Abstract
Prior studies have demonstrated dysfunctions within the core neurocognitive networks (the executive control [ECN], default mode [DMN] and salience [SN] networks) in late-life depression (LLD). Whether inter-network dysfunctional connectivity is present in LLD, and if such disruptions are associated with core symptom dimensions is unknown. A cross-sectional resting-state functional connectivity magnetic resonance imaging investigation was conducted of LLD (n=39) and age- and gender-equated healthy comparison (HC) (n=29) participants. Dual regression independent component analysis approach was used to identify components that represented the ECN, DMN and SN. The intrinsic inter-network connectivity was compared between LLD and HC participants and the relationship of inter-network connectivity abnormalities with dimensional measures was examined. Relative to HC participants, LLD subjects showed decreased inter-network connectivity between the bilateral ECN and default mode subcortical (thalamus, basal ganglia and ventral striatum) networks, and the left ECN and SN insula component; and increased inter-network connections between the left ECN and posterior DMN and salience (dorsal anterior cingulate) network components. Distinct internetwork connectivity abnormalities correlated with depression and anxiety severity, and executive dysfunction in LLD participants. LLD subjects also showed pronounced intra-network connectivity differences within the ECN, whereas fewer but significant DMN and SN disruptions were also detected. Investigating the intrinsic inter-network functional connectivity could provide a mechanistic framework to better understand the neural basis that underlies core symptom dimensions in LLD. Inter-network connectivity measures have the potential to be neuroimaging biomarkers of symptom dimensions comprising LLD, and may assist in developing symptom-specific treatment algorithms.
Keywords: Late-life depression, symptom dimensions, resting-state functional connectivity, ICA, inter-network, brain networks
INTRODUCTION
Late-life depression (LLD) is a recurrent, clinically heterogeneous syndrome that currently is defined by specific psychiatric symptom dimensions, as well as multidomain cognitive dysfunction. LLD is associated with delayed treatment response, leaving many to suffer from persistent emotional distress, poor medical and functional outcomes, cognitive decline, increased suicide rates, and premature mortality (Naismith et al., 2012). Substantial literature suggests that poor treatment outcomes in the depressed elderly are associated with greater symptom severity and cognitive impairment, specifically persistent executive function and episodic memory impairments (Alexopoulos et al., 2005; Andreescu et al., 2007; Gildengers et al., 2005; Sheline et al., 2010a). However, current knowledge regarding the neural substrates of categorically defined LLD provides a limited perspective on the neurobiological mechanisms underlying multiple symptom dimensions comprising this disorder. Regardless, the aberrant frontal-subcortical neural circuitry, which is thought to be driven by underlying cerebrovascular ischemia and neurodegenerative processes, may contribute to clinical features of poor treatment response in LLD (Aizenstein et al., 2014; Taylor et al., 2013). Frontal, striatal, and limbic regional abnormalities could simultaneously impair function in multiple brain networks and explain the considerable variability seen in the clinical manifestation of LLD.
Converging evidence supports brain network dysfunction as a model of the potential neural mechanisms involved in the pathogenesis of LLD’s clinical heterogeneity (Li et al., 2015; Tadayonnejad and Ajilore, 2014). The three core neurocognitive networks, the executive control (ECN), default mode (DMN), and salience (SN) neuronal systems are considered relevant contributors to the abnormal behaviors and impaired cognitive processes observed in depression (Mulders et al., 2015). Signs of executive dysfunction frequently accompany LLD (Taylor et al., 2013), and the ECN plays a critical role in such functions, including working memory, cognitive control, judgment, and decision-making in the context of goal-directed behaviors. Using seed-based resting-state functional connectivity magnetic resonance imaging (R-fcMRI) technique, diminished functional connectivity (Fc) within the ECN is observed in LLD and is predictive of poor treatment response, persistent depressive symptoms and executive dysfunction (Alexopoulos et al., 2012). LLD is also associated with enhanced DMN Fc in symptomatic (Alexopoulos et al., 2012; Eyre et al., 2016) but not remitted (Sexton et al., 2012) patients. Intrinsic ECN and DMN Fc changes differ based on antidepressant response, and could serve as early markers of treatment response variability in LLD (Karim et al., 2016). The DMN regions primarily mediate episodic and autobiographical memory, self-monitoring, and related social cognitive functions (Buckner et al., 2008). These same DMN areas also play an important role in emotional regulation and are linked to impaired self-referential processing, negativity bias, and increased rumination during depressive episodes (Sheline et al., 2009). Finally, the SN is sensitive to salient environmental events and is involved in interoceptive awareness and emotional experiences (Menon and Uddin, 2010). Disrupted SN connectivity has been detected in major depression, and may be reflective of disease severity and increased somatization (Manoliu et al., 2013; Paulus and Stein, 2006). These investigations focus on intra-network Fc disruptions in LLD, but there is a dearth of R-fcMRI studies that examine the interactions between the aforementioned functional networks in LLD. The ECN, DMN, and SN are densely interconnected and, therefore, dysfunction in any one of these networks may also disrupt other functional systems resulting in the clinical heterogeneity seen in LLD.
To further understand the brain network abnormalities underlying the clinical heterogeneity of LLD, it is imperative to examine how network dysfunction relates to the different symptom dimensions comprising LLD. It is plausible that the neurobiological mechanisms underlying symptom dimensions may not cleanly map to the neural correlates of the categorically defined LLD diagnosis. Such an approach brings the field of LLD research one step closer to the goals of the Research Domain Criteria (RDoC) project, a National Institute of Mental Health initiative that aims to develop novel ways to classify psychiatric syndromes based on neurobiological measures and behavioral dimensions.
By applying the dual regression independent component analysis (ICA) approach on R-fcMRI data, we first aimed to determine the inter-network Fc between ECN, DMN, and SN in individuals with LLD. The ICA method extracts low frequency signal fluctuations at rest and from a single data-driven analysis allows simultaneous estimation of multiple spatially independent intrinsic networks that correspond to those established by task-based functional MRI studies (Beckmann et al., 2005; Biswal et al., 2010; Smith, S.M. et al., 2009). Secondly, we examined the association of inter-network Fc abnormalities with specific symptom dimensions that are commonly encountered in persons with LLD (i.e., depressive and anxiety symptoms, executive functioning and episodic memory). We hypothesized that intrinsic ECN connectivity abnormalities with sub-networks of the DMN and SN would differentiate LLD from nondepressed cognitively healthy comparison (HC) subjects. We further postulated that the observed inter-network Fc abnormalities would correlate with depression and anxiety severity and greater executive dysfunction in LLD. Finally, we performed a voxelwise ICA analysis in the ECN, DMN, and SN to examine intra-network Fc differences in the regions implicated in emotional regulation that distinguish LLD from HC subjects.
METHODS AND MATERIALS
Participants
We enrolled 68 participants aged 60 years and older into the current study. The participant groups included patients with LLD (n = 39) and age- and gender-equated HC (n = 29) participants. All LLD subjects were recruited from the Medical College of Wisconsin (MCW) Geriatric Psychiatry and Memory Disorders Clinics. The HC subjects were recruited from the community using advertisements. The MCW Institutional Review Board approved this study, and written informed consent was obtained.
The core neuropsychological battery administered to all participants included the Mini-Mental State Examination (MMSE) (Folstein et al., 1975); Mattis Dementia Rating Scale-2 (MDRS-2) (Lucas et al., 1998); education-adjusted Logical Memory II Delayed Paragraph Recall (LMII-DR) subscale from the Wechsler Memory Scale-Revised (Wechsler, 1987); Physical Self Maintenance Scale/Instrumental Activities of Daily Living (PSMS/IADL) (Lawton and Brody, 1969); 30-item Yesavage Geriatric Depression Scale (GDS) (Yesavage et al., 1982); diagnostic assessment for Axis 1 disorders, including the depression module from the Structured Clinical Interview for DSM IV Disorders (SCID) (First et al., 2002); modified Hachinski Ischemic Scale (HIS); and Hamilton Anxiety Scale (HAM-A) (Hamilton, 1959). The neuropsychiatric and functional scales were chosen based on their ability to characterize cognitive functioning and depression severity in prior LLD studies (Butters et al., 2004).
Inclusion/exclusion criteria
All participants met the following criteria: MMSE score > 24, age- and education-corrected Mayo Clinic’s Older American Normative Studies (MOANS)-scaled score of ≥ 5 on MDRS-2, no dementia diagnosis, and HIS < 4.
LLD
Specific inclusion criteria for the LLD patients included a GDS score ≥ 10 and SCID depression module positive for major depression. The objective of this study was to investigate the neurobiological correlates of multi-domain symptoms that mimic a clinically representative sample of LLD patients. Therefore, we did not exclude participants with significant anxiety or mild cognitive impairment as long as the primary diagnosis was LLD, which is consistent with previous studies (Butters et al., 2004; Mulsant et al., 2001).
HC
The eligibility criteria were similar, except these participants had to be cognitively normal and could not be on psychoactive medications or meet lifetime criteria for any psychiatric disorders.
Exclusion criteria included past or current history of concurrent Axis 1 psychiatric disorders, such as psychotic or bipolar disorders; alcohol or substance abuse/dependence during the past five years; active suicidality; a history of neurological disease, including Parkinson’s disease, dementia, multiple sclerosis, seizures, or stroke; head injury with loss of consciousness; MRI contraindications; and unstable chronic medical conditions.
MRI data acquisition and preprocessing
All MRI data were obtained using a GE 3T MR750 scanner with a standard transmit-receive quadrature head coil. Participants were instructed to close their eyes and relax during the scans. All participants underwent an 8-minute task-free R-fcMRI scan, TR/TE = 2000 ms/25 ms using a single-shot gradient echo planar sequence, and a 6-min high-resolution 3D anatomical scan using a spoiled gradient-recalled echo sequence.
SPM8 (http://www.fil.ion.ucl.ac.uk/spm) software and the VBM8 (http://www.neuro.uni-jena.de/vbm) toolbox were used to process the SPGR anatomical scans. Individual high-resolution T1-weighted image was segmented into gray matter (GM), white matter (WM), and cerebral spinal fluid (CSF) components. Whole brain volumes for each type of tissue component were summed to obtain the intracranial volume (ICV). The gray matter ratio (GMR) was then calculated as a fraction between GM volume (GMV) and ICV.
AFNI software (http://afni.nimh.nih.gov), FSL software (http://www.fmrib.ox.ac.uk), and MATLAB programs (The MathWorks Inc., Natick, MA) were utilized to process R-fcMRI data. Preprocessing steps were based on previously published procedures described by Biswal et al (Biswal et al., 2010). Briefly, the slice-timing corrected images were corrected for nuisance signals such as motion, heart rate, respiration, WM, and CSF. A band-pass filter (0.01 ~ 0.1 Hz) and a 5-mm Gaussian smooth kernel were applied to the images followed by spatial normalization to the Montreal Neurological Institute (MNI) template space.
Additional imaging parameters and preprocessing steps are described in the supplement.
MRI data analysis
First, the preprocessed R-fcMRI data from all participants were temporally concatenated. The concatenated group data was used in the group-level ICA to generate group-level independent components (IC) for all participants using FSL’s MELODIC function (Beckmann et al., 2005). The total number of IC was fixed at 20 based on recent publications on ICA using R-fcMRI (Biswal et al., 2010; Smith, S.M. et al., 2009; Wang et al., 2013). To ensure consistency of the 20 ICs, group ICA was repeated eight times using randomly resampled data (Biswal et al., 2010). Specifically, a meta-analysis was completed using all repetitions of the group ICA components to extract 20 spatially independent components. The dual regression method was then used to reconstruct the IC maps for each individual using the 20 group ICs. The first regression model was used to regress the group IC template with the individual participants’ 4D data to produce a set of time courses. Using each of these unique time courses as the temporal predictor, a second regression model was used to estimate the subject-specific spatially independent maps corresponding to the 20 ICs (Biswal et al., 2010; Filippini et al., 2009; Smith, S.M. et al., 2009; Wang et al., 2013).
Defining inter-network functional connectivity matrix
After the dual-regression ICA analysis, we selected seven of the 20 ICs to indicate three intrinsic networks of interest: the ECN, DMN, and SN (Table S1). We then performed a partial correlation analysis among these seven ICs using their associated time courses, which resulted in a 7 × 7 symmetric intrinsic network connectivity (INC) matrix for each participant. Each of the 21 unique cells in the matrix reflects the internetwork Fc between a pair of ICs. The diagonal values of each INC matrix were excluded from the following statistical analyses because they all have the same value, one.
Voxelwise ICA analysis
In order to examine the spatial extent of differences in the three intrinsic networks, voxel-level analyses were performed (Wang et al., 2013). Specifically, the data from the seven ICs were combined and averaged to generate the voxel-wise z-score maps for each of the three intrinsic Fc networks based on their associated ICs. The ECN map included two ICs, which represented the left and right ECN (regions included the dorsolateral prefrontal cortex [DLPFC] and the superior parietal lobe [SPL]). The DMN map included three ICs, which represented the subcortical (thalamus and striatum), and anterior and posterior portions of the DMN (medial prefrontal cortex [mePFC], inferior parietal lobe [IPL], posterior cingulate cortex [PCC], and parahippocampal gyrus [PHG]). Finally, the SN map included two ICs, which represented the dorsal anterior cingulate cortex (dACC) and insula.
An anatomical mask, which contained a priori defined ROIs, was applied to each subject’s Fc map to limit the number of voxels being compared. The a priori ROIs were selected based on regions of the emotional regulation circuitry (Fusar-Poli et al., 2009; Li et al., 2015), which also included areas that are integral to the ECN, DMN, SN, and others implicated in emotional processing (Table S2). The Wake Forest University PickAtlas toolbox (http://fmri.wfubmc.edu/software/pickatlas) was used to create the anatomical ROI mask (Figure S1).
Statistical analysis
Demographic information (age and education), except gender (using χ2-test), and neuropsychological measurements were compared between the LLD and HC groups using two-sample t-tests (SPSS software version 18.0 [SPSS Inc., Chicago, IL]).
The group-mean INC matrix was obtained by averaging across participants within each study group. A two-sample t-test was used to determine the differences in the partial correlation values for each matrix cell, between LLD and HC groups, while controlling for age, gender, education, and GMR. False discovery rate (FDR) correction (q < 0.05) was applied to account for multiple comparison errors. Multiple linear regression analyses were used to investigate the neural correlates of depressive and anxiety symptoms, episodic memory and executive functioning in the LLD group. The clinical measures (i.e., GDS, HAM-A, LMII-DR, and Initiation/Perseveration subscale of the MDRS-2 [MDRS-2 Init/Pers]) were used as dependent variables. Separate models were used for each behavior, and clinical measures were regressed with unique partial correlation values that showed differences between LLD and HC in the INC matrix while controlling for age, gender, education, and GMR. Multiple linear regression analyses were performed to examine the relationships of age of depression onset (independent variable) with the INC abnormalities (dependent variable) in the LLD group.
A voxel-level two-sample t-test was performed to determine the intra-network Fc differences between LLD and HC groups in the ECN, DMN, and SN limited to voxels within the a priori ROIs (Figure S1, Table S2), while controlling for age, gender, education, and voxelwise GMV. Monte Carlo simulation (3dClustSim) was adopted to correct for the false-positive findings. To account for three comparisons, the statistical threshold was set at p < 0.0167 and cluster size > 664 mm3.
RESULTS
Demographics
The LLD and HC groups did not show significant differences in age, gender, and education (p > 0.05) (Table 1). The differences in the neuropsychiatric characteristics are summarized in Table 1.
Table 1.
Demographics and clinical characteristics
| HC (n = 29) Mean ± SD |
LLD (n = 39) Mean ± SD |
p-value | |
|---|---|---|---|
| Age | 71.79 ± 6.95 | 68.62 ± 6.45 | 0.080 |
| Gender (F/M) | 17/12 | 30/9 | 0.120 |
| Education | 15.24 ± 2.61 | 14.46 ± 2.76 | 0.239 |
| Age of onset | N/A | 35.66 ± 18.74 | |
| Neuropsychiatric measurements | |||
| GDS | 1.83 ± 1.75 | 17.00 ± 5.36 | <0.001 |
| MMSE | 28.83 ± 1.31 | 27.79 ± 1.47 | 0.003 |
| HAM-A | 1.14 ± 1.15† | 10.00 ± 4.33 | <0.001 |
| Memory Recall Scores | |||
| Immediate | 14.55 ± 3.28 | 11.69 ± 4.69 | 0.004 |
| Delayed | 12.97 ± 3.08 | 9.64 ± 5.49 | 0.002 |
| MDRS-2 raw scores | |||
| Attention | 36.45 ± 0.57 | 36.41 ± 0.82 | 0.822 |
| Init/Pers | 36.48 ± 1.12 | 35.13 ± 3.87 | 0.044 |
| Construction | 5.97 ± 0.19 | 5.97 ± 0.16 | 0.838 |
| Conceptualization | 37.76 ± 1.27 | 36.64 ± 2.45 | 0.018 |
| Memory | 23.76 ± 1.12 | 22.94 ± 2.67 | 0.085 |
| Total | 140.48 ± 2.43 | 137.10 ± 6.44 | 0.004 |
| Current antidepressants (%) | |||
| No antidepressant | N/A | 3 (7.7) | |
| SSRI or SNRI monotherapy | N/A | 21 (53.9) | |
| NDRI or MIRZ monotherapy | N/A | 5 (12.8) | |
| Combination treatment | N/A | 10 (25.6) |
Notes
HAM-A score was not available for one HC subject; the Mean and SD are therefore based on the remaining 28 subjects.
Abbreviations – HC, healthy comparison; LLD, late-life depression; SD, standard deviation; F/M, female/male; GDS, geriatric depression scale; MMSE, mini-mental state examination; HAM-A, Hamilton anxiety scale; MDRS-2, Mattis dementia rating scale-2; Init/Pers, Initiation/Preservation; SSRI: selective serotonin reuptake inhibitor; SNRI: serotonin norepinephrine reuptake inhibitor; NDRI: norepinephrine dopamine reuptake inhibitor; MIRZ: mirtazapine
Intrinsic network connectivity matrix group differences
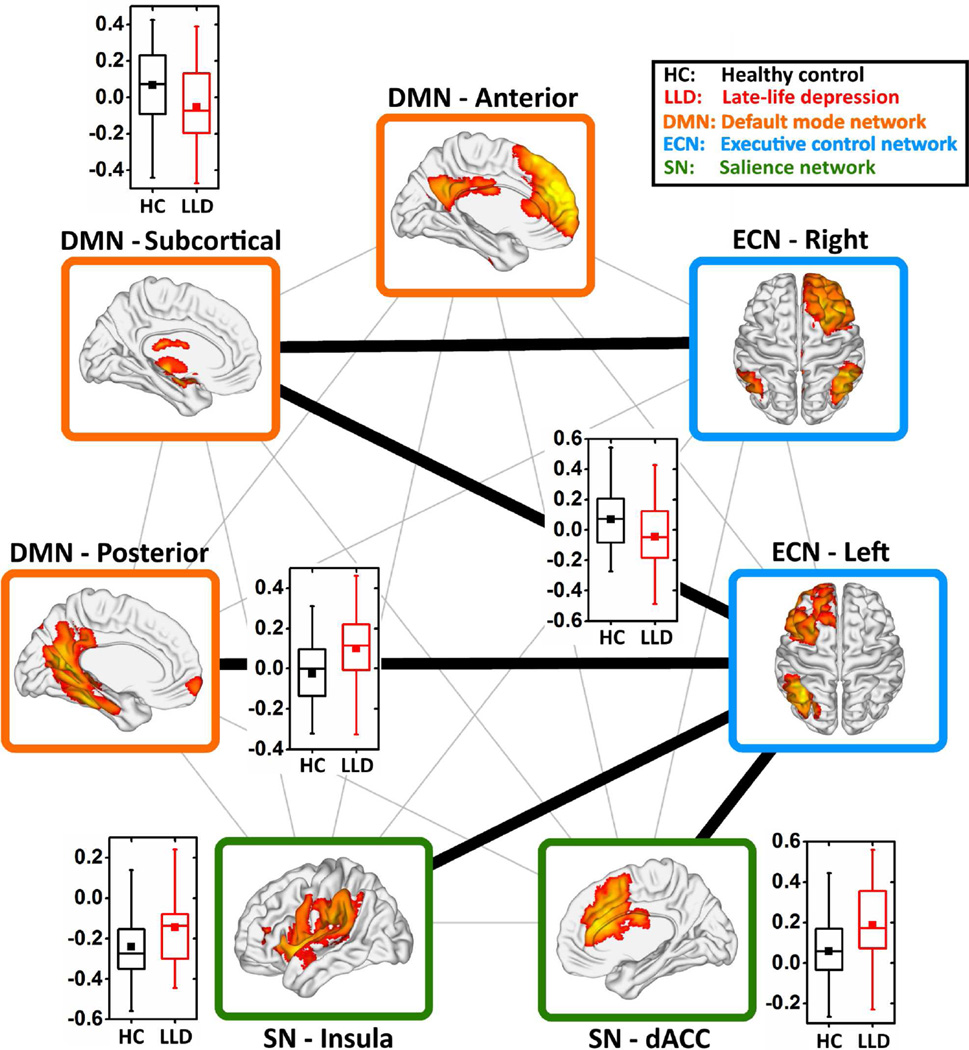
After controlling for covariates, the LLD subjects showed decreased positive inter-network Fc between the bilateral ECN and DMN subcortical components, and decreased negative Fc between the left ECN and SN insula when compared to the HC participants. Also, the LLD subjects showed increased inter-network Fc between the left ECN and posterior DMN with a reversal from negative to positive connectivity, and increased positive connectivity between the left ECN and SN dACC (FDR corrected, q < 0.05) (Figure 1). The age of depression onset did not correlate with INC abnormalities in the LLD group.
Figure 1. Disrupted inter-network intrinsic connectivity in late-life depression.
The inter-network INC was calculated as the partial-correlation among the unique time courses of the seven independent components, which were selected to indicate the three intrinsic functional connectivity networks (i.e. ECN, blue color; DMN, orange color; and SN, green color). The thick black lines between each pair of components represent functional connectivity differences between the HC and LLD groups. The box plots show the details of the between-group comparisons (two-sample t-test, FDR-corrected q < 0.05). The vertical axes represent the functional connectivity. The black and red boxes represent the HC and LLD groups, respectively. Abbreviations: ECN, executive control network; DMN, default mode network; SN, salience network; FDR, false discovery rate; HC, healthy comparison; LLD, late-life depression.
Relationships between INC abnormalities and behaviors in LLD
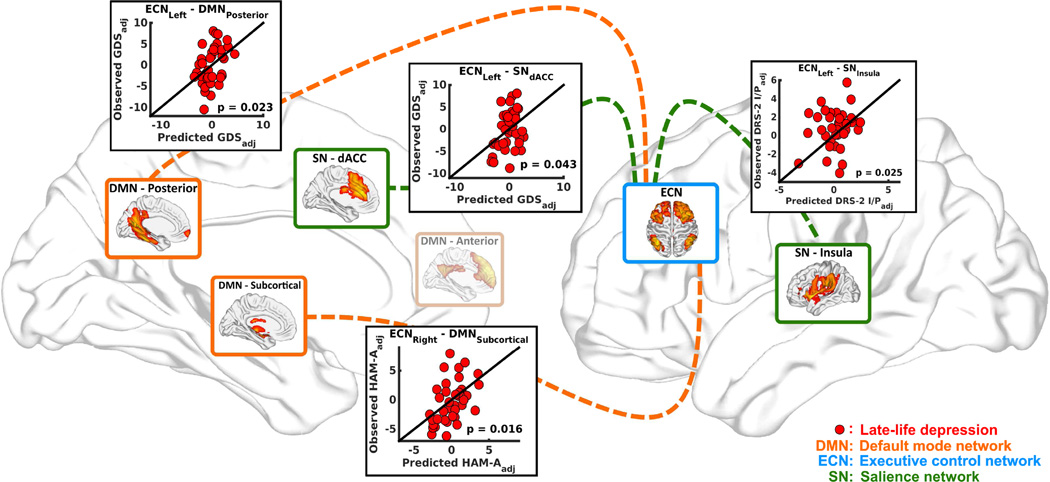
Multiple linear regression analyses revealed the associations between dimensional measures and abnormal INCs in the LLD group (Figure 2). The INC between the left ECN and posterior DMN (R2 = 0.17, p = 0.023) and the left ECN and SN dACC (R2 = 0.13, p = 0.043) correlated with the GDS scores. The INC between the left ECN and SN insula correlated with the MDRS-2 Init/Pers scores (R2 = 0.16, p = 0.025). The INC between the right ECN and subcortical DMN correlated with the HAM-A scores (R2 = 0.18, p = 0.016). Education (R2 = 0.32, p = 0.001) and GMR (R2 = 0.13, p = 0.042) were associated with LMII-DR in the LLD group; however, no INC abnormalities correlated with memory scores.
Figure 2. Relationships between the multi-domain symptom measurements and abnormal INC.
Distinct inter-network functional connectivity abnormalities in the LLD group predicted symptom dimension scores (i.e., GDS, HAM-A, LMII-DR, and MDRS-2 Init/Pers). The linear regression models were adjusted for age, gender, education, and whole-brain GMR. Each scatter plot shows predicted symptom dimension score based on internetwork functional connectivity value (x-axis) plotted against observed symptom dimension score (y-axis). Each red dot represents individual LLD participant. Abbreviations: INC, intrinsic network connectivity; LLD, late-life depression; GDS, geriatric depression scale; HAM-A, Hamilton anxiety scale; LMII-DR, education-adjusted Logical Memory II Delayed Paragraph Recall subscale from the Wechsler Memory Scale-Revised; MDRS-2 Init/Pers, Mattis Dementia Rating Scale – 2 Initiation/Perseveration subscale; dACC, dorsal anterior cingulate.
Voxelwise intra-network intrinsic Fc comparisons
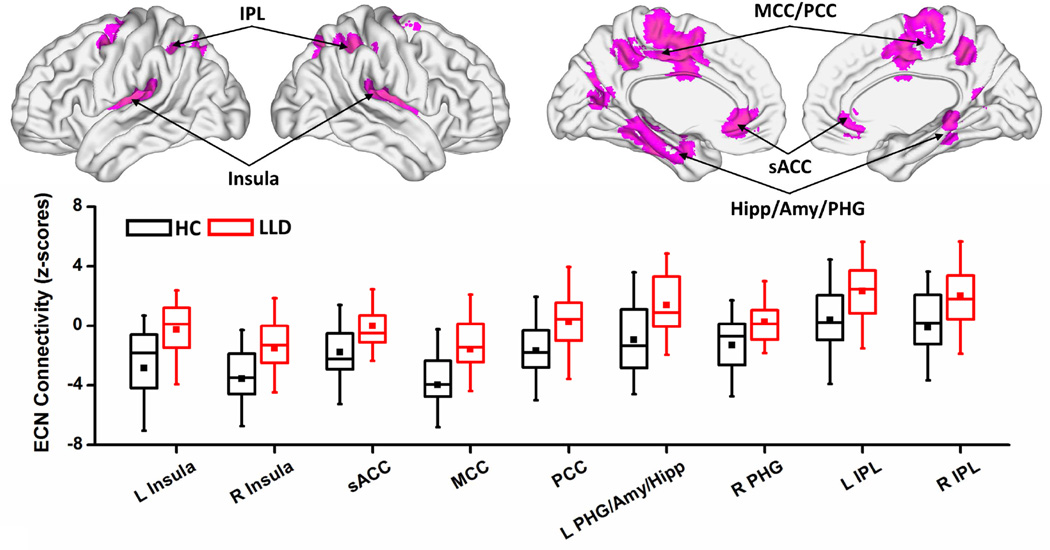
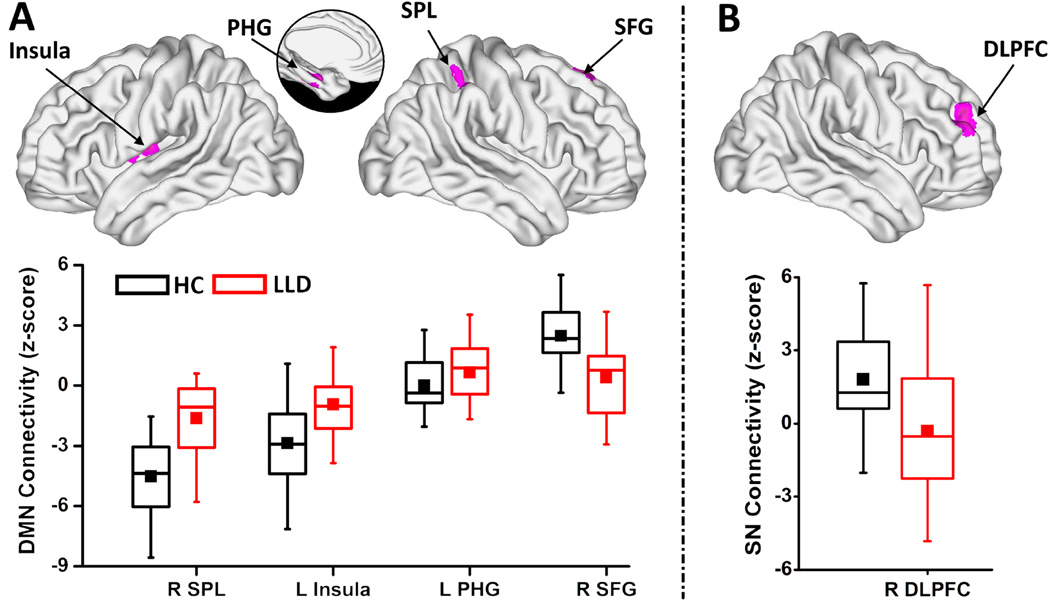
The one-sample voxelwise patterns for LLD and HC groups are included in the supplement (Figure S2). Relative to HC participants, LLD subjects showed the following voxel-level abnormalities in the three networks (Table 2; Figures 3 and 4):
ECN: Decreased negative Fc in the bilateral insula, subgenual anterior cingulate cortex (sACC), and middle cingulate cortex (MCC); decreased negative Fc and a reversal of Fc patterns to positive in the bilateral PCC and left medial temporal lobe (MTL) cluster, which includes PHG, hippocampus (Hipp), and amygdala (Amy). The bilateral IPL showed increased positive Fc (Figure 3).
DMN: Decreased negative Fc in the left insula and right SPL; increased connectivity in the left PHG; and decreased positive DMN connectivity in the right superior frontal gyrus (SFG) (Figure 4A).
SN: Decreased positive Fc in the right DLPFC (Figure 4B).
Table 2.
Voxelwise two-sample t-test results for the INC differences
| Regions Side | Side | Cluster size (mm3) |
Coordinates (RAI) |
z-score | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Executive Control Network | ||||||
| Decreased Negative FC | ||||||
| Insula | L | 4864 | 40 | 18 | 14 | 4.57 |
| R | 4504 | −38 | 2 | 8 | 3.85 | |
| sACC | L/R | 1952 | 2 | −42 | −2 | 3.42 |
| MCC | L/R | 18816 | 12 | 17 | 38 | 4.17 |
| Decreased Negative FC (reversal to positive FC) | ||||||
| PCC | L/R | 8768 | 15 | 44 | 38 | 4.64 |
| PHG | L | 5936* | 28 | 16 | −30 | 4.14 |
| Amy | L | * | 20 | 7 | −20 | 2.86 |
| Hipp | L | * | 21 | 26 | −15 | 2.98 |
| PHG | R | 1392 | −28 | 38 | −6 | 3.64 |
| Increased Positive FC | ||||||
| IPL | L | 664 | 36 | 46 | 42 | 3.94 |
| R | 2272 | −44 | 46 | 52 | 3.39 | |
| Default Mode Network | ||||||
| Decreased Negative FC | ||||||
| SPL | R | 886 | −48 | 44 | 56 | 4.68 |
| Insula | L | 1080 | 42 | 0 | 8 | 3.71 |
| Increased Positive FC | ||||||
| PHG | L | 888 | 26 | 16 | 30 | 3.16 |
| Decreased Positive FC | ||||||
| SFG | R | 568 | −12 | −30 | 62 | −3.84 |
| Salience Network | ||||||
| Decreased Positive FC | ||||||
| DLPFC | R | 944 | −26 | −46 | 30 | −3.16 |
regions in the same cluster.
Abbreviations – INC: intrinsic network connectivity; FC: functional connectivity, sACC: subgenual anterior cingulate cortex, MCC: middle cingulate cortex, PCC: posterior cingulated cortex, PHG: parahippocampal gyrus, Amy: amygdala, Hipp: hippocampus, IPL: inferior parietal lobe, SPL: superior parietal lobe, SFG: superior frontal gyrus, DLPFC: dorsolateral prefrontal cortex, L/R: left/right.
Figure 3. Voxelwise intra-network functional connectivity differences between HC and LLD groups in the ECN.
Voxelwise two-sample t-test (top, magenta colored regions) shows the spatial extent of the intra-network abnormalities in the ECN (p < 0.0167 and 3dClustSim corrected cluster size > 664 mm3). The box plots (bottom) indicate the quantitative data distribution of the individual groups (HC: black color and LLD: red color). In the box plots, the bands, from the top, represent the 75%, median, and 25%, respectively. The square in each box indicates the mean and whiskers shows the first standard deviation of the data. Abbreviations: HC, healthy comparison; LLD, late-life depression; L/R, left/right; sACC, subgenual anterior cingulate cortex; MCC, middle cingulate cortex; PCC, posterior cingulate cortex; PHG, parahippocampal gyrus; Amy, amygdala; Hipp, hippocampus; IPL, inferior parietal lobe.
Figure 4. Voxelwise intra-network functional connectivity differences between HC and LLD groups in the DMN and SN.
Voxelwise two-sample t-tests (top, magenta colored regions) show the spatial extent of the intra-network abnormalities in the (A) DMN and (B) SN (p < 0.0167 and 3dClustSim corrected cluster size > 664 mm3). The box plots (bottom) indicate the quantitative data distribution of the individual groups (HC: black color and LLD: red color). In the box plots, the bands, from the top, represent the 75%, median, and 25%, respectively. The square in each box indicates the mean and whiskers shows the first standard deviation of the data. Abbreviations: HC, healthy comparison; LLD, late-life depression; L/R, left/right; SPL, superior parietal lobe; PHG, parahippocampal gyrus; SFG, superior frontal gyrus; DLPFC, dorsolateral prefrontal cortex.
DISCUSSION
Our primary finding is that abnormal inter-network Fc of the ECN with the posterior DMN and SN components differentiated LLD from nondepressed older adults. Distinct inter-network connectivity abnormalities in LLD were associated with symptom dimensions commonly linked to poor treatment response, i.e., depressive and anxiety symptom severity and executive dysfunction. These findings provide novel evidence into the neurophysiologic mechanisms that are associated with the heterogeneous clinical presentation of LLD.
Decreased ECN-subcortical INC correlates with anxiety severity
In LLD, both the left and right ECN components encompassing the DLPFC and SPL showed decreased Fc with a subcortical network consisting of the bilateral basal ganglia, ventral striatum, and thalamus. Our results are thus concordant with the fronto-striatal disconnection hypothesis of LLD. Previous task-based functional MRI in LLD studies testing this theory found DLPFC and parietal region hypoactivation, but mainly in individuals with late-onset depression (Aizenstein et al., 2005; Wang et al., 2008). Conversely, the striatal response to tasks that probe fronto-subcortical circuitry has produced mixed findings (Aizenstein et al., 2005; Naismith et al., 2010). In an R-fcMRI study, diminished ECN connectivity predicted treatment non-remission in LLD when the seed was placed in the left DLPFC, though abnormalities did not include subcortical regions (Alexopoulos et al., 2012). To our knowledge, this present study is the first to show inter-network ECN-subcortical hypoconnectivity in LLD. However, we did not find age of onset to correlate with the observed INC abnormalities. Notably, the right ECN-subcortical network hypoconnectivity correlated with anxiety severity in the LLD group. Since the presence of anxiety has been associated with delayed treatment response and lower remission rates in LLD patients (Andreescu et al., 2007; Greenlee et al., 2010), the abnormal ECN-subcortical Fc observed here could potentially serve as a marker of treatment response in LLD. Prior studies have shown hypofunctioning in the ECN during the performance of neutral tasks that involve response-conflict in individuals with high-trait anxiety (Bishop, 2009). Interestingly, while increased ECN Fc with the affective limbic regions has been demonstrated in patients with generalized anxiety disorder (Etkin et al., 2009), this finding was stronger in less anxious patients. Common and disorder-specific vulnerabilities during implicit regulation of emotional conflict that involve ECN regions have also been reported in major depression and generalized anxiety disorder (Etkin and Schatzberg, 2011). Therefore, our preliminary findings of diminished ECN-subcortical Fc may not only provide insight into the network correlates of anxiety symptoms in LLD but also represent an imaging endophenotype of severe anxiety symptoms across categorically classified late-life psychiatric disorders (Etkin et al., 2013).
Increased left ECN connectivity with posterior DMN and SN dACC correlates with overall depression severity
Our results further showed a loss of anticorrelation and a reversal to positive inter-network connectivity in LLD between the left ECN and posterior DMN component comprising bilateral PCC, IPL, and MTL regions. Enhanced DMN Fc is demonstrated in LLD (Alexopoulos et al., 2012; Eyre et al., 2016; Wu et al., 2011), which may be due to aging-related brain changes (Smith, G.S. et al., 2009; Wu et al., 2011). The failure to deactivate DMN to an affective reappraisal task (Sheline et al., 2009) and the enhanced Fc within this resting-state network (Sheline et al., 2010b) could be linked to abnormal self-referential processing and negative rumination in major depression. However, our results are in contrast with a previous investigation that detected diminished left ECN-posterior DMN connectivity in treatment-resistant depression (Abbott et al., 2013). We believe these inconsistencies are driven by several factors. For instance, the depressed cohort in Abbott et al.’s study included both mid- and late-life treatment resistant patients who met criteria for electroconvulsive therapy. In contrast, we enrolled only LLD patients and although our sample experienced persistent symptoms despite a therapeutic antidepressant trial, most were not treatment resistant. Also, in order to mimic a LLD cohort commonly encountered in clinical practice, we enrolled a heterogeneous sample that experienced varying levels of cognitive dysfunction, depression, and anxiety severity. Additionally, the methodology used to extract independent components and the brain regions included in the left ECN component were different in both studies, which could have contributed to the discrepancies.
We found that increased left ECN-posterior DMN connectivity was associated with overall depression severity in LLD. This finding is intriguing as impaired posterior DMN deactivation correlates with depressive symptoms in older adults with subsyndromal depression (Woo et al., 2009). A previous R-fcMRI investigation also showed that greater Fc between the left DLPFC and posterior DMN Fc was associated with higher depressive symptoms and better cognitive performance in older nondemented individuals (Goveas et al., 2011). Whether the direction of temporal correlations between the left ECN and DMN will serve as a marker of antidepressant treatment resistance in LLD requires further exploration. Aberrant ECN-DMN interactions may still be driven by the degree of cognitive dysfunction and age-related brain changes. The increased connectivity between the ECN and the posterior DMN in LLD may also reflect biases toward enduring negative ruminative thoughts at the cost of engaging with the external world, which should be examined in future investigations.
The increased connectivity between the left ECN and SN dACC component that we observed in LLD patients also correlated with depression severity. The dACC has extensive reciprocal connections with the prefrontal and parietal cortices, especially the DLPFC; together, they play a crucial role in cognitive control, especially by influencing cognitively demanding information processing, and response selection and inhibition. Both structural and functional dACC abnormalities may predict antidepressant response in LLD (Alexopoulos et al., 2012; Gunning et al., 2009). Functional dACC abnormalities are associated with apathy (Alexopoulos et al., 2013), a prevalent clinical feature of LLD. In LLD, apathy is also associated with poor antidepressant treatment outcome and persistence of depression (Lavretsky et al., 1999). Therefore, abnormal ECN-SN dACC inter-network connectivity may contribute to apathy in those with severe forms of LLD.
Decreased left ECN-salience insula connectivity correlates with executive dysfunction
The left ECN’s inter-network connectivity with the SN insula showed decreased connectivity in LLD, which is consistent with previous findings in major depression (Manoliu et al., 2013). Manoliu et al demonstrated both decreased intra-network insula Fc and inter-network negative connectivity between the left ECN and SN’s insula in middle-aged depressed patients. Decreased effective connections from the insula to the ECN were present in those with melancholic depression (Hyett et al., 2015). The insula, which plays a crucial role in the orientation of attention to the internally and externally relevant salient stimuli, guides behaviors associated with social, affective, and higher-order mental processes. The insula subcomponent is also considered an important hub that mediates the dynamic switching between ECN-dependent goal directed behaviors and DMN-controlled self-referential processes (Sridharan et al., 2008). Remarkably, the aberrant ECN-SN insula connectivity observed in the current study correlated with executive dysfunction in LLD patients. Persistent executive dysfunction is one of the core features associated with depression severity and poor antidepressant response in LLD. The impaired ECN-SN insula interactions could be driving abnormal switching between the ECN and DMN in LLD, thereby contributing to depression severity and dysexecutive behaviors.
Decreased ECN-subcortical and ECN-insula connectivity might be driven by increased WM hyperintensity (WMH) burden in the prefrontal-striatal circuitry and damage to the associated WM tracts. Therefore, the increases in positive inter-network connections of the left ECN with posterior DMN and SN dACC might be a compensatory process to make up for the diminished network interactions in patients with LLD. Although we accounted for whole brain GMR in our analyses, the influences of WMH burden and regional gray matter volumes on functional network interactions in LLD remain unclear.
Voxelwise intra-network functional connectivity findings
Consistent with our inter-network connectivity findings, we found intra-network Fc abnormalities predominantly in the ECN among depressed patients. The ECN functional connections in the insula were decreased, whereas a reversal to positive connectivity in the posterior default mode regions was detected. These results are consistent with the inter-network connectivity abnormalities seen in the present study. Alexopoulos et al. found decreased Fc in the ECN in LLD nonremitters compared with remitters. The intra-ECN Fc during the depressive episode predicted apathy and dysexecutive behaviors at the study endpoint (Alexopoulos et al., 2012). In the present study, we also found abnormal intra-network Fc in the SN and DMN in LLD subjects, though the imbalance within these networks was less pronounced than the ECN. Our findings of decreased SN Fc in the right DLPFC are concordant with the LLD literature. In depressed elderly, diminished functional connectivity between the right DLPFC and dACC during a cognitive control task was noted to partially improve with antidepressant treatment (Aizenstein et al., 2009). Increased DMN Fc in the PHG is also consistent with the functional imaging findings in LLD (Alexopoulos et al., 2012). However, we also found diminished DMN Fc in the salience and the cognitive control regions. The increased WMH burden may perhaps account for the decreased DMN Fc seen in LLD (Wu et al., 2011).
Limitations
Our interpretation that aberrant inter-network connectivity is related to multidomain symptom severity is based on cross-sectional data, and, therefore, should be considered preliminary and for hypothesis-generating purposes. The cross-sectional study design precludes us from making any causal inferences. For instance, we are unable to comment as to whether the observed network abnormalities are related to the depressive state, and if the aberrant connectivity will resolve with treatment. Our sample size is modest, and, therefore, these findings need to be replicated in larger cohorts. We did not correct for multiple comparisons when examining the brain-behavior relationships; therefore, these results should be interpreted with caution. Identifying the influences of age-related brain changes, especially regional brain volumes and WMH burden on the intra- and inter-network connectivity abnormalities observed here are beyond the scope of this study. Although an ideal study design would have included antidepressant-free participants, the fact that most of our LLD patients were treatment nonremitters and had moderate to severe depression raises ethical concerns regarding withholding medications for research purposes. Spurious correlations in R-fcMRI data are thought to persist even after performing motion regression. As such, we compared the six rigid-body head displacement motion parameters between LLD and HC groups but found no differences (p > 0.05; see Figure S3).
Conclusions
Our results support the neurophysiological underpinnings of LLD to be abnormal inter-network connections of the ECN with components of the default mode and salience neuronal systems. Our findings further highlight impairments in the ECN’s interactions with other neurocognitive networks to underlie clinical features that have previously been shown to be associated with poor treatment response in LLD. Future research with larger sample sizes that incorporate experimental paradigms to probe the neurocognitive networks of interest coupled with comprehensive assessments of multiple symptom dimensions relevant to treatment response will further delineate the brain-behavior relationships in LLD. Additionally, diverse late-life psychiatric disorders may share common vulnerability in the within-ECN Fc and ECN’s interactions with other brain networks that could relate to symptoms (e.g., depressive and anxiety symptoms, and executive dysfunction) that transcend diagnostic boundaries (Etkin et al., 2013; McTeague et al., 2016). Multimodal imaging investigations should be conducted to determine the relationship between inter-network functional connectivity abnormalities and structural disruptions in LLD. Future work should also examine the utility of inter-network functional connectivity as potential imaging biomarkers of treatment response in patients with LLD.
Supplementary Material
Acknowledgments
We thank Ms. Lydia Washechek, B.A., for editorial assistance, Ms. Stacy Claesges, B.A., for subject recruitment and Mr. Yu Liu, M.S., for MRI technical support. Dr. Goveas has obtained written permission from those mentioned here in the acknowledgement section.
Funding/Support: This work was supported by Alzheimer’s Association New Investigator Research Grant NIRG-11-204070 (JSG), Advancing Healthier Wisconsin Endowment an endowment of the Medical College of Wisconsin (JSG), the Brain and Behavior Research Foundation (formerly NARSAD) Young Investigator program (JSG); Extendicare (now RECALL) Foundation (JSG), R01 AD20279 (SJL) from the National Institute on Aging, and 8UL1TR000055 from the Clinical and Translational Science Award program of the National Center for Advancing Translational Sciences.
Role of the Sponsors/Funders: The sponsors/funders had no role in the design and conduct of the study; in the collection, management, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors’ contributions:
Drs. Wenjun Li and Joseph S. Goveas had full access to all of the study data and take responsibility for its integrity and the data analysis accuracy.
Study concept and design: Li W, Wang Y, Ward BD, Li SJ, Goveas JS.
Acquisition of data: Li W, Antuono PG, Goveas JS.
Analysis and interpretation of data: Li W, Wang Y, Ward BD, Goveas JS.
Drafting of the manuscript: Li W, Goveas JS.
Critical revision of the manuscript for important intellectual content: Li W, Wang Y, Ward BD, Antuono P, Li SJ, Goveas JS.
Statistical analysis: Li W, Ward BD.
Obtained funding: Li SJ, Goveas JS.
Administrative, technical and material support: Antuono PG, Li SJ, Goveas JS.
Study supervision: Antuono P, Goveas JS.
FINANCIAL DISCLOSURES
Conflict of Interest Disclosures:
Drs. W. Li, Wang, and Mr. Ward report no biomedical financial interests or potential conflicts of interest. Within the past five years, Dr. Antuono has served on the speaker bureaus of Novartis and Pfizer. Dr. Antuono reports research support from Myriad, Glaxo Smith Kline, Pfizer, ICON, Premier Rach, Octa Pharma, Eisai, Bristol Myers Squibb, Janssen, Baxter and Elan; and Dr. Shi-Jiang Li reports research grant funding from the National Institute on Aging and Pfizer; in addition, he has served as a consultant for Bristol-Meyers Squibb and BrainSymphonics, LLC. Dr. Goveas reports grant support from the Alzheimer’s Association International grant program, Brain and Behavior Research Foundation (formerly NARSAD) Young Investigator program, Extendicare (now RECALL) Foundation, and Advancing Healthier Wisconsin Endowment for Research to MCW.
References
- Abbott CC, Lemke NT, Gopal S, Thoma RJ, Bustillo J, Calhoun VD, Turner JA. Electroconvulsive therapy response in major depressive disorder: a pilot functional network connectivity resting state FMRI investigation. Frontiers in psychiatry. 2013;4:10. doi: 10.3389/fpsyt.2013.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizenstein HJ, Butters MA, Figurski JL, Stenger VA, Reynolds CF, 3rd, Carter CS. Prefrontal and striatal activation during sequence learning in geriatric depression. Biological psychiatry. 2005;58(4):290–296. doi: 10.1016/j.biopsych.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Aizenstein HJ, Butters MA, Wu M, Mazurkewicz LM, Stenger VA, Gianaros PJ, Becker JT, Reynolds CF, 3rd, Carter CS. Altered functioning of the executive control circuit in late-life depression: episodic and persistent phenomena. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2009;17(1):30–42. doi: 10.1097/JGP.0b013e31817b60af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizenstein HJ, Khalaf A, Walker SE, Andreescu C. Magnetic resonance imaging predictors of treatment response in late-life depression. J Geriatr Psychiatry Neurol. 2014;27(1):24–32. doi: 10.1177/0891988713516541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos GS, Hoptman MJ, Kanellopoulos D, Murphy CF, Lim KO, Gunning FM. Functional connectivity in the cognitive control network and the default mode network in late-life depression. Journal of affective disorders. 2012;139(1):56–65. doi: 10.1016/j.jad.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos GS, Hoptman MJ, Yuen G, Kanellopoulos D, Seirup JK, Lim KO, Gunning FM. Functional connectivity in apathy of late-life depression: a preliminary study. Journal of affective disorders. 2013;149(1–3):398–405. doi: 10.1016/j.jad.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos GS, Kiosses DN, Heo M, Murphy CF, Shanmugham B, Gunning-Dixon F. Executive dysfunction and the course of geriatric depression. Biol Psychiatry. 2005;58(3):204–210. doi: 10.1016/j.biopsych.2005.04.024. [DOI] [PubMed] [Google Scholar]
- Andreescu C, Lenze EJ, Dew MA, Begley AE, Mulsant BH, Dombrovski AY, Pollock BG, Stack J, Miller MD, Reynolds CF. Effect of comorbid anxiety on treatment response and relapse risk in late-life depression: controlled study. Br J Psychiatry. 2007;190:344–349. doi: 10.1192/bjp.bp.106.027169. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philosophical transactions of the Royal Society of London. Series B Biological sciences. 2005;360(1457):1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SJ. Trait anxiety and impoverished prefrontal control of attention. Nat Neurosci. 2009;12(1):92–98. doi: 10.1038/nn.2242. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S, Dogonowski AM, Ernst M, Fair D, Hampson M, Hoptman MJ, Hyde JS, Kiviniemi VJ, Kotter R, Li SJ, Lin CP, Lowe MJ, Mackay C, Madden DJ, Madsen KH, Margulies DS, Mayberg HS, McMahon K, Monk CS, Mostofsky SH, Nagel BJ, Pekar JJ, Peltier SJ, Petersen SE, Riedl V, Rombouts SA, Rypma B, Schlaggar BL, Schmidt S, Seidler RD, Siegle GJ, Sorg C, Teng GJ, Veijola J, Villringer A, Walter M, Wang L, Weng XC, Whitfield-Gabrieli S, Williamson P, Windischberger C, Zang YF, Zhang HY, Castellanos FX, Milham MP. Toward discovery science of human brain function. Proc Natl Acad Sci U S A. 2010;107(10):4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Butters MA, Whyte EM, Nebes RD, Begley AE, Dew MA, Mulsant BH, Zmuda MD, Bhalla R, Meltzer CC, Pollock BG, Reynolds CF, 3rd, Becker JT. The nature and determinants of neuropsychological functioning in late-life depression. Arch Gen Psychiatry. 2004;61(6):587–595. doi: 10.1001/archpsyc.61.6.587. [DOI] [PubMed] [Google Scholar]
- Etkin A, Gyurak A, O'Hara R. A neurobiological approach to the cognitive deficits of psychiatric disorders. Dialogues Clin Neurosci. 2013;15(4):419–429. doi: 10.31887/DCNS.2013.15.4/aetkin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Prater KE, Schatzberg AF, Menon V, Greicius MD. Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Arch Gen Psychiatry. 2009;66(12):1361–1372. doi: 10.1001/archgenpsychiatry.2009.104. [DOI] [PubMed] [Google Scholar]
- Etkin A, Schatzberg AF. Common abnormalities and disorder-specific compensation during implicit regulation of emotional processing in generalized anxiety and major depressive disorders. Am J Psychiatry. 2011;168(9):968–978. doi: 10.1176/appi.ajp.2011.10091290. [DOI] [PubMed] [Google Scholar]
- Eyre HA, Yang H, Leaver AM, Van Dyk K, Siddarth P, Cyr NS, Narr K, Ercoli L, Baune BT, Lavretsky H. Altered resting-state functional connectivity in late-life depression: A cross-sectional study. Journal of affective disorders. 2016;189:126–133. doi: 10.1016/j.jad.2015.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, Matthews PM, Beckmann CF, Mackay CE. Distinct patterns of brain activity in young carriers of the APOE- 4 allele. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(17):7209–7214. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-1V TR Axis 1 disorders, research version, non-patient edition (SCID-I/NP) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S, Benedetti F, Abbamonte M, Gasparotti R, Barale F, Perez J, McGuire P, Politi P. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J Psychiatry Neurosci. 2009;34(6):418–432. [PMC free article] [PubMed] [Google Scholar]
- Gildengers AG, Houck PR, Mulsant BH, Dew MA, Aizenstein HJ, Jones BL, Greenhouse J, Pollock BG, Reynolds CF., 3rd Trajectories of treatment response in late-life depression: psychosocial and clinical correlates. J Clin Psychopharmacol. 2005;25(4 Suppl 1):S8–S13. doi: 10.1097/01.jcp.0000161498.81137.12. [DOI] [PubMed] [Google Scholar]
- Goveas J, Xie C, Wu Z, Douglas Ward B, Li W, Franczak MB, Jones JL, Antuono PG, Yang Z, Li SJ. Neural correlates of the interactive relationship between memory deficits and depressive symptoms in nondemented elderly: resting fMRI study. Behavioural brain research. 2011;219(2):205–212. doi: 10.1016/j.bbr.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenlee A, Karp JF, Dew MA, Houck P, Andreescu C, Reynolds CF., 3rd Anxiety impairs depression remission in partial responders during extended treatment in late-life. Depress Anxiety. 2010;27(5):451–456. doi: 10.1002/da.20672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning FM, Cheng J, Murphy CF, Kanellopoulos D, Acuna J, Hoptman MJ, Klimstra S, Morimoto S, Weinberg J, Alexopoulos GS. Anterior cingulate cortical volumes and treatment remission of geriatric depression. Int J Geriatr Psychiatry. 2009;24(8):829–836. doi: 10.1002/gps.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32(1):50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Hyett MP, Breakspear MJ, Friston KJ, Guo CC, Parker GB. Disrupted effective connectivity of cortical systems supporting attention and interoception in melancholia. JAMA psychiatry. 2015;72(4):350–358. doi: 10.1001/jamapsychiatry.2014.2490. [DOI] [PubMed] [Google Scholar]
- Karim HT, Andreescu C, Tudorascu D, Smagula SF, Butters MA, Karp JF, Reynolds C, Aizenstein HJ. Intrinsic functional connectivity in late-life depression: trajectories over the course of pharmacotherapy in remitters and non-remitters. Mol Psychiatry. 2016 doi: 10.1038/mp.2016.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavretsky H, Lesser IM, Wohl M, Miller BL, Mehringer CM. Clinical and neuroradiologic features associated with chronicity in late-life depression. Am J Geriatr Psychiatry. 1999;7(4):309–316. [PubMed] [Google Scholar]
- Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. The Gerontologist. 1969;9(3):179–186. [PubMed] [Google Scholar]
- Li W, Ward BD, Xie C, Jones JL, Antuono PG, Li SJ, Goveas JS. Amygdala network dysfunction in late-life depression phenotypes: Relationships with symptom dimensions. J Psychiatr Res. 2015;70:121–129. doi: 10.1016/j.jpsychires.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas JA, Ivnik RJ, Smith GE, Bohac DL, Tangalos EG, Kokmen E, Graff-Radford NR, Petersen RC. Normative data for the Mattis Dementia Rating Scale. J Clin Exp Neuropsychol. 1998;20(4):536–547. doi: 10.1076/jcen.20.4.536.1469. [DOI] [PubMed] [Google Scholar]
- Manoliu A, Meng C, Brandl F, Doll A, Tahmasian M, Scherr M, Schwerthoffer D, Zimmer C, Forstl H, Bauml J, Riedl V, Wohlschlager AM, Sorg C. Insular dysfunction within the salience network is associated with severity of symptoms and aberrant inter-network connectivity in major depressive disorder. Front Hum Neurosci. 2013;7:930. doi: 10.3389/fnhum.2013.00930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTeague LM, Goodkind MS, Etkin A. Transdiagnostic impairment of cognitive control in mental illness. J Psychiatr Res. 2016;83:37–46. doi: 10.1016/j.jpsychires.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain structure & function. 2010;214(5–6):655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulders PC, van Eijndhoven PF, Schene AH, Beckmann CF, Tendolkar I. Resting-state functional connectivity in major depressive disorder: A review. Neurosci Biobehav Rev. 2015;56:330–344. doi: 10.1016/j.neubiorev.2015.07.014. [DOI] [PubMed] [Google Scholar]
- Mulsant BH, Pollock BG, Nebes R, Miller MD, Sweet RA, Stack J, Houck PR, Bensasi S, Mazumdar S, Reynolds CF., 3rd A twelve-week, double-blind, randomized comparison of nortriptyline and paroxetine in older depressed inpatients and outpatients. Am J Geriatr Psychiatry. 2001;9(4):406–414. [PubMed] [Google Scholar]
- Naismith SL, Lagopoulos J, Ward PB, Davey CG, Little C, Hickie IB. Fronto-striatal correlates of impaired implicit sequence learning in major depression: an fMRI study. J Affect Disord. 2010;125(1–3):256–261. doi: 10.1016/j.jad.2010.02.114. [DOI] [PubMed] [Google Scholar]
- Naismith SL, Norrie LM, Mowszowski L, Hickie IB. The neurobiology of depression in later-life: clinical, neuropsychological, neuroimaging and pathophysiological features. Prog Neurobiol. 2012;98(1):99–143. doi: 10.1016/j.pneurobio.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. An insular view of anxiety. Biol Psychiatry. 2006;60(4):383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Sexton CE, Allan CL, Le Masurier M, McDermott LM, Kalu UG, Herrmann LL, Maurer M, Bradley KM, Mackay CE, Ebmeier KP. Magnetic resonance imaging in late-life depression: multimodal examination of network disruption. Arch Gen Psychiatry. 2012;69(7):680–689. doi: 10.1001/archgenpsychiatry.2011.1862. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, Mintun MA, Wang S, Coalson RS, Raichle ME. The default mode network and self-referential processes in depression. Proc Natl Acad Sci U S A. 2009;106(6):1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Pieper CF, Barch DM, Welsh-Bohmer K, McKinstry RC, MacFall JR, D'Angelo G, Garcia KS, Gersing K, Wilkins C, Taylor W, Steffens DC, Krishnan RR, Doraiswamy PM. Support for the vascular depression hypothesis in late-life depression: results of a 2-site, prospective, antidepressant treatment trial. Arch Gen Psychiatry. 2010a;67(3):277–285. doi: 10.1001/archgenpsychiatry.2009.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Price JL, Yan Z, Mintun MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci U S A. 2010b;107(24):11020–11025. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GS, Kramer E, Ma Y, Kingsley P, Dhawan V, Chaly T, Eidelberg D. The functional neuroanatomy of geriatric depression. Int J Geriatr Psychiatry. 2009;24(8):798–808. doi: 10.1002/gps.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF. Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009;106(31):13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(34):12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadayonnejad R, Ajilore O. Brain network dysfunction in late-life depression: a literature review. Journal of geriatric psychiatry and neurology. 2014;27(1):5–12. doi: 10.1177/0891988713516539. [DOI] [PubMed] [Google Scholar]
- Taylor WD, Aizenstein HJ, Alexopoulos GS. The vascular depression hypothesis: mechanisms linking vascular disease with depression. Mol Psychiatry. 2013;18(9):963–974. doi: 10.1038/mp.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Krishnan KR, Steffens DC, Potter GG, Dolcos F, McCarthy G. Depressive state- and disease-related alterations in neural responses to affective and executive challenges in geriatric depression. Am J Psychiatry. 2008;165(7):863–871. doi: 10.1176/appi.ajp.2008.07101590. [DOI] [PubMed] [Google Scholar]
- Wang Y, Risacher SL, West JD, McDonald BC, Magee TR, Farlow MR, Gao S, O'Neill DP, Saykin AJ. Altered default mode network connectivity in older adults with cognitive complaints and amnestic mild cognitive impairment. J Alzheimers Dis. 2013;35(4):751–760. doi: 10.3233/JAD-130080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Manual for the Wechsler Memory Scale-Revised. San Antonio: Psychological Corporation; 1987. [Google Scholar]
- Woo SL, Prince SE, Petrella JR, Hellegers C, Doraiswamy PM. Modulation of a human memory circuit by subsyndromal depression in late life: a functional magnetic resonance imaging study. Am J Geriatr Psychiatry. 2009;17(1):24–29. doi: 10.1097/JGP.0b013e318180056a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Andreescu C, Butters MA, Tamburo R, Reynolds CF, 3rd, Aizenstein H. Default-mode network connectivity and white matter burden in late-life depression. Psychiatry research. 2011;194(1):39–46. doi: 10.1016/j.pscychresns.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.