Abstract
Introduction
Psoriasis is a chronic inflammatory disorder with significant morbidity and mortality, but a persistent gap appears to exist for the adequate treatment of patients with moderate to severe disease. As the extent of under-treatment is unknown, we attempted to determine overall treatment patterns and estimate under-treatment using a large database.
Methods
Data from the US National Health and Wellness Survey was used to estimate the proportion of patients with mild, moderate or severe psoriasis. The proportion with moderate to severe disease was estimated by excluding those with mild disease, and projecting this to the total insured US population, weighted by age and gender. Using US health plan claims data, patient totals by treatment type were determined between October 1, 2007 and September 30, 2012. Patients had to be continuously enrolled in a health plan and be ≥18 years at the end of the analysis window. Psoriasis was confirmed if patients had at least one claim of any type of psoriasis except psoriatic arthropathy (ICD-9 code 696.1). A monthly treatment history, classified by biologic, traditional oral systemic, phototherapy and topical therapy, was recorded for each patient.
Results
There were an estimated 1.7 million insured US patients with moderate to severe psoriasis. Of these, 1 million (59%) were not treated for their condition in the preceding year. Among 695,488 patients who were treated for psoriasis in the preceding year, 346,201 were currently receiving treatment and 349,287 had lapsed treatment. Of the patients lapsed and currently treated in this period, the numbers who received each treatment type were 156,409 (biologic), 222,657 (traditional oral systemic), 22,911 (phototherapy), and 293,511 (topical). A limitation of the study was that only insurance claims were analyzed.
Conclusion
Moderate to severe psoriasis remains persistently untreated or under-treated. We suggest that potential barriers preventing access to care be explored.
Funding
This study was sponsored by Pfizer Inc.
Keywords: Biologics, Healthcare claims data, Phototherapy, Psoriasis, Real-world data, Topical therapy, Traditional oral systemics
Introduction
Psoriasis is a chronic inflammatory disorder with significant morbidity and mortality. It affected approximately 7.4 million US adults in 2013, with an estimated prevalence of 3.2% [1]. The financial burden of psoriasis is considerable, with an estimated annual cost to the US of $112 billion (2013 USD) [2]. The burden also extends to physical, psychological, and quality of life impairment [3].
Current treatment guidelines for psoriasis recommend topical therapies for mild disease, either as monotherapy or in combination with phototherapy, and traditional oral systemic agents (e.g., methotrexate), or biologic agents (e.g., anti-tumor necrosis factor inhibitors) for moderate to severe disease [4–8]. Phototherapy is also recommended as one of the therapies for moderate to severe psoriasis [9]. However, despite guidelines, inadequate treatment of patients, absence of treatment, or unsatisfactory disease control, remain key concerns for healthcare professionals [10]. Recent survey data suggested that a gap persists in the appropriate treatment of patients with moderate to severe psoriasis [11, 12].
Patients with psoriasis tend to cycle through multiple treatment options, which are often added, switched, re-started, or discontinued, but real-world data describing treatment patterns and treatment flow are scarce. In surveys by the National Psoriasis Foundation (NPF) involving 5604 patients between 2003 and 2011, the proportion of untreated patients was approximately one-third each in those with mild, moderate, or severe psoriasis, respectively [10]. Determining these patterns is important to the prescribing physician because it provides the foundation for understanding the treatment journey that patients undertake in accessing relief from this distressing condition.
Health claims databases provide an appropriate source of insight into the management of psoriasis as they capture information from healthcare professionals who are caring for individual insured patients, including records of diagnoses, prescriptions and procedures, dates of prescription fills and refills, treatment changes, and gaps where no prescription has been given. These databases are typically large and, therefore, representative of the regional or national population.
We used health plan claims data to determine overall treatment patterns and areas of under-treatment in insured US patients with moderate to severe plaque psoriasis over a 5-year period. In this study, we investigated whether patients with moderate to severe psoriasis are under-treated.
Methods
Classification of Disease Severity
Health claims data do not include information on severity; therefore, this information was obtained by estimating the proportion of mild psoriasis patients out of the total psoriasis population included in the US National Health and Wellness Survey [11]. The National Health and Wellness Survey is a large, international, patient-self-reported database of general health as well as disease-specific information, collected annually; the most recent sample includes information from the 2009 survey of 75,000 US patients [13]. To obtain the proportion of patients with moderate to severe psoriasis, patients with mild disease were excluded. Respondents in this survey self-reported their psoriasis severity having been asked the question: “According to the NPF, the palm of the hand equals 1 percent of the skin. Thinking about this, please estimate the percent of your body surface your psoriasis currently affects, 1 Mild (less than 3% body coverage), 2 Moderate (3–10% body coverage), 3 Severe (more than 10% body coverage).”
Filtering Process for Comorbidities
To correct for subjects with, and those treated for, related comorbidities (e.g., psoriatic arthritis), patients with certain concurrent inflammatory conditions were retained on a fair-share basis (e.g., half of patients with an additional inflammatory condition and one-third of the patients with two additional inflammatory conditions were retained).
Data Sources and Patient Population
With regard to the insured population, longitudinal US health plan claims data were obtained from IMS Health (LifeLink PharMetrics Plus™, Waltham, MA, USA) covering patients continuously enrolled in covered commercial healthcare insurance plans for the 5-year period from October 1, 2007 to September 30, 2012. To be eligible for inclusion, data were from patients aged 18 years or older at the end of this analysis window. All data obtained from this source were Health Insurance Portability and Accountability Act compliant and exempt from institutional review board approval.
Patients were considered to have psoriasis if they had at least one claim listing International Classification of Diseases, Ninth Revision (ICD-9) code 696.1 (“all types of psoriasis except psoriatic arthropathy”) during the 5-year analysis window. Patients were included if they had continuous longitudinal data available (i.e., had been continuously enrolled for 5 years in any of the covered plans and had gender and age data available). Patients with a gap in enrollment information were excluded.
Projection to the US Population
Based on the proportion of patients with psoriasis in the health plan claims database, we projected patient numbers to reflect the total insured US population [14] (Fig. 1). Collation of health-plan claims created a large sample that required weighting adjustments to be applied on the basis of age and gender to correct for bias and non-sampling [15].
Fig. 1.
Patients and projection to US population
Total disease load was calculated by extrapolating from the psoriasis population, diagnosed by ICD-9 code 696.1, to include undiagnosed patients, previously reported as 0.4% of the US population [16]. No confidence intervals were available from the latter source, and all estimates in this investigation were calculated without rounding to provide granular information.
Data Processing
All drugs and procedures of interest (Table 1) were processed to construct a treatment history for each patient. Definitions of terms are listed in Table 2. Standardized days of service (duration) were set for each prescription but not for procedures (Table 3).
Table 1.
Drugs and treatments of interest in the study
| Biologic | Traditional oral systemic | Topical |
|---|---|---|
| Licensed for psoriasis | Traditional oral systemics of interest | Phototherapy |
| Adalimumab | Acitretin | PUVA |
| Etanercept | Cyclosporine | UVB |
| Infliximab | Methotrexate | |
| Ustekinumab | Prednisone |
| Other biologics | Other traditional systemics | Other topical |
|---|---|---|
| Abatacept | Azathioprine | Various |
| Alefacept (Withdrawn) | Hydroxychloroquine | |
| Anakinra | Hydroxyurea | |
| Certolizumab pegol | Isotretinoin | |
| Efalizumab (Withdrawn) | Leflunomide | |
| Golimumab | Mycophenolate mofetil | |
| Rituximab | Sulfasalazine | |
| Tocilizumab | Thioguanine |
PUVA psoralen and ultraviolet A therapy; UVB ultraviolet type B therapy
Table 2.
Definition of terms
| Term | Definition |
|---|---|
| Naïve | A patient who did not have a treatment of interest at a given time point but had a claim with a psoriasis diagnosis (ICD-9 code 696.1) at some point during the analysis window |
| Days of service | Length of period of treatment or procedure awarded by the prescription (31 days if not recorded or <31 days). Procedures were assigned 31 or 61 days of service |
| Grace period | Additional 61 days added to days of service to account for poor compliance/adherence/concordance and to prevent very short switches out of and back into treatment |
| Lapsed | A patient who received a treatment of interest during the analysis window but was not on a treatment of interest at a given time point beyond the grace period |
| Lapsed biologic/systemic-experienced | Patients who had previously received a traditional biologic or systemic treatment but were only receiving topical treatment at a given time point beyond the grace period |
| Compliance | Adherence to treatment plan during the analysis window |
| Switch | Change in treatment status (i.e., starting, re-starting, or changing treatment) |
| Untreated | Not treated in the 5-year window with any of the drugs of interest |
ICD-9 International Classification of Diseases, Ninth Revision
Table 3.
Standardized duration of prescriptions (days of service)
| Prescription | Durationa (days) |
|---|---|
| Adalimumab | 31 |
| Alefacept | 84 |
| Cyclosporine | 31 |
| Efalizumab | 84 |
| Etanercept | 31 |
| Infliximab | 56 |
| Methotrexate | 31 |
| Phototherapy | 31 |
| Triamcinolone | 31 |
| Ustekinumabb | 84 |
aAll prescriptions were allocated a minimum duration of 31 days
bPrescriptions were set to 84 days due to the erratic nature of the prescriptions observed in the data
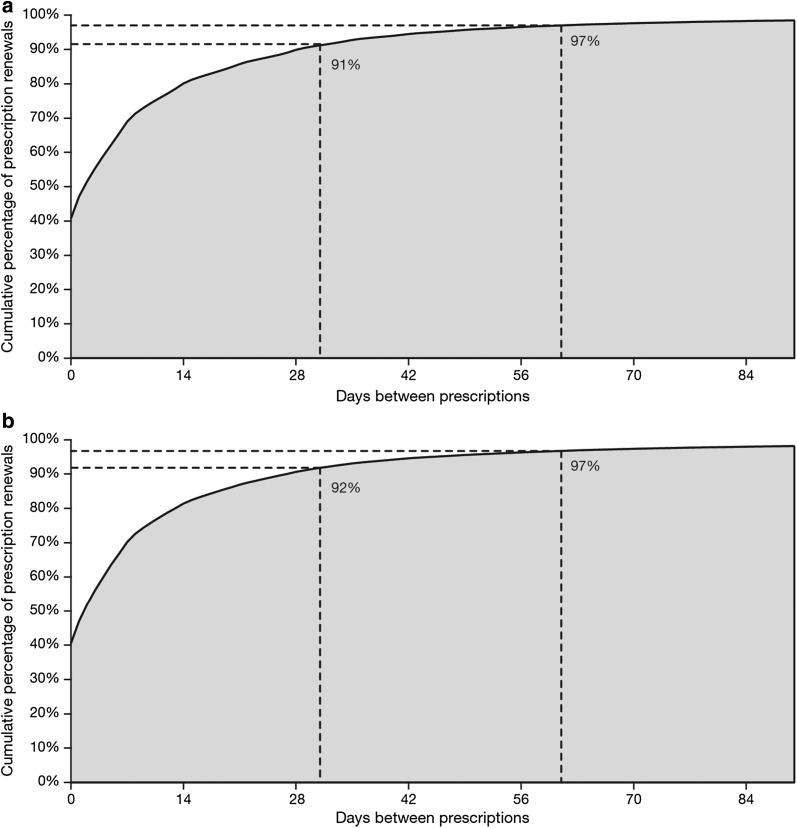
Given that most patients were not fully compliant with treatment, we considered both the duration of treatment and a grace period. Treatment was categorized by month, such that if the claim date (date the prescription was taken to the pharmacy or the procedure was administered) plus days of service (minimum 31 days) plus 61 days grace extended on to or beyond the 15th of a month, treatment was recorded for that month. The number of days grace (31 days) required to meet high levels of continuous use was similar across different treatment classifications (Fig. 2).
Fig. 2.
Number of days grace required to meet high levels of continuous use. Graphs show the cumulative percentages of a etanercept and b methotrexate prescription renewals (30-day prescriptions) over time (days since the end of the last prescription)
When a prescription/procedure began before the end of a previous prescription/procedure for the same drug, the newer prescription took precedence and the remainder of the old prescription was disregarded. If two or more biologic prescriptions were in the same month, the one with the most recent start date was retained and the other was excluded.
Treatment Categories
Treatments for all patients were identified on a monthly basis to permit the full treatment history to be assessed.
Treatments were classified in the mutually exclusive hierarchy of biologic, traditional oral systemic, phototherapy, topical, lapsed, or naïve, as defined in Table 2. A treatment group that was higher in the hierarchy could include concurrent treatment with a therapy in a lower group in the hierarchy (e.g., a biologic patient also receiving phototherapy and topical therapy). Any patients treated with a biologic at any time during our 5-year analysis were retained. Traditional oral systemic patients were categorized as those with or without biologic experience at a point in time (e.g., a patient taking a traditional oral systemic who had previously taken a biologic during the 5-year timeframe was categorized as a traditional oral systemic patient with biologic experience). The lapsed patient group was divided into those with previous biologic exposure, traditional oral systemic agent exposure, and any biologic or traditional oral systemic exposure.
Results
Patient Population
In the 5-year analysis window, health plan claims data identified 8,871,114 continuously enrolled patients, 923,073 (10.4%) of whom were diagnosed with psoriasis (ICD-9 code 696.1). Among these patients, 141,502 (15.3%) had continuous longitudinal data available and 46,369 (5.0%) met all of the inclusion criteria (Fig. 1). During the 5-year period, approximately 2.9 million prescriptions were recorded, with the most common being topical treatment (1.7 million prescriptions) and etanercept (0.4 million prescriptions), followed by methotrexate, adalimumab, and acitretin.
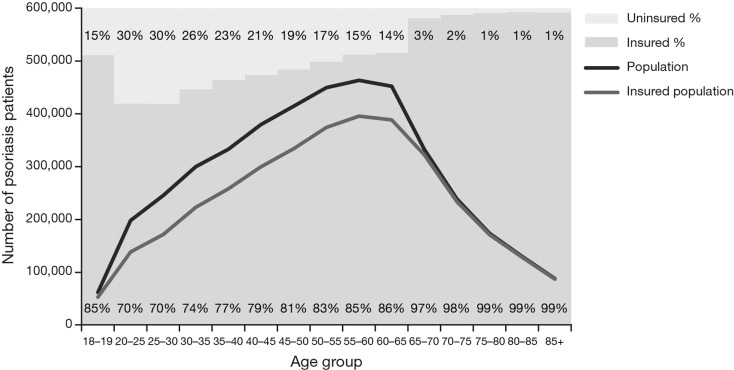
During the analysis period, the number of patients with psoriasis was highest in the sixth to seventh decade of life and then declined (Fig. 3). The number was higher in females than males, attributable to women below the age of 40 years; after 40 years of age, the numbers with psoriasis were similar in both genders. Also, as the population aged, a higher proportion of patients acquired medical insurance. At younger ages, more women than men were identified with psoriasis, although the difference narrowed up to age 60 years and then disappeared (Fig. 3).
Fig. 3.
Weighted number of patients with psoriasis (in 2011) by age category in the total population and insured US population. Weighting was by age and gender to the US insured population using US census data and numbers of people of each age and gender enrolled in the health plan database. Solid bars show the proportion of patients with and without insurance in each age category
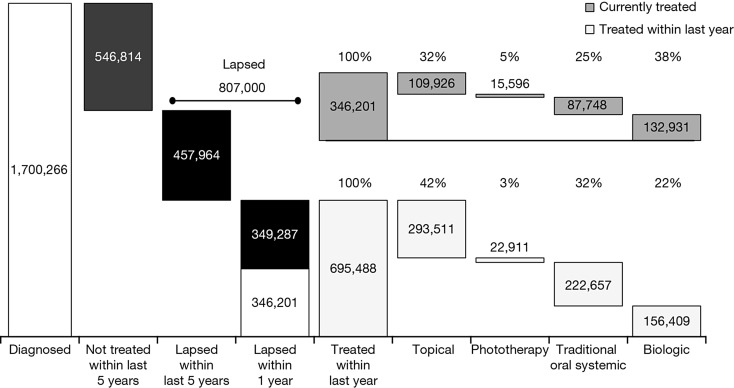
The eligible population of 46,369 was projected on to the US population with a resulting estimate of 5,957,740 patients with psoriasis. Assigning disease severity by treatment types, excluding patients with mild disease (4,065,721) and those prescribed treatment for a related inflammatory comorbidity (191,753), resulted in an estimated 1,700,266 insured patients with moderate to severe psoriasis in the 5-year analysis window (Fig. 4). Of these, 546,814 (32.2%) were diagnosed but not treated.
Fig. 4.
Projected insured US population diagnosed with moderate to severe psoriasis. Bars show proportions in each treatment category
In the year prior to September 2012, there were 346,201 (of the 695,488 treated patients) who were currently receiving treatment for psoriasis and another 349,287 who had lapsed treatment. Furthermore, of the patients treated (lapsed + currently treated) in the prior year, 156,409 (22.5%), 222,657 (32.0%), 22,911 (3.3%), and 293,511 (42.2%) received a biologic, traditional oral systemic, phototherapy, or a topical treatment, respectively (Fig. 4). Most (83%) of the 132,931 patients with psoriasis currently receiving a biologic were on biologic monotherapy, with 17% receiving a combination of a biologic and systemic therapy, which was consistent over the analysis period.
Longitudinal Data
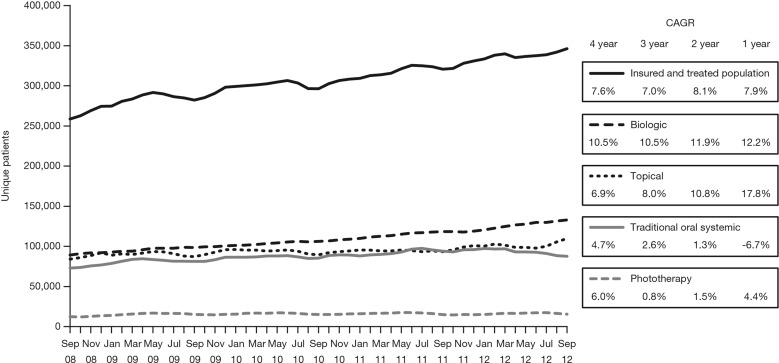
The available longitudinal data indicated an increase in the use of all treatment types from September 2008 to September 2012 with increases of 10.5, 4.7, 6.0, and 6.9% in biologic, traditional oral systemic, phototherapy, and topical treatments, respectively (Fig. 5).
Fig. 5.
Longitudinal data showing trends in the use of four treatment types for moderate to severe psoriasis from September 2008 to September 2012. CAGR compound annual growth rate. Patients receiving topical therapy who were exposed to traditional oral systemic treatment were classified as “lapsed”, as defined in Table 3
Treatment Dynamics
In the year prior to September 2012, an estimated 438,000 patients stopped their psoriasis treatment, another 384,000 re-started a psoriasis treatment, and 807,000 experienced a lapse in treatment (currently unpublished).
Discussion
Our analysis is one of the first systematic investigations to use health plan claims data to examine the number of patients with moderate to severe psoriasis and patterns of treatment in the US longitudinally over a 5-year period. This was achieved by identifying an eligible population of 46,369 insurance patients with moderate to severe disease and projecting this cohort to reflect the estimated 5.9 million insured US patients with psoriasis, of whom 1.7 million had moderate to severe disease. Although only around 1% of patients fulfilled all inclusion criteria, the resultant >46,000 patients represents a large patient population. The weighted estimate of 5.9 million represents 2.3% of the total US insured population (2012) of 206.2 million [17].
In the 5-year period, approximately one-quarter of patients (0.46 million) had received, but subsequently lapsed, from their treatment. The characteristics of the lapsed from treatment group have been investigated in a separate analysis (currently unpublished). One-third appeared to be untreated, as shown by the 0.55 million who were diagnosed but not treated—a proportion that is worthy of further investigation. Untreated patients never received any of the specified drugs of interest in the 5-year window.
The proportion who were untreated was comparable with that identified by the NPF biannual surveys between 2003 and 2011, although different methodology was used (i.e., claims data vs. survey questionnaires). Estimates were between 24% and 35% for untreated moderate psoriasis and between 9% and 30% for untreated severe psoriasis in the NPF surveys [10]. In contrast to results from the NPF surveys, phototherapy was the least frequently used category of treatment (NPF: 33% peak in 2014 vs. 8% in the preceding year in our study), although the two analyses were not directly comparable. This difference may be due to the limited visibility of phototherapy within the claims database. The most frequently used treatment in the present study was topical therapy alone (74%) compared with approximately one-third (30%) of NPF survey patients with moderate psoriasis and one-fifth (21%) with severe psoriasis [10]. The reason for the difference may be methodological or attributed to the one-off nature of topical therapy use.
Including patients treated within the preceding year and patients who were currently treated, biologics and traditional oral systemic therapies accounted for 60% (n = 289,340) and 55% (n = 310,405) of treatments, respectively. We observed that, although the use of biologics was growing, there remained a consistent proportion of patients on traditional oral systemic drugs. Overall, 60 different treatment types were recorded in the database, indicating the complexity and variability of patient disease loads.
When selecting a therapy, the clinician has to account not only for the benefit-risk profile of the prescribed medication but also patient preferences and payor procedures. Reimbursement impacts treatment decisions and the availability of oral and injectable alternatives is crucial to meet patients’ needs and insurance coverage. In a published study, patients preferred oral therapy to phototherapies in a structured interview setting [18]. Patients with longer disease duration attached greater importance to duration of benefit, whereas patients on oral therapy were more concerned with magnitude of benefit in a conjoint analysis study [19]. Treatment guidelines recommend topical therapy for mild disease, yet topical treatment was either currently being used in 32% of patients or had been used in the previous 12 months (42%) in our analysis of patients with moderate to severe disease, suggesting that patients were receiving inadequate therapy, based on the claims data.
An advantage of analyzing claims data is the ability to monitor the start, end, and re-start of a treatment as well as the switch from one treatment to another. The dynamics of treatment transitions have been investigated in a separate analysis (currently unpublished).
Health claims data track patients individually from clinics based on insurance coverage. Population estimates are relevant to the present analysis in that they are also individual-based. The proportion of moderate to severe patients with psoriasis may be higher in estimates of clinic samples than in the general population. We estimated the number of patients with moderate to severe psoriasis using claims data from a 5-year timeframe. In our investigation, there was no visibility of uninsured patients and treatments not associated with a claim. In the uninsured population, the burden may be particularly pronounced in the areas of non-treatment and under-treatment. Furthermore, we calculated total disease load by extrapolating from the number of patients diagnosed with psoriasis to include undiagnosed patients using a population-based approach [16]. We acknowledge that this did not align perfectly with the population we analyzed, but was the best source of data available on the undiagnosed population in the US.
We assumed that patients who were prescribed treatment actually went on to take the medication. Furthermore, we included grace periods to take into account discontinuous prescriptions. However, topical treatment and phototherapy may be underestimated in this analysis in cases when the use of both therapies lies outside the recordable scope of a claims database (e.g., the use of an over-the-counter topical treatment or subsequent use of phototherapy at home).
Although we chose the ICD-9 code 696.1, which encompassed all types of psoriasis except psoriatic arthropathy, there was still the potential for misclassification since the 696.1 code is not specific to plaque psoriasis. We excluded other 696 ICD-9 codes to minimize the level of similar, non-plaque psoriasis indications.
The true extent of the under-treatment among the psoriasis population is likely even greater than in this analysis, which examined only the insured population. In the current healthcare environment, the uninsured and the newly insured patients with psoriasis, who have yet to gain access to providers with expertise in treating psoriasis, comprise a large proportion of the overall psoriasis population. Therefore, in the US, the under-treatment of patients with moderate to severe psoriasis is unfortunately highly prevalent and severe.
Conclusion
In conclusion, this large health-plan-claims-based study showed that 40.9% of 1.7 million eligible US patients were receiving therapy for moderate to severe psoriasis in the 12 months up to September 2012. In that period, in approximately half (50.2%) of patients, treatment lapsed. Of those currently treated or treated within the last year, 42% had received topical therapy only. Our findings indicate that under-treatment of psoriasis is common, and, therefore, we suggest that the potential barriers preventing patients from accessing care, and the reasons for under-treatment, should be explored in future studies.
Acknowledgements
The study and article processing charges were funded by Pfizer Inc. Both Pfizer and non-Pfizer authors have participated in the study design, data collection, data analysis, and open scientific discussion of the data, its interpretation, and the development of the associated manuscript. Hernan Valdez and Gene Wallenstein, employees of Pfizer Inc, provided intellectual input during the manuscript development. The views and opinions expressed within the manuscript are those of all authors and do not necessarily represent those of the funding organization. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. AWA, JWK, SR, CM, HT, and MK were involved in the conception and design of the work. JWK and SR were involved in data acquisition and analyses. All authors were involved in data interpretation and manuscript drafting, reviewing, and development. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published. Medical writing support, under the direction of the authors, was provided by Neil Cockburn of Complete Medical Communications and was funded by Pfizer Inc. Joaquim Nascimento of Vanguard Strategy provided intellectual input during the manuscript development. Data from this work were presented at meetings of the American Academy of Dermatology-Winter 2015, Dermatology Nurses’ Association 2015, and National Psoriasis Foundation 2015.
Disclosures
AWA is a consultant for AbbVie, Amgen, Eli Lilly, Janssen, Merck, and Pfizer Inc. JWK was an employee of Vanguard Strategy, London, UK, at the time of the study, and was contracted to Pfizer Inc. SR was an employee of Vanguard Strategy, London, UK, at the time of the study, and was contracted to Pfizer Inc. CM is an employee and shareholder of Pfizer Inc. HT is an employee and shareholder of Pfizer Inc. MK is an employee and shareholder of Pfizer Inc.
Compliance with Ethics Guidelines
This was a retrospective study using Health Insurance Portability and Accountability Act compliant health-plan claims data, exempt from Institutional Review Board requirements.
Data Availability
The datasets during and/or analyzed in the current study are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced content
To view enhanced content for this article go to www.medengine.com/Redeem/F417F06020C5259F.
Contributor Information
Huaming Tan, Email: Huaming.Tan@pfizer.com.
Mandeep Kaur, Email: Mandeep.Kaur2@pfizer.com.
References
- 1.Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512–516. doi: 10.1016/j.jaad.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 2.Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: a systematic review. JAMA Dermatol. 2015;151:651–658. doi: 10.1001/jamadermatol.2014.3593. [DOI] [PubMed] [Google Scholar]
- 3.Augustin M, Radtke MA. Quality of life in psoriasis patients. Expert Rev Pharmacoecon Outcomes Res. 2014;14:559–568. doi: 10.1586/14737167.2014.914437. [DOI] [PubMed] [Google Scholar]
- 4.Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 1. Overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826–850. doi: 10.1016/j.jaad.2008.02.039. [DOI] [PubMed] [Google Scholar]
- 5.Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 4. Guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61:451–485. doi: 10.1016/j.jaad.2009.03.027. [DOI] [PubMed] [Google Scholar]
- 6.Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643–659. doi: 10.1016/j.jaad.2008.12.032. [DOI] [PubMed] [Google Scholar]
- 7.Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: Section 5. Guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. J Am Acad Dermatol. 2010;62:114–135. doi: 10.1016/j.jaad.2009.08.026. [DOI] [PubMed] [Google Scholar]
- 8.Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 6. Guidelines of care for the treatment of psoriasis and psoriatic arthritis: case-based presentations and evidence-based conclusions. J Am Acad Dermatol. 2011;65:137–174. doi: 10.1016/j.jaad.2010.11.055. [DOI] [PubMed] [Google Scholar]
- 9.Menter A, Augustin M, Signorovitch J, et al. The effect of adalimumab on reducing depression symptoms in patients with moderate to severe psoriasis: a randomized clinical trial. J Am Acad Dermatol. 2010;62:812–818. doi: 10.1016/j.jaad.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 10.Armstrong AW, Robertson AD, Wu J, Schupp C, Lebwohl MG. Undertreatment, treatment trends, and treatment dissatisfaction among patients with psoriasis and psoriatic arthritis in the United States: findings from the national psoriasis foundation surveys, 2003–2011. JAMA Dermatol. 2013;149:1180–1185. doi: 10.1001/jamadermatol.2013.5264. [DOI] [PubMed] [Google Scholar]
- 11.Kantar Health. National Health and Wellness Survey. http://stage.kantarhealth.com/docs/datasheets/Kantar_Health_NHWS_datasheet%20.pdf?sfvrsn=38. Accessed Mar 30 2015.
- 12.Kang B, Ayodele L. Immune and inflammatory disorders study: psoriasis August 2012. Burlington, MA, USA: Decision Resources; 2012. pp. 1–198. [Google Scholar]
- 13.Gupta S, Goren A, Phillips AL, Stewart M. Self-reported burden among caregivers of patients with multiple sclerosis. Int J MS Care. 2012;14:179–187. doi: 10.7224/1537-2073-14.4.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.United States Census Bureau. Health Insurance Historical Tables––HIB Series HIB-2. Health Insurance Coverage Status and Type of Coverage––All Persons by Age and Sex: 1999 to 2012. Abstract 2012.
- 15.Brick JM, Kalton G. Handling missing data in survey research. In: Armitage P, Berry G, editors. Statistical methods in medical research. Oxford: Blackwell Scientific Publications; 1996. pp. 215–238. [DOI] [PubMed] [Google Scholar]
- 16.Kurd SK, Gelfand JM. The prevalence of previously diagnosed and undiagnosed psoriasis in US adults: results from NHANES 2003-2004. J Am Acad Dermatol. 2009;60:218–224. doi: 10.1016/j.jaad.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeNavas-Walt C, Proctor BD, Smith JC. Income, poverty, and health insurance coverage in the United States: 2012. United States Census Bureau: Washington DC; 2013.
- 18.Opmeer BC, Heydendael VM, deBorgie CA, et al. Patients with moderate-to-severe plaque psoriasis preferred oral therapies to phototherapies: a preference assessment based on clinical scenarios with trade-off questions. J Clin Epidemiol. 2007;60:696–703. doi: 10.1016/j.jclinepi.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 19.Schaarschmidt ML, Umar N, Schmieder A, et al. Patient preferences for psoriasis treatments: impact of treatment experience. J Eur Acad Dermatol Venereol. 2013;27:187–198. doi: 10.1111/j.1468-3083.2011.04440.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets during and/or analyzed in the current study are available from the corresponding author on reasonable request.