Abstract
Morphometric assessments, such as muscle density and body fat distribution, have emerged as strong predictors of cardiovascular risk and post-operative morbidity and mortality. To date, no study has examined morphometric mortality risk prediction among kidney transplant (KT) candidates. KT candidates, waitlisted 2008–2009 were identified (n=96) and followed to the earliest of transplant, death, or administrative end of study. Morphometric measures, including abdominal adipose tissue, paraspinous and psoas muscle composition, and aortic calcification were measured from CTs. Risk of waitlist mortality was examined using Cox proportional hazards regression. On adjusted analyses, radiologic measures remained independently and significantly associated with lower waitlist mortality; the addition of radiologic measures significantly improved model predictive ability over models containing traditional risk factors alone (net reclassification index: 0.56, 95% CI: 0.31–0.75). Higher psoas muscle attenuation (indicative of leaner muscle) was associated with decreased risk of death (aHR: 0.93, 95% CI: 0.91–0.96, p< 0.001); and for each unit increase in lean paraspinous volume there was an associated 2% decreased risk for death (aHR: 0.98, 95%CI: 0.96–0.99, p=0.03). Radiologic measures of lean muscle mass, such as psoas muscle attenuation and paraspinous lean volume, may improve waitlist mortality risk prediction and candidate selection.
Keywords: kidney transplantation, morphometric analysis, risk prediction, survival analysis, waitlist mortality
INTRODUCTION
There is a large unmet need for donor kidneys with over 100,000 patients currently waiting for a deceased donor kidney transplant, yet each year only 11,000 receive a transplant.1 With annual death rates among waitlist registrants approaching 15%, many die before receiving a kidney transplant.2
Current kidney transplant selection practices involve a series of evaluations intended to assess perioperative morbidity and mortality, particularly cardiovascular risk, such that allocation of a scarce resource is optimized.3,4 However, these assessments are costly and often imprecise, as waitlist mortality remains high in large part due to cardiovascular events. Identification of alternative methodologies for predicting morbidity and mortality among waitlisted ESRD patients is a priority. In fact, a 2012 US consensus conference convened to evaluate “Transplant Program Quality and Surveillance”, including a review of the limitations of existing data on pre-transplant metrics and an evaluation of the needs for more accurate predictive tools for waitlist mortality. The conference concluded that more data on waiting list mortality risk and outcomes should be provided.5
Only 51% of dialysis patients are still alive three years after initiation of treatment for end-stage renal disease (ESRD), illustrating the extreme vulnerability of these patients compared to the general population.6 Although comorbidity and disability are associated with mortality and hospitalization in ESRD,6–11 their high prevalence in the ESRD population limits their ability to inform risk prediction.7,8,12–14 Other metrics such as elevated cardiac troponin T (cTnT) have been studied to refine risk prediction in this population,15–17 showing a 1.7-fold increased risk for mortality among waitlist candidates (hazard ratio (HR): 1.73; 95%CI: 1.25–2.39, p=0.01).16 However, the test was associated with low sensitivity (70%) and specificity (69%), and is not routinely performed as part of the kidney transplant evaluation process.16 Moreover, while novel, these additional metrics, such as cTnT, identified to date require additional testing, cost, and time, and as such, may not be practical additions to an already expensive and intense kidney transplantation evaluation process.
Morphometric assessments, such as muscle density and visceral fat volume, have emerged as strong predictors of cardiovascular risk and post-operative morbidity and mortality.18–21 Morphometric analyses do not require any additional testing, as these measurements are made using preoperative computed tomography (CT) imaging routinely obtained as part of many transplant center selection practices. To date, no study has examined morphometric risk prediction and mortality among waitlisted ESRD patients.22,23 Given the significant knowledge gap regarding risk prediction among kidney waitlist candidates, we leveraged the second largest kidney transplant waiting list in the US and examined the relationship between morphometric measures and waitlist mortality.
PATIENTS AND METHODS
Data Source
The study used data from our center’s transplant registry, which includes data on all kidney waitlisted candidates and transplant recipients at our center. The use of these data and a waiver of informed consent were approved by our Institutional Review Board (Protocol Number: X140509006).
Study Population
Adult kidney transplant candidates listed at UAB between 2008 and 2009 with CT imaging of their abdomen and pelvis performed at the time of evaluation were identified (n=320). Candidates whose CT scan quality permitted complete analysis of all morphometric measurements of interest were included (n=96).
Traditional Risk Factors
Patient characteristics traditionally associated with cardiovascular mortality were abstracted from the medical record. These traditional measures included age, race, BMI, length of time spent on dialysis, comorbidities such as diabetes, hypertension, and peripheral vascular disease (PVD), social history, and family history of diabetes, hypertension, and chronic kidney disease (CKD)/ESRD. These factors were abstracted from the record on or closest to the date of listing for transplant.
Morphometric Measures
The same methods employed for morphometric assessment of participants in the Coronary Artery Risk Development in Young Adults (CARDIA) study were used in this cohort. The base CT scanning protocol utilized in numerous large studies (Multi-Ethnic Study of Atherosclerosis, Framingham, Family Heart Study, Jackson Heart Study, and Epidemiology of Diabetes Interventions and Complications) and published by CARDIA was used.24–26 Digital Imaging and Communications in Medicine (DICOM) images from CT were analyzed by skilled analysts, blinded to all clinical information, at Vanderbilt University on a dedicated imaging processing workstation with custom programmed subroutines (Osirix, Pixmeo Bernex, Switzerland) and a dedicated pen computing display (Cintiq, Wacom Technology Corporation Vancouver, WA). Abdominal adipose tissues were volumetrically measured from a block of slices centered at the L4–5 vertebral bodies extending for 30 mm in the z-axis (head-to-foot). Specific Hounsfield units (HU) thresholds are used to discriminate between fat (−190 to −30 HU) and muscle (−30 to −160). Trained analysts defined the boundaries of the air/skin, subcutaneous fat/muscular fascia and peritoneum creating anatomically defined regions of interest (ROI). For the entire abdomen (all tissue deep to the skin), the intra-abdominal space (tissue within the peritoneal cavity) and the subcutaneous space (tissue deep to the skin but superficial to the muscular fascia) the volume [mm3] and mean tissue attenuation [mean CT number] of adipose and lean tissue based on the specific CT number thresholds was calculated. The ratio of visceral to subcutaneous adipose tissue was calculated directly.
Additional segmentations of the psoas and paraspinous skeletal muscles were performed and corresponding volumes of fat and lean tissue calculated within a 30mm block of slices centered at L3–4. Calcified atherosclerotic plaque was measured in the infrarenal abdominal aorta and common iliac arteries and reported as an Agatston Score (Aquarius Workstation, TeraRecon, Foster City, CA), as previously described.24,26,27
Exploratory Data Analyses
Characteristics were compared by candidate status – death on the waiting list, transplantation, still waiting. Continuous variables were analyzed using Kruskal Wallis tests, and categorical variables were examined using chi-square tests of independence.
Outcome Ascertainment
The primary outcome was waitlist mortality. Death dates were abstracted from the medical record, obtained from the United Network for Organ Sharing, and supplemented by information from the Centers for Medicare and Medicaid Services and the Limited Access Death Master File available from the National Technical Information Service. Exposure time began at time of waitlisting to the earlier of patient death, transplantation, or administrative end of study (July 1, 2015).
Survival Analyses
Risk of mortality was examined using multivariable Cox proportional hazards regression. Factors found to be significant on univariate analyses, as well as traditional factors known to be associated with cardiovascular mortality, were considered for model development, with the most parsimonious model chosen by minimizing Akaike’s Information Criterion. Analysis considering transplantation as a competing risk was performed using the Fine and Gray method, and inferences were confirmed (Supplemental Table 1).28 All analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC), Stata version 14 (StataCorp, College Station, TX), and R version 3.2.5 (R Foundation for Statistical Computing, Vienna, Austria).
Model discrimination and calibration
The discriminative ability of the standard Cox model was calculated with Harrell’s C index. To assess calibration of the model, we stratified patients by quantile of risk and compared observed and expected waitlist mortality using the Groennesby and Borgan score test.29,30 To assess improvements in discrimination between a model containing only traditional risk factors with a model containing both traditional and morphometric factors, we used an extension of the Net Reclassification Improvement (NRI) index from R package survIDINRI, estimating NRI using 200 resampling perturbations and assessing the values at 3 years post-listing for transplant.31,32
Sensitivity Analyses
Additional models were built, including models chosen by backwards, forwards, and stepwise selection and factors significant at an alpha of < 0.10 on univariate analyses. Active status on the waitlist was considered, and results were found to be similar to the competing risks model (Supplemental Table 2). Models considering other traditional risk factors were generated (Supplemental Table 3). Inferences of the reported parsimonious model were confirmed.
RESULTS
Kidney Transplant Candidate Characteristics
The cohort at time of being added to the waitlist had a median age of 50.7 years (IQR: 40.1–58.0), 57% male, 52% African American, 52% were blood group O, and the median time spent on dialysis prior to evaluation for transplant was 0.63 years (interquartile range (IQR): 0.0–2.30). Comorbid conditions were common, including diabetes 44%, hypertension 90%, coronary artery disease 39%, and PVD 4%. Nine percent of the cohort was infected with hepatitis C, and 22% had a history of prior transplant. After a median of 3.9 years of follow-up (IQR: 1.3–6.1), 39 patients died while waiting, 26 patients were transplanted (13 received deceased donor transplants and 13 living donor transplants), and 31 were still awaiting kidney transplant. Among those transplanted, median waiting time was 0.9 years (IQR: 0.3–3.1).
Waitlist Mortality
We explored traditional risk factors known to be associated with increased risk for cardiovascular morbidity and mortality (Table 1). Candidates that died while waiting were older than those who achieved transplant or remained on the waitlist (54.5 yrs vs. 47.6 yrs vs. 44.9 yrs, respectively, p=0.01) and had longer median time on dialysis prior to evaluation compared to those who achieved transplant (1.1yrs vs. 0.13yrs, p< 0.001). BMI ≥ 30kg/m2 was more common among those remaining alive on the waitlist (60% vs. 31% for those transplanted and 28% for those who died while waiting, p=0.02). Additionally, African American candidates were more likely to die (54%) or remain on the waitlist (71%) than be transplanted (27%, p=0.004).
Table 1.
Traditional risk factors of waitlist candidates by candidate status at time of listing.
| Parameter | Total (n=96) |
Transplanted (n=26) |
Died on Waitlist (n=39) |
Still Waiting (n=31) |
|
|---|---|---|---|---|---|
| N(%) | N(%) | N(%) | N(%) | p | |
| Age, median (IQR) | 50.7 (40.1–58.0) | 47.6 (36.3–60.5) | 54.5 (47.2–61.1) | 44.9 (38.7–51.8) | 0.01 |
| Male sex | 55 (57) | 18 (69) | 19 (49) | 18 (58) | 0.26 |
| Race | |||||
| Non African American | 46 (48) | 19 (73) | 18 (46) | 9 (29) | 0.004 |
| African American | 50 (52) | 7 (27) | 21 (54) | 22 (71) | |
| BMI (kg/m2), median (IQR) | 28.0 (24.0–33.4) | 26.5 (24.0–30.0) | 27.1 (22.0–32.0) | 31.5 (27.0–35.0) | 0.07 |
| BMI ≥ 30 | 37 (39) | 8 (31) | 11 (28) | 18 (60) | 0.02 |
| Time on dialysis (yrs), median (IQR) |
0.63 (0.0–2.3) | 0.13 (0.0–0.7) | 1.10 (0.3–4.3) | 0.92 (0.0–2.5) | 0.002 |
| On dialysis | 69 (72) | 13 (50) | 33 (85) | 23 (74) | 0.009 |
| Blood type | |||||
| A | 26 (27) | 6 (23) | 12 (31) | 8 (26) | 0.58 |
| B | 16 (17) | 3 (12) | 7 (18) | 6 (19) | |
| AB | 4 (4) | 0 (0) | 3 (8) | 1 (3) | |
| O | 50 (52) | 17 (65) | 17 (44) | 16 (52) | |
| Diabetes | |||||
| Type I | 9 (9) | 5 (19) | 3 (8) | 1 (3) | 0.25 |
| Type II | 33 (34) | 6 (23) | 16 (41) | 11 (36) | |
| None | 54 (56) | 15 (58) | 20 (51) | 19 (61) | |
| Hypertension | 86 (90) | 24 (92) | 33 (85) | 29 (95) | 0.49 |
| Coronary artery disease | 37 (39) | 8 (31) | 16 (41) | 13 (43) | 0.63 |
| Peripheral vascular disease | 4 (4) | 0 (0) | 4 (10) | 0 (0) | 0.06 |
| Hepatitis C | 9 (9) | 1 (4) | 6 (15) | 2 (7) | 0.27 |
| Prior transplant | 21 (22) | 2 (8) | 12 (31) | 7 (23) | 0.08 |
| Ever smoked | 52 (57) | 14 (56) | 23 (62) | 15 (52) | 0.69 |
| Alcohol use | 15 (16) | 6 (23) | 5 (13) | 4 (13) | 0.56 |
| Family history of diabetes | 34 (35) | 9 (35) | 15 (39) | 10 (32) | 0.86 |
| Family history of hypertension | 39 (41) | 8 (31) | 19 (49) | 12 (39) | 0.34 |
| Family history of CKD | 36 (38) | 11 (42) | 15 (39) | 10 (32) | 0.73 |
| QTC, median (IQR) | 461 (433–481) | 444 (416–469) | 466 (441–481) | 464.5 (438–485) | 0.13 |
| Ejection fraction, median (IQR) | 55.0 (51–61) | 55.0 (52–61) | 55.5 (51–63) | 55.0 (51–60) | 0.91 |
| Albumin, median (IQR) | 3.50 (3.1–3.8) | 3.70 (3.3–3.8) | 3.30 (3.1–3.7) | 3.50 (2.9–3.7) | 0.14 |
| HDL cholesterol, median (IQR) | 42.0 (32–55) | 37.0 (28–55) | 48.0 (39–63) | 39.0 (33–51) | 0.06 |
| LDL cholesterol, median (IQR) | 78.0 (59–101) | 80.5 (68–117) | 71.0 (53–94) | 80.0 (54–111) | 0.23 |
| Triglycerides, median (IQR) | 124.0 (85–204) | 112.0 (78–188) | 120.5 (73–205) | 129.0 (100–204) | 0.57 |
| Serum total protein, median (IQR) |
7.0 (6.4–7.7) | 6.8 (6.3–7.2) | 7.1 (6.3–8.0) | 7.3 (6.5–7.5) | 0.41 |
| Fasting blood glucose, median (IQR) |
107.5 (93–155) | 111.5 (99–155) | 111.0 (88–158) | 103.0 (92–155) | 0.82 |
We also explored previously reported morphometric measures associated with increased risk for cardiovascular morbidity and mortality (Table 2). Total abdominal, visceral and subcutaneous fat volumes showed no association with waitlist mortality although abdominal fat attenuation was slightly higher in those that died on the WL. Skeletal muscle composition of the paraspinous and psoas muscles was strongly associated with waitlist mortality in unadjusted analyses. Lean and total muscle attenuation were each lower in those who died than either those transplanted or still awaiting transplant for both the paraspinous and psoas muscles. These lower attenuations were consistent with higher fat volume to total muscle volume ratios in those who died compared to those who were transplanted or were still alive awaiting transplant. Abdominal aortoiliac calcified plaque prevalence and calcified plaque burden (higher Agatston scores) were higher in those who died awaiting transplant.
Table 2.
Distribution of morphometric risk factors for mortality among waitlist candidates by candidate status.
| Parameter | Total (n=96) |
Transplanted (n= 26) |
Died on Waitlist (n= 39) |
Still Waiting (n= 31) |
p |
|---|---|---|---|---|---|
| Abdominal fat measures | |||||
| Total abdominal measures | |||||
| Abdomen total volume, cm3 | 2222.5 (1681.0, 2812.7) |
2086.1 (1680.7, 2563.5) |
2017.4 (1605.8, 2936.2) |
2596.7 (1935.0, 2879.2) |
0.18 |
| Total fat volume, cm3 | 1098.7 (712.8, 1843.9) |
1098.7 (724.1, 1277.5) |
959.3 (673.6, 1963.4) |
1605.2 (909.4, 1852.0) |
0.3 |
| Total fat attenuation, HU | −88.9 (−94.8, −78.9) |
−88.9 (−94.7, −77.0) |
−85.3 (−91.4, −76.8) |
−92.6 (−97.7, −87.0) |
0.04 |
| Total muscle volume, cm3 | 864.8 (749.7, 977.5) |
907.7 (779.4, 1087.8) |
834.7 (683.6, 929.5) |
864.8 (750.1, 1057.9) |
0.11 |
| Total muscle attenuation, HU | 30.9 (26.1, 34.7) |
30.4 (26.3, 33.3) |
28.1 (23.1, 36.3) |
31.9 (28.1, 34.9) |
0.19 |
| Visceral | |||||
| Fat volume, cm3 | 297.6 (139.6, 468.2) |
335.5 (136.8, 444.9) |
282.6 (126.1, 394.1) |
359.2 (140.9, 633.0) |
0.3 |
| Fat attenuation, HU | −81.5 (−90.3, −73.5) |
−83.6 (−90.4, −73.7) |
−77.2 (−89.1, −72.5) |
−86.8 (−93.0, −73.6) |
0.49 |
| Subcutaneous | |||||
| Subcutaneous fat volume, cm3 | 706.2 (398.2, 1063.8) |
636.3 (383.9, 767.3) |
515.2 (339.5, 1121.1) |
828.8 (510.2, 1154.1) |
0.13 |
| Subcutaneous fat attenuation, HU | −94.5 (−101.6, −84.9) |
−94.3 (−101.9, −80.4) |
−92.8 (−96.6, −79.7) |
−97.0 (−102.9, −91.1) |
0.08 |
| Abdominal muscle measures | |||||
| Paraspinous | |||||
| Total volume, cm3 | 71.6 (63.9, 81.3) | 70.3 (64.9, 81.5) | 68.0 (62.4, 74.9) | 77.6 (71.1, 83.6) | 0.03 |
| Total attenuation, HU | 27.4 (12.2, 40.3) | 32.9 (28.4, 43.0) | 14.8 (3.4, 28.6) | 30.9 (17.7, 41.5) | < 0.001 |
| Fat volume, cm3 | 6.0 (3.4, 9.8) | 4.0 (3.2, 5.3) | 8.9 (4.3, 14.5) | 5.7 (3.1, 8.6) | < 0.001 |
| Fat attenuation, HU | −60.4 (−64.8, −56.8) | −59.6 (−65.8, −56.9) | −60.9 (−64.1, −55.9) | −60.7 (−67.3, −56.8) | 0.71 |
| Muscle lean volume, cm3 | 64.2 (55.9, 74.3) | 64.9 (59.3, 74.4) | 57.3 (51.6, 64.3) | 70.9 (63.5, 79.0) | 0.001 |
| Muscle lean attenuation, HU | 35.2 (23.8, 45.2) | 40.6 (34.4, 47.6) | 25.6 (19.4, 34.8) | 38.0 (24.7, 46.6) | < 0.001 |
| Ratio of fat to total volume | 0.09 (0.05, 0.14) | 0.06 (0.05, 0.08) | 0.13 (0.06, 0.21) | 0.09 (0.04, 0.11) | < 0.001 |
| Ratio of lean to total volume | 0.91 (0.86, 0.95) | 0.94 (0.92, 0.95) | 0.87 (0.79, 0.94) | 0.91 (0.89, 0.96) | < 0.001 |
| Psoas | |||||
| Total volume, cm3 | 30.2 (22.6, 36.7) | 32.3 (24.4, 36.7) | 25.0 (19.5, 35.2) | 31.1 (26.7, 38.5) | 0.04 |
| Total attenuation, HU | 37.6 (27.7, 44.3) | 40.5 (35.1, 46.5) | 28.0 (20.0, 38.3) | 42.0 (35.7, 46.9) | < 0.001 |
| Fat volume, cm3 | 1.3 (0.6, 1.8) | 1.2 (0.5, 1.5) | 1.5 (0.9, 2.9) | 1.0 (0.5, 1.6) | 0.02 |
| Fat attenuation, HU | −56.1 (−59.8, −52.2) | −57.1 (−60.5, −51.9) | −55.6 (−60.9, −52.6) | −56.0 (−59.1, −50.8) | 0.69 |
| Muscle lean volume, cm3 | 28.9 (21.5, 35.2) | 31.4 (22.5, 35.3) | 22.1 (17.7, 34.6) | 30.1 (26.2, 37.7) | 0.01 |
| Muscle lean attenuation, HU | 41.1 (34.9, 47.0) | 44.9 (38.5, 49.1) | 35.3 (28.8, 42.6) | 45.0 (39.6, 50.3) | < 0.001 |
| Ratio of fat to total volume | 0.04 (0.02, 0.07) | 0.03 (0.01, 0.05) | 0.06 (0.03, 0.11) | 0.03 (0.01, 0.04) | < 0.001 |
| Ratio of lean to total volume | 0.96 (0.93, 0.98) | 0.97 (0.95, 0.99) | 0.94 (0.89, 0.97) | 0.97 (0.96, 0.99) | < 0.001 |
| Abdominal aortic calcification | |||||
| AAC, total, Agatston units | 561.1 (0.0, 3159.6) |
100.0 (0.0, 1486.8) |
866.0 (292.8, 6145.3) |
206.0 (0.0, 2042.6) |
0.02 |
| Aortic calcium visualized, N (%) | 66 (689) | 16 (62) | 33 (85) | 17 (55) | 0.02 |
On unadjusted analyses, longer time on dialysis was significantly associated with mortality (HR: 1.12, 95%CI: 1.01–1.23, p=0.03), as was older candidate age (HR: 1.04, 95%CI: 1.01–1.08, p=0.007). Additionally, candidate BMI ≥30kg/m2 was associated with a 2.38-fold lower risk of death (HR: 0.42; 95%CI: 0.21–0.85, p=0.02). Total abdominal fat attenuation was significantly lower among waitlist candidates who died compared to those transplanted (−85.3 HU vs. −88.9 HU); and for each HU decrease in total abdominal fat attenuation there was an associated 3% increased risk of waitlist mortality (HR: 1.03, 95%CI: 1.00–1.05, p=0.02). Several abdominal muscle measures correlated with waitlist mortality. Psoas lean volume (22.1cm3 vs. 31.4cm3, p=0.01) and attenuation (35.3 HU vs. 44.9 HU, p<0.001) were significantly lower among candidates who died waiting compared to those transplanted. Greater psoas lean muscle mass was associated with decreased risk of waitlist mortality (volume HR: 0.93, 95%CI: 0.90–0.97, p<0.001; attenuation HR: 0.92, 95%CI: 0.89–0.95, p<0.001). Similar trends were observed with measures of paraspinous lean volume and attenuation. Abdominal aortic calcification (AAC) Agatston score was significantly higher among waitlist candidates who died compared to those transplanted (866 Agatston units (AU) vs. 100 AU); and prevalent AAC was associated with a 2.64-fold increased risk of waitlist mortality (HR: 2.64, 95%CI: 1.11–6.31, p=0.03) (Table 3).
Table 3.
Unadjusted risk of waitlist mortality.
| Parameter | HR | 95% CI | p-value |
|---|---|---|---|
| Traditional | |||
| Age (per 1-year increase) | 1.04 | 1.01–1.08 | 0.007 |
| African American race | 0.72 | 0.38–1.36 | 0.31 |
| BMI ≥ 30 kg/m2 | 0.42 | 0.21–0.85 | 0.02 |
| Dialysis time (per 1-year increase) | 1.12 | 1.01–1.23 | 0.03 |
| On dialysis | 2.40 | 1.01–5.73 | 0.05 |
| Morphometric | |||
| Total abdominal fat attenuation, HU | 1.03 | 1.00–1.05 | 0.02 |
| Paraspinous muscle total volume, cm3 | 0.97 | 0.95–0.99 | 0.01 |
| Paraspinous muscle total attenuation, HU | 0.95 | 0.93–0.97 | < 0.001 |
| Paraspinous muscle fat volume, cm3 | 1.08 | 1.04–1.11 | < 0.001 |
| Paraspinous muscle lean volume, cm3 | 0.95 | 0.93–0.97 | < 0.001 |
| Paraspinous muscle lean attenuation, HU | 0.94 | 0.91–0.96 | < 0.001 |
| Psoas muscle total volume, cm3 | 0.95 | 0.91–0.98 | 0.002 |
| Psoas muscle total attenuation, HU | 0.93 | 0.91–0.95 | < 0.001 |
| Psoas muscle fat volume, cm3 | 1.38 | 1.14–1.68 | 0.001 |
| Psoas muscle lean volume, cm3 | 0.93 | 0.90–0.97 | < 0.001 |
| Psoas muscle lean attenuation, HU | 0.92 | 0.89–0.95 | < 0.001 |
| Abdominal aortic calcification, Agatston units | 1.00 | 1.00–1.00 | 0.08 |
| Presence of abdominal aortic calcification | 2.64 | 1.11–6.31 | 0.03 |
After adjusting for multiple factors, including both traditional and morphometric measures, radiologic measures remained the strongest independent risk factors associated with waitlist mortality. Specifically, for each HU increase in psoas muscle attenuation (indicative of leaner muscle mass) there was an associated 7% decreased risk of death (adjusted HR: 0.93, 95%CI: 0.91–0.96, p< 0.001) (Table 4 & Figure 1). For each HU increase in total fat attenuation (or abdominal fat infiltration), there was an associated 3% increased risk of waitlist mortality (aHR: 1.03, 95%CI: 1.00–1.06, p=0.02), and for each HU increase in paraspinous muscle lean volume there was an associated 2% decreased risk of death (aHR: 0.98, 95%CI: 0.96–0.99, p=0.03).
Table 4.
Adjusted hazard ratio of kidney waitlist mortality.
| Model 1 – Traditional Factors | Model 2 – CT Measures | Model 3 – Traditional & CT | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | P | HR | 95% CI | p | |
| Age, per 1-year increase | 1.05 | 1.02–1.09 | 0.001 | 1.04 | 1.00–1.08 | 0.03 | |||
| Years on dialysis, per 1-year increase | 1.14 | 1.03–1.26 | 0.01 | 1.12 | 0.99–1.25 | 0.06 | |||
| Total fat attenuation, per 1 unit increase in HU |
1.03 | 1.01–1.06 | 0.006 | 1.03 | 1.00–1.06 | 0.02 | |||
| Paraspinous total volume, per 1-unit increase in cm3 |
0.99 | 0.97–1.01 | 0.19 | 0.98 | 0.96–0.99 | 0.03 | |||
| Psoas total attenuation, per 1-unit increase in HU |
0.93 | 0.90–0.95 | < 0.001 | 0.93 | 0.91–0.96 | < 0.001 | |||
Net Reclassification Index=0.555 (95% CI: 0.31–0.75, p < 0.001); AIC=277.420, calibration p-value=0.33, Harrell’s c statistic=0.795
Figure 1.
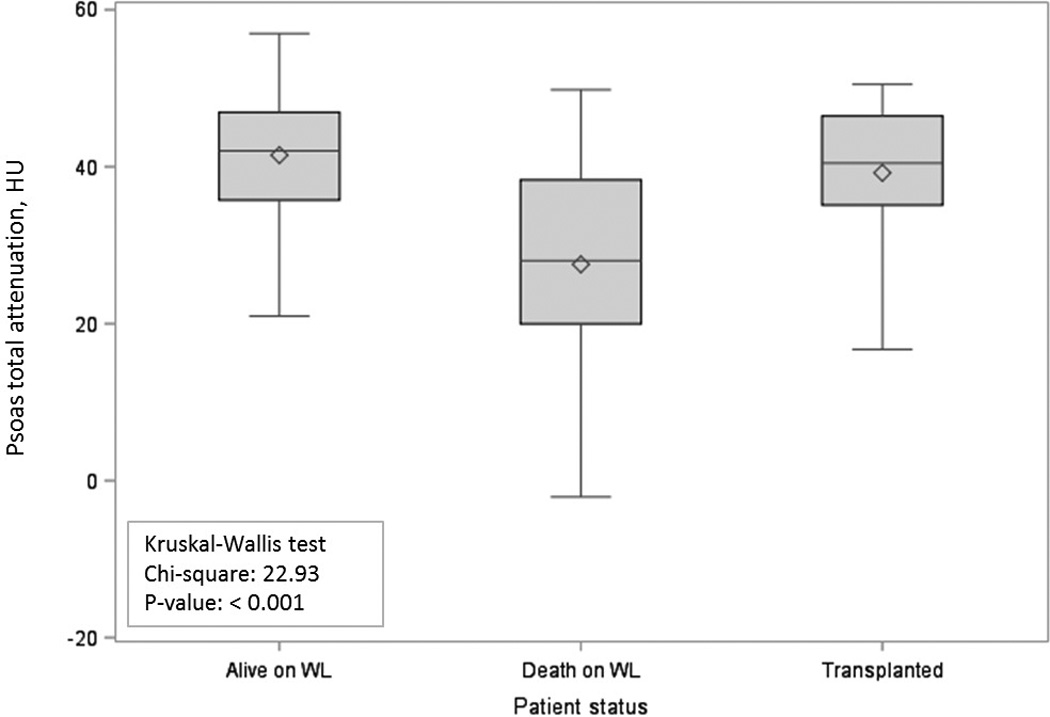
Total psoas attenuation in Hounsfield units by candidate status.
When the discriminative ability of a model containing only the traditional risk factors was compared to that including both the traditional and the morphometric factors, the model including both was shown to have an NRI of 0.555 (95%CI: 0.31–0.75, p < 0.001), indicating that the predictive ability of the model for waitlist mortality was improved significantly by including radiologic measures.
DISCUSSION
In this single center study of kidney transplant waitlist candidates, we found that morphometric measures of lean abdominal mass as measured by CT imaging were independently associated with waitlist mortality. Specifically, higher psoas muscle attenuation or greater lean muscle mass was protective, such that for each HU increase in attenuation there was a 7% decrease in risk of death. Additionally, greater volume of the lean paraspinous muscle was associated with a 2% lower risk of death, and higher fat attenuation was associated with a 3% increased risk. These findings were independent of factors traditionally known to be associated with increased risk of waitlist mortality including candidate age and length of time on dialysis, and suggest that morphometric measures of sarcopenia using radiologic imaging may improve kidney waitlist mortality risk prediction and candidate selection.
Sarcopenia describes the lean mass reduction typically seen with aging and is a component of frailty.33,34 It is estimated that 42% of dialysis patients meet criteria for frailty, and among those dialysis patients older than 50 years the incidence is greater than 50%.10,35 Frailty in the dialysis population is associated with a 2.6-fold increased risk of mortality and a 1.43-fold increased risk of hospitalization independent of age, comorbidity or disability.35,36 Sarcopenia increases progressively along with loss of renal function in chronic kidney disease,37 and represents a significant component of the increased mortality risk seen among frail dialysis patients. In particular, morphometric measures including mid arm muscle circumference (lean mass surrogate) and triceps skin-fold thickness (fat mass surrogate) have been associated with all-cause mortality in hemodialysis patients.38,39 With an NRI of 0.555 for a model with morphometric factors compared to traditional factors alone, our findings also suggest that measurements such as lean muscle mass and volume are strongly associated with mortality risk and lend further support for incorporation of morphometric measures into mortality risk prediction tools for ESRD waitlist candidates.
Our study is unique in that it used novel methods for assessing mortality risk among kidney waitlist candidates. To date no study of kidney waitlist candidates has used CT-derived morphometric measures of sarcopenia to assess risk of waitlist mortality. ESRD patients undergoing evaluation for transplant listing often undergo cross-sectional imaging during the evaluation process to evaluate vascular anatomy, which can be used to assess sarcopenia. Measurements of psoas muscle density and paraspinous lean volume are precise and reproducible and can be obtained at no extra cost and add no additional time to an often laborious evaluation process. Interestingly, other studies have examined various non-radiologic measures of frailty and sarcopenia in the kidney waitlist candidate population and have demonstrated increased mortality risk with increasing frailty and progressive sarcopenia; yet none of those measures have garnered widespread acceptance by the transplant community and are not routinely incorporated into current evaluation practices or standards. Many of those measures, including measures of sarcopenia such as mid-arm muscle circumference and triceps skin-fold thickness, are subjective and susceptible to provider error, as the methods lack the necessary consistency and precision to be reliably included in risk prediction tools. In contrast, CT-derived morphometric measures introduce objective measures of sarcopenia, and as suggested by our results, may prove to be better risk prediction tools for waitlist mortality.
Inferences based on the results of our study must take into account several limitations specific to single center retrospective studies. Alternative provider-based measures of sarcopenia, such as gait, grip strength, mid-arm muscle circumference or triceps skin-fold thickness, were not measured as a part of our center’s evaluation process. As a result, a direct comparison of the predictive ability of radiographic and provider-based measures of sarcopenia could not be studied. Additionally, this study was designed as a pilot project. Due to resource constraints and variability of CT image quality, we were only able to obtain complete morphometric data on 96 patients, thus introducing the possibility for selection bias. Larger studies will be necessary to confirm our findings.
To date, this is the first study to examine the association between radiographic morphometric measures of sarcopenia and kidney waitlist mortality. Our findings suggest that muscle size and density are independently associated with kidney waitlist mortality, and that higher lean muscle mass may be protective. Radiologic measures of sarcopenia, such as psoas muscle attenuation and paraspinous lean volume, may help to improve waitlist mortality risk adjustment and prediction and candidate informed consent.
Supplementary Material
Acknowledgments
This research was supported in part by the National Institutes of Health grant numbers K24-DK101828 (PI: Segev) and K23-DK103918 (PI: Locke) and the University of Alabama at Birmingham Faculty Development Grant Program (PI: Locke).
Footnotes
CONFLICT OF INTEREST
The authors of this manuscript declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
Dr. Jayme Locke oversaw the design of the study, secured funding, coordinated data collection and analysis, and wrote the manuscript. Dr. Jeff Carr participated in study design, contributed to data collection and management of the radiologic measures, and participated in manuscript revision. Ms. Nair conducted the analysis of the radiologic measures and participated in manuscript revision. Mr. Terry oversaw data collection and management of the radiologic measures and participated in manuscript revision. Mrs. Reed conducted the statistical analyses and participated in manuscript revision. Mr. Smith conducted the medical record data collection and participated in manuscript preparation. Dr. Segev participated in the design of the study and participated in manuscript revision. Dr. Lewis participated in the design of the study and participated in manuscript revision.
REFERENCES
- 1.Organ Procurement and Transplantation Network and Scientific Registry of Transplant Recipients. Annual Report of the US Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients. 2013 [Google Scholar]
- 2.Matas AJ, Smith JM, Skeans MA, et al. OPTN/SRTR 2013 Annual Data Report: kidney. Am J Transplant. 2015;15(Suppl 2):1–34. doi: 10.1111/ajt.13195. [DOI] [PubMed] [Google Scholar]
- 3.Bunnapradist S, Danovitch GM. Evaluation of Adult Kidney Transplant Candidates. American Journal of Kidney Diseases. 2007;50:890–898. doi: 10.1053/j.ajkd.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 4.Friedewald JJ, Samana CJ, Kasiske BL, et al. The kidney allocation system. Surg Clin North Am. 2013;93:1395–1406. doi: 10.1016/j.suc.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Kasiske BL, McBride MA, Cornell DL, et al. Report of a consensus conference on transplant program quality and surveillance. Am J Transplant. 2012;12:1988–1996. doi: 10.1111/j.1600-6143.2012.04130.x. [DOI] [PubMed] [Google Scholar]
- 6.United States Renal Data System. 2013 Atlas of CKD & ESRD. [Accessed August 2, 2016];Annual Data Report 2013. http://www.usrds.org/atlas.aspx. [Google Scholar]
- 7.Goodkin DA, Bragg-Gresham JL, Koenig KG, et al. Association of comorbid conditions and mortality in hemodialysis patients in Europe, Japan, and the United States: the Dialysis Outcomes and Practice Patterns Study (DOPPS) J Am Soc Nephrol. 2003;14:3270–3277. doi: 10.1097/01.asn.0000100127.54107.57. [DOI] [PubMed] [Google Scholar]
- 8.Gomez AT, Kiberd BA, Royston JP, et al. Comorbidity burden at dialysis initiation and mortality: A cohort study. Can J Kidney Health Dis. 2015;2:34. doi: 10.1186/s40697-015-0068-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McAdams-DeMarco MA, Suresh S, Law A, et al. Frailty and falls among adult patients undergoing chronic hemodialysis: a prospective cohort study. BMC Nephrol. 2013;14:1–5. doi: 10.1186/1471-2369-14-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johansen KL, Chertow GM, Jin C, Kutner NG. Significance of frailty among dialysis patients. J Am Soc Nephrol. 2007;18:2960–2967. doi: 10.1681/ASN.2007020221. [DOI] [PubMed] [Google Scholar]
- 11.Chang TI, Paik J, Greene T, Miskulin DC, Chertow GM. Updated comorbidity assessments and outcomes in prevalent hemodialysis patients. Hemodial Int. 2010;14:478–485. doi: 10.1111/j.1542-4758.2010.00468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carrero JJ, de Mutsert R, Axelsson J, et al. Sex differences in the impact of diabetes on mortality in chronic dialysis patients. Nephrol Dial Transplant. 2011;26:270–276. doi: 10.1093/ndt/gfq386. [DOI] [PubMed] [Google Scholar]
- 13.Longenecker JC, Coresh J, Powe NR, et al. Traditional cardiovascular disease risk factors in dialysis patients compared with the general population: the CHOICE Study. J Am Soc Nephrol. 2002;13:1918–1927. doi: 10.1097/01.asn.0000019641.41496.1e. [DOI] [PubMed] [Google Scholar]
- 14.Miskulin D. Characterizing comorbidity in dialysis patients: principles of measurement and applications in risk adjustment and patient care. Perit Dial Int. 2005;25:320–332. [PubMed] [Google Scholar]
- 15.Lentine KL, Hurst FP, Jindal RM, et al. Cardiovascular risk assessment among potential kidney transplant candidates: approaches and controversies. Am J Kidney Dis. 2010;55:152–167. doi: 10.1053/j.ajkd.2009.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hickson LJ, Cosio FG, El-Zoghby ZM, et al. Survival of patients on the kidney transplant wait list: relationship to cardiac troponin T. Am J Transplant. 2008;8:2352–2359. doi: 10.1111/j.1600-6143.2008.02395.x. [DOI] [PubMed] [Google Scholar]
- 17.Lentine KL, Costa SP, Weir MR, et al. Cardiac disease evaluation and management among kidney and liver transplantation candidates: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. J Am Coll Cardiol. 2012;60:434–480. doi: 10.1016/j.jacc.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 18.Englesbe MJ, Lee JS, He K, et al. Analytic morphomics, core muscle size, and surgical outcomes. Ann Surg. 2012;256:255–261. doi: 10.1097/SLA.0b013e31826028b1. [DOI] [PubMed] [Google Scholar]
- 19.Englesbe MJ, Terjimanian MN, Lee JS, et al. Morphometric age and surgical risk. J Am Coll Surg. 2013;216:976–985. doi: 10.1016/j.jamcollsurg.2013.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waits SA, Kim EK, Terjimanian MN, et al. Morphometric age and mortality after liver transplant. JAMA Surg. 2014;149:335–340. doi: 10.1001/jamasurg.2013.4823. [DOI] [PubMed] [Google Scholar]
- 21.Ding J, Hsu FC, Harris TB, et al. The association of pericardial fat with incident coronary heart disease: the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Clin Nutr. 2009;90:499–504. doi: 10.3945/ajcn.2008.27358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okamoto T, Morimoto S, Ikenoue T, Furumatsu Y, Ichihara A. Visceral fat level is an independent risk factor for cardiovascular mortality in hemodialysis patients. Am J Nephrol. 2014;39:122–129. doi: 10.1159/000358335. [DOI] [PubMed] [Google Scholar]
- 23.Yoon HE, Park BG, Hwang HS, et al. The prognostic value of abdominal aortic calcification in peritoneal dialysis patients. Int J Med Sci. 2013;10:617–623. doi: 10.7150/ijms.5773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.VanWagner LB, Ning H, Lewis CE, et al. Associations between nonalcoholic fatty liver disease and subclinical atherosclerosis in middle-aged adults: the Coronary Artery Risk Development in Young Adults Study. Atherosclerosis. 2014;235:599–605. doi: 10.1016/j.atherosclerosis.2014.05.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu J, Musani SK, Bidulescu A, et al. Fatty liver, abdominal adipose tissue and atherosclerotic calcification in African Americans: the Jackson Heart Study. Atherosclerosis. 2012;224:521–525. doi: 10.1016/j.atherosclerosis.2012.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reis JP, Loria CM, Lewis CE, et al. Association between duration of overall and abdominal obesity beginning in young adulthood and coronary artery calcification in middle age. JAMA. 2013;310:280–288. doi: 10.1001/jama.2013.7833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuipers AL, Zmuda JM, Carr JJ, et al. Association of volumetric bone mineral density with abdominal aortic calcification in African ancestry men. Osteoporos Int. 2014;25:1063–1069. doi: 10.1007/s00198-013-2486-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. JASA. 1999;94:496–509. [Google Scholar]
- 29.Gronnesby JK, Borgan O. A method for checking regression models in survival analysis based on the risk score. Lifetime Data Anal. 1996;2:315–328. doi: 10.1007/BF00127305. [DOI] [PubMed] [Google Scholar]
- 30.Hosmer DW, Lemeshow LS, May S. Applied Survival Analysis: Regression Modeling of Time-to-Event Data. Hoboken, NJ: Wiley-Interscience; 2008. pp. 187–195. [Google Scholar]
- 31.Pencina MJ, D'Agostino RB, Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uno H, Tian L, Cai T, Kohane IS, Wei LJ. A unified inference procedure for a class of measures to assess improvement in risk prediction systems with survival data. Stat Med. 2013;32:2430–2442. doi: 10.1002/sim.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14:392–397. doi: 10.1016/j.jamda.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McAdams-DeMarco MA, Law A, Salter ML, et al. Frailty as a novel predictor of mortality and hospitalization in individuals of all ages undergoing hemodialysis. J Am Geriatr Soc. 2013;61:896–901. doi: 10.1111/jgs.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bao Y, Dalrymple L, Chertow GM, Kaysen GA, Johansen KL. Frailty, dialysis initiation, and mortality in end-stage renal disease. Arch Intern Med. 2012;172:1071–1077. doi: 10.1001/archinternmed.2012.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buford TW, Anton SD, Judge AR, et al. Models of accelerated sarcopenia: critical pieces for solving the puzzle of age-related muscle atrophy. Ageing Res Rev. 2010;9:369–383. doi: 10.1016/j.arr.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Noori N, Kopple JD, Kovesdy CP, et al. Mid-arm muscle circumference and quality of life and survival in maintenance hemodialysis patients. Clin J Am Soc Nephrol. 2010;5:2258–2268. doi: 10.2215/CJN.02080310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang CX, Tighiouart H, Beddhu S, et al. Both low muscle mass and low fat are associated with higher all-cause mortality in hemodialysis patients. Kidney Int. 2010;77:624–629. doi: 10.1038/ki.2009.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


