Abstract
Early childhood is a time of rapid developmental changes in sleep, cognitive control processes, and the regulation of emotion and behavior. This experimental study examined sleep-dependent effects on response inhibition and self-regulation, as well as whether acute sleep restriction moderated the association between these processes. Preschool children (N = 19; 45.6 ± 2.2 months; 11 female) followed a strict sleep schedule for at least 3 days before each of 2 morning behavior assessments: baseline (habitual nap/night sleep) and sleep restriction (missed nap/delayed bedtime). Response inhibition was evaluated via a go/no-go task. Twelve self-regulation strategies were coded from videotapes of children while attempting an unsolvable puzzle. We then created composite variables representing adaptive and maladaptive self-regulation strategies. Although we found no sleep-dependent effects on response inhibition or self-regulation measures, linear mixed-effects regression showed that acute sleep restriction moderated the relationship between these processes. At baseline, children with better response inhibition were more likely to use adaptive self-regulation strategies (e.g., self-talk, alternate strategies), and those with poorer response inhibition showed increased use of maladaptive self-regulation strategies (e.g., perseveration, fidgeting); however, response inhibition was not related to self-regulation strategies following sleep restriction. Our results showing a sleep-dependent effect on the associations between response inhibition and self-regulation strategies indicate that adequate sleep facilitates synergy between processes supporting optimal social-emotional functioning in early childhood. Although replication studies are needed, findings suggest that sleep may alter connections between maturing emotional and cognitive systems, which have important implications for understanding risk for or resilience to developmental psychopathology.
INTRODUCTION
Early childhood is a sensitive period marked by rapid changes in executive function (EF) and self-regulation (Bell & Deater-Deckard, 2007; Carlson, 2005; Kochanska, Coy, & Murray, 2001; Zelazo et al., 2003), two key underlying processes necessary to achieve key developmental outcomes such as social-emotional adjustment (Riggs, Jahromi, Razza, Dillworth-Bart, & Mueller, 2006) and school readiness (Bierman, Nix, Greenberg, Blair, & Domitrovich, 2008; Blair & Diamond, 2008; McClelland & Cameron, 2012). Self-regulation generally refers to the processes that enable an individual to control one’s behavior, attention, and emotion, especially in pursuit of a goal or when posed with a challenge (Raffaelli, Crockett, & Shen, 2005). EF is viewed as an overarching construct encompassing complex cognitions with three core components: working memory, mental flexibility, and response inhibition (Huizinga, Dolan, & Van Der Molen, 2006; Miyake et al., 2000). Although some consider response inhibition an aspect of self-regulation (Kochanska, 1997), we and many others (Blair & Ursache, 2011; Blair, Zelazo, & Greenberg, 2005; Hofmann, Schmeichel, & Baddeley, 2012; Posner & Rothbart, 1998) characterize response inhibition (a component of EF) as a related but distinct process from self-regulation because it is less emotionally charged and more cognitive in nature. Deficits in EF skills and the inability to self-regulate behavior when challenged are hallmarks of many child psychopathologies including externalizing behaviors such as attention deficit/hyperactivity disorder (ADHD) and impulsivity (e.g., if a child cannot inhibit his or her behavior when required in a classroom setting; Barkley, 1997; Gaub & Carlson, 1997), as well as internalizing behaviors such as anxiety (e.g., if a child lacks strategies to manage an emotionally challenging situation; Zahn-Waxler, Klimes-Dougan, & Slattery, 2000).
The relationship between EF and self-regulation is considered bidirectional (Blair & Dennis, 2010; Blair & Ursache, 2011). For example, EF skills facilitate self-regulation strategies by organizing thoughts and behaviors in goal-directed ways (Fuster, 2000; Ochsner & Gross, 2005). Likewise, taxing self-regulation may impair EF by depleting resources for complex cognition, especially during times of high mental “load” (Fuster, 2002). Furthermore, the interplay of EF and self-regulation is increasingly recognized as significant for optimal health outcomes, as the poor integration of emotion and cognitive processes is a risk factor for maladjustment and school failure (Eysenck, Derakshan, Santos, & Calvo, 2007) and has been suggested as a potential pathway contributing to developmental psychopathology (Blair & Dennis, 2010). Finally, an established developmental science literature suggests that both intrinsic child characteristics (e.g., age, sex, stress reactivity, temperament; Carlson & Wang, 2007; Hongwanishkul, Happaney, Lee, & Zelazo, 2005; Hughes & Ensor, 2008; Quas et al., 2014; Rothbart, Posner, & Kieras, 2006) and environmental factors (e.g., socioeconomic status, poverty, quality of caregiving; Blair, 2010; Blair & Raver, 2012) play a role in the development of EF skills and self-regulation, as well as in their association. Data from a number of studies indicate that sleep may also influence the interconnections between these developmental processes (Zohar, Tzischinsky, Epstein, & Lavie, 2005; reviewed in Dahl, 1996; Walker & Harvey, 2010).
Although sleep is increasingly recognized as an important factor in the cognitive and affective dimensions fundamental to EF and self-regulation in older children, adolescents, and adults (Baum et al., 2014; Hagger, 2010; Mauss, Troy, & LeBourgeois, 2013; Sadeh, Gruber, & Raviv, 2002; Talbot, McGlinchey, Kaplan, Dahl, & Harvey, 2010), well-controlled research that “simulates” experimentally how young children lose sleep in the real world is scarce. A few published findings suggest that insufficient sleep may disrupt the behavioral components that enable optimal cognitive and emotional functioning (e.g., response inhibition, self-regulation) during early childhood, the school-age years, and adolescence. For example, experimental and quasi-experimental data indicate that acute daytime sleep loss in regularly napping preschoolers leads to moderate-to-large decrements in objective assessments of self-regulation, emotion processing, and learning (Berger, Miller, Seifer, Cares, & LeBourgeois, 2012; Kurdziel, Duclos, & Spencer, 2013; Miller, Seifer, Crossin, & LeBourgeois, 2015). In addition, kindergarten and primary school-age students experiencing chronic experimental sleep restriction (i.e., multiple nights) show increased impulsivity and emotional lability (Gruber, Cassoff, Frenette, Wiebe, & Carrier, 2012), decreased brain processing under high cognitive “load” (Molfese et al., 2013), and/or impaired neurobehavioral functioning (i.e., attention, working memory, processing speed); however, such effects are not consistent across studies and are commonly task dependent. For example, Vriend et al. (2013) reported reduced emotion regulation and performance on memory tasks but no differences in performance on multiple tasks assessing attention after 4 nights of 1-hr sleep restriction (compared to baseline) in 8- to 12-year-olds. Similarly, in a study of 8- to 15-year-olds, Fallone, Acebo, Arnedt, Seifer, and Carskadon (2001) found increased inattentive behaviors but no change in performance on tasks measuring response inhibition or sustained attention after chronic sleep restriction (5 nights of 4 hr of sleep) as compared to sleep optimization. Finally, in a study by Sadeh, Gruber, and Raviv (2003), 1 hr of sleep restriction across 3 nights led to worse performance on a simple reaction time task but did not significantly change performance on other tasks measuring working memory, attention, or response inhibition in fourth to sixth graders (9–12 years of age). Such variable findings in older children and adolescents suggest that studying the sleep-dependent effects on cognitive and emotional processes (e.g., response inhibition, self-regulation) that are central to healthy development in early childhood is warranted.
Longitudinal and cross-sectional data indicate dramatic shifts in the timing, duration, and quality of sleep between the toddler and kindergarten years: Total 24-hr sleep duration decreases by about 20%, daytime napping declines, and evening behavioral sleep problems (e.g., sleep onset delay, bedtime resistance) commonly emerge (Beltramini & Hertzig, 1983; Iglowstein, Jenni, Molinari, & Largo, 2003; Kataria, Swanson, & Trevathan, 1987; Zuckerman, Stevenson, & Bailey, 1987; reviewed in Honaker & Meltzer, 2014). Insufficient sleep is also prevalent: Approximately 30% of parents report that their toddler or preschooler does not get enough sleep (National Sleep Foundation, 2004), and objective actigraphic data indicate that young children on average obtain less than the recommended 10–13 hr of sleep (Acebo et al., 2005). Further, a number of observational studies suggest links between sleep in early childhood and initial indicators of developmental psychopathology. For example, parent-reported short sleep duration and sleep problems during the preschool years are associated with concurrent reports of behavioral and emotional disturbance, including anxiety, depression, hyperactivity, and impulsivity (Bates, Viken, Alexander, Beyers, & Stockton, 2002; Goodlin-Jones, Tang, Liu, & Anders, 2009; Lavigne et al., 1999; Reid, Hong, & Wade, 2009). Longitudinal data also suggest that the consequences of sleep problems and insufficient sleep in early childhood may persist into the school-age years and adolescence, thus posing a risk for later mood and attentional problems (Gregory & O’Connor, 2002; Touchette et al., 2007; Wong, Brower, & Zucker, 2009).
Although links between poor or insufficient sleep and psychopathology are well documented (Walker & Harvey, 2010), very few studies have focused on the underlying mechanisms contributing to such relationships in childhood. The published data demonstrating sleep-dependent effects on response inhibition and self-regulation have examined them independent of each other; however, the cognitive processes involved in response inhibition are intricately bound with the emotional processes involved in self-regulation during a challenge (Bell & Deater-Deckard, 2007; Bell & Wolfe, 2004). Also, poorly connected cognitive and emotional processes have been recognized in psychopathologies during childhood including depression (Blair & Dennis, 2010; Hayden, Klein, Durbin, & Olino, 2006), anxiety disorders (Mennin, Heimberg, Turk, & Fresco, 2002), and ADHD (Barkley, 1997). Further, observational data indicate that sleep moderates the relationship between psychological processes such as emotional insecurity or intelligence and outcomes such as adjustment and academic achievement during childhood (El-Sheikh, Buckhalt, Keller, Cummings, & Acebo, 2007; Erath, Tu, Buckhalt, & El-Sheikh, 2015). Although the literature supports the need for well-controlled experimental research examining the degree to which sleep moderates associations between cognitive and emotional processes underlying developmental psychopathology, such investigations are scarce.
The current study addresses this impetus and several significant gaps in understanding the interplay between sleep, response inhibition, and self-regulation in early childhood. First, as late bedtimes are a risk factor for poor emotional and behavioral outcomes (Asarnow, McGlinchey, & Harvey, 2014; Bates et al., 2002), we utilized an experimental protocol that “mimicked” this type of sleep loss in preschool children by first establishing stable baseline sleep and then introducing sleep loss with randomly assigned timing. With this protocol, our aim was to extend our previously published data showing that nap deprivation resulted in nonadaptive self-regulation strategies in toddlers (Miller et al., 2015). Second, we examined whether acute sleep restriction produced differences in not only self-regulation strategies but also response inhibition. Finally, we utilized an integrative approach supported by the developmental science literature (Bell & Deater-Deckard, 2007; Bell & Wolfe, 2004; Blair, 2002; Gray, 1990; Leventhal & Scherer, 1987) to explore whether acute sleep loss would moderate associations between EF and self-regulation strategies.
METHOD
Participant Recruitment and Screening
Participants were 19 healthy 40- to 48-month-old children (45.6 ± 2.2 months; 11 female; 89% Caucasian, 11% mixed race) who were enrolled in an ongoing longitudinal study of the codevelopment and coregulation of sleep; circadian rhythms; and multiple cognitive, behavioral, and health outcomes. Children entered the study at ages 30–36 months; this within-subjects analysis includes data from the children who completed the 2nd year of the study at ages 40–48 months. Families were recruited from the Boulder, CO, area via community outreach events, flyers, and website advertisements. Parents were 36.3 ± 5.0 years of age (mother) and 39.1 ± 5.5 years (father), attained an education level ranging from high school to graduate degree (for mothers, 50% completed some college or a 4-year college degree, 50% graduate degree; for fathers, 17% completed high school, 44% some college or a 4 year college degree, 39% graduate degree), and average annual family income was $109,444. A telephone-screening interview and questionnaires were used to evaluate study eligibility. Study inclusion required that children were healthy, typically developing, sleeping on a regular sleep–wakefulness schedule, and reportedly napping three times or more per week. Children were excluded based on the following criteria: cosleeping (i.e., bed sharing); travel beyond two time zones within 3 months of the study; regular use of medications affecting sleep or alertness; reported or diagnosed sleep problems; developmental disabilities, neurologic/metabolic disorders, chronic medical conditions, lead poisoning, or head injury involving loss of consciousness; conceptual age < 35 weeks or > 45 weeks; low birth weight (< 5.5 lb); or a family history (first degree) of diagnosed narcolepsy, psychosis, or bipolar disorder. We rescreened children to confirm study criteria before their 40- to 48-month-old assessments; however, because napping naturally declines across early childhood (Iglowstein et al., 2003; Weissbluth, 1995), we expected that some children would be sleeping only at night (i.e., not napping; criteria > 1 month). Thus, for this analysis, participants were not required to take naps (26%; n = 5 not napping). Of the 27 children who met criteria at rescreening, 25 completed both behavior assessments. Of these, six were not included in this analysis due to noncompliance (n = 2) or failure to demonstrate understanding of the response inhibition task (n = 4). Thus, our final sample included 19 preschoolers. Parents signed an informed consent form approved by the University of Colorado Institutional Review Board. Study procedures were performed in accordance with the Declaration of Helsinki. Parents were compensated with cash, and children received a U.S. savings bond and small nonmonetary gifts.
Protocol
Children followed an individualized, strict sleep schedule for at least 3 days based on their habitual sleep–wakefulness pattern (i.e., napping or not napping). This standard lead-in interval is important to promote stabilization of the circadian system and to optimize sleep duration before the experimental sleep protocol. In-home behavior assessments were completed in the morning following two conditions (Figure 1): baseline (children maintained their individual strict sleep schedule) and sleep restriction (16 hr of prior wakefulness; no nap and about a 3-hr bedtime delay; scheduled morning wake time). Conditions were counterbalanced, with an intervening 5 or more days on the sleep schedule between assessments. Both assessments occurred 3 hr after habitual morning wake time (10:01 ± 00:18) to minimize the effects of sleep inertia and to simulate a time when children are likely to be engaging in activities at preschool. The response inhibition task was always administered before the self-regulation task.
FIGURE 1.
Sample protocol for an exemplary child following a stabilization sleep schedule (20:00 bedtime, 07:00 wake time, and 12:30–14:00 afternoon nap opportunity). Note. Black bars represent time in bed, gray bars represent time awake, and white boxes indicate the timing of behavior assessments (baseline, sleep restriction). Behavior assessments took place 3 hr after regularly scheduled morning wake time.
Adherence to study rules was monitored through daily correspondence via telephone or e-mail and verified with wrist actigraphy and sleep diaries. In the event of a protocol violation (i.e., accidental nap; sleep patterns deviating > 15 min from set schedule; illness; use of medications affecting sleep and/or alertness; caffeine consumption), assessments were rescheduled after an additional 3 or more days on the sleep schedule. Assessments for eight children (42%) were rescheduled due to protocol violations.
Actigraphy
We utilized standard laboratory procedures for collecting, checking, and scoring actigraphy data, as detailed in our previous publications (Berger et al., 2012; LeBourgeois et al., 2013; Simpkin et al., 2014). Daily sleep diary questions asked about lights-off and wake times for nap and nighttime sleep and intervals when the actigraph was off (Akacem et al., 2015). The actigraph (model AW Spectrum) was worn on the child’s nondominant wrist and provided continuous recordings of sleep/wakefulness states by measurement of motor activity (Philips/Respironics, Pittsburg, PA). For each sleep episode, three actigraph variables were derived: (a) time in bed—minutes from lights-off to lights-on, (b) sleep duration—minutes from sleep start to sleep end, and (c) sleep efficiency—% of sleep epochs between sleep start and sleep end time. Although actigraphy measures were not primary outcomes in this study, they were used to verify sleep schedule protocol compliance (Table 2).
TABLE 2.
Actigraphic Sleep Measures for the First 4 Days, and 24 hr Before Each Behavior Assessment
|
Baseline |
Sleep Restriction |
Statistics |
|||||
|---|---|---|---|---|---|---|---|
| M | SD | M | SD | t | d | p | |
| 4 Days Before Assessments | |||||||
| Nap Lights-Off Time | 13:13 | 0:35 | 13:12 | 0:23 | 0.18 | 0.03 | .86 |
| Nap Wake Time | 14:52 | 0:34 | 14:47 | 0:32 | 0.73 | 0.14 | .48 |
| Night Lights-Off Time | 20:08 | 0:24 | 20:04 | 0:23 | 1.28 | 0.16 | .22 |
| Morning Wake Time | 06:39 | 0:22 | 06:42 | 0:26 | −1.00 | −0.16 | .33 |
| 24 hr Time in Bed (hr) | 11.5 | 0.6 | 11.7 | 0.5 | −1.49 | −0.27 | .15 |
| 24 hr Sleep Duration (hr) | 10.7 | 0.6 | 10.9 | 0.5 | −0.95 | −0.17 | .36 |
| 24 hr Sleep Efficiency (%) | 88.3 | 3.5 | 89.0 | 3.6 | −0.77 | −0.19 | .45 |
| 24 hr Before Assessments | |||||||
| Nap Lights-Off Time | 13:17 | 0:32 | — | — | — | — | — |
| Nap Wake Time | 14:52 | 0:41 | — | — | — | — | — |
| Nights Lights-Off Time | 20:07 | 0:28 | 22:34 | 0:30 | −14.50 | −5.11 | <.01 |
| Morning Wake Time | 06:43 | 0:27 | 06:54 | 0:25 | −1.47 | −0.39 | .16 |
| Time in Bed (hr) | 11.7 | 0.9 | 8.3 | 0.5 | 13.71 | 4.85 | <.01 |
| Sleep Duration (hr) | 10.9 | 0.5 | 8.1 | 0.5 | 12.87 | 4.99 | <.01 |
| Sleep Efficiency (%) | 89.5 | 3.2 | 90.1 | 4.0 | −0.67 | −0.17 | .51 |
Behavior Assessments and Coding
Our experimental protocol included objective assessment of response inhibition and adaptive, as well as maladaptive self-regulation strategies reflecting the ability to cope with a challenge. The setup for behavior tasks included a child-size chair and table with a video camera positioned to capture the child’s body from the chest up. The response inhibition task was administered on a touch screen computer.
Response Inhibition
Response inhibition was measured via a standard go/no-go task (Willoughby, Wirth, Blair, & Family Life Project, 2012). In this task, a line drawing of one of seven possible animals appeared on the touch-screen computer. Children were instructed to touch a green button on the screen as fast as they could every time an animal appeared, except when the animal was a pig (no-go trials). Up to three training trials were administered first to ensure adequate understanding of task demands. If a child failed all three training trials, the task was discontinued. Eight no-go trials were dispersed among 40 total animal trials. Response inhibition performance was quantified as the % correct inhibitions on no-go trials (out of eight possible; see Table 3).
TABLE 3.
Descriptive Statistics of Response Inhibition and Adaptive and Maladaptive Self-Regulation
|
Baseline |
Sleep Restriction |
Statistics |
|||||
|---|---|---|---|---|---|---|---|
| Variable | M | SD | M | SD | z | d | p |
| Response Inhibition (%) | 78.29 | 13.07 | 81.58 | 22.96 | −0.95 | −0.18 | .34 |
| Adaptive SR (z score) | −0.09 | 0.33 | −0.08 | 0.44 | −1.01 | 0.15 | .31 |
| Maladaptive SR (z score) | 0.05 | 0.55 | 0.05 | 0.38 | −0.56 | 0.00 | .57 |
Note: SR = self-regulation.
Self-Regulation
Self-regulation was assessed through administration of our previously published task used to measure young children’s behavioral responses to challenge (Berger et al., 2012; Miller et al., 2015) and was adapted from procedures by Smiley and Dweck (1994). The task included an age-appropriate but unsolvable puzzle in which there was a wrong piece that prevented task completion. When all pieces but the misfit piece were successfully placed, children attempted the puzzle for 1 additional min and then were prompted by the examiner to “finish the puzzle” and asked “Why can’t you finish the puzzle?” and “What can you do to finish the puzzle?” This task was designed to resemble a challenge that preschoolers may encounter in their daily lives. Children were videotaped during the task, and adaptive and maladaptive self-regulation strategies were later coded as defined in the literature (Berhenke, Miller, Brown, Seifer, & Dickstein, 2011; Smiley & Dweck, 1994) and based on our prior published work (Miller et al., 2015). Self-regulation strategies were coded from videotapes by trained researchers using The Observer XT version 11.0 (Noldus Technologies, Wageningen, The Netherlands). We observed high intercoder correlations for all coded strategies (> 90%). Coders consulted with an expert reviewer (coauthor ALM) for consensus coding as needed.
Twelve self-regulation strategies were coded during the puzzle task (Table 1). The percent time each strategy was used was calculated during the 5 s after the child was prompted to “finish the puzzle,” as our previous work indicates that this portion of the task marks the time of most salient challenge and elicits the strongest effects on emotion expression and self-regulation (Berger et al., 2012; Miller et al., 2015). Coded self-regulation strategies were based on and modified from previous work (Berhenke et al., 2011; Miller et al., 2015) and included both adaptive and maladaptive responses to the puzzle challenge. Adaptive self-regulation strategies involved a child attempting to actively address the challenge and problem solve (i.e., healthy skepticism, solicit help, social referencing, self-talk, alternate strategies, cognitive reappraisal). Strategies that reflected a limited ability to effectively cope with the challenge were considered maladaptive (i.e., negative self-appraisal, focusing on the misfit piece, perseveration, fidgeting, insistence on completion). Composite scores of adaptive and maladaptive self-regulation were computed by averaging the z scores of relevant self-regulation strategies based on our theoretical understanding of these adaptive and maladaptive self-regulation strategies and our previously published work (Miller et al., 2015). A negative correlation between adaptive and maladaptive self-regulation composite scores (r = −.49, p = .03) indicates that when children use adaptive self-regulation strategies, they are less likely to use maladaptive strategies. This moderate correspondence between adaptive and nonadaptive strategies indicates convergence on the latent construct “self-regulation” yet highlights the importance of distinct self-regulatory dimensions.
TABLE 1.
Coded Self-Regulation Strategies
| Strategy Definition | |
|---|---|
| Adaptive Self-Regulation | |
| Cognitive Reappraisal | Child attempts to reframe the task to view it in more positive manner (e.g., “It’s okay; we can do the other pieces without it.”) |
| Alternate Strategies | Child uses appropriate problem-solving strategies to attempt to fit the wrong piece (e.g., rotating the wrong piece in the spot, trying to put the piece in a different spot, etc.) |
| Self-Talk | Child talks to himself or herself (any type verbalization not addressed to someone else) |
| Solicit Help | Child directly asks experimenter for help with the task (e.g., “Can you help me?”) |
| Healthy Skepticism | Child makes comment that indicates that she or he understands that something is wrong with the puzzle (e.g., “This piece doesn’t go here”) |
| Social Referencing | Child looks to another person for information about how to respond, think or feel about an environmental event or stimuli |
| Comfort Seeking | Child initiates interaction with another person in the hope of obtaining comfort, not help with the task |
| Maladaptive Self-Regulation | |
| Focus on Wrong Piece | Child fixates on the wrong piece and may ignore other pieces |
| Insistence on Completion | Child insists puzzle is finished—may accept or ignore that the wrong piece does not fit |
| Negative Self-Appraisal | Child attributes trouble with the task to personal attributes (i.e., “This is too hard for me”) |
| Perseveration | Child is focused on a task element that is no longer (or was never) productive (e.g., attempting to cram the wrong piece into a spot) |
| Fidgeting | Any repetitive, purposeless motion of the legs, arms, hands, buttocks, or trunk; reflects worry and anxiety |
| Disruptive Behavior | Child acts in a way that is disruptive or aggressive (yelling, kicking, etc.) |
Analysis
Descriptive features of the distribution of each behavioral outcome were first inspected for normality (presented as M, SD). Because the behavioral data were positively skewed in the sleep restriction condition, we employed Wilcoxon matched-pairs signed-rank tests (two-tailed) to examine sleep-dependent differences in response inhibition and self-regulation composites. In addition, we used Wilcoxon matched-pairs signed-rank tests (two-tailed) to verify that there were no order (learning) effects from one assessment to the other on either the go/no-go task or any of the self-regulation strategies used during the unsolvable puzzle task. Linear mixed-effects regression was then used to determine whether sleep moderated the relationship between response inhibition and adaptive and maladaptive self-regulation. With this analysis, each subject served as his or her own control. We chose to examine one dimension of EF, response inhibition, not only based on the appreciation that inhibitory control is the most elementary aspect of EF (Carlson & Wang, 2007; Jahromi & Stifter, 2008) but also because it is integral to the development of self-regulation in early childhood (Riggs et al., 2006). Although the relationship between EF and self-regulation is bidirectional, cognitive control processes are considered the cornerstone of coping and regulation of behavior; thus, we selected adaptive and maladaptive self-regulation strategies as our outcomes of interest and included composite scores of these as the dependent variables in separate models. Response inhibition, sleep condition, and the interaction between response inhibition and sleep condition were included as independent variables in each model. Finally, Pearson correlations (two-tailed) were computed to examine the association between response inhibition and adaptive and maladaptive self-regulation in each condition (linearity inspected with scatterplots). The alpha level for all analyses was set at .05. All statistical analyses were performed in R version 3.1.1 (R Core Team, Vienna, Austria).
RESULTS
Sleep Schedule and Protocol Verification
We found no significant differences between children’s average actigraphic sleep measures (i.e., lights-off time, wake time, time in bed, sleep efficiency; Table 2) during the first 4 days before each behavior assessment. Because children did not nap and stayed up late during the sleep restriction condition, we expected differences in sleep during the 24 hr preceding each behavior assessment. Average lights-off time was 2.5 hr later on the night before the sleep restriction condition relative to the baseline condition; however, morning wake time did not differ, as expected by the stipulated experimental protocol. Children spent approximately 3.4 hr less time in bed and had shorter sleep durations of about 2.7 hr during the sleep restriction relative to the baseline condition. Sleep efficiency was similar between conditions (p > .05).
We also used actigraphy data to determine whether sleep measures differed between napping (n = 14) and non-napping children (n = 5). Although 24-hr sleep duration was similar between these two groups (p > .05), non-napping children slept 39 min longer at night than those who napped (t = 2.4, d = 1.19, p = .03). The amount of sleep loss in the sleep restriction condition relative to baseline was similar between napping groups (p > .05). Thus, our sleep manipulation presented a similar challenge to all participants, whether they were regularly napping or not. We found no differences in response inhibition and self-regulation performance between napping and non-napping children in either condition or in changes between conditions (all ps > .05).
Response Inhibition and Self-Regulation
Wilcoxon matched-pairs signed-rank tests showed no significant effect of order on any behavioral measure (response inhibition or self-regulation), indicating that there was no learning effect between assessments (all ps > .05). Wilcoxon matched-pairs signed-rank tests revealed no significant effect of sleep restriction on response inhibition or adaptive or maladaptive self-regulation strategies (Table 3). We did observe interindividual differences in children’s responses to sleep restriction, with some children performing better in the baseline condition, whereas others improved after sleep restriction. With regard to response inhibition, 26% performed better in baseline and 63% better in sleep restriction. For adaptive self-regulation, 59% used more in baseline and 37% used more in sleep restriction. Finally, with reference to maladaptive self-regulation, 68% used less in baseline and 32% used less in sleep restriction.
Results from the linear mixed-effects regression models are summarized in Table 4. The interaction between response inhibition performance and sleep condition significantly predicted adaptive and maladaptive self-regulation (adaptive: β = −1.35, p = .041; maladaptive: β = 2.60, p < .001), indicating that sleep restriction moderated the association between response inhibition and adaptive and maladaptive self-regulation.
TABLE 4.
Linear Mixed-Effects Models
|
Adaptive SR |
Maladaptive SR |
|||||
|---|---|---|---|---|---|---|
| Variable | β | SE (β) | z | β | SE (β) | z |
| Response Inhibition | 2.63 | 1.22 | 2.16* | −4.85 | 1.26 | −3.86*** |
| Sleep Condition | 1.01 | 0.53 | 1.89 | −2.05 | 0.54 | −3.80*** |
| Response Inhibition × Sleep Condition |
−1.35 | 0.66 | −2.05* | 2.60 | 0.67 | 3.87*** |
Note: SR = self-regulation.
p < .05.
p < .001.
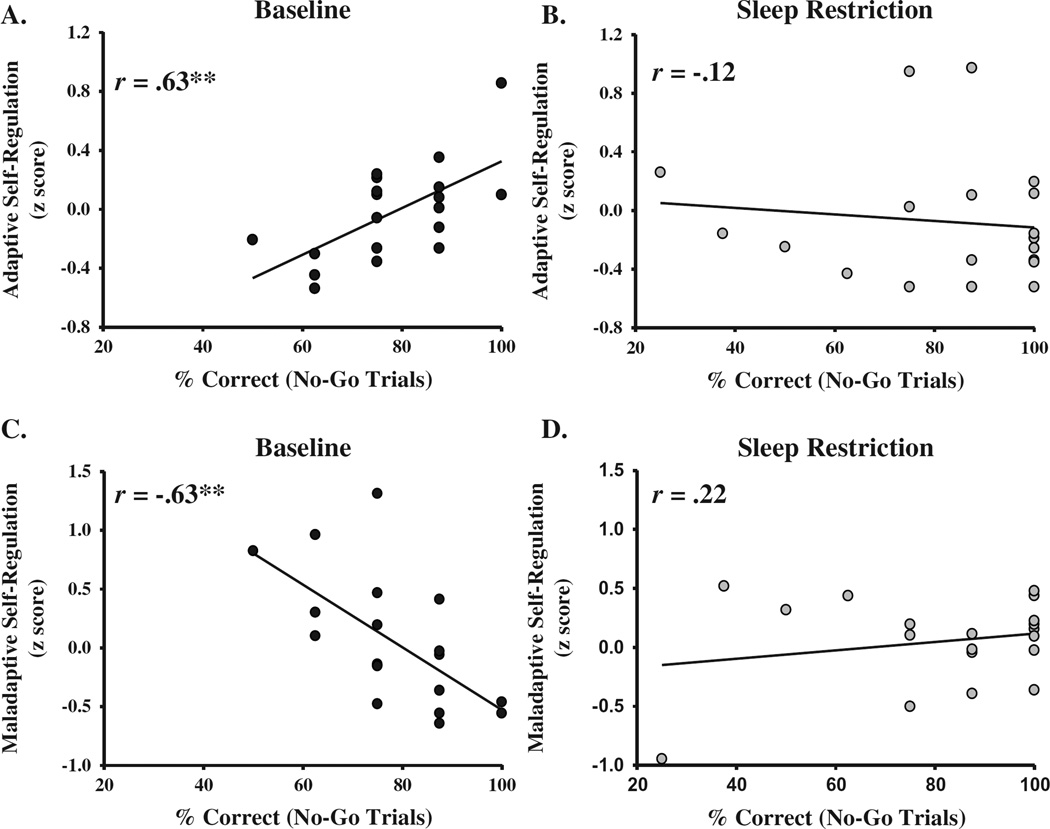
Figure 2 provides scatterplots of the associations between response inhibition performance and self-regulation composites. In the baseline condition, we found a strong correlation between response inhibition performance and adaptive self-regulation (r = .63, p = .004; Figure 2A), such that children with better response inhibition used more adaptive self-regulation strategies. However, a similar association between response inhibition and adaptive self-regulation in the sleep restriction condition was not observed (r = –.12, p = .63; Figure 2B). We also found a strong negative association between response inhibition performance and maladaptive self-regulation in the baseline condition (r = −.63, p = .004; Figure 2C). Children exhibiting poor response inhibition were more likely to use maladaptive self-regulation. Again, this association was not observed following sleep restriction (r = .22, p = .37; Figure 2D).
FIGURE 2.
Scatterplots of associations between response inhibition performance (% correct on no-go trials) and adaptive and maladaptive self-regulation composite scores in the baseline and sleep restriction conditions. Note: *p < .05. **p < .01.
DISCUSSION
This study utilized a well-controlled experimental protocol to examine sleep-dependent effects on response inhibition and self-regulation (i.e., adaptive strategies, nonadaptive strategies) separately, as well as to determine whether acute sleep loss moderated the association between response inhibition and self-regulation in early childhood. In our sample of healthy, good sleeping 40- to 48-month-olds, acute sleep restriction of about 2.7 hr did not reveal a main effect on response inhibition performance or self-regulation strategies during a challenge task. Sleep loss, however, did alter the strength of the interconnection between these measures of cognitive and emotional control. That is, response inhibition was strongly associated with self-regulation when children were well rested but not following sleep restriction. This finding suggests that acute sleep loss across 1 day and night leads to a disconnect between key processes that underlie a number of childhood outcomes, including social-emotional adjustment, school readiness, and mood. The results of this original investigation build upon existing observational, clinical, and other experimental data supporting the importance of sleep for healthy cognitive and emotional development. They also lay the foundation for additional experimental studies that are needed to replicate and extend our results and thus increase understanding of sleep-related pathways that may promote mood, learning, and attentional disorders. Findings are discussed with regard to the importance of sleep for healthy systemic interactions and risk for developmental psychopathology.
Sleep Moderates the Association Between EF and Self-Regulation
An established literature indicates that cognitive and emotional processes are independent predictors of child functioning in academic and social contexts (Bierman et al., 2008; Blair & Diamond, 2008; Bridgett, Oddi, Laake, Murdock, & Bachmann, 2013; McClelland & Cameron, 2012; Riggs et al., 2006); however, expanding developmental science and theoretical frameworks suggest the importance of studying the predictors of the interconnections across multiple systems (Bauer, Quas, & Boyce, 2002; Bridgett et al., 2013; Quas et al., 2014). Indeed, EF skills are proposed to underlie the capacity to self-regulate in response to stress or challenge (Hofmann et al., 2012). Furthermore, a growing literature shows that interactions among these different stress-response systems together shape child functioning and that patterns of intersystem connection are influenced by environmental variables such as SES (Blair, 2010; Quas et al., 2014). In addition, few observational data suggest that associations between EF and self-regulation during early childhood may be altered by child characteristics. One such study found that verbal ability and age moderated the relationship between inhibitory control and emotion regulation measures (Carlson & Wang, 2007). Others recognize that differing temperaments may influence how recruiting EF skills for cognitive control may contribute to self-regulation (Fox & Calkins, 2003; Hongwanishkul et al., 2005). To date, few published studies have considered how sleep loss as a stressor may disrupt the connections between EF and self-regulation in early childhood, and to our knowledge none have utilized an experimental approach. Thus, our data make an important contribution to both the child development and sleep literatures and suggest that sleep is a significant yet underappreciated state that may be critical for healthy integration of multiple regulatory systems.
In this study, we found no main effects of acute sleep loss on measures of response inhibition and self-regulation; however, we did observe a sleep-dependent moderation of the association between these two performance measures. Although these findings may appear contradictory, they align with propositions made in two theoretical models and neuroimaging findings in adults. First, nearly two decades ago, Dahl (1996) described a developmental framework for studying the interplay between regulatory systems (e.g., sleep, attention, affect, arousal) based on overlap in clinical, behavioral, and physiological domains, as well as the importance of the prefrontal cortex (PFC) in modulating the integration of such processes (Dahl, 1996). According to this model, sleep loss poses a relatively small challenge to cognition in “isolated” conditions; however, when such cognitive demands are presented in the context of demanding emotional or social situations, decrements in self-regulation are more likely. Second, Zohar and colleagues’ cognitive-energy model proposes that sleep loss may reduce cognitive energy stores and impair the ability to call upon these resources to promote regulation of emotion and behavior (Zohar et al., 2005).
In addition, data from functional neuroimaging studies performed in the past decade provide strong evidence that sleep plays a significant role in altering interconnections between brain regions involved in the regulation of emotion (reviewed in Goldstein & Walker, 2014). For example, a recent study performed in adults showed that all-night sleep deprivation causes a functional disconnect between the mPFC and amygdala during an emotionally salient task. Such findings suggest that when adults obtain adequate sleep, the PFC is tightly coupled with the amygdala and thus can exert inhibitory control over emotions, whereas without sleep this connectivity and associated cognitive control is diminished (Yoo, Gujar, Hu, Jolesz, & Walker, 2007). Our behavioral data indicating a decoupling between response inhibition and self-regulation with sleep loss map onto these neurophysiological data and suggest that sleep may be especially important for developing connectivity between cognitive and emotion centers of the brain. Indeed, although the neural bases of EF and self-regulation in early childhood are not fully understood, some structural and functional evidence exists. For example, accumulating neuroimaging data indicates that the development of EF and self-regulation is attributed to maturation of the connectivity between cognitive and emotional brain regions (e.g., Belden, Luby, Pagliaccio, & Barch, 2014; Bell & Wolfe, 2004; Cacioppo & Berntson, 1999; Gabard-Durnam et al., 2014; Gee et al., 2013; Luking et al., 2011). The developing functional links between EF and self-regulation are, in part, due to maturation of the PFC, which is reciprocally connected to the amygdala and provides cognitive control of emotion and behavior (Bush, Luu, & Posner, 2000). Of interest, recent resting state functional MRI data indicate that connectivity between the mPFC and amygdala increases between the preschool years and adulthood; however, the functional coupling of such brain regions critical for the regulation of arousal and emotion is not significant in early childhood; adultlike connectivity first emerges at around 10 years of age (Gabard-Durnam et al., 2014). Thus, developmental changes in functional connectivity between regions assumed critical for EF-self-regulation interactions during childhood may make this pathway especially vulnerable to disruption; however, to our knowledge, data on the functional connectivity of emotional and cognitive brain regions after sleep loss do not exist.
Sleep Restriction as a Probe for Understanding Sleep Function in Childhood
Previous experimental studies examining relationships between sleep and aspects of emotion, self-regulation, and neurodevelopment have produced mixed results. For example, Sadeh and colleagues reported that three consecutive nights of 1-hr sleep restriction in kindergarten students impaired tasks measuring simple processing speed, whereas performance on more complex cognitive tasks was preserved (Sadeh et al., 2003). In another study, 5 nights of sleep restriction resulted in increased inattention but no significant decrements in response inhibition in 8- to 15-year-olds (Fallone et al., 2001). Such discrepant findings have been attributed primarily to factors such as the task type or the level of cognitive or emotional load elicited by the task or the specific task type (Drummond et al., 1999; Franzen, Buysse, Dahl, Thompson, & Siegle, 2009; Molfese et al., 2013; Randazzo, Muehlbach, Schweitzer, & Walsh, 1998; Vriend et al., 2013). Such explanations may also apply to our study, as children were older than those in our previous work, thus potentially making the unsolvable puzzle task less challenging.
Our data showing no acute sleep-dependent effects on response inhibition or self-regulation strategies in preschoolers also suggest that the level of “challenge” to the homeostatic sleep system as determined by prescribed experimental protocols may also provide important clues for interpreting mixed results. For instance, quasi-experimental data in preschoolers indicate that napping is important for learning, but only for children who habitually meet part of their 24-hr sleep need with a daytime nap (Kurdziel et al., 2013). We have also shown that nap deprivation of about 90 min in regularly napping toddlers leads to decrements in emotion processing (Berger et al., 2012) and self-regulation strategies (Miller et al., 2015), with effect sizes similar to studies of adults after 1 night of total sleep deprivation. In the current study, however, we utilized a slightly different protocol that simulated another way that children may lose sleep in the real world. We extended their wakefulness by 16 hr, including no napping and a bedtime delay of about 3 hr, and then performed behavioral assessments the following morning when children were likely to be in preschool engaged in social and learning activities. Although we utilized the same challenge task (unsolvable puzzle) as in our previous nap deprivation work (Berger et al., 2012; Miller et al., 2015), our current results differed from these nap-dependent findings. This may imply that nap deprivation poses a robust challenge in regularly napping children when assessed in the afternoon at a time of high homeostatic sleep load (Jenni & LeBourgeois, 2006), whereas our current approach allowed children to obtain some sleep (about 8 hr) and thus potentially “recover” in part from sleep loss. Thus, other protocols such as chronic sleep restriction or sleep promotion in children experiencing insufficient sleep may be warranted to understand the role of sleep in independent aspects of cognition and emotion in early childhood.
Our counterbalanced design produced no order effects (p > .05); however, close visual inspection indicated large interindividual variability in children’s response to sleep restriction. Some children had better response inhibition and self-regulation in the baseline condition, whereas others showed the opposite response to acute sleep loss. Additional research is necessary to identify potential behavioral, physiological, and social factors that may explain why some children experienced performance decrements after sleep restriction and some did not. The need for understanding individual differences in the sensitivity to acute and chronic sleep loss in adolescence and adulthood is an area of keen interest, with safety, academic, and mental health implications (Leproult et al., 2003; Van Dongen & Belenky, 2009); however, little is known about brain and behavioral markers that differentiate young children’s sleep-dependent responses and whether they track susceptibility or resilience to sleep loss across the life span. Data from El-Sheikh and colleagues (2007) suggested that race and socioeconomic status may moderate associations between sleep quality and cognitive functioning during childhood; however, additional research on other factors that may contribute to children’s sensitivity to sleep loss (e.g., sex, chronotype) using well-controlled experimental designs is needed, especially given that early childhood is a sensitive period in neurobehavioral development.
Implications for Developmental Psychopathology
Our results indicating that sleep loss alters the behavioral integration between EF and self-regulation in early childhood has important implications for healthy development, as well as developmental psychopathology (Dahl, 1996). EF and self-regulation are considered foundations of school readiness and are independently associated with emotional and behavioral problems. For example, children with difficulties regulating behavior and emotion are more likely to have unhealthy social relationships (Eisenberg et al., 1995) and poor academic outcomes (Blair, 2002). In addition, EF skills are associated with childhood externalizing problems (Lewis, Lamm, Segalowitz, Stieben, & Zelazo, 2006; Riggs et al., 2006) and depression and anxiety. A lack of integration between cognitive and emotional processes has been identified in those with depression (Blair & Dennis, 2010; Hayden et al., 2006). Further, the inability to recruit EF skills to regulate emotions effectively is a key aspect of anxiety disorders (Mennin et al., 2002). Poor EF (inhibitory control and working memory) and the inability to recruit such mechanisms for self-regulation are central to ADHD (Barkley, 1997). The behavioral manifestation of experimental sleep restriction resembles ADHD-like symptoms in healthy children (Fallone et al., 2001). Taken together, we propose that the disruption of neurological and behavioral pathways underlying emotion–cognition links may be mechanisms by which insufficient sleep accelerates the progression of multiple mental health disorders, including anxiety, depression, and ADHD. Furthermore, our data highlight the need for studying multiple systems in tandem and including sleep as part of models for understanding predictors and outcomes of the connectivity between EF and self-regulation as cornerstones of mental health.
Limitations and Future Directions
In this study, we employed a rigorous experimental design and strict study criteria, which increased control of nuisance variables known to influence our outcomes of interest. Although this approach likely diminished the external validity of our findings, it did provide important insights for future research directions. We chose previously published tasks of response inhibition and self-regulation that approximate the situations that children likely encounter in daily life; however, they were administered in a “lab-type” home setting. This approach limits the generalizability of our findings to real-world settings such as preschool, where learning activities present dynamic challenges incorporating EF skills and self-regulation in a social context. We observed a positive skew in the distribution of the response inhibition measure, which may reflect a ceiling effect due to our sample of generally high-functioning children and therefore may have reduced our ability to detect significant sleep-dependent changes due to reduced variability. Experimental sleep studies that utilize observational behavioral assessments of self-regulation in real-world preschool settings are a rich area for future investigation (Ferrier, Bassett, & Denham, 2014; Ponitz, McClelland, Matthews, & Morrison, 2009). Furthermore, we enrolled a sample including only healthy children with no sleep, behavioral, emotional, or developmental problems and whose family context allowed for stable sleep schedules. Our sample was also primarily Caucasian, well educated, and of middle to upper socioeconomic class. Given the high prevalence of sleep and behavior problems in early childhood (Beltramini & Hertzig, 1983; Briggs-Gowan, Carter, Skuban, & Horwitz, 2001; Kataria et al., 1987; Zuckerman et al., 1987), their co-occurrence (Goodlin-Jones et al., 2009; Lavigne et al., 1999; Reid et al., 2009), and disparities in the sleep health of children living in poverty or of minority status (Acebo et al., 2005; Stein, Mendelsohn, Obermeyer, Amromin, & Benca, 2001), we propose the need for additional experimental studies across diverse groups.
Additional areas of fruitful investigation in uncovering the interplay between sleep and developmental substrates are numerous. First, as sleep is a modifiable health-risk behavior, future research could utilize education programs for parents and teachers to promote healthy sleep or behavioral interventions for preschoolers suffering from insufficient sleep (Garrison, 2014). Some intervention programs, such as Head Start REDI (Bierman et al., 2008; Blair & Razza, 2007) or Tools of the Mind (Bodrova & Leong, 2007; Diamond, Barnett, Thomas, & Munro, 2007), seek to improve emotional competence and academic success by targeting EF and self-regulation. Our findings suggest that sleep should be incorporated in these programs in order to promote maximum benefits. Second, we targeted only one aspect of EF (i.e., response inhibition) and did not examine discrete facial measures of emotion expression (Berger et al., 2012). Thus, future sleep-related studies should incorporate additional dimensions of cognitive control (i.e., working memory, mental flexibility) and emotion processing in their interaction with self-regulation (Ferrier et al., 2014). Third, as insufficient sleep is considered a stressor that influences cognitive control, emotion processing, and self-regulation, additional experimental sleep studies that incorporate physiological measures of sympathetic and parasympathetic activation with behavior may provide key insights into how such systems work in tandem. Finally, as previously noted, our behavioral results map onto sleep-dependent functional brain connectivity changes in adults (Yoo et al., 2007), highlighting the need for examining the neurophysiological mechanisms that may account for a behavioral disconnect between EF and self-regulation when children experience insufficient sleep. Such data may also offer novel insights into individual differences in the sensitivity to sleep loss in early childhood and across development.
Acknowledgments
FUNDING
Funding for this study was provided by the National Institute of Mental Health: K01-MH066139, R01-MH086566.
Contributor Information
Allyson M. Schumacher, Department of Integrative Physiology, University of Colorado Boulder
Alison L. Miller, Department of Health Behavior and Health Education, The University of Michigan School of Public Health
Sarah E. Watamura, Department of Psychology, University of Denver
Salome Kurth, Department of Integrative Physiology, University of Colorado Boulder.
Jonathan M. Lassonde, Department of Integrative Physiology, University of Colorado Boulder
Monique K. LeBourgeois, Department of Integrative Physiology, University of Colorado Boulder
REFERENCES
- Acebo C, Sadeh A, Seifer R, Tzischinsky O, Hafer A, Carskadon MA. Sleep/wake patterns derived from activity monitoring and maternal report for healthy 1- to 5-year-old children. Sleep. 2005;28(12):1568–1577. doi: 10.1093/sleep/28.12.1568. [DOI] [PubMed] [Google Scholar]
- Akacem LD, Simpkin CT, Carskadon MA, Wright KP, Jr, Jenni OG, Achermann P, LeBourgeois MK. The timing of the circadian clock and sleep differ between napping and non-napping toddlers. PLoS ONE. 2015;10(4):e0125181. doi: 10.1371/journal.pone.0125181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asarnow LD, McGlinchey E, Harvey AG. The effects of bedtime and sleep duration on academic and emotional outcomes in a nationally representative sample of adolescents. Journal of Adolescent Health. 2014;54(3):350–356. doi: 10.1016/j.jadohealth.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychological Bulletin. 1997;121(1):65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Bates JE, Viken RJ, Alexander DB, Beyers J, Stockton L. Sleep and adjustment in preschool children: Sleep diary reports by mothers relate to behavior reports by teachers. Child Development. 2002;73(1):62–75. doi: 10.1111/1467-8624.00392. [DOI] [PubMed] [Google Scholar]
- Bauer AM, Quas JA, Boyce WT. Associations between physiological reactivity and children’s behavior: Advantages of a multisystem approach. Journal of Developmental & Behavioral Pediatrics. 2002;23(2):102–113. doi: 10.1097/00004703-200204000-00007. [DOI] [PubMed] [Google Scholar]
- Baum KT, Desai A, Field J, Miller LE, Rausch J, Beebe DW. Sleep restriction worsens mood and emotion regulation in adolescents. Journal of Child Psychology and Psychiatry. 2014;55(2):180–190. doi: 10.1111/jcpp.12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belden AC, Luby JL, Pagliaccio D, Barch DM. Neural activation associated with the cognitive emotion regulation of sadness in healthy children. Developmental Cognitive Neuroscience. 2014;9:136–147. doi: 10.1016/j.dcn.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell MA, Deater-Deckard K. Biological systems and the development of self-regulation: Integrating behavior, genetics, and psychophysiology. Journal of Developmental & Behavioral Pediatrics. 2007;28(5):409–420. doi: 10.1097/DBP.0b013e3181131fc7. [DOI] [PubMed] [Google Scholar]
- Bell MA, Wolfe CD. Emotion and cognition: An intricately bound developmental process. Child Development. 2004;75(2):366–370. doi: 10.1111/j.1467-8624.2004.00679.x. [DOI] [PubMed] [Google Scholar]
- Beltramini AU, Hertzig ME. Sleep and bedtime behavior in preschool-aged children. Pediatrics. 1983;71(2):153–158. [PubMed] [Google Scholar]
- Berger RH, Miller AL, Seifer R, Cares SR, LeBourgeois MK. Acute sleep restriction effects on emotion responses in 30- to 36-month-old children. Journal of Sleep Research. 2012;21(3):235–246. doi: 10.1111/j.1365-2869.2011.00962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berhenke A, Miller AL, Brown E, Seifer R, Dickstein S. Observed emotional and behavioral indicators of motivation predict school readiness in Head Start graduates. Early Childhood Research Quarterly. 2011;26(4):430–441. doi: 10.1016/j.ecresq.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierman KL, Nix RL, Greenberg MT, Blair C, Domitrovich CE. Executive functions and school readiness intervention: Impact, moderation, and mediation in the head start REDI program. Development and Psychopathology. 2008;20(3):821–843. doi: 10.1017/S0954579408000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C. School readiness. Integrating cognition and emotion in a neurobiological conceptualization of children’s functioning at school entry. American Psychologist. 2002;57(2):111–127. doi: 10.1037//0003-066x.57.2.111. [DOI] [PubMed] [Google Scholar]
- Blair C. Stress and the development of self-regulation in context. Child Development Perspectives. 2010;4(3):181–188. doi: 10.1111/j.1750-8606.2010.00145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, Dennis T. An optimal balance: The integration of emotion and cognition in context. In: Calkins SD, Bell MA, editors. Child development at the intersection of emotion and cognition. Washington, DC: American Psychological Association; 2010. pp. 17–35. [Google Scholar]
- Blair C, Diamond A. Biological processes in prevention and intervention: The promotion of self-regulation as a means of preventing school failure. Development and Psychopathology. 2008;20(3):899–911. doi: 10.1017/S0954579408000436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, Raver CC. Child development in the context of adversity: Experiential canalization of brain and behavior. American Psychologist. 2012;67(4):309–318. doi: 10.1037/a0027493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, Razza RP. Relating effortful control, executive function, and false belief understanding to emerging math and literacy ability in kindergarten. Child Development. 2007;78(2):647–663. doi: 10.1111/j.1467-8624.2007.01019.x. [DOI] [PubMed] [Google Scholar]
- Blair C, Ursache A. A bidirectional model of executive functions and self-regulation. Handbook of Self-Regulation: Research, Theory, and Applications. 2011;2:300–320. [Google Scholar]
- Blair C, Zelazo PD, Greenberg MT. The measurement of executive function in early childhood. Developmental Neuropsychology. 2005;28(2):561–571. doi: 10.1207/s15326942dn2802_1. [DOI] [PubMed] [Google Scholar]
- Bodrova E, Leong DJ. Tools of the mind. Upper Saddle River, NJ: Pearson; 2007. [Google Scholar]
- Bridgett DJ, Oddi KB, Laake LM, Murdock KW, Bachmann MN. Integrating and differentiating aspects of self-regulation: Effortful control, executive functioning, and links to negative affectivity. Emotion. 2013;13(1):47–63. doi: 10.1037/a0029536. [DOI] [PubMed] [Google Scholar]
- Briggs-Gowan MJ, Carter AS, Skuban EM, Horwitz SM. Prevalence of social-emotional and behavioral problems in a community sample of 1- and 2-year-old children. Journal of the American Academy of Child & Adolescent Psychiatry. 2001;40(7):811–819. doi: 10.1097/00004583-200107000-00016. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4(6):215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Berntson GG. The affect system architecture and operating characteristics. Current Directions in Psychological Science. 1999;8(5):133–137. [Google Scholar]
- Carlson SM. Developmentally sensitive measures of executive function in preschool children. Developmental Neuropsychology. 2005;28(2):595–616. doi: 10.1207/s15326942dn2802_3. [DOI] [PubMed] [Google Scholar]
- Carlson SM, Wang TS. Inhibitory control and emotion regulation in preschool children. Cognitive Development. 2007;22(4):489–510. [Google Scholar]
- Dahl RE. The regulation of sleep and arousal: Development and psychopathology. Development and Psychopathology. 1996;8(01):3–27. [Google Scholar]
- Diamond A, Barnett WS, Thomas J, Munro S. Preschool program improves cognitive control. Science. 2007;318(5855):1387–1388. doi: 10.1126/science.1151148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond SP, Brown GG, Stricker JL, Buxton RB, Wong EC, Gillin JC. Sleep deprivation-induced reduction in cortical functional response to serial subtraction. Neuroreport. 1999;10(18):3745–3748. doi: 10.1097/00001756-199912160-00004. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Fabes RA, Murphy B, Maszk P, Smith M, Karbon M. The role of emotionality and regulation in children’s social functioning: A longitudinal study. Child Development. 1995;66(5):1360–1384. [PubMed] [Google Scholar]
- El-Sheikh M, Buckhalt JA, Keller PS, Cummings EM, Acebo C. Child emotional insecurity and academic achievement: The role of sleep disruptions. Journal of Family Psychology. 2007;21(1):29–38. doi: 10.1037/0893-3200.21.1.29. [DOI] [PubMed] [Google Scholar]
- Erath SA, Tu KM, Buckhalt JA, El-Sheikh M. Associations between children’s intelligence and academic achievement: The role of sleep. Journal of Sleep Research. 2015;24(5):510–513. doi: 10.1111/jsr.12281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenck MW, Derakshan N, Santos R, Calvo MG. Anxiety and cognitive performance: Attentional control theory. Emotion. 2007;7(2):336–353. doi: 10.1037/1528-3542.7.2.336. [DOI] [PubMed] [Google Scholar]
- Fallone G, Acebo C, Arnedt JT, Seifer R, Carskadon MA. Effects of acute sleep restriction on behavior, sustained attention, and response inhibition in children. Perceptual and Motor Skills. 2001;93(1):213–229. doi: 10.2466/pms.2001.93.1.213. [DOI] [PubMed] [Google Scholar]
- Ferrier DE, Bassett HH, Denham SA. Relations between executive function and emotionality in preschoolers: Exploring a transitive cognition-emotion linkage. Frontiers in Psychology. 2014;5:487. doi: 10.3389/fpsyg.2014.00487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox NA, Calkins SD. The development of self-control of emotion: Intrinsic and extrinsic influences. Motivation and Emotion. 2003;27(1):7–26. [Google Scholar]
- Franzen PL, Buysse DJ, Dahl RE, Thompson W, Siegle GJ. Sleep deprivation alters pupillary reactivity to emotional stimuli in healthy young adults. Biological Psychology. 2009;80(3):300–305. doi: 10.1016/j.biopsycho.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JM. Executive frontal functions. Experimental Brain Research. 2000;133(1):66–70. doi: 10.1007/s002210000401. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Physiology of executive functions: The perception-action cycle. In: Stuss DT, Knight RT, editors. Principles of frontal lobe function. New York, NY: Oxford University Press; 2002. pp. 96–108. [Google Scholar]
- Gabard-Durnam LJ, Flannery J, Goff B, Gee DG, Humphreys KL, Telzer E, Tottenham N. The development of human amygdala functional connectivity at rest from 4 to 23years: A cross-sectional study. NeuroImage. 2014;95:193–207. doi: 10.1016/j.neuroimage.2014.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison MM. Predictors of treatment success in behavioral sleep intervention among preschool children. Sleep. 2014;37:A303. [Google Scholar]
- Gaub M, Carlson CL. Behavioral characteristics of DSM-IV ADHD subtypes in a school-based population. Journal of Abnormal Child Psychology. 1997;25(2):103–111. doi: 10.1023/a:1025775311259. [DOI] [PubMed] [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, Tottenham N. A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. The Journal of Neuroscience. 2013;33(10):4584–4593. doi: 10.1523/JNEUROSCI.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein AN, Walker MP. The role of sleep in emotional brain function. Annual Review of Clinical Psychology. 2014;10:679–708. doi: 10.1146/annurev-clinpsy-032813-153716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodlin-Jones B, Tang K, Liu J, Anders TF. Sleep problems, sleepiness and daytime behavior in preschool-age children. Journal of Child Psychology and Psychiatry. 2009;50(12):1532–1540. doi: 10.1111/j.1469-7610.2009.02110.x. [DOI] [PubMed] [Google Scholar]
- Gray JA. Brain systems that mediate both emotion and cognition. Cognition & Emotion. 1990;4(3):269–288. [Google Scholar]
- Gregory AM, O’Connor TG. Sleep problems in childhood: A longitudinal study of developmental change and association with behavioral problems. Journal of the American Academy of Child & Adolescent Psychiatry. 2002;41(8):964–971. doi: 10.1097/00004583-200208000-00015. [DOI] [PubMed] [Google Scholar]
- Gruber R, Cassoff J, Frenette S, Wiebe S, Carrier J. Impact of sleep extension and restriction on children’s emotional lability and impulsivity. Pediatrics. 2012;130(5):e1155–e1161. doi: 10.1542/peds.2012-0564. [DOI] [PubMed] [Google Scholar]
- Hagger MS. Sleep, self-regulation, self-control and health. Stress and Health. 2010;26(3):181–185. [Google Scholar]
- Hayden EP, Klein DN, Durbin CE, Olino TM. Positive emotionality at age 3 predicts cognitive styles in 7-year-old children. Development and Psychopathology. 2006;18(2):409–423. doi: 10.1017/S0954579406060226. [DOI] [PubMed] [Google Scholar]
- Hofmann W, Schmeichel BJ, Baddeley AD. Executive functions and self-regulation. Trends in Cognitive Sciences. 2012;16(3):174–180. doi: 10.1016/j.tics.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Honaker SM, Meltzer LJ. Bedtime problems and night wakings in young children: An update of the evidence. Paediatric Respiratory Reviews. 2014;15(4):333–339. doi: 10.1016/j.prrv.2014.04.011. [DOI] [PubMed] [Google Scholar]
- Hongwanishkul D, Happaney KR, Lee WS, Zelazo PD. Assessment of hot and cool executive function in young children: Age-related changes and individual differences. Developmental Neuropsychology. 2005;28(2):617–644. doi: 10.1207/s15326942dn2802_4. [DOI] [PubMed] [Google Scholar]
- Hughes C, Ensor R. Does executive function matter for preschoolers’ problem behaviors? Journal of Abnormal Child Psychology. 2008;36(1):1–14. doi: 10.1007/s10802-007-9107-6. [DOI] [PubMed] [Google Scholar]
- Huizinga M, Dolan CV, Van Der Molen MW. Age-related change in executive function: Developmental trends and a latent variable analysis. Neuropsychologia. 2006;44(11):2017–2036. doi: 10.1016/j.neuropsychologia.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Iglowstein I, Jenni OG, Molinari L, Largo RH. Sleep duration from infancy to adolescence: Reference values and generational trends. Pediatrics. 2003;111(2):302–307. doi: 10.1542/peds.111.2.302. [DOI] [PubMed] [Google Scholar]
- Jahromi LB, Stifter CA. Individual differences in preschoolers’ self-regulation and theory of mind. Merrill-Palmer Quarterly. 2008;54(1):125–150. [Google Scholar]
- Jenni OG, LeBourgeois MK. Understanding sleep-wake behavior and sleep disorders in children: The value of a model. Current Opinion in Psychiatry. 2006;19(3):282–287. doi: 10.1097/01.yco.0000218599.32969.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataria S, Swanson MS, Trevathan GE. Persistence of sleep disturbances in preschool children. The Journal of Pediatrics. 1987;110(4):642–646. doi: 10.1016/s0022-3476(87)80571-1. [DOI] [PubMed] [Google Scholar]
- Kochanska G. Mutually responsive orientation between mothers and their young children: Implications for early socialization. Child Development. 1997;68(1):94–112. [PubMed] [Google Scholar]
- Kochanska G, Coy KC, Murray KT. The development of self-regulation in the first four years of life. Child Development. 2001;72(4):1091–1111. doi: 10.1111/1467-8624.00336. [DOI] [PubMed] [Google Scholar]
- Kurdziel L, Duclos K, Spencer RM. Sleep spindles in midday naps enhance learning in preschool children. Proceedings of the National Academy of Sciences. 2013;110(43):17267–17272. doi: 10.1073/pnas.1306418110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavigne JV, Arend R, Rosenbaum D, Smith A, Weissbluth M, Binns HJ, Christoffel KK. Sleep and behavior problems among preschoolers. Journal of Developmental & Behavioral Pediatrics. 1999;20(3):164–169. doi: 10.1097/00004703-199906000-00005. [DOI] [PubMed] [Google Scholar]
- LeBourgeois MK, Carskadon MA, Akacem LD, Simpkin CT, Wright KP, Jr, Achermann P, Jenni OG. Circadian phase and its relationship to nighttime sleep in toddlers. Journal of Biological Rhythms. 2013;28(5):322–331. doi: 10.1177/0748730413506543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leproult R, Colecchia EF, Berardi AM, Stickgold R, Kosslyn SM, Van Cauter E. Individual differences in subjective and objective alertness during sleep deprivation are stable and unrelated. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2003;284(2):R280–R290. doi: 10.1152/ajpregu.00197.2002. [DOI] [PubMed] [Google Scholar]
- Leventhal H, Scherer K. The relationship of emotion to cognition: A functional approach to a semantic controversy. Cognition & Emotion. 1987;1(1):3–28. [Google Scholar]
- Lewis MD, Lamm C, Segalowitz SJ, Stieben J, Zelazo PD. Neurophysiological correlates of emotion regulation in children and adolescents. Journal of Cognitive Neuroscience. 2006;18(3):430–443. doi: 10.1162/089892906775990633. [DOI] [PubMed] [Google Scholar]
- Luking KR, Repovs G, Belden AC, Gaffrey MS, Botteron KN, Luby JL, Barch DM. Functional connectivity of the amygdala in early-childhood-onset depression. Journal of the American Academy of Child & Adolescent Psychiatry. 2011;50(10):1027–1041. doi: 10.1016/j.jaac.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauss IB, Troy AS, LeBourgeois MK. Poorer sleep quality is associated with lower emotion-regulation ability in a laboratory paradigm. Cognition & Emotion. 2013;27(3):567–576. doi: 10.1080/02699931.2012.727783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland MM, Cameron CE. Self-regulation in early childhood: Improving conceptual clarity and developing ecologically valid measures. Child Development Perspectives. 2012;6(2):136–142. [Google Scholar]
- Mennin DS, Heimberg RG, Turk CL, Fresco DM. Applying an emotion regulation framework to integrative approaches to generalized anxiety disorder. Clinical Psychology: Science and Practice. 2002;9(1):85–90. [Google Scholar]
- Miller AL, Seifer R, Crossin R, LeBourgeois MK. Toddler’s self-regulation strategies in a challenge context are nap-dependent. Journal of Sleep Research. 2015;24(3):279–287. doi: 10.1111/jsr.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: A latent variable analysis. Cognitive Psychology. 2000;41(1):49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Molfese DL, Ivanenko A, Key AF, Roman A, Molfese VJ, O’Brien LM, Hudac CM. A one-hour sleep restriction impacts brain processing in young children across tasks: Evidence from event-related potentials. Developmental Neuropsychology. 2013;38(5):317–336. doi: 10.1080/87565641.2013.799169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Sleep Foundation. Children and sleep. 2004 Retrieved from https://sleepfoundation.org/sleep-polls-data/sleep-in-america-poll/2004-children-and-sleep.
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9(5):242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ponitz CC, McClelland MM, Matthews JS, Morrison FJ. A structured observation of behavioral self-regulation and its contribution to kindergarten outcomes. Developmental Psychology. 2009;45(3):605–619. doi: 10.1037/a0015365. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK. Attention, self-regulation and consciousness. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 1998;353(1377):1915–1927. doi: 10.1098/rstb.1998.0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quas JA, Yim IS, Oberlander TF, Nordstokke D, Essex MJ, Armstrong JM, Boyce WT. The symphonic structure of childhood stress reactivity: Patterns of sympathetic, parasympathetic, and adrenocortical responses to psychological challenge. Development and Psychopathology. 2014;26(4, Pt.1):963–982. doi: 10.1017/S0954579414000480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffaelli M, Crockett LJ, Shen Y-L. Developmental stability and change in self-regulation from childhood to adolescence. The Journal of Genetic Psychology. 2005;166(1):54–76. doi: 10.3200/GNTP.166.1.54-76. [DOI] [PubMed] [Google Scholar]
- Randazzo AC, Muehlbach MJ, Schweitzer PK, Walsh JK. Cognitive function following acute sleep restriction in children ages 10-14. Sleep. 1998;21(8):861–868. [PubMed] [Google Scholar]
- Reid GJ, Hong RY, Wade TJ. The relation between common sleep problems and emotional and behavioral problems among 2- and 3-year-olds in the context of known risk factors for psychopathology. Journal of Sleep Research. 2009;18(1):49–59. doi: 10.1111/j.1365-2869.2008.00692.x. [DOI] [PubMed] [Google Scholar]
- Riggs NR, Jahromi LB, Razza RP, Dillworth-Bart JE, Mueller U. Executive function and the promotion of social-emotional competence. Journal of Applied Developmental Psychology. 2006;27(4):300–309. [Google Scholar]
- Rothbart MK, Posner MI, Kieras J. Temperament, attention, and the development of self-regulation. In: McCartney K, Phillips D, editors. Blackwell handbook of early childhood development. Malden, MA: Blackwell; 2006. pp. 338–357. [Google Scholar]
- Sadeh A, Gruber R, Raviv A. Sleep, neurobehavioral functioning, and behavior problems in school-age children. Child Development. 2002;73(2):405–417. doi: 10.1111/1467-8624.00414. [DOI] [PubMed] [Google Scholar]
- Sadeh A, Gruber R, Raviv A. The effects of sleep restriction and extension on school-age children: What a difference an hour makes. Child Development. 2003;74(2):444–455. doi: 10.1111/1467-8624.7402008. [DOI] [PubMed] [Google Scholar]
- Simpkin CT, Jenni OG, Carskadon MA, Wright KP, Jr, Akacem LD, Garlo KG, LeBourgeois MK. Chronotype is associated with the timing of the circadian clock and sleep in toddlers. Journal of Sleep Research. 2014;23(4):397–405. doi: 10.1111/jsr.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley PA, Dweck CS. Individual differences in achievement goals among young children. Child Development. 1994;65(6):1723–1743. doi: 10.1111/j.1467-8624.1994.tb00845.x. [DOI] [PubMed] [Google Scholar]
- Stein MA, Mendelsohn J, Obermeyer WH, Amromin J, Benca R. Sleep and behavior problems in school-aged children. Pediatrics. 2001;107(4):e60–e60. doi: 10.1542/peds.107.4.e60. [DOI] [PubMed] [Google Scholar]
- Talbot LS, McGlinchey EL, Kaplan KA, Dahl RE, Harvey AG. Sleep deprivation in adolescents and adults: Changes in affect. Emotion. 2010;10(6):831–841. doi: 10.1037/a0020138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touchette É, Petit D, Séguin JR, Boivin M, Tremblay RE, Montplaisir JY. Associations between sleep duration patterns and behavioral/cognitive functioning at school entry. Sleep. 2007;30(9):1213. doi: 10.1093/sleep/30.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dongen HPA, Belenky G. Individual differences in vulnerability to sleep loss in the work environment. Industrial Health. 2009;47(5):518–526. doi: 10.2486/indhealth.47.518. [DOI] [PubMed] [Google Scholar]
- Vriend JL, Davidson FD, Corkum PV, Rusak B, Chambers CT, McLaughlin EN. Manipulating sleep duration alters emotional functioning and cognitive performance in children. Journal of Pediatric Psychology. 2013;38(10):1058–1069. doi: 10.1093/jpepsy/jst033. [DOI] [PubMed] [Google Scholar]
- Walker MP, Harvey AG. Obligate symbiosis: Sleep and affect. Sleep Medicine Reviews. 2010;14(4):215–217. doi: 10.1016/j.smrv.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Weissbluth M. Naps in children: 6 months-7 years. Sleep. 1995;18(2):82–87. doi: 10.1093/sleep/18.2.82. [DOI] [PubMed] [Google Scholar]
- Willoughby MT, Wirth RJ, Blair CB, Family Life Project I. Executive function in early childhood: Longitudinal measurement invariance and developmental change. Psychological Assessment. 2012;24(2):418–431. doi: 10.1037/a0025779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong MM, Brower KJ, Zucker RA. Childhood sleep problems, early onset of substance use and behavioral problems in adolescence. Sleep Medicine. 2009;10(7):787–796. doi: 10.1016/j.sleep.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo S-S, Gujar N, Hu P, Jolesz FA, Walker MP. The human emotional brain without sleep—A prefrontal amygdala disconnect. Current Biology. 2007;17(20):R877–R878. doi: 10.1016/j.cub.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Zahn-Waxler C, Klimes-Dougan B, Slattery MJ. Internalizing problems of childhood and adolescence: Prospects, pitfalls, and progress in understanding the development of anxiety and depression. Development and Psychopathology. 2000;12(03):443–466. [PubMed] [Google Scholar]
- Zelazo PD, Muller U, Frye D, Marcovitch S, Argitis G, Boseovski J, Sutherland A. The development of executive function in early childhood. Monographs of the Society for Research in Child Development. 2003;68(3):vii–137. doi: 10.1111/j.0037-976x.2003.00260.x. [DOI] [PubMed] [Google Scholar]
- Zohar D, Tzischinsky O, Epstein R, Lavie P. The effects of sleep loss on medical residents’ emotional reactions to work events: A cognitive-energy model. Sleep. 2005;28(1):47–54. doi: 10.1093/sleep/28.1.47. [DOI] [PubMed] [Google Scholar]
- Zuckerman B, Stevenson J, Bailey V. Sleep problems in early childhood: Continuities, predictive factors, and behavioral correlates. Pediatrics. 1987;80(5):664–671. [PubMed] [Google Scholar]