Abstract
Purpose
Tranexamic acid (TXA) is effective in reducing blood loss in primary total knee replacement. Almost all studies used an intravenous form or a topical form. The aim of this study was to assess the blood sparing efficacy and the safety of oral TXA.
Materials and Methods
All patients with primary total knee replacement performed in our institute from January 2015 to October 2015 were eligible. Oral TXA group was given 1 g oral TXA 2 hours before induction of anesthesia and 6 hours and 12 hours postoperatively. The control group was not given TXA.
Results
There were 94 cases in the oral TXA group and 95 cases in the control group. There was no difference in the baseline characteristics. The oral TXA group had a significantly lower hemoglobin drop (1.7 g/dL vs. 2.5 g/dL), lower drain output (154 mL vs. 203 mL), lower hidden blood loss (244 mL vs. 423 mL) and lower total blood loss (398 mL vs. 626 mL). There was no difference in transfusion rate (1.1% vs. 3.2%) and thromboembolic complication. There was no infection or mortality in both groups.
Conclusions
Oral TXA is effective in reducing blood loss in primary total knee replacement. It is a safe alternative to the intravenous or topical form.
Keywords: Knee, Arthroplasty, Tranexamic acid, Oral, Blood loss
Introduction
Tranexamic acid (TXA) has been shown to be effective in reducing blood loss, hemoglobin (Hb) drop and blood transfusion in primary total knee arthroplasty1–5). Most studies used the intravenous form6–9) or the intraarticular topical form10–12). Both forms were shown to be equally effective13,14). While TXA is very safe to use with no evident increase in mortality or life threatening thromboembolic events4,15,16), drug allergy with anaphylactic shock has been reported with the intravenous form17). The topical form carries the theoretical risk of periprosthetic infection due to needle contamination and may aggravate sepsis18). Also the effect of topical application on cementation and the longevity of prosthesis are still not confirmed. On the other hand, oral form has been shown to be equally19–24) or even more effective25) in primary total knee and hip arthroplasty to reduce blood loss, Hb drop and blood transfusion. It was estimated to be more cost effective than the intravenous form21,25). Despite these apparent advantages, only six clinical studies reported the use of oral TXA in total knee arthroplasty in English literature to our knowledge. Only two randomized controlled trials (RCTs) compared oral TXA with no treatment19,22), and both had small case numbers.
The aim of this study was to assess primarily the blood sparing efficacy of the oral form of TXA in primary total knee arthroplasty and secondarily the safety of the drug. The null hypothesis was that there was no difference in blood loss in patients with oral TXA compared with those without.
Materials and Methods
The study was a prospective RCT. All patients with primary total knee arthroplasty performed in our institute from January 2015 to October 2015 were eligible for selection. Cases were allocated by random number into a trial group (oral TXA group) or a control group. The oral TXA group was given 1 g oral TXA 2 hours before induction of anesthesia and then two more doses 6 hours and 12 hours postoperatively. The control group was not given TXA. Allocations were blinded to the surgeons and outcome assessors.
Exclusion criteria were patients with absence of written informed consent, bilateral arthroplasties, complicated primary total knee arthroplasty with previous osteotomy, simultaneous fracture fixation, implant removal or bone grafting, thromboembolic diseases, presence of clotting disorder or current treatment with an antiplatelet agent, anticoagulant or deep vein thrombosis (DVT) prophylaxis in the perioperative period, renal disease and history of allergy to TXA.
All arthroplasties were performed through the medial parapatellar approach with the use of a tourniquet, a posterior-stabilized implant, a bone plug in the intramedullary canal or navigation without canal violation, cementation, hemostasis with a tourniquet on, a compression bandage, suction pressure drainage at 200 mmHg for 24 hours, and a foot pump for DVT prophylaxis.
The primary outcome measure was Hb drop. Secondary outcome measures included intraoperative blood loss, drain output, total blood loss (TBL), hidden blood loss, transfusion requirement, thromboembolic complications, cerebrovascular or cardiovascular complications and 30-day mortality. All cases had Doppler ultrasonography on postoperative day 7 to detect any proximal DVT.
The lowest postoperative Hb level during hospital stay was used for comparison and calculation of Hb drop. Estimated blood volume (BV) was calculated using the Nadler et al.26) method while TBL was calculated according to the Hb balance method: TBL=BV×(Hbi−Hbe)/Hbi where Hbi (g/dL) was the preoperative serum Hb level, and Hbe (g/dL) was the postoperative serum Hb level.
Hidden blood loss was the difference between TBL and drain output. Measurement of intraoperative blood loss was not done because the routine use of tourniquet for the whole procedure had resulted in negligible amount. The serum Hb level for transfusion trigger was 8 g/dL. The aim of blood transfusion was to top up the serum Hb level to 10 g/dL.
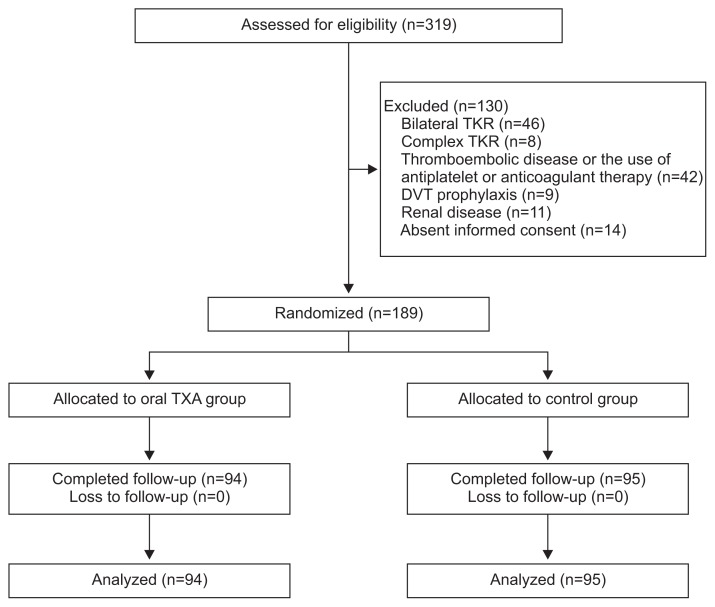
There were 319 patients with primary total knee arthroplasties performed in the period from January 2015 to October 2015 (Fig. 1). Forty-six patients had bilateral arthroplasties, 8 had complex arthroplasty with previous osteotomy, simultaneous fracture fixation, implant removal or bone grafting. Forty-two had a history of thromboembolic disease or were on current treatment with an antiplatelet agent or an anticoagulant, 9 had DVT prophylaxis, 11 had renal disease, and 14 had absence of written informed consent. They were excluded from the study. After exclusion, the remaining 189 patients were randomly allocated into the oral TXA group (n=94) and the control group (n=95). All allocated patients completed follow-up. The mean follow-up duration was 8.2 months (range, 4 to 13 months).
Fig. 1.
Consolidated Standards of Reporting Trials (CONSORT) flow diagram of patients. TKR: total knee replacement, DVT: deep vein thrombosis, TXA: tranexamic acid.
The mean age was 70 in the oral TXA group and 68 in the control group (p<0.05) (Table 1). There was no other significant difference between two groups in the baseline characteristics including body weight, height, body mass index, preoperative Hb, estimated BV (according to the method by Nadler et al.26)) and operation time.
Table 1.
Baseline Characteristics
| Characteristic | Oral TXA group (n=94) | Control group (n=95) | p-value |
|---|---|---|---|
| Age (yr) | 70±8 | 68±8 | 0.04a) |
| Sex (F), mean (%) | 67.4 | 69.1 | 0.793 |
| Body weight (kg) | 66.1±11.7 | 67.3±11.5 | 0.502 |
| Height (m) | 1.54±0.08 | 1.54±0.09 | 0.902 |
| BMI (kg/m2) | 27.7±4.1 | 28.4±4.3 | 0.299 |
| Preoperative Hb (g/dL) | 13.6±1.3 | 13.8±1.2 | 0.270 |
| Blood volume (mL) | 3,035±514 | 3,079±487 | 0.545 |
| Operation time (min) | 90±18 | 92±15 | 0.394 |
Values are presented as mean±standard deviation.
TXA: tranexamic acid, BMI: body mass index, Hb: hemoglobin.
p<0.05.
1. Statistical Analysis
The sample size was aimed at around 90 in each group based on the following calculations.
The primary outcome measure used for calculation was postoperative Hb drop, which was found to be around 2.5 g/dL (standard deviation 1 g/dL) without TXA according to recent studies10–12) and historical data of our institute. The reported postoperative Hb drop after intravenous or topical TXA was around 1.5–2.0 g/dL10–12). Based on the assumption that oral form was as effective as intravenous form, 2.0 g/dL was used for calculation to ensure adequate power of analysis. A two-tailed test was used with an alpha value of 0.05 and a power of 0.90. The attrition rate due to patient refusal was assumed to be 5%. Statistical analysis was performed with IBM SPSS ver. 20.0 (IBM Co., Armonk, NY, USA). Univariate analysis of numerical data was performed with Student t-test. p<0.05 was considered statistically significant. The study was approved by the Institutional Review Board.
Results
On the outcome measures (Table 2), the oral TXA group had a significantly higher postoperative Hb level (11.9 g/dL vs. 11.2 g/dL), lower Hb drop (1.7 g/dL vs. 2.5 g/dL), lower drain output (154 mL vs. 203 mL), lower hidden blood loss (244 mL vs. 423 mL) and lower TBL (398 mL vs. 626 mL). There was no difference in the transfusion rate (1.1% vs. 3.2%) and the rate of thromboembolic complications between groups. There was no cerebrovascular or cardiovascular complication in both groups. No infection or 30-day mortality occurred in both groups.
Table 2.
Outcome Measures
| Characteristic | Oral TXA group (n=94) | Control group (n=95) | p-value |
|---|---|---|---|
| Postoperative Hb (g/dL) | 11.9±1.4 | 11.2±1.3 | 0.001a) |
| Hb drop (g/dL) | 1.7±0.8 | 2.5±0.9 | <0.001a) |
| Drain (mL) | 154±77 | 203±77 | <0.001a) |
| Hidden blood loss (mL) | 244±193 | 423±270 | <0.001a) |
| Total blood loss (mL) | 398±186 | 626±265 | <0.001a) |
| Transfusion rate (%) | 1.06 | 3.16 | 0.621 |
| Length of stay (day) | 5.9±2.2 | 5.8±1.7 | 0.612 |
| Proximal DVT (%) | 1.1 | 0 | 0.497 |
| PE (%) | 1.1 | 1.1 | 1.000 |
| Infection (%) | 0 | 0 | - |
| Mortality (%) | 0 | 0 | - |
Values are presented as mean±standard deviation.
TXA: tranexamic acid, Hb: hemoglobin, DVT: deep vein thrombosis, PE: pulmonary embolism.
p<0.05.
One proximal DVT occurred in the oral TXA group and one pulmonary embolism occurred in each group. The patient with proximal DVT in the oral TXA group was also one of the patients with pulmonary embolism. Both patients with pulmonary embolism presented with respiratory distress and the diagnosis was confirmed with contrast spiral computed tomography of the thorax. They were successfully treated with oxygen support and anticoagulant.
Discussion
The most significant finding of the present study is the significant reduction of Hb drop, drain output, hidden blood loss and actual TBL postoperatively with the use of oral TXA compared with no anti-fibrinolytic drug treatment. There were only six studies on oral TXA in total knee arthroplasty in English literature. Two were cohort studies on both total knee and total hip arthroplasties23,25), two were RCTs comparing the oral versus intravenous form20,21), only the remaining two were RCTs comparing the oral form versus no treatment19,22). The present study may be the third and the largest RCT in English literature to compare the blood sparing efficacy of oral TXA with no treatment in primary total knee arthroplasty.
Zohar et al.20) was the first to report less drain output, less transfusion requirement and lower hematocrit drop in total knee arthroplasty compared with no treatment; Bradshaw et al.19) reported similar findings. Irwin et al.25) reported less transfusion requirement compared with the intravenous form in a heterogeneous group of patients with total knee or total hip arthroplasty while McGrath et al.23) reported less Hb drop and transfusion compared with no treatment also in a heterogenous knee and hip replacement population. Recently, Alipour et al.22) reported less hematocrit drop and less blood loss compared with no treatment in total knee arthroplasty, while Fillingham et al.21) reported equivalence between the oral and intravenous forms in Hb drop and blood loss. The use of intravenous or topical TXA in total knee arthroplasty is well-supported by various meta-analyses and RCTs. The blood sparing efficacy was reflected in reduction in Hb or hematocrit drop and reduction in blood loss and transfusion requirement. Hematocrit drop reduction was reported to be around 0.0211,20) while Hb drop reduction was around 0.8–1.4 g/dL10,11). The present study was able to show a reduction of Hb drop of 0.8 g/dL. Similarly, McGrath et al.23) showed a significant reduction of Hb drop of 0.76 g/dL with oral TXA.
The mean reduction in blood loss with intravenous or topical TXA was around 125 mL intraoperatively, 250 mL postoperatively and around 170–500 mL in total blood loss1,3,5). The present study showed a reduction of 179 mL in hidden blood loss and 228 mL in total blood loss. Similarly, Alipour et al.22) reported a significant reduction of 224 mL at 24 hours postoperatively with oral TXA. Both results suggested oral form having comparable blood sparing efficacy as the intravenous or topical form.
There was no significant reduction of transfusion requirement in the present study (1.1% vs. 3.2%). The low transfusion requirement in both groups could be explained by the high postoperative Hb level (11.9 g/dL and 11.2 g/dL, respectively) which was much higher than the transfusion trigger of 8 g/dL. In contrast with the high baseline transfusion rate (15.7%) reported by Mc-Grath et al.23), the low baseline transfusion rate (3.2%) of the present study would render the difference insignificant. Nevertheless, Irwin et al.25) even reported a lower risk of transfusion with oral TXA compared with intravenous TXA (odds ratio 0.48).
The cost of using oral TXA has been shown to be much lower than the intravenous form. Irwin et al.25) estimated a reduction of USD 2.59 per patient. Fillingham et al.21) reported the cost per patient for intravenous TXA was USD 47–108 as opposed to USD 14 for oral TXA, which was equivalent to 3.4 to 7.7 times reduction in cost for the oral form. In the present study, the cost for a single shot of 1 g intravenous or topical TXA was around USD 8.34, while it was only around USD 0.55 for three doses of 1 g oral TXA. This was equivalent to 15 times reduction in drug cost or an absolute saving of USD 7.80 per patient.
There is no consensus on the regimen of oral TXA in literature. Pilbrant et al.27) estimated the bioavailability to be around 34% and elimination in blood occurred mostly within 8 hours. His study demonstrated that a dose of 2 g oral form produced higher plasma concentration than 1 gm intravenous form at six hours after administration in a 70 kg volunteer. The present study used a regimen of 1 g oral TXA 2 hours before induction of anesthesia and then two doses at 6 hours and 12 hours postoperatively in patients with a mean body weight of 66 kg. This was similar to the regimen described by Zohar et al.20). The only difference was that there were three postoperative doses instead of two. Irwin et al.25) described a regimen of a single dose of 25 mg/kg 2 hours before induction of anesthesia; it was equivalent to 1.65 g for the average body weight of 66 kg in the present study. In contrast, Bradshaw et al.19) used a regimen of four 1.5 g doses given six-hourly with the first three doses given preoperatively. Since it has been shown that fibrinolysis peaks 6 hours after the end of surgery and is maintained for about 18 hours after surgery28), the single dose described by Irwin et al.25) raises the risk of inadequate drug plasma concentration by 18 hours. From the perspective of balancing risks and benefits, the 4-dose regimen seems too long in view of the theoretic risk of DVT. The present study therefore used a regimen with duration in the medium range.
Although the intravenous form has been shown to be safe and effective, there is a real risk of anaphylactic shock17). So far, the allergic reactions reported for the oral form were milder and slower onset29,30). The oral form in this context seems to be a better choice than the intravenous form, provided drug efficacies are similar. The present study successfully demonstrated a significant blood sparing efficacy of the oral form, which is comparable to that of the intravenous form in various studies.
Topical administration has become popular recently because theoretically it could avoid thromboembolic risk associated with systemic administration. However, it has increased the theoretical risk of periprosthetic infection due to bacterial contamination during needle aspiration and dilution for the drug. An alarming report by Klak et al.18) showed that TXA could aggravate staphylococcal septic arthritis and sepsis. Based on this theoretical risk of introducing infection by direct application of drug to the joint and the potential of even aggravating sepsis, we advocate the use of oral form considering similar efficacies between the two forms.
The present study did not show significant difference with the intravenous or topical form in the incidence of postoperative complications including thromboembolic events. However, this could be due to the inadequate sample size which was based on calculation of the therapeutic effect size rather than the adverse effect size of the drug. All previous studies on TXA suffer similar limitations such that more clinical trials are called for to accumulate adequate pooled sample size for analysis for the thromboembolic risk. Future RCTs on oral TXA should compare the efficacy between different forms of drug. Meta-analyses on oral TXA are lacking and required to address the drug safety issue.
Conclusions
The present study shows that oral TXA is effective in blood sparing in terms of reduction of Hb drop, drain output, hidden blood loss and total blood loss. It is safe to use and is an alternative to the intravenous or topical form.
Footnotes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
References
- 1.Yang ZG, Chen WP, Wu LD. Effectiveness and safety of tranexamic acid in reducing blood loss in total knee arthroplasty: a meta-analysis. J Bone Joint Surg Am. 2012;94:1153–9. doi: 10.2106/JBJS.K.00873. [DOI] [PubMed] [Google Scholar]
- 2.Alshryda S, Sukeik M, Sarda P, Blenkinsopp J, Haddad FS, Mason JM. A systematic review and meta-analysis of the topical administration of tranexamic acid in total hip and knee replacement. Bone Joint J. 2014;96:1005–15. doi: 10.1302/0301-620X.96B8.33745. [DOI] [PubMed] [Google Scholar]
- 3.Zhang H, Chen J, Chen F, Que W. The effect of tranexamic acid on blood loss and use of blood products in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2012;20:1742–52. doi: 10.1007/s00167-011-1754-z. [DOI] [PubMed] [Google Scholar]
- 4.Poeran J, Rasul R, Suzuki S, Danninger T, Mazumdar M, Opperer M, Boettner F, Memtsoudis SG. Tranexamic acid use and postoperative outcomes in patients undergoing total hip or knee arthroplasty in the United States: retrospective analysis of effectiveness and safety. BMJ. 2014;349:g4829. doi: 10.1136/bmj.g4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Panteli M, Papakostidis C, Dahabreh Z, Giannoudis PV. Topical tranexamic acid in total knee replacement: a systematic review and meta-analysis. Knee. 2013;20:300–9. doi: 10.1016/j.knee.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 6.Good L, Peterson E, Lisander B. Tranexamic acid decreases external blood loss but not hidden blood loss in total knee replacement. Br J Anaesth. 2003;90:596–9. doi: 10.1093/bja/aeg111. [DOI] [PubMed] [Google Scholar]
- 7.Molloy DO, Archbold HA, Ogonda L, McConway J, Wilson RK, Beverland DE. Comparison of topical fibrin spray and tranexamic acid on blood loss after total knee replacement: a prospective, randomised controlled trial. J Bone Joint Surg Br. 2007;89:306–9. doi: 10.1302/0301-620X.89B3.17565. [DOI] [PubMed] [Google Scholar]
- 8.Alvarez JC, Santiveri FX, Ramos I, Vela E, Puig L, Escolano F. Tranexamic acid reduces blood transfusion in total knee arthroplasty even when a blood conservation program is applied. Transfusion. 2008;48:519–25. doi: 10.1111/j.1537-2995.2007.01564.x. [DOI] [PubMed] [Google Scholar]
- 9.Aguilera X, Martinez-Zapata MJ, Bosch A, Urrutia G, Gonzalez JC, Jordan M, Gich I, Maymo RM, Martínez N, Monllau JC, Celaya F, Fernandez JA. Efficacy and safety of fibrin glue and tranexamic acid to prevent postoperative blood loss in total knee arthroplasty: a randomized controlled clinical trial. J Bone Joint Surg Am. 2013;95:2001–7. doi: 10.2106/JBJS.L.01182. [DOI] [PubMed] [Google Scholar]
- 10.Wong J, Abrishami A, El Beheiry H, Mahomed NN, Roderick Davey J, Gandhi R, Syed KA, Muhammad Ovais Hasan S, De Silva Y, Chung F. Topical application of tranexamic acid reduces postoperative blood loss in total knee arthroplasty: a randomized, controlled trial. J Bone Joint Surg Am. 2010;92:2503–13. doi: 10.2106/JBJS.I.01518. [DOI] [PubMed] [Google Scholar]
- 11.Roy SP, Tanki UF, Dutta A, Jain SK, Nagi ON. Efficacy of intra-articular tranexamic acid in blood loss reduction following primary unilateral total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2012;20:2494–501. doi: 10.1007/s00167-012-1942-5. [DOI] [PubMed] [Google Scholar]
- 12.Konig G, Hamlin BR, Waters JH. Topical tranexamic acid reduces blood loss and transfusion rates in total hip and total knee arthroplasty. J Arthroplasty. 2013;28:1473–6. doi: 10.1016/j.arth.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aguilera X, Martinez-Zapata MJ, Hinarejos P, Jordan M, Leal J, Gonzalez JC, Monllau JC, Celaya F, Rodriguez-Arias A, Fernandez JA, Pelfort X, Puig-Verdie Ll. Topical and intravenous tranexamic acid reduce blood loss compared to routine hemostasis in total knee arthroplasty: a multicenter, randomized, controlled trial. Arch Orthop Trauma Surg. 2015;135:1017–25. doi: 10.1007/s00402-015-2232-8. [DOI] [PubMed] [Google Scholar]
- 14.Gomez-Barrena E, Ortega-Andreu M, Padilla-Eguiluz NG, Perez-Chrzanowska H, Figueredo-Zalve R. Topical intraarticular compared with intravenous tranexamic acid to reduce blood loss in primary total knee replacement: a doubleblind, randomized, controlled, noninferiority clinical trial. J Bone Joint Surg Am. 2014;96:1937–44. doi: 10.2106/JBJS.N.00060. [DOI] [PubMed] [Google Scholar]
- 15.Duncan CM, Gillette BP, Jacob AK, Sierra RJ, Sanchez-Sotelo J, Smith HM. Venous thromboembolism and mortality associated with tranexamic acid use during total hip and knee arthroplasty. J Arthroplasty. 2015;30:272–6. doi: 10.1016/j.arth.2014.08.022. [DOI] [PubMed] [Google Scholar]
- 16.Gillette BP, DeSimone LJ, Trousdale RT, Pagnano MW, Sierra RJ. Low risk of thromboembolic complications with tranexamic acid after primary total hip and knee arthroplasty. Clin Orthop Relat Res. 2013;471:150–4. doi: 10.1007/s11999-012-2488-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lucas-Polomeni MM, Delaval Y, Menestret P, Delaval P, Ecoffey C. A case of anaphylactic shock with tranexamique acid (Exacyl) Ann Fr Anesth Reanim. 2004;23:607–9. doi: 10.1016/j.annfar.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 18.Klak M, Anakkala N, Wang W, Lange S, Jonsson IM, Tarkowski A, Jin T. Tranexamic acid, an inhibitor of plasminogen activation, aggravates staphylococcal septic arthritis and sepsis. Scand J Infect Dis. 2010;42:351–8. doi: 10.3109/00365540903510690. [DOI] [PubMed] [Google Scholar]
- 19.Bradshaw AR, Monoghan J, Campbell D. Oral tranexamic acid reduces blood loss in total knee replacement arthroplasty. Curr Orthop Prac. 2012;23:209–12. doi: 10.1097/BCO.0b013e318247f1d5. [DOI] [Google Scholar]
- 20.Zohar E, Ellis M, Ifrach N, Stern A, Sapir O, Fredman B. The postoperative blood-sparing efficacy of oral versus intravenous tranexamic acid after total knee replacement. Anesth Analg. 2004;99:1679–83. doi: 10.1213/01.ANE.0000136770.75805.19. [DOI] [PubMed] [Google Scholar]
- 21.Fillingham YA, Kayupov E, Plummer DR, Moric M, Gerlinger TL, Della Valle CJ. The James A. Rand Young Investigator’s Award: a randomized controlled trial of oral and intravenous tranexamic acid in total knee arthroplasty: the same efficacy at lower cost? J Arthroplasty. 2016;31(9 Suppl):26–30. doi: 10.1016/j.arth.2016.02.081. [DOI] [PubMed] [Google Scholar]
- 22.Alipour M, Tabari M, Keramati M, Zarmehri AM, Makhmalbaf H. Effectiveness of oral tranexamic acid administration on blood loss after knee artroplasty: a randomized clinical trial. Transfus Apher Sci. 2013;49:574–7. doi: 10.1016/j.transci.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 23.McGrath S, Yates P, Prosser G. Oral tranexamic acid in hip and knee arthroplasty: a prospective cohort study. Open J Orthop. 2014;4:215–20. doi: 10.4236/ojo.2014.48035. [DOI] [Google Scholar]
- 24.Lee QJ, Chang WY, Wong YC. Blood-sparing efficacy of oral tranexamic acid in primary total hip arthroplasty. J Arthroplasty. 2017;32:139–42. doi: 10.1016/j.arth.2016.06.058. [DOI] [PubMed] [Google Scholar]
- 25.Irwin A, Khan SK, Jameson SS, Tate RC, Copeland C, Reed MR. Oral versus intravenous tranexamic acid in enhanced-recovery primary total hip and knee replacement: results of 3000 procedures. Bone Joint J. 2013;95:1556–61. doi: 10.1302/0301-620X.95B11.31055. [DOI] [PubMed] [Google Scholar]
- 26.Nadler SB, Hidalgo JH, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51:224–32. [PubMed] [Google Scholar]
- 27.Pilbrant A, Schannong M, Vessman J. Pharmacokinetics and bioavailability of tranexamic acid. Eur J Clin Pharmacol. 1981;20:65–72. doi: 10.1007/BF00554669. [DOI] [PubMed] [Google Scholar]
- 28.Blanie A, Bellamy L, Rhayem Y, Flaujac C, Samama CM, Fontenay M, Rosencher N. Duration of postoperative fibrinolysis after total hip or knee replacement: a laboratory follow-up study. Thromb Res. 2013;131:e6–11. doi: 10.1016/j.thromres.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 29.Kaku Y, Ito T, Kudo K, Kido-Nakahara M, Nakahara T, Moroi Y, Furue M. Generalized fixed drug eruption induced by tranexamic acid. Eur J Dermatol. 2014;24:408–9. doi: 10.1684/ejd.2014.2354. [DOI] [PubMed] [Google Scholar]
- 30.Imbesi S, Nettis E, Minciullo PL, Di Leo E, Saija A, Vacca A, Gangemi S. Hypersensitivity to tranexamic acid: a wide spectrum of adverse reactions. Pharm World Sci. 2010;32:416–9. doi: 10.1007/s11096-010-9415-8. [DOI] [PubMed] [Google Scholar]