Abstract
Objective
Neurologic deterioration associated with cerebral edema (CE) in diabetic ketoacidosis (DKA) is typically sudden in onset, progresses rapidly and requires emergent treatment. The utility of brain imaging by head computed tomography (CT) in decisions to treat for CE has not been previously studied. The objective of this study was to describe the characteristics of pediatric patients with DKA who develop altered mental status (AMS) and evaluate the role of head CT in this cohort.
Design
Retrospective analysis of clinical, biochemical, and radiological data.
Setting
Tertiary care children’s hospital (2004–2010).
Patients
686 admissions of patients (<26 years) with diabetic ketoacidosis.
Main Results
AMS was documented during 96 of 686 DKA admissions (14%). Compared with alert patients, those with AMS were younger (median 12.0 vs. 13.1 years; p=0.007) and more acidotic (pH 7.04 vs. 7.19; p<0.001), with higher serum osmolality (328 vs. 315 mOsm/kg; p<0.001) and longer hospital lengths of stay (4.5 vs. 3 days; p=0.002). Head CT was performed during 60 of 96 DKA admissions with AMS (63%), 16 (27%) of which had abnormal results. Hyperosmolar therapy for CE was given during 23 of the 60 admissions (38%), during which 12 (52%) had normal head CT results, 8 of these12 (67%) after CE treatment and 4 (33%) before. Of the 11 admissions with abnormal head CT results that received hyperosmolar therapy, 4 head CTs (36%) occurred after hyperosmolar treatment and 7 (64%) before. For the 11 admissions with head CT before CE treatment, there was a median 2-hour delay between head CT and hyperosmolar therapy.
Conclusions
In this single-center retrospective study, there was no evidence that decisions about treatment of patients with DKA and suspected CE were enhanced by head CT, and head CT may have led to a significant delay in hyperosmolar therapy.
Keywords: Diabetic ketoacidosis, brain edema, computerized tomography
Introduction
Diabetic ketoacidosis (DKA) remains the most common catastrophic complication of Type 1 Diabetes Mellitus (T1DM) and occurs in 25–40% of children with this disease [1]. Mental status abnormalities are reported in up to 15% of children with DKA [2, 3] and have been associated with evidence of cerebral edema (CE) on magnetic resonance imaging (MRI) [4]. These studies have led to the notion that CE is not a rare phenomenon in children with DKA, but occurs frequently with varying severity. Symptomatic CE in DKA likely represents the most severe manifestation of a common phenomenon [5] and is defined by clinical diagnostic criteria including abnormal motor or verbal responses to pain, decorticate posture, and abnormal neurogenic respiratory patterns [6]. Though rare, with a reported incidence of 0.5–4% in children with DKA in national population studies [1–3, 7–9], symptomatic CE in DKA is a life-threatening complication that carries a high morbidity and a mortality rate of 21–24% [1, 8, 10] and is associated with rapid neurologic deterioration requiring emergent treatment [11]. Current clinical practice guidelines for DKA focus on rapid clinical recognition and treatment of CE in DKA [5], with brain imaging recommended only “after treatment for cerebral edema has been started, to rule out other possible intracerebral causes of neurologic deterioration especially thrombosis or hemorrhage, which may benefit from specific therapy” [5].
The reliance on brain imaging by head computed tomography (CT) to make decisions regarding therapy for CE during an episode of DKA has several important limitations. Over half of children with DKA, regardless of neurologic symptoms, have narrowing of the lateral ventricles apparent on head imaging several hours after the initiation of therapy, suggesting that subclinical cerebral edema occurs frequently in this setting [4]. On the other hand, up to 40% of children with clinically apparent cerebral edema may demonstrate no acute abnormality in their initial head CT [6]. Although head CT may be a useful adjunct to neurological evaluation in some clinical situations, it poses risks that are not negligible in children with DKA. Increasing use of CT has potentially adverse effects of radiation induced malignancy, especially on the growing child [13–15]. In children younger than 15 years of age, cumulative ionizing radiation doses from repeated head CTs increases the risk of brain tumors and leukemia [16].
The discordance between imaging findings and clinical presentation, the cost and risks associated with exposure to unnecessary radiation and the uncertain prevalence of intracerebral pathologies other than cerebral edema raise questions about the utility of head CT in the acute management of a child with DKA who develops altered mental status (AMS). The objectives of this study were to describe the characteristics of patients with DKA who develop altered mental status and to examine the role of head CT in treatment decisions for suspected cerebral edema. We also set out to describe the frequency of imaging and range of head CT findings in patients with DKA and AMS. Our primary hypothesis was that decisions about treatment of patients with diabetic ketoacidosis and suspected cerebral edema are made based upon clinical findings, regardless of head CT findings.
Materials and Methods
All patients with an ICD-9 code for DKA (250.1, 250.2, or 250.3) admitted to Boston Children’s Hospital from 2004–2010 were initially identified from hospital billing records. Charts were reviewed to ensure that patients had a diagnosis of DKA defined by a clinical history consistent with diabetes, a venous or arterial pH ≤7.30 or a serum bicarbonate ≤15 mmol/L, and moderate to large ketonuria on a urine test strip. In order to ascertain all cases of concern for cerebral edema in DKA, pharmacy records were also obtained for all patients who received 3% hypertonic saline or mannitol over the same time period and cross-referenced with patients with DKA as defined above. Electronic medical records during DKA admission were reviewed and demographic information, onset of diabetes, vital signs, Glasgow Coma Scale (GCS) score, nursing and physician clinical notes, laboratory values and attending radiologist head CT reports were collected for each patient.
Patients were classified as having AMS based on nursing and physician documentation. Each patient with AMS was assigned a neurologic symptom score, according to previously published literature [17], based on descriptions of the patient’s clinical status in clinical notes (attending physician, resident and/or nursing) as well as GCS score (1 = irritable, disoriented, confused, or GCS 13–15; 2 = lethargic, somnolent, or GCS 11–12; 3 = stuporous, purposeful response to pain, or GCS 8–10; 4 = abnormal or absent purposeful response to pain or GCS 6–7; 5 = focal neurologic finding, fixed and dilated pupil(s), respiratory arrest, or GCS 3–5) and vital signs data. The study was approved by the Boston Children’s Hospital Institutional Review Board.
Statistical analysis
The primary unit of analysis was DKA admission (or DKA admission with AMS), as 56 patients had more than one DKA episode requiring admission over the study period. All regression analyses accounted for multiple DKA admissions per patient using generalized estimating equations (GEEs). Differences between AMS and alert patients were assessed using linear or logistic regression as appropriate. For DKA admissions with AMS, logistic regression was used to compare the characteristics of patients with and without head CTs performed, as well as with and without treatment for CE. Multivariable stepwise logistic regression was then used to further evaluate the association of demographic and clinical variables with the use of head CT in patients with AMS. Potential covariates included age at admission; gender; onset of diabetes; neurologic symptom score; initial pH, serum bicarbonate, BUN, glucose, and hemoglobin values; and treatment for CE before head CT. A significance level of 0.1 was required for covariates to enter and stay in the model. If more than one head CT was performed during admission, the first head CT was used in analyses. For admissions with both head CT and hyperosmolar therapy for CE, the duration between time head CT performed and time treatment given was calculated. A p value of less than 0.05 was considered significant in the multivariable model. Analyses were performed using SAS (version 9.4, SAS Institute, Cary, NC).
Results
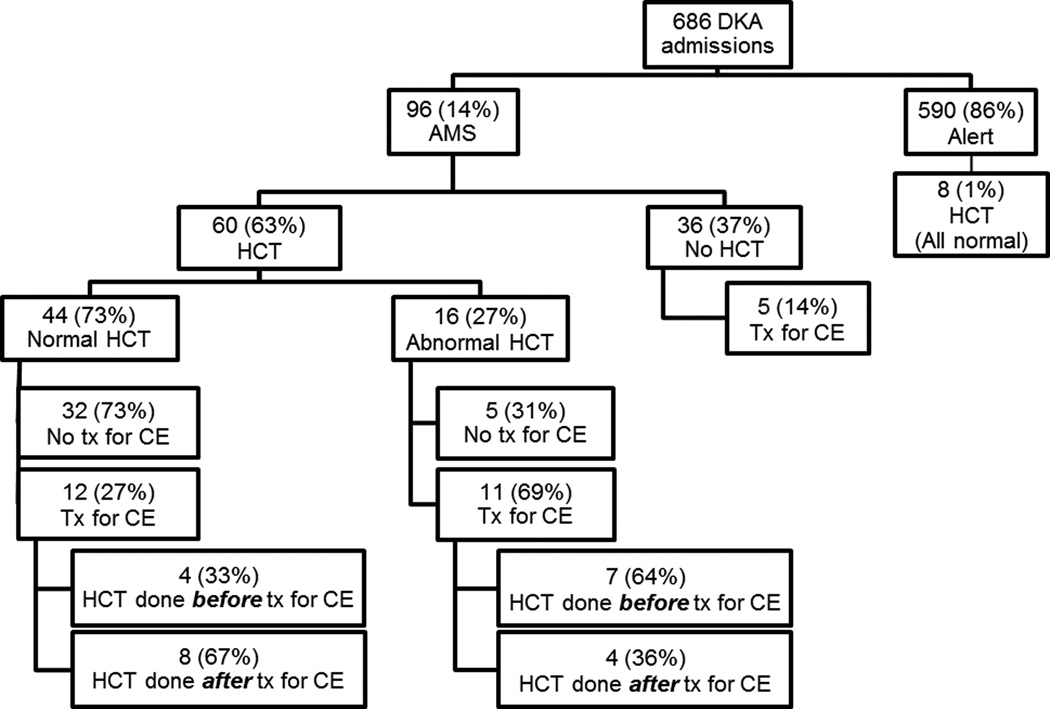
We identified 686 admissions for DKA, during 96 (14%) of which patients developed AMS (Figure 1). Patients who developed AMS were significantly younger compared with alert patients (median 12.0 vs. 13.1 years; p = 0.007; Table 1). There were no statistically significant differences in gender or prevalence of new onset T1DM between the AMS and alert groups. On admission, patients with AMS were significantly more acidotic with lower serum bicarbonate and higher serum osmolality and had higher BUN, blood glucose levels, and anion gap (all p < 0.001). Initial sodium levels were similar between groups. Patients with AMS had longer hospital lengths of stay compared to alert patients (median [interquartile range (IQR)] 4.5 [4–5] vs. 3 [3–4]; p = 0.002). There were no deaths in our cohort during the study period.
Figure 1. Distribution of DKA admissions by mental status category, HCT, and treatment for CE.
AMS, altered mental status; CE, cerebral edema; DKA, diabetic ketoacidosis; HCT, head computed tomography; tx, treatment.
TABLE 1.
Characteristics of Diabetic Ketoacidosis Patients by Mental Status
| Characteristic | Altered Mental Status (n = 96) |
Alert (n = 590) |
pa |
|---|---|---|---|
| Age at admission, median (IQR), years | 12.0 (8.5–14.7) | 13.1 (9.6–16.6) | 0.007 |
| Female, no. (%) | 53 (55) | 321 (54) | 0.89 |
| New onset T1DM, no. (%) | 55 (57) | 276 (47) | 0.07 |
| Initial laboratory values (from outside hospital or BCH), median (IQR) |
|||
| pH | 7.04 (6.94–7.14) | 7.19 (7.12–7.25) | <0.001 |
| pCO2, mmHg | 21 (14–27) | 28 (22–33) | <0.001 |
| Sodium, mmol/L | 133 (128–137) | 133 (130–136) | 0.39 |
| Serum bicarbonate, mmol/L | 5 (4–8) | 10 (7–12) | <0.001 |
| BUN, mg/dL | 23 (17–33) | 17 (13–22) | <0.001 |
| Glucose, mg/dL | 618 (499–796) | 506 (395–658) | <0.001 |
| Anion gap, mmol/L | 30 (26–34) | 27 (23–30) | <0.001 |
| Serum osmolarity, mOsm/kg | 328 (311–348) | 315 (305–328) | <0.001 |
| Hemoglobin, g/dL | 14.0 (12.6–15.3) | 14.6 (13.6–15.8) | 0.005 |
| Intubated, no. (%) | 4 (4) | 0 | <0.001 |
| Head CT performed, no. (%) | 60 (63) | 8 (1) | <0.001 |
| Treated for cerebral edema, no. (%) | 28 (29) | 0 | <0.001 |
AMS, altered mental status; BCH, Boston Children’s Hospital; BUN, blood urea nitrogen; CT, computed tomography; IQR, interquartile range; T1DM, Type 1 diabetes mellitus.
Missing data <2% except for anion gap (87 AMS, 411 alert), serum osmolarity (69 AMS, 386 alert), and hemoglobin (95 AMS, 449 alert).
P values for the comparison between groups were calculated using linear and logistic regression, accounting for multiple admissions per patient using generalized estimating equations for continuous and binary variables, respectively. Where there was a zero count in the alert group, the p value was calculated using Fisher’s exact test.
Head CTs were performed during 68 DKA admissions overall (10%). As expected, patients with AMS were more likely to undergo head imaging than alert patients (N=60 [63%] vs. 8 [1%]; p < 0.001; Table 1). Among DKA admissions with AMS, there were no statistically significant differences in patient demographics or clinical symptoms between those who received head imaging and those who did not, including prevalence of new onset diabetes, initial laboratory values, and need for intubation (Table 2). The neurologic symptom scores were not significantly different for patients with AMS who underwent head CT and those who did not receive head imaging (median [IQR] 2 [2–3] vs. 2 [1.5–2]; p = 0.10). The lowest pH during hospital admission in AMS patients with head CT was lower than in those without imaging (7.00 [6.92–7.10] vs. 7.04 [6.97–7.19]; p = 0.04).
TABLE 2.
Characteristics of Diabetic Ketoacidosis Patients with Altered Mental Status by Imaging Decision
| Characteristic | Head CT (n = 60) |
No Head CT (n = 36) |
pa |
|---|---|---|---|
| Age at admission, median (IQR), years | 11.6 (9.3–14.7) | 12.5 (4.4–14.9) | 0.20 |
| Female, no. (%) | 33 (55) | 20 (56) | 0.96 |
| New onset T1DM, no. (%) | 34 (57) | 21 (58) | 0.88 |
| Neurologic symptom score, no. (%)b | |||
| 1 | 6 (10) | 9 (25) | |
| 2 | 36 (60) | 23 (64) | |
| 3 | 11 (18) | 2 (6) | |
| 4 | 2 (3) | 0 | |
| 5 | 5 (8) | 2 (6) | |
| Median (IQR) | 2 (2–3) | 2 (1.5–2) | 0.10 |
| Initial laboratory values (from outside hospital or BCH), median (IQR) |
|||
| pH | 7.01 (6.93–7.12) | 7.05 (6.98–7.20) | 0.06 |
| pCO2, mmHg | 21 (13–26) | 22 (15–29) | 0.19 |
| Sodium, mmol/L | 134 (129–137) | 132 (126–138) | 0.63 |
| Serum bicarbonate, mmol/L | 6 (4–8) | 5 (4–8) | 0.95 |
| BUN, mg/dL | 24 (17–31) | 22 (18–34) | 0.88 |
| Glucose, mg/dL | 629 (480–847) | 615 (526–717) | 0.23 |
| Anion gap, mmol/L | 31 (25–34) | 29 (27–34) | 0.88 |
| Serum osmolarity, mOsm/kg | 329 (311–362) | 326 (311–340) | 0.06 |
| Hemoglobin, g/dL | 14.3 (13.2–15.3) | 12.8 (12.3–15.4) | 0.22 |
| Intubated, no. (%) | 3 (5) | 1 (3) | 0.60 |
BCH, Boston Children’s Hospital; BUN, blood urea nitrogen; CT, computed tomography; IQR, interquartile range; T1DM, Type 1 diabetes mellitus.
Missing data <2% except for anion gap (58 CT, 29 no CT) and serum osmolarity (45 CT, 24 no CT).
P values for the comparison between groups were calculated using logistic regression, accounting for multiple admissions per patient using generalized estimating equations.
The neurological symptom score ranges from 1 to 5, with higher values indicating more neurological deterioration.
Hyperosmolar therapy was given for treatment of suspected CE in 4% (N=28) of all DKA admissions, all of which presented with AMS (29% of DKA admissions with AMS). Patients with AMS who received hyperosmolar treatment had higher neurological symptom scores and, on admission, were more acidotic and had lower pCO2 and sodium levels compared to those who did not receive treatment (Supplemental Table 1).
Head CT results
Head CTs were performed during 8 admissions where patients were described as alert, all of which were read as normal without evidence of CE or intracranial pathology. Head CTs were obtained for headache in 7 of these admissions and a change in mental status in 1 admission. None of these patients received treatment for CE.
Of the 60 head CTs performed in admissions with AMS, 44 (73%) were normal. Of the 16 head CTs with abnormal findings, 8 (50%) had diffuse cerebral edema without herniation, 7 (44%) had mild cerebral edema, and 1 (6%) had mild ventriculomegaly. There were no intracranial pathological events besides CE for any admissions. In particular, there were no cerebral thromboses, ischemic events or intracerebral hemorrhages. AMS was the primary indication for obtaining a head CT (98%), with asymmetric pupils (2%) as a rare indication.
Hyperosmolar treatment for CE and head CT
Patients were not treated for CE during 37 admissions (62%) with AMS where head CTs were performed. Of these, 5 (14%) had an abnormal head CT. As detailed in Supplemental Table 2, the treating team determined that neurologic symptoms did not warrant treatment despite abnormal head CT findings.
During 23 AMS admissions (38%) where head CT was performed, patients received treatment for CE with hyperosmolar therapy. The head CTs were normal in 12 of these admissions (52%), with 8 (67%) after CE treatment and 4 (33%) before. The head CTs had abnormal results in the other 11 admissions (48%), with 4 (36%) performed after CE treatment and 7 (64%) before.
In the 12 admissions (52%) where head CT was performed after hyperosmolar treatment, the median time between treatment and head CT was 70 minutes (IQR 38–156). Of these, 4 head CTs were abnormal (3 had diffuse cerebral edema and 1 had mild cerebral edema) and 8 head CTs were normal. Of the patients with abnormal head CT results after initial treatment for CE 3 out of 4 (75%) received a second or more doses of hyperosmolar agent. Of the 8 patients with a normal head CT after initial treatment for CE, 5 (63%) also received a second or more doses of hyperosmolar treatment.
In the 11 (48%) admissions where head CT was obtained before hyperosmolar treatment, the median time between head CT and treatment was 115 minutes (IQR range 58–354) and for the patients with abnormal head CTs 58 minutes (53–115).
During 5 AMS admissions, patients were treated for suspected CE and did not receive a head CT due to clinical improvement following treatment.
Discussion
In this study, patients with DKA developed AMS during 14% of admissions. Concordant with other large population studies of DKA [1, 4, 10, 18], these patients were significantly younger, more acidotic and had a higher anion gap, BUN, and serum glucose, as well as longer hospital length of stay. Head CTs were performed during 10% of all DKA admissions and 63% of DKA admissions with AMS. Suspected CE in DKA resulting in treatment with hyperosmolar therapies occurred in 4% of all admissions (29% of admissions with AMS), which is consistent with a recently published national population study from our group utilizing the Pediatric Health Information Systems (PHIS) database [9]. In our cohort, patients with AMS and clinical suspicion of CE sufficient to warrant treatment with hyperosmolar therapies were equally as likely to have a normal head CT (52%) as an abnormal one (48%). Furthermore, in some cases, treatment was given despite normal head CT results. Most importantly, however, patients who received a head CT prior to treatment for cerebral edema had a median 2-hour delay in treatment.
Patients with radiologic evidence of CE on head CT who do not have acute neurological symptoms are unlikely to be treated for CE (Supplemental Table 2), while patients in whom there is clinical concern for CE will likely be treated with hyperosmolar therapies regardless of findings on head CT. Taken together, these findings lend credence to current guidelines that recommend brain imaging should be considered for patients with suspected CE only after treatment for CE has been initiated in order to avoid delay in treatment [5]. In addition, because the majority (67%) of head CTs performed after treatment for CE were normal, our study suggests that in some of these cases, head CT could have been avoided thereby decreasing the risks associated with unnecessary radiation exposure. In our study, more than half of the patients (63%) with a normal head CT after initial treatment for CE received a second or more doses of hyperosmolar treatment suggesting they were treated based on clinical criteria rather than CT findings. Documentation in medical record supports this, with persistence of altered mental status cited as the reason for subsequent treatment. Furthermore, in the one patient with an abnormal head CT that received only one dose of hyperosmolar agent prior to CT, treatment was not repeated despite abnormal head CT results as there was documented improvement in mental status following head CT. While our sample size is small, our data suggests that treatment for suspected CE with hyperosmolar therapy is dependent on clinical status and not head CT results and there is limited utility in obtaining a head CT in patients who respond quickly to hyperosmolar therapy. It has been estimated that for every 600,000 abdominal and head CT examinations performed in children younger than 15 years, 500 of the exposed patients will die from cancer attributable to CT radiation [15]. In many clinical scenarios the benefits of obtaining a head CT outweigh the associated risks. However, in patients with T1DM who are likely to have more than one admission for DKA, frequent imaging with head CT may pose an even more significant risk. Consideration should be given to the balance between the added risks associated with radiation exposure, transport to head CT and costs, and the likelihood that imaging will change clinical management in the absence of a focal neurologic deficit.
The previously reported incidence of other intracranial pathology in patients with DKA who develop AMS was not replicated in this study. In our cohort, which included 60 head CTs during DKA admissions with AMS, 16 of which were abnormal, there were no patients with thrombosis, ischemia, or hemorrhage. To our knowledge, this is the largest analysis of head CTs obtained during the course of DKA admissions to date, therefore, these differences are likely not explained by underdiagnoses due to lack of imaging. Previous reports describing the prevalence of other intracerebral events during an episode of DKA, such as thrombosis and ischemic events, are derived from case series of patients who were selected based on poor outcomes [6, 12] with up to 10% of patients reported to have thrombosis, infection or hemorrhage [12] and findings of subarachnoid or intraventricular hemorrhages, edema and hemorrhages, or focal brain injury reported in 52% of head CTs in another series [6]. Because of their selection bias, these studies likely overestimate the incidence of lesions other than diffuse cerebral edema and may be contributing to the frequency of head CTs in this population. A larger, multicenter study is needed to determine the actual incidence of other intracerebral events previously described with DKA such as venous thrombosis, cerebral hemorrhage or infarct, to identify the difference in clinical presentation which could help delineate which patients unequivocally need brain imaging, and to ascertain which imaging modality is likely to provide the most diagnostic accuracy at the least risk to the patient.
This retrospective review was conducted in a freestanding children’s hospital and may not reflect care in community hospitals or other settings. Decisions regarding acquisition of a head CT and treatment for suspected cerebral edema were based on clinical criteria and it is possible that these decisions were influenced by variables not collected in our database. The patients included in our study were admitted prior to publication of the current guidelines, however, the recommendations suggesting that head imaging should be only be performed after a patient has been treated for suspected cerebral edema have remained largely unchanged in guidelines from 2000 and 2009 [5, 19, 20]. Due to inconsistencies in charting of the time of onset of altered mental status, the “delay in treatment” with hyperosmolar therapy was calculated from the time a head CT was ordered to the time hyperosmolar therapy was given. Thus, the true “delay in treatment” may be underestimated. While we collected data on a large number of patients admitted with DKA, a relatively smaller subset had head CTs performed or were treated for suspected cerebral edema, thus some analyses may be underpowered. Lastly, multiple unblinded radiologists interpreted the head CTs, which may have resulted in bias. Nonetheless, this was the information available to the treating clinician in real time.
Conclusions
In this retrospective study, there was no evidence that decisions about treatment of patients with DKA and suspected CE were enhanced by head CT results, and in fact, head CT resulted in a significant delay in hyperosmolar therapy. Our findings reinforce published guidelines [5, 19, 20] that suggest that treatment with hyperosmolar therapies in DKA should occur as soon as cerebral edema is suspected. Future studies are needed to assess the prevalence of other intracranial pathologies such as thrombosis or ischemia in DKA patients with altered mental status and to determine if imaging should be reserved for patients who fail to improve after treatment for CE or who have focal neurologic deficits.
Supplementary Material
Acknowledgments
Financial Disclosures: Research reported in this publication was supported by the National Institutes of Health (T32 HD 75727-1). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funding institutions did not contribute to the collection, management, analysis, and interpretation of the data; nor preparation, review, or approval of the manuscript.
References
- 1.Glaser N, Barnett P, McCaslin I, et al. Risk factors for cerebral edema in children with diabetic ketoacidosis. The Pediatric Emergency Medicine Collaborative Research Committee of the American Academy of Pediatrics. N Engl J Med. 2001 Jan 25;344(4):264–269. doi: 10.1056/NEJM200101253440404. [DOI] [PubMed] [Google Scholar]
- 2.Bello FA, Sotos JF. Cerebral oedema in diabetic ketoacidosis in children. Lancet. 1990 Jul 7;336(8706):64. doi: 10.1016/0140-6736(90)91587-z. [DOI] [PubMed] [Google Scholar]
- 3.Duck SC, Wyatt DT. Factors associated with brain herniation in the treatment of diabetic ketoacidosis. J Pediatr. 1988 Jul;113(1 Pt 1):10–14. doi: 10.1016/s0022-3476(88)80521-3. [DOI] [PubMed] [Google Scholar]
- 4.Glaser NS, Wootton-Gorges SL, Buonocore MH, et al. Frequency of sub-clinical cerebral edema in children with diabetic ketoacidosis. Pediatr Diabetes. 2006 Apr;7(2):75–80. doi: 10.1111/j.1399-543X.2006.00156.x. [DOI] [PubMed] [Google Scholar]
- 5.Wolfsdorf JI, Allgrove J, Craig ME, et al. Diabetic ketoacidosis and hyperglycemic hyperosmolar state. Pediatr Diabetes. 2014 Sep;15(Suppl 20):154–179. doi: 10.1111/pedi.12165. [DOI] [PubMed] [Google Scholar]
- 6.Muir AB, Quisling RG, Yang MC, Rosenbloom AL. Cerebral edema in childhood diabetic ketoacidosis: natural history, radiographic findings, and early identification. Diabetes Care. 2004 Jul;27(7):1541–1546. doi: 10.2337/diacare.27.7.1541. [DOI] [PubMed] [Google Scholar]
- 7.Wolfsdorf J, Glaser N, Sperling MA. Diabetic ketoacidosis in infants, children, and adolescents: A consensus statement from the American Diabetes Association. Diabetes Care. 2006 May;29(5):1150–1159. doi: 10.2337/diacare.2951150. [DOI] [PubMed] [Google Scholar]
- 8.Lawrence SE, Cummings EA, Gaboury I, Daneman D. Population-based study of incidence and risk factors for cerebral edema in pediatric diabetic ketoacidosis. J Pediatr. 2005 May;146(5):688–692. doi: 10.1016/j.jpeds.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 9.Decourcey DD, Steil GM, Wypij D, Agus MS. Increasing use of hypertonic saline over mannitol in the treatment of symptomatic cerebral edema in pediatric diabetic ketoacidosis: an 11-year retrospective analysis of mortality*. Pediatr Crit Care Med. 2013 Sep;14(7):694–700. doi: 10.1097/PCC.0b013e3182975cab. [DOI] [PubMed] [Google Scholar]
- 10.Edge JA, Hawkins MM, Winter DL, Dunger DB. The risk and outcome of cerebral oedema developing during diabetic ketoacidosis. Arch Dis Child. 2001 Jul;85(1):16–22. doi: 10.1136/adc.85.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watts W, Edge JA. How can cerebral edema during treatment of diabetic ketoacidosis be avoided? Pediatr Diabetes. 2014 Jun;15(4):271–276. doi: 10.1111/pedi.12155. [DOI] [PubMed] [Google Scholar]
- 12.Rosenbloom AL. Intracerebral crises during treatment of diabetic ketoacidosis. Diabetes Care. 1990 Jan;13(1):22–33. doi: 10.2337/diacare.13.1.22. [DOI] [PubMed] [Google Scholar]
- 13.Brenner DJ. Estimating cancer risks from pediatric CT: going from the qualitative to the quantitative. Pediatr Radiol. 2002 Apr;32(4):228-221. doi: 10.1007/s00247-002-0671-1. discussion 242-224. [DOI] [PubMed] [Google Scholar]
- 14.Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N Engl J Med. 2007 Nov 29;357(22):2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 15.Brenner D, Elliston C, Hall E, Berdon W. Estimated risks of radiation-induced fatal cancer from pediatric CT. AJR Am J Roentgenol. 2001 Feb;176(2):289–296. doi: 10.2214/ajr.176.2.1760289. [DOI] [PubMed] [Google Scholar]
- 16.Pearce MS, Salotti JA, Little MP, et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet. 2012 Aug 4;380(9840):499–505. doi: 10.1016/S0140-6736(12)60815-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marcin JP, Glaser N, Barnett P, et al. Factors associated with adverse outcomes in children with diabetic ketoacidosis-related cerebral edema. J Pediatr. 2002 Dec;141(6):793–797. doi: 10.1067/mpd.2002.128888. [DOI] [PubMed] [Google Scholar]
- 18.Edge JA, Ford-Adams ME, Dunger DB. Causes of death in children with insulin dependent diabetes 1990–96. Arch Dis Child. 1999 Oct;81(4):318–323. doi: 10.1136/adc.81.4.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolfsdorf J, Craig ME, Daneman D, et al. Diabetic ketoacidosis in children and adolescents with diabetes. Pediatr Diabetes. 2009 Sep;10(Suppl 12):118–133. doi: 10.1111/j.1399-5448.2009.00569.x. [DOI] [PubMed] [Google Scholar]
- 20.Swift PGF, editor. ISPAD (International Society for Pediatric and Adolescent Diabetes) consensus guidelines for the management of type 1 diabetes mellitus in children and adolescents. Zeist, Netherlands: Medforum; 2000. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.