Abstract
In the gastrointestinal tract, the tug of war for iron may provide a new way to vaccinate. Recent work shows that immunizing mice with siderophores (small molecules microbes produce to capture iron) foils pathogen colonization and may instead allow a commensal to expand.
Keywords: siderophore, iron, Salmonella, vaccine
Microbes and mammals both require the enzyme cofactor iron. During infection, the competition for iron is so intense that microbial iron acquisition systems are major determinants of virulence. One strategy bacteria and fungi use to acquire iron in the host is to secrete small molecules called siderophores which bind Fe3+ with extremely high affinity (association constants can exceed 1050) [1]. Microbes then capture the iron–siderophore complex with a specific membrane receptor and release the iron into the cytosol. Siderophore biosynthesis or utilization can be inhibited by small molecules, which have potential for development as antimicrobials [2]. Another iron-based antimicrobial approach exploits siderophores that have been naturally or synthetically conjugated to antibiotics, tricking microbes into taking up the antibiotic along with the siderophore [3]. A third siderophore-based strategy, priming the host to make anti-siderophore antibodies that may prevent microbes from acquiring iron, has recently taken a giant leap forward [4].
While siderophores are not themselves immunogenic, a siderophore conjugated to an immunogenic carrier protein stimulates mice to produce siderophore-specific antibodies within three weeks [5]. This and other observations suggested that vaccinating against siderophores may confer protection against pathogens that require them. Recently a team of microbiologists and chemists took the next major step [4]. Sassone-Corsi et al. conjugated the siderophore enterobactin (Ent) to an immunogenic carrier protein. Salmonella enterica requires Ent for virulence in mice [6]. The carrier protein, cholera toxin subunit B (CTB) is an adjuvant that promotes mucosal inflammation [7]. The researchers delivered CTB-Ent or CTB alone to mouse nasal mucosal tissue twice, two weeks apart (Figure 1). One week later, significant levels of Ent-specific immunoglobulin A (IgA) antibodies appeared in the feces of CTB-Ent but not CTB immunized mice.
Figure 1. Siderophore Immunization and Challenge Strategy.
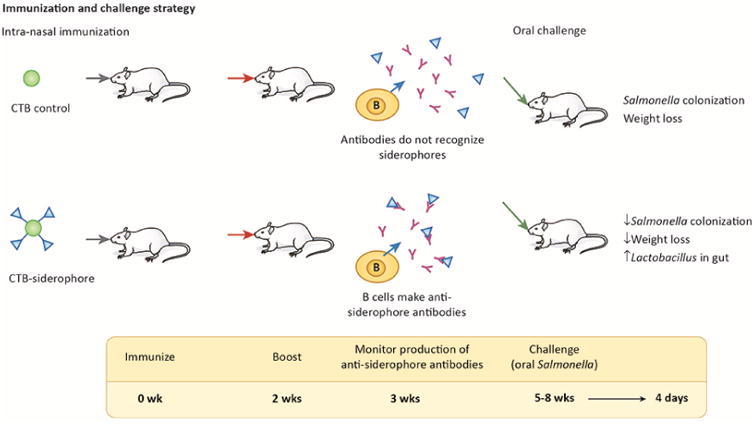
At zero and two weeks, control CTB or siderophore-conjugated CTB (CTB-Ent, approximately four Ent molecules per CTB) was delivered to mice intra-nasally (100 μg/mouse) by Sassone-Corsi et al. [4]. After three weeks, IgA antibodies specific for siderophores were observed in the feces of CTB-Ent immunized mice, but not in CTB immunized mice. After five to eight weeks, the mice were challenged in a model of Salmonella colitis, which requires pre-treatment with the antibiotic streptomycin [8], followed by inoculation with 109 streptomycin-resistant Salmonella. Four days later, at the peak of inflammatory response and iron limitation, tissue colonization, body weight, and the gut microbiota were compared.
Next, the Sassone-Corsi team [4] examined whether vaccination confers resistance to infection with Salmonella. Five to eight weeks after immunization, they pre-treated mice orally with the antibiotic streptomycin to increase susceptibility to Salmonella [8]. They then challenged orally with 109 bacteria [4]. By the fourth day after infection, the CTB-Ent-immunized mice contained 10-fold fewer Salmonella in their intestines. Similar results were obtained in a different mouse line, in mice housed under different conditions and in mice intraperitoneally immunized with CTB-Ent. In addition, the researchers observed an encouraging negative correlation between Salmonella intestinal load and levels of anti-Ent IgA in individual mice. Altogether, the data suggest that infection could be reduced by optimizing vaccination, and that antibodies to siderophores may prevent Salmonella from capturing enough siderophore-bound iron to thrive in the gut.
The researchers also examined whether gut inflammation or the gut microbiota differ in CTB versus CTB-Ent immunized mice. The basis for this inquiry is the survival advantage that Salmonella and other facultative anaerobes have in the inflamed gut due to availability of alternative electron acceptors, such as nitrate and tetrathionate [9]. As expected, histopathology and molecular markers revealed no significant inflammation four days after infection or mock-infection. In contrast, infection resulted in inflammation in both CTB and CTB-Ent immunized mice, suggesting Salmonella had access to alternative electron acceptors under both conditions. However, Salmonella growth in the gut also requires iron captured by siderophores [6], consistent with the failure of CTB-Ent immunized mice to support Salmonella colonization. Instead, it appears that in these mice, commensal Lactobacillus species expand upon challenge with Salmonella. Lactobacillus thrive in an inflamed gut for unknown reasons, but do not scavenge enterobactin [10]. These data highlight that in a complex ecosystem, inhibition of one organism, in this case a pathogen, may open up a niche for another organism, in this case, fortunately, a commensal.
In summary, immunization against siderophores can protect the host from a gut pathogen that depends upon siderophores to replicate. This exciting find has broad potential for bacterial and fungal pathogens and merits further study. Key remaining questions are whether IgA antibodies are necessary and sufficient for protection and whether natural transmission of the pathogen is reduced, as anticipated. In addition, it is important to determine whether microbes have the capacity to acquire or evolve resistance to anti-siderophore antibodies. Nevertheless, anti-siderophore vaccines have tremendous potential because they could minimize the spread of siderophore-requiring pathogens in food animals and in people.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cassat JE, Skaar EP. Iron in infection and immunity. Cell Host & Microbe. 2013;13:509–519. doi: 10.1016/j.chom.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lamb AL. Breaking a pathogen's iron will: inhibiting siderophore production as an antimicrobial strategy. Biochim Biophys Acta. 2015;1854:1054–1070. doi: 10.1016/j.bbapap.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braun V, et al. Sideromycins: tools and antibiotics. Biometals. 2009;22:3–13. doi: 10.1007/s10534-008-9199-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sassone-Corsi M, et al. Siderophore-based immunization strategy to inhibit growth of enteric pathogens. Proc Natl Acad Sci USA. 2016;113:13462–13467. doi: 10.1073/pnas.1606290113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergeron RJ, et al. Vibriobactin antibodies: a vaccine strategy. J Med Chem. 2009;52:3801–3813. doi: 10.1021/jm900119q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raffatellu M, Bäumler AJ. Salmonella's iron armor for battling the host and its microbiota. Gut Microbes. 2010;1:70–72. doi: 10.4161/gmic.1.1.10951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holmgren J, et al. Mucosal adjuvants and anti-infection and anti-immunopathology vaccines based on cholera toxin, cholera toxin B subunit and CpG DNA. Immunol Lett. 2005;97:181–188. doi: 10.1016/j.imlet.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 8.Barthel M, et al. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect Immun. 2003;71:2839–2858. doi: 10.1128/IAI.71.5.2839-2858.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winter SE, et al. Host-derived nitrate boosts growth of E. coli in the inflamed gut. Science. 2013;339:708–711. doi: 10.1126/science.1232467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Behnsen J, et al. The cytokine IL-22 promotes pathogen colonization by suppressing related commensal bacteria. Immunity. 2014;40:262–273. doi: 10.1016/j.immuni.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


