pancreatic β-cell insulin secretion is another potential mechanism by which ozone could cause hyperglycemia and glucose intolerance. We evaluated the effect of acute and subchronic ozone exposure on β-cell function in rats by determining baseline and glucose-stimulated insulin release into the circulation. No differences were observed in serum insulin levels between air and 1.00 ppm ozone at baseline prior to I.P. glucose injection at 1 wk-D1 (Fig. 4A). However 30 min after glucose injection, insulin levels were significantly increased in air-exposed animals, whereas ozone-exposed rats showed no insulin increase in response to glucose (Fig. 4A). Neither baseline nor glucose-stimulated insulin release in the 0.25 ppm ozone-exposed rats were determined at this time point. Subchronic ozone exposure showed similar insulin responses compared to acute exposure. At 12 wk-D1, baseline serum insulin levels was trending to decrease with 1.00 ppm ozone exposure relative to the air group (p = 0.1018; Fig. 4B). Bolus glucose injection led to marked increases in serum insulin in air and 0.25 ppm ozone-exposed rats, suggesting a robust response of pancreatic β-cells to release insulin when circulating glucose levels were increased. This glucose-induced increase in insulin levels was completely abolished in rats exposed to 1.00 ppm ozone (Fig. 4B). Glucose-stimulated insulin secretion was not determined in rats after the 1 wk recovery period following 13 wks of ozone exposure. Although the insulin levels in 1.00 ppm exposed rats were markedly lower relative to air or 0.25 ppm groups at 13 wk, this difference did not reach statistical significance using a 2-way ANOVA method due to large intragroup variability and small group size (n = 3–5; Fig. 4C). However, when insulin mean values for the air and 1.00 ppm ozone were compared directly with an unpaired t-test, there was a significant (p < 0.05) decrease. This ozone-induced decrease was less pronounced after a 1wk recovery period (Fig. 4C).
Fig. 4.
Acute and subchronic ozone exposure inhibits glucose-stimulated β-cell insulin secretion. Serum insulin levels were measured immediately following 1 day air or 1.00 ppm ozone exposure (A; 1 wk-D1, a separate cohort (cohort 3) of rats was used for this testing and exposures were performed for only 4 h) and at 12 wk-D1 (B). Insulin levels were measured at baseline (0 min) and 30 min after I.P. injection of glucose (20% D-glucose; 2 g/kg). Insulin levels were not determined (N.D.) in 0.25 ppm ozone group during acute exposure. (C) Insulin levels were also determined immediately after necropsy at 13wk and 13wk + 1wk recovery. Values for A and B indicate mean ± SEM (n = 6–10) while values for C indicate mean ± SEM (n = 3–5). *Indicates significant ozone effect when compared to matching air group (*p < 0.05). † Indicates significant effect of glucose injection compared to baseline levels.
3.4. Subchronic episodic ozone exposure does not produce liver or muscle insulin resistance
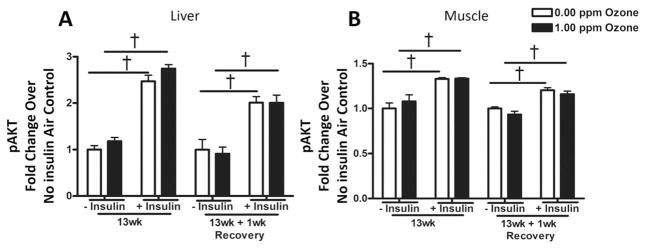
To measure tissue-specific insulin resistance, we measured pAKT (and total), which mediates insulin-induced cellular glucose uptake, at 13 wk and after a 13 wk + 1 wk recovery in the liver and muscle from air and 1.00 ppm ozone-exposed rats. Half the rats (n = 4–5) for each, 0.00 and 1.00 ppm exposure groups, were injected with insulin 10–15 min prior to necropsy to increase the sensitivity of detecting levels of pAKT in response to high insulin. The validity of the pAKT measurements was confirmed by significant increases in the levels of pAKT in rats injected with insulin prior to necropsy (Fig. 5). No changes were noted in the pAKT levels in the liver (Fig. 5A) or muscle (Fig. 5B) following ozone exposure in rats with or without insulin injection, suggesting that insulin-mediated glucose uptake was not altered by ozone exposure in these tissues.
Fig. 5.
The effect of subchronic ozone exposure and 1wk recovery on insulin signaling in liver and muscle tissue. Half of the rats from each group were I.P. injected with 1.0 IU insulin/kg body weight 10–15 min prior to blood collection (necropsy) to enhance phosphorylation of AKT (pAKT), which mediates glucose uptake through insulin receptors. The levels of total AKT and pAKT were measured in the (A) liver and (B) muscle extracts, and the relative pAKT levels were normalized to non-insulin injected air control group at each respective time point. Values indicate mean ± SEM (n = 4–5). †Indicates significance between insulin injected and non-insulin injected rats (p < 0.05).
3.5. Subchronic episodic ozone exposure alters circulating stress hormone levels
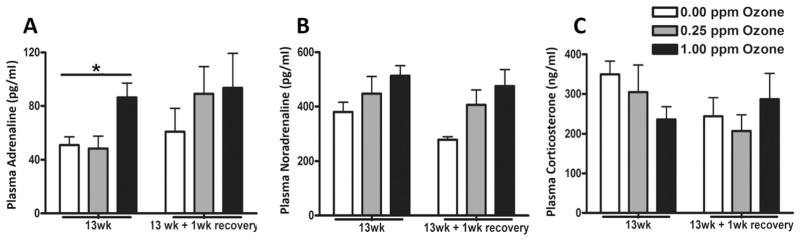
To determine if these stress hormones are still elevated following a subchronic episodic exposure, adrenaline, noradrenaline, and corticosterone plasma levels were measured in rats exposed to air or ozone for 13 wk and after a 1 wk recovery period. Serum adrenaline levels were significantly increased at 13 wk following 1.00 ppm ozone; however, no ozone effects were noted following a 1 wk recovery period (Fig. 6A). Noradrenaline (Fig. 6B) and corticosterone (Fig. 6C) changes were statistically insignificant between exposure groups.
Fig. 6.
Subchronic ozone exposure changes circulating stress hormones. Stress hormones were measured in plasma samples collected using EDTA as an anticoagulant in rats exposed to air or ozone (0.25 and 1.00 ppm) immediately after final exposure (13wk) and following a 1wk recovery. Stress hormones included (A) adrenaline, (B) noradrenaline and (C) corticosterone. Values indicate mean ± SEM (n = 8–10). *Indicates significant ozone effect when compared to matching air group (*p < 0.05).
3.6. The effect of subchronic ozone exposure on circulating proinflammatory cytokines and metabolic biomarkers
To determine if proinflammatory cytokines might be increased after subchronic exposure in WKY rats, we examined serum levels of cytokines at 13 wk. No significant increases were observed in IL-6, TNF-α or IL-1β; however, small increases were observed in serum IFN-γ, IL-4, and IL-10 after subchronic ozone exposure (Table 1).
Table 1.
Serum levels of proinflammatory cytokine biomarkers in WKY rats after subchronic exposure to air or 1.00 ppm ozone.
| Exposure
| ||
|---|---|---|
| Biomarker | Air | Ozone |
| IL-1β (pg/ml) | 63.37 ± 4.58 | 70.19 ± 7.74 |
| IL-4 (pg/ml) | 6.85 ± 0.15 | 7.32 ± 0.12** |
| IL-5 (pg/ml) | 23.96 ± 5.65 | 16.25 ± 2.28 |
| IL-6 (pg/ml) | 210.52 ± 9.01 | 229.62 ± 4.68 |
| IL-10 (pg/ml) | 81.00 ± 2.45 | 88.91 ± 1.38** |
| IFN-γ (pg/ml) | 26.99 ± 0.92 | 29.48 ± 0.49* |
| KC-GRO (pg/ml) | 191.21 ± 23.36 | 195.66 ± 34.85 |
| TNF-α (pg/ml) | 3.88 ± 0.35 | 4.57 ± 0.45 |
Serum samples obtained after 13 wk (cohort 1) air and 1.00 ppm ozone (mean ± SEM; n = 8) were analyzed using a Mesoscale Discovery Inc. cytokine multiplex panel kit.
Indicates significant ozone effect when compared to matching air group (*p ≤ 0.05, **p ≤ 0.01). Note that half of rats per group were injected with 1 U/kg insulin, 10–15 min prior to necropsy.
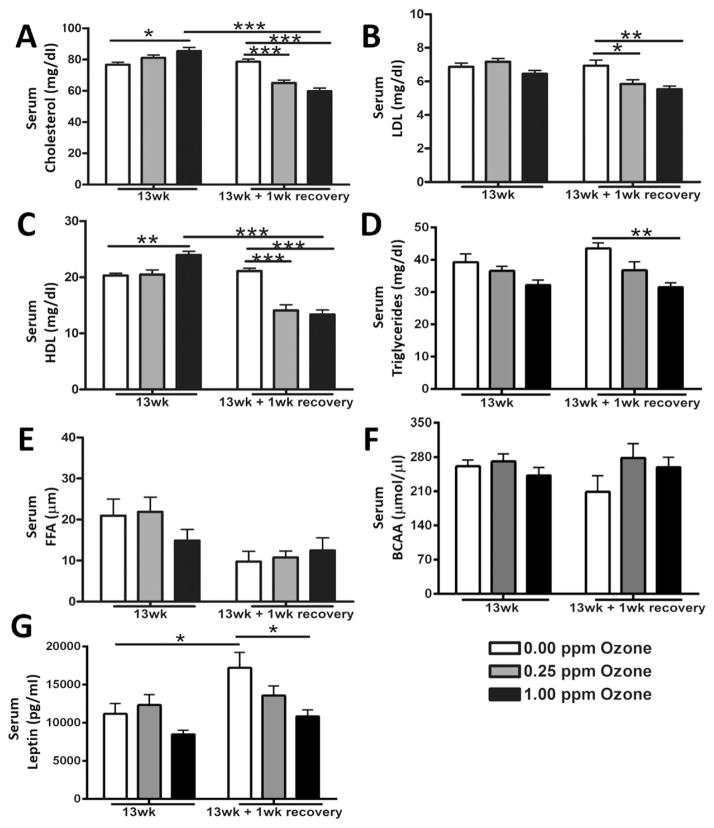
We have previously shown that acute ozone exposure elevated circulating total cholesterol, NEFAs and BCAA in rats. To determine if these changes would persist during a subchronic exposure, we evaluated the serum levels of these metabolic biomarkers. At 13 wk, 1.00 ppm ozone significantly increased total cholesterol (Fig. 7A). However, after a 1 wk recovery, serum cholesterol levels were decreased in both ozone-exposed groups when compared to air control (Fig. 7A). LDL was not significantly changed at the 13wk time point after ozone exposure, however, these levels were significantly decreased in ozone-exposed rats after the recovery period (Fig. 7B). HDL was significantly elevated in the 1.00 ppm ozone-exposed rats at 13 wk (Fig. 7C). In the recovery group, HDL serum levels were significantly lower in the ozone-exposed rats compared to the air (Fig. 7C). Triglycerides were not significantly affected by ozone at 13 wk but were found to be decreased in the 1.00 ppm ozone group after recovery (Fig. 7D). Neither the levels of NEFAs (Fig. 7E) nor BCAA (Fig. 7F) were significantly changed by ozone at any time point. There were no significant increases in leptin at 13 wk, with levels decreased after recovery in rats exposed to 1.00 ppm ozone (Fig. 7G).
Fig. 7.
Subchronic ozone-induced changes in serum metabolic markers. Changes in circulating metabolites and leptin were measured in rats exposed to air, 0.25 ppm, or 1.00 pm ozone at 13 wk and after 1wk recovery. The biomarkers included (A) total cholesterol, (B) low density lipoprotein (LDL), (C) high density lipoprotein (HDL), (D) triglycerides, (E) non-esterified fatty acids (NEFAs), (F) branched chain amino acids (BCAA) and (G) leptin. Values indicate mean ± SEM (n = 8–10). *Indicates differences between groups (*p < 0.05, **p < 0.01, ***p < 0.001).
3.7. Subchronic episodic ozone exposure induces lung injury and inflammation
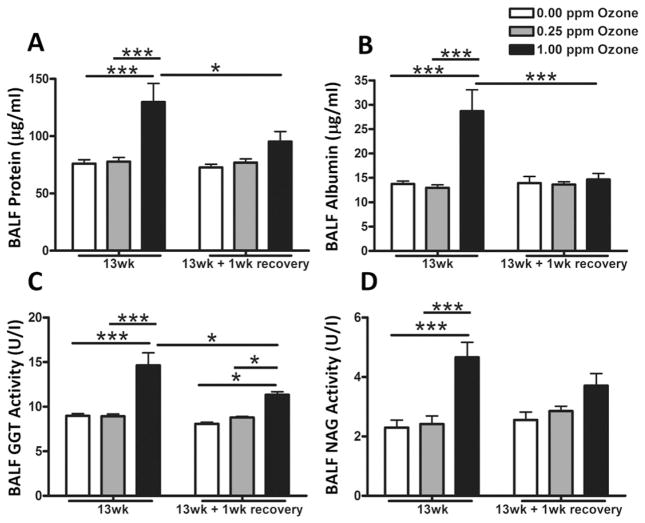
To determine if subchronic episodic exposure would cause persistent lung changes, BALF was evaluated for injury and inflammation biomarkers. Both total protein and albumin, markers of lung permeability, were significantly elevated at 1.00 ppm ozone when compared to air at 13wk but this effect was largely reversed following recovery (Fig. 8A & B). Rats exposed to 1.00 ppm ozone showed significant increases in BALF GGT activity at 13wk, which persisted when the animals were allowed a 1 wk recovery period (Fig. 8C). NAG activity was also increased in rats exposed to 1.00 ppm ozone at the 13 wk, however, this effect was attenuated after recovery (Fig. 8D).
Fig. 8.
Subchronic ozone induced lung injury as determined by the assessment of bronchoalveolar lavage fluid (BALF). Lung injury markers were determined immediately after air, 0.25 ppm or 1.00 ppm ozone exposure at 13 wk and following a 1 wk recovery. BALF was analyzed for lung injury markers: (A) total protein, (B) albumin, (C) γ-glutamyl transferase (GGT) activity and (D) N-acetyl glucosaminidase (NAG) activity. Values indicate mean ± SEM (n = 8–10). *Indicates differences between groups (*p ≤ 0.05, ***p ≤ 0.001).
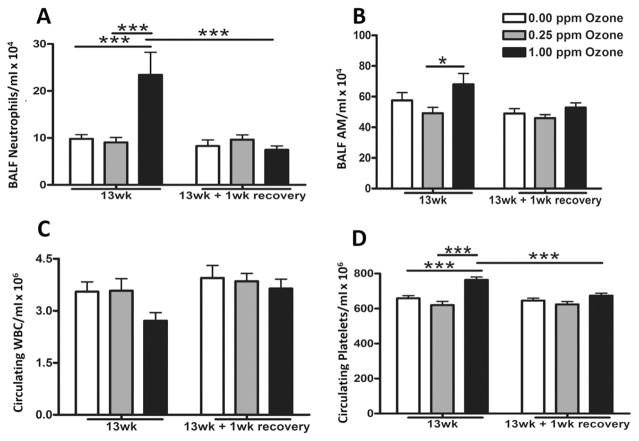
Lung neutrophilic inflammation was increased after 13 wk of ozone exposure compared to air and 0.25 ppm groups. This ozone effect on neutrophils was not observed in the recovery group (Fig. 9A). The number of BALF alveolar macrophages were also increased in rats exposed to 1.00 ppm ozone relative to 0.25 ppm at 13 wk with levels returning to baseline following 1 wk recovery (Fig. 9B). Interestingly, circulating white blood cells were not significantly decreased by the subchronic ozone exposure (Fig. 9C). 1.00 ppm ozone exposure for 13 wk increased circulating platelets, but this effect was reversed following the recovery (Fig. 9D).
Fig. 9.
Subchronic ozone induced lung inflammation as determined by the assessment of cells in bronchoalveolar lavage fluid (BALF). Lung inflammation was determined by analysis of cells in bronchoalveolar lavage fluid (BALF) immediately after air, 0.25 ppm or 1.00 ppm ozone exposure at 13 wk and following a 1 wk recovery. BALF total cell count and cell differentials were performed to quantify (A) neutrophils and (B) alveolar macrophages (AM). Circulating (C) white blood cells (WBC) and (D) platelets were quantified using a Beckman-Coulter AcT blood analyzer. Values indicate mean ± SEM (n = 8–10). *Indicates differences between groups (*p ≤ 0.05, ***p ≤ 0.001).
4. Discussion
We have previously shown that acute ozone exposure induces sympathetic and HPA-mediated systemic stress response characterized by hyperglycemia, glucose intolerance, lipidemia, and elevations in BCAA, FFA, and circulating stress hormones (Miller et al., 2015, 2016b). Humans are exposed episodically to ozone throughout their lifetime which may cause a chronic stress response activation. Chronic elevations of stress hormones have been implicated in metabolic dysfunction and are postulated to lead to insulin resistance (Kelly and Ismail, 2015). However, the persistence of chronic stress and metabolic derangement, and their contribution to insulin resistance have not been examined after subchronic ozone exposure. The main objectives of this study were to determine: (1) if ozone-induced acute metabolic and stress effects would persist after subchronic episodic exposure, and (2) if subchronic ozone exposure will lead to liver and muscle insulin resistance.
Our data show that, as observed after an acute exposure, 3 days/wk of subchronic episodic ozone exposure for 13 wk induced fasting hyperglycemia, glucose intolerance, and increased circulating cholesterols and adrenaline levels, but these effects were reversible upon a 1 wk recovery period. No ozone-induced changes were observed in serum FFA and BCAA, suggesting the likelihood of tolerance development to repeated weekly exposures. We show that no insulin resistance occurred in the liver or muscle tissues after a 13 wk ozone exposure. However, basal circulating insulin levels were decreased and glucose-stimulated pancreatic insulin release was abolished after subchronic ozone exposure, suggesting insulin insufficiency in ozone-exposed rats. Increases in lung injury and inflammation were still present at 13 wk but these effects were reversible after a 1 wk recovery period. Overall, our data demonstrate that ozone-induced pulmonary, metabolic and stress effects persist through 13wk episodic ozone exposure, however, these effects are reversible upon a short recovery of 1wk. Further, subchronic ozone exposure diminishes glucose-stimulated insulin release while decreasing the baseline levels over time without the impairment of peripheral insulin resistance.
Three potential mechanisms may explain hyperglycemia and glucose intolerance after acute and subchronic episodic ozone exposure: 1) increased glucose release from the liver through gluconeogenesis, 2) decreased glucose uptake by peripheral tissues, and 3) decreased release of insulin from pancreatic β-cells. We observed that acute ozone exposure increased hepatic gluconeogenesis but did not impair insulin-mediated glucose clearance from the circulation, indicating no peripheral insulin resistance. In contrast, hepatic gluconeogenesis was not altered during subchronic exposure while insulin effectively cleared circulating glucose. More importantly, both acute and subchronic ozone exposures abolished glucose-mediated β-cell insulin secretion, likely contributing to glucose intolerance.
Hepatic gluconeogenesis is activated by different physiological conditions, such as fasting or exercise, and is tightly regulated by the availability of gluconeogenic precursors and phosphorylation/expression of gluconeogenic enzymes. Moreover, gluconeogenesis is further regulated by the availability of insulin (Girard, 2006) and stress hormones (Altuna et al., 2006). It is postulated that insulin can directly or indirectly inhibit gluconeogenesis by specifically targeting hepatic glucose production or suppressing the release of glucagon from pancreatic α-cells, the main stimulator of gluconeogenesis. Insulin can also suppress the formation of gluconeogenic precursors through the inhibition of adipose lipolysis and skeletal muscle proteolysis (Girard, 2006). Adrenaline, through β2-adrenergic receptors, can enhance hepatic glucose output by increasing endogenous cyclic AMP (cAMP), which subsequently activates protein kinase A that stimulates fructose bisphosphatase 2 and cAMP response element binding dependent activation of phosphoenolpyruvate carboxykinase and inhibits phosphofructokinase 2 (Valera et al., 1994; Burgess et al., 2004). The ozone-induced increases in gluconeogenesis thus, could be explained by the increased levels of adrenaline together with decreased insulin levels and glucose-mediated insulin release.
Pancreatic β-cell insulin secretion is regulated at many levels (central and local) by factors including endocannabinoids (Jourdan et al., 2016), glucocorticoids (Kuo et al., 2015), circulating glucose and fatty acids (Oh, 2015), micro RNAs (Esguerra et al., 2014), adrenaline (Iwanir and Reuveny, 2008; Gibson et al., 2006), and many more. We observed that acute and subchronic ozone exposure diminished glucose-mediated pancreatic β-cell insulin secretion even though circulating glucose levels remained elevated in rats. Since this effect is associated with increased circulating adrenaline during acute (Miller et al., 2015) and chronic exposure to ozone as seen in this study, it is conceivable that diminished insulin secretion in the present study could be due to the effect of adrenaline on pancreatic β-cells. Catecholamines have been proposed to act through different members of the α-adrenergic family of receptors and impair insulin secretion through changes in cAMP and/or Ca2+ levels, hyperpolarization of the pancreatic β-cells or glucagon release from pancreatic α-cells (Peterhoff et al., 2003). It has been shown that glucose-induced ERK1/2 activation and subsequent insulin secretion are inhibited by adrenaline through the α2-adrenergic receptors (Gibson et al., 2006). However, it cannot be ascertained from our study if other factors played a role in inhibiting insulin secretion during ozone exposure. Since basal levels of insulin were not affected after acute exposure, but were decreased after subchronic ozone exposure, it is likely that the diminished insulin release could contribute to the observed persistent hyperglycemia and glucose intolerance following the 13 wk subchronic exposure.
Our previous study showed that subchronic ozone exposure is associated with increased levels of insulin in 24 month but not 4 month old Brown Norway rats (Gordon et al., 2013). As observed before, in this study too, acute or subchronic ozone exposure did not elevate insulin levels in young WKY rats. On the contrary, serum insulin levels decreased in ozone-exposed rats relative to air group (Fig. 4), suggesting that there might be a tendency to develop insulin deficiency if the length of exposures increases with age in rats. More importantly, our data show no peripheral insulin resistance in the muscle or liver after ozone exposure. Incidentally, exposure to ozone has been associated with TID in pregnancy and in children (Hathout et al., 2002, 2006; Beyerlein et al., 2015; Malmqvist et al., 2015). Our data show that insulin resistance is not the cause of ozone-induced hyperglycemia, glucose intolerance, or the activation of gluconeogenesis observed in our study. These findings contradict the Vella et al. (2015) study involving a 16 h ozone inhalation, which showed insulin resistance in the muscle as determined by activation of the c-Jun. N-terminal kinase pathway. This discrepancy could be due to the very long single exposure in the Vella paper as opposed to the episodic subchronic exposure in our study. The possibility that ozone may contribute to the susceptibility of TID by producing β-cell dysfunction requires further investigation.
Since long-term particulate matter exposures have been shown to induce systemic inflammation and peripheral insulin resistance (Rajagopalan and Brook, 2012), we examined a number of circulating cytokines after subchronic ozone exposure in this study. No increases were observed in IL-6, TNF-α or IL-1β. Small increases were observed in serum IFN-γ, IL-4, and IL-10 after subchronic ozone exposure in our study (Table 1). Our previous studies involving long-term ozone exposure have also failed to demonstrate increases in circulating proinflammatory cytokines such as IL-6 and TNF-α (Gordon et al., 2013; Bass et al., 2013). Since ozone-exposure was not associated with insulin resistance, the contribution of systemic inflammation in insulin resistance, which is observed after particulate matter exposure, could not be addressed in this study involving ozone.
Although we have shown that acute ozone exposure increases circulating FFA and BCAA (Miller et al., 2015), elevations in these circulating metabolites were not noted after subchronic ozone exposure. This could be due to attenuation of response upon episodic ozone exposure. Total cholesterol and HDL, however, were higher in rats after subchronic ozone exposure. As in the case with acute exposure, increased adrenaline could alter cholesterol metabolism (Kunihara and Oshima, 1983) leading to cholesterol increases in the circulation. The increases in cholesterols and triglycerides noted after the 13 wk ozone exposure and subsequent decreases following recovery may indicate lipid redistribution in peripheral tissues, which will be important to examine in future studies.
Our exposure protocol differed somewhat from previous subchronic studies we have done using ozone (Kodavanti et al., 2011; Gordon et al., 2013, 2014; Bass et al., 2013) in that this study involved 3 days/wk exposure as opposed to 1 or 2 days/wk exposure. This was done with the understanding that while the first two days of weekly ozone could produce pronounced pulmonary injury and systemic response (Miller et al., 2015), 3 consecutive days of exposure was expected to attenuate these ozone-induced effects. Attenuation, often referred to as adaptation or tolerance, of ozone-induced pulmonary injury, inflammation, and function after daily repeated exposure is fairly well demonstrated in humans (Jörres et al., 2000; Schelegle et al., 2003) and in animals (Kirschvink et al., 2002; van Bree et al., 2002). The degree of attenuation depends on the ozone concentration and the temporality of exposure and it has been shown that the adaptation to weekly 2–3 day ozone exposure is lost within 1wk upon re-exposure (Gordon et al., 2013, 2014). Rats exposed to ozone for 6 h/day, 2 days/wk led to partial attenuation relative to acute exposure in pulmonary and systemic metabolic effects when determined after 13 wk exposures (Snow et al., 2016; Bass et al., 2013), suggesting that weekly exposure for several wks will also dampen the degree of pulmonary and systemic effects observed after the first wk. In this study we wanted to determine if during weekly exposure, the effects observed on the first day would be attenuated on the third day. Further, to determine whether repeated induction of tolerance over 13 wk would lead to failure in this response. We noted that GTT and PTT when performed on the third day in a given week showed marked reduction in ozone-induced hyperglycemia, glucose intolerance as well as gluconeogenesis. The pulmonary injury and inflammation found after day 3 post 13 wks exposure was dampened relative to the effects after an acute exposure (Miller et al., 2015). This suggests that upon repeated weekly exposure some systemic and pulmonary responses are partially attenuated. The phenomenon of adaptation or tolerance might involve many factors including the type of response being examined, the degree of injury/inflammation at a given concentration, and the exposure protocol. Thus, the interpretation of any findings becomes highly complex. Nevertheless, one could speculate that central mechanisms might be playing an important role in the ozone adaptation phenomenon. Since we have shown that catecholamines and glucocorticoids are potentially involved in these ozone-induced pulmonary and metabolic effects (Miller et al., 2016b), it is likely that adaptive mechanisms may involve altered stress response signaling during subchronic exposure. Chronic stress activation can lead to the adaptive response “habituation”, involving neural plasticity and downregulation of glucocorticoid and β-adrenergic receptors in the central nervous system and in the periphery leading to reduced stress-mediated responses (Herman, 2013).
One of the critical questions we wanted to address is the reversibility of effects upon discontinuation of ozone exposure. The persistence of the effects, including insulin insufficiency, can have implications in chronic disease outcomes. As noted, pulmonary and systemic effects observed immediately following the 13 wk exposure were largely reversed, although circulating insulin tended to remain low in ozone exposed rats relative to air exposed rats following the 1 wk recovery. Interestingly, the circulating lipids showed a reversed pattern of change, suggesting that while most effects are not persistent upon discontinuation of exposure, lipid redistribution might remain an important outcome of chronic episodic exposure and needs to be further examined in light of liver and muscle diseases and their link to pulmonary complications.
In conclusion, we demonstrate that subchronic weekly episodic ozone exposure induces persistent pulmonary injury/inflammation, hyperglycemia, glucose intolerance, and increases in cholesterols. We further show that while peripheral insulin resistance did not occur by subchronic episodic ozone exposure, it had a major impact on β-cell insulin secretion in response to circulating glucose, likely mediated by increased circulating adrenaline levels. This, together with reduced circulating insulin levels after subchronic ozone exposure, supports a link between chronic ozone and TID. Despite the fact that most subchronic ozone-induced changes are reversible upon termination exposure, the question remains about lipid redistribution in peripheral tissues and chronic episodic pollution effects. Overall, these data provide further insight into the mechanism(s) of how subchronic ozone exposure may contribute to insulin insufficiency and produce other systemic impairments.
Footnotes
Disclaimer: The research described in this article has been reviewed by the National Health and Environmental Effects Research Laboratory, U.S. Environmental Protection Agency, and approved for publication. Approval does not signify that the contents necessarily reflect the views and policies of the Agency, nor does the mention of trade names of commercial products constitute endorsement or recommendation for use.
Acknowledgments: We thank Dr. Christopher Gordon and Dr. Colette Miller of the US EPA for their critical review of this manuscript. We also like to thank Ms. Virginia Bass (UNC, Chapel Hill, NC) for her help in the experiment. Late Mr. Dock Terrell is acknowledged for his help in performing ozone exposures. This research was supported by the US-EPA-University of North Carolina Cooperative Trainee Agreement (#CR-83515201), and UNC-NIEHS Toxicology Training Grant (T32 ES007126). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Transparency document
The Transparency document related to this article can be found in the online version.
References
- Altuna ME, Lelli SM, San Martin de Viale LC, Damasco MC. Effect of stress on hepatic 11beta-hydroxysteroid dehydrogenase activity and its influence on carbohydrate metabolism. Can J Physiol Pharmacol. 2006;10:977–984. doi: 10.1139/y06-046. [DOI] [PubMed] [Google Scholar]
- Arner P, Langin D. Lipolysis in lipid turnover, cancer cachexia, and obesity-induced insulin resistance. Trends Endocrinol Metab: TEM. 2014;25:255–262. doi: 10.1016/j.tem.2014.03.002. [DOI] [PubMed] [Google Scholar]
- Bass V, Gordon CJ, Jarema KA, Macphail RC, Cascio WE, Phillips PM, Ledbetter AD, Schladweiler MC, Andrews D, Miller D, Doerfler DL, Kodavanti UP. Ozone induces glucose intolerance and systemic metabolic effects in young and aged brown Norway rats. Toxicol Appl Pharmacol. 2013;273:551–560. doi: 10.1016/j.taap.2013.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyerlein A, Krasmann M, Thiering E, Kusian D, Markevych I, D’Orlando O, Warncke K, Jochner S, Heinrich J, Ziegler AG. Ambient air pollution and early manifestation of type 1 diabetes. Epidemiology. 2015;26:e31–e32. doi: 10.1097/EDE.0000000000000254. [DOI] [PubMed] [Google Scholar]
- Bjorntorp P, Holm G, Rosmond R. Hypothalamic arousal, insulin resistance and type 2 diabetes mellitus. Diabet Med. 1999;1:373–383. doi: 10.1046/j.1464-5491.1999.00067.x. [DOI] [PubMed] [Google Scholar]
- Block ML, Calderon-Garciduenas L. Air pollution: mechanisms of neuroinflammation and CNS disease. Trends Neurosciences. 2009;32:506–516. doi: 10.1016/j.tins.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess SC, Hausler N, Merritt M, Jeffrey FM, Storey C, Milde A, Koshy S, Lindner J, Magnuson MA, Malloy CR, Sherry AD. Impaired tricarboxylic acid cycle activity in mouse livers lacking cytosolic phosphoenolpyruvate carboxykinase. J Biol Chem. 2004;279:48941–48949. doi: 10.1074/jbc.M407120200. [DOI] [PubMed] [Google Scholar]
- Cai D. Neuroinflammation and Neurodegeneration in Overnutrition-Induced Diseases. TEM, Trends Endocrinology Metabolism. 2013:40–47. doi: 10.1016/j.tem.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campen MJ, McDonald JD, Reed MD, Seagrave J. Fresh gasoline emissions, not paved road dust, alter cardiac repolarization in ApoE−/− mice. Cardiovasc Toxicol. 2006:199–210. doi: 10.1385/ct:6:3:199. [DOI] [PubMed] [Google Scholar]
- Delaunay F, Khan A, Cintra A, Davani B, Ling ZC, Andersson A, Ostenson CG, Gustafsson J, Efendic S, Okret S. Pancreatic beta cells are important targets for the diabetogenic effects of glucocorticoids. J Clin Invest. 1997;100:2094–2098. doi: 10.1172/JCI119743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esguerra JL, Mollet IG, Salunkhe VA, Wendt A, Eliasson L. Regulation of pancreatic Beta cell stimulus-secretion coupling by microRNAs. Genes (Basel) 2014;4:1018–1031. doi: 10.3390/genes5041018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnerty K, Choi JE, Lau A, Davis-Gorman G, Diven C, Seaver N, Linak WP, Witten M, McDonagh PF. Instillation of coarse ash particulate matter and lipopolysaccharide produces a systemic inflammatory response in mice. J Toxicol Environ Health A. 2007;70:1957–1966. doi: 10.1080/15287390701549229. [DOI] [PubMed] [Google Scholar]
- Friedman TC, Mastorakos G, Newman TD, Mullen NM, Horton EG, Costello R, Papadopoulos NM, Chrousos GP. Carbohydrate and lipid metabolism in endogenous hypercortisolism: shared features with metabolic syndrome X and NIDDM. Endocr J. 1996;43:645–655. doi: 10.1507/endocrj.43.645. [DOI] [PubMed] [Google Scholar]
- Gibson TB, Lawrence MC, Gibson CJ, Vanderbilt CA, McGlynn K, Arnette D, Chen W, Collins J, Naziruddin B, Levy MF, Ehrlich BE, Cobb MH. Inhibition of glucose-stimulated activation of extracellular signal-regulated protein kinases 1 and 2 by epinephrine in pancreatic beta-cells. Diabetes. 2006;4:1066–1073. doi: 10.2337/diabetes.55.04.06.db05-1266. [DOI] [PubMed] [Google Scholar]
- Girard J. Insulin’s effect on the liver: “direct or indirect? continues to be the question. J Clin Invest. 2006;116:302–304. doi: 10.1172/JCI27743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon CJ, Jarema KA, Lehmann JR, Ledbetter AD, Schladweiler MC, Schmid JE, Ward WO, Kodavanti UP, Nyska A, MacPhail RC. Susceptibility of adult and senescent Brown Norway rats to repeated ozone exposure: an assessment of behavior, serum biochemistry and cardiopulmonary function. Inhal Toxicol. 2013;25:141–159. doi: 10.3109/08958378.2013.764946. [DOI] [PubMed] [Google Scholar]
- Gordon CJ, Johnstone AF, Aydin C, Phillips PM, MacPhail RC, Kodavanti UP, Ledbetter AD, Jarema KA. Episodic ozone exposure in adult and senescent Brown Norway rats: acute and delayed effect on heart rate, core temperature and motor activity. Inhal Toxicol. 2014;26:380–390. doi: 10.3109/08958378.2014.905659. [DOI] [PubMed] [Google Scholar]
- Gottipolu RR, Wallenborn JG, Karoly ED, Schladweiler MC, Ledbetter AD, Krantz T, Linak WP, Nyska A, Johnson JA, Thomas R, Richards JE, Jaskot RH, Kodavanti UP. One-month diesel exhaust inhalation produces hypertensive gene expression pattern in healthy rats. Environ Health Perspect. 2009;117:38–46. doi: 10.1289/ehp.11647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathout EH, Beeson WL, Nahab F, Rabadi A, Thomas W, Mace JW. Role of exposure to air pollutants in the development of type 1 diabetes before and after 5 yr of age. Pediatr Diabetes. 2002;3:184–188. doi: 10.1034/j.1399-5448.2002.30403.x. [DOI] [PubMed] [Google Scholar]
- Hathout EH, Beeson WL, Ischander M, Rao R, Mace JW. Air pollution and type 1 diabetes in children. Pediatr Diabetes. 2006;7:81–87. doi: 10.1111/j.1399-543X.2006.00150.x. [DOI] [PubMed] [Google Scholar]
- Herman JP. Neural control of chronic stress adaptation. Front Behav Neurosci. 2013;7:61. doi: 10.3389/fnbeh.2013.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwanir S, Reuveny E. Adrenaline-induced hyperpolarization of mouse pancreatic islet cells is mediated by G protein-gated inwardly rectifying potassium (GIRK) channels. Pflugers Arch. 2008;6:1097–1108. doi: 10.1007/s00424-008-0479-4. [DOI] [PubMed] [Google Scholar]
- Jörres RA, Holz O, Zachgo W, Timm P, Koschyk S, Müller B, Grimminger F, Seeger W, Kelly FJ, Dunster C, Frischer T, Lubec G, Waschewski M, Niendorf A, Magnussen H. The effect of repeated ozone exposures on inflammatory markers in bronchoalveolar lavage fluid and mucosal biopsies. Am J Respir Crit Care Med. 2000;161:1855–1861. doi: 10.1164/ajrccm.161.6.9908102. [DOI] [PubMed] [Google Scholar]
- Jourdan T, Godlewski G, Kunos G. Endocannabinoid regulation of β-cell functions: implications for glycemic control and diabetes. Diabetes Obes Metab. 2016;18:549–557. doi: 10.1111/dom.12646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly SJ, Ismail M. Stress and type 2 diabetes: a review of how stress contributes to the development of type 2 diabetes. Annu Rev Public Health. 2015;36:441–462. doi: 10.1146/annurev-publhealth-031914-122921. [DOI] [PubMed] [Google Scholar]
- Kim JH, Hong YC. GSTM1, GSTT1, and GSTP1 polymorphisms and associations between air pollutants and markers of insulin resistance in elderly Koreans. Environ Health Perspect. 2012;120:1378–1384. doi: 10.1289/ehp.1104406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschvink N, Fiévez L, Bureau F, Degand G, Maghuin-Rogister G, Smith N, Art T, Lekeux P. Adaptation to multiday ozone exposure is associated with a sustained increase of bronchoalveolar uric acid. Free Radic Res. 2002;36:23–32. doi: 10.1080/10715760210169. [DOI] [PubMed] [Google Scholar]
- Kodavanti UP, Thomas R, Ledbetter AD, Schladweiler MC, Shannahan JH, Wallenborn JG, Lund AK, Campen MJ, Butler EO, Gottipolu RR, Nyska A, Richards JE, Andrews D, Jaskot RH, McKee J, Kotha SR, Patel RB, Parinandi NL. Vascular and cardiac impairments in rats inhaling ozone and diesel exhaust particles. Environ Health Perspect. 2011;119:312–318. doi: 10.1289/ehp.1002386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooter IM, Boere AJ, Fokkens PH, Leseman DL, Dormans JA, Cassee FR. Response of spontaneously hypertensive rats to inhalation of fine and ultrafine particles from traffic: experimental controlled study. Part Fibre Toxicol. 2006;3:7. doi: 10.1186/1743-8977-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunihara M, Oshima T. Effects of epinephrine on plasma cholesterol levels in rats. J Lipid Res. 1983;24:639–644. [PubMed] [Google Scholar]
- Kuo T, McQueen A, Chen TC, Wang JC. Regulation of glucose homeostasis by glucocorticoids. Adv Exp Med Bio. 2015;872:99–126. doi: 10.1007/978-1-4939-2895-8_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque S, Surace MJ, McDonald J, Block ML. Air pollution & the brain: subchronic diesel exhaust exposure causes neuroinflammation and elevates early markers of neurodegenerative disease. J Neuroinflammation. 2011;8:105. doi: 10.1186/1742-2094-8-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Ying Z, Harkema J, Sun Q, Rajagopalan S. Epidemiological and experimental links between air pollution and type 2 diabetes. Toxicol Pathol. 2013;41:361–373. doi: 10.1177/0192623312464531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Fonken LK, Wang A, Maiseyeu A, Bai Y, Wang TY, Maurya S, Ko YA, Periasamy M, Dvonch T, Morishita M, Brook RD, Harkema J, Ying Z, Mukherjee B, Sun Q, Nelson RJ, Rajagopalan S. Central IKKβ inhibition prevents air pollution mediated peripheral inflammation and exaggeration of type II diabetes. Part Fibre Toxicol. 2014;11:53. doi: 10.1186/s12989-014-0053-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmqvist E, Larsson HE, Jönsson I, Rignell-Hydbom A, Ivarsson SA, Tinnerberg H, Stroh E, Rittner R, Jakobsson K, Swietlicki E, Rylander L. Maternal exposure to air pollution and type 1 diabetes–accounting for genetic factors. Environ Res. 2015;140:268–274. doi: 10.1016/j.envres.2015.03.024. [DOI] [PubMed] [Google Scholar]
- Mendez R, Zheng Z, Fan Z, Rajagopalan S, Sun Q, Zhang K. Exposure to fine airborne particulate matter induces macrophage infiltration, unfolded protein response, and lipid deposition in white adipose tissue. Am J Trans Res. 2013;5:224–234. [PMC free article] [PubMed] [Google Scholar]
- Miller DB, Karoly ED, Jones JC, Ward WO, Vallanat BD, Andrews DL, Schladweiler MC, Snow SJ, Bass VL, Richards JE, Ghio AJ, Cascio WE, Ledbetter AD, Kodavanti UP. Inhaled ozone (O)-induces changes in serum metabolomic and liver transcriptomic profiles in rats. Toxicol Appl Pharmacol. 2015;286:65–79. doi: 10.1016/j.taap.2015.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DB, Ghio AJ, Karoly ED, Bell LN, Snow SJ, Madden MC, Soukup J, Cascio WE, Gilmour MI, Kodavanti UP. Ozone exposure increases circulating stress hormones and lipid metabolites in humans. Am J Respir Crit Care Med. 2016a;193:1382–1391. doi: 10.1164/rccm.201508-1599OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DB, Snow SJ, Schladweiler MC, Richards JE, Ghio AJ, Ledbetter AD, Kodavanti UP. Acute ozone-induced pulmonary and systemic metabolic effects are diminished in Adrenalectomized rats. Toxicol Sci. 2016b;150:312–322. doi: 10.1093/toxsci/kfv331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokoena ML, Harvey BH, Viljoen F, Ellis SM, Brink CB. Ozone exposure of flinders sensitive line rats is a rodent translational model of neurobiological oxidative stress with relevance for depression and antidepressant response. Psychopharmacol (Berl) 2015;232:2921–2938. doi: 10.1007/s00213-015-3928-8. [DOI] [PubMed] [Google Scholar]
- Montero I, Orbe J, Varo N, Beloqui O, Monreal JI, Rodriguez JA, Diez J, Libby P, Paramo JA. C-reactive protein induces matrix metalloproteinase-1 and -10 in human endothelial cells: implications for clinical and subclinical atherosclerosis. J Am Coll Cardiol. 2006;47:1369–1378. doi: 10.1016/j.jacc.2005.10.070. [DOI] [PubMed] [Google Scholar]
- Mutlu GM, Green D, Bellmeyer A, Baker CM, Burgess Z, Rajamannan N, Christman JW, Foiles N, Kamp DW, Ghio AJ, Chandel NS, Dean DA, Sznajder JI, Budinger GR. Ambient particulate matter accelerates coagulation via an IL-6-dependent pathway. J Clin Invest. 2007;117:2952–2961. doi: 10.1172/JCI30639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa Y, Hiura Y, Sawamura H, Iwai N. Inhalation exposure to carbon black induces inflammatory response in rats. Circ J. 2008;72:144–149. doi: 10.1253/circj.72.144. [DOI] [PubMed] [Google Scholar]
- Nonogaki K. New insights into sympathetic regulation of glucose and fat metabolism. Diabetologia. 2000;43(5):533–549. doi: 10.1007/s001250051341. http://dx.doi.org/10.1007/s001250051341. [DOI] [PubMed] [Google Scholar]
- Nosadini R, Del Prato S, Tiengo A, Valerio A, Muggeo M, Opocher G, Mantero F, Duner E, Marescotti C, Mollo F, Belloni F. Insulin resistance in Cushing’s syndrome. J Clin Endocrinol Metab. 1983;57:529–536. doi: 10.1210/jcem-57-3-529. [DOI] [PubMed] [Google Scholar]
- Nurkiewicz TR, Porter DW, Barger M, Castranova V, Boegehold MA. Particulate matter exposure impairs systemic microvascular endothelium-dependent dilation. Environ Health Perspect. 2004;112:1299–1306. doi: 10.1289/ehp.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh YS. Mechanistic insights into pancreatic beta-cell mass regulation by glucose and free fatty acids. Anat Cell Biol. 2015;48:16–24. doi: 10.5115/acb.2015.48.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson JF, Bachireddy C, Shyamprasad S, Goldfine AB, Brownstein JS. Association between fine particulate matter and diabetes prevalence in the U.S. diabet. Care. 2010;33:2196–2201. doi: 10.2337/dc10-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry RJ, Samuel VT, Petersen KF, Shulman GI. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature. 2014;510(7503):84–91. doi: 10.1038/nature13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterhoff M, Sieg A, Brede M, Chao CM, Hein L, Ullrich S. Inhibition of insulin secretion via distinct signaling pathways in alpha2-adrenoceptor knockout mice. Eur J Endocrinol. 2003;149:343–350. doi: 10.1530/eje.0.1490343. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Brook RD. Air pollution and type 2 diabetes: mechanistic insights. Diabetes. 2012;61:3037–3045. doi: 10.2337/db12-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rask E, Olsson T, Soderberg S, Andrew R, Livingstone DE, Johnson O, Walker BR. Tissue-specific dysregulation of cortisol metabolism in human obesity. J Clin Endocrin Metab. 2001;86:1418–1421. doi: 10.1210/jcem.86.3.7453. [DOI] [PubMed] [Google Scholar]
- Schelegle ES, Walby WF, Alfaro MF, Wong VJ, Putney L, Stovall MY, Sterner-Kock A, Hyde DM, Plopper CG. Repeated episodes of ozone inhalation attenuates airway injury/repair and release of substance P, but not adaptation. Toxicol Appl Pharmacol. 2003;186:127–142. doi: 10.1016/s0041-008x(02)00026-1. [DOI] [PubMed] [Google Scholar]
- Snow SJ, Gordon CJ, Bass VL, Schladweiler MC, Ledbetter AD, Jarema KA, Phillips PM, Johnstone AF, Kodavanti UP. Age-related differences in pulmonary effects of acute and subchronic episodic ozone exposures in Brown Norway rats. Inhal Toxicol. 2016;28:313–323. doi: 10.3109/08958378.2016.1170910. [DOI] [PubMed] [Google Scholar]
- Stafoggia M, Forastiere F, Faustini A, Biggeri A, Bisanti L, Cadum E, Cernigliaro A, Mallone S, Pandolfi P, Serinelli M, Tessari R, Vigotti MA, Perucci CA, EpiAir G. Susceptibility factors to ozone-related mortality: a population-based case-crossover analysis. Am J Respir Crit Care Med. 2010;182:376–384. doi: 10.1164/rccm.200908-1269OC. [DOI] [PubMed] [Google Scholar]
- Sun L, Liu C, Xu X, Ying Z, Maiseyeu A, Wang A, Allen K, Lewandowski RP, Bramble LA, Morishita M, Wagner JG, Dvonch JT, Sun Z, Yan X, Brook RD, Rajagopalan S, Harkema JR, Sun Q, Fan Z. Ambient fine particulate matter and ozone exposures induce inflammation in epicardial and perirenal adipose tissues in rats fed a high fructose diet. Part Fibre Toxicol. 2013;10:43. doi: 10.1186/1743-8977-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surwit RS, Schneider MS. Role of stress in the etiology and treatment of diabetes mellitus. Psychosom Med. 1993;55:380–393. doi: 10.1097/00006842-199307000-00005. [DOI] [PubMed] [Google Scholar]
- Thiering E, Heinrich J. Epidemiology of air pollution and diabetes. Trends Endocrinol Metab TEM. 2015;26:384–394. doi: 10.1016/j.tem.2015.05.002. [DOI] [PubMed] [Google Scholar]
- Valera A, Pujol A, Pelegrin M, Bosch F. Transgenic mice overexpressing phosphoenolpyruvate carboxykinase develop non-insulin-dependent diabetes mellitus. Proc Natl Acad Sci U S A. 1994;91:9151–9154. doi: 10.1073/pnas.91.19.9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bree L, Dormans JA, Koren HS, Devlin RB, Rombout PJ. Attenuation and recovery of pulmonary injury in rats following short-term, repeated daily exposure to ozone. Inhal Toxicol. 2002;14:883–900. doi: 10.1080/08958370290084674. [DOI] [PubMed] [Google Scholar]
- Vella RE, Pillon NJ, Zarrouki B, Croze ML, Koppe L, Guichardant M, Pesenti S, Chauvin MA, Rieusset J, Géloën A, Soulage CO. Ozone exposure triggers insulin resistance through muscle c-Jun N-terminal kinase activation. Diabetes. 2015;64:1011–1124. doi: 10.2337/db13-1181. [DOI] [PubMed] [Google Scholar]
- Xu X, Liu C, Xu Z, Tzan K, Zhong M, Wang A, Lippmann M, Chen LC, Rajagopalan S, Sun Q. Long-term exposure to ambient fine particulate pollution induces insulin resistance and mitochondrial alteration in adipose tissue. Toxicol Sci. 2011;124:88–98. doi: 10.1093/toxsci/kfr211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanobetti A, Schwartz J. Ozone and survival in four cohorts with potentially predisposing diseases. Am J Respir Crit Care Med. 2011;184:836–841. doi: 10.1164/rccm.201102-0227OC. [DOI] [PMC free article] [PubMed] [Google Scholar]