Abstract
Objective
The neutrophil-to-lymphocyte ratio (NLR) has been used as a surrogate marker of systemic inflammation. We sought to investigate the association between NLR and wound healing in diabetic wounds.
Methods
The outcomes of 120 diabetic foot ulcers in 101 patients referred from August 2011 to December 2014 were examined retrospectively. Demographic, patient-specific, and wound-specific variables as well as NLR at baseline visit were assessed. Outcomes were classified as ulcer healing, minor amputation, major amputation, and chronic ulcer.
Results
The subjects’ mean age was 59.4 ± 13.0 years, and 67 (66%) were male. Final outcome was complete healing in 24 ulcers (20%), minor amputation in 58 (48%) and major amputation in 16 (13%), and 22 chronic ulcers (18%) at the last follow-up (median follow-up time, 6.8 months). In multivariate analysis, higher NLR (odds ratio, 13.61; P = .01) was associated with higher odds of nonhealing.
Conclusions
NLR can predict odds of complete healing in diabetic foot ulcers independent of wound infection and other factors.
Diabetic foot ulcer is a debilitating complication of diabetes mellitus and is the main cause of lower extremity amputations in the diabetic population.1–3 Predicting wound healing outcome can lead to better intervention and lower rates of amputation.4,5 Several tests have been shown to predict wound healing in the diabetic foot.5 The neutrophil-to-lymphocyte ratio (NLR) has recently been shown to predict overall and disease-specific mortality and chemotherapy response in cancer patients.6–8 NLR is also shown to be associated with complexity of peripheral arterial disease,9 systemic endothelial dysfunction,10 and cardiovascular diseases.11 No study has investigated the predicting value of NLR for wound healing outcome in diabetic foot ulcers. The objective of this study was to evaluate the association between NLR and treatment outcomes in diabetic foot ulcers.
METHODS
Patients
The protocol was approved by the Institutional Review Board of the Oregon Health & Science University, and informed consent was waived. Subjects referred to the vascular surgery clinic with diabetic foot ulcer from August 2011 to December 2014 were included in the study. After exclusion of those with missing data on their baseline neutrophil or lymphocyte count, 120 ulcers in 101 patients were studied. The ulcers were observed until complete healing, amputation (minor or major), or last clinical follow-up. The last follow-up date in chronic wounds was February 2016.
Definitions
Outcome of ulcers was classified as complete wound healing, minor amputation, major amputation, and chronic wounds. Ulcers that did not reach complete healing or amputation during the follow-up period were considered chronic wounds; ulcers that reached complete healing without amputation were considered complete healing. For analysis purposes, any amputation or chronic wounds were considered nonhealing ulcers. Amputations below the ankle were classified as minor amputations, and amputations higher than this level were defined as major amputations.
Wound infection was defined on the basis of the Wound, Ischemia, and foot Infection (WIfI) criteria or a report of a positive result of wound tissue culture.12
Peripheral arterial disease was determined by meeting one of the following criteria:
Ankle-brachial index <0.92
Toe-brachial index <0.6
Undergoing revascularization as part of current ulcer treatment
Time to heal was measured from the baseline evaluation on referral.
Statistical analysis
Numerical data were summarized as mean ± standard deviation, and categorical variables were summarized as frequency (percentage). Independent samples t-test and one-way analysis of variance were used to compare mean values between two and more than two groups, respectively. In cases of significantly different variances between groups (determined by the Bartlett test for equal variances), the analysis of variance test was replaced with a W test. To evaluate univariate and multivariate associations between NLR and wound healing, binomial generalized estimating equations logit models were constructed with healed/not healed wounds as outcome variable. There were multiple observations in some cases, so we set the patients’ unique id as the identifier cluster variable. Then, standardized values of NLR and other numerical variables were entered in generalized estimating equation models to cope with the inherent patient effect on the results. The multivariate model was adjusted for patients’ age, gender, body mass index, smoking status, type of diabetes, systolic blood pressure, serum glucose levels, wound age, wound infection, affected limb, and presence of peripheral arterial disease on referral. We determined the optimal cutoff value for NLR for predicting ulcer outcome with maximum sensitivity and specificity using a nonparametric receiver operating characteristic curve by Youden method. All analyses were done using Stata InterCooled 14.1 for Windows (StataCorp LP, College Station, Tex). P value < .05 was considered significant.
RESULTS
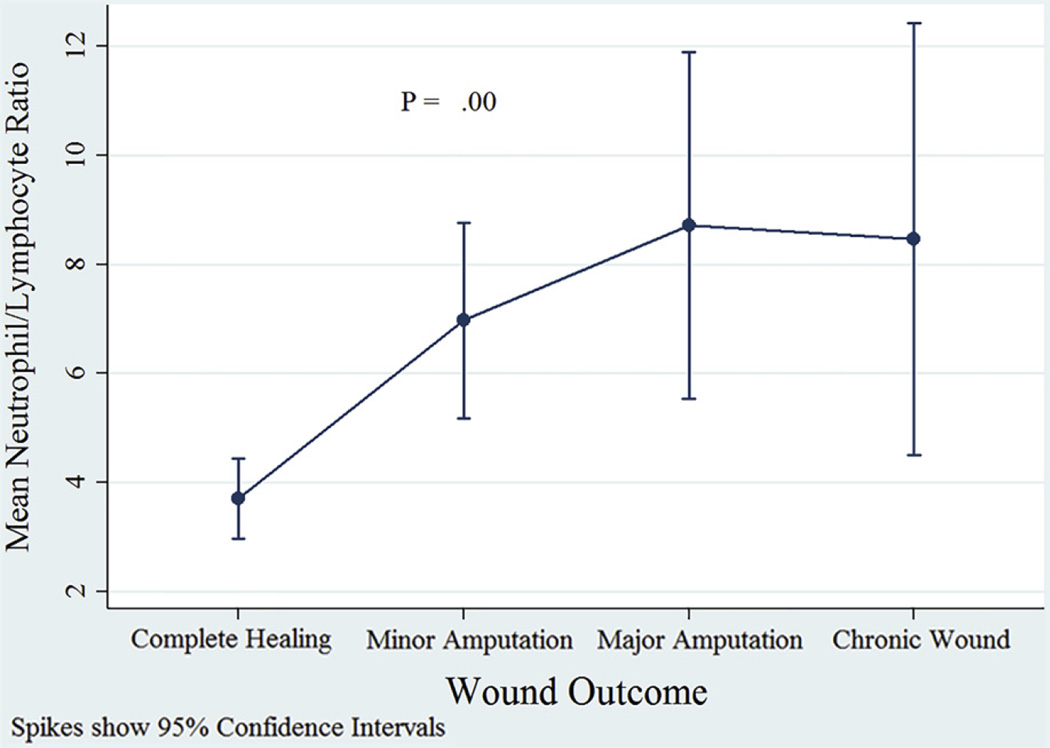
Table I shows the baseline characteristics of the study population and a comparison with excluded subjects. The patients’ mean age was 59.4 ± 13.0 years, and 67 (66%) of them were male (male-to-female ratio, 2:1). Of the 120 ulcers, 24 (20%) healed, 58 (48%) underwent minor amputation, 16 (13%) underwent major amputation, and 22 (18%) became chronic wounds. Indications for major amputations were as follows: osteomyelitis (n = 5 [31%]), sepsis (n = 2 [12.5%]), wound infection (n = 2 [12.5%]), nonhealing ulcer (n = 4 [25%]), and gangrene (n = 3 [19%]). Indications for minor amputations were as follows: osteomyelitis (n = 16 [28%]), wound infection (n = 13 [22%]), nonhealing ulcer (n = 15 [26%]), and gangrene (n = 14 [24%]). Median time to heal in the complete healing group was 4.3 (0.3–19.5) months, and median follow-up time in the nonhealing group was 6.8 (0.3–50.1) months. Mean NLR was 3.70 ± 1.75, 6.97 ± 6.82, 8.72 ± 5.97, and 8.46 ± 8.94 in complete healing, minor amputation, major amputation, and chronic wounds, respectively (P = .00, W test; Fig 1).
Table I.
Baseline characteristics of the study population and comparison with excluded subjects
| Study populationa |
Excluded populationa |
P valueb | |
|---|---|---|---|
| Patients | (n = 101) | (n = 38) | |
| Age, years | 59.4 ± 13.0 | 64.0 ± 12.4 | .06 |
| Gender | |||
| Female | 34 (34) | 14 (37) | .72 |
| Male | 67 (66) | 24 (63) | |
| BMI, kg/m2 | 33.3 ± 10.3 | 31.0 ± 5.8 | .10 |
| Type of DM | |||
| Type 1 | 18 (18) | 6 (16) | .78 |
| Type 2 | 83 (82) | 32 (84) | |
| Smoking status | |||
| Never smoked | 37 (37) | 12 (32) | .75 |
| Past smoker | 38 (38) | 17 (45) | |
| Current smoker | 25 (25) | 9 (23) | |
| Ulcers | (n = 120) | (n = 44) | |
| Wound age, days | 103.8 ± 213.2 | 100.4 ± 157.4 | .91 |
| Affected side | |||
| Right | 58 (48) | 23 (52) | .65 |
| Left | 62 (52) | 21 (48) | |
| Infection | |||
| Yes | 70 (58) | 17 (39) | .02 |
| No | 50 (42) | 27 (61) | |
| Peripheral arterial diseasec |
|||
| Yes | 62 (52) | 26 (59) | .40 |
| No | 58 (48) | 18 (41) | |
| Vascular intervention | |||
| Yes | 32 (27) | 11 (25) | .83 |
| No | 88 (73) | 33 (75) | |
| NLR | 6.82 ± 6.66 | ||
| Blood glucose on referral, mg/dL |
209.7 ± 124.5 | 162.1 ± 83.6 | .03 |
| Systolic blood pressure on referral, mm Hg |
133.3 ± 23.9 | 132.1 ± 18.6 | .77 |
| Outcome | |||
| Complete healing | 24 (20) | 12 (27) | .55 |
| Minor amputation | 58 (48) | 20 (45) | |
| Major amputation | 16 (14) | 3 (7) | |
| Chronic wound | 22 (18) | 9 (21) | |
| Time to heal, months | 7.3 ± 6.2 | 5.9 ± 4.3 | .48 |
| Chronic wound follow-up time, months |
12.3 ± 13.6 | 7.0 ± 5.2 | .13 |
BMI, Body mass index; DM, diabetes mellitus; NLR, neutrophil-to-lymphocyte ratio.
Numerical data are summarized as mean ± standard deviation, and categorical measures are shown as frequency (percentage).
Numerical measures are compared with independent samples t-test, and frequencies are compared using χ2 test.
Defined as ankle-brachial index <0.92 or toe-brachial index <0.6 or vascular intervention for current ulcer treatment.
Fig 1.
A comparison of mean neutrophil-to-lymphocyte ratio (NLR) in different wound outcome groups; the P value shows the result of W test.
On association analysis, higher NLRs were significantly associated with higher rates of wound nonhealing in both univariate (odds ratio [OR], 5.30; 95% confidence interval [CI], 1.38–20.28; P = .01) and multivariate models (OR, 13.60; 95% CI, 1.87–98.91; P = .01). Table II shows univariate and multivariate-adjusted associations between wound healing and NLR.
Table II.
Univariate and multivariate-adjusted associations between wound healing and neutrophil-to-lymphocyte ratio (NLR)
| Univariate modela | Multivariate modela | |||
|---|---|---|---|---|
| OR (95% CI) | P valueb | OR (95% CI) | P valueb | |
| NLR | 5.30 (1.38–20.28) | .01 | 13.61 (1.87–98.91) | .01 |
| Patient factorsc | ||||
| Age, years | 0.73 (0.45–1.16) | .18 | 0.48 (0.22–1.07) | .07 |
| Gender, male vs female | 1.50 (0.60–3.75) | .39 | 1.89 (0.52–6.80) | .33 |
| Smoking, compared with nonsmoker | ||||
| Current smoker | 5.40 (1.11–26.34) | .04 | 10.32 (1.45–73.65) | .02 |
| Former smoker | 1.52 (0.58–3.99) | .40 | 1.28 (0.36–4.49) | .70 |
| BMI, kg/m2 | 0.86 (0.57–1.30) | .47 | 0.56 (0.29–1.08) | .08 |
| Type of diabetes, type 2 vs type 1 | 0.87 (0.26–2.85) | .81 | 5.23 (0.69–39.75) | .11 |
| Baseline plasma glucose, mg/dL | 1.51 (0.86–2.66) | .15 | 2.17 (0.78–6.05) | .14 |
| Peripheral arterial disease, yes vs no | 1.11 (0.45–2.79) | .81 | 5.12 (0.60–43.44) | .13 |
| Vascular intervention, yes vs no | 0.67 (0.25–1.75) | .41 | 0.59 (0.09–4.00) | .59 |
| Wound factorsc | ||||
| Side of wound, left foot vs right foot | 1.65 (0.67–4.09) | .28 | 4.09 (1.11–15.05) | .03 |
| Infection, yes vs no | 2.90 (1.15–7.32) | .02 | 2.74 (0.55–13.63) | .22 |
| Wound age, days | 1.19 (0.69–2.08) | .53 | 1.67 (0.74–3.79) | .22 |
| Wound size, extensive vs smalld | 0.92 (0.37–2.28) | .85 | 0.49 (0.12–1.92) | .30 |
| Wound depth, deep vs shallowd | 2.88 (1.05–7.88) | .04 | 3.06 (0.60–15.64) | .18 |
BMI, Body mass index; CI, confidence interval; OR, odds ratio.
Generalized estimation equation models with patients’ id as the cluster variable.
Statistically significant associations are shown in bold.
Standardized values of the numerical measures are applied in all models.
Based on the Society for Vascular Surgery Wound, Ischemia, and foot Infection (WIfI) classification system.
Data regarding glycated hemoglobin (HbA1c) levels are missing in 21 of 120 (17.5%) of the cases in our study. Therefore, we decided to include the closest fasting blood glucose levels to the baseline evaluation in the models. Looking at the associations between HbA1c and our main exposure (NLR) and outcome (healing) variables using the available data, HbA1c was a significant predictor of wound healing (OR, 1.59; 95% CI, 1.14–2.22; P = .01). However, it was not significantly associated with NLR values (β coefficient ± standard error, −0.18 ± 0.26; P = .50).
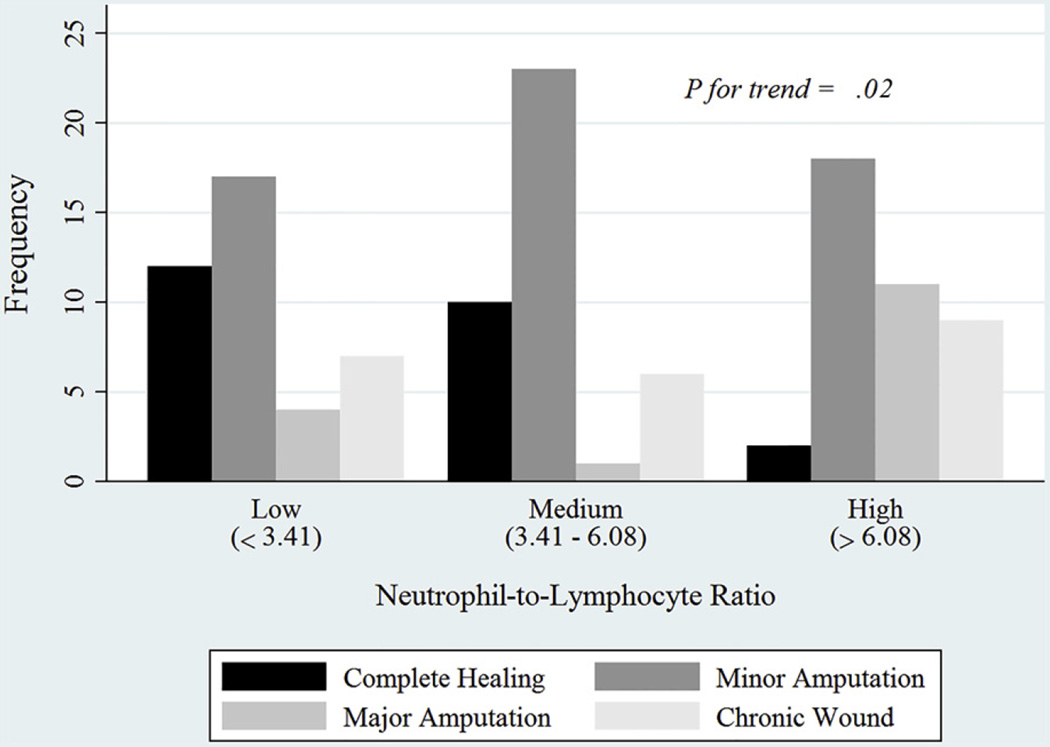
To reinforce the linear relationship between NLR and ulcer healing, NLR was categorized into three groups with equal numbers of observations. There was a significant trend of decreasing rate of complete wound healing with increasing orders of NLR categories. In fact, 30.0% of the ulcers in the lower NLR tertile healed; this rate was 25.0% for the middle NLR tertile and 5.0% for the higher tertile (P for trend, .02; Fig 2).
Fig 2.
Wound outcomes by tertiles of neutrophil-to-lymphocyte ratio (NLR).
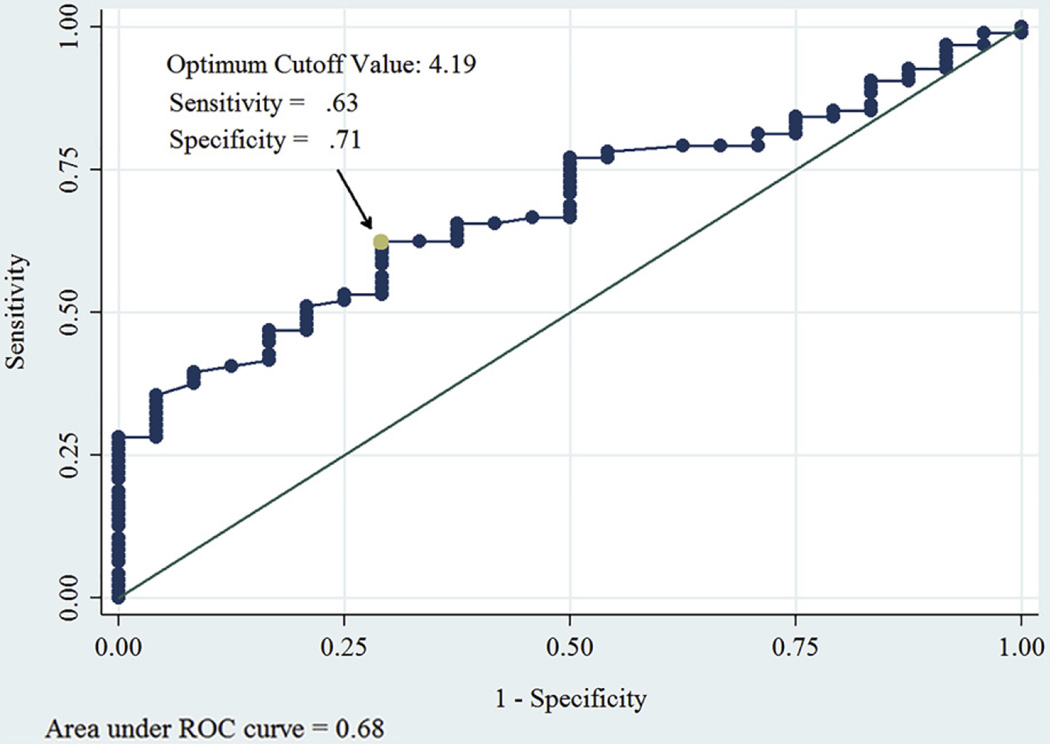
We then sought to determine the optimal NLR value to predict wound healing with maximal sensitivity and specificity. The optimal cutoff value of NLR for categorization of wound healing outcome (healed/not healed) was estimated as 4.19 (Youden J ± standard error, 0.33 ± 0.10). Sensitivity of the test at this threshold was 0.63, and the specificity was 0.71 (Fig 3).
Fig 3.
Receiver operating characteristic (ROC) curve showing the optimum cutoff value of neutrophil-to-lymphocyte ratio (NLR) to predict wound healing in diabetic foot ulcer.
DISCUSSION
Our study shows that an increased NLR is independently associated with higher odds of nonhealing in patients with diabetic foot ulcer even after adjusting for wound infection and many risk factors.
The NLR is a result of dividing the number of neutrophils to lymphocytes acquired by the differential blood cell count test. It is an inexpensive and more accessible way of evaluating immune system activity compared with many other wound-specific markers, such as matrix metalloproteinases or growth factors. NLR is also stable and resistant to environmental and physiologic changes, such as dehydration, physical activity, and blood sample handling, that affect the results of other markers.13 NLR has been shown to predict outcome in many diseases. It has been shown to have prognostic value to predict survival in terminal cancer14 and cardiac mortality in stable coronary artery disease.15 Lou et al16 and Shiny et al17 showed that NLR can be used as an indicator of systemic inflammation in diabetic patients. In other studies, an increased NLR has been associated with a high risk of critical limb ischemia with a high OR even after adjusting for main cardiovascular risk factors.18,19
The predictive value of NLR in diabetic wounds has not been well studied. Clinical and experimental studies demonstrate defects in neutrophil and lymphocyte activities in diabetes.20–22 NLR is a marker that evaluates both excitatory and inhibitory activities of the immune system. Inflammatory mediators from neutrophils cause endothelial damage; in contrast, lymphocytes have a modulatory effect on neutrophils as well as an antiatherosclerotic role.23 A high NLR can represent endothelial damage and dysfunction as a result of higher neutrophilic activity.24 This endothelial dysfunction can lead to worse outcomes in the setting of diabetic wounds.25 Recently, some studies have shown that NLR can be an indicating marker of infection and its severity.26,27 However, in our study, after adjusting for several risk factors including wound infection, blood glucose levels, and cardiovascular risk factors, NLR could predict complete healing with OR of 3.45 (P = .01). This result emphasizes the value of NLR in assessing inflammatory mechanisms other than infection in predicting outcome in diabetic foot ulcers.
Different threshold values of NLR have been proposed to predict outcome in different settings. The suggested values generally have been higher than 5. In one study, patients with ischemic limb ischemia and NLR >5 had higher mortality rates during 5 years of follow-up.28 In another study, NLR ≥5.25 predicted all-cause mortality with sensitivity and specificity of 69% and 71%, respectively, in patients with chronic critical limb ischemia.19 Also in another study, preprocedural NLR ≥5.2 predicted early (within 30 days after surgery) and midterm (total) amputation with sensitivity and specificity of 83% and 64% and 63% and 63%, respectively.29 Also, NLR >5.67 predicted mortality in acute ischemic stroke with sensitivity and specificity of 81.7% and 65.8%, respectively.30 Our study suggests that the optimal NLR value for predicting diabetic foot ulcer healing is 4.2; however, this value by itself cannot predict diabetic foot ulcer healing with high sensitivity and specificity (63% and 71%). This study is the first study to show the predictability of NLR for treatment outcome in diabetic foot ulcers. Appropriate inclusion and exclusion criteria make our results reliable and reproducible.
As limitations, our study has a relatively small sample size and is retrospective. The retrospective nature of this study also made it difficult to determine the exact size and depths of wounds, and therefore the full effect of wound size and depth may not be apparent in our statistical model. The NLR by itself cannot predict healing of diabetic foot ulcer with high sensitivity and specificity (63% and 71%; area under the curve, 0.68). However, it is one independent factor of diabetic foot ulcer healing that may be important in the ability to predict wound healing when it is added to other known factors, such as wound infection, wound size, and wound ischemia. In addition, treatment of diabetic ulcers was at the discretion of the treating surgeon; therefore, there was variability in use of débridement, offloading, dressing, and vascular procedures. Therefore, the confounding effect of differences in treatment cannot be determined from this study. Also, excluding subjects with lower infection rates and lower blood glucose levels could potentially affect the results. However, including patients with worse glycemic control and wound infection can underestimate the predictability of NLR rather than overestimate it.
CONCLUSIONS
Our study shows that NLR is an independent predictor of outcome in diabetic foot ulcers. NLR <4.2 can predict complete wound healing with sensitivity of 63% and specificity of 71%.
Acknowledgments
N.V. is supported by NIH Postdoctoral Fellowship Award T32-HL094294.
Footnotes
Author conflict of interest: none.
Presented at the poster session at the 2016 Vascular Annual Meeting of the Society for Vascular Surgery, National Harbor, Md, June 8–11, 2016.
AUTHOR CONTRIBUTIONS
Conception and design: NV, YJ, GL, RM, NA, GM, AA
Analysis and interpretation: NV, YJ, GL, RM, NA, GM, AA
Data collection: NV, YJ, GL, RM, NA, GM, AA
Writing the article: NV, YJ, GL, RM, NA, GM, AA
Critical revision of the article: NV, YJ, GL, RM, NA, GM, AA
Final approval of the article: NV, YJ, GL, RM, NA, GM, AA
Statistical analysis: NV, YJ, GL, RM, NA, GM, AA
Obtained funding: Not applicable
Overall responsibility: NV
REFERENCES
- 1.Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet. 2005;366:1719–1724. doi: 10.1016/S0140-6736(05)67698-2. [DOI] [PubMed] [Google Scholar]
- 2.Dunbar GL, Hellenberg DA, Levitt NS. Diabetes mellitus and non-traumatic lower extremity amputations in four public sector hospitals in Cape Town, South Africa, during 2009 and 2010. S Afr Med J. 2015;105:1053–1056. doi: 10.7196/SAMJ.2015.v105i12.9276. [DOI] [PubMed] [Google Scholar]
- 3.Namgoong S, Jung S, Han SK, Jeong SH, Dhong ES, Kim WK. Risk factors for major amputation in hospitalised diabetic foot patients. Int Wound J. 2016;13(Suppl 1):13–19. doi: 10.1111/iwj.12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeffcoate WJ, Harding KG. Diabetic foot ulcers. Lancet. 2003;361:1545–1551. doi: 10.1016/S0140-6736(03)13169-8. [DOI] [PubMed] [Google Scholar]
- 5.Wang Z, Hasan R, Firwana B, Elraiyah T, Tsapas A, Prokop L, et al. A systematic review and meta-analysis of tests to predict wound healing in diabetic foot. J Vasc Surg. 2016;63(Suppl):29S.e2–36S.e2. doi: 10.1016/j.jvs.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Dirican N, Karakaya YA, Gunes S, Daloglu FT, Dirican A. Association of intratumoral tumor infiltrating lymphocytes and neutrophil-to-lymphocyte ratio are an independent prognostic factor in non-small cell lung cancer. Clin Respir J. doi: 10.1111/crj.12417. [published online ahead of print November 30, 2015] [DOI] [PubMed] [Google Scholar]
- 7.Hasegawa S, Eguchi H, Tomokuni A, Tomimaru Y, Asaoka T, Wada H, et al. Pre-treatment neutrophil to lymphocyte ratio as a predictive marker for pathological response to preoperative chemoradiotherapy in pancreatic cancer. Oncol Lett. 2016;11:1560–1566. doi: 10.3892/ol.2015.4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagasaki T, Akiyoshi T, Fujimoto Y, Konishi T, Nagayama S, Fukunaga Y, et al. Prognostic impact of neutrophil-to-lymphocyte ratio in patients with advanced low rectal cancer treated with preoperative chemoradiotherapy. Dig Surg. 2015;32:496–503. doi: 10.1159/000441396. [DOI] [PubMed] [Google Scholar]
- 9.Aykan AÇ, Hatem E, Kalaycioğlu E, Karabay CY, Zehir R, Gokdeniz T, et al. Neutrophil-to-lymphocyte ratio may be a marker of peripheral artery disease complexity. Anatol J Cardiol. doi: 10.5152/AnatolJCardiol.2015.6240. [published online ahead of print November 26, 2015] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez-Urbistondo D, Beltran A, Beloqui O, Huerta A. The neutrophil-to-lymphocyte ratio as a marker of systemic endothelial dysfunction in asymptomatic subjects. Nefrologia. 2016;36:397–403. doi: 10.1016/j.nefro.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 11.Afari ME, Bhat T. Neutrophil to lymphocyte ratio (NLR) and cardiovascular diseases: an update. Expert Rev Cardiovasc Ther. 2016;14:573–577. doi: 10.1586/14779072.2016.1154788. [DOI] [PubMed] [Google Scholar]
- 12.Mills JL, Sr, Conte MS, Armstrong DG, Pomposelli FB, Schanzer A, Sidawy AN, et al. The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System: risk stratification based on wound, ischemia, and foot infection (WIfI) J Vasc Surg. 2014;59:220–234. e1–e2. doi: 10.1016/j.jvs.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 13.DiGangi C. Neutrophil-lymphocyte ratio: predicting cardiovascular and renal complications in patients with diabetes. J Am Assoc Nurse Pract. 2016;28:410–414. doi: 10.1002/2327-6924.12366. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura Y, Watanabe R, Katagiri M, Saida Y, Katada N, Watanabe M, et al. Neutrophil/lymphocyte ratio has a prognostic value for patients with terminal cancer. World J Surg Oncol. 2016;14:148. doi: 10.1186/s12957-016-0904-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papa A, Emdin M, Passino C, Michelassi C, Battaglia D, Cocci F. Predictive value of elevated neutrophil-lymphocyte ratio on cardiac mortality in patients with stable coronary artery disease. Clin Chim Acta. 2008;395:27–31. doi: 10.1016/j.cca.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 16.Lou M, Luo P, Tang R, Peng Y, Yu S, Huang W, et al. Relationship between neutrophil-lymphocyte ratio and insulin resistance in newly diagnosed type 2 diabetes mellitus patients. BMC Endocr Disord. 2015;15:9. doi: 10.1186/s12902-015-0002-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shiny A, Bibin YS, Shanthirani CS, Regin BS, Anjana RM, Balasubramanyam M, et al. Association of neutrophil-lymphocyte ratio with glucose intolerance: an indicator of systemic inflammation in patients with type 2 diabetes. Diabetes Technol Ther. 2014;16:524–530. doi: 10.1089/dia.2013.0264. [DOI] [PubMed] [Google Scholar]
- 18.Gary T, Pichler M, Belaj K, Hafner F, Gerger A, Froehlich H, et al. Neutrophil-to-lymphocyte ratio and its association with critical limb ischemia in PAOD patients. PLoS One. 2013;8:e56745. doi: 10.1371/journal.pone.0056745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spark JI, Sarveswaran J, Blest N, Charalabidis P, Asthana S. An elevated neutrophil-lymphocyte ratio independently predicts mortality in chronic critical limb ischemia. J Vasc Surg. 2010;52:632–636. doi: 10.1016/j.jvs.2010.03.067. [DOI] [PubMed] [Google Scholar]
- 20.Bagdade JD, Root RK, Bulger RJ. Impaired leukocyte function in patients with poorly controlled diabetes. Diabetes. 1974;23:9–15. doi: 10.2337/diab.23.1.9. [DOI] [PubMed] [Google Scholar]
- 21.Pradhan L, Nabzdyk C, Andersen ND, LoGerfo FW, Veves A. Inflammation and neuropeptides: the connection in diabetic wound healing. Expert Rev Mol Med. 2009;11:e2. doi: 10.1017/S1462399409000945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alba-Loureiro TC, Hirabara SM, Mendonca JR, Curi R, Pithon-Curi TC. Diabetes causes marked changes in function and metabolism of rat neutrophils. J Endocrinol. 2006;188:295–303. doi: 10.1677/joe.1.06438. [DOI] [PubMed] [Google Scholar]
- 23.Azab B, Daoud J, Naeem FB, Nasr R, Ross J, Ghimire P, et al. Neutrophil-to-lymphocyte ratio as a predictor of worsening renal function in diabetic patients (3-year follow-up study) Ren Fail. 2012;34:571–576. doi: 10.3109/0886022X.2012.668741. [DOI] [PubMed] [Google Scholar]
- 24.Unlu M, Arslan Z. The relation between neutrophil-lymphocyte ratio and endothelial dysfunction. Angiology. 2015;66:694. doi: 10.1177/0003319715584879. [DOI] [PubMed] [Google Scholar]
- 25.Tam JC, Ko CH, Lau KM, To MH, Kwok HF, Siu WS, et al. Enumeration and functional investigation of endothelial progenitor cells in neovascularization of diabetic foot ulcer rats with a Chinese 2-herb formula. J Diabetes. 2015;7:718–728. doi: 10.1111/1753-0407.12230. [DOI] [PubMed] [Google Scholar]
- 26.Farah R, Khamisy-Farah R. Association of neutrophil to lymphocyte ratio with presence and severity of gastritis due to Helicobacter pylori infection. J Clin Lab Anal. 2014;28:219–223. doi: 10.1002/jcla.21669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lowsby R, Gomes C, Jarman I, Lisboa P, Nee PA, Vardhan M, et al. Neutrophil to lymphocyte count ratio as an early indicator of blood stream infection in the emergency department. Emerg Med J. 2015;32:531–534. doi: 10.1136/emermed-2014-204071. [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez-Fajardo JA, Brizuela-Sanz JA, Aguirre-Gervas B, Merino-Diaz B, Del Rio-Sola L, Martin-Pedrosa M, et al. Prognostic significance of an elevated neutrophil-lymphocyte ratio in the amputation-free survival of patients with chronic critical limb ischemia. Ann Vasc Surg. 2014;28:999–1004. doi: 10.1016/j.avsg.2013.06.037. [DOI] [PubMed] [Google Scholar]
- 29.Tasoglu I, Cicek OF, Lafci G, Kadirogullari E, Sert DE, Demir A, et al. Usefulness of neutrophil/lymphocyte ratio as a predictor of amputation after embolectomy for acute limb ischemia. Ann Vasc Surg. 2014;28:606–613. doi: 10.1016/j.avsg.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 30.Tokgoz S, Kayrak M, Akpinar Z, Seyithanoglu A, Guney F, Yuruten B. Neutrophil lymphocyte ratio as a predictor of stroke. J Stroke Cerebrovasc Dis. 2013;22:1169–1174. doi: 10.1016/j.jstrokecerebrovasdis.2013.01.011. [DOI] [PubMed] [Google Scholar]