Abstract
Lyme borreliosis (LB) is the most prevalent arthropod-borne infectious disease in North America and many countries of the temperate Northern Hemisphere. It is associated with local and systemic manifestations and has persistent post-treatment health complications in some individuals. Innate and acquired immunity-related inflammation is likely to play a critical role in both host defense against Borrelia burgdorferi and disease severity. Large-scale analytical approaches to quantify gene expression (transcriptomics), proteins (proteomics) and metabolites (metabolomics) in LB have recently emerged with a potential to advance the development of disease biomarkers in early, disseminated and posttreatment disease stages. These technologies may permit defining the disease stage and facilitate its early detection to improve diagnosis. They will also likely allow elucidating the underlying molecular pathways to aid in identifying molecular targets for therapy. This article reviews the findings within the field of omics relevant to LB and its prospective utility in developing an array of biomarkers that can be employed in LB diagnosis and detection particularly at the early disease stages.
Keywords: Biomarkers, Omics, Diagnosis, Inflammation, Innate immunity, Lyme disease
Introduction
Lyme disease—also known as Lyme borreliosis (LB)—can be caused in humans by at least three genospecies of the Borrelia burgdorferi sensu lato complex, B. burgdorferi, B. garinii and B. afzelii. In the USA and southern Canada, B. burgdorferi sensu stricto cause flu-like illness at early disease stages that can later develop to Lyme arthritis and other long-term complications [1]. LB is initiated by the bacterial infection following a bite from an infected Ixodes scapularis or Ixodes pacificus blacklegged tick. Presently, LB is the most common vector-borne disease in North America and Europe [1]. Over 30,000 cases are reported in the US annually [2]. However, actual prevalence estimates are thought to be at least ten times as high because of underreporting [3]. In Canada, an increased incidence of LB by ~six-fold—from 128 to 707 cases—was noted between 2009 and 2015 [4].
Symptoms of early LB (stage 1) usually begin 1–2 weeks after a tick bite with a proportion of patients developing the characteristic erythema migrans (EM) rash that can last 4 weeks or longer and may be accompanied by fatigue, malaise, fever, chills, myalgia and headache. If untreated, bacteria may then disseminate systemically via the lymphatic system or blood to the joints, nervous system and cardiovascular system. Symptoms of early disseminated LB (stage 2) may occur weeks to months after the tick bite and may include numbness, Bell’s palsy, palpitations, chest pain or shortness of breath. Approximately 6 months after infection, patients may present with joint pain and swelling, and synovial fluid findings that suggest an inflammatory process. Months to years after the initial tick bite, LB can progress to the late disseminated stage (stage 3), which may result in substantial morbidity, primarily from chronic arthritis. Indeed, arthritis usually manifests during the late disease stage and occurs in up to 60% of untreated patients. Neurologic and cardiac involvements have been also described. Cardiac involvement usually occurs within 1 to 2 months after infection with Lyme carditis as a less common complication of the systemic LB disease [for review, see Ref. 5]. As the innate and adaptive immune responses develop following the infection, patients may recover during the early disease phase without antibiotic therapy. LB patients treated with antibiotics in the early stages do not develop detectable antibodies [6, 7]. Most patients who are not treated in early LB go on to suffer early disseminated LB with manifestation of neuroborreliosis (e.g., Bell’s palsy and meningitis), multiple EM lesions and, less commonly, myocarditis [8–10]. These stages and characteristics are based on the guidelines developed by the Infectious Diseases Society of America (IDSA) [9]. The IDSA LB guidelines have been delisted recently by the US National Guideline Clearinghouse (NGC) as they do not conform to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology endorsed by the Institute of Medicine (IOM). The presently listed LB guidelines by NGC are those of the International Lyme and Associated Diseases Society (ILADS) guidelines [11].
Inflammation, induced by either the spirochete or its antigens in the affected tissues, is thought to play a major role in LB pathogenesis at both the early and late disease stages [12, 13]. Early inflammatory responses distinguish patients from healthy controls and diverge from those of other diseases with overlapping clinical features [12]. The final outcome of infection, however, is dependent on the intricate interaction between the pathogen and the host immune response [12, 13]. Therefore, elucidating the extent of alteration in the host inflammatory and immunological pathways at the early stages of host-pathogen interaction may provide an insight into the potential mechanisms rendering B. burgdorferi-infected subjects susceptible to disseminated LB and, perhaps, the later development of post-treatment Lyme disease syndrome (PTLDS). It may also facilitate characterizing an array of biomarkers for various disease stages that can serve as targets for new diagnostic techniques and assist in development of therapies [13].
Early LB is usually diagnosed by the recognition of an EM skin lesion as detectable antibodies are not present at the very early disease stage in many patients [14]. However, other skin lesions can be confused with EM, e.g., southern tick-associated rash illness, tick-bite hypersensitivity reactions and some cutaneous fungal infections [6, 15, 16]. Several laboratory-based molecular and immunologic approaches for detection of B. burgdorferi sensu lato and diagnosis of LB have been developed over the past 3 decades [17]. These included tests for direct detection of the spirochete, the detection of specific antibodies using whole-cell lysates, recombinant antigens or peptide antigens in enzyme immunoassays (EIA), or nucleic acid amplification from peripheral blood samples [for review, see 17]. At early disease stages, detection of B. burgdorferi antibodies or using PCR-based approaches in peripheral blood samples were proved unsatisfactory [17]. Currently, an antibody-based diagnostic method is widely utilized in clinical practice, and a two-tier approach for serologic testing—using EIA followed by immunoblotting for IgM and IgG—is recommended [18]. The approach is based on antibody detection and is highly specific and sensitive in patients with late manifestations of LB but exhibits a moderate sensitivity (29%–40%) in those in early disease [14, 17]. Recent evidence, however, suggests that serological testing can be poor, even in LB patients who were culture-positive for B. burgdorferi [19]. The current status of LB serological testing emphasizes the need for more sophisticated approaches such as omics technologies at all disease stages.
These limitations, together with the possible misdiagnosis of EM lesions by clinicians, necessitate the development of an improved test for the detection of LB, particularly at the early disease stages. Non-antibody-based methodologies have been proposed as a novel approach for the detection of spirochetes or assessing the responses to the pathogen [17]. If these methods improve the established diagnostic tests by having higher specificity and sensitivity, they will enhance patient management and may obviate repeated testing and help alleviate controversies and subjectivities over LB diagnosis [14].
Driven by marked improvement in analytical platforms, increasing resolution and sensitivity, high-throughput capabilities and reduced cost, the use of omics approaches has grown exponentially in recent years [20]. Omics methodologies have allowed elucidating mechanisms of pathogenesis for numerous disease-causing agents and facilitated discovery of disease biomarkers (biosignature) and response to prevention or therapy [20–24]. It has the potential to assess the effects of a particular factor on many molecules including thousands of mRNAs, proteins, metabolites, imprinting of genes, alternative splicing of mRNAs and mutations [22]. The present article provides a comprehensive evaluation and review of the omics technologies employed to study biomarkers and biosignatures of early LB stages in human. The contributions of the individual omics analytical platform to understanding disease etiology is presented, with a goal to provide a background on their respective abilities in identifying a panel of inflammatory mediators as biomarkers for early disease detection and diagnosis.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by the author.
Inflammation in the Early Stages of Lyme Disease
Following exposure to foreign microbial, chemical or physical agents, the first line of host defense is the activation of the innate immune response, which results in inflammatory reactions to mediate damage repair, isolate or eliminate the infectious factor and re-establish homeostasis [25, 26]. The initiation of innate immunity-related inflammatory reactions relies on the pattern recognition receptors (PRRs) such as the Toll-like receptors (TLRs). TLRs are type I transmembrane proteins that have an extracellular domain containing leucine-rich repeats (LRRs) and a cytoplasmic tail with a conserved Toll/interleukin-1 (IL-1) receptor (TIR) domain [27]. Additional pathogen recognition occurs by nucleotide oligomerization domain (NOD)-like receptors (NLRs) and C-type lectin (CTL) receptors (CLR). TLRs recognize structurally conserved pathogen-associated molecular patterns (PAMPs) [28–30] and trigger a downstream signaling cascade that activates the transcription factor NF-κB. Activation of NF-κB elicits stimulation of cytokine synthesis, upregulation of adhesion molecule expression and generation of reactive oxygen species [30–33].
Proinflammatory cytokines, such as TNF-α, IL-6, IL-8 and IL-1β, are produced predominantly by activated macrophages and are involved in the upregulation of inflammatory reactions. Early stages of LB infection are linked to the synthesis of several of these monocyte-derived cytokines that play a critical role in disease pathogenesis [34–38, see below]. Proinflammatory cytokines activate phagocytes to recognize and eliminate pathogens and facilitate attracting other immune cells to the site of infection. Furthermore, these cytokines induce T cell polarization leading to production of IFN-γ by Th1 lymphocytes and IL-17 by Th17 cells [39]. During early B. burgdorferi infection, IL-1β is produced in high concentrations by monocytes/macrophages [40–42], a synthesis that is triggered primarily by the peptidoglycan molecules of the bacterial cell wall [43]. Levels of IL-1β were higher in synovial fluid and tissue of patients with post-treatment Lyme arthritis compared to their counterparts who recovered after the antibiotic treatment [42]. Although the role of IL-1β is yet to be fully understood and is controversial—together with other cytokines—at post infection and PTLDS [11], it was thought to be related to the induction of a IL-17/Th17 response against the spirochetes and the subsequent synthesis of IL-22 [40]. Thus, IL-17/Th17 response augments the immune activation upon microbial recognition [40] with IL-1β controlling the production of IL-17A, IL-17F, IL-17AF, IL-21, IL-22 and IL-26. These products of Th17, particularly IL-22, are critical factors in the development of the Borrelia antigen-induced arthritis in animal models [44, 45]. IL-1β blockade was, therefore, associated with a disrupted Th17 response and IL-17 levels [35]. IL-22 (and IFN-γ) was detected in the skin of individuals with EM [46], and IL-17 was found in higher levels in synovial cells from Lyme arthritis patients [47] and patients with neuroborreliosis [48] than subjects with earlier disease stages.
Antiinflammatory cytokines are immunoregulatory molecules that control the response to proinflammatory cytokines and play a critical physiologic role in the systemic inflammatory states. Major antiinflammatory cytokines include IL-1 receptor antagonist (IL-1Ra), IL-4, IL-5, IL-10, IL-11 and IL-13. Several studies from human and animal models demonstrated that Th2 (synthesis of the antiinflammatory IL-4, -5, -10 and -13) is more predominant than Th1 within the target organ following the exposure to Borrelia [38, 40, 49]. Indeed, human monocytes exposed to B. burgdorferi outer surface protein A (OspA) and the intact spirochetes synthesized high levels of IL-10 [50], which, in turn, inhibited the function of monocytes, macrophages and Th1 cells and reduced their migration through endothelial cells [51]. IL-12 and IL-18, which are secreted by antigen-presenting cells (APCs) to induce Th1, were also elevated in cerebrospinal fluid from patients with neuroborreliosis [52]. Studies in Borrelia-infected mice [53] and patients with neuroborreliosis [54] have shown that a rapid IFN-γ response provides a more beneficial outcome than a slower or no responses. However, this instantaneous response was associated with a subsequent IL-4 production [53, 54], indicating that a Th1 response, although critical for spirochetal eradication, can consequently contribute to tissue damage and persistent inflammation if unregulated.
Interaction of TLRs with B. burgdorferi Osps is critical in early stages of LB pathogenesis [1, 36, 55, 56] and was thought to mediate both short- and long-term disease outcomes [57–59]. A number of single-nucleotide polymorphisms (SNPs) in the TLR genes [13] and their downstream factors [60, 61] were recently proposed to modulate the host response to infection. These SNPs alter the TLR signaling patterns and may have an impact on the clinical manifestations of bacterial, fungal and viral infections [62]. For example, TLR1 Ile602Ser was linked to elevated proinflammatory cytokine levels and a more effective Th1-like response (i.e., the microbicidal action of IFN-γ) in LB patients [63] at early disease stages. TLR2 Arg753Gln polymorphism, however, provided protection against the development of late disease stage [64]. PBMCs with TLR1 Arg80Thr, Asn248Ser, and Ile602Ser and TLR6 Ser249Pro had a significantly lower synthesis of proinflammatory cytokines compared to their wild-type counterparts [65].
TLR1 T1805G (Ile602Ser), TLR2 G2258A (Arg753Gln) and TLR5 C1174T (Arg395Stop) were examined in patients with different LB symptoms including EM and antibiotic-responsive and refractory arthritis [63]. These SNPs were associated with decreasing numbers of plasma membrane TLRs (TLR1 T1805G and TLR2 G2258A) or with abrogation of the cellular flagellin signaling pathway (TLR5 C1174T) leading to an overall impairment of the TLR pathway and a disrupted state of cytokine synthesis [63]. Patients with antibiotic-refractory arthritis had ~two-fold higher frequency of TLR1 Ile602Ser (T1805G) compared to those with EM (OR = 1.9; p = 0.05) [63]. This status of antibiotic-refractory Lyme arthritis occurs when there is persistence of synovitis for at least 3 months after antibiotic treatment, despite expulsion of viable B. burgdorferi from the affected area [65]. Similarly, SNPs in TLR8 were proposed to lead to immunodeficiency syndromes and may be associated with an increased risk of severe clinical manifestations following B. burgdorferi infection [66, 67]. In contrast to the increased risk of Lyme arthritis associated with TLR1 Ile602Ser (T1805G), TLR2 Arg293Gln (A2258G) was shown to be protective [64]. One study demonstrated that the frequency of TLR2 Arg753Gln (A2258G) is lower in LB patients compared to matched controls (OR = 0.39, p = 0.03). In this study, patients with stage 3 LB (i.e., late persistent Lyme arthritis) had a further lower frequency of Arg753Gln (A2258G) compared to the matched controls (OR = 0.15, p = 0.003), suggesting a protective effect of TLR2 Arg293Gln in Lyme arthritis [64]. Other TLR gene polymorphisms such as TLR5 (Arg395Stop) and TLR6 (Ser249Pro) were identified to have a functional significance in host-pathogen interaction during both early and late LB stages [13, 63, 64, 68].
In general, after initial recognition of Borrelia by TLR2/TLR1 heterodimers, the first stage in the innate immunity-related inflammation is phagocytosis. This leads to a robust proinflammatory cytokine synthesis. TLRs, known to recognize nucleic acids (e.g., TLR7, 8 and 9), might also recognize Borrelia RNA or DNA. This would result in the production of a type I IFN signature, a process to which NLRs may contribute [39]. Production of various cytokines critical to the pathogenesis of LB, e.g., IL-1β, IFN-γ and IL-17, is subsequently induced. In particular, IL-1β was demonstrated to be associated with the acute and chronic inflammatory processes seen in LB [39].
Omics Biosignature in the Early Stages of Lyme Disease
Omics technologies permit examining the differences in DNA, RNA, proteins, metabolites and other molecules between and among species. These molecular profiles may vary with cell or tissue exposure to chemicals, drugs or pathological agents and thus have potential use in elucidating disease etiology, detection and potential preventive approaches. Omics assessments are often conducted in a high-throughput manner to produce large data sets on functional, structural and/or response-related alterations within a particular body compartment, e.g., cell, tissue or fluid. As previously stated, “these new methods have already facilitated significant advances in our understanding of the molecular responses to cell and tissue damage, and of perturbations in functional cellular systems” [69]. Furthermore, the integrated approach implemented in omics can enable a comprehensive delineation of the genetic control to cellular functions and responses to alterations.
The contributions of an individual omics platform to recognizing LB etiology and the potential of these techniques in identifying a panel of biomarkers for early disease detection and diagnosis present distinct challenges given the paucity of existing information. For example, in humans, no genome-wide association study has been conducted yet on LB with a small number of reports existing on other omics techniques. Highlighted below is, therefore, the available information from transcriptomics, metabolomics and inflammatomics studies specifically at the early disease stages.
Transcriptomics in Lyme Disease Patients
Transcriptomic analysis aims to describe and quantify RNA species such as mRNAs, non-coding RNAs and small RNAs and their variations in response to external stimuli or disease. Expression profiling by microarrays has been widely used to detect variations in the expression of many, but not all, transcribed genes under both normal and perturbed conditions. In an attempt to gain insights into the molecular basis of acute LB and the ensuing development of post-treatment symptoms, a recent longitudinal transcriptome study was conducted on LB patients enrolled at the time of diagnosis and followed at 3 weeks and 6 months post-antibiotic treatment [70]. At the time of diagnosis, the transcriptomes of LB patients revealed a total of 1235 differentially expressed genes compared to the matched controls. Among those, the expression of 37 genes was up- or downregulated above the significant threshold of two-fold. Three weeks following the completion of a standard course antibiotic treatment, 1060 genes were differentially expressed with only 17 above the 2-fold threshold [70].
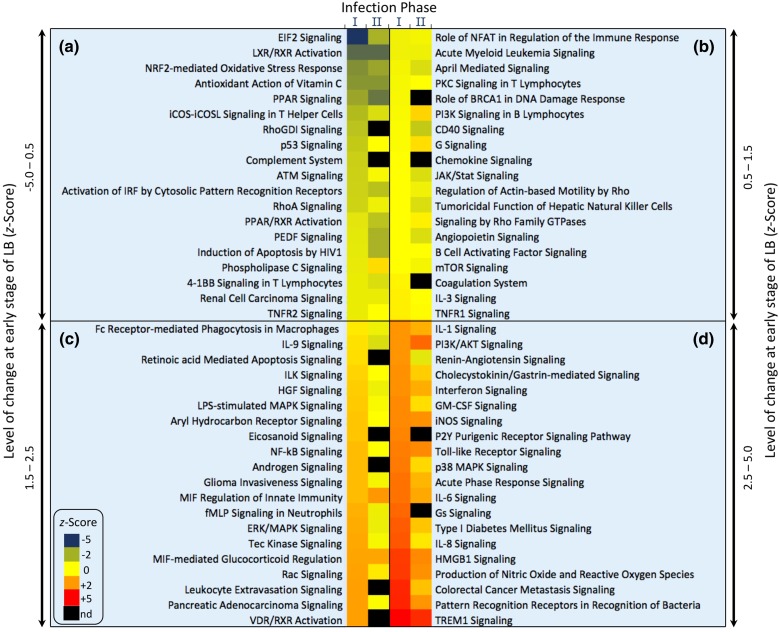
The differentially expressed genes at both the time of diagnosis (panel I, Fig. 1) and at 3 weeks following the completion of treatment (panel II, Fig. 1) were found to influence ~80 different pathways, the majority of which were linked to the innate immunity-related inflammation (Fig. 1). Analysis of the pathways modulated by these differentially expressed genes revealed activation of the inflammatory response, immune cell trafficking and hematologic system pathways. Of the ten most altered pathways, eight were directly related to the host immune response. Specifically, the eukaryotic initiation factor 2 (eIF2) signaling pathway was downregulated at diagnosis. eIF2 signaling plays a central role in modulating translation initiation and protein synthesis and elongation in response to cellular stress [71]. Functional disruption and downregulation of the eIF2 pathway was noted with a number of intracellular bacterial pathogens [72]. However, Borrelia spirochetes do not enter cells during infection or express eIF2 inhibitors [73]. Conversely, some evidence demonstrates that B. burgdorferi can invade various cell types in vitro [74]. Therefore, it is not known whether the downregulation of the eIF2 pathway in LB patients is caused by Borrelia-mediated immune dysregulation or is simply a host response to limit tissue injury [70]. Further studies are needed to assess whether eIF2 inhibitors may be potential targets for inflammatory responses in LB as proposed previously for other pathological disorders [72].
Fig. 1.
Heat map of pathways modified at the early stages of Lyme disease [70]. Pathways found to be up- or downregulated at Lyme disease diagnosis (stage I) and 3 weeks post-treatment with a standard course of antibiotics (stage II). Levels of change and the corresponding color scheme were extrapolated from the reported z-scores. Based on the level of change (z-scores) of stage I, the 78 modulated pathways were rearranged into four categories: z-score = −5.0 to 0.5 (panel a), 0.5–1.5 (panel b), 1.5–2.5 (panel c) and 2.5–5.0 (panel d). Data were inferred from the supplementary materials of the original study [70]
Transcriptional upregulation was prominent in TLR1, TLR2, TLR4, TLR7 and TLR8 during the early stages of LB, i.e., at diagnosis [70] together with a lack of activation of the inflammatory T cell apoptotic and B-cell developmental pathways [70]. This broad upregulation of the TLRs reflects a general increase in their regulatory activity rather than a direct association with B. burgdorferi proteins. In this respect, the most critical upstream regulators in LB at early stages were the proinflammatory (IFN-γ, IL-1β, and TNF-α) and antiinflammatory (IL-6, IL-10) cytokines together with NF-κB and the immunoglobulin complex [70]. TNF-α was the common upstream regulator of the TLR-signaling and the TREM1 (triggering receptor expressed on myeloid cells-1) pathway, an amplifier of the immune and inflammatory response [75]. Modulation of TREM1 impacts a number of inflammatory conditions, including septic shock and acute dengue virus infection [25, 26]. It is worth noting that only MIAT (myocardial infarction-associated transcript), CCDC163P (coiled-coil domain containing 163, pseudogene), ZNF266 (zinc finger protein 266) and GPR15 (G-protein coupled receptor 15) were found to be differentially expressed in patients with persistent LB symptoms compared to those with resolved disease [70].
Transcriptomics in Macrophages
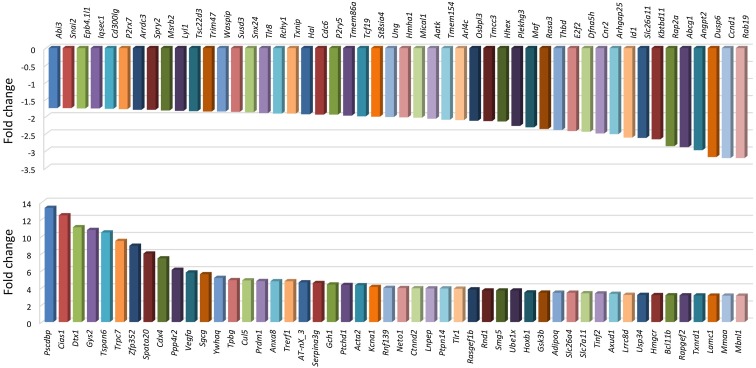
The transcriptomic findings in LB patients are supported by earlier studies from mouse J774 macrophages stimulated with live B. burgdorferi spirochetes [76]. Transcriptome profiling in these cells revealed that spirochetes had significantly upregulated the expression of 347 gene transcripts and downregulated ~700 others (with over a two-fold change). Among these genes, B. burgdorferi specifically altered the expression of an array of innate immunity- and inflammation-related genes to trigger the production of inflammatory mediators via recognition of TLRs (Fig. 2). Some of these genes include chemokine (C-X-C motif) ligand genes (e.g., Cxcl2 and Cxcl10), genes that encode monocyte-derived chemokines (e.g., Ccl2, Ccl5 and Ccl9), proinflammatory cytokine genes (e.g., Tnf and ILs) and TLR genes (TLR1 and TLR2) [66, 76, 85, 86]. Induction of effectors of the adaptive immune system, such as CD40 and CD86, which drive T-cell activation and proliferation, was also prominent [77] as well as IFN-α/IFN-ß-inducible genes and a number of downstream factors including NFκB and interleukins [76]. Overall, the transcriptomic biosignature of the differentially expressed genes and pathways was persistent during early stages of LB infection [70, 76]. This observation was demonstrated both in vivo [70] and in vitro [76], suggesting that a clinical diagnostic test for LB based on host gene expression can be a feasible approach for diagnosis of early disease stages. Furthermore, this approach can be employed during the period between infection and appearance of detectable antibody, a time window of a current diagnostic gap and subjectivity of clinical-based diagnosis [14, 17].
Fig. 2.
Differentially expressed gene transcripts in response to B. burgdorferi [76]. The selected ones are the top 50 downregulated (upper panel) or upregulated (lower panel) gene transcripts. Genes were considered to be differentially expressed when exhibiting ≥2-fold change, compared with unstimulated cells. Mouse macrophages were treated with live B. burgdorferi for 4 h. Data were extrapolated from the supplementary materials of the original study [76]
Metabolomics in Lyme Disease Patients
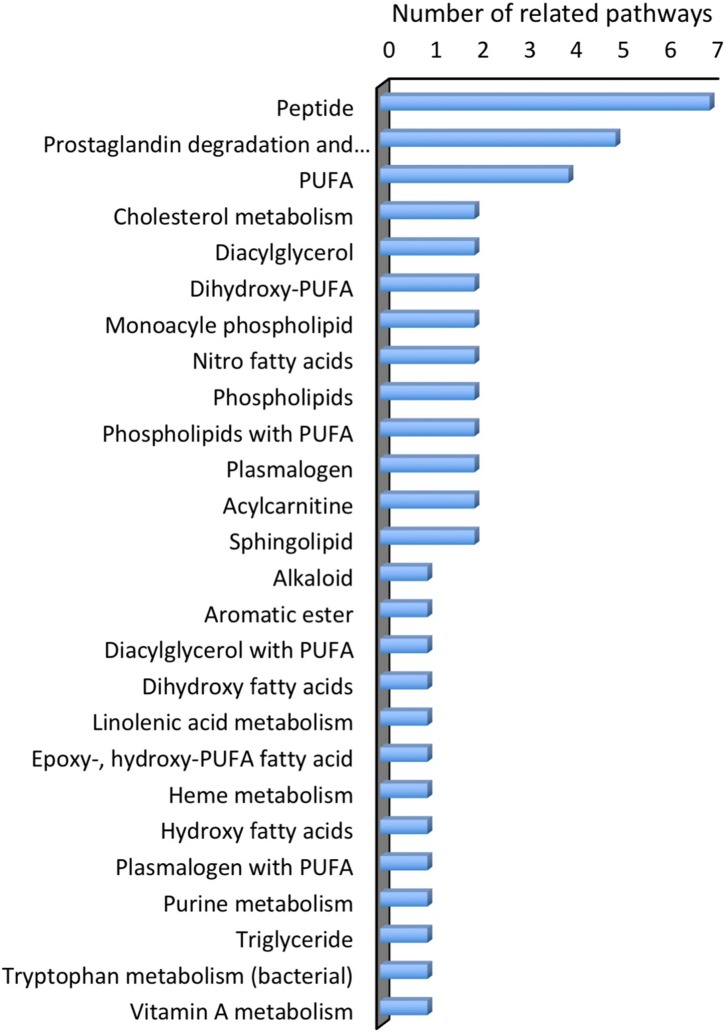
Metabolomics is the analysis of the whole metabolome (low molecular weight molecules) under a given set of physiological, environmental and/or clinical conditions [20, 21, 78]. To develop a metabolic biosignature that identifies LB patients at early disease stages and classifies them from non-patients, serum samples from patients and healthy controls were recently analyzed for small molecule metabolites [14]. The generation of a metabolic biosignature was based on the hypothesis that the inflammatory responses at the early disease stage is distinguished from that in healthy controls and of other conditions with similar clinical features [14]. Together with statistical modeling, proteomic analysis allowed for the initial chemical identification of 95 molecular features that resulted in 49 assigned putative chemical structures (Fig. 3). The identified metabolites included: 11 polyunsaturated fatty acids (PUFAs) or lipids with PUFAs, and related to these, 6 products of prostaglandin metabolism; 8 structures of fatty acid or cholesterol metabolism; sphingolipids; plasmalogens; products of tryptophan, purine and heme metabolism; an endogenous alkaloid and 7 peptides. This metabolic biosignature permitted distinguishing early LB patients from healthy controls with a sensitivity of 88% and a specificity of 95%. In this study, sera were collected from early LD patients and healthy controls. Other disease sera were also collected for metabolic biosignature comparison with LB from patients with infectious mononucleosis, fibromyalgia, severe periodontitis and syphilis. The study revealed a shift in the abundance of selected metabolites in patients with early LD as compared to healthy controls and patients diagnosed with other diseases [14]. The majority of the putatively identified metabolites in the early LB biosignature were lipid or lipophilic structures, suggesting that B. burgdorferi infection elicits alterations in markers of the inflammatory response as well as lipid mediators [14]. This inflammatory pathway is, however, related to prostaglandin synthesis and cyclooxygenase cascades [14] rather than innate immune-associated inflammation (Fig. 3). Since the host inflammatory responses initiated by B. burgdorferi lead to the clinical manifestations of this disease [79], the observed metabolic profile was proposed to reflect a host response that emerges rapidly following infection. In support, innate immunity-related inflammatory markers were significantly increased in LB patients at the pre-treatment stage compared to healthy controls with no inflammatory conditions and changes were associated with greater rates of lymphopenia, elevated liver enzymes and a higher number of disease symptoms although they had higher rates of seroconversion [12].
Fig. 3.
Molecular features assigned putative chemical structures for the metabolic biosignature of Lyme disease [14]. The molecular features were assigned according to the number of chemical pathways related to each molecular feature. Data were extrapolated from the supplementary materials of the original study [14]
The findings of the differentially expressed genes and pathways identified by transcriptomics in LB patients [70] (Fig. 1) were consistent with those described in vitro in mouse macrophage cells [76] (Fig. 2) and were further validated by the outcome of a number of metabolic analyses in both human and cell culture models [12, 14, 76, 80]. In mouse J774 macrophages stimulated with live B. burgdorferi, the inflammatory marker mRNA gene transcripts induced by spirochetes were examined at the protein level [76]. Genotype-phenotype matching was observed in these cells, as the 18 cytokines/chemokines that exhibited mRNA transcript upregulation resulted in increased levels of IL-1α, IL-1β, IFN-γ, CCL5 and IL-9 in stimulated macrophages compared to the unstimulated cells [76]. Furthermore, early response to live Borrelia spirochetes was examined in whole blood cells from 21 patients with different clinical outcomes of LB [80]. In asymptomatic seropositive LB affected subjects, an increased numbers of TNF-α-secreting dendritic cells and elevated levels of IL-12 were observed compared to seronegative controls or patients with PTLDS. The proinflammatory and antibacterial TNF-α and IL-12 are capable of inducing Th1 responses [81, 82], and their secretion in asymptomatic subjects supports their role in the early resolution of LB conditions. Other innate cytokines (e.g., IL-1β, IL-6, IL-8, IL-10) were also detectable early in Borrelia-stimulated whole blood cells [80]. It can be suggested, therefore, that the levels of serum chemokines and the expression of their respective genes may be informative biomarkers for early stages of LB that can also relate to specific disease manifestations.
Inflammatomics in Lyme Disease Patients
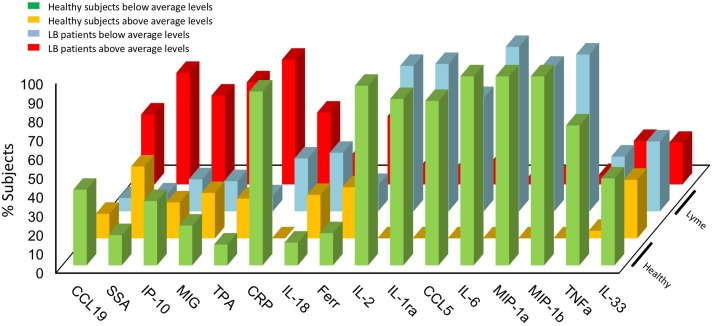
A recent study evaluated the levels of 58 immune mediators and 7 acute phase markers from sera of patients diagnosed with acute LB and matched controls [12]. Elevated levels of monocyte-derived chemokines (CCL19, CXCL9, CXCL10), acute phase inflammatory reactants such as CRP and serum amyloid A (SAA), several IL-1 cytokine family members (IL-1Ra, IL-18, IL-33), inflammatory cytokines (TNF-α and IL-6) and the T cell cytokine IL-2 were observed in patients with acute LB. In that study, the levels of CXCL9 and CXCL10 were coordinately increased in the LB patients, particularly in a subgroup displaying an overall elevated level of inflammatory markers (see below), and was associated with induced liver enzymes [12]. It is known that EM lesions, the primary site of inflammation and bacterial replication in early LB, express high levels of CXCL9 and CXCL10 [46, 83]. Taken together, this observation and the association between CXCL9/CXCL10 levels and lymphopenia both indicate that the infection-induced tissue inflammation and chemokine production stimulate the recruitment of activated effector T cells from the blood into the site of infection [12].
Close inspection of these findings indicated that a higher percentage of LB patients was found to have concentrations of inflammatory markers above the average levels compared to healthy controls (Fig. 4). On the other hand, an increased percentage of healthy subjects were noted to have levels of inflammatory markers below the average values compared to LB patients (Fig. 4). Patients with acute LB also exhibited upregulation of acute phase reactants such as CRP and SAA. CRP is a short pentraxin that acts as a fluid phase pattern recognition protein [84] whereas SAA is a serum lipoprotein that recognizes bacteria by interacting with the Osps [85]. Infection with B. burgdorferi apparently stimulates the coordinated production of CRP and SAA along with IL-6 [86, 87] and elevated serum liver enzymes during the acute stage of LB [12]. Other changes in cellular markers included decreased CD57 lymphocytes in patients with persistent LB [88] and increased C3a and C4a at 96 h following infection, i.e., during the acute disease stage [89]. Collectively, these cytokines and chemokines generate a novel signature that clearly distinguishes patients with acute LB from normal controls [12]. These observations were also noted in the mouse J774 macrophages stimulated with live B. burgdorferi spirochetes [76] and in whole blood cells from patients with various clinical outcomes of LB [80]. This analysis has permitted the description of a cytokine signature associated with early stages of infection and allowed for identification of two distinctive cytokine profiles of two subsets of patients who significantly diverged in symptom presentation. The two subgroups were either displaying elevated levels of cytokines and chemokines during the early disease stage or exhibiting levels of inflammatory mediators that cluster around those in normal controls [80]. This distinction may be relevant to the host’s response to B. burgdorferi infection and several PTLDS. Furthermore, the detection of a subgroup of LB patients who have low levels of immune mediators could represent a set of hyporesponsive subjects who can immunologically clear the infection with minimum inflammatory response [80].
Fig. 4.
Percentage of Lyme disease patients with modified levels of inflammatory markers compared to healthy controls [12]. Data were calculated as the percentage of subjects above or below the average level of the given inflammatory marker by determining the fold change in each Lyme disease patient (n = 44) and healthy controls (n = 23). Data were extrapolated by image analysis of the heat map presenting the levels of immune mediators in the original article [12]
Potential of Omics in Lyme Disease: Conclusion
The use of the omics approach permits the acquisition of large-scale data sets with the aim of identifying biomarkers or biosignature of a disease and/or elucidating functional or pathological mechanisms [20, 21]. This high throughput technology has been utilized recently in LB and facilitated the characterization of a distinctive disease biosignature, particularly at the early disease stages [12, 14, 70, 76]. The use of omics techniques together with targeted marker analysis have identified an array of gene transcripts and a number of secreted inflammatory mediators as candidates of a refined biosignature or biomarkers for the early recognition of LB [12, 14, 70, 76]. The low sensitivity of serologic testing in the early stages of LB is a consequence of the time it takes to develop a humoral immune response [90, 91]. In contrast, inflammation reflects the instantaneous response of the innate immune system to infection [12, 53, 54].
Omics studies facilitated the identification of a range of cytokines and chemokines along the innate immunity pathway for their role in the onset and resolution of LB [12]. Specifically, transcriptomic [70] and metabolomic [14] analyses have uncovered multiple previously undescribed pathways, genes, proteins and metabolic factors that may be utilized in the future as biomarkers for diagnosis and may constitute prospective targets for new therapies. Furthermore, analysis of the related chemokines and cytokines in LB patients [12] permitted identifying two subsets of patients with distinct disease phenotypes who differ in symptoms, liver involvement, lymphocyte levels and status of seroconversion. These changes are involved in disease pathogenesis and can be utilized to develop disease markers. When integrated, these findings may assist in developing specific immunotherapeutic approaches in relation to response to infection in addition to their potential in diagnosis. However, although levels of serum cytokines and chemokines may be informative biomarkers for early LB stages, some of these factors have a short serum half-life. In fact, recent evidence for the instability of certain inflammatory marker RNA species [92] may preclude the utility of these factors in disease early detection. However, reliable diagnostic testing using these biomarkers, particularly at early disease stage, can still be employed if an integrative approach is considered with a number of long-term genomic, proteomic and/or metabolomic biomarkers that can be characterized at various diseases stages.
Technical advances in microarray, gene expression analysis, mass spectrometry and bioinformatics offer an exciting prospect for future discovery of diagnostic and prognostic markers in LB disease. The substantial agreement between the information gathered from the transcriptomics, proteomics and metabolomics studies on the role of inflammatory mediators in the early stages of LB provides unprecedented opportunity to develop a panel of biomarkers for diagnosis, disease subtyping and response to therapy. However, a number of propositions are warranted for these prospects to advance, particularly toward using inflammatory markers as an LB diagnostic platform deployable into clinical settings. Larger studies with increased sampling resolution and various LB disease stages are needed, perhaps through a multinational collaborative effort that encompasses various strains of Borrelia species. This effort should be of a longitudinal nature to evaluate gene, protein and metabolite expressions and levels along the natural history of the disease. Moreover, functional studies are necessary to identify a specific set of inflammatory genes or mediators that can be employed in LB diagnosis. However, prior to such a biosignature characterization, stringent criteria should be introduced to ensure the most robust biomarkers are identified and utilized. Furthermore, variability in the assessment of disease biosignature should be eliminated or minimized, and establishing a system of suitability protocols is an essential step in the refinement and standardization of the analytical procedures before their application to a clinical setting. In parallel, multidisciplinary teams and collaborative efforts are necessary in view of the nature of an omics approach. Omics techniques include signal detection (microarray, mass spectra, etc.), preprocessing (subtraction of background, peak detection, analysis of expression, etc.), data normalization and identification of differentially expressed molecules (genes, peptides, metabolites, etc.) together with powerful statistical and computational techniques. All such competences need to be assembled and directed to provide large-scale discovery in the diagnosis of LB. Finally, a comprehensive set of post-analysis data is yet to be interrogated to facilitate a “one-stop” multidimensional biomarker discovery. Integration of different omics platforms into a single study population will allow a global systemic approach to elucidate the mechanisms of LB development and provide novel tools for diagnosis and prognosis.
Acknowledgements
This work and the article processing charges were funded by the Public Health Agency of Canada. The author meets the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, takes responsibility for the integrity of the work as a whole and has given final approval for the version to be published.
Disclosures
Alaa Badawi declares no conflict of interest.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by the author.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced content To view enhanced content for this article go to http://www.medengine.com/Redeem/5327F06058F9862F.
References
- 1.Radolf JD, Caimano MJ, Stevenson B, Hu LT. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat Rev Microbiol. 2012;10:87–99. doi: 10.1038/nrmicro2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Center for Disease Control and Prevention. How many people get Lyme disease? http://www.cdc.gov/lyme/stats/humancases.html (accessed, June 28, 2016).
- 3.Hinckley AF, Connally NP, Meek JI, Johnson BJ, Kemperman MM, Feldman KA, et al. Lyme disease testing by large commercial laboratories in the United. Clin Infect Dis. 2014;59:676–681. doi: 10.1093/cid/ciu397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Government of Canada. Lyme disease. http://www.healthycanadians.gc.ca (accessed: June 20, 2016).
- 5.Wright WF, Riedel DJ, Talwani R. Diagnosis and management of lyme disease. Am Fam Phys. 2012;85:1086–1093. [PubMed] [Google Scholar]
- 6.Wormser GP. Clinical practice. Early Lyme disease. N Engl J Med. 2006;354:2794–2801. doi: 10.1056/NEJMcp061181. [DOI] [PubMed] [Google Scholar]
- 7.Aucott J, Morrison C, Munoz B, Rowe PC, Schwarzwalder A, West SK. Diagnostic challenges of early Lyme disease: lessons from a community case series. BMC Infect Dis. 2009;9:79. doi: 10.1186/1471-2334-9-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention Three sudden cardiac deaths associated with Lyme carditis: United States, November 2012-July 2013. Morb Mortal Wkly Rep. 2013;62:993–996. [PMC free article] [PubMed] [Google Scholar]
- 9.Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, Klempner MS, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43:1089–1134. doi: 10.1086/508667. [DOI] [PubMed] [Google Scholar]
- 10.Ogden NH, Lindsay LR, Morshed M, Sockett PN, Artsob H. The emergence of Lyme disease in Canada. Can Med Assoc J. 2009;180:1221–1224. doi: 10.1503/cmaj.080148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cameron DJ, Johnson LB, Maloney EL. Evidence assessments and guideline recommendations in Lyme disease: the clinical management of known tick bites, erythema migrans rashes and persistent disease. Expert Rev Anti Infect Ther. 2014;12:1103–1135. doi: 10.1586/14787210.2014.940900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soloski MJ, Crowder LA, Lahey LJ, Wagner CA, Robinson WH, Aucott JN. Serum inflammatory mediators as markers of human Lyme disease activity. PLoS One. 2014;9:e93243. doi: 10.1371/journal.pone.0093243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rahman S, Shering M, Ogden NH, Lindsay R, Badawi A. Toll-like receptor cascade and gene polymorphism in host-pathogen interaction in Lyme disease. J Inflamm Res. 2016;9:91–102. doi: 10.2147/JIR.S104790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Molins CR, Ashton LV, Wormser GP, Hess AM, Delorey MJ, Mahapatra S, et al. Development of a metabolic biosignature for detection of early Lyme disease. Clin Infect Dis. 2015;60:1767–1775. doi: 10.1093/cid/civ185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stanek G, Wormser GP, Gray J, Strle F. Lyme borreliosis. Lancet. 2012;379:461–473. doi: 10.1016/S0140-6736(11)60103-7. [DOI] [PubMed] [Google Scholar]
- 16.Dandache P, Nadelman RB. Erythema migrans. Infect Dis Clin North Am. 2008;22:235–260. doi: 10.1016/j.idc.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 17.Aguero-Rosenfeld ME, Wang G, Schwartz I, Wormser GP. Diagnosis of Lyme borreliosis. Clin Microbiol Rev. 2005;18:484–509. doi: 10.1128/CMR.18.3.484-509.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. Morb Mortal Wkly Rep 1995; 44:590–1. [PubMed]
- 19.Weitzner E, McKenna D, Nowakowski J, Scavarda C, Dornbush R, Bittker S, et al. Long-term assessment of post-treatment symptoms in patients with culture-confirmed early Lyme disease. Clin Infect Dis. 2015;61:1800–1806. doi: 10.1093/cid/civ735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wheelock CE, Goss VM, Balgoma D, Nicholas B, Brandsma J, Skipp PJ, et al. Application of omics technologies to biomarker discovery in inflammatory lung diseases. Eur Resp J. 2013;42:802–825. doi: 10.1183/09031936.00078812. [DOI] [PubMed] [Google Scholar]
- 21.Dulin D, Lipfert J, Moolman MC, Dekker NH. Studying genomic processes at the single- molecule level: introducing the tools and applications. Nat Rev Genet. 2012;14:9–22. doi: 10.1038/nrg3316. [DOI] [PubMed] [Google Scholar]
- 22.Pielaat A, Barker GC, Hendriksen P, Peijnenburg A and Kuile BH. A foresight study on emerging technologies: State of the art of omics technologies and potential applications in food and feed safety. REPORT 1: Review on the state of art of omics technologies in risk assessment related to food and feed safety. EFSA supporting publication 2013: EN-495, 126 pp. (Available online: http://www.efsa.europa.eu/publications).
- 23.Vranova M, Halin C. Lymphatic vessels in inflammation. J Clin Cell Immunol. 2014;5:250. doi: 10.4172/2155-9899.1000250. [DOI] [Google Scholar]
- 24.Ferrer-Acosta Y, González M, Fernández M, Washington AV. Emerging roles for platelets in inflammation and disease. J Infect Dis Ther. 2014;2:149. doi: 10.4172/2332-0877.1000149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takeda K, Akira S. TLR signaling pathways. Semin Immunol. 2004;16:3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 26.Beutler B. Innate immunity: an overview. Mol Immunol. 2004;40:845–859. doi: 10.1016/j.molimm.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 27.Choe J, Kelker MS, Wilson IA. Crystal structure of human toll-like receptor 3 (TLR3) ectodomain. Science. 2005;309:581–585. doi: 10.1126/science.1115253. [DOI] [PubMed] [Google Scholar]
- 28.Albiger B, Dahlberg S, Henriques-Normark B, Normark S. Role of the innate immune system in host defence against bacterial infections: focus on the Toll-like receptors. J Intern Med. 2007;261:511–528. doi: 10.1111/j.1365-2796.2007.01821.x. [DOI] [PubMed] [Google Scholar]
- 29.Kumar S, Ingle H, Prasad DV, Kumar H. Recognition of bacterial infection by innate immune sensors. Crit Rev Microbiol. 2013;39:229–246. doi: 10.3109/1040841X.2012.706249. [DOI] [PubMed] [Google Scholar]
- 30.Wooten RM, Modur VR, McIntyre TM, Weis JJ. Borrelia burgdorferi outer membrane protein A induces nuclear translocation of nuclear factor-kappa B and inflammatory activation in human endothelial cells. J Immunol. 1996;157:4584–4590. [PubMed] [Google Scholar]
- 31.Ma Y, Seiler KP, Tai KF, Yang L, Woods M, Weis JJ. Outer surface lipoproteins of Borrelia burgdorferi stimulate nitric oxide production by the cytokine-inducible pathway. Infect Immun. 1994;62:3663–3671. doi: 10.1128/iai.62.9.3663-3671.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma Y, Weis JJ. Borrelia burgdorferi outer surface lipoproteins OspA and OspB possess B-cell mitogenic and cytokine-stimulatory properties. Infect Immun. 1993;61:3843–3853. doi: 10.1128/iai.61.9.3843-3853.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morrison TB, Weis JH, Weis JJ. Borrelia burgdorferi outer surface protein A (OspA) activates and primes human neutrophils. J Immunol. 1997;158:4838–4845. [PubMed] [Google Scholar]
- 34.Miller LC, Isa S, Vannier E, Georgilis K, Steere AC, Dinareloo CA. Live Borrelia burgdorferi preferentially activate interleukin-1 beta gene expression and protein synthesis over the interleukin-1 receptor antagonist. J Clin Invest. 1992;90:906–912. doi: 10.1172/JCI115966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oosting M, Van de Veerdonk FL, Kanneganti TD, Strum P, Verschueren I, Berende A, et al. Borrelia species induce inflammasome activation and IL-17 production through a caspase-1-dependent mechanism. Eur J Immunol. 2011;41:172–181. doi: 10.1002/eji.201040385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petzke MM, Brooks A, Krupna MA, Mordue D, Schwartz I. Recognition of Borrelia burgdorferi, the Lyme disease spirochete, by TLR7 and TLR9 induces a type I IFN response by human immune cells. J Immunol. 2009;183:5279–5292. doi: 10.4049/jimmunol.0901390. [DOI] [PubMed] [Google Scholar]
- 37.Porat R, Poutsiaka DD, Miller LC, Granowitz EV, Dinarello CA. Interleukin-1 (IL-1) receptor blockade reduces endotoxin and Borrelia burgdorferi stimulated IL-8 synthesis in human mononuclear cells. FASEB J. 1992;6:2482–2486. doi: 10.1096/fasebj.6.7.1532945. [DOI] [PubMed] [Google Scholar]
- 38.Salazar JC, Duhnam-Ems S, La Vake C, Cruz AR, Moore MW, Caimano MJ et al. Activation of human monocytes by live Borrelia burgdorferi generates TLR2-dependent and -independent responses which include induction of IFN-beta. PLoS Pathog 2009; 5. doi:10.1371/journal.ppat.1000444. [DOI] [PMC free article] [PubMed]
- 39.Oosting M, Buffen K, van der Meer JWM, Netea MG, Joosten LAB. Innate immunity networks during infection with Borrelia burgdorferi. Crit Rev Microbiol. 2016;42:233–244. doi: 10.3109/1040841X.2014.929563. [DOI] [PubMed] [Google Scholar]
- 40.Bachmann M, Horn K, Rudloff I, Goren I, Holdener M, Chrosten U, et al. Early production of IL-22 but not IL-17 by peripheral blood mononuclear cells exposed to live Borrelia burgdorferi: the role of monocytes and interleukin-1. PLoS Pathog 2010;6. doi:10.1371/journal.ppat.1001144. [DOI] [PMC free article] [PubMed]
- 41.Oosting M, Berende A, Sturm P, Ter Hofstede HJ, de Jong DJ, Kanneganti TD, et al. Recognition of Borrelia burgdorferi by NOD2 is central for the induction of an inflammatory reaction. J Infect Dis. 2010;201:1849–1858. doi: 10.1086/652871. [DOI] [PubMed] [Google Scholar]
- 42.Shin JJ, Glickstein LJ, Steere AC. High levels of inflammatory chemokines and cytokines in joint fluid and synovial tissue throughout the course of antibiotic-refractory Lyme arthritis. Arthritis Rheum. 2007;56:1325–1335. doi: 10.1002/art.22441. [DOI] [PubMed] [Google Scholar]
- 43.Beck G, Benach JL, Habicht GS. Isolation, preliminary chemical characterization, and biological activity of Borrelia burgdorferi peptidoglycan. Biochem Biophys Res Commun. 1990;167:89–95. doi: 10.1016/0006-291X(90)91734-A. [DOI] [PubMed] [Google Scholar]
- 44.Burchill MA, Nardelli DT, England DM, DeCoster DJ, Christopherson JA, Callister SM, et al. Inhibition of interleukin-17 prevents the development of arthritis in vaccinated mice challenged with Borrelia burgdorferi. Infect Immun. 2003;71:3437–3442. doi: 10.1128/IAI.71.6.3437-3442.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuo J, Nardelli DT, Warner TF, Callister SM, Schell RF. Interleukin-35 enhances Lyme arthritis in Borrelia-vaccinated and -infected mice. Clin Vaccine Immunol. 2011;18:1125–1132. doi: 10.1128/CVI.00052-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jones KL, Muellegger RR, Means TK, Lee M, Glickstein LJ, Damle N, et al. Higher mRNA levels of chemokines and cytokines associated with macrophage activation in erythema migrans skin lesions in patients from the United States than in patients from Austria with Lyme borreliosis. Clin Infect Dis. 2008;46:85–92. doi: 10.1086/524022. [DOI] [PubMed] [Google Scholar]
- 47.Codolo G, Amedei A, Steere AC, Papinutto E, Cappon A, Polenghi A, et al. Borrelia burgdorferi NapA-driven Th17 cell inflammation in lyme arthritis. Arthritis Rheum. 2008;58:3609–3617. doi: 10.1002/art.23972. [DOI] [PubMed] [Google Scholar]
- 48.Henningsson AJ, Tjernberg I, Malmvall BE, Forsberg P, Ernerudh J. Indications of Th1 and Th17 responses in cerebrospinal fluid from patients with Lyme neuroborreliosis: a large retrospective study. J Neuroinflammation. 2011;8:36. doi: 10.1186/1742-2094-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ekerfelt C, Ernerudh J, Bunikis J, Vrethem M, Aagesen J, Roberg M, et al. Compartmentalization of antigen specific cytokine responses to the central nervous system in CNS borreliosis: secretion of IFN-γ predominates over IL-4 secretion in response to outer surface proteins of Lyme disease Borrelia spirochetes. J Neuroimmunol. 1997;79:155–162. doi: 10.1016/S0165-5728(97)00118-5. [DOI] [PubMed] [Google Scholar]
- 50.Giambartolomei GH, Dennis VA, Lasater BL, Philipp MT. Induction of pro- and anti-inflammatory cytokines by Borrelia burgdorferi lipoproteins in monocytes is mediated by CD14. Infect Immun. 1990;67:140–147. doi: 10.1128/iai.67.1.140-147.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lisinski TJ, Furie MB. Interleukin-10 inhibits proinflammatory activation of endothelium in response to Borrelia burgdorferi or lipopolysaccharide but not interleukin-1beta or tumor necrosis factor alpha. J Leukoc Biol. 2002;72:503–511. [PubMed] [Google Scholar]
- 52.Grusell M, Widhe M, Ekerfelt C. Increased expression of the Th1-inducing cytokines interleukin-12 and interleukin-18 in cerebrospinal fluid but not in sera from patients with Lyme neuroborreliosis. J Neuroimmunol. 2002;131:173–178. doi: 10.1016/S0165-5728(02)00255-2. [DOI] [PubMed] [Google Scholar]
- 53.Kang I, Barthold SW, Persing DH, Bockenstedt LK. T-helpercell cytokines in the early evolution of murine Lyme arthritis. Infect Immun. 1997;65:3107–3111. doi: 10.1128/iai.65.8.3107-3111.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Widhe M, Jarefors S, Ekerfelt C, Vrethem M, Bergstrom S, Forsberg P, et al. Borrelia-specific interferon-gamma and interleukin-4 secretion in cerebrospinal fluid and blood during Lyme borreliosis in humans: association with clinical outcome. J Infect Dis. 2004;189:1881–1891. doi: 10.1086/382893. [DOI] [PubMed] [Google Scholar]
- 55.Berende A, Oosting M, Kullberg BJ, Netea MG, Joosten LA. Activation of innate host defense mechanisms by Borrelia. Eur Cytokine Netw. 2010;21:7–18. doi: 10.1684/ecn.2009.0179. [DOI] [PubMed] [Google Scholar]
- 56.Bernardino AL, Myers TA, Alvarez X, Hasegawa A, Philipp MT. Toll-like receptors: insights into their possible role in the pathogenesis of Lyme neuroborreliosis. Infect Immun. 2008;76:4385–4395. doi: 10.1128/IAI.00394-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Batsford S, Dunn J, Mihatsch M. Outer surface lipoproteins of Borrelia burgdorferi vary in their ability to induce experimental joint injury. Arthritis Rheum. 2004;50:2360–2369. doi: 10.1002/art.20337. [DOI] [PubMed] [Google Scholar]
- 58.DuChateau BK, Munson EL, England DM, Lovrich SD, Callister SM, Jensen JR, et al. Macrophages interact with enriched populations of distinct T lymphocyte subsets for the induction of severe destructive Lyme arthritis. J Leukoc Biol. 1999;65:162–170. doi: 10.1002/jlb.65.2.162. [DOI] [PubMed] [Google Scholar]
- 59.Brigl M, Bry L, Kent SC, Gumperz JE, Brenner MB. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat Immunol. 2003;4:1230–1237. doi: 10.1038/ni1002. [DOI] [PubMed] [Google Scholar]
- 60.Gautam JK, Comeau LD, Krueger JK, Smith MF. Structural and functional evidence for the role of the TLR2 DD loop in TLR1/TLR2 heterodimerization and signaling. J Biol Chem. 2006;281:30132–30142. doi: 10.1074/jbc.M602057200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hawn TR, Misch E, Dunstan SJ, Thwaites GE, Lan NT, Quy HT, et al. A common human TLR1 polymorphism regulates the innate immune response to lipopeptides. Eur J Immunol. 2007;37:2280–2289. doi: 10.1002/eji.200737034. [DOI] [PubMed] [Google Scholar]
- 62.Frazão JB, Errante PR, Condino-Neto A. Toll-like receptors’ pathway disturbances are associated with increased susceptibility to infections in humans. Arch Immunol Ther Exp (Warsz) 2013;61:427–443. doi: 10.1007/s00005-013-0243-0. [DOI] [PubMed] [Google Scholar]
- 63.Strle K, Shin JJ, Glickstein LJ, Steere AC. Association of a Toll-like receptor 1 polymorphism with heightened Th1 inflammatory responses and antibiotic-refractory Lyme arthritis. Arthritis Rheum. 2012;64:1497–1507. doi: 10.1002/art.34383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schröder NW, Diterich I, Zinke A, Eckert J, Draing C, von Baehr V, et al. Heterozygous Arg753Gln polymorphism of human TLR-2 impairs immune activation by Borrelia burgdorferi and protects from late stage Lyme disease. J Immunol. 2005;175:2534–2540. doi: 10.4049/jimmunol.175.4.2534. [DOI] [PubMed] [Google Scholar]
- 65.Sellati TJ, Sahay B, Wormser GP. The toll of a TLR1 polymorphism in Lyme disease: a tale of mice and men. Arthritis Rheum. 2012;64:1311–1315. doi: 10.1002/art.34386. [DOI] [PubMed] [Google Scholar]
- 66.Lin YT, Verma A, Hodgkinson CP. Toll-like receptors and human diseases: lessons from single nucleotide polymorphisms. Curr Genom. 2012;13:633–645. doi: 10.2174/138920212803759712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cervantes JL, Hawley KL, Benjamin SJ, Weinerman B, Luu SM, Salazar JC. Phagosomal TLR signaling upon Borrelia burgdorferi infection. Front Cell Infect Microbiol. 2014;4:55. doi: 10.3389/fcimb.2014.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Oosting M, Ter Hofstede H, Sturm P, Adema GJ, Kullberg B-J, van der Meer JWM, et al. TLR1/TLR2 heterodimers play an important role in the recognition of Borrelia spirochetes. PLoS One. 2011;6:e25998. doi: 10.1371/journal.pone.0025998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aardema MJ, MacGregor JT. Toxicology and genetic toxicology in the new era of “toxicogenomics”: impact of “-omics” technologies. Mutat Res. 2002;499:13–25. doi: 10.1016/S0027-5107(01)00292-5. [DOI] [PubMed] [Google Scholar]
- 70.Bouquet J, Soloski MJ, Swei A, Cheadle C, Federman S, Billaud JN, et al. Longitudinal transcriptome analysis reveals a sustained differential gene expression signature in patients treated for acute Lyme disease. MBio. 2016;7:e00100–e00116. doi: 10.1128/mBio.00100-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Park SH, Moon Y. Integrated stress response-altered proinflammatory signals in mucosal immune-related cells. Immunopharmacol Immunotoxicol. 2013;35:205–214. doi: 10.3109/08923973.2012.742535. [DOI] [PubMed] [Google Scholar]
- 72.Fontana MF, Banga S, Barry KC, Shen X, Tan Y, Luo ZQ, et al. Secreted bacterial effectors that inhibit host protein synthesis are critical for induction of the innate immune response to virulent Legionella pneumophila. PLoS Pathog. 2011;7:e1001289. doi: 10.1371/journal.ppat.1001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Embers ME, Ramamoorthy R, Philipp MT. Survival strategies of Borrelia burgdorferi, the etiologic agent of Lyme disease. Microbes Infect. 2004;6:312–318. doi: 10.1016/j.micinf.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 74.Livengood JA, Gilmore RD., Jr Invasion of human neuronal and glial cells by an infectious strain of Borrelia burgdorferi. Microbes Infect. 2006;8:2832–2840. doi: 10.1016/j.micinf.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 75.Sharif O, Knapp S. From expression to signaling: roles of TREM-1 and TREM-2 in innate immunity and bacterial infection. Immunobiology. 2008;213:701–713. doi: 10.1016/j.imbio.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 76.Gautam A, Dixit S, Philipp MT, Singh SR, Morici LA, Kaushal D, et al. Interleukin-10 alters effector functions of multiple genes induced by Borrelia burgdorferi in macrophages to regulate Lyme disease inflammation. Infect Immun. 2011;79:4876–4892. doi: 10.1128/IAI.05451-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 78.Fiehn O. Combining genomics, metabolome analysis, and biochemical modelling to understand metabolic networks. Comp Funct Genom. 2001;2:155–168. doi: 10.1002/cfg.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schröder NW, Eckert J, Stübs G, Schumann RR. Immune responses induced by spirochetal outer membrane lipoproteins and glycolipids. Immunobiology. 2008;213:329–340. doi: 10.1016/j.imbio.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 80.Sjöwall J, Carlsson A, Vaarala O, Bergström S, Ernerudh J, Forsberg P, et al. Innate immune responses in Lyme borreliosis: enhanced tumour necrosis factor-α and interleukin-12 in asymptomatic individuals in response to live spirochetes. Clin Exper Immunol. 2005;141:89–98. doi: 10.1111/j.1365-2249.2005.02820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pasparakis M, Alexopoulou L, Douni E, Kollias G. Tumour necrosis factors in immune regulation: everything that’s interesting is…new! Cytokine Growth Factor Rev. 1996;7:223–229. doi: 10.1016/S1359-6101(96)00031-7. [DOI] [PubMed] [Google Scholar]
- 82.Trinchieri G, Pflanz S, Kastelein RA. The IL-12 family of heterodimeric cytokines. new players in the regulation of T cell responses. Immunity. 2003;19:641–644. doi: 10.1016/S1074-7613(03)00296-6. [DOI] [PubMed] [Google Scholar]
- 83.Salazar JC, Pope CD, Sellati TJ, Feder HM, Jr, Kiely TG, Dardick KR, et al. Coevolution of markers of innate and adaptive immunity in skin and peripheral blood of patients with erythema migrans. J Immunol. 2003;171:2660–2670. doi: 10.4049/jimmunol.171.5.2660. [DOI] [PubMed] [Google Scholar]
- 84.Bottazzi B, Doni A, Garlanda C, Mantovani A. An integrated view of humoral innate immunity: pentraxins as a paradigm. Annu Rev Immunol. 2010;28:157–183. doi: 10.1146/annurev-immunol-030409-101305. [DOI] [PubMed] [Google Scholar]
- 85.Shah C, Hari-Dass R, Raynes JG. Serum amyloid A is an innate immune opsonin for Gram-negative bacteria. Blood. 2006;108:1751–1757. doi: 10.1182/blood-2005-11-011932. [DOI] [PubMed] [Google Scholar]
- 86.Fujita T. Evolution of the lectin-complement pathway and its role in innate immunity. Nat Rev Immunol. 2002;2:346–353. doi: 10.1038/nri800. [DOI] [PubMed] [Google Scholar]
- 87.Bode JG, Albrecht U, Haussinger D, Heinrich PC, Schaper F. Hepatic acute phase proteins–regulation by IL-6- and IL-1-type cytokines involving STAT3 and its crosstalk with NF-kappaB-dependent signaling. Eur J Cell Biol. 2012;91:496–505. doi: 10.1016/j.ejcb.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 88.Stricker RB1, Winger EE. Decreased CD57 lymphocyte subset in patients with chronic Lyme disease. Immunol Lett. 2001;76:43–8. [DOI] [PubMed]
- 89.Shoemaker RC, Giclas PC, Crowder C, House D, Glovsky MM. Complement split products C3a and C4a are early markers of acute lyme disease in tick bite patients in the United States. Int Arch Allergy Immunol. 2008;146:255–261. doi: 10.1159/000116362. [DOI] [PubMed] [Google Scholar]
- 90.Dressler F, Whalen JA, Reinhardt BN, Steere AC. Western blotting in the serodiagnosis of Lyme disease. J Infect Dis. 1993;167:392–400. doi: 10.1093/infdis/167.2.392. [DOI] [PubMed] [Google Scholar]
- 91.Nowalk AJ, Gilmore RD, Jr, Carroll JA. Serologic proteome analysis of Borrelia burgdorferi membrane-associated proteins. Infect Immun. 2006;74:3864–3873. doi: 10.1128/IAI.00189-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Herjan T, Novotny M, Hamilton TA. Diversity in sequence-dependent control of GRO chemokine mRNA half-life. J Leukoc Biol. 2013;93:895–904. doi: 10.1189/jlb.0812370. [DOI] [PMC free article] [PubMed] [Google Scholar]