Abstract
Activity-dependent pruning of synaptic contacts plays a critical role in shaping neuronal circuitry in response to the environment during postnatal brain development. Although there is compelling evidence that shrinkage of dendritic spines coincides with synaptic long-term depression (LTD), and that LTD is accompanied by synapse loss, whether NMDA receptor (NMDAR)-dependent LTD is a required step in the progression toward synapse pruning is still unknown. Using repeated applications of NMDA to induce LTD in dissociated rat neuronal cultures, we found that synapse density, as measured by colocalization of fluorescent markers for pre- and postsynaptic structures, was decreased irrespective of the presynaptic marker used, post-treatment recovery time, and the dendritic location of synapses. Consistent with previous studies, we found that synapse loss could occur without apparent net spine loss or cell death. Furthermore, synapse loss was unlikely to require direct contact with microglia, as the number of these cells was minimal in our culture preparations. Supporting a model by which NMDAR-LTD is required for synapse loss, the effect of NMDA on fluorescence colocalization was prevented by phosphatase and caspase inhibitors. In addition, gene transcription and protein translation also appeared to be required for loss of putative synapses. These data support the idea that NMDAR-dependent LTD is a required step in synapse pruning and contribute to our understanding of the basic mechanisms of this developmental process.
Keywords: synapse, development, confocal microscopy, neuron culture, dendritic spine, phosphatase, caspase, protein synthesis, transcription
Introduction
In many brain regions, synapse number initially increases and subsequently decreases over the course of postnatal development1. These changes in synapse number represent a period of time during which the rate of synapse formation exceeds that of pruning, followed by a net loss of synapses as the rate of pruning overtakes that of synapse formation2–6. With increasing age, the turnover rate of synapses, usually inferred from dendritic spine instability, declines in several brain regions3,7,8. The loss of synapses on a large scale may be one way neuronal circuits are sculpted in response to experience. During early postnatal and adolescent development, this experience- and activity-dependent process is required for the refinement and proper functioning of neuronal circuits; disruption of synapse pruning can lead to dysfunction underlying some neurodevelopmental and psychiatric disorders9–11. For example, many disorders in the autism spectrum are thought to be caused by pruning deficits12–14. Conversely, schizophrenia is often accompanied by excessive pruning of synaptic connections, particularly in prefrontal cortical areas15–18. In addition, a gene recently identified as a risk factor for schizophrenia, C4, encoding complement protein C4, has also been linked to synapse elimination in the lateral geniculate nucleus19.
Supporting the idea that synapse pruning requires neuronal activity, and that it is not simply due to a lack of neuronal activity, is evidence showing that loss of dendritic spines and functional connections is often greater with more activity in the form of visual experience7,20,21. Although the precise mechanisms underlying activity-dependent synapse elimination in the developing brain remain unknown, the idea that repeated synapse weakening by long-term depression (LTD) is a trigger for this synapse loss has been strengthened with experimental support 22–26. Interestingly, although spine shrinkage accompanies LTD, the two phenomena can be dissociated, suggesting that the same initiating events (i.e., NMDA receptor activation) can trigger both distinct signaling pathways27–29. Similarly, spine loss does not always accompany synapse loss, suggesting that the two processes might occur through independent mechanisms23,24. Some evidence suggests that synapses on the smallest spines are most susceptible to separation23 (but see26).
NMDA receptor-dependent LTD (NMDAR-LTD) can be induced in a variety of experimental models, including in vivo, acutely prepared hippocampal slices, hippocampal slices maintained in cultures, as well as dissociated cortical neurons23,30–32. Most typically, LTD is induced with low frequency (0.5–3 Hz) afferent stimulation30, but it can also be induced with application of NMDA (chemLTD)33,34. NMDAR-dependent LTD requires serine-threonine phosphatase and caspase activity35,36, as well as mRNA and/or protein synthesis37,38, however it is distinct from mGluR-dependent LTD, which requires protein synthesis but not necessarily phosphatase activity39–42. Both types of synaptic depression have been shown to cause dendritic spine shrinkage/loss26–29,43–45, axonal bouton shrinkage/retraction24, and/or changes in miniature synaptic currents12,32,42. The process of experimentally-induced synapse loss can take place over a wide range of timeframes, on the order of minutes/hours23,24 to days/weeks26,46–50. We hypothesized that the specific signaling cascades that are required for NMDAR-LTD can initiate synapse loss (separation of pre- and post-synaptic structures). This is an important point, as LTD-like mechanisms offer the advantage of synapse specificity, similar to that of LTP30. Interestingly, biasing plasticity toward LTP with active CaMKII reduces synaptic contact turnover51, suggesting that LTP and LTD counter each other in terms of synapse stability.
To begin to address whether LTD is a mechanistic prerequisite for synapse pruning, we established an in vitro model of LTD-induced synapse loss by activation of NMDA receptors. We also investigated whether pharmacological inhibition of LTD-related signaling can prevent synapse loss in a model of synapse pruning in cultured rat cortical neurons.
Materials and Methods
Animal Use
The protocols for animal use in this study were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the institution’s Animal Care and Use Committee.
Reagents
The following reagents were used in this study: N-methyl-D-aspartic acid (Sigma, M-3262), FK506 (Tocris, 3631), calyculin A (LC Labs, C-3987), okadaic acid (LC Labs, O-5857), fostriecin (Santa Cruz Biotechnology, sc-202160), actinomycin D (Sigma, A-9415), anisomycin (Sigma, A-5862), cycloheximide (Sigma), Z-DEVD-FMK (Tocris, 2166), natural mouse laminin (Life Technologies, 23017-015), fetal bovine serum (HyClone, SH30910), poly-D-lysine (Sigma, P6407), DME medium (Life Technologies, 11995-065), Neurobasal medium (Life Technologies, 21103-049), B27 Supplement (Life Technologies, 17504-044), GlutaMAX (Life Technologies, 35050-061), 5-fluoro-2′deoxyuridine (FUDR; Sigma, F0503), uridine (Sigma, U3003), Hanks Balanced Salt Solution (HBSS; Invitrogen 14025076 and 14175079), bovine serum albumin (BSA; Sigma, A7030), DMSO (Sigma, D2650), paraformaldehyde (Electron Microscopy Sciences, 157-8), propidium iodide (Sigma, P-4170), and Prolong Gold Antifade Mounting Medium (Invitrogen, P36934).
Fostriecin, cycloheximide, and NMDA were dissolved in water. Calyculin A, okadaic acid, FK506, z-DEVD-FMK, actinomycin D and anisomycin were dissolved in DMSO. The final concentration of DMSO was no more than 0.1%. All of the above were prepared as concentrated stock solutions, stored at −20°C, diluted to their indicated final concentrations in culture medium, and allowed to equilibrate in a 37°C incubator for 1 hour before each experiment. A modified Sindbis virus encapsulating a gene for eGFP was prepared by NIEHS Viral Vector Core Laboratory and stored at −80°C as concentrated stocks until ready to use52.
Preparation of Cultured Neurons
Mixed neuronal cultures were prepared from embryonic day 18 Sprague-Dawley rat brains. Hippocampal and cortical tissue pieces were mechanically disrupted by gentle trituration in HBSS, washed, and resuspended in HBSS. Dissociated neurons were plated at low-density (~80,000) on poly-D-lysine (25 μg/ml)- and laminin (2 mg/ml)-coated 12mm glass coverslips or MatTek dishes in DMEM with 10% fetal bovine serum and grown at 37°C. Half of the medium was replaced 3–4 days later, and then every 3–4 days with serum-free Neurobasal medium plus 2% NS21 made in-house53 or B27 supplement (Life Technologies; in a limited number of experiments), and 1% GlutaMAX. FUDR was added at 4 days in vitro (DIV) to inhibit proliferation of non-neuronal cells. Microglia cultures prepared as described by Harry et al.54 were generously provided by C. McPherson.
Electrophysiological recordings
Whole-cell patch-clamp recordings were performed on either unlabeled neurons or those expressing eGFP. Neurons were perfused at 1 ml min−1 at room temperature in ACSF consisting of 124 mM NaCl, 2.5 mM KCl, 2 mM MgCl2, 2 mM CaCl2, 1.25 mM NaH2PO4, 26 mM NaHCO3, 17 mM D-glucose, 0.5 mM picrotoxin and 0.001 mM TTX. Patch electrodes (3–7 MΩ) were filled with 120 mM potassium gluconate, 10 mM KCl, 3 mM MgCl2, 0.5 mM EGTA, 40 mM HEPES, 2 mM Na2ATP, 0.3 mM NaGTP, with pH adjusted to 7.2 by NaOH. Neurons were voltage clamped at −60 mV and mEPSCs recorded. mEPSCs were detected and analyzed using the Mini Analysis program (version 6.0.7, Synaptosoft). Series resistance was monitored throughout the experiments and data from those cells with greater than 20% change were excluded from the analyses. Baseline recordings were acquired for 3 minutes from cultured neurons on coverslips (18–22 DIV), followed by 20 μM NMDA with 20 μM glycine in artificial cerebrospinal fluid (ACSF) for 8 minutes55. Ten minutes after washout, miniature EPSCs were recorded for 3 additional minutes from the same cell for comparison to the baseline recordings.
Viral Infection
Dissociated cultures were infected with modified Sindbis-eGFP52 at 18–20 DIV, as this range was long enough for the neurons to develop mature spines56–58 (Figure 1A, 1B). Cells on coverslips were treated with virus diluted in conditioned media according to a series of pilot studies in which distribution of neurons expressing GFP was compared and the chosen working dilution resulted in neuronal GFP expression across roughly 25–50% of the coverslip by 16–20 hours post-infection, the time of the experiments. Antibodies against eGFP were used in addition to maximize fluorescent labeling of dendritic spines (described below).
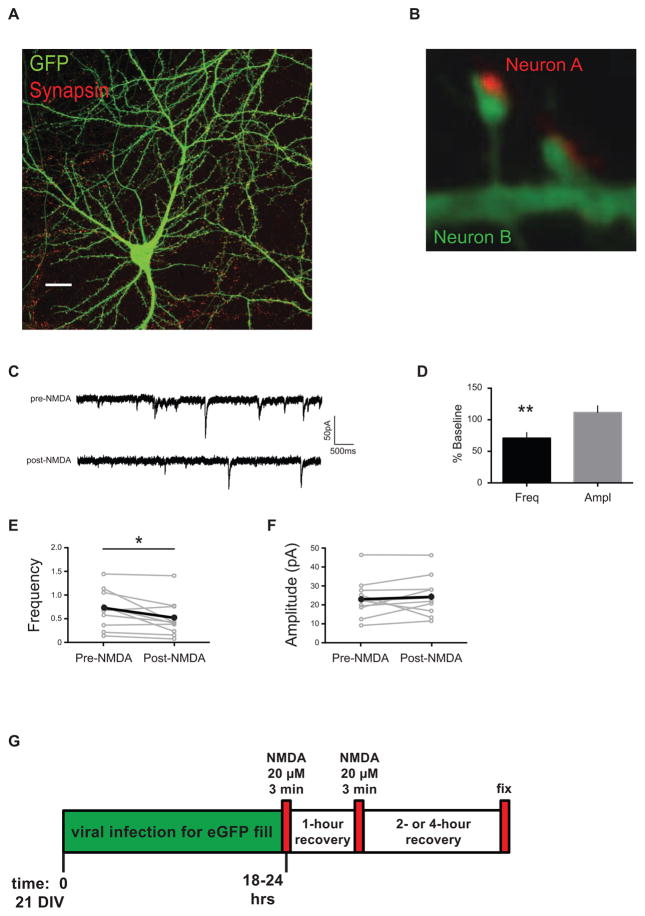
Figure 1. Experimental design.
(A) Dissociated neuronal cultures at 18 – 22 days DIV have fully-branched dendrites studded with mature spines that can be visualized with fluorescent markers using confocal microscopy, and the changes in synapse density can then be quantified. Scale bar, 20 μm. (B) High magnification confocal images of dendrites with spiny synapses. Synapsin antibody staining (red) in the presynaptic terminal of Neuron A is apposed to the GFP-labeled spines and dendrite (green) of postsynaptic Neuron B. NMDA treatment of dissociated neuronal cultures causes a decrease in frequency of miniature EPSCs. (C) Representative traces from mEPSCs recorded from cultured neurons pre- and post-NMDA treatment. (D) Data from cells, normalized to baseline, after NMDA treatment shows a decrease in mEPSC frequency, but no change in amplitude (n = 10 cells, 3 biological samples). Paired analyses of mEPSC recordings from individual cells pre-NMDA and post-NMDA treatments: (E) frequency and (F) amplitude. Some points/error bars are obscured by overlying symbols with similar values. * p freq = 0.031. (G) Schematic diagram of experimental timeline showing that dissociated neurons were cultured for 21 days, infected with a modified Sindbis-eGFP to fill some of the cells, treated twice with the LTD-inducing drug, NMDA (20μM for 3 minutes), allowed to recover two or four hours, and fixed. Cells were then stained for synapse markers (immunofluorescence), imaged, and putative synapse numbers determined.
Immunocytochemistry
Staining of putative synapses was generally performed as described previously59. Two or four hours following experimental treatment, cells were fixed in warm 4% paraformaldehyde (PFA)/2.5% sucrose in phosphate-buffered saline (PBS) for 10 minutes and made permeable with 0.2% Triton X-100 in PBS for 5 minutes. After blocking in 5% BSA/PBS for 30 minutes at room temperature, cells were stained overnight at 4°C with antibodies diluted in blocking buffer, washed 3 times in PBS, stained with the corresponding AlexaFluor 488, 568, or 633 secondary antibodies for 1 hr at room temperature, and washed 3 times in PBS before mounting on slides with Prolong Gold Antifade Mounting Medium (Invitrogen). The following primary antibodies were used at the respective dilutions: chicken anti-GFP (1:40,000, Gene-Tex, GTX13970), rabbit anti-synapsin 1 (1:1000, Millipore, AB1543P), rabbit anti-bassoon (1:500, Cell Signaling, D63B6), mouse anti-PSD95 (1:250, NeuroMab, 75–028), chicken anti-MAP2 (1:2000, Abcam, ab5392), rabbit anti-Iba1 (1:1000, Wako). AlexaFluor goat anti-mouse 488, goat anti-rabbit 568, and goat anti-chicken 633 secondary antibodies (Invitrogen) were used at 1:500 dilutions. To confirm specificity of staining, omission of primary antibody was used as a negative control and showed only negligible staining.
Imaging and Analysis
Pyramidal neurons with a spiny appearance, in contrast to the non-spiny type with relatively unbranched dendrites that are thought to belong to GABAergic neurons60, were chosen specifically to study putative excitatory glutamatergic synapses. Fluorescent images from neuronal cultures were acquired with a Zeiss LSM 510-UV meta confocal microscope (Carl Zeiss Inc, Oberkochen, Germany) using either a Plan-NEOFLUAR 40X/1.3 Oil DIC or a Plan-APOCHROMAT 63X/1.4 Oil DIC objective. Confocal images were acquired and then an optical zoom of 4 was applied for viewing synapses in high-resolution z-stacks. Z-stack images were collapsed into 2D maximum intensity projections before analyzing fluorescence of dendrites using Zen 2010 (Zeiss) for cropping, MetaMorph software (Molecular Devices) for counting, and ImageJ (NIH) for display adjustment. PSD-95- or GFP-labeled spines visible in the green channel were counted manually, followed by visualization of red puncta (synapsin- or bassoon-labeled presynaptic terminals) that were directly adjacent or otherwise contacting spines or the dendritic shafts. Thus, putative synapses were defined as colocalized presynaptic synapsin or bassoon (red), and postsynaptic GFP or PSD-95 (green) fluorescence. Synapses were counted along 20-μm traced segments of dendrites, with proximal dendritic synapses measured 20 – 50 μm from the soma, and distal synapses defined as those > 100 μm from the soma. Note that fluorescence colocalization here indicates only that the two structures (pre- and postsynaptic) are closer than can be resolved in our conditions. Mean numbers of synapses in each condition were normalized to the appropriate vehicle- or mock-treated controls within experiments, then grouped and averaged across experiments. A minimum of three neurons per condition was imaged in each experiment. No attempt was made to quantify shape or size of spines. Slides were coded to ensure blind acquisition and analysis of images.
In some experiments, neurons were treated with MitoTracker Red (Invitrogen, 1 nM in DMSO) to reveal mitochondria, and were imaged using the structured illumination technique on a Zeiss Elyra PS.1 super-resolution microscope (Carl Zeiss Inc, Oberkochen, Germany). A Plan-APOCHROMAT 63X/1.4 Oil DIC objective was used to collect Z-stack images, which were subsequently processed with the SIM processing module of Zeiss Zen 2012.
Experimental Design
To investigate the signaling mechanisms underlying synapse pruning, we first sought to establish an appropriate in vitro model of LTD that also induces synaptic elimination. Three-week-old cultured neurons exhibit highly-branched dendrite morphology, with many fully decorated with mature spines, thus providing an ideal timeframe for imaging synapses56–58. Neurons fixed after virus-eGFP infection, and labeled with validated markers for pre- and post-synaptic structures were used as a measure of synapse density61 (Figure 1A). For example, staining with antibodies raised against synapsin in the presynaptic terminal of Neuron A (red) is apposed to the GFP-labeled spines and dendrite of postsynaptic Neuron B (green) (Figure 1B). Consistent with many studies that established the pharmacological induction of LTD (chemLTD) in cultured neurons32,55,62–65, we replicated a previous report that a brief exposure to NMDA can reduce the frequency of miniature excitatory synaptic currents55 (Figure 1C – 1F), which is one indicator of synaptic depression. This NMDA treatment induced a reduction in mEPSC frequency but not amplitude (Figure 1D: Mini frequency recordings from n = 10 cells, 3 biological samples, and shown as a percentage of the pre-drug baseline; frequency 70.94 ± 8.89%, p = 0.0097; amplitude 111.5 ± 10.6%, p = 0.305), consistent with previous reports for chemLTD in culture preparations55,66. Input resistances were unchanged, ruling out overt breakdown of the membrane (112.92 ± 7.65%, p = 0.38). Thus, brief, low-dose NMDA application to our mixed cortical cultures reduces excitatory transmission as reported previously (Figure 1E – 1F: mean ± SEM of paired analyses of recordings from individual cells pre-NMDA and post-NMDA treatments; frequencypre 0.71 ± 0.13, amplitudepre 23.33 ± 3.28 pA; frequencypost 0.50 ± 0.12, amplitudepost 24.66 ± 3.35 pA; n = 10 cells; * pfreq = 0.031). Although this treatment included added glycine, together with the NMDA in ACSF, we note that the Neurobasal media used in the imaging experiments contains 0.4 mM glycine. Therefore, additional glycine was not included in subsequent experiments. To summarize, our experimental design entailed stimulating neurons at ~21 DIV with NMDA to induce synaptic depression and determining the number of putative synapses on neurons expressing GFP (Figure 1G).
Pharmacological Treatments
To pharmacologically induce LTD (chemLTD) for subsequent image analysis of GFP-expressing neurons, cultures on coverslips (~ 21 DIV) were either mock-treated with culture medium only, or treated with 20 μM NMDA dissolved in culture medium, which was removed after 3 minutes. Cells were then returned to NMDA-free conditioned medium for 1 hour and were subjected to a second treatment, unless indicated (Figure 1G). Cells were exposed to pharmacological inhibitors 10 – 45 minutes prior to and during NMDA addition. In cases where inhibitors were dissolved in DMSO as vehicle, the vehicle alone was used in the control medium. To inhibit protein phosphatase activity, cultures were pre-incubated with serine-threonine phosphatase inhibitors at concentrations selected from published reports, and only those concentrations that did not cause overt neuronal toxicity (blebbing), as assessed in low magnification images, or detachment (floating cells), were used in further studies: calyculin A (CA, 1 – 5 nM,10 minutes prior to NMDA), okadaic acid (OA, 1 – 20 nM, 10 minutes prior to NMDA), fostriecin (fos, 10 – 100 nM, 10 minutes prior to NMDA), or FK506 (10 – 100 nM, 20 minutes prior to NMDA)]. To inhibit mRNA and protein synthesis, we used actinomycin D (actino, 10 nM – 1 μM, 20 minutes prior to NMDA) for transcription inhibition, and anisomycin (aniso, 1 – 20 μM, 20 minutes prior to NMDA), or cycloheximide (CHX, 60 μM, 20 minutes prior to NMDA) for translation inhibition. Similarly, for caspase-3 inhibition, cultures were incubated with z-DEVD-FMK (DEVD, 10 μM, 45 minutes prior to NMDA), as described67. Enzyme activity was not measured. Neuronal cell death was assessed by incubating cultured neurons for 30 minutes – 1 hour at 37°C in propidium iodide (PI; 5 μg/ml)-containing media before imaging and analysis68. PI uptake by nuclei was visualized at 20X magnification using a Zeiss Axio-Observer Z1 epi-fluorescence microscope. Images were then imported into MetaMorph image analysis software (Molecular Devices) where the ‘Count Nuclei’ application was used to count the total number of PI-positive cells.
Statistical Analysis
All experiments were performed on at least three independent neuronal cultures (biological samples) prepared from separate litters of pups. Error bars represent standard errors of the means (SEM). Statistical evaluations were performed using either unpaired student t-tests, or one-way analyses of variance (ANOVAs) followed by appropriate between-group comparisons (Graphpad Prism 6 and InStat, San Diego, CA). All levels of significance represent two-tailed values. Significance was placed at p < 0.05.
Results
NMDA induces synapse loss in culture
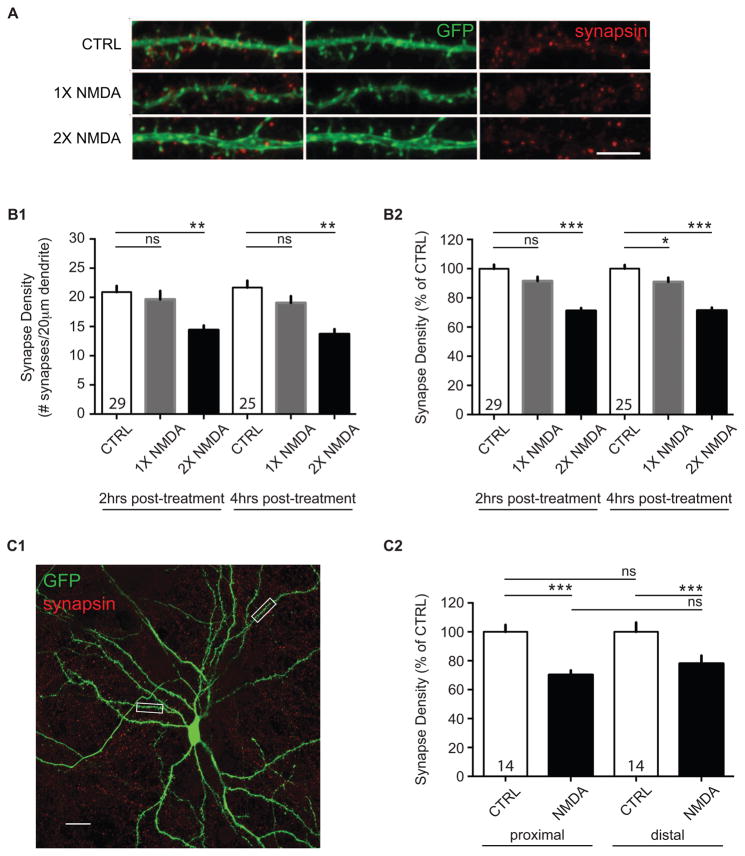
Repeated periods of LTD-inducing stimulation can be accompanied by synapse loss23,50, and bath application of NMDA can induce synaptic depression in slices and in culture preparations29,32–34,48,55,62,69, as well as a rapid loss of dendritic spines70. We therefore tested whether repeated applications of NMDA would influence the number of synaptic connections in dissociated cultures by determining the density of fluorescently-labeled presynaptic terminals (synapsin61) co-localized with dendritic spines of fluorescently-labeled neurons (GFP52; Figure 2A). We found that brief applications of NMDA (20 μM for 3 minutes) induced a lasting decrease in the number of putative synaptic contacts (Figure 2B1). Two applications of NMDA were required for reliable detection of synapse loss, though, as one application induced a much smaller loss that was less consistent across individual dendrites (Figure 2B2). the reduction in synapse density was apparent at two hours post-NMDA treatment and did not reverse or progress further at four hours post-treatment (ANOVA; two hours: F(2,17) = 10.38; p = 0.0011; n = 20 biological samples; four hours: F(2,9) = 14.81; p = 0.0014; n = 12 biological samples); or normalized to same day mock-treatment branches in control coverslips (ANOVA; two hours: F(2,222) = 34.15; p < 0.0001; n = 225 dendritic segments; four hours: F(2,277) = 38.92; p < 0.0001; n = 280 dendritic segments). Thus, we chose to focus our efforts to measure synapse changes on the two-hour post-recovery timeframe.
Figure 2. NMDA-induced LTD results in synapse elimination.
(A) Confocal images of neurons after treatments with media alone (CTRL), or one (1X NMDA) or two (2X NMDA) treatments with NMDA showing fluorescent immunoreactive staining of the presynaptic bouton (red, synapsin) and postsynaptic spine (green, GFP) in 21 DIV cultured dissociated neurons. Scale bar, 5 μm. (B1) Synapse loss occurs reliably after two NMDA treatments in dissociated neurons. Quantification of synapse density (averaged means of # of synapses/20 μm dendrite) in mock-treated (CTRL), once-treated (1X NMDA), and twice-treated (2X NMDA) cultured cells. (B2) Quantification of normalized synapse density (% of CTRL) in mock-treated (CTRL), once-treated (1X NMDA), and twice-treated (2X NMDA) cultured cells. Data show that synapse density changes at 2 hrs or 4 hrs post-NMDA treatment are similar. N = at least three independent biological samples. Values within bars represent sample sizes (# neurons). Error bars represent SEM. Significance from control (CTRL): * p < 0.05, ** p < 0.01, *** p < 0.001; ns, not significant. (C1) NMDA-mediated synapse loss occurs similarly at both proximal and distal synapses. Representative confocal image of a GFP-expressing neuron, with 20 μm-boxed regions marking examples of proximal (20 – 50 μm from the soma) and distal (> 100 μm from the soma) dendritic areas used in analyses shown in (C2). Scale bar, 20 μm. (C2) Quantification of normalized synapse density for proximal and distal synapses in mock-treated (CTRL) and treated (NMDA) cells. Data are averaged means of synapses from at least three biological samples. Values within bars represent sample sizes (# neurons). Error bars represent SEM. Significance: *** p < 0.001; ns, not significant.
The effect of repeated NMDA applications on synapse density was not significantly different between proximal and distal dendrites (Figure 2C1: 20 – 50 μm, or > 100 μm from the cell body, respectively), indicating that synapse loss does not appear to differ in these two dendritic locales (Figure 2C2: unpaired t-test; control (CTRL) vs. NMDA, proximal: 70.29 ± 3.10%, n = 16 – 21 dendritic segments; distal, 78.10 ± 5.47%, n = 15–16 dendritic segments).
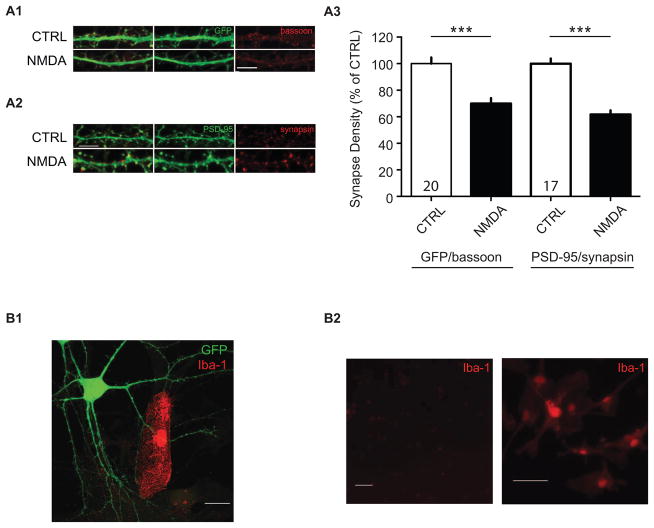
Presynaptic and postsynaptic markers are generally stable in the early phases of NMDA-induced LTD70, and our use of eGFP-filled neurons was intended to avoid confounding degradation of specific proteins after NMDA treatment71. Nevertheless, to confirm our findings using an alternative method of labeling synaptic contacts, we stained for the presynaptic protein, bassoon, and the postsynaptic protein, PSD-95 (Figures 3A1, 3A2). We found that similar to our observations using synapsin/GFP to label synapses, two treatments with NMDA induced a significant loss of putative synaptic contacts measured with bassoon as the presynaptic and GFP as the postsynaptic labels, or with synapsin together with PSD-95 (Figure 3A3; synapse density normalized to CTRL, GFP/bassoon: 69.95 ± 4.11%, n = 29 – 32 dendritic segments, p < 0.0001; PSD-95/synapsin: 61.72 ± 3.11%, n = 24 – 37 dendritic segments, p < 0.0001). We conclude that protein degradation of pre- or postsynaptic markers is unlikely to underlie our observations.
Figure 3. NMDA-induced synapse loss is not likely to be due to loss of synapsin or GFP.
NMDA-induced synapse loss is still observed using an alternative presynaptic marker, bassoon (A1), or postsynaptic marker, PSD-95 (A2). Confocal images after 0 (CTRL) and 2 NMDA treatments showing fluorescent immunoreactive staining using alternative markers of (A1) the presynaptic bouton (red, bassoon), and (A2) the postsynaptic spine, PSD-95. Scale bar, 5 μm. (A3) Quantification of normalized synapse density from neurons stained with alternative antibody combinations, anti-GFP/anti-bassoon, and anti-PSD95/anti-synapsin. Data are averaged means of synapses from at least three independent experiments. Values within bars represent sample sizes (# neurons). Error bars represent SEM. Significance from control (CTRL): *** p < 0.001. (B1) Synapse pruning is unlikely to be a result of microglia action. Example image of an Iba1-positive microglial cell contacting a GFP-expressing neuron. Scale bar, 20 μm. (B2) Relative Iba-1-immunoreactivity in neuronal cultures using our neuron culture protocol (left), and microglia-enriched cultures grown in serum-containing media (Iba1-positive control, right). Scale bars, 50 μm.
To address possible concerns that repeated exposure to NMDA renders neurons vulnerable to excitotoxic cell death72, we stained the cultures with propidium iodide (PI), a marker of disrupted cell membranes. Two hours following two applications of NMDA, we found no difference in the number of neurons staining positive for PI, compared with mock-treated controls (unpaired t-test, CTRL: 100%, NMDA: 100.8 ± 7.94%, n = 4 – 5 biological samples, p = 0.91). Even when cultures were assessed 24 hours after drug treatment, no significant difference in PI staining was observed (unpaired t-test, CTRL: 100%, NMDA: 89.2 ± 24.3%, n = 4 – 5 biological samples, p = 0.63). Consistent with previous reports36,73, these data confirm that the synapse losses we observed were not a result of excitotoxicity induced by NMDA. Finally, to determine whether the virus and/or the eGFP made the neurons more susceptible to NMDA-induced cell death, we looked at neuronal cultures infected with Sindbis-eGFP that were PI-positive. Again, we found no significant changes in PI uptake between the mock-treated neurons and those treated twice with NMDA at two hours after NMDA application (unpaired t-test, CTRL: 100%, NMDA: 128.5 ± 18.7%, n = 3 biological samples, p = 0.20) and 24 hours post-NMDA treatment (CTRL: 100%, NMDA: 106.3 ± 18.3%, n = 3 biological samples, p = 0.70). Together these data indicate that two brief applications of NMDA, of the same concentration and duration that can induce LTD, induce loss of putative synapses lasting at least four hours that is unlikely to be attributed to protein (label) degradation, dendritic location, or neuronal death.
Because microglia have been proposed to be critical for synapse pruning74,75, we sought to determine whether this cell type could contribute to the synapse loss we observe after NMDA treatment. In our cultures, cells are grown for four days in serum-containing media, which supports glial proliferation during the critical early stages of neuronal development. Subsequent FUDR exposure and feeding with serum-free Neurobasal media arrests further glial growth. To first determine whether microglia appear within proximity of the dendrites we assessed, we stained our cultures using an antibody for a microglia-specific protein ionized calcium binding adaptor molecule 1 (Iba-1)76. Cultures plated with ≈80,000 neurons/coverslip contained only rare Iba-1-stained cells (in the range 8–28/coverslip in three biological samples), and fewer in contact with eGFP-expressing neurons (Figure 3B1). A microglia-enriched culture served as a positive control for the antibody (Figure 3B2). Because microglia did not appear in sufficient numbers to contact the synapses we sampled, we attempted no further study on the topic.
Relationship between spine shrinkage and synapse loss
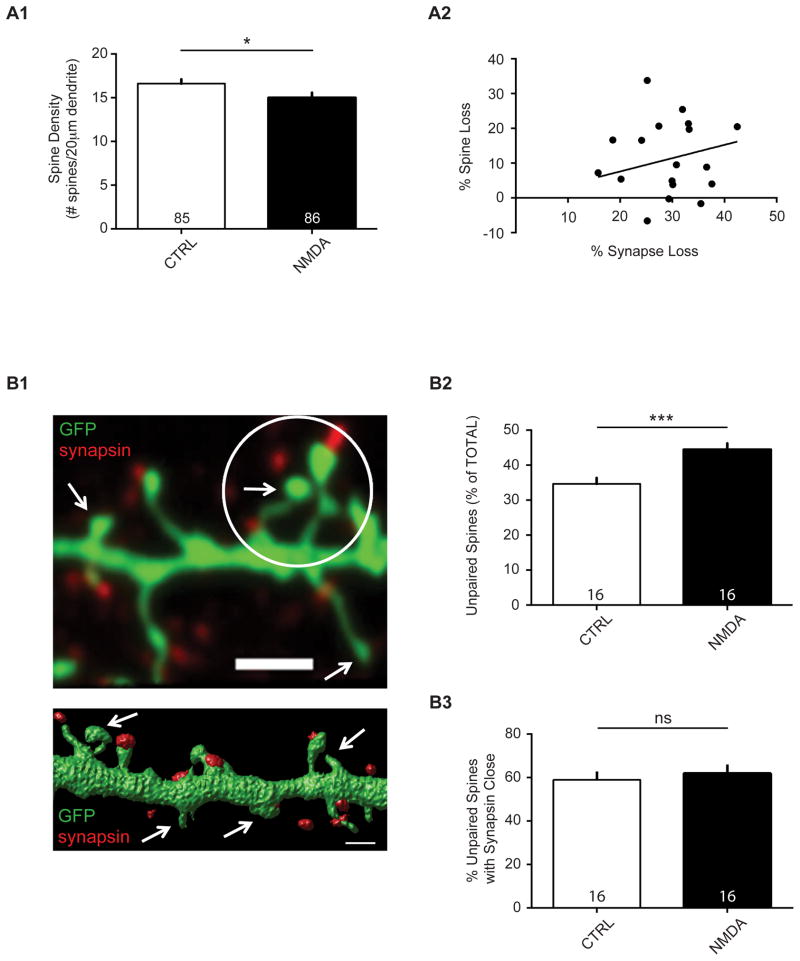
NMDA receptor-dependent LTD has long been known to be accompanied by spine shrinkage, and NMDA can induce spine collapse27,28,70,77. Because synaptic contacts consist of a dense network of extracellular matrix molecules, a simple shrinkage or even complete collapse of a spine may, or may not result in a loss of contact with a presynaptic terminal. In many cases, synapse loss may occur without spine shrinkage, and vice versa23. To test then whether the degree of spine shrinkage or loss was correlated with the synapse loss reported here, we compared the amount of synapse loss to the amount of spine loss in individual experiments. This analysis also would address whether spine shrinkage below the level of detection is a likely explanation for our observation of an apparent synapse loss. We found that, consistent with previous findings, NMDA treatment resulted in modest net spine loss (Figure 4A1: CTRL, 16.76 ± 0.48; NMDA, 15.11 ± 0.52; n = 16 biological samples, p = 0.025). However, a linear regression analysis indicated that there was no correlation between spine loss and synapse loss (Figure 4A2: linear regression, R2 = 0.014; p = 0.636). Thus, in many cases synapse loss could occur without a corresponding loss of spines.
Figure 4. NMDA treatment results in spine loss.
(A1) Data are averaged means of spine density (# spines/20 μm dendrite) in cultured neurons after mock or NMDA treatment from 16 independent experiments. Values within bars represent sample sizes (# neurons). Error bars represent SEM. Significance from control (CTRL): * p = 0.025. (A2) Spine loss is not correlated with putative synapse loss. Data are averaged means of spine and synapse numbers, expressed as % spine loss and % synapse loss, in cultured neurons after mock or NMDA treatment from 16 independent experiments. R2 = 0.014; p = 0.636. (B1) Confocal image (top; scale bar, 2 μm) and surface rendering (bottom; scale bar, 1 μm) of spiny dendritic sections (GFP-expressing) with punctate antibody staining to endogenous synapsin protein. Arrows indicate examples of ‘unpaired spines’. Circle marks a 2-μm radius region of interest for an ‘unpaired spine with synapsin close’. Center of the white circle is on an unpaired spine head center; any unpaired synapsin within the circle denotes ‘synapsin close’. (B2) NMDA treatment increases the number of unpaired spines (without synapsin, as a % of the total number of spines; ***p = 0.0004), but not (B3) unpaired/nonsynaptic spines with synapsin close (ns, not significant; p = 0.61). Values within bars represent sample sizes (# neurons). Error bars represent SEM. Significance from control (CTRL): *** p < 0.001; ns, not significant.
To determine whether we could infer that a separation of synaptic structures had indeed occurred after repeated NMDA treatment, we sought to assess the frequency of unconnected synaptic partners and instances of ‘unpaired’ spines. Although each dendritic spine is generally thought to contain a synapse4,78, some studies have reported spines with and without presynaptic partners79,80, and we also observe instances of the same. Indeed, in our culture preparation and staining conditions, over 30 percent of spines had no apparent presynaptic partner within the resolution of our imaging capability (i.e. fluorescence appearing co-localized; Figure 4B1). However, nearly 60 percent of these unpaired spines had synapsin-positive puncta within 2 μm of the center of the spine head, possibly indicative of undetected synaptic structure or recently separated contact. We found that NMDA treatment results in a significant increase in the number of unpaired spines, as might be expected if synaptic structures were separated without overt loss of spines (Figure 4B2: CTRL, 34.66 ± 1.71%; NMDA, 44.45 ± 1.78%, n = 16 biological samples, p = 0.0004). However, NMDA treatment caused no change in the percentage of these unpaired spines with synapsin-positive puncta nearby (Figure 4B3: CTRL, 59.06 ± 3.82%; NMDA, 61.95 ± 4.11%; n = 16 biological samples, p = 0.61). We conclude from these data that two NMDA treatments result in a net synapse loss that cannot be accounted for by spine loss alone. This loss appears to be reflected by an increase in the number of spines without presynaptic partners.
Role of LTD-related signaling in NMDA-induced synapse loss
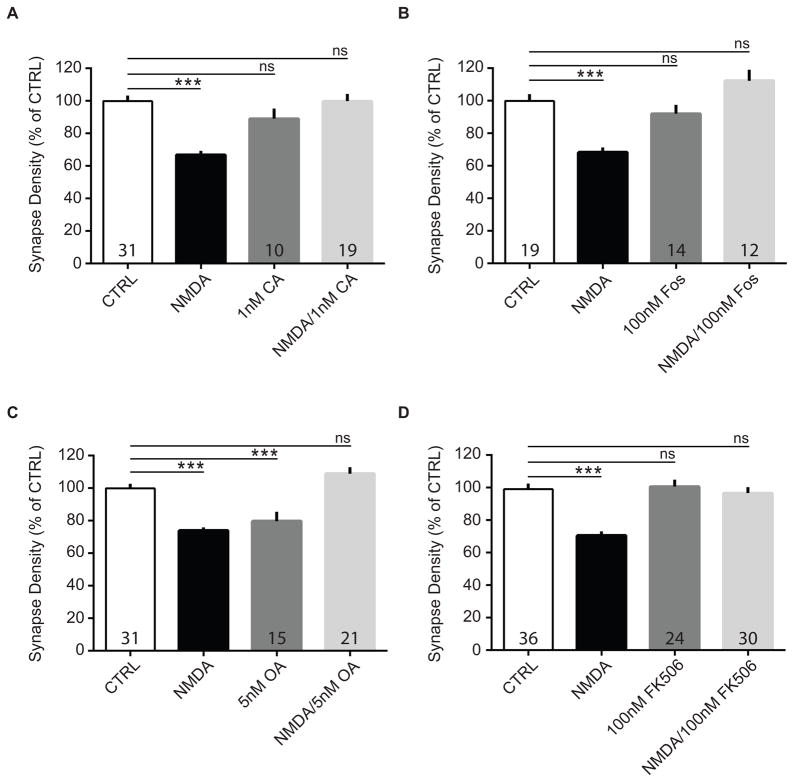
Depression of synaptic responses and NMDA-induced spine loss have been shown to be prevented by inhibitors of protein phosphatases, consistent with the finding that glutamatergic synaptic proteins are dephosphorylated by serine-threonine protein phosphatases PP1, PP2A, and PP2B, resulting in AMPA receptor internalization34,35,55,70,81–87. We therefore tested whether phosphatase activity was critical for synapse loss induced by NMDA in our cultures. To block protein phosphatase activity, cultures were pre-incubated for 10 – 20 minutes with inhibitors of the serine/threonine-specific phosphatases PP1/PP2A and PP2B. Cultures were then treated simultaneously with NMDA and one of the phosphatase inhibitors, calyculin A (CA, 1 nM), okadaic acid (OA, 5 nM), fostriecin (fos, 100 nM), or FK506 (100 nM), or inhibitor alone. After washout and a one-hour recovery period, cells received a second treatment and were fixed two hours later. We found that the NMDA-induced reduction in putative synapses was significantly attenuated in all cases (Figure 5A: CTRL, 100.0 ± 4.42%; NMDA, 62.68 ± 2.92%; 1 nM CA, 89.08 ± 6.29%; NMDA + CA, 99.90 ± 4.46%; ANOVA, F(3, 156) = 20.21, p < 0.0001; n = 3 – 5 biological samples; Figure 5B: CTRL, 100.0 ± 4.87%; NMDA, 68.36 ± 3.29%; 100 nM Fos, 92.01 ± 5.50%; NMDA + Fos, 112.30 ± 6.85%; ANOVA, F(3, 122) = 13.95, p < 0.0001; n = 3 – 4 biological samples; Figure 5C: CTRL, 100.0 ± 3.28%; NMDA, 73.04 ± 2.29%; 5 nM OA, 79.77 ± 5.77%; NMDA + OA, 108.90 ± 3.93%; ANOVA, F(3, 197) = 22.39, p < 0.0001; n = 3 – 5 biological samples; Figure 5D: CTRL, 100.0 ± 3.59%; NMDA, 71.74 ± 2.77%; 100 nM FK506, 100.66 ± 4.16%; NMDA + FK506, 96.64 ± 3.63%; ANOVA, F(3, 238) = 16.32, p < 0.0001; n = 7 biological samples). These results strongly suggest that PP1/PP2A and PP2B activity, which are critical for NMDAR-dependent LTD, are also necessary for NMDA-mediated synapse pruning. Interestingly, okadaic acid alone consistently induced a loss of synaptic contacts, which was apparently mitigated by the NMDA treatments (Figure 5C). Thus, it remains unknown whether okadaic acid-induced synapse loss is prevented by NMDA treatment, or whether NMDA-induced loss is prevented by okadaic acid. In summary, our findings are strongly suggestive of a role for protein phosphatases in NMDA-induced reductions in synapse density in vitro, and support the idea that NMDA-LTD may be a trigger for synapse pruning in vivo.
Figure 5. Phosphatase inhibitors prevent NMDA-induced synapse pruning.
Cultured neurons were treated twice with NMDA (20 μM for 3 min) alone, NMDA plus a serine-threonine phosphatase inhibitor (CA, fos, OA, or FK506), inhibitor alone, or vehicle-/mock-treated, and processed for immunocytochemistry after 2 hrs. Quantification of the effects of (A) CA, (B) fos, (C) OA, or (D) FK-506 on NMDA-induced changes in synapse density from neurons stained with GFP and synapsin. Data are averaged means of synapses from at least three biological samples. Values within bars represent sample sizes (# neurons). Error bars represent SEM. Significance from control (CTRL): *** p < 0.001; ns, not significant.
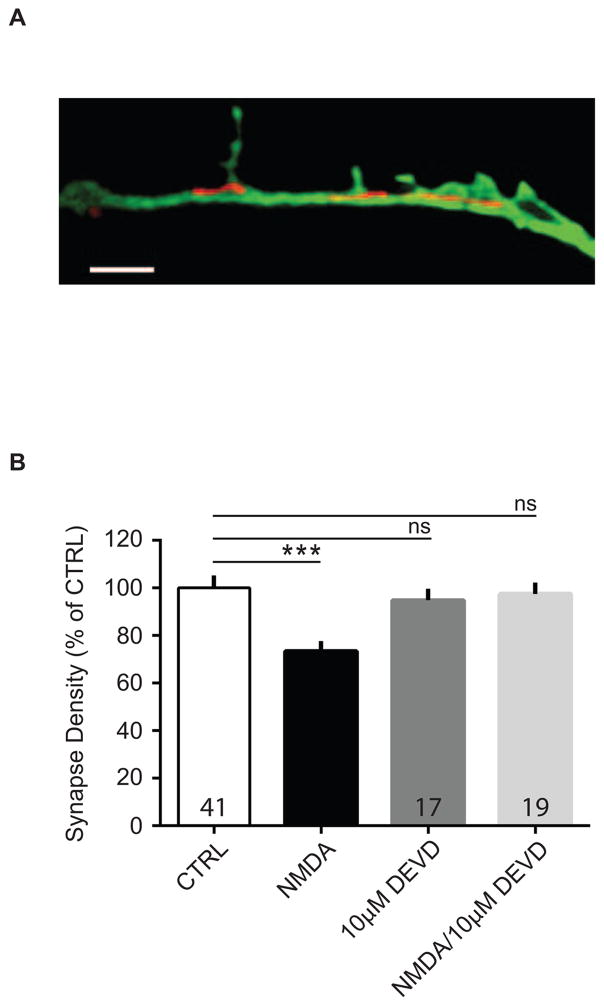
Caspases have recently been found not only to function in apoptosis, but also are required for LTD136,88,89. In addition, we found that MitoTracker Red labeled substantial numbers of mitochondria in live eGFP-filled dendrites, as imaged with structured illumination microscopy (3D-SIM) (Figure 6A). We therefore reasoned that if LTD is required for synapse pruning, then inhibition of caspase-3 activity should similarly inhibit synapse loss induced with NMDA treatment. Thus, we asked whether blockade of LTD with a caspase inhibitor also occludes NMDA-induced pruning. We found that 10 μM z-DEVD-FMK, a caspase-3 inhibitor, applied prior to and during NMDA treatment of cultured neurons prevented NMDA-induced synapse loss (Figure 6B: CTRL, 100.0 ± 5.28%; NMDA, 73.48 ± 4.18%; 10 μM DEVD, 94.86 ± 4.84%; NMDA + DEVD, 97.56 ± 4.81%; ANOVA, F(3, 151) = 6.613, p = 0.0003; n = 3 biological samples). The caspase-3 inhibitor alone had no significant effect on synapse number. Interestingly, although one group found no effect of pan-caspase inhibitor, z-VAD-FMK, on NMDA-mediated F-actin loss in spines77, Erturk, et al. reported that inhibition of caspase-3 activity prevented spine shrinkage from NMDA-induced LTD67. Overall, these data suggest that synapse loss occurred in a caspase-dependent manner, further supporting a model by which synapse pruning is triggered by NMDAR-LTD.
Figure 6. Caspase-3 inhibition blocks NMDA-induced synapse elimination.
(A) 3-D SIM image of MitoTracker Red-stained mitochondria in a dendrite from a GFP-expressing cultured neuron. Scale bar, 2 μm. (B) Cultured neurons were treated twice with NMDA (20 μM for 3 min) alone, NMDA plus caspase-3 inhibitor, z-DEVD-FMK (DEVD), DEVD alone, or vehicle-treated, and processed for immunocytochemistry after 2 hrs. Quantification of the effect of DEVD on NMDA-induced changes in synapse density from neurons stained with GFP and synapsin. Data are averaged means of synapses from three biological samples. Values within bars represent sample sizes (# neurons). Error bars represent SEM. Significance from control (CTRL): *** p < 0.001.
Role of transcription and translation in synapse loss
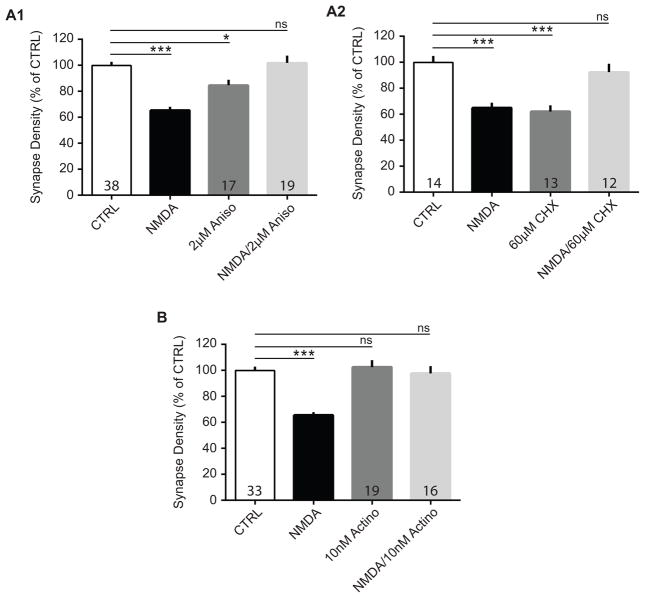
To examine the importance of new protein or mRNA synthesis in the mechanisms underlying synapse pruning, we tested cultures that were treated with NMDA and protein synthesis inhibitors. Protein synthesis has been shown to be important for LTD in several studies37,39,90, but whether or not translation is required for synapse pruning in cortical neurons has not been investigated. To block protein synthesis activity, cultures were pre-incubated 20 minutes with inhibitors at concentrations that did not cause neuronal toxicity in pilot experiments. Cells were then treated simultaneously with NMDA and one of two protein synthesis inhibitors that use different mechanisms to block peptide elongation (anisomycin (aniso), 2 μM; or cycloheximide (CHX), 60 μM). We found that the reduction of synaptic contacts in NMDA-treated cultures appeared to be prevented by application of either anisomycin or cycloheximide. Interestingly though, as in the case with OA, treatment of cells with protein synthesis inhibitors alone reduced synapse numbers as well (Figure 7A1: CTRL, 100.0 ± 3.19%; NMDA, 66.20 ± 2.85%; Aniso, 84.69 ± 4.14%; NMDA + Aniso, 101.90 ± 5.68%; ANOVA, F(3, 203) = 19.11, p < 0.0001; n = 4 – 6 biological samples; Figure 7A2: CTRL, 100.0 ± 4.96%; NMDA, 64.93 ± 3.95%; CHX, 62.19 ± 4.68%; NMDA + CHX, 92.46 ± 6.35%; ANOVA, F(3, 107) = 14.28, p < 0.0001; n = 3 biological samples).
Figure 7. Protein synthesis and gene transcription inhibition prevent NMDA-mediated synapse loss.
Cultured neurons were treated twice with NMDA (20 μM for 3min) alone, NMDA plus a protein synthesis or transcription inhibitor, inhibitor alone, or vehicle-/mock-treated, and processed for immunocytochemistry after 2 hrs. Quantification of the effects of (A1) anisomycin (aniso), (A2) cycloheximide (CHX), or (B) actinomycin D (actino) on NMDA-induced changes in synapse density from neurons stained with GFP and synapsin. Data are averaged means of synapses from at least three biological samples. Values within bars represent sample sizes (# neurons). Error bars represent SEM. Significance from control (CTRL): * p < 0.05, *** p < 0.001.
To determine whether synapse loss requires synthesis of new mRNA, or whether existing pools of mRNA are sufficient, we tested whether the transcription inhibitor actinomyosin D (actino, 10 nM) prevented NMDA-induced synapse pruning. Indeed, we found that the reduction of synaptic contacts in NMDA-treated cultures is prevented by the application of actinomycin D (Figure 7B: CTRL, 100.0 ± 3.34%; NMDA, 66.06 ± 3.29%; actino, 102.60 ± 5.32%; NMDA + actino, 97.73 ± 5.55%; ANOVA, F(3, 159) = 13.36, p < 0.0001; n = 4 – 5 biological samples). These data indicate that gene transcription, which may be required for LTD, is also required for synapse loss38 (but see43,90). Further, because this drug treatment did not cause synapse loss on its own, these data suggest that effects of protein synthesis inhibitors on synapse number are independent of new mRNA synthesis.
Discussion
Synapse numbers increase during early postnatal development in most mammals, and are thought to decrease with neuronal activity during experience91. Despite the growing knowledge of the molecules important for synapse pruning19,43,74,92–100, the mechanisms by which neuronal activity regulates this process in relation to LTD in cortical structures remains unknown. Several models involving neuronal activity have been proposed, including competition for growth factors or other molecules92, heterosynaptic-type interactions, where highly active synapses drive pruning at the expense of unused synapses101,102, homeostatic-type mechanisms103, and homosynaptic depression of synaptic transmission, such as in LTD7,26. Alternative, but not mutually exclusive, models for synapse loss posit that microglia or astrocytes consume synaptic components74, 75, 104–110 (but see111). Also unknown is whether the mechanisms underlying developmental loss of synapses at the neuromuscular junction are similar to those in cortical structures112. Although we cannot rule out the idea that some of these other mechanisms play a role in cortical synapse elimination, our study adds to the evidence that LTD-inducing stimulation can also induce synapse pruning.
Perhaps the best evidence to date that LTD-like mechanisms are required for synapse pruning in the central nervous system is that decreases in synapse number are impaired by genetic manipulations that also inhibit LTD. Some examples include knockout of components of the major histocompatibility complex (MHC1), where LTD and/or ocular dominance plasticity are also impaired94,95,113–115, and deletion of different subunits of NMDARs, which results in increases in synapse number 93,116–119 (but see120). However, whether these manipulations impact development of synapses or whether they directly prevent synapse pruning is often ambiguous121–123.
Here, we tested whether signaling pathways more traditionally associated with synaptic plasticity, particularly those closely linked to LTD, also were required for synapse pruning. To do this, we first sought to establish a model of LTD in vitro that also induced synaptic elimination. We found that repeated, but not single, applications of NMDA induced reliable loss of putative synaptic contacts, consistent with previous findings showing that repeated electrically-induced LTD was accompanied by synapse elimination in slice cultures23,24,26. The loss of synaptic structures was apparent at two hours after drug application and was not dependent on position in relation to the cell soma or on degradation of pre- or post-synaptic markers. Also, although NMDA is known to be excitotoxic to neurons at high doses and extended exposures124,125, NMDA treatment (2 × 20 μM for 3 minutes) did not cause a significant increase in cellular death, as measured by propidium iodide staining. Moreover, although we cannot rule out a role in the pruning process for astrocytes, or factors released from non-neuronal cells, we found that microglia were too sparse in our cultures to play a role requiring their direct contact with individual synapses. Thus, our model is suitable for testing our hypothesis that signaling pathways required for LTD are also required for synapse pruning.
Role of LTD-linked mechanisms
We do note that both NMDA-LTD and the putative synapse loss reported here require protein phosphatase activity and caspase activity, indicating that this model of synapse elimination has some mechanistic similarities with typical LTD. For example, LTD and glutamate receptor dephosphorylation and/or internalization are blocked by phosphatase and caspase inhibitors35,36,85. Moreover, inhibition of caspase-3 prevents NMDA-induced spine shrinkage67.
Parsing the contributions of phosphatases PP1 and/or PP2A, PP4/5/6 by inhibitors calyculin A, fostriecin, or okadaic acid is challenging, as these drugs have broad, overlapping specificities and inhibit enzyme activity at different IC50 values126–128. Although work in other neuronal systems found no inhibitory effects of CA and OA on NMDA-LTD33,55, the concentrations used here (CA, 1 nM; OA, 5 nM; fos, 100 nM) were of varying magnitudes lower, and likely impacted the phosphatases that were blocked. Future studies such as those using selective knockout of the individual phosphatases may be able to distinguish between PP1/PP2A on the basis of their differential sensitivity to concentration, cellular permeation properties, timing of activity, and subcellular location129–131. Interestingly, although FK506 blocks NMDA-induced AMPAR internalization in dissociated cultures33,55, FK506 treatment alone may actually increase dendritic spine density and complexity of branching132. Additionally, there may be interactions between PP1, PP2A, and PP2B133. Future studies testing whether AMPAR internalization, which is required for LTD and ocular dominance plasticity25, is required for pruning will be essential for determining whether the actual ‘readout’ of LTD is also critical for this type of synapse loss.
The role of protein synthesis in LTD clearly lacks consensus; several studies have shown no effect of protein synthesis inhibitors on LTD induced with low-frequency stimulation39,134, whereas others have observed inhibitor sensitivity37,38,135. Interestingly, anisomycin has been reported to activate the p38 MAPK pathway to induce LTD134, an effect not replicated with cycloheximide. Thus, the NMDA-mimicking effect of anisomycin or cycloheximide pretreatment on synapse loss preclude our making any definitive conclusions about the requirement for protein synthesis in synapse pruning (although see similar results reported with anisomycin at the neuromuscular junction136). Our results do raise the intriguing possibility that proteins made from newly synthesized mRNA drive pruning, whereas proteins made from existing pools of mRNA are required for synapse maintenance. Supporting this idea are data from ‘tagging’ experiments under conditions of protein synthesis inhibition suggesting that some plasticity-related proteins/mRNA are limited137. Here though, we show that when all synapses are activated with NMDA, some synaptic contacts are lost, but that NMDA action oddly protects against loss due to protein synthesis inhibition. Our experiments cannot address whether a synapse-specific LTD-type mechanism, or an LTP-at-the-expense-of-others type mechanism, is responsible. Supporting the latter, though, is evidence that (post-synaptic) neuronal activity can lead to synaptic weakening after several hours138, although no LTP is induced to compete in a way hypothesized by this model. Interestingly, several different miRNAs related to LTD are induced with NMDA treatment and can be regulated locally in dendrites43. Our LTD-inducing treatment also was unlikely to have engaged the signaling molecule Jacob, which is recruited to the nucleus under primarily LTP-inducing stimulation139. In either case, local protein synthesis, possibly involving regulation by phosphatases, may be serving a critical role in maintaining synapses140.
Several possible mechanisms for NMDA-mediated synapse pruning remain to be tested. For example, in a study of synapse elimination in C. elegans, and in rat cortical cultures, E3 ubiquitin ligases play a role141,142. In addition, extracellular proteases could be involved in pruning143, either serving in a signaling capacity such as in a culture model of the developing neuromuscular junction144, or as a way of breaking down the extracellular matrix in the synaptic cleft by matrix metalloproteinases145. Related to extracellular matrix is Sema5B, which when proteolytically processed, can induce synapse pruning146. Furthermore, evidence of the effects of environmental exposure on synaptic remodeling (e.g. bisphenol A and other endocrine disrupters of brain function) is mounting147. Our model of NMDA-induced synapse pruning easily can be applied to study the role of these possible mechanisms.
Role of spine loss in synapse pruning
Dendritic spines, and likely synapses on them, are dynamic throughout the brain and their numbers can be manipulated by environmental enrichment or NMDAR blockade, depending on the brain region92,120. However, it is important to distinguish between the mechanisms required for functional weakening (i.e. LTD; AMPA receptor internalization) and those related to structure (i.e. spine shrinkage, spine loss, and separation of pre- and post-synaptic structures), as any or all of these processes may occur as a result of LTD initiation by NMDARs or mGluRs46. Strong evidence has been presented that LTD induction can cause spine shrinkage28,44,148,149, although the signaling required for LTD can be dissociated from those required for spine shrinkage27. Likewise, although it is assumed that spine shrinkage would lead to eventual loss of spines resulting in synapse loss, examples of LTD-related synapse separation have been observed to occur in the absence of spine loss or shrinkage23,24. Our data showing little correlation between spine loss and synapse loss are also in agreement with this idea (Figure 4A2). Consistent also with this dissociation between spine shrinkage and synapse loss is our observation that the percentage of ‘unpaired’ spines increased with NMDA treatment (Figure 4B2). These data are suggestive of presynaptic terminals disconnecting from spines that remained intact. Alternatively, we cannot rule out that NMDA treatment resulted in the appearance of new spines lacking presynaptic partners, but we note that many of these ‘unpaired’ spines were structurally mature in that they had no resemblance to filopodia, arguing against this possibility. Because as yet no markers of recently separated synaptic structures exist to measure this process directly, we therefore conclude that spine shrinkage/loss is unlikely to be a requisite step in synapse loss, but that LTD may still be an initiating event.
Synapse loss in disease
These studies were performed at three weeks in culture, and so the results are most relevant to developing cortical structures and developmental disorders such as those discussed above. Nevertheless, synapse elimination may also have relevance to normal brain function in adulthood and to age- and disease-related cognitive decline. For example, dendritic spine remodeling, including elimination, can occur after fear conditioning, extinction, and reconditioning in layer V neurons of mouse frontal association cortex150. Synapse loss by stress hormones also is well documented151, but whether its mechanism is related to LTD is unknown. In addition, this study may have implications in diseases such as Alzheimer’s disease (AD) and Parkinson’s disease, where synapse loss is profound142,152. Phosphatase inhibition blocks synapse loss in a mouse model of AD153, so it is conceivable that NMDARs and LTD-like mechanisms underlie AD-related synapse loss as well154,155 (but see also156). We therefore look forward to a deeper understanding of the possible role for LTD in mechanisms underlying activity-dependent synapse pruning in both developmental and disease contexts.
Highlights.
NMDA applications induce LTD and synapse pruning in cultured rat neurons
NMDA-mediated decreases in synapse density require LTD-associated signaling
Synapse loss is independent of spine loss, dendrite location, or direct contact with microglia
NMDAR-LTD is likely a required step in an in vitro model of synapse pruning
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute of Environmental Health Sciences, National Institutes of Health (ES 100221). We thank Grace Kissling for advice on the statistics, Ji-yeon Hwang for preparation of neuronal cultures, Christopher McPherson for microglia cultures, Thomas Helton for comments on the manuscript, and Negin Martin and the Viral Vector Core Laboratory for preparation of Sindbis virus.
Footnotes
CONTRIBUTIONS: S.M.D. and M.A.H. conceived and designed the studies. S.M.D. and M.A.H. wrote the manuscript. M.A.H., and M.Z. conducted experiments and analyzed data. C.J.T. designed analyses of confocal imaging data. S.M.D. supervised the project.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rakic P, Bourgeois JP, Eckenhoff MF, Zecevic N, Goldman-Rakic PS. Concurrent overproduction of synapses in diverse regions of the primate cerebral cortex. Science. 1986;232:232–235. doi: 10.1126/science.3952506. [DOI] [PubMed] [Google Scholar]
- 2.Huttenlocher PR. Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Res. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- 3.Holtmaat AJGD, et al. Transient and Persistent Dendritic Spines in the Neocortex In Vivo. Neuron. 2005;45:279–291. doi: 10.1016/j.neuron.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Alvarez VA, Sabatini BL. Anatomical and Physiological Plasticity of Dendritic Spines. Annu Rev Neurosci. 2007;30:79–97. doi: 10.1146/annurev.neuro.30.051606.094222. [DOI] [PubMed] [Google Scholar]
- 5.Glantz LA, Gilmore JH, Hamer RM, Lieberman JA, Jarskog LF. Synaptophysin and postsynaptic density protein 95 in the human prefrontal cortex from mid-gestation into early adulthood. Neuroscience. 2007;149:582–591. doi: 10.1016/j.neuroscience.2007.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen C, Regehr WG. Developmental remodeling of the retinogeniculate synapse. Neuron. 2000;28:955–966. doi: 10.1016/s0896-6273(00)00166-5. [DOI] [PubMed] [Google Scholar]
- 7.Elston GN, Oga T, Fujita I. Spinogenesis and Pruning Scales across Functional Hierarchies. J Neurosci. 2009;29:3271–3275. doi: 10.1523/JNEUROSCI.5216-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qiao Q, et al. Long-term stability of axonal boutons in the mouse barrel cortex. Devel Neurobio. 2015:n/a–n/a. doi: 10.1002/dneu.22311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang HJ, et al. Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat Med. 2012;18:1–7. doi: 10.1038/nm.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim IH, et al. Disruption of Arp2/3 Results in Asymmetric Structural Plasticity of Dendritic Spines and Progressive Synaptic and Behavioral Abnormalities. J Neurosci. 2013;33:6081–6092. doi: 10.1523/JNEUROSCI.0035-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Penzes P, Buonanno A, Passafaro M, Sala C, Sweet RA. Developmental vulnerability of synapses and circuits associated with neuropsychiatric disorders. J Neurochem. 2013;126:165–182. doi: 10.1111/jnc.12261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pfeiffer BE, et al. Fragile X Mental Retardation Protein Is Required for Synapse Elimination by the Activity-Dependent Transcription Factor MEF2. Neuron. 2010;66:191–197. doi: 10.1016/j.neuron.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang G, et al. Loss of mTOR-Dependent Macroautophagy Causes Autistic-like Synaptic Pruning Deficits. Neuron. 2014;83:1131–1143. doi: 10.1016/j.neuron.2014.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Auerbach BD, Osterweil EK, Bear MF. Mutations causing syndromic autism define an axis of synaptic pathophysiology. Nature. 2011;480:63–68. doi: 10.1038/nature10658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glantz LA, Lewis DA. Decreased Dendritic Spine Density on Prefrontal Cortical Pyramidal Neurons in Schizophrenia. Arch Gen Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- 16.McGlashan TH, Hoffman RE. Schizophrenia as a disorder of developmentally reduced synaptic connectivity. Arch Gen Psychiatry. 2000;57:637–648. doi: 10.1001/archpsyc.57.7.637. [DOI] [PubMed] [Google Scholar]
- 17.Selemon LD, Zecevic N. Schizophrenia: a tale of two critical periods for prefrontal cortical development. Transl Psychiatry. 2015;5:e623. doi: 10.1038/tp.2015.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calabrò M, et al. Genes involved in pruning and inflammation are enriched in a large mega-sample of patients affected by Schizophrenia and Bipolar Disorder and controls. Psychiatry Res. 2015;228:945–949. doi: 10.1016/j.psychres.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sekar A, et al. Schizophrenia risk from complex variation of complement component 4. Nature. 2016;530:177–183. doi: 10.1038/nature16549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reiter HO, Stryker MP. Neural plasticity without postsynaptic action potentials: less-active inputs become dominant when kitten visual cortical cells are pharmacologically inhibited. Proc Natl Acad Sci USA. 1988;85:3623–3627. doi: 10.1073/pnas.85.10.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rittenhouse CD, Shouval HZ, Paradiso MA, Bear MF. Monocular deprivation induces homosynaptic long-term depression in visual cortex. Nature. 1999;397:347–350. doi: 10.1038/16922. [DOI] [PubMed] [Google Scholar]
- 22.Coleman JE, et al. Rapid structural remodeling of thalamocortical synapses parallels experience-dependent functional plasticity in mouse primary visual cortex. J Neurosci. 2010;30:9670–9682. doi: 10.1523/JNEUROSCI.1248-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bastrikova N, Gardner GA, Reece JM, Jeromin A, Dudek SM. Synapse elimination accompanies functional plasticity in hippocampal neurons. Proc Natl Acad Sci USA. 2008;105:3123–3127. doi: 10.1073/pnas.0800027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Becker N, Wierenga CJ, Fonseca R, Bonhoeffer T, Nägerl UV. LTD induction causes morphological changes of presynaptic boutons and reduces their contacts with spines. Neuron. 2008;60:590–597. doi: 10.1016/j.neuron.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 25.Yoon BJ, Smith GB, Heynen AJ, Neve RL, Bear MF. Essential role for a long-term depression mechanism in ocular dominance plasticity. Proc Natl Acad Sci USA. 2009;106:9860–9865. doi: 10.1073/pnas.0901305106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wiegert JS, Oertner TG. Long-term depression triggers the selective elimination of weakly integrated synapses. Proc Natl Acad Sci USA. 2013;110:E4510–9. doi: 10.1073/pnas.1315926110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou Q, Homma KJ, Poo MM. Shrinkage of Dendritic Spines Associated with Long-Term Depression of Hippocampal Synapses. Neuron. 2004;44:749–757. doi: 10.1016/j.neuron.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 28.Oh WC, Hill TC, Zito K. Synapse-specific and size-dependent mechanisms of spine structural plasticity accompanying synaptic weakening. Proc Natl Acad Sci USA. 2013;110:E305–12. doi: 10.1073/pnas.1214705110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He K, Lee A, Song L, Kanold PO, Lee HK. AMPA receptor subunit GluR1 (GluA1) serine-845 site is involved in synaptic depression but not in spine shrinkage associated with chemical long-term depression. J Neurophysiol. 2011;105:1897–1907. doi: 10.1152/jn.00913.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dudek SM, Bear MF. Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-D-aspartate receptor blockade. Proc Natl Acad Sci USA. 1992;89:4363–4367. doi: 10.1073/pnas.89.10.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heynen AJ, Abraham WC, Bear MF. Bidirectional modification of CA1 synapses in the adult hippocampus in vivo. Nature. 1996;381:163–166. doi: 10.1038/381163a0. [DOI] [PubMed] [Google Scholar]
- 32.Carroll RC, Lissin DV, von Zastrow M, Nicoll RA, Malenka RC. Rapid redistribution of glutamate receptors contributes to long-term depression in hippocampal cultures. Nat Neurosci. 1999;2:454–460. doi: 10.1038/8123. [DOI] [PubMed] [Google Scholar]
- 33.Kameyama K, Lee HK, Bear MF, Huganir RL. Involvement of a postsynaptic protein kinase A substrate in the expression of homosynaptic long-term depression. Neuron. 1998;21:1163–1175. doi: 10.1016/s0896-6273(00)80633-9. [DOI] [PubMed] [Google Scholar]
- 34.Lee HK, Kameyama K, Huganir RL, Bear MF. NMDA induces long-term synaptic depression and dephosphorylation of the GluR1 subunit of AMPA receptors in hippocampus. Neuron. 1998;21:1151–1162. doi: 10.1016/s0896-6273(00)80632-7. [DOI] [PubMed] [Google Scholar]
- 35.Mulkey RM, Herron CE, Malenka RC. An essential role for protein phosphatases in hippocampal long-term depression. Science. 1993;261:1051–1055. doi: 10.1126/science.8394601. [DOI] [PubMed] [Google Scholar]
- 36.Li Z, et al. Caspase-3 Activation via Mitochondria Is Required for Long-Term Depression and AMPA Receptor Internalization. Cell. 2010;141:859–871. doi: 10.1016/j.cell.2010.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sajikumar S, Frey JU. Anisomycin inhibits the late maintenance of long-term depression in rat hippocampal slices in vitro. Neurosci Lett. 2003;338:147–150. doi: 10.1016/s0304-3940(02)01400-3. [DOI] [PubMed] [Google Scholar]
- 38.Kauderer BS, Kandel ER. Capture of a protein synthesis-dependent component of long-term depression. Proc Natl Acad Sci USA. 2000;97:13342–13347. doi: 10.1073/pnas.97.24.13342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huber KM, Kayser MS, Bear MF. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science. 2000;288:1254–1257. doi: 10.1126/science.288.5469.1254. [DOI] [PubMed] [Google Scholar]
- 40.Huber KM, Roder JC, Bear MF. Chemical induction of mGluR5- and protein synthesis-dependent long-term depression in hippocampal area CA1. J Neurophysiol. 2001;86:321–325. doi: 10.1152/jn.2001.86.1.321. [DOI] [PubMed] [Google Scholar]
- 41.Fitzjohn SM, Kingston AE, Lodge D, Collingridge GL. DHPG-induced LTD in area CA1 of juvenile rat hippocampus; characterisation and sensitivity to novel mGlu receptor antagonists. Neuropharmacol. 1999;38:1577–1583. doi: 10.1016/s0028-3908(99)00123-9. [DOI] [PubMed] [Google Scholar]
- 42.Casimiro TM, et al. mGluR and NMDAR activation internalize distinct populations of AMPARs. Mol Cell Neurosci. 2011;48:1–10. doi: 10.1016/j.mcn.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu Z, et al. miR-191 and miR-135 are required for long-lasting spine remodelling associated with synaptic long-term depression. Nat Comm. 2014;5:1–17. doi: 10.1038/ncomms4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nägerl UV, Eberhorn N, Cambridge SB, Bonhoeffer T. Bidirectional Activity-Dependent Morphological Plasticity in Hippocampal Neurons. Neuron. 2004;44:759–767. doi: 10.1016/j.neuron.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 45.Hsieh H, et al. AMPAR Removal Underlies Aβ-Induced Synaptic Depression and Dendritic Spine Loss. Neuron. 2006;52:831–843. doi: 10.1016/j.neuron.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hasegawa S, Sakuragi S, Tominaga-Yoshino K, Ogura A. Dendritic spine dynamics leading to spine elimination after repeated inductions of LTD. Sci Rep. 2015;5:7707–6. doi: 10.1038/srep07707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Egashira Y, et al. Involvement of the p75NTR signaling pathway in persistent synaptic suppression coupled with synapse elimination following repeated long-term depression induction. J Neurosci Res. 2010;88:3433–3446. doi: 10.1002/jnr.22505. [DOI] [PubMed] [Google Scholar]
- 48.Kamikubo Y, et al. Long-lasting synaptic loss after repeated induction of LTD: independence to the means of LTD induction. Eur J Neurosci. 2006;24:1606–1616. doi: 10.1111/j.1460-9568.2006.05032.x. [DOI] [PubMed] [Google Scholar]
- 49.Shinoda Y, Kamikubo Y, Egashira Y, Tominaga-Yoshino K, Ogura A. Repetition of mGluR-dependent LTD causes slowly developing persistent reduction in synaptic strength accompanied by synapse elimination. Brain Res. 2005;1042:99–107. doi: 10.1016/j.brainres.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 50.Shinoda Y, Tanaka T, Tominaga-Yoshino K, Ogura A. Persistent Synapse Loss Induced by Repetitive LTD in Developing Rat Hippocampal Neurons. PLoS ONE. 2010;5:e10390–6. doi: 10.1371/journal.pone.0010390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pratt KG, Taft CE, Burbea M, Turrigiano GG. Dynamics underlying synaptic gain between pairs of cortical pyramidal neurons. Devel Neurobio. 2008;68:143–151. doi: 10.1002/dneu.20577. [DOI] [PubMed] [Google Scholar]
- 52.Jeromin A, Yuan LL, Frick A, Pfaffinger P, Johnston D. A modified Sindbis vector for prolonged gene expression in neurons. J Neurophysiol. 2003;90:2741–2745. doi: 10.1152/jn.00464.2003. [DOI] [PubMed] [Google Scholar]
- 53.Chen Y, et al. NS21: re-defined and modified supplement B27 for neuronal cultures. J Neurosci Meth. 2008;171:239–247. doi: 10.1016/j.jneumeth.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harry GJ, Tyler K, d’Hellencourt CL, Tilson HA, Maier WE. Morphological alterations and elevations in tumor necrosis factor-alpha, interleukin (IL)-1alpha, and IL-6 in mixed glia cultures following exposure to trimethyltin: modulation by proinflammatory cytokine recombinant proteins and neutralizing antibodies. Toxicol Appl Pharmacol. 2002;180:205–218. doi: 10.1006/taap.2002.9390. [DOI] [PubMed] [Google Scholar]
- 55.Beattie EC, et al. Regulation of AMPA receptor endocytosis by a signaling mechanism shared with LTD. Nat Neurosci. 2000;3:1291–1300. doi: 10.1038/81823. [DOI] [PubMed] [Google Scholar]
- 56.Dailey ME, Smith SJ. The dynamics of dendritic structure in developing hippocampal slices. J Neurosci. 1996;16:2983–2994. doi: 10.1523/JNEUROSCI.16-09-02983.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Papa M, Bundman MC, Greenberger V, Segal M. Morphological analysis of dendritic spine development in primary cultures of hippocampal neurons. J Neurosci. 1995;15:1–11. doi: 10.1523/JNEUROSCI.15-01-00001.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boyer C, Schikorski T, Stevens CF. Comparison of hippocampal dendritic spines in culture and in brain. J Neurosci. 1998;18:5294–5300. doi: 10.1523/JNEUROSCI.18-14-05294.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Glynn MW, McAllister AK. Immunocytochemistry and quantification of protein colocalization in cultured neurons. Nat Protoc. 2006;1:1287–1296. doi: 10.1038/nprot.2006.220. [DOI] [PubMed] [Google Scholar]
- 60.Benson DL, Watkins FH, Steward O, Banker G. Characterization of GABAergic neurons in hippocampal cell cultures. J Neurocytol. 1994;23:279–295. doi: 10.1007/BF01188497. [DOI] [PubMed] [Google Scholar]
- 61.Micheva KD, Busse B, Weiler NC, O’Rourke N, Smith SJ. Single-Synapse Analysis of a Diverse Synapse Population: Proteomic Imaging Methods and Markers. Neuron. 2010;68:639–653. doi: 10.1016/j.neuron.2010.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lu W, et al. Activation of synaptic NMDA receptors induces membrane insertion of new AMPA receptors and LTP in cultured hippocampal neurons. Neuron. 2001;29:243–254. doi: 10.1016/s0896-6273(01)00194-5. [DOI] [PubMed] [Google Scholar]
- 63.Ehlers MD. Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron. 2000;28:511–525. doi: 10.1016/s0896-6273(00)00129-x. [DOI] [PubMed] [Google Scholar]
- 64.Lee SH, Liu L, Wang YT, Sheng M. Clathrin adaptor AP2 and NSF interact with overlapping sites of GluR2 and play distinct roles in AMPA receptor trafficking and hippocampal LTD. Neuron. 2002;36:661–674. doi: 10.1016/s0896-6273(02)01024-3. [DOI] [PubMed] [Google Scholar]
- 65.Snyder EM, et al. Role for A kinase-anchoring proteins (AKAPS) in glutamate receptor trafficking and long term synaptic depression. J Biol Chem. 2005;280:16962–16968. doi: 10.1074/jbc.M409693200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lissin DV, Carroll RC, Nicoll RA, Malenka RC, von Zastrow M. Rapid, activation-induced redistribution of ionotropic glutamate receptors in cultured hippocampal neurons. J Neurosci. 1999;19:1263–1272. doi: 10.1523/JNEUROSCI.19-04-01263.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Erturk A, Wang Y, Sheng M. Local Pruning of Dendrites and Spines by Caspase-3-Dependent and Proteasome-Limited Mechanisms. J Neurosci. 2014;34:1672–1688. doi: 10.1523/JNEUROSCI.3121-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lau AC, Cui H, Tymianski M. The use of propidium iodide to assess excitotoxic neuronal death in primary mixed cortical cultures. Methods Mol Biol. 2007;399:15–29. doi: 10.1007/978-1-59745-504-6_2. [DOI] [PubMed] [Google Scholar]
- 69.Atkins CM, Davare MA, Oh MC, Derkach V, Soderling TR. Bidirectional regulation of cytoplasmic polyadenylation element-binding protein phosphorylation by Ca2+/calmodulin-dependent protein kinase II and protein phosphatase 1 during hippocampal long-term potentiation. J Neurosci. 2005;25:5604–5610. doi: 10.1523/JNEUROSCI.5051-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Halpain S, Hipolito A, Saffer L. Regulation of F-actin stability in dendritic spines by glutamate receptors and calcineurin. J Neurosci. 1998;18:9835–9844. doi: 10.1523/JNEUROSCI.18-23-09835.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Colledge M, et al. Ubiquitination regulates PSD-95 degradation and AMPA receptor surface expression. Neuron. 2003;40:595–607. doi: 10.1016/s0896-6273(03)00687-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Choi DW. Excitotoxic cell death. Dev Neurobiol. 1992;23:1261–1276. doi: 10.1002/neu.480230915. [DOI] [PubMed] [Google Scholar]
- 73.Shehata M, Matsumura H, Okubo-Suzuki R, Ohkawa N, Inokuchi K. Neuronal Stimulation Induces Autophagy in Hippocampal Neurons That Is Involved in AMPA Receptor Degradation after Chemical Long-Term Depression. J Neurosci. 2012;32:10413–10422. doi: 10.1523/JNEUROSCI.4533-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stevens B, et al. The Classical Complement Cascade Mediates CNS Synapse Elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 75.Schafer DP, et al. Microglia Sculpt Postnatal Neural Circuits in an Activity and Complement-Dependent Manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kraft AD, McPherson CA, Harry GJ. Association Between Microglia, Inflammatory Factors, and Complement with Loss of Hippocampal Mossy Fiber Synapses Induced by Trimethyltin. Neurotox Res. 2016:1–14. doi: 10.1007/s12640-016-9606-8. [DOI] [PubMed] [Google Scholar]
- 77.Graber S, Maiti S, Halpain S. Cathepsin B-like proteolysis and MARCKS degradation in sub-lethal NMDA-induced collapse of dendritic spines. Neuropharmacology. 2004;47:706–713. doi: 10.1016/j.neuropharm.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 78.Nimchinsky EA, Sabatini BL, Svoboda K. Structure and function of dendritic spines. Annu Rev Physiol. 2002;64:313–353. doi: 10.1146/annurev.physiol.64.081501.160008. [DOI] [PubMed] [Google Scholar]
- 79.Arellano JI, Espinosa A, Fairén A, Yuste R, DeFelipe J. Non-synaptic dendritic spines in neocortex. Neuroscience. 2007;145:464–469. doi: 10.1016/j.neuroscience.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 80.Portera-Cailliau C, Pan DT, Yuste R. Activity-regulated dynamic behavior of early dendritic protrusions: evidence for different types of dendritic filopodia. J Neurosci. 2003;23:7129–7142. doi: 10.1523/JNEUROSCI.23-18-07129.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee HK, Barbarosie M, Kameyama K, Bear MF, Huganir RL. Regulation of distinct AMPA receptor phosphorylation sites during bidirectional synaptic plasticity. Nature. 2000;405:955–959. doi: 10.1038/35016089. [DOI] [PubMed] [Google Scholar]
- 82.Mulkey RM, Endo S, Shenolikar S, Malenka RC. Involvement of a calcineurin/inhibitor-1 phosphatase cascade in hippocampal long-term depression. Nature. 1994;369:486–488. doi: 10.1038/369486a0. [DOI] [PubMed] [Google Scholar]
- 83.Thiels E, Norman ED, Barrionuevo G, Klann E. Transient and persistent increases in protein phosphatase activity during long-term depression in the adult hippocampus in vivo. Neuroscience. 1998;86:1023–1029. doi: 10.1016/s0306-4522(98)00135-3. [DOI] [PubMed] [Google Scholar]
- 84.Morishita W, et al. Regulation of synaptic strength by protein phosphatase 1. Neuron. 2001;32:1133–1148. doi: 10.1016/s0896-6273(01)00554-2. [DOI] [PubMed] [Google Scholar]
- 85.Delgado JY, et al. NMDA receptor activation dephosphorylates AMPA receptor glutamate receptor 1 subunits at threonine 840. J Neurosci. 2007;27:13210–13221. doi: 10.1523/JNEUROSCI.3056-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mauna JC, Miyamae T, Pulli B, Thiels E. Protein phosphatases 1 and 2A are both required for long-term depression and associated dephosphorylation of cAMP response element binding protein in hippocampal area CA1 in vivo. Hippocampus. 2011;21:1093–1104. doi: 10.1002/hipo.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Oh MC, Derkach VA, Guire ES, Soderling TR. Extrasynaptic Membrane Trafficking Regulated by GluR1 Serine 845 Phosphorylation Primes AMPA Receptors for Long-term Potentiation. J Biol Chem. 2006;281:752–758. doi: 10.1074/jbc.M509677200. [DOI] [PubMed] [Google Scholar]
- 88.Snigdha S, Smith ED, Prieto GA, Cotman CW. Caspase-3 activation as a bifurcation point between plasticity and cell death. Neurosci Bull. 2012;28:14–24. doi: 10.1007/s12264-012-1057-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jiao S, Li Z. Nonapoptotic function of BAD and BAX in long-term depression of synaptic transmission. Neuron. 2011;70:758–772. doi: 10.1016/j.neuron.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Manahan-Vaughan D, Kulla A, Frey JU. Requirement of translation but not transcription for the maintenance of long-term depression in the CA1 region of freely moving rats. J Neurosci. 2000;20:8572–8576. doi: 10.1523/JNEUROSCI.20-22-08572.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yang G, Pan F, Gan WB. Stably maintained dendritic spines are associated with lifelong memories. Nature. 2009;462:920–924. doi: 10.1038/nature08577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bian WJ, Miao WY, He SJ, Qiu Z, Yu X. Coordinated Spine Pruning and Maturation Mediated by Inter-Spine Competition for Cadherin/Catenin Complexes. Cell. 2015;162:808–822. doi: 10.1016/j.cell.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 93.Kehoe LA, et al. GluN3A Promotes Dendritic Spine Pruning and Destabilization during Postnatal Development. J Neurosci. 2014;34:9213–9221. doi: 10.1523/JNEUROSCI.5183-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Datwani A, et al. Classical MHCI Molecules Regulate Retinogeniculate Refinement and Limit Ocular Dominance Plasticity. Neuron. 2009;64:463–470. doi: 10.1016/j.neuron.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Adelson JD, et al. Developmental Sculpting of Intracortical Circuits by MHC Class I H2-Db and H2-Kb. Cer Cortex. 2014:1–11. doi: 10.1093/cercor/bhu243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bochner DN, et al. Blocking PirB up-regulates spines and functional synapses to unlock visual cortical plasticity and facilitate recovery from amblyopia. Sci Transl Med. 2014;6:258ra140–258ra140. doi: 10.1126/scitranslmed.3010157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Orefice LL, Shih CC, Xu H, Waterhouse EG, Xu B. Control of spine maturation and pruning through proBDNF synthesized and released in dendrites. Mol Cell Neurosci. 2016;71:66–79. doi: 10.1016/j.mcn.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Woolfrey KM, et al. Epac2 induces synapse remodeling and depression and its disease-associated forms alter spines. Nat Neurosci. 2009;12:1275–1284. doi: 10.1038/nn.2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pielarski KN, et al. Asymmetric N-cadherin expression results in synapse dysfunction, synapse elimination, and axon retraction in cultured mouse neurons. PLoS ONE. 2013;8:e54105. doi: 10.1371/journal.pone.0054105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Talantova M, et al. Aβ induces astrocytic glutamate release, extrasynaptic NMDA receptor activation, and synaptic loss. Proc Natl Acad Sci USA. 2013;110:E2518–27. doi: 10.1073/pnas.1306832110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu Y, Fields RD, Fitzgerald S, Festoff BW, Nelson PG. Proteolytic activity, synapse elimination, and the Hebb synapse. J Neurobiol. 1994;25:325–335. doi: 10.1002/neu.480250312. [DOI] [PubMed] [Google Scholar]
- 102.Buffelli M, et al. Genetic evidence that relative synaptic efficacy biases the outcome of synaptic competition. Nature. 2003;424:430–434. doi: 10.1038/nature01844. [DOI] [PubMed] [Google Scholar]
- 103.Lee KJ, Lee Y, Rozeboom A, Lee JY, Udagawa N, Hoe HS, Pak DT. Requirement for Plk2 in Orchestrated Ras and Rap Signaling, Homeostatic Structural Plasticity, and Memory. Neuron. 2011;69:957–973. doi: 10.1016/j.neuron.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Paolicelli RC, et al. Synaptic Pruning by Microglia Is Necessary for Normal Brain Development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- 105.Mishra A, Kim HJ, Shin AH, Thayer SA. Synapse Loss Induced by Interleukin-1β Requires Pre- and Post-synaptic Mechanisms. J Neuroimm Pharmacol. 2012;7:571–578. doi: 10.1007/s11481-012-9342-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ji K, Akgul G, Wollmuth LP, Tsirka SE. Microglia actively regulate the number of functional synapses. PLoS ONE. 2013;8:e56293. doi: 10.1371/journal.pone.0056293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kettenmann H, Kirchhoff F, Verkhratsky A. Microglia: New Roles for the Synaptic Stripper. Neuron. 2013;77:10–18. doi: 10.1016/j.neuron.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 108.Chung WS, et al. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature. 2013;504:394–400. doi: 10.1038/nature12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chung WS, Allen NJ, Eroglu C. Astrocytes Control Synapse Formation, Function, and Elimination. Cold Spring Harb Perspect Biol. 2015;7:a020370–18. doi: 10.1101/cshperspect.a020370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bahrini I, Song JH, Diez D, Hanayama R. Neuronal exosomes facilitate synaptic pruning by up-regulating complement factors in microglia. Sci Rep. 2015;5:7989–8. doi: 10.1038/srep07989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cheng TW, et al. Emergence of Lamina-Specific Retinal Ganglion Cell Connectivity by Axon Arbor Retraction and Synapse Elimination. J Neurosci. 2010;30:16376–16382. doi: 10.1523/JNEUROSCI.3455-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Development: Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat Rev Neurosci. 2001;2:791–805. doi: 10.1038/35097557. [DOI] [PubMed] [Google Scholar]
- 113.Huh GS, et al. Functional Requirement for Class I MHC in CNS Development and Plasticity. Science. 2000;290:2155–2159. doi: 10.1126/science.290.5499.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Djurisic M, et al. PirB regulates a structural substrate for cortical plasticity. Proc Natl Acad Sci USA. 2013;110:20771–20776. doi: 10.1073/pnas.1321092110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lee H, et al. Synapse elimination and learning rules co-regulated by MHC class I H2-Db. Nature. 2014;509:195–200. doi: 10.1038/nature13154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Adesnik H, Li G, During MJ, Pleasure SJ, Nicoll RA. NMDA receptors inhibit synapse unsilencing during brain development. Proc Natl Acad Sci USA. 2008;105:5597–5602. doi: 10.1073/pnas.0800946105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Roberts AC, et al. Downregulation of NR3A-Containing NMDARs Is Required for Synapse Maturation and Memory Consolidation. Neuron. 2009;63:342–356. doi: 10.1016/j.neuron.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ohno T, et al. Specific involvement of postsynaptic GluN2B-containing NMDA receptors in the developmental elimination of corticospinal synapses. Proc Natl Acad Sci USA. 2010;107:15252–15257. doi: 10.1073/pnas.0906551107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fiuza M, González-González I. GluN3A expression restricts spine maturation via inhibition of GIT1/Rac1 signaling. Proc Natl Acad Sci USA. 2013;110:20807–20812. doi: 10.1073/pnas.1312211110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Attardo A, Fitzgerald JE, Schnitzer MJ. Impermanence of dendritic spines in live adult CA1 hippocampus. Nature. 2015;523:592–596. doi: 10.1038/nature14467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Glynn MW, et al. MHCI negatively regulates synapse density during the establishment of cortical connections. Nat Neurosci. 2011;14:442–451. doi: 10.1038/nn.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Goda Y, Davis GW. Mechanisms of synapse assembly and disassembly. Neuron. 2003;40:243–264. doi: 10.1016/s0896-6273(03)00608-1. [DOI] [PubMed] [Google Scholar]
- 123.Park M, et al. CYY-1/Cyclin Y and CDK-5 Differentially Regulate Synapse Elimination and Formation for Rewiring Neural Circuits. Neuron. 2011;70:742–757. doi: 10.1016/j.neuron.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Karpova A, et al. Encoding and Transducing the Synaptic or Extrasynaptic Origin of NMDA Receptor Signals to the Nucleus. Cell. 2013;152:1119–1133. doi: 10.1016/j.cell.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 125.Qiu J, et al. Mitochondrial calcium uniporter Mcu controls excitotoxicity and is transcriptionally repressed by neuroprotective nuclear calcium signals. Nat Comm. 2013;4:2034. doi: 10.1038/ncomms3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sheppeck JE, Gauss CM, Chamberlin AR. Inhibition of the Ser-Thr phosphatases PP1 and PP2A by naturally occurring toxins. Bioorg Med Chem. 1997;5:1739–1750. doi: 10.1016/s0968-0896(97)00146-6. [DOI] [PubMed] [Google Scholar]
- 127.Favre B, Turowski P, Hemmings BA. Differential inhibition and posttranslational modification of protein phosphatase 1 and 2A in MCF7 cells treated with calyculin-A, okadaic acid, and tautomycin. J Biol Chem. 1997;272:13856–13863. doi: 10.1074/jbc.272.21.13856. [DOI] [PubMed] [Google Scholar]
- 128.Beaumont V, Zhong N, Fletcher R, Froemke RC, Zucker RS. Phosphorylation and local presynaptic protein synthesis in calcium- and calcineurin-dependent induction of crayfish long-term facilitation. Neuron. 2001;32:489–501. doi: 10.1016/s0896-6273(01)00483-4. [DOI] [PubMed] [Google Scholar]
- 129.Bordelon JR, et al. Differential localization of protein phosphatase-1alpha, beta and gamma1 isoforms in primate prefrontal cortex. Cer Cortex. 2005;15:1928–1937. doi: 10.1093/cercor/bhi070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Allen PB, Ouimet CC, Greengard P. Spinophilin, a novel protein phosphatase 1 binding protein localized to dendritic spines. Proc Natl Acad Sci USA. 1997;94:9956–9961. doi: 10.1073/pnas.94.18.9956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Slupe AM, Merrill RA, Strack S. Determinants for Substrate Specificity of Protein Phosphatase 2A. Enzyme Res. 2011;2011:398751. doi: 10.4061/2011/398751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rozkaine A, Hyman BT, Spires-Jones TL. Calcineurin inhibition with FK506 ameliorates dendritic spine density deficits in plaque-bearing Alzheimer model mice. Neurobiol Dis. 2011;41:650–654. doi: 10.1016/j.nbd.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Winder DG, Sweatt JD. Roles of serine/threonine phosphatases in hippocampal synaptic plasticity. Nat Rev Neurosci. 2001;2:461–474. doi: 10.1038/35081514. [DOI] [PubMed] [Google Scholar]
- 134.Xiong W, et al. Anisomycin activates p38 MAP kinase to induce LTD in mouse primary visual cortex. Brain Res. 2006;1085:68–76. doi: 10.1016/j.brainres.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 135.Linden DJ. A protein synthesis-dependent late phase of cerebellar long-term depression. Neuron. 1996;17:483–490. doi: 10.1016/s0896-6273(00)80180-4. [DOI] [PubMed] [Google Scholar]
- 136.McCann CM, Nguyen QT, Santo Neto H, Lichtman JW. Rapid synapse elimination after postsynaptic protein synthesis inhibition in vivo. J Neurosci. 2007;27:6064–6067. doi: 10.1523/JNEUROSCI.0627-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fonseca R, Vabulas RM, Hartl FU, Bonhoeffer T, Nägerl UV. A balance of protein synthesis and proteasome-dependent degradation determines the maintenance of LTP. Neuron. 2006;52:239–245. doi: 10.1016/j.neuron.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 138.Bukalo O, Campanac E, Hoffman DA, Fields RD. Synaptic plasticity by antidromic firing during hippocampal network oscillations. Proc Natl Acad Sci USA. 2013;110:5175–5180. doi: 10.1073/pnas.1210735110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Behnisch T, et al. Nuclear Translocation of Jacob in Hippocampal Neurons after Stimuli Inducing Long-Term Potentiation but Not Long-Term Depression. PLoS ONE. 2011;6:e17276–12. doi: 10.1371/journal.pone.0017276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Liu-Yesucevitz L, et al. Local RNA translation at the synapse and in disease. J Neurosci. 2011;31:16086–16093. doi: 10.1523/JNEUROSCI.4105-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ding M, Chao D, Wang G, Shen K. Spatial regulation of an E3 ubiquitin ligase directs selective synapse elimination. Science. 2007;317:947–951. doi: 10.1126/science.1145727. [DOI] [PubMed] [Google Scholar]
- 142.Helton TD, Otsuka T, Lee MC, Mu Y, Ehlers MD. Pruning and loss of excitatory synapses by the parkin ubiquitin ligase. Proc Natl Acad Sci USA. 2008;105:19492–19497. doi: 10.1073/pnas.0802280105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Liu Y, Fields RD, Festoff BW, Nelson PG. Proteolytic action of thrombin is required for electrical activity-dependent synapse reduction. Proc Natl Acad Sci USA. 1994;91:10300–10304. doi: 10.1073/pnas.91.22.10300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jia M, Li M, Dunlap V, Nelson PG. The thrombin receptor mediates functional activity-dependent neuromuscular synapse reduction via protein kinase C activation in vitro. J Neurobiol. 1999;38:369–381. [PubMed] [Google Scholar]
- 145.Huntley GW. Synaptic circuit remodelling by matrix metalloproteinases in health and disease. Nat Rev Neurosci. 2012;13:743–757. doi: 10.1038/nrn3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.O’Connor TP, et al. Semaphorin 5B mediates synapse elimination in hippocampal neurons. Neural Dev. 2009;4:18–19. doi: 10.1186/1749-8104-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hajszan T, Leranth C. Bisphenol A interferes with synaptic remodeling. Neuroendocrinol. 2010;31:519–530. doi: 10.1016/j.yfrne.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Calabrese B, Saffin JM, Halpain S. Activity-Dependent Dendritic Spine Shrinkage and Growth Involve Downregulation of Cofilin via Distinct Mechanisms. PLoS ONE. 2014;9:e94787–18. doi: 10.1371/journal.pone.0094787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Stein IS, Gray JA, Zito K. Non-Ionotropic NMDA Receptor Signaling Drives Activity-Induced Dendritic Spine Shrinkage. J Neurosci. 2015;35:12303–12308. doi: 10.1523/JNEUROSCI.4289-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lai CSW, Franke TF, Gan WB. Opposite effects of fear conditioning and extinction on dendritic spine remodelling. Nature. 2012;483:87–91. doi: 10.1038/nature10792. [DOI] [PubMed] [Google Scholar]
- 151.Tata DA, Marciano VA, Anderson BJ. Synapse loss from chronically elevated glucocorticoids: Relationship to neuropil volume and cell number in hippocampal area CA3. J Comp Neurol. 2006;498:363–374. doi: 10.1002/cne.21071. [DOI] [PubMed] [Google Scholar]
- 152.Koffie RM, et al. Oligomeric amyloid beta associates with postsynaptic densities and correlates with excitatory synapse loss near senile plaques. Proc Natl Acad Sci USA. 2009;106:4012–4017. doi: 10.1073/pnas.0811698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cavallucci V, et al. Calcineurin Inhibition Rescues Early Synaptic Plasticity Deficits in a Mouse Model of Alzheimer’s Disease. Neuromol Med. 2013;15:541–548. doi: 10.1007/s12017-013-8241-2. [DOI] [PubMed] [Google Scholar]
- 154.Wei W, et al. Amyloid beta from axons and dendrites reduces local spine number and plasticity. Nat Neurosci. 2009;13:190–196. doi: 10.1038/nn.2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Li S, et al. Soluble oligomers of amyloid Beta protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron. 2009;62:788–801. doi: 10.1016/j.neuron.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hong S, et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science. 2016;352:712–716. doi: 10.1126/science.aad8373. [DOI] [PMC free article] [PubMed] [Google Scholar]