Abstract
Exposure of murine and human tissues to ionizing radiation (IR) induces the expression of p16INK4a, a tumor suppressor gene and senescence/aging biomarker. Increased p16INK4a expression is often delayed several weeks post exposure to IR. In this context, it remains unclear if it occurs to suppress aberrant cellular growth of potentially transformed cells or is simply a result of IR-induced loss of tissue homeostasis. To address this question, we used a conditional p16INK4a null mouse model and determined the impact of p16INK4a inactivation long-term post exposure to IR. We found that, in vitro, bone marrow stromal cells exposed to IR enter DNA replication following p16INK4a inactivation. However, these cells did not resume growth; instead, they mostly underwent cell cycle arrest in G2. Similarly, delayed inactivation of p16INK4a in mice several weeks post exposure to IR resulted in increased BrdU incorporation and cancer incidence. In fact, we found that the onset of tumorigenesis was similar whether p16INK4a was inactivated before or after exposure to IR. Overall, our results suggest that IR-induced p16INK4a dependent growth arrest is reversible in mice and that sustained p16INK4a expression is necessary to protect against tumorigenesis.
Keywords: senescence, p16INK4a, ionizing irradiation, tumor suppressor, cell cycle
INTRODUCTION
Exposure to ionizing radiation (IR) leads to an increase in p16INK4a expression in various murine tissues 16, 31. Similarly, p16INK4a expression is also elevated in skin biopsies of leukemia survivors previously exposed to radiation therapy and in T cells collected from breast cancer survivors treated with anthracycline-based chemotherapy 17, 23. Several other inducers of p16INK4a have also been described such as oncogenic signalling and telomere dysfunction 22. Most of these inducers seem to have in common the activation of a DNA damage/stress response that in some instances may prelude downstream neoplastic conversion 3, 27, 32.
Loss of p16INK4a is observed in many human cancers and predisposes mice to tumorigenesis 24, 25. In fact, p16INK4a is a cyclin dependent kinase inhibitor that acts by preventing the phosphorylation of the retinoblastoma (pRb) family proteins and ultimately cell cycle progression 26. Following IR-induced DNA damage, it is believed that most cells will alt cell cycle progression by a mechanism that entails primarily an ATM/p53/p21 cascade 33. On the other hand, expression of p16INK4a is more complex as it seems to occur in a delayed manner to DNA damage or oncogenic signalling 13, 29, 32. For example, normal fibroblasts exposed to IR in vitro induce transient but rapid (within hours) upregulation of p53 and p21 protein levels, while p16INK4a expression is not detected until several days later 13, 20. The reason for this delayed increase in p16INK4a expression following DNA damage is unknown. One hypothesis is that exposure to IR may induce neoplastic stress that later induce p16INK4a in an indirect manner 2, 32. Alternatively, p16INK4a expression may rise in response to the accumulation of reactive oxygen species or as a bystander effect of IR-induced loss of tissue homeostasis 10, 11, 32.
Whether induced following exposure to IR or during normal aging, expression of p16INK4a seems to occur preferentially into possibly exhausted progenitor and stem cell populations, preventing adequate tissue renewal 12, 14, 18, 28, 30, 31. For example, we recently showed increased neurogenesis in the irradiated mouse brain in absence of p16INK4a expression (Le et al. submitted). Thus, while p16INK4a expression prevents damaged cells from proliferating, it likely also diminishes the regenerative potential of aged/irradiated tissues. In the absence of reliable markers, it remains unknown whether irradiated cells expressing p16INK4a are truly senescent in vivo or maintained in check long term. However, we believe that exposure to IR is likely to lead to senescence in most cells either directly through a persistent DNA damage response or by forcing premature exhaustion of cycling progenitor cells.
In this context, the development of strategies that would prevent or limit p16INK4a expression in progenitor/stem cells becomes attractive, as it may allow better tissue regeneration in cancer survivors. In support of this approach, it was shown that p53/Arf activity is not necessary to protect mice from IR-induced lymphoma 5. In fact, only transient (as short as six days) p53 and p19Arf expression was sufficient to protect against development of cancer. Whether transient or sustained p16INK4a expression is necessary to exert a similar tumor suppressive effect remains unknown. Actually, it is unknown if the delayed IR-induced p16INK4a expression occurs to prevent neoplastic progression. We answered this question using a conditional p16INK4a null mouse model and showed that while the inactivation of p16INK4a stimulates cell cycle progression in irradiated cells and tissues, its long-term expression is necessary to protect against IR-induced cancer.
RESULTS AND DISCUSSION
Irradiated bone marrow stromal cells do not resume growth following p16INK4a deletion
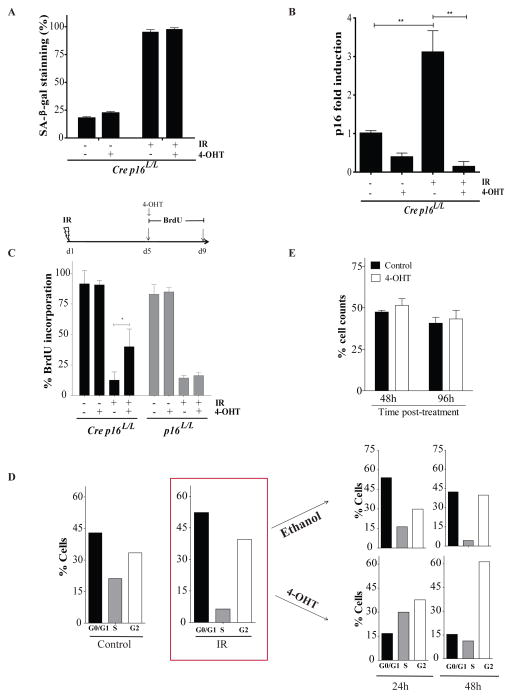
We first examined in vitro the role of p16INK4a in preventing cell cycle progression and proliferation following exposure to IR. We chose to use bone marrow derived stromal cells for our primary cell cultures as we found that these cells do not transform easily in vitro when compared to mouse embryonic fibroblasts which grow robustly in presence of a high level p16INK4a expression 19. Bone marrow stromal cells (defined as Cre p16L/L) were derived from p16INK4a specific conditional allele transgenic mice expressing Cre-ERT2 recombinase under the human ubiquitin C (UBC) promoter 21. We found that exposure to 10 Gy resulted in over 90% of the cells to express the senescence-associated β-galactosidase (SAβ-gal) biomarker (Figure 1a and Supplementary Figure 1a). In contrast, about 20% of non-irradiated control cells had SAβ-gal staining. As expected, treatment of these cells with 4-hydroxy tamoxifen (4-OHT) on day 5 following exposure to IR efficiently reduced expression of p16INK4a both at the RNA and protein levels (Figure 1c and Supplementary Figure 1b). However, while deletion of p16INK4a expression did not reduce the proportion of cells staining positive for SAβ-gal, it allowed a fraction of these cells to resume cell cycle and to incorporate BrdU (Figure 1c). Importantly, no increase in BrdU incorporation was observed in bone marrow stromal cells lacking the Cre recombinase treated with 4-OHT (defined as p16L/L, Figure 1c). Cell cycle analysis performed five days post exposure to IR, a time at which the senescence phenotype is already initiated, showed that stromal cells are arrested in both G1 and G2 (Figure 1d). Treatment of these irradiated cell populations with 4-OHT, but not the control vehicle, induced a proportion of cells to progress in S and G2 phases with a greater proportion of cells in G2 being detected at 48 hours post treatment. Finally, we observed no increase in the total cell number up to 96 hours post 4-OHT treatment (Figure 1e). These results suggest that deletion of p16INK4a in irradiated stromal cell allows for cell cycle re-entry in a significant fraction of cells but that these cells fail to resume growth in vitro.
Figure 1.
Deletion of p16INK4a in irradiated bone marrow stromal cells allows for cell cycle progression but not cell growth. (a) Proportion of cells staining positive for SA-β-galactosidase (SA-β-gal) 9 days post-exposure or not to 10 Gy IR. Where indicated, cells were treated (+) or not (−) with 100nM 4-Hydroxy Tamoxifen (4-OHT) overnight on day 5 post-IR. Bone marrow stromal cells expressing or not the Cre recombinase (defined as Cre p16L/L or p16L/L respectively) were isolated has previously described4 from the femur of p16INK4a specific conditional allele transgenic mice. Cells were used at low passage (less than 3) and cultured in DMEM containing 10% fetal bovine serum under low (3%) oxygen concentration. (b) Differential mRNA expression levels of p16INK4a as determined by qPCR in Cre p16L/L cells treated as described above. Shown is fold increase in p16INK4a expression normalized to 18S. Student t-test (** p < 0.01). q-PCR was performed using SYBR GREEN PCR SensiMix™ low ROX kit (Quantance, CA, USA) using the following primers for p16INK4a and S18 genes F5′AACTCTTTCGGTCGTACCCC3′, R5′GCGTGCTTGAGCTGAAGCTA3′ and F5′TCAACTTTCGATGGTAGTCGCCGT3′, R5′TCCTTGGATGTGGTAGCCGTTTCT3′ respectively. (c) Proportion of cells incorporating BrdU (4-day pulse) 5 days after exposure or not to IR as determined by immunostaining (BrdU antibody catalogue number 347583, BD Biosciences, USA). Inactivation of p16INK4a by 4-OHT was initiated simultaneously with addition of BrdU. Student t-test (* p < 0.05). (d) Cell cycle analysis of Cre p16L/L cells before and 5 days post exposure to IR as determined by flow cytometry. Irradiated cells were then treated with 4-OHT or ethanol (vehicle) and cell cycle analysed again 24 and 48 hours later. Shown are results of a representative experiment from n=3 independent cell populations. (e) Cre p16L/L cells were irradiated and 5 day later the cells were treated with 4-OHT or its vehicle. The proportion of viable cells was determined 48 and 96 hours later. Data are expressed as mean ± SD of n=3 independent cell populations.
Increase BrdU incorporation in mice tissues following deletion of p16INK4a
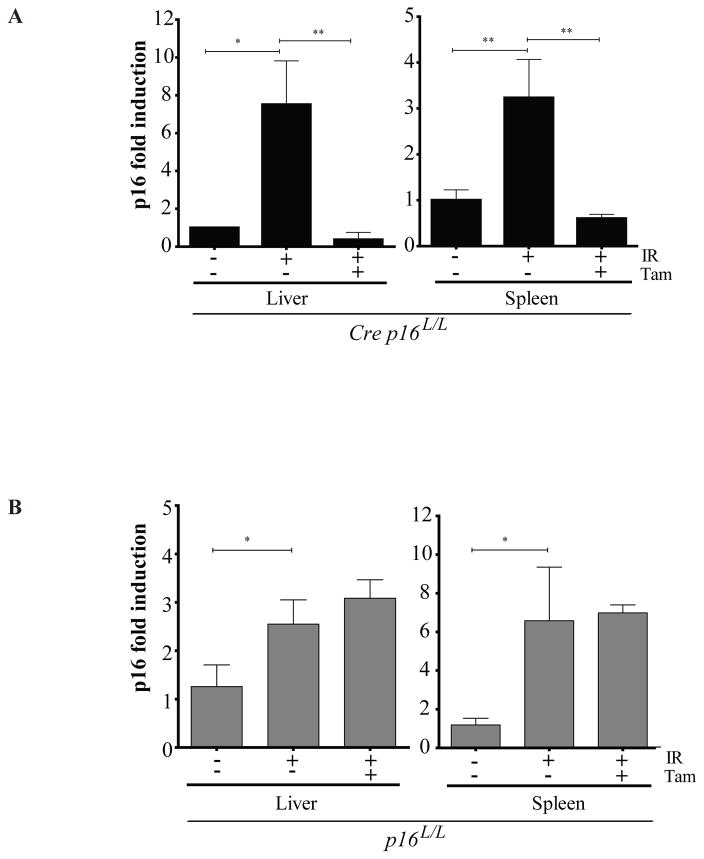
Murine stromal cells are known to be sensitive to in vitro growth conditions and can undergo telomere and p16INK4a independent premature senescence19. Therefore, it is not surprising to see about 20% of the early passaged cells (<3) to stain positive for SAβ-gal in absence of IR despite being cultured under low (3%) oxygen concentration (Figure 1a). In this context, we believe that the absence of cell proliferation following p16INK4a inactivation in vitro could be the result of a premature stress-induced senescence and thus may not adequately represent the in vivo situation. To address this issue, we irradiated Cre p16L/L mice at the sub lethal dose of 2.5 Gy and then waited 8 weeks for p16INK4a expression to increase. We had previously performed time course studies and found that a minimum of 6–8 weeks is necessary to observe robust IR-induced p16INK4a expression in mouse tissues 16. Treatment of Cre p16L/L mice with tamoxifen for 5 days resulted in efficient (50–80%) recombination and consequent reduction of IR-induced p16INK4a expression in both liver and spleen (Figure 2a and Supplementary Figure 2). As expected, no decrease in p16INK4a expression was observed in Cre deficient mice injected with tamoxifen (Figure 2b).
Figure 2.
Conditional deletion of IR-induced p16INK4a expression in mice. 8–10 weeks old mice were irradiated at the dose of 2.5 Gy (total body irradiation using a Faxitron CP-160 at a rate of 1 Gy/min) and 8 weeks later they were treated (+) or not (−) with Tamoxifen (Tam) at a dose of 200 mg/kg (diluted in a mixture 1:50 of ethanol and corn oil respectively) by gavage for 5 consecutive days to inactivate p16INK4a. Expression of p16INK4a relative to 18S was determined by qPCR on liver and spleen tissues collected from Cre p16L/L (a) or p16L/L (b) mice. n=5 mice per group. Data are expressed as mean ± SD. Student t-test * p < 0.05 and ** p < 0.01.
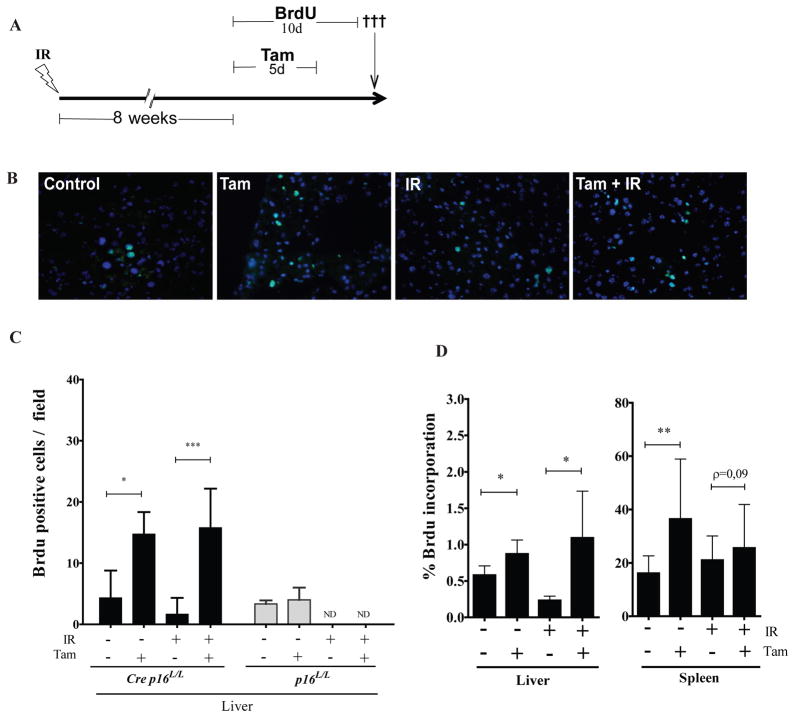
In line with our in vitro data, we found that liver cryosections collected from tamoxifen treated Cre p16L/L mice showed marked increase in BrdU incorporation (4–8 fold) independently of whether mice were previously exposed or not to IR (Figure 3a–c). Not surprisingly, we found that liver from irradiated mice had incorporated lower levels of BrdU and that treatment of Cre deficient mice with tamoxifen did not increase BrdU levels. However, these results also showed that p16INK4a expression in relatively young (18 weeks old) non irradiated mice is sufficient to restrict cell cycle progression in a high proportion of cells in the liver. To confirm these results, we made single cell suspension from control and irradiated livers, and determined BrdU incorporation using flow cytometry. Again, we found that p16INK4a inactivation leads to increase BrdU incorporation (Figure 3d). Likewise, inactivation of p16INK4a led to a significant increase in BrdU incorporation in the spleen but failed to do so in previously irradiated tissues (Figure 3d). Further analysis revealed that increase in BrdU incorporation in the spleen was restricted to cells of non-hematopoietic origin (defined as negative for the CD45 marker - see Supplementary Figure S3b). It is unclear at the moment why such a high proportion (25–35%) of non-hematopoietic splenic cell, but not liver cells, incorporated BrdU upon p16INK4a inactivation (Supplementary Figure S3). Such a high proportion of splenic stromal cells expressing p16INK4a may help explain previous results from our laboratory showing lymphopoiesis is INK4a/ARF-dependent4. In fact, we have shown that the absence of INK4a/ARF expression leads to a non-cell-autonomous increase in B cells and common lymphoid progenitor cell populations in the spleen 4. However, whether there is a direct relationship between p16INK4a expression in the spleen stroma and altered lymphopoiesis remains to be determined.
Figure 3.
Increase BrdU incorporation in irradiated mouse tissues following deletion of p16INK4a. (a) Schematic of the experiment. Cre p16 L/L mice were irradiated or not at a dose of 2.5 Gy (total body irradiation). 8 weeks later, mice were treated or not with Tam by gavage for 5 consecutive days. Beginning with the first Tam injection, mice also received daily intraperitoneally injection of BrdU (50 mg/kg) for a total of 10 consecutive days. (b) Representative images from liver cryosections treated as indicated showing the incorporation of BrdU in green and nuclei in blue (stained with DAPI). The BrdU antibody used was from BD Biosciences (catalogue number 347583). (c) Number of cells incorporating BrdU was determined by counting manually immunostained liver sections collected from both Cre p16 L/L and p16 L/L mice treated as described in a. Data are expressed as mean ± SD of at least 5 randomly selected fields (40X) obtained from a minimum of 4 mice per group. ND (not determined). (d) Proportion of cells incorporating BrdU from dissociated liver and spleen tissues collected from Cre p16 L/L mice as determined on single cell suspensions by flow cytometry using the BrdU flow kit (catalogue number 559619 from BD Bioscences, USA) and analyzed using a BD-LSRFortesa. Data are expressed as mean ± SD. Dissociated cell samples were collected from a minimum of 4 mice per group and analysed individually. Student t-test * p < 0.05 and ** p < 0.01.
Sustained p16INK4a expression is necessary to limit cancer incidence
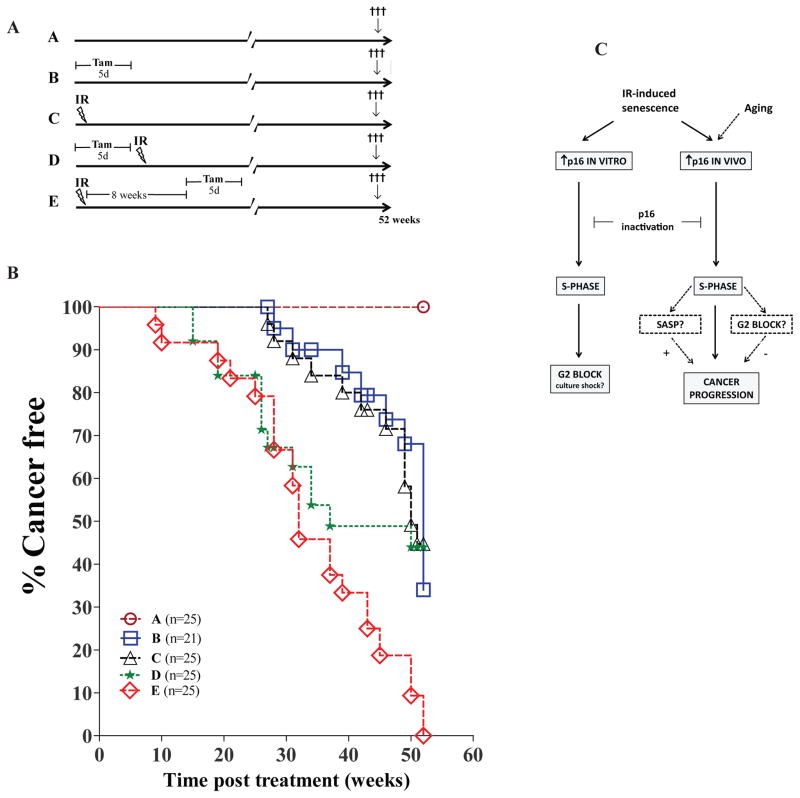
We have shown that p16INK4a expression is increased in tissues long-term following exposure to IR and that this limits cell cycle progression. Yet, we don’t know if this expression occurs as a tumor suppressive mechanism or simply as a bystander effect to genotoxic stress. Neither do we know if persistent expression of p16INK4a is necessary to protect against cancer development or if transient expression would be sufficient to induce an irreversible growth arrest in damaged cells. In light of these possibilities, inhibition of p16INK4a functions after damage could favour tissue regeneration without increasing the risk of developing cancer. Hence, to test this hypothesis, we injected conditional p16INK4a null mice with tamoxifen for 5 days either before or after exposure to 2.5 Gy irradiation and monitored tumor incidence over one year (a schematic of the different groups used is shown in Figure 4a). Inactivation of p16INK4a alone (group B) or exposure to IR alone (group C) was shown to induce cancer in about 60% of mice (Figure 4b). In contrast, none of the untreated mice (group A) had develop cancer during that time. Inactivation of p16INK4a before exposure to IR (group D) increased cancer incidence with only about half the mice alive 30 weeks post treatment. More importantly, mice that received tamoxifen 8 weeks post exposure to IR (group E), removing sustained p16INK4a expression long after damage induction, displayed a significant increase in cancer incidence with only about half the mice alive 30 weeks post treatment. In fact, inactivation of p16INK4a after exposure to IR, compared to inactivation before IR, seemed to worsen the incidence of cancer one-year post treatment. Analysis of tissues revealed that inactivation of p16INK4a had only a modest impact on the type of cancer occurring with a high proportion of mice in all groups developing mostly (50–84%) lymphomas (Supplementary Figure S4a). Furthermore, PCR analysis showed that randomly selected tumours derived from all groups had deleted p16INK4a, even in mice not treated with tamoxifen (Supplementary Figure S4b). More importantly, none of the analyzed tumors seemed to have concomitantly deleted p19ARF gene (Supplementary Figure S4b). Overall, these results suggest that sustained IR-induced p16INK4a expression is necessary to protect against cancer progression. These results are similar to what was observed following the deletion of p539, suggesting an equivalent role for p16INK4a in maintaining tumorigenic cells in check. Yet, these results are in opposition to a model where transient expression of p53 (6 days only) was shown to be sufficient to protect mice from IR-induced lymphoma 5. Reason for such discrepancy is unclear but likely involves variation in the models used (germline vs somatic inactivation).
Figure 4.
Sustained p16INK4a expression is necessary to protect against cancer. (a) 8–12 weeks old Cre p16 L/L male and female mice were randomly distributed in n=21–25 mice per group and sacrificed 52 weeks post treatment. In group A, mice were left untreated. In groups B and C, mice received respectively Tam for 5 days or a single dose of 2.5 Gy total body irradiation. In group D, mice were first treated with Tam for 5 days and then immediately irradiated as in group C. In group E, mice were first irradiated and 8 weeks later received Tam for 5 days. (b) Kaplan/Meier curves showing cancer free survival of mice treated as described in a. Mice were sacrificed 52 weeks post treatment or once they had reach a distress point in accordance to our institutional animal guideline, whatever happened first. An autopsy was performed at the time of sacrificed and, when possible, tumor type was identified. Groups D and E were not statistically different (Wilcoxon test). Groups B and C were statistically different (p<0.001) from group E but not from group D (p=0.09 and p=0.06 respectively). (c) Schematic describing the expected role played by p16INK4a following exposure to IR. Inactivation of p16INK4a in irradiated cells in vitro leads to cell cycle re-entry and subsequent block in G2 that may or may not be dependent on cell culture conditions. Upon inactivation of p16INK4a in vivo, following irradiation or normal chronological aging, increase S phase and cancer progression is observed. Whether a G2 block occurs and the extent by which the SASP may contributes to cancer progression is unknown.
Many reasons may explain why a transient 8 weeks p16INK4a response, at the time of damage, failed to protect, if not worsen, cancer progression. First, the simplest explanation would be that p16INK4a-induced senescence/growth arrest is reversible in mice, a phenotype also previously observed in mouse embryo fibroblasts following the inactivation of p538. Second, it may be possible that the accumulation of p16INK4a positive cells, which occurs in group E but not in group D, is detrimental to cancer free survival, especially several weeks following IR. This may be possible if the accumulation of damaged cells in irradiated tissues favours cancer development through, for example, the secretion of inflammatory cytokines 6, 15. However, cytokine arrays performed on serum and spleen lysates collected from mice 8 weeks after IR did not show any meaningful changes compared to age-matched non irradiated animals (Supplementary Tables 1). Nonetheless, we speculate that it is possible that variation in certain cytokines, either not measured in these arrays or undetectable at the systemic level, may still have an impact, in the splenic or bone marrow microenvironment (for example). Still, the fact that inactivation of p16INK4a 8 weeks after exposure to IR did not somehow delay cancer incidence was very surprising. Third, we cannot rule out the possibility that tumors may have arisen from irradiated cells that had not yet increase p16INK4a expression prior to tamoxifen treatment, avoiding the need to bypass senescence. However, once again, one would have expected a reduction in cancer incidence in the event that cancer progression is stochastic and not limited to a subtype of cells which have delayed (more than 8 weeks) or do not at all increase p16INK4a expression upon IR.
Overall, IR-induced p16INK4a expression is necessary to maintain growth arrest long-term, in at least a subset of oncogenically activated cells. In fact, inactivation of p16INK4a in these cells may have directly lead to cancer progression and the G2 cell cycle arrest we observed in vitro is likely a culture artefact that does not occur in vivo (see Figure 4c). We speculate that if a G2 block would have occurred in mice, it would have been expected to at least delay cancer incidence, which it did not. Still, the scenario of a G2 block occurring in vivo may be reconcilable with our data if the protective effect of cell cycle block is masked by the pro-tumorigenic inflammatory phenotype. Direct elimination of damaged cells and their secretory phenotype using newly developed mice strains containing a suicide gene under the control of the p16INK4a promoter may help resolve this question 1, 3, 7.
In conclusion, it will be interesting to determine if there is a link between the development of lymphoma and the proportion of senescent cells observed in the spleen. We believe it is conceivable that senescent splenic stromal cells act in a non-autonomous manner to foster the development of lymphoma, the same way we previously showed they act on lymphopoiesis 4. Also, given the apparent necessity for sustained p16INK4a expression to protect against cancer progression, we believe it is of utmost importance to identify the inducers of p16INK4a at the molecular level. The identification and subsequent modulation of these inducers may make it possible to increase the regeneration of irradiated/aged tissues without increasing the risk of developing cancer.
Supplementary Material
Acknowledgments
We are grateful to the members of Dr Elie Haddad’s laboratory, flow cytometry and animal facility for providing technical support. We also would like to thank Dr Francis Rodier and Mohamad El Harris for the critical reading of the manuscript. This work was supported by a grant from the Canadian Institute of Health Research #MOP-341566 to C.M.B. L.P. has been supported by a student fellowship from the Fondation des Étoiles. C.M.B. is supported by a scientist award from the Fonds de recherche du Québec - Santé.
Footnotes
CONFLICT OF INTERST
No financial interest/relationships relating to the topic of this article have been declared.
References
- 1.Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beausejour CM, Krtolica A, Galimi F, Narita M, Lowe SW, Yaswen P, et al. Reversal of human cellular senescence: roles of the p53 and p16 pathways. Embo J. 2003;22:4212–4222. doi: 10.1093/emboj/cdg417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burd CE, Sorrentino JA, Clark KS, Darr DB, Krishnamurthy J, Deal AM, et al. Monitoring tumorigenesis and senescence in vivo with a p16(INK4a)-luciferase model. Cell. 2013;152:340–351. doi: 10.1016/j.cell.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carbonneau CL, Despars G, Rojas-Sutterlin S, Fortin A, Le O, Hoang T, et al. Ionizing radiation-induced expression of INK4a/ARF in murine bone marrow-derived stromal cell populations interferes with bone marrow homeostasis. Blood. 2012;119:717–726. doi: 10.1182/blood-2011-06-361626. [DOI] [PubMed] [Google Scholar]
- 5.Christophorou MA, Ringshausen I, Finch AJ, Swigart LB, Evan GI. The pathological response to DNA damage does not contribute to p53-mediated tumour suppression. Nature. 2006;443:214–217. doi: 10.1038/nature05077. [DOI] [PubMed] [Google Scholar]
- 6.Coppe JP, Patil CK, Rodier F, Sun Y, Munoz DP, Goldstein J, et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Demaria M, Ohtani N, Youssef SA, Rodier F, Toussaint W, Mitchell JR, et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Developmental cell. 2014;31:722–733. doi: 10.1016/j.devcel.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dirac AM, Bernards R. Reversal of senescence in mouse fibroblasts through lentiviral suppression of p53. J Biol Chem. 2003;278:11731–11734. doi: 10.1074/jbc.C300023200. [DOI] [PubMed] [Google Scholar]
- 9.Hinkal G, Parikh N, Donehower LA. Timed somatic deletion of p53 in mice reveals age-associated differences in tumor progression. PLoS One. 2009;4:e6654. doi: 10.1371/journal.pone.0006654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ito K, Hirao A, Arai F, Matsuoka S, Takubo K, Hamaguchi I, et al. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature. 2004;431:997–1002. doi: 10.1038/nature02989. [DOI] [PubMed] [Google Scholar]
- 11.Ito K, Hirao A, Arai F, Takubo K, Matsuoka S, Miyamoto K, et al. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med. 2006;12:446–451. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- 12.Janzen V, Forkert R, Fleming HE, Saito Y, Waring MT, Dombkowski DM, et al. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature. 2006;443:421–426. doi: 10.1038/nature05159. [DOI] [PubMed] [Google Scholar]
- 13.Johmura Y, Shimada M, Misaki T, Naiki-Ito A, Miyoshi H, Motoyama N, et al. Necessary and sufficient role for a mitosis skip in senescence induction. Mol Cell. 2014;55:73–84. doi: 10.1016/j.molcel.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Krishnamurthy J, Ramsey MR, Ligon KL, Torrice C, Koh A, Bonner-Weir S, et al. p16INK4a induces an age-dependent decline in islet regenerative potential. Nature. 2006;443:453–457. doi: 10.1038/nature05092. [DOI] [PubMed] [Google Scholar]
- 15.Krtolica A, Parrinello S, Lockett S, Desprez PY, Campisi J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc Natl Acad Sci U S A. 2001;98:12072–12077. doi: 10.1073/pnas.211053698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le ON, Rodier F, Fontaine F, Coppe JP, Campisi J, DeGregori J, et al. Ionizing radiation-induced long-term expression of senescence markers in mice is independent of p53 and immune status. Aging Cell. 2010;9:398–409. doi: 10.1111/j.1474-9726.2010.00567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marcoux S, Le ON, Langlois-Pelletier C, Laverdiere C, Hatami A, Robaey P, et al. Expression of the senescence marker p16INK4a in skin biopsies of acute lymphoblastic leukemia survivors: a pilot study. Radiation oncology. 2013;8:252. doi: 10.1186/1748-717X-8-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Molofsky AV, Slutsky SG, Joseph NM, He S, Pardal R, Krishnamurthy J, et al. Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature. 2006;443:448–452. doi: 10.1038/nature05091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parrinello S, Samper E, Krtolica A, Goldstein J, Melov S, Campisi J. Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts. Nat Cell Biol. 2003;5:741–747. doi: 10.1038/ncb1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robles SJ, Adami GR. Agents that cause DNA double strand breaks lead to p16INK4a enrichment and the premature senescence of normal fibroblasts. Oncogene. 1998;16:1113–1123. doi: 10.1038/sj.onc.1201862. [DOI] [PubMed] [Google Scholar]
- 21.Ruzankina Y, Pinzon-Guzman C, Asare A, Ong T, Pontano L, Cotsarelis G, et al. Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss. Cell Stem Cell. 2007;1:113–126. doi: 10.1016/j.stem.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salama R, Sadaie M, Hoare M, Narita M. Cellular senescence and its effector programs. Genes Dev. 2014;28:99–114. doi: 10.1101/gad.235184.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanoff HK, Deal AM, Krishnamurthy J, Torrice C, Dillon P, Sorrentino J, et al. Effect of cytotoxic chemotherapy on markers of molecular age in patients with breast cancer. J Natl Cancer Inst. 2014;106:dju057. doi: 10.1093/jnci/dju057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Serrano M, Lee H, Chin L, Cordon-Cardo C, Beach D, DePinho RA. Role of the INK4a locus in tumor suppression and cell mortality. Cell. 1996;85:27–37. doi: 10.1016/s0092-8674(00)81079-x. [DOI] [PubMed] [Google Scholar]
- 25.Sharpless NE, Bardeesy N, Lee KH, Carrasco D, Castrillon DH, Aguirre AJ, et al. Loss of p16Ink4a with retention of p19Arf predisposes mice to tumorigenesis. Nature. 2001;413:86–91. doi: 10.1038/35092592. [DOI] [PubMed] [Google Scholar]
- 26.Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 27.Sorrentino JA, Krishnamurthy J, Tilley S, Alb JG, Jr, Burd CE, Sharpless NE. p16INK4a reporter mice reveal age-promoting effects of environmental toxicants. J Clin Invest. 2014;124:169–173. doi: 10.1172/JCI70960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sousa-Victor P, Gutarra S, Garcia-Prat L, Rodriguez-Ubreva J, Ortet L, Ruiz-Bonilla V, et al. Geriatric muscle stem cells switch reversible quiescence into senescence. Nature. 2014;506:316–321. doi: 10.1038/nature13013. [DOI] [PubMed] [Google Scholar]
- 29.Stein GH, Drullinger LF, Soulard A, Dulic V. Differential roles for cyclin-dependent kinase inhibitors p21 and p16 in the mechanisms of senescence and differentiation in human fibroblasts. Mol Cell Biol. 1999;19:2109–2117. doi: 10.1128/mcb.19.3.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Deursen JM. The role of senescent cells in ageing. Nature. 2014;509:439–446. doi: 10.1038/nature13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y, Schulte BA, LaRue AC, Ogawa M, Zhou D. Total body irradiation selectively induces murine hematopoietic stem cell senescence. Blood. 2006;107:358–366. doi: 10.1182/blood-2005-04-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamakoshi K, Takahashi A, Hirota F, Nakayama R, Ishimaru N, Kubo Y, et al. Real-time in vivo imaging of p16Ink4a reveals cross talk with p53. J Cell Biol. 2009;186:393–407. doi: 10.1083/jcb.200904105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou BB, Elledge SJ. The DNA damage response: putting checkpoints in perspective. Nature. 2000;408:433–439. doi: 10.1038/35044005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.