Abstract
Chickpea starch was subjected to triple retrogradation cycle with time intervals of 24, 48 and 72 h. The impact on in vitro digestibility, functional, pasting and structural characteristics was investigated. Compared to native chickpea starch, the resistant starch (RS) content of triple retrograded starch was significantly increased with increased retrogradation time whereas slowly digestible starch content was decreased. Water binding capacity and solubility of triple retrograded starch were significantly increased whereas swelling power and pasting properties were decreased. Triple retrograded starches showed B type and B + V type crystalline structure. After triple retrogradation, the organised granular structure was disrupted, irregularly shaped particles were formed showing porous, coarse, filamentous network structure. FT-IR spectra perceived a slight change in percentage intensity of C–H stretch of triple retrograded starches (TRSs). Triple retrogradation was observed to be a promising methods for RS product.
Keywords: Triple retrogradation, In vitro digestibility, Chickpea starch, Structural properties, Pasting properties
Introduction
Chickpea (Cicer arietinum L.) is that the largest created food legume in South Asia and also the third largest created food legume globally (Gaur et al. 2010). Starch is the most abundant carbohydrate in chickpea and is taken into account to be competitive within the food industry (Goni and Valentín-Gamazo 2003). Resistant starch (RS) has recently gained attention as a purposeful food ingredient, as a result of its potential health benefits and useful properties in foods (Sajilata et al. 2006). RS has been classified into four general subtypes namely type I (RS1), type II (RS2), type III (RS3) and type IV (RS4) on the basis of the reason for resistance to digestion (Englyst et al. 1992).
Starch associations by the retrogradation method manufactured the thermally stable enzyme-resistant starch, which is classified as the RS3 type (Englyst et al. 1992). Among the ways used to increase RS3 yield, acid hydrolysis of amylomaize starch (Vasanthan and Bhatty 1998) and repeated freeze-thawings (Chung et al. 2003) are the most widely investigated. In general, several ways involving physical, chemical and enzymatic transformations are used to change the properties of starch that enhance health attributes and/or minimise defects in the structure. Starch retrogradation, as well as short retrogradation and long run retrogradation, is an inevitable development that gelatinized starch changes from associate amorphous state to a crystalline area (Tian et al. 2009). The formation of RS is influenced by a variety of things together with the amylose content and chain length of molecules, autoclaving (gelatinization) temperature, storage (retrogradation) time period and temperature of starch gels (Eerlingen et al. 1993).
Ashwar et al. (2016) reported that reduction of swelling index and low pasting properties enhanced the resistant starch (30.31–38.65 %) in treated rice starch samples throughout autoclaving-retrogradation treatment. A significant raise in water solubility and water binding capacity was discovered as a result of heating and autoclaving methods in RS production (Ozturk et al. 2011). Results of investigations show that RS preparations are suitable for food products that need high water binding capacity (Ozturk et al. 2011). Hence the objective of the study was to determine the effect of triple retrogradation on characteristics of resistant starch.
Materials and methods
Materials
Desi chickpea (Cicer arietinum L.) was obtained from Tamil Nadu Agricultural University, Coimbatore. Starch was isolated from chickpea by the method of Vasanthan (2001). This isolated starch was used for the preparation of triple retrograded starch. Analytical grade chemicals and reagents were used for analysis.
Preparation of triple retrograded chickpea starch
Triple retrograded chickpea starch was prepared in triplicate according to the method of Xie et al. (2014) with slight modifications. Triple retrogradation treatments can be described as follows: chickpea starch (25.0 g) and distilled water (50 ml) were placed in a 150 ml glass container, sealed, and heated in boiling water at 121 °C for 30 min. The resultant gel was hermetically sealed and stored at 4 °C for 24, 48, and 72 h respectively to perform one cycle of retrogradation treatment. The retrograded samples were heated in boiling water for 30 min again and subjected to three cycles of retrogradation treatment with time intervals of 24, 48 and 72 h. Under these conditions, chickpea starch was treated successively by triple retrogradation to perform 3 cycles. After treatment, each of resulting gel was cut into pieces (<5 cm in length and thickness), then oven dried at 45 °C temperature for 4–6 h. All samples were milled to pass through a 100-mesh sieve to obtain the starch products. The moisture contents of the triple retrograded starches (TRSs) were in the range of 11.14–11.31 %.
In vitro digestibility profiles of native chickpea starch and triple retrograded starches
The in vitro digestibility of the starch samples was analyzed by the standard method of Englyst et al. (1999) with some modification. Briefly, starch sample (200 mg, dry base) was hydrolyzed by a mixed enzyme solution of porcine pancreatic α-amylase (290 U/ml) and amyloglucosidase (15 U/ml), where U is defined as the amount of enzyme that liberates 1.0 mg glucose from starch in 1 min at pH 5.2 and 37 °C. Phosphate buffer (15 ml, 0.2 mol/l, pH 5.2) and five glass balls (10 mm in diameter) were added to each of the conical tubes containing the sample (200 mg, dry forms). After equilibration at 37 °C for 5 min, the enzyme solution (5 ml) was added to the sample tube, followed by incubation in a water bath at 37 °C with shaking (170 rpm). Aliquots (0.5 ml) were taken at intervals of 20 and 120 min and mixed with 4 ml of 80 % ethanol to deactivate the enzymes. The mixed solution was centrifuged at 2000 rpm for 10 min, and the glucose content in the supernatant was measured using the glucose oxidase/peroxidase (GOPOD) assay kit (Englyst et al. 1992).
Functional properties of native chickpea starch and triple retrograded starches
Water binding capacity
The procedure described by the method of Yamazaki (1954) with slight modification. Starch (5.0 g., dry basis) was added to 75 ml distilled water in a tarred 100 ml centrifuge bottle. The bottle was stoppered and agitated on a wrist-action shaker for 1 h. It was then centrifuged for 10 min at 2200×g, the water was decanted, and the bottle was tipped up and allowed to drain for 10 min more. The bottle was weighed and the amount of water held by the starch was determined. The water binding capacity was calculated from the formula below
Swelling power and starch solubility
Swelling power and starch solubility of the starches were determined by the method of Gani et al. (2010). Starch suspensions (1 %,w/v) were prepared in a flask, heated to 90 °C for 30 min with shaking every 5 min and left for cooling to room temperature and centrifuged for 15 min at 3000×g. The supernatant was decanted and the residual volume was determined. The solid part was dried in an oven for 2 h at 130 °C.
Pasting properties of native chickpea starch and triple retrograded starches
Pasting properties of native chickpea starches and triple retrograded starches were evaluated with Rapid Visco Analyzer (RVA) (RVA Tech Master, Perten Instruments, Japan) according to the method described by Noda et al. (2004).
Structural properties of native chickpea starch and triple retrograded starches
X-ray diffraction (XRD)
The procedure described earlier (Surendra Babu et al. 2015b) was followed with slight modification. X-ray diffraction patterns of starch samples were obtained using a Powder X-ray diffractometer (Rigaku Mini Hex-II, Japan). X-ray intensity of starch was affected by moisture content samples were conditioned to 75 % relative humidity before taking the X-ray diffraction pattern. The graphs were plotted between the 2θ angles of 10 and 60 and smoothed with the software PowderX (Chinese Academy of Sciences, Beijing). The percentage crystallinity of the starches was calculated by using the method of Nara and Komiya (1983) with the Origin-version 6.0 software (Microcal Software, Inc., Northampton, MA 01060 USA).
Scanning electron microscope (SEM)
The procedure described earlier (Surendra Babu et al. 2015a) was followed with slight modification. Starch granules were observed using a Scanning Electron Microscope (SEM) (JEOL-Model 6390, Japan). The samples were fixed with a conductive tape of copper of double glue, which was covered with a layer of coal of 20 nm thickness. It was deposited at vacuum with an evaporator in a JEOL electron microscope. Later on, it was covered with a layer of gold of 50 nm of thickness in the ionizer of metals JEOL. This was observed in the microscope and registered photographically. Film pieces were mounted on aluminum stubs using a double-sided tape and then coated with a layer of gold (40–50 nm), allowing surface and cross-section visualization. All samples were examined using an accelerating voltage of 5 kV.
FT-IR spectroscopy
The procedure described earlier (Surendra Babu et al. 2015a) with slight modification. The infra-red spectra of all the starch samples were attained with an FT-IR spectrometer (Brucker, Tensor-27). The equipment was operated with a resolution of 2.0 cm−1 and scanning range of 4000–370 cm−1. The sample was mixed with KBr (1:100, w/w) before acquisition and the background value from pure KBr was acquired before the sample was scanned.
Statistical analysis
The data were analysed using an SPSS version 16.0. The mean and standard deviation of the triplicate analyse of the samples were calculated. Analysis of Variance (ANOVA) was performed to determine significant differences between the means, while the means were separated using the least significant difference (LSD).
Results and discussion
In vitro digestibility profiles of native chickpea starch (NCPS) and triple retrograded starches
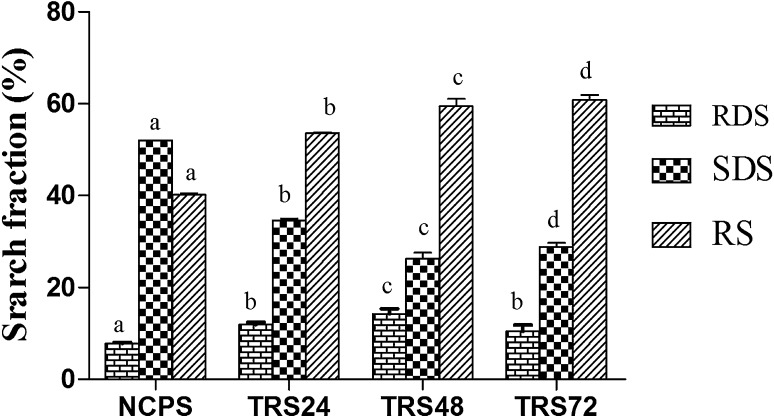
Rapidly digestible starch (RDS), slowly digestible starch (SDS) and resistant starch (RS) contents of the NCPS and TRSs (TRS24, TRS48 and TRS72) are presented in Fig. 1. The results showed that RS content of TRS24h, TRS48h and TRS78h were 53.54, 59.42 and 60.75 % in respectively and it was significantly (P < 0.05) higher than native chickpea starch (40.16 %). Hughes et al. (2009) also reported that RS content of NCPS was in the range of 24.14–40.57 %. The RS content of TRSs increased with increased retrogradation time from 24 to 72 h. The RDS content of TRS24 (11.93 %), TRS48 (14.25 %) and TRS72 (10.43 %), was significantly (P < 0.05) higher than NCPS (7.78 %) whereas SDS content of TRSs such as TRS24 (34.55 %), TRS48 (26.31 %) and TRS72 (28.81 %) was significantly (P < 0.05) less than NCPS (52.05 %). Chung et al. (2009) reported increase of RDS, RS and decrease of SDS when unmodified corn, pea and lentil starches were subjected to annealing and heat-moisture treatment. Dundara and Gocmen (2013) reported that the RS yield of starch increased from 24.94 % (24 h-storage time) to 30.41 % (72 h-storage time) at an autoclaving temperature of 145 °C. The increase in RDS and decrease in SDS on triple retrogradation may have been due to disruption of double helices forming the starch crystallites at the granule surface and/or to crystallite reorientation (Chung et al. 2009). The increase in RS and decrease in SDS during modifications suggested the partial transformation of SDS to RS. Eerlingen et al. (1993) reported that yield of RS largely depended on storage time and temperature and that storage temperature influenced the type of RS crystals (A or B, X-ray diffraction pattern) formation. Vasanthan and Bhatty (1998) studied the impact of retrogradation and annealing on the resistant starch content of amylomaize, barley, field pea, and lentil starches and the study indicated that the RS content of retrograded starch gels (6–9 %, w/w) were higher than the native starches (3–5 %, w/w).
Fig. 1.
In vitro digestibility profiles of native chickpea starch and triple retrograded starches. All values are means of triplicates ± standard deviation. Different letters on top of the bars indicate significant differences (P < 0.05) among treatments in % starch fractions. RDS, SDS, RS indicate rapidly digestible starch, slowly digestible starch and resistant starch respectively. NCPS native chickpea starch, TRS24h, TRS48h and TRS72h represent triple retrograded starches 24, 48 and 72 h
Functional properties of NCPS and triple retrograded starches
Functional properties including WBC, Solubility and swelling power of NCPS and TRSs are presented in Table 1.
Table 1.
WBC (%), solubility (%), swelling power (g/g), crystallinity, crystallinity type and diffraction peaks of native chickpea starch and TRSs
| Samples | WBC (%) | Solubility (%) | Swelling power (g/g) | Crystallinity (%) | Crystallinity type | Diffraction peaks |
|---|---|---|---|---|---|---|
| NCPS | 93.59 ± 3.12a | 2.33 ± 0.57a | 9.54 ± 0.23a | 25.44 | C | 15.5, 17.6, 23.8 |
| TRS24 | 141.91 ± 8.87b | 10.73 ± 0.05b | 7.06 ± 0.11b | 28.08 | B | 14.5, 17.0, 18.6, 22.1, 24.1 |
| TRS48 | 153.53 ± 0.87c | 11.26 ± 0.15bd | 6.73 ± 0.15c | 31.81 | B + V | 14.3, 17.1, 19.6, 22.2, 24.4 |
| TRS72 | 159.59 ± 5.32c | 11.6 ± 0.43 cd | 6.43 ± 0.15c | 33.89 | B + V | 14.4, 17.3, 19.7, 22.4, 24.1 |
WBC water binding capacity, TRSs Triple retrograded starches
All values are the mean of triplicates ± standard deviation. Means in the same column with different letters are significantly different (P < 0.05)
Water binding capacity
Water binding capacity of NCPS, TRS24h, TRS48h and TRS72h was 93.59, 141.91, 153.53 and 159.59 % respectively. This result was consistent with Polesi and Sarmento (2011) who studied that RS from chickpea and observed the similar effect on hydrolysis and heat treatment water absorption index. An increased water binding capacity was observed in retrograded starches may be the result of a change in molecular structure or any other mechanisms leading to an easier mobility of the starch, where leaching of starch was also observed (Govindasamy et al. 1996).
Solubility and swelling power
The solubility of TRS was significantly (P < 0.05) higher than NCPS whereas swelling power was reduced. Similarly increased water solubility and decreased swelling power were observed by Reddy et al. (2013) for kidney bean starch. The solubility of the treated starches was increased with increase in storage time from 24 to 72 h whereas swelling power was decreased. Water solubility is often used as a degradation indicator of molecular components. The increase in solubility might have occured as a result of changes in the molecular structure or as an independent mechanism that results in the mobility of the starch elements, leading to the leaching of carbohydrates from molecules involved (Govindasamy et al. 1996). Consequently, the treatment of chickpea starch with retrogradation may lead to the depolymerization of amylopectin, making the starch lose the ability to hold absorbed water and swell (Wang and Wang 2003).
Pasting properties of NCPS and triple retrograded starches
The pasting properties of the NCPS and TRSs are summarized in Table 2. Significant differences (P < 0.05) in pasting properties among NCPS and TRSs were determined. Triple retrograded starches had lower peak, trough, breakdown, final, setback viscosities and peak time, whereas NCPS showed the highest values for these parameters. Among TRSs TRS48h showed the highest value for these parameters except peak time. These observations were in agreement with those reported by Ashwar et al. (2016) in RS from the rice. RS content of NCPS was less moreover viscosity of the starch was high. Triple retrogradation increased the RS content; consequently viscosity was decreased in TRSs. Gelencser et al. (2008) also stated that there was an inverse proportionality between RS content and viscosity of the starches. The partial hydrolysis of starch produces short linear and branched chains from crystalline and amorphous regions, which results in a product with reduced paste viscosity and low ability to form a gel (Polesi and Sarmento 2011). Significant reduction in swelling index of treated starch could be responsible for lowering of peak viscosity (Ashwar et al. 2016).
Table 2.
Pasting properties of native chickpea starch and triple retrograded starches
| Pasting properties | NCPS | TRS24 | TRS48 | TRS72 |
|---|---|---|---|---|
| Peak viscosity (cP) | 4453 ± 267.27a | 72.66 ± 4.04b | 79.33 ± 4.50b | 68.33 ± 6.11b |
| Trough (cP) | 2861 ± 223.54a | 68 ± 3.60b | 74 ± 4.58b | 63.66 ± 6.02b |
| Breakdown (cP) | 1592 ± 243.21a | 4.66 ± 1.15b | 5.33 ± 1.15b | 4.66 ± 0.57b |
| Final viscosity (cP) | 5603 ± 264.17a | 105 ± 6b | 123.33 ± 15.94b | 103 ± 21.93b |
| Setback (cP) | 2742 ± 112.86a | 37 ± 2.64b | 49.33 ± 11.93b | 39.33 ± 16.19b |
| Peak time (min) | 4.22 ± 0.10a | 6.97 ± 0.04b | 6.8 ± 0.17b | 6.95 ± 0.07b |
| Pasting temperature (°C) | 72.35 ± 0.47 | nd | nd | nd |
All values are the mean of triplicates ± standard deviation. Means in the same row with different letters are significantly different (P < 0.05)
NCPS Native chickpea starch and Triple retrograded starches (24h, 48h and 78h)
Structural properties of NCPS and triple retrograded starches
XRD and relative crystallinity
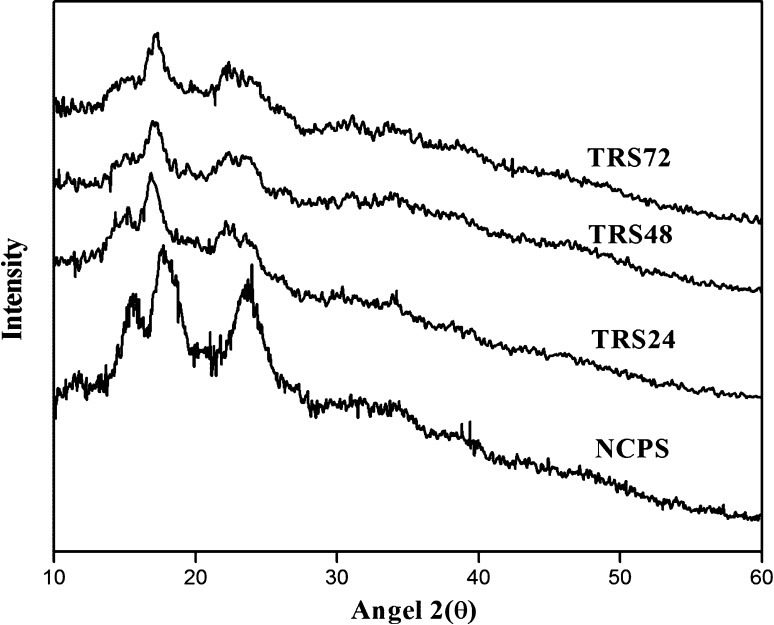
The X-ray diffraction pattern of NCPS and TRSs is presented in Fig. 2. NCPS showed type-C crystallinity pattern (Table 1), with intermediate intensity peak at diffraction angles of 2θ = 15.5° and strong peaks at 2θ = 17.6° and 23.8°. This pattern is characteristic of starch from legume and consists of a mixture of both types A and B crystalline structures (Huang et al. 2007). TRS24 showed type-B crystallinity pattern (Table 1) with intensity peaks at diffraction angles of 2θ = 14.5°, 17.0°, 18.6°, 22.1° and 24.1°. This result was agreed with the results of Polesi and Sarmento (2011) who reported that type-B crystallinity pattern for chickpea retrograded starches. TRSs (TRS48 and TRS72) showed a mixture of B-type and V-type crystalline pattern (Table 1) with intensity peaks at diffraction angles of 2θ = 14.3° and 14.4°, 17.1° and 17.3°, 19.6° and 19.7°, 22.2° and 22.4°, 24.4° and 24.1° respectively. This was consistent with results of Miao et al. (2009) who reported a mixture of B and V-type crystalline pattern for RS of waxy rice. It could be observed that the peak 2θ = 23.8° of NCPS disappears and the doublet emerges at 2θ = 22.1° and 24.1° in TRSs; these results strongly indicated that the change occurred in the crystalline starch structure. A similar pattern of change was observed by Leong et al. (2007) for sago starch (Metroxylon sago) treated with pullulanase, autoclaved and retrograded. The change in crystalline pattern was consistent with a previous report during the preparation of resistant starch from maize starch, the appearance of a peak at 19.77° 2θ might contribute to the disappearance of an amorphous region based on amylopectin, which might be caused by the retrogradation that occured at a low temperature (Frost et al. 2009). The degrees of crystallinity were higher in TRSs than NCPS. This result was in agreement with the results of Zhou et al. (2014) who stated that increasing crystallinity pattern in dual modified indica rice RS. This was due to transformation of amorphous amylose into a double helix structure during the autoclaving and cooling processes, and this in turn, resulted in a more orderly crystallite (Zhang et al. 2014).
Fig. 2.
X-ray diffract gram of native chickpea starch (NCPS) and triple retrograded starches (TRS for 24, 48 and 72h)
Scanning electron microscopy (SEM)
NCPS showed large oval shaped granules, small spherical shaped granules and a smooth granular surface of starch with no evidence of any fissures (Fig. 3). Similar observations for starches from Indian chickpea cultivars have been reported by Singh et al. (2004). The shape of the starch granules in the different legumes varied from oval to elliptical or spherical (Singh et al. 2008). Triple retrogradation influenced the granule morphology of NCPS. After triple retrogradation, the granular structure was disrupted, some granules with little holes on the surface appeared and adhered together to form coarse, filamentous network structure. Morphological changes occurred, and the irregularly shaped particles formed a white porous network. Similar results were reported by Zhang and Jin (2011). TRSs showed more crystallinity and formed a larger structure. This was attributed to retrogradation of more amylose chains, which resulted in the reorganization of the starch structure into a helical complex. This increased a density of the crystal structure greatly increased its resistance to enzyme attack (Zhang and Jin 2011).
Fig. 3.
Scanning electron microscopy (SEM) images of native chickpea starch (NCPS) and triple retrograded starches (TRS24, TRS48 and TRS72)
Fourier transform infrared (FTIR) spectroscopy
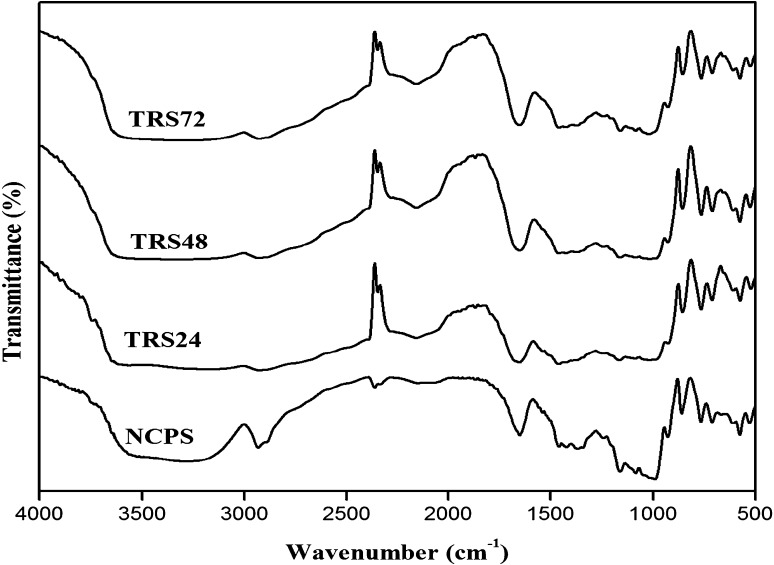
The FT-IR spectra of NCPS and TRSs are illustrated in Fig. 4. In the present study, all the characteristic peaks observed in NCPS were also noticed in TRSs. The absorption intensity of TRSs in the band from 800 to 1200 cm−1 was weaker than that of NCPS; this indicated that certain conformation changes had occurred in retrograded starches. This might be due to reflects of C–C, C–OH and C–H stretching vibration (Zhang et al. 2010). Changes in starch polymer conformation, and the process of hydration (Wei et al. 2011). The peak at 900–950 cm−1 in the infrared spectrum of all starch samples was an evidence for the vibration of the glycosidic linkage. The absorption band at 1367.22 cm−1 (corresponding to the bending vibration of –CH2OH) of TRSs were narrower than that of NCPS. This was due to changes in the combinations of hydrogen bonds generated during the formation of RS which might have contributed to this difference (Zhang et al. 2014). The sharp band at 2932 cm−1 was a characteristic of C–H stretches associated with the ring hydrogen atoms. The percentage intensity of C–H stretch of TRSs was decreased slightly compared to NCPS. This trend was more pronounced in TRS48h and TRS24h. Intensity changes in C–H stretch range might be attributed to the change in the amylose and amylopectin content of starch molecule (Young 1984). The amplitude of TRSs in the absorption bands between 3100 and 3700 cm−1 (corresponding to the –OH group) was different from that of NCPS. It was due to the gelatinization and retrogradation process, a more precise bonding combination of internal and intermolecular hydrogen might cause the change of amplitude (Flores-Morales et al. 2012).
Fig. 4.
The FT-IR spectra of native chickpea starch and triple retrograded starches
Conclusion
The resistant starch yield of foods depends on the botanical source of the starch and the processing methods. The study indicate that production of RS increased by triple retrogradation. TRS72 found to contain more RS water binding capacity, solubility and lower swelling power and pasting profiles amongst TRSs. The crystalline type changed from C (NCPS) to B (TRS24) and B + V (TRS48 and TRS72). Irregularly shaped particles formed porous, coarse network in TRSs. FT-IR spectra perceived a slight change in percentage intensity of C–H stretch of triple retrograded starches. These findings suggest structural changes within the starch granules were caused by triple retrogradation which to an increase in the RS content.
Acknowledgments
The authors gratefully acknowledge to the Department of Food Science and Nutrition for providing University Research Fellow (URF) and Tamil Nadu Agricultural University, Coimbatore, for providing desi chickpea seeds.
References
- Ashwar BA, Gani A, Wani IA, Shah A, Masoodi FA, Saxena Production of resistant starch from rice by dual autoclaving-retrogradation treatment: in vitro digestibility, thermal and structural characterization. Food Hydrocoll. 2016;56:108–117. doi: 10.1016/j.foodhyd.2015.12.004. [DOI] [Google Scholar]
- Chung HJ, Jeong HY, Lim ST. Effects of acid hydrolysis and defatting on crystallinity and pasting properties of freeze-thawed high amylose corn starch. Carbohydr Polym. 2003;54:449–455. doi: 10.1016/j.carbpol.2003.05.001. [DOI] [Google Scholar]
- Chung HJ, Liu Q, Hoover R. Impact of annealing and heat-moisture treatment on rapidly digestible, slowly digestible and resistant starch levels in native and gelatinized corn, pea and lentil starches. Carbohydr Polym. 2009;75:436–447. doi: 10.1016/j.carbpol.2008.08.006. [DOI] [Google Scholar]
- Dundara AN, Gocmen D. Effects of autoclaving temperature and storing time on resistant starch formation and its functional and physicochemical properties. Carbohydr Polym. 2013;97:764–771. doi: 10.1016/j.carbpol.2013.04.083. [DOI] [PubMed] [Google Scholar]
- Eerlingen RC, Crombez M, Delcour JA. Enzyme-resistant starch. I. Quantitative and qualitative influence of incubation time and temperature of autoclaved starch on the resistant starch formation. Cereal Chem. 1993;70(3):339–344. [Google Scholar]
- Englyst HN, Kingman SM, Cummings JH. Classification and measurement of nutritionally important starch fractions. Eur J Clin Nutr. 1992;46(2):33–50. [PubMed] [Google Scholar]
- Englyst KN, Englyst HN, Hudson GJ, Cole TJ, Cummings JH. Rapidly available glucose in foods: an in vitro measurement that reflects the glycemic response. Am J Clin Nutr. 1999;69:448–454. doi: 10.1093/ajcn/69.3.448. [DOI] [PubMed] [Google Scholar]
- Flores-Morales A, Jiménez-Estrada M, Mora-Escobedo R. Determination of the structural changes by FT-IR, Raman, and CP/MAS 13 C NMR spectroscopy on retrograded starch of maize tortillas. Carbohydr Polym. 2012;87(1):61–68. doi: 10.1016/j.carbpol.2011.07.011. [DOI] [PubMed] [Google Scholar]
- Frost K, Kaminski D, Kirwan G, Lascaris E, Shanks R. Crystallinity and structure of starch using wide angle X-ray scattering. Carbohydr Polym. 2009;78(3):543–548. doi: 10.1016/j.carbpol.2009.05.018. [DOI] [Google Scholar]
- Gani A, Haq SS, Masoodi FA, Broadway AA, Gani A. Phisico-chemical, morphological and pasting properties of starches extracted from water chestnuts (Trapa natans) from three lakes of Kashmir, India. Braz Arch Biol Technol. 2010;53(3):731–740. doi: 10.1590/S1516-89132010000300030. [DOI] [Google Scholar]
- Gaur PM, Tripathi S, Gowda CLL, Ranga Rao GV, Sharma HC, Pande S, Sharma M (2010) Chickpea seed production manual. Patancheru 502 324, Andhra Pradesh, India. International Crops Research Institute for the Semi-Arid Tropics (ICRISAT), p 28
- Gelencser T, Juhasz R, Hodsagi M, Gergely SZ, Salgo A. Comparative study of native and resistant starches. Acta Aliment. 2008;37:255–270. doi: 10.1556/AAlim.37.2008.2.11. [DOI] [Google Scholar]
- Goni I, Valentín-Gamazo C. Chickpea flour ingredient slows glycemic response to pasta in healthy volunteers. Food Chem. 2003;81:511–515. doi: 10.1016/S0308-8146(02)00480-6. [DOI] [Google Scholar]
- Govindasamy S, Campanella OH, Oates CG. High moisture twin-screw extrusion of sago starch: 1. Influence on granule morphology and structure. Carbohydr Polym. 1996;30:215–286. doi: 10.1016/S0144-8617(96)00024-0. [DOI] [Google Scholar]
- Huang J, Schols HA, van Soest JJG, Jin Z, Sulmann E, Voragen AGJ. Physicochemical properties and amylopectin chain profiles of cowpea, chickpea and yellow pea starches. Food Chem. 2007;101:1338–1345. doi: 10.1016/j.foodchem.2006.03.039. [DOI] [Google Scholar]
- Hughes T, Hoover R, Liu Q, Donner E, Chibbar R, Jaiswal S. Composition, morphology, molecular structure, and physicochemical properties of starches from newly released chickpea (Cicer arietinum L.) cultivars grown in Canada. Food Res Int. 2009;42:627–635. doi: 10.1016/j.foodres.2009.01.008. [DOI] [Google Scholar]
- Leong YH, Karim AA, Norziah MH. Effect of pullulanase debranching of sago (Metroxylon sagu) starch at sub gelatinization temperature on the yield of resistant starch. Starch/Starke. 2007;59:21–32. doi: 10.1002/star.200600554. [DOI] [Google Scholar]
- Miao M, Jiang B, Zhang T. Effect of pullulanase debranching and recrystallization on structure and digestibility of waxy maize starch. Carbohydr Polym. 2009;76(2):214–221. doi: 10.1016/j.carbpol.2008.10.007. [DOI] [Google Scholar]
- Nara S, Komiya T. Studies on the relationship between water saturated state and crystallinity by the diffraction method for moistened potato starch. Starch-Starke. 1983;35:407–410. doi: 10.1002/star.19830351202. [DOI] [Google Scholar]
- Noda T, Tsuda S, Mori M, Takigawa S, Endo CM, Saito K. The effect of harvested dates on the starch properties of various potato cultivars. Food Chem. 2004;86:119–125. doi: 10.1016/j.foodchem.2003.09.035. [DOI] [Google Scholar]
- Ozturk S, Koksel H, Ng PKW. Production of resistant starch from acid modified amylotype starches with enhanced functional properties. J Food Eng. 2011;103:156–164. doi: 10.1016/j.jfoodeng.2010.10.011. [DOI] [Google Scholar]
- Polesi LF, Sarmento SBS. Structural and physicochemical characterization of RS prepared using hydrolysis and heat treatments of chickpea starch. Starch/Starke. 2011;63:226–235. doi: 10.1002/star.201000114. [DOI] [Google Scholar]
- Reddy CK, Suriya M, Haripriya S. Physico-chemical and functional properties of resistant starch prepared from red kidney beans (Phaseolus vulgaris.L) starch by enzymatic method. Carbohydr Polym. 2013;95:220–226. doi: 10.1016/j.carbpol.2013.02.060. [DOI] [PubMed] [Google Scholar]
- Sajilata MG, Singhal RS, Kulkarni PR. Resistant starch—a review. Compr Rev Food Sci F. 2006;5:1–17. doi: 10.1111/j.1541-4337.2006.tb00076.x. [DOI] [PubMed] [Google Scholar]
- Singh N, Sandhu KS, Kaur M. Characterization of starches separated from Indian chickpea (Cicer arietinum L.) cultivars. J Food Eng. 2004;63:441–449. doi: 10.1016/j.jfoodeng.2003.09.003. [DOI] [Google Scholar]
- Singh N, Nakaura Y, Inouchi N, Nishinari K. Structure and viscoelastic properties of starches separated from different legumes. Starch/Stärke. 2008;60:349–357. doi: 10.1002/star.200800689. [DOI] [Google Scholar]
- Surendra Babu A, Parimalavalli R, Jagannadham K, Sudhakara Rao J. Chemical and structural properties of sweet potato starch treated with organic and inorganic acid. J Food Sci Technol. 2015;52(9):5745–5753. doi: 10.1007/s13197-014-1650-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surendra Babu A, Parimalavalli R, Rudra SG. Effect of citric acid concentration and hydrolysis time on physicochemical properties of sweet potato starches. Int J Biol Macromol. 2015;80:557–565. doi: 10.1016/j.ijbiomac.2015.07.020. [DOI] [PubMed] [Google Scholar]
- Tian Y, Li Y, Jin Z, Xu X. Anovel molecular simulation method for evaluating the endothermic transition of amylose recrystallite. Eur Food Res Technol. 2009;229:853–858. doi: 10.1007/s00217-009-1124-y. [DOI] [Google Scholar]
- Vasanthan T (2001) Overview of laboratory isolation of starch from plant materials. Current Protocols in Food Analytical Chemistry E:E2:E2.1. doi:10.1002/0471142913.fae0201s00
- Vasanthan T, Bhatty RS. Enhancement of resistant starch (RS3) in amylomaize, barley, field pea and lentil starches. Starch/Starke. 1998;50:286–291. doi: 10.1002/(SICI)1521-379X(199807)50:7<286::AID-STAR286>3.0.CO;2-O. [DOI] [Google Scholar]
- Wang Y, Wang L. Physicochemical properties of common and waxy corn starches oxidized by different levels of sodium hypochlorite. Carbohydr Polym. 2003;52:207–217. doi: 10.1016/S0144-8617(02)00304-1. [DOI] [Google Scholar]
- Wei C, Qin F, Zhou W, Xu B, Chen C, Chen Y. Comparison of the crystalline properties and structural changes of starches from high-amylose transgenic rice and its wild type during heating. Food Chem. 2011;128(3):645–652. doi: 10.1016/j.foodchem.2011.03.080. [DOI] [Google Scholar]
- Xie YY, Hu XP, Jin ZY, Xu XM, Chen HQ. Effect of repeated retrogradation on structural characteristics and in vitro digestibility of waxy potato starch. Food Chem. 2014;163:219–225. doi: 10.1016/j.foodchem.2014.04.102. [DOI] [PubMed] [Google Scholar]
- Yamazaki WT. Interrelations among bread dough absorption, cookie diameter, protein content, and alkaline water retention capacity of soft winter wheat flours. Cereal Chem. 1954;31:135–142. [Google Scholar]
- Young AH. Fractionation of starch. In: Whistler RL, BeMiller JN, Paschall EF, editors. Starch: chemistry and technology. Orlando: Academic; 1984. pp. 249–283. [Google Scholar]
- Zhang H, Jin Z. Preparation of resistant starch by hydrolysis of maize starch with pullulanase. Carbohydr Polym. 2011;83(2):865–867. doi: 10.1016/j.carbpol.2010.08.066. [DOI] [Google Scholar]
- Zhang J, Chen F, Liu F, Wang ZW. Study on structural changes of microwave heat-moisture treated resistant Canna edulis Ker starch during digestion in vitro. Food Hydrocoll. 2010;24(1):27–34. doi: 10.1016/j.foodhyd.2009.07.005. [DOI] [Google Scholar]
- Zhang Y, Zeng H, Wang Y, Zeng S, Zheng B. Structural characteristics and crystalline properties of lotus seed resistant starch and its prebiotic effects. Food Chem. 2014;155:311–318. doi: 10.1016/j.foodchem.2014.01.036. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Meng S, Chen D, Zhu X, Yuan H. Structure characterization and hypoglycemic effects of dual modified resistant starch from indica rice starch. Carbohydr Polym. 2014;103:81–86. doi: 10.1016/j.carbpol.2013.12.020. [DOI] [PubMed] [Google Scholar]