Abstract
Background
Chronic increasing in arterial afterload may be an important trigger for left ventricular (LV) remodeling and dysfunction that lead to heart failure (HF). Racial differences in the predisposition to HF are well described, but the underlying mechanisms remain unclear.
Objective
We evaluated the racial differences in arterial elastance (Ea), which reflects the arterial afterload faced by the LV, and its associations with cardiac structure and function. We hypothesize that the LV in blacks displays heightened afterload sensitivity as compared to whites.
Methods
We studied 5727 community-based, elderly Atherosclerosis Risk in Community (ARIC) Study participants (22% black), who underwent echocardiography between 2011 and 2013.
Results
Blacks were younger (75 ± 5 vs 76 ± 5 years old), more frequently women (66 vs 57%), and had higher prevalence of obesity (46 vs 31%), hypertension (94 vs 80%), and diabetes mellitus (47 vs 34%) than whites. Adjusting for these baseline differences, Ea was higher among blacks (1.96 ± 0.01 vs 1.80 ± 0.01 mmHg/mL). In blacks, Ea was associated with greater LV remodeling (LV mass index, β = 3.21 ± 0.55 g/m2, p<0.001) and higher LV filling pressures (E/e′ ratio, β = 0.42 ± 0.11, p<0.001). These relationships were not observed in whites (LV mass, β = 0.16 ± 0.32 g/m2, p=0.61, p for interaction <0.001; E/e′ratio, β = −0.32 ± 0.06, p<0.001, p for interaction <0.001)..
Conclusion
These community-based data suggest that black Americans display heightened afterload sensitivity as a stimulus for LV structural and functional remodeling, which may contribute to their greater risk of HF, as compared to white Americans.
Keywords: hypertension, arterial elastance, left ventricle hypertrophy, cardiac remodeling, race
INTRODUCTION
Black Americans have the highest incidence of heart failure (HF) among racial groups in the United States. Although this has been in part attributed to race-related differences in socioeconomic status and prevalence of hypertension and diabetes,(1–3) reasons for the higher incidence of HF among blacks are largely unexplained. For instance, racial differences in the incidence of HF could result from differences in the incidence of coronary artery disease, but blacks still have higher incidence of HF after excluding cases preceded by a myocardial infarction (MI).(1)
The development of HF has been linked to abnormalities in ventricular-arterial coupling, which is represented by the ratio between arterial and ventricular elastance.(4) Arterial elastance (Ea) is a lumped parameter that incorporates mean and pulsatile components of the systemic vascular properties and reflects the total arterial afterload.(5) High arterial afterload may be a trigger for changes in left ventricle structure and function, which are predecessors of clinical HF.(6–9) Arterial afterload appears to be higher among blacks than whites, as a result of racial differences in arterial stiffness and intravascular volume.(10–12) In parallel, left ventricular hypertrophy is more common among blacks with hypertension.(13) However, it is uncertain whether this is out of proportion to arterial afterload.
Therefore, we assessed the racial differences in Ea and its associations with cardiac structure and function in a large biracial community-based study of an elderly population, among whom these differences are expected to be more noticeable as a consequence of the long-term cardiac effects of arterial afterload.
METHODS
Study population
The Atherosclerosis Risk in Communities (ARIC) Study is an ongoing, prospective observational study. The original cohort recruited 15,792 individuals from 45 to 64 years old in four communities in the United States (Forsyth County, NC; Jackson, MS; Minneapolis, MN; and Washington County, MD) between 1897 and 1989. After baseline evaluation, the participants underwent four subsequent follow-up visits. At the time of the Visit 5 – between 2011 and 2013 - 10,740 participants were alive, 6538 attended and 6118 had an echocardiogram performed. Population characteristics, sampling, design, procedures and detailed study rationale have been previously published.(14)
Race was self-reported by a questionnaire. Hypertension was defined as a systolic blood pressure (average between 2nd and 3rd measures) ≥140 mmHg, diastolic blood pressure (average between 2nd and 3rd measures) ≥90 mmHg or if taking anti-hypertensive medication in any of the ARIC visits. Diabetes mellitus was defined as present if a participant self-reported a physician diagnosis of diabetes, was taking medication for diabetes, had a fasting blood glucose level ≥126 mg/dL, or had a non-fasting blood glucose level ≥ 200 mg/dL in any of the ARIC visits. Prevalent HF at visit 5 was defined as an adjudicated HF hospitalization since 2005, HF hospitalization with International Classification of Diseases ninth revision (ICD-9) code 428x before 2005 or self-report of HF or treatment for HF with at subsequent confirmation of self-report by treating physician.(15)
From the 6118 participants, we excluded subjects with moderate or severe aortic valve disease (stenosis or insufficiency, n=78), atrial fibrillation at the time of echocardiography (n=267), those whose race was neither white nor black (n=6), and those with missing Ea (n=40) resulting in 5727 individuals for the present analysis. Institutional review boards from each center approved the study protocol and all participants provided written informed consent.
Echocardiography
All studies were obtained using a dedicated Philips IE33 Ultrasound system by trained sonographers according to a dedicated echocardiographic protocol.(16) All measurements were performed according to American Society of Echocardiography (ASE) guidelines in a dedicated echocardiography core laboratory.(17) Details about the design and protocol of the ARIC visit 5 echo study, including reproducibility data, have been previously published.(16)
LV dimensions and wall thickness were obtained from the parasternal long-axis view according to the recommendations of the ASE.(18) LV mass was determined by the linear method and all measurements were indexed to body surface area (BSA) when appropriate. LV mass was also indexed to height2.7, which may have some advantages over indexing to BSA in obese patients. (19) LV hypertrophy was defined as LV mass index (LV mass/BSA) >115g/m2 for men or >95 g/m2 for women.(18) LV outflow tract (LVOT) diameter was obtained from the parasternal long-axis view and stroke volume (SV) was calculated by the product of LVOT area and LVOT velocity-time integral.
We assessed LV diastolic function through pulse wave Doppler of mitral inflow (E, A velocities, E/A ratio) and peak septal and lateral mitral annular relaxation tissue Doppler velocities (e′). Left atrial (LA) volume was measured by the method of disks using apical 4- and 2-chamber views at an end-systolic frame preceding mitral valve opening and indexed to BSA (LA volume index).
Assessment of arterial function and ventricular-arterial coupling
Ea was calculated by end-systolic pressure (ESP) divided by SV.(20) Using the brachial blood pressure (BP) measurement at the time of echocardiographic examination, ESP was estimated by 0.9 x systolic BP.(7–9,20,21) We also estimated the steady and pulsatile components of arterial load.(22) Systemic vascular resistance index (SVRI) was estimated by mean arterial pressure multiplied by 80 and divided by cardiac index and total arterial compliance (TAC) by the ratio between SV and pulse pressure.(23,24) Carotid-femoral pulse wave velocity (cfPWV) was measured using a ColinVP-1000 plus system (Omron Co., Komaki, Japan).(25)
LV end-systolic elastance (Ees) was determined by the single beat method(21) using LVEF, brachial BP, SV, pre-ejection period, and total ejection period. The later two were obtained from pulse-wave Doppler of LVOT flow. In order to correct for the effects of chronic LV remodeling, we normalized Ees for the ratio of LV mass to volume as previously described.(26)
LV systolic function
LV volumes and the LV ejection fraction (LVEF) were assessed by the modified Simpson’s rule. Speckle tracking analysis was performed using TomTec Cardiac Performance Analysis package. Global longitudinal strain was obtained from apical 4-chamber and 2-chamber views. Peak systolic mitral annular velocity (s′) was also obtained by tissue Doppler imaging.
European ancestry among Blacks
Proportion of European ancestry was estimated in black participants using genotyping methods in the ARIC Study, described previously.(27) Briefly, genotyping was performed on stored DNA from visit 1 using the Illumina BeadLab platform at the Center for Inherited Disease Research (Johns Hopkins University, Baltimore).(28) Blind duplicate genotypes were performed, as part of a quality control program, and had a mismatch rate of 0.1%. Samples were eliminated from the analysis due to low call, sex mismatch, duplicity, excess of heterozygous genotypes or estimated proportion of European Ancestry higher than 0.85. ANCESTRYMAP software was used to estimate the proportion of European Ancestry for each individual. We classified black participants as below or above median European Ancestry.
Statistical analysis
We compared clinical differences between black and white participants using unpaired t test or Chi-square test, accordingly. We compared measures of cardiac structure and function between race groups, adjusting for potential confounders, including age, sex body mass index, use of anti-hypertensive medications, diabetes mellitus, heart rate and history of coronary heart disease (CHD) or HF. These covariates were selected based on a priori knowledge, as they are potential confounders for the associations with cardiac structure and function.(29,30) We included anti-hypertensive medication, rather than diagnosis of hypertension, to account for the drug effect on measures of arterial elastance. Because LV concentricity influences Ees, analysis of race-differences in Ees and Ea/Ees ratio were further adjusted by LV mass/end-diastolic volume. We also performed a separate analysis excluding body mass index from the model for measures of cardiac structure that were indexed by body surface area or height (Supplemental Material). We assessed the independent association between Ea and measures of cardiac structure and function with linear regression adjusting for same baseline clinical parameters. Then, we tested for effect modification by race, via an Ea-race interaction term, and for effect modification by European Ancestry among blacks, via an Ea-European Ancestry - treated as continuous variable - interaction term. To evaluate if the effect modification by European Ancestry among blacks was influenced by socioeconomic status, we further adjusted for low annual income in visit 4 (<$50,000) and educational level at visit 1 (low, mid-level or high, as described elsewhere) and presented as supplementary material.(31) Echo measures of cardiac structure and function for which there was a clear Ea-race interaction (p<0.001) were subsequently analyzed for curvilinear associations using adjusted restricted cubic spline models. For all other analyses, p-values<0.05 were considered to be statistically significant. There were no additional adjustments for multiple testing.
Finally, we performed two separate analysis: inverse probability weighting in order to correct for the possible effects of selective attrition,(32) and exclusion of individuals with a previous diagnosis of HF. Analyses were performed using Stata version 14 (Stata Corp, College Station, TX).
RESULTS
Study participants
The 5727 study participants were aged 66 to 90 years old, 59% women, and 22% were black. Compared to whites, black participants were more frequently women and had a higher prevalence of obesity (46 vs 31%, p < 0.001), and diabetes mellitus but lower prevalence of CHD. The prevalence of hypertension was very high in this elderly population, particularly among blacks, but the hypertension was defined using a very sensitive criteria (see methods section). Even so, blacks were more likely to use anti-hypertensive medication during visit 5 than whites. Also, blacks displayed higher systolic BP, diastolic BP, and heart rate during the echocardiogram (Table 1). The prevalence of HF was slightly higher among blacks.
Table 1.
Participants characteristics according to race
| Blacks n=1286 |
Whites n=4441 |
p value | |
|---|---|---|---|
| Age, y | 75.0 ± 4.9 | 76.1 ± 5.1 | < 0.001 |
| Women, n(%) | 854 (66) | 2523 (57) | < 0.001 |
| Height, cm | 165 ± 9 | 165 ± 10 | 0.96 |
| Weight, kg | 83 ± 18 | 77 ± 17 | < 0.001 |
| Body mass index, kg/m2 | 30 ± 7 | 28 ± 5 | < 0.001 |
| Body surface area, m2 | 1.90 ± 0.21 | 1.84 ± 0.22 | < 0.001 |
| Hypertension, n(%) | 1209 (94) | 3545 (80) | < 0.001 |
| Anti-hypertensive use, n(%) | 1117 (87) | 3127 (71) | < 0.001 |
| Diabetes mellitus, n(%) | 607 (47) | 1521 (34) | < 0.001 |
| Current smokers, n(%) | 86 (7) | 249 (6) | 0.21 |
| CHD, n(%) | 117 (9) | 680 (16) | < 0.001 |
| Heart failure, n(%) | 73 (6) | 160 (4) | < 0.001 |
| Systolic BP, mmHg | 135 ± 19 | 129 ± 17 | < 0.001 |
| Diastolic BP, mmHg | 70 ± 11 | 65 ± 10 | < 0.001 |
| Pulse pressure, mmHg | 65 ± 16 | 64 ± 14 | 0.07 |
| Heart rate, bpm | 64 ± 11 | 62 ± 10 | < 0.001 |
BP – blood pressure; CHD – coronary heart disease
Cardiac structure and function
Compared to whites, blacks presented slightly larger left ventricle volumes and lower LV mass, but similar prevalence of LV hypertrophy, after adjusting for age, sex, BMI, use of anti-hypertensive medication, diabetes mellitus, and presence of CHD or HF (Table 2). Blacks also exhibited lower measures of LV systolic function, including LVEF, longitudinal strain and s′ velocity.
Table 2.
Differences in cardiac function and structure between blacks and whites adjusted for clinical covariates
| Unadjusted | Adjusted* | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Black n=1286 |
White n=4441 |
p value | Black n=1257 |
White n=4233 |
p value | |
| Cardiac structure | ||||||
| EDVI, mL/m2 | 44.2 ± 0.3 | 43.9 ± 0.2 | 0.44 | 45.2 ± 0.3 | 43.8 ± 0.2 | < 0.001 |
| LV mass/BSA, g/m2 | 78.1 ± 0.6 | 79.6 ± 0.3 | 0.023 | 77.7 ± 0.5 | 79.6 ± 0.3 | 0.003 |
| LV mass/height2.7, g/m2.7 | 38.3 ± 0.3 | 37.8 ± 0.2 | 0.15 | 36.9 ± 0.3 | 38.2 ± 0.1 | < 0.001 |
| LV mass/EDV, mg/mL | 1.82 ± 0.01 | 1.87 ± 0.01 | 0.001 | 1.76 ± 0.01 | 1.88 ± 0.01 | < 0.001 |
| LV hypertrophy, % | 12 | 10 | 0.079 | 10 | 10 | 0.88 |
| LA volume index, mL/m2 | 25.9 ± 0.2 | 25.4 ± 0.1 | 0.034 | 26.1 ± 0.2 | 25.3 ± 0.1 | 0.001 |
| Systolic function | ||||||
| Ejection fraction, % | 64.7 ± 0.2 | 65.6 ± 0.1 | < 0.001 | 64.5 ± 0.2 | 65.7 ± 0.1 | < 0.001 |
| Peak longitudinal strain, % | − 17.52 ± 0.07 | − 18.14 ± 0.04 | < 0.001 | − 17.60 ± 0.07 | −18.11 ± 0.04 | < 0.001 |
| s′, cm/s | 6.77 ± 0.04 | 7.03 ± 0.02 | < 0.001 | 6.76 ± 0.04 | 7.05 ± 0.02 | < 0.001 |
| Stroke volume, mL | 65.0 ± 0.4 | 67.4 ± 0.2 | < 0.001 | 64.8 ± 0.4 | 67.5 ± 0.2 | <0.001 |
| Cardiac output, L/min | 4.35 ± 0.03 | 4.36 ± 0.02 | 0.67 | 4.22 ± 0.03 | 4.40 ± 0.02 | <0.001 |
| Diastolic function | ||||||
| E wave, cm/s | 65.5 ± 0.5 | 66.6 ± 0.3 | 0.072 | 64.9 ± 0.5 | 66.7 ± 0.3 | 0.003 |
| A wave, cm/s | 82.6 ± 0.5 | 79.8 ± 0.3 | < 0.001 | 80.6 ± 0.5 | 80.3 ± 0.3 | 0.61 |
| Deceleration time, ms | 199 ± 1 | 208 ± 1 | < 0.001 | 200 ± 1 | 208 ± 1 | < 0.001 |
| E/A ratio | 0.82 ± 0.008 | 0.87 ± 0.004 | < 0.001 | 0.84 ± 0.008 | 0.87 ± 0.004 | 0.001 |
| e′, cm/s | 6.17 ± 0.04 | 6.28 ± 0.02 | 0.022 | 6.24 ± 0.04 | 6.28 ± 0.02 | 0.44 |
| E/e′ ratio | 11.5 ± 0.1 | 11.3 ± 0.1 | 0.20 | 11.27 ± 0.11 | 11.34 ± 0.06 | 0.65 |
| Arterial function and Ventricular-arterial coupling | ||||||
| Ea, mmHg/mL | 1.95 ± 0.01 | 1.81 ± 0.01 | < 0.001 | 1.96 ± 0.01 | 1.80 ± 0.01 | < 0.001 |
| SVRI, dyne.s.cm-5.m2 | 3378 ± 24 | 3064 ± 13 | < 0.001 | 3410 ± 24 | 3056 ± 13 | < 0.001 |
| TAC, mL/mmHg | 1.05 ± 0.009 | 1.10 ± 0.005 | < 0.001 | 1.04 ± 0.009 | 1.10 ± 0.005 | < 0.001 |
| cfPWV, m/s | 12.4 ± 0.11 | 11.6 ± 0.06 | < 0.001 | 12.4 ± 0.11 | 11.6 ± 0.06 | < 0.001 |
| LV Ees, mmHg/mL | 2.92 ± 0.02 | 2.83 ± 0.01 | 0.001 | 2.93 ± 0.02 | 2.81 ± 0.01 | < 0.001 |
| LV Ees/M/V, mmHg/g | 1.70 ± 0.02 | 1.60 ± 0.01 | < 0.001 | 1.75 ± 0.02 | 1.59 ± 0.01 | < 0.001 |
| Ea/Ees | 0.70 ± 0.005 | 0.66 ± 0.003 | < 0.001 | 0.70 ± 0.005 | 0.66 ± 0.003 | < 0.001 |
BSA – body surface area, cfPWV – carotid-femoral pulse wave velocity, Ea – arterial elastance, EDV – end diastolic volume, EDVI – end-diastolic volume index, Ees – end-systolic elastance, Ees/M/V – end-systolic elastance normalized for mass/volume ratio, LA – left atrium, LV – left ventricle, SVRI – systemic vascular resistance index, TAC – total arterial compliance
Continuous variables are presented as mean ± SE values
Adjusted analysis by age, sex, body mass index, use of anti-hypertensive, diabetes mellitus, heart rate and prevalent coronary heart disease or heart failure. Left ventricle mass/end diastolic volume ratio was added in the model for LV Ees and Ea/Ees ratio comparisons.
LV early diastolic relaxation velocity (e′), which is relatively load-independent, and estimated LV filling pressures (E/e′ ratio) were similar between race groups, but peak E wave velocity, deceleration time, and E/A ratio were lower among blacks compared to whites.
Arterial function and ventricular-arterial coupling
Ea was higher, indicating higher arterial load, among blacks than among whites (table 2). SVRI was higher and TAC was lower among blacks, suggesting that both steady and pulsatile components of arterial load were implicated in such racial differences. cfPWV was slightly higher among blacks than whites, indicating elevated arterial stiffness. Ventricular stiffness was greater among blacks, as reflected by higher LV Ees after taking into account LV concentricity (table 2).
Association between arterial elastance and cardiac structure and function
We found that the association between Ea and measures of cardiac structure and function significantly differs between blacks and whites. Ea was positively associated with LV end diastolic volume and LV mass among blacks, but not among whites (Table 3). Overall, high Ea was associated with lower measures of systolic function, including longitudinal strain, s′ and LVEF, and race modified the association between Ea and LVEF.
Table 3.
Race-specific associations of arterial elastance with cardiac structure and function, after adjusting for confounders
| Black n=1257 |
p value | White N=4233 |
p value | p for interaction | |
|---|---|---|---|---|---|
| Cardiac structure | |||||
| EDVI, mL/m2 | 1.56 ± 0.28 | <0.001 | −0.33 ± 0.17 | 0.048 | <0.001 |
| LV mass/BSA, g/m2 | 3.21 ± 0.55 | <0.001 | 0.16 ± 0.32 | 0.61 | <0.001 |
| LV mass/height2.7, g/m2.7 | 1.80 ± 0.27 | < 0.001 | 0.36 ± 0.15 | 0.020 | < 0.001 |
| LV mass/EDV, mg/mL | 0.02 ± 0.01 | 0.12 | 0.02 ± 0.01 | 0.007 | 0.90 |
| LA volume index, mL/m2 | 0.03 ± 0.22 | 0.87 | −0.92 ± 0.13 | <0.001 | <0.001 |
| Systolic function | |||||
| Ejection fraction, % | −2.23 ± 0.18 | <0.001 | −1.38 ± 0.10 | <0.001 | <0.001 |
| Peak longitudinal strain, % | 0.74 ± 0.07 | <0.001 | 0.59 ± 0.04 | <0.001 | 0.20 |
| s′, cm/s | −0.32 ± 0.03 | <0.001 | −0.23 ± 0.02 | <0.001 | 0.34 |
| Diastolic function | |||||
| E wave, cm/s | −1.68 ± 0.47 | <0.001 | −4.00 ± 0.30 | <0.001 | 0.003 |
| A wave, cm/s | −2.41 ± 0.48 | <0.001 | −3.36 ± 0.31 | <0.001 | 0.15 |
| Deceleration time, ms | −4.06 ± 1.16 | <0.001 | −4.48 ± 0.77 | <0.001 | 0.58 |
| E/A ratio | 0.01 ± 0.01 | 0.12 | −0.01 ± 0.005 | 0.011 | 0.06 |
| e′, cm/s | −0.33 ± 0.04 | <0.001 | −0.22 ± 0.02 | <0.001 | 0.028 |
| E/e′ ratio | 0.42 ± 0.11 | <0.001 | −0.32 ± 0.06 | <0.001 | <0.001 |
BSA – body surface area, EDV – end diastolic volume, EDVI – end-diastolic volume index, LA – left atrium, LV – left ventricle
Values are the coefficient (β ± SE) for each 1 SD increase in arterial elastance adjusted by age, sex, body mass index, use of anti-hypertensive, diabetes mellitus, heart rate and prevalent coronary heart disease or heart failure.
Likewise, we found that high Ea was associated with worse LV diastolic function in blacks than in whites. Although Ea was negatively associated with e′ in both race groups, this association was steeper among blacks, i.e. among individuals with high Ea, e′ was lower in blacks than in whites. Furthermore, high Ea was associated with higher E/e′ ratio and LA volume index, indicating higher filling pressures, in blacks than in whites (Table 3). These findings were similar when we analyzed with inverse probability weighting (Supplement tables 1 and 2) or after excluding participants with diagnosis of HF (Supplement tables 3 and 4).
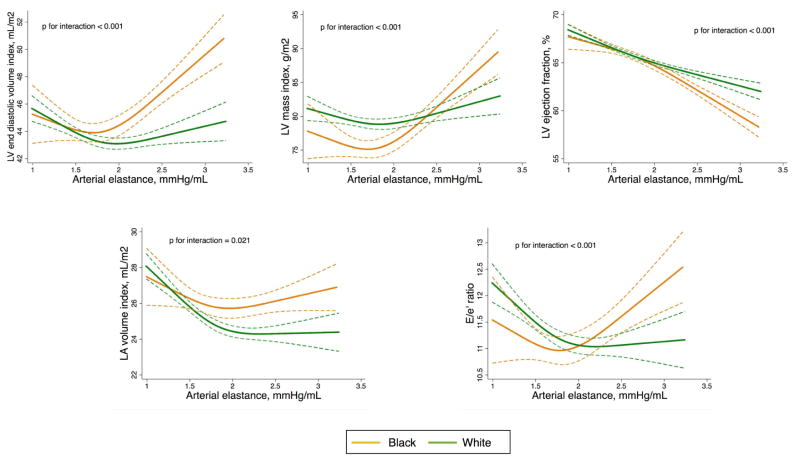
We also observed non-linear relationships between Ea and cardiac function and structure and how they differed between blacks and whites. In Figure 1, we see that these relationships clearly diverged between race groups for measures of Ea above 2 mmHg/mL: blacks with high Ea displayed higher LV mass and volume, higher left atrium volume and higher E/e′ ratio compared to whites with similar levels of Ea.
Figure 1.
Race-specific associations of arterial elastance with measures of cardiac structure and function.
LV – left ventricle, LA – left atrium. Adjusted plot for age, sex, body mass index, use of anti-hypertensive, diabetes mellitus, heart rate and prevalence of coronary heart disease or heart failure. Dashed lines show 95% prediction brands.
The association between Ea and cardiac structure and function also differed according to genetic ancestry among blacks. While blacks with below median (0.153) European Ancestry had direct associations between Ea and LV mass, LV and LA volumes and E/e′ ratio, these associations significantly attenuated among blacks with above median European Ancestry, and were more similar to the associations observed among white participants (table 4). These differences persisted after adjusting for annual income and educational level (Supplementary table 5).
Table 4.
Race-specific associations of arterial elastance with cardiac structure and function in elderly accounting for European Ancestry among blacks
| Black | White | |||
|---|---|---|---|---|
|
|
||||
| Below median PEA* n=513 |
Above median PEA* n=526 |
N=4213 | p for interaction | |
| EDVI, mL/m2 | 2.57 ± 0.46y | 0.47 ± 0.43 | −0.33 ± 0.17z | <0.001 |
| LV mass/BSA, g/m2 | 5.47 ± 0.85y | 0.35 ± 0.87 | 0.16 ± 0.32 | <0.001 |
| LV mass/height2.7, g/m2.7 | 2.81 ± 0.42y | 0.55 ± 0.44 | 0.36 ± 0.15z | < 0.001 |
| LA volume index, mL/m2 | 0.83 ± 0.32z | −0.83 ± 0.37z | −0.92 ± 0.13y | <0.001 |
| Ejection fraction, % | −2.14 ± 0.26y | −2.18 ± 0.29y | −1.38 ± 0.10y | 0.001 |
| E/e′ ratio | 0.67 ± 0.17y | 0.22 ± 0.18 | −0.32 ± 0.06y | <0.001 |
BSA – body surface area, LA – left atrium, LV – left ventricle, EDVI – end-diastolic volume index, PEA – proportion of European Ancestry.
Values are the coefficient (β ± SE) for each 1 SD increase in arterial elastance adjusted by age, sex, body mass index, use of anti-hypertensive, diabetes mellitus, heart rate and prevalent coronary heart disease or heart failure.
PEA was 0.11 ± 0.03 and 0.25 ± 0.10 for blacks with below and above median PEA groups, respectively.
p < 0.001;
p < 0.05
DISCUSSION
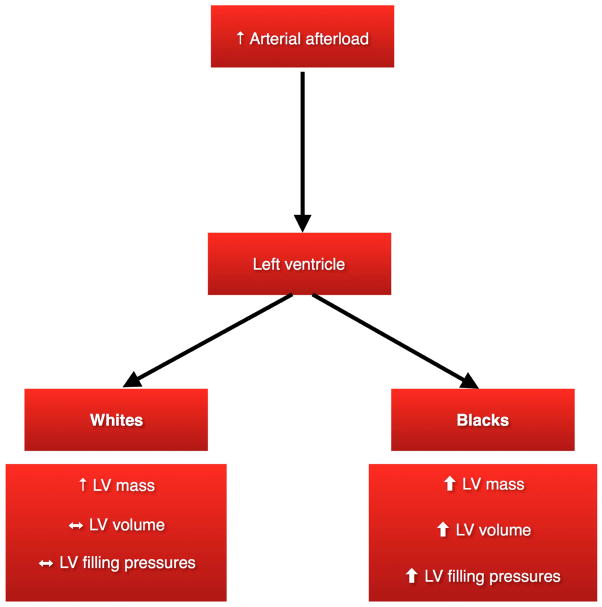
In this large biracial cohort of elderly individuals, we showed for the first time that the association of Ea with cardiac structure and function differs between blacks and whites. Not only do blacks display higher arterial afterload (Ea) as compared to whites, they also display more adverse changes in cardiac structure and function as Ea increases compared to whites, indicating greater vulnerability to high afterload (Figure 2). These data may help to explain why blacks with hypertension are at greater risk of cardiovascular complications. (1,7,33) This in turn suggests that intensive BP reduction, which lowers Ea and has been proven to reduce cardiovascular events, may be even more important among blacks to reduce the burden of cardiovascular disease.(34)
Figure 2.
Race-specific changes in left ventricle structure and function in response of high arterial afterload
LV - Left ventricle
Previous studies have suggested that the higher prevalence of LV hypertrophy among blacks cannot be attributed exclusively to their higher predisposition to hypertension, in concordance with our findings. (13,35–37) Blacks have higher LV mass than their white counterparts after adjusting for body size, severity and duration of hypertension.(13) It has been postulated that there are racial differences in the cardiac adaptation to increased peripheral resistance.(38) Peripheral resistance and systemic blood pressure are closely related to LV systolic pressure, which is directly related to myocardial wall stress, and high peripheral resistance and arterial stiffness may be a trigger for LV hypertrophy and dysfunction.(22,39,40) Ea is a composite parameter that better reflects the net impact of the arterial afterload towards the ventricle. Our results suggest that blacks develop more cardiac remodeling and dysfunction compared to whites with similar levels of Ea.
Whether an inherent racial susceptibility or environmental factors are implicated in the higher predisposition of blacks to LV remodeling is uncertain. Racial differences in health outcomes may be partly explained by disparities in socioeconomic status. For instance, low income and low education level have been associated with higher arterial stiffness and LV mass, and can mediate the race disparities.(10,41,42) On the other hand, racial differences in arterial stiffness are observed among people free of traditional cardiovascular risk factors and at very young ages, suggesting racial differences on arterial function and structure early in life.(11,43)
We found that the association of high Ea with cardiac remodeling and dysfunction attenuates among blacks with above median European Ancestry, even after adjusting for socioeconomic status, which suggests a genetic basis to explain these racial differences. Accordingly, the genetic profile rather than self-reported race was associated with arterial stiffness in the Multi-Ethnic Study of Atherosclerosis.(44) Blacks are more likely to carry a minor corin I555(P568) allele, which has been associated with enhanced cardiac hypertrophic response to pressure-overload.(45,46) Even so, we must interpret these results with caution because the contribution of genetic ancestry to these phenotypic differences may still reflect environmental factors to some extent.
Furthermore, the association between Ea and cardiac remodeling can be unrelated to increased afterload triggering LV hypertrophy. LV mass may increase prior to the development of overt hypertension,(47) suggesting that the factors that promote LV hypertrophy may be unconnected to high BP. For instance, either norepinephrine or angiotensin II may directly affect the LV size independently of BP.(48,49) It is uncertain, however, whether these factors play a role in the higher predisposition of blacks to LV hypertrophy.
Our study suggests that blacks have both higher Ea and greater afterload sensitivity for cardiac remodeling and dysfunction compared to whites, which may elucidate part of their higher predisposition to cardiovascular diseases, particularly HF.(1) Hence, blacks are more likely not only to develop hypertension – the clinical expression of high Ea – but to experience its harmful effects on the heart. Recently, it was shown that treatment targeting a systolic BP to less than 120 mmHg, compared with less than 140 mmHg, results in lower rates of cardiovascular events among patients at high cardiovascular risk.(34) Together, these data imply that intensive BP lowering may be particularly advantageous among blacks, and increase even further the priority for better control of BP in Black Americans, an enormous unmet public health need.
Our study has some limitations that deserve attention. This is a cross-sectional study, which prevents us from establishing a temporal sequence of the cardiovascular abnormalities. Racial differences in the association between Ea and cardiac remodeling can merely reflect the racial disparities in the hypertension course. Besides, there may be survivor bias in this cross-sectional study, and only approximately 60% of participants who were alive at visit 5 underwent echocardiogram. We addressed this by performing inverse probability weighting analysis and found consistent results. In addition, although our results may provide mechanistic insights that help to explain why blacks have higher predisposition to HF, we did not explore new mechanisms, but the racial differences in ventricular-arterial coupling. Even though an increase in Ea should result in an increase in LV wall stress, we did not directly measure LV wall stress. We used noninvasive methods to assess Ea and Ees, which have greater variability compared with invasive measurements. Nevertheless, Doppler measures are feasible parameters to evaluate in large population-based studies and have been previously validated against gold-standard invasive methods. Finally, we cannot definitively exclude that the differences and relationships we observed are not due to other unmeasured confounding variables.
CONCLUSION
In this large community-based study, we demonstrated that the association of Ea with cardiac structure and function diverge between black and white Americans. These results suggest that blacks are more susceptible to changes in cardiac structure and function triggered by increased arterial load, which may explain their higher predisposition to develop HF. Further studies are needed to evaluate whether specific therapies that have impact on arterial afterload can further reduce the cardiovascular risk among blacks.
Supplementary Material
COMPETENCY IN MEDICAL KNOWLEDGE
Hypertension leads to changes in cardiac structure and function, which precede the clinical manifestations of cardiovascular diseases, triggered by high arterial afterload. High arterial afterload appears to cause more cardiac remodeling among blacks, compared to whites. This helps to explain why blacks have higher risk of developing cardiovascular diseases than whites.
TRANSLATIONAL OUTLOOK
Further studies are needed to evaluate: 1) Which therapies, including anti-hypertensive drugs, can better reduce the arterial afterload; and 2) Whether different classes of anti-hypertensive drugs have differential effect on the incidence of cardiovascular events according to race.
Acknowledgments
ACKNOWLEDGMENTS/FUNDING SOURCES
The authors thank the staff and participants of the ARIC study for their important contributions. The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute (NHLBI) contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). This work was also supported by NHLBI cooperative agreement NHLBI-HC-11-08 [SDS], grants R00-HL-107642 [SC] and K08-HL-116792 [AMS]; American Heart Association grant 14CRP20380422[AMS]; grant from the Ellison Foundation [SC]; National Institutes of Health grant T32 HL094301-06[SH]. AG was supported by the Portuguese Foundation for Science and Technology Grant HMSP-ICS/007/2012; W.N.J. was supported by the Brazilian National Council for Scientific and Technological Development Grant 249481/2013-8; and MMF was supported by Lemann Foundation.
ABREVIATIONS LIST
- ARIC
Atherosclerosis Risk in Community
- BP
Blood Pressure
- BMI
Body Mass Index
- BSA
Body Surface Area
- CHD
Coronary Heart Disease
- Ea
Arterial elastance
- HF
Heart Failure
- LV
Left Ventricle
- LVEF
Left Ventricle Ejection Fraction
- MI
Myocardial Infarction
Footnotes
Conflicts of interest: None to declare
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bahrami H, Kronmal R, Bluemke DA, et al. Differences in the incidence of congestive heart failure by ethnicity: the multi-ethnic study of atherosclerosis. Arch Intern Med. 2008;168:2138–2145. doi: 10.1001/archinte.168.19.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang PP, Chambless LE, Shahar E, et al. Incidence and survival of hospitalized acute decompensated heart failure in four US communities (from the Atherosclerosis Risk in Communities Study) Am J Cardiol. 2014;113:504–510. doi: 10.1016/j.amjcard.2013.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yancy CW. Heart failure in African Americans. Am J Cardiol. 2005;96:3i–12i. doi: 10.1016/j.amjcard.2005.07.028. [DOI] [PubMed] [Google Scholar]
- 4.Kawaguchi M, Hay I, Fetics B, Kass DA. Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: implications for systolic and diastolic reserve limitations. Circulation. 2003;107:714–720. doi: 10.1161/01.cir.0000048123.22359.a0. [DOI] [PubMed] [Google Scholar]
- 5.Segers P, Stergiopulos N, Westerhof N. Relation of effective arterial elastance to arterial system properties. Am J Physiol Heart Circ Physiol. 2002;282:H1041–1046. doi: 10.1152/ajpheart.00764.2001. [DOI] [PubMed] [Google Scholar]
- 6.Writing Committee M. Yancy CW, Jessup M, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 7.Borlaug BA, Redfield MM, Melenovsky V, et al. Longitudinal changes in left ventricular stiffness: a community-based study. Circ Heart Fail. 2013;6:944–952. doi: 10.1161/CIRCHEARTFAILURE.113.000383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wohlfahrt P, Redfield MM, Lopez-Jimenez F, et al. Impact of general and central adiposity on ventricular-arterial aging in women and men. JACC Heart Fail. 2014;2:489–499. doi: 10.1016/j.jchf.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wohlfahrt P, Redfield MM, Melenovsky V, Lopez-Jimenez F, Rodeheffer RJ, Borlaug BA. Impact of chronic changes in arterial compliance and resistance on left ventricular ageing in humans. European journal of heart failure. 2015;17:27–34. doi: 10.1002/ejhf.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Din-Dzietham R, Couper D, Evans G, Arnett DK, Jones DW. Arterial stiffness is greater in African Americans than in whites: evidence from the Forsyth County, North Carolina, ARIC cohort. Am J Hypertens. 2004;17:304–313. doi: 10.1016/j.amjhyper.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Morris AA, Patel RS, Binongo JNG, et al. Racial differences in arterial stiffness and microcirculatory function between Black and White Americans. J Am Heart Assoc. 2013;2:e002154. doi: 10.1161/JAHA.112.002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chrysant SG, Danisa K, Kem DC, Dillard BL, Smith WJ, Frohlich ED. Racial differences in pressure, volume and renin interrelationships in essential hypertension. Hypertension. 1979;1:136–41. doi: 10.1161/01.hyp.1.2.136. [DOI] [PubMed] [Google Scholar]
- 13.Kizer JR, Arnett DK, Bella JN, et al. Differences in left ventricular structure between black and white hypertensive adults: the Hypertension Genetic Epidemiology Network study. Hypertension. 2004;43:1182–1188. doi: 10.1161/01.HYP.0000128738.94190.9f. [DOI] [PubMed] [Google Scholar]
- 14.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 15.Rosamond WD, Chang PP, Baggett C, et al. Classification of heart failure in the atherosclerosis risk in communities (ARIC) study: a comparison of diagnostic criteria. Circ Heart Fail. 2012;5:152–9. doi: 10.1161/CIRCHEARTFAILURE.111.963199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah AM, Cheng S, Skali H, et al. Rationale and design of a multicenter echocardiographic study to assess the relationship between cardiac structure and function and heart failure risk in a biracial cohort of community-dwelling elderly persons: the Atherosclerosis Risk in Communities study. Circ Cardiovasc Imaging. 2014;7:173–181. doi: 10.1161/CIRCIMAGING.113.000736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Picard MH, Adams D, Bierig SM, et al. American Society of Echocardiography recommendations for quality echocardiography laboratory operations. J Am Soc Echocardiogr. 2011;24:1–10. doi: 10.1016/j.echo.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 18.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39. e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Chirinos JA, Segers P, De Buyzere ML, et al. Left ventricular mass: allometric scaling, normative values, effect of obesity, and prognostic performance. Hypertension. 2010;56:91–98. doi: 10.1161/HYPERTENSIONAHA.110.150250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelly RP, Ting CT, Yang TM, et al. Effective arterial elastance as index of arterial vascular load in humans. Circulation. 1992;86:513–521. doi: 10.1161/01.cir.86.2.513. [DOI] [PubMed] [Google Scholar]
- 21.Chen CH, Fetics B, Nevo E, et al. Noninvasive single-beat determination of left ventricular end-systolic elastance in humans. J Am Coll Cardiol. 2001;38:2028–2034. doi: 10.1016/s0735-1097(01)01651-5. [DOI] [PubMed] [Google Scholar]
- 22.Coutinho T, Borlaug BA, Pellikka PA, Turner ST, Kullo IJ. Sex differences in arterial stiffness and ventricular-arterial interactions. J Am Coll Cardiol. 2013;61:96–103. doi: 10.1016/j.jacc.2012.08.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chemla D, Hébert JL, Coirault C, et al. Total arterial compliance estimated by stroke volume-to-aortic pulse pressure ratio in humans. Am J Physiol. 1998;274:H500–505. doi: 10.1152/ajpheart.1998.274.2.H500. [DOI] [PubMed] [Google Scholar]
- 24.Lam CS, Roger VL, Rodeheffer RJ, et al. Cardiac structure and ventricular-vascular function in persons with heart failure and preserved ejection fraction from Olmsted County, Minnesota. Circulation. 2007;115:1982–90. doi: 10.1161/CIRCULATIONAHA.106.659763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyer ML, Tanaka H, Palta P, et al. Correlates of Segmental Pulse Wave Velocity in Older Adults: The Atherosclerosis Risk in Communities (ARIC) Study. Am J Hypertens. 2015:hpv079. doi: 10.1093/ajh/hpv079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gaasch WH, Little WC. Assessment of left ventricular diastolic function and recognition of diastolic heart failure. Circulation. 2007;116:591–593. doi: 10.1161/CIRCULATIONAHA.107.716647. [DOI] [PubMed] [Google Scholar]
- 27.Cheng C-Y, Reich D, Coresh J, et al. Admixture mapping of obesity-related traits in African Americans: the Atherosclerosis Risk in Communities (ARIC) Study. Obesity (Silver Spring) 2010;18:563–572. doi: 10.1038/oby.2009.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fan JB, Oliphant A, Shen R, et al. Highly parallel SNP genotyping. Cold Spring Harb Symp Quant Biol. 2003;68:69–78. doi: 10.1101/sqb.2003.68.69. [DOI] [PubMed] [Google Scholar]
- 29.Nadruz W, Jr, Claggett B, Goncalves A, et al. Smoking and Cardiac Structure and Function in the Elderly: The ARIC Study (Atherosclerosis Risk in Communities) Circ Cardiovasc Imaging. 2016;9:e004950. doi: 10.1161/CIRCIMAGING.116.004950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goncalves A, Jhund PS, Claggett B, et al. Relationship between alcohol consumption and cardiac structure and function in the elderly: the Atherosclerosis Risk In Communities Study. Circ Cardiovasc Imaging. 2015:8. doi: 10.1161/CIRCIMAGING.114.002846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fretz A, Schneider ALC, McEvoy JW, et al. The Association of Socioeconomic Status With Subclinical Myocardial Damage, Incident Cardiovascular Events, and Mortality in the ARIC Study. Am J Epidemiol. 2016;183:452–461. doi: 10.1093/aje/kwv253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gottesman RF, Rawlings AM, Sharrett AR, et al. Impact of differential attrition on the association of education with cognitive change over 20 years of follow-up: the ARIC neurocognitive study. Am J Epidemiol. 2014;179:956–966. doi: 10.1093/aje/kwu020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J. 2011;32:670–679. doi: 10.1093/eurheartj/ehq426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.A Randomized Trial of Intensive versus Standard Blood-Pressure Control. New England Journal of Medicine. 2015;373:2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang J, Chen W, Ruan L, Toprak A, Srinivasan SR, Berenson GS. Differential effect of elevated blood pressure on left ventricular geometry types in black and white young adults in a community (from the Bogalusa Heart Study) Am J Cardiol. 2011;107:717–722. doi: 10.1016/j.amjcard.2010.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drazner MH, Dries DL, Peshock RM, et al. Left ventricular hypertrophy is more prevalent in blacks than whites in the general population: the Dallas Heart Study. Hypertension. 2005;46:124–129. doi: 10.1161/01.HYP.0000169972.96201.8e. [DOI] [PubMed] [Google Scholar]
- 37.Gardin JM, Wagenknecht LE, Anton-Culver H, et al. Relationship of cardiovascular risk factors to echocardiographic left ventricular mass in healthy young black and white adult men and women. The CARDIA study. Coronary Artery Risk Development in Young Adults. Circulation. 1995;92:380–387. doi: 10.1161/01.cir.92.3.380. [DOI] [PubMed] [Google Scholar]
- 38.Dunn FG, Oigman W, Sungaard-Riise K, et al. Racial differences in cardiac adaptation to essential hypertension determined by echocardiographic indexes. J Am Coll Cardiol. 1983;1:1348–1351. doi: 10.1016/s0735-1097(83)80150-8. [DOI] [PubMed] [Google Scholar]
- 39.Fernandes VRS, Polak JF, Cheng S, et al. Arterial stiffness is associated with regional ventricular systolic and diastolic dysfunction: the Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28:194–201. doi: 10.1161/ATVBAHA.107.156950. [DOI] [PubMed] [Google Scholar]
- 40.Chaturvedi N, Bulpitt CJ, Leggetter S, et al. Ethnic differences in vascular stiffness and relations to hypertensive target organ damage. J Hypertens. 2004;22:1731–1737. doi: 10.1097/00004872-200409000-00017. [DOI] [PubMed] [Google Scholar]
- 41.Dekkers C, Treiber FA, Kapuku G, Van Den Oord EJCG, Snieder H. Growth of left ventricular mass in African American and European American youth. Hypertension. 2002;39:943–951. doi: 10.1161/01.hyp.0000015612.73413.91. [DOI] [PubMed] [Google Scholar]
- 42.Thurston RC, Matthews KA. Racial and socioeconomic disparities in arterial stiffness and intima media thickness among adolescents. Soc Sci Med. 2009;68:807–813. doi: 10.1016/j.socscimed.2008.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Collins RT, Somes GW, Alpert BS. Differences in arterial compliance among normotensive adolescent groups: Collins arterial compliance in adolescents. Pediatr Cardiol. 2008;29:929–934. doi: 10.1007/s00246-008-9239-7. [DOI] [PubMed] [Google Scholar]
- 44.Wassel CL, Jacobs DR, Duprez DA, et al. Association of self-reported race/ethnicity and genetic ancestry with arterial elasticity: the Multi-Ethnic Study of Atherosclerosis (MESA) J Am Soc Hypertens. 2011;5:463–472. doi: 10.1016/j.jash.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dries DL, Victor RG, Rame JE, et al. Corin gene minor allele defined by 2 missense mutations is common in blacks and associated with high blood pressure and hypertension. Circulation. 2005;112:2403–2410. doi: 10.1161/CIRCULATIONAHA.105.568881. [DOI] [PubMed] [Google Scholar]
- 46.Rame JE, Drazner MH, Post W, et al. Corin I555(P568) allele is associated with enhanced cardiac hypertrophic response to increased systemic afterload. Hypertension. 2007;49:857–864. doi: 10.1161/01.HYP.0000258566.95867.9e. [DOI] [PubMed] [Google Scholar]
- 47.Post WS, Larson MG, Levy D. Impact of left ventricular structure on the incidence of hypertension. The Framingham Heart Study. Circulation. 1994;90:179–185. doi: 10.1161/01.cir.90.1.179. [DOI] [PubMed] [Google Scholar]
- 48.Harrap SB, Dominiczak AF, Fraser R, et al. Plasma angiotensin II, predisposition to hypertension, and left ventricular size in healthy young adults. Circulation. 1996;93:1148–1154. doi: 10.1161/01.cir.93.6.1148. [DOI] [PubMed] [Google Scholar]
- 49.Schlaich MP, Kaye DM, Lambert E, Sommerville M, Socratous F, Esler MD. Relation between cardiac sympathetic activity and hypertensive left ventricular hypertrophy. Circulation. 2003;108:560–565. doi: 10.1161/01.CIR.0000081775.72651.B6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.