Abstract
The changes in chemical composition, antioxidant activity and fatty acid composition of lentil flour after dehulling, germination and cooking of seeds were investigated. Dehulling showed no significant effect on protein content, however, protein content decreased in most of the varieties after germination and cooking. Total soluble sugars (TSS) content increased significantly after dehulling (2.0–41.64 %) and cooking (2.08–31.07 %) whereas, germination had no significant effect on TSS content. Total lipids increased significantly after dehulling (21.56–42.86 %) whereas, it decreased significantly after germination (2.97–26.52 %) and cooking (23.05–58.63 %). Cooking was more effective than other methods in reducing trypsin inhibitors (80.51–85.41 %). Dehulling was most effective in reducing tannins (89.46–92.99 %) and phytic acid (52.63–60.00 %) content over raw seed. Myristic, palmitic, stearic, oleic and linoleic acid content decreased while linolenic acid content increased after dehulling. Dehulling, germination and cooking decreased the content of antioxidant metabolite (gallic acid, catechin and quercetin) and also antioxidant activities. Raw samples followed by germinated samples showed the highest concentrations of phytochemicals responsible for antioxidant activity and also the antioxidant capacities. Present study showed germination and cooking would be useful in formulation and development of lentil based functional foods for human health benefits.
Keywords: Lentil, Dehulling, Germination, Nutrients, Antinutrients, Antioxidant properties
Introduction
Pilses are major source of dietary nutrients. Among pulses, lentil (Lens culinaris L.), a widely grown grain and dietary staple in many Middle Eastern, European, South American, African and Asian countries like India has been found good in nutritional quality as well as an excellent dietary source of phytochemicals (including lipophilic and hydrophilic compounds) possessing high antioxidant capacity (Aguilera et al. 2010). Although lentil has excellent nutritional quality, however it also contains certain anti-nutritional constituents such as trypsin inhibitors, phytic acid, tannins that could limit their protein and carbohydrate utilization. Trypsin inhibitors are low molecular weight proteins capable of binding to and inactivating the digestive enzyme, trypsin while phytic acid lowers the bioavailability of minerals (Reddy et al. 1984). Tannins are known to form complexes with proteins, which are reported to be responsible for low protein digestibility and decreased amino acid availability (Adsule and Kadam 1989). Traditional processing methods of pulses such as soaking, dehulling and germination are sometimes used to reduce or eliminate the anti-nutrients those affect protein absorption. The seed coat (hull) of pulses is often indigestible and may have a bitter taste. Dehulling has been reported to improve the palatability and taste of chickpea, pigeon-pea and lentil and reduce polyphenols or tannins, mostly present in the seed coat of pulses (Wang 2008). Germination is one of the most simple, common and effective processes for improving the nutritional quality of pulses by the reduction of anti-nutritive compounds and augmenting the levels of free amino acids, available carbohydrates, dietary fiber, and other components (Vidal-Valverde et al. 2002). Lentils are usually cooked by a domestic boiling process before consumption. The cooking process not only improves the flavor and palatability of lentils but also affects the bio-accessibility and bio-availability of nutrients (Xu and Chang 2008).
There are many studies that reported bioactive components in raw lentils and attributed potential health benefits to the antioxidant activities of hydrophilic phytochemicals such as phenolics (Xu and Chang 2008). Literature is available on processing techniques which improves the palatability and bio-availability of nutrients but available information on dehulling, germination, cooking and their effects on phytochemicals (nutritional and antinutritional), antioxidant properties and fatty acid composition of lentil is rather scarce. Therefore, the current investigation aimed of determining how dehulling, germination as well as cooking affect the nutritional composition (total protein, total soluble sugars, total lipids and starch), anti-nutrients (tannins, phytic acid and trypsin inhibitor), antioxidant metabolites along with antioxidant activities and fatty acid composition of five varieties of lentil. These findings are expected to give insight into the possible utilization of lentil as new functional food.
Materials and methods
Plant materials
The experimental materials consisted of five varieties (VL Masoor 125, VL Masoor 133, VL 142, VL Masoor 507, VL Masoor 514) of lentil. Among these varieties, varieties VL Masoor 125, VL Masoor 133, VL 142 were microsperma type whereas, VL Masoor 507, VL Masoor 514 were macrosperma type. Samples were cleaned to remove foreign material and damaged seeds prior to processing.
Methods
Processing of lentil samples
Processing of samples was done in one batch and processed samples were dried in an oven at 50 ± 5 °C for 16–18 h. Processed samples along with raw (undehulled) seeds were ground into flour and were stored in airtight containers for further analysis.
Germination
Seeds were cleaned, washed and soaked in 4–5 volumes of water (22–25 °C) for 12 h at room temperature. Then water was drained out and seeds were allowed to germinate under a wet muslin cloth for 48 h.
Cooking
Seed (50 g) were soaked in distilled water 1:4 (seed:water, w/v) for 12 h at room temperature. After draining the water, seed were transferred to a perforated container and cooked in a boiling water bath. From the cooked samples, water was drained and seeds were dried.
Dehulling
Seeds (50 g) were soaked in distilled water 1:4 (seed:water, w/w) for 12 h at room temperature. At the end of the period, water was drained out and the seed were manually dehulled.
Chemical analysis
The nitrogen content was estimated by Kjeldhal method (AOAC 2005) based on the assumption that plant proteins contain 16 g/100 g nitrogen. Protein content was calculated using the formula, protein = nitrogen × 6.25. Total sugar content (TSS) was determined colorimetrically by the anthrone method (Hedge and Hofreiter 1962). Gravimetric method by Bligh and Dyer (1959) was used for determination of total fat content. Tannin was determined colorimetrically following the AOAC method (AOAC 2005). Phytic acid (PA) contents of defatted flours were determined by the method of Haug and Lantzsch (1983). The phytic acid content was calculated from a calibration curve using phytate phosphorus salt in the range of 10–50 µg. Trypsin inhibitor activity (TIA) was determined colorimetrically using a spectrophotometer at 410 nm (Smith et al. 1980).
Fatty acid profile
Oil from samples was extracted in hexane by soxhlet apparatus (Extraction unit, E-816, Buchi). Methyl esters were obtained by a two-step catalytic process according to the method of Ghadge and Raheman (2005). Fatty acid composition was determined using an Agilent 7860A gas chromatograph (GC) equipped with a flame ionization detector (FID) and an auto sampler. Peak separation was performed on a DB-225 capillary column (diameter—250 µm, length—30 m, film thickness—0.25 µm) from Agilent Technologies. The carrier gas was nitrogen set to a constant gas flow of 1.2 ml/min at 160 °C initial temperature. 1 µl of sample was injected at a 20:1 split ratio into the column with the following temperature conditions: 160 °C for 2 min; raised from 150 to 220 °C at 6 °C/min. Both inlet and detector were set to 230 °C. Fatty acid composition was determined by identifying and calculating relative peak area per cent by GC post run analysis EZChrom elite compact software.
HPLC-PAD analysis
Fine powders of raw and processed samples (1.0 g) were extracted in 85 % methanol at 35 °C for 12 h and filtered through Whatman filter paper No. 1. The extract solutions stored in amber bottles at 4 °C served as the working solution (10 mg/ml) for determination of gallic acid, catechin di-hydrate, quercetin and antioxidant activities. All samples were filtered through a 0.22 µm cellulose acetate filter before injection. The standards: Gallic acid, Catechins di-hydrate and Quercetin were procured from Sigma, USA. Methanol, Acetonitrile and water were HPLC grade.
The chromatographic system is provided by a 996 photodiode-array detector (Waters, Milford, MA, USA) and a column Spherisorb ODS2 C18 (300 × 3.9 mm). The mobile phase was methanol:acetonitrile:water (60:20:20 v/v/v). The mobile phase was filtered through a 0.22 µm membrane filter. Then deaerated ultrasonically prior to use. Best resolution and sensitivity of RP-HPLC separation was obtained for Gallic acid, Catechins di-hydrate and Quercetin at 290 nm. Flow rate and injection volume were 1.0 ml/min and 10 µl. The chromatographic peaks of the analytes were confirmed by comparing their retention time and UV spectra with those of the reference standards.
Determination of anti-oxidative properties
The total antioxidant activity (TA) of lentil methanolic extract was estimated using the phosphomolybdenum method of Prieto et al. (1999) based on the reduction of Mo (VI) to Mo (V) by the sample analyte and subsequent formation of specific green phosphate/Mo (V) compounds. The ferric reducing antioxidant power (FRAP) was assayed following the method of Benzie and Strain (1996). A standard curve of trolox (10–100 µM) was prepared. Total antioxidant activity and ferric reducing antioxidant power were expressed as µM trolox equivalent/gram dry weight (µM TE/g DW). The reducing power was determined according to the method of Huda-Faujan et al. (2009).
Statistical analysis
The analysis was carried out in three replicates for all determinations. The statistical analyses were performed using the statistical package SPSS (Version No 10). Analyses of variance (ANOVA) were performed and significance of each group was verified with one-way analysis of variance followed by Duncan’s multiple range test (p < 0.05). For multifactorial comparison, principal component analysis (PCA) was used to display the correlation among the various parameters and their relationship with the different lentil varieties. Varimax rotation was performed to produce orthogonal transformations to the reduced factors to identify the high and low correlations better. Multifactorial analysis was carried out using the XLStat-Pro 7.5 (Addinsoft, New York, USA).
Results and discussion
Effect of dehulling, germination and cooking on nutritive properties
Table 1 shows that total protein content in raw samples ranged from 19.08 (VL 142) to 25.46 (VL Masoor 133) g/100 g. The results were comparable with findings of Kaur and Sandhu (2010) for lentil. Seed processing had no significant effect on protein content. After dehulling, the total protein content ranged from 18.53 (VL 142) to 24.19 (VL Masoor 133) g/100 g. Most of the varieties showed a decrease in protein content after germination and cooking. Decrease in protein content after germination and cooking could be due to the increase in level of protease during germination (Torres et al. 2007). In contrast, some studies (Ghumman et al. 2016; Vasishtha et al. 2014) reported increase in soluble protein content on germination and on cooking respectively, as compared to unprocessed grain for chickpea. Total soluble sugars (TSS) content increased significantly (p < 0.05) after dehulling and cooking (Table 1) whereas, germination has no significant effect on TSS content. TSS content in raw samples ranged from 15.51 g/100 g in VL Masoor 507 to 19.47 g/100 g in VL Masoor 125. In dehulled samples, it ranged from 19.86 g/100 g (VL Masoor 125) to 26.26 g/100 g (VL 142). In cooked samples, the highest content of TSS (25.52 g/100 g) was found for VL Masoor 125 whereas; the lowest (18.62 g/100 g) was recorded for VL Masoor 514. Dehulling resulted in 2.0–41.64 % increase in total soluble sugars content whereas cooking caused a 3.0–6.7 % increase in TSS content. TSS level improved significantly after dehulling which could be due to removal of hull portion that have less content of soluble sugars. A previous study in horse gram showed a significant increase in sugars content after germination (Pal et al. 2016).
Table 1.
Effect of dehulling, germination and cooking on total protein, total soluble sugars, total lipids and starch contents of lentil flours (on dry weight basis/100 g)
| Variety | Total protein (g) | Total soluble sugars (g) | Total lipids (g) | Starch (g) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R | DH | G | C | R | DH | G | C | R | DH | G | C | R | DH | G | C | |
| VL 125 | 23.19b | 23.30a
(+0.47) |
22.36a,b
(−3.58) |
20.29b,c
(−12.51) |
19.47a | 19.86b
(+2.00) |
17.81a
(−8.53) |
25.52a
(+31.07) |
2.69a,b | 3.27b
(21.56) |
2.61b
(−2.97) |
2.07a
(−23.05) |
29.01a | 33.43a
(15.25) |
26.13a
(−9.93) |
19.00a,b
(−34.51) |
| VL 133 | 25.46a | 24.19a
(−4.99) |
22.84a
(−10.29) |
23.83a
(−6.40) |
16.90a,b | 20.74b
(+22.72) |
18.97a
(+12.25) |
19.48b
(+15.27) |
2.79a,b | 3.58b
(28.32) |
2.05c
(−26.52) |
1.81a
(−35.13) |
24.93b | 30.44a,b
(22.10) |
21.44b
(−14.00) |
13.92c
(−44.16) |
| VL 142 | 19.08d | 18.53c
(−2.88) |
20.11c
(+5.40) |
18.77c
(−1.62) |
18.54a,b | 26.26a
(+41.64) |
17.01a
(−8.25) |
19.81b
(+6.85) |
3.58a | 4.36a,b
(21.79) |
3.23a
(−9.78) |
2.01a
(−43.85) |
21.53c | 30.71a,b
(42.64) |
15.64c
(−27.36) |
17.79b
(−17.37) |
| VL 507 | 23.22b | 23.20a
(−0.09) |
22.40a,b
(−3.53) |
24.15a
(+4.01) |
15.51b | 21.62b
(+39.39) |
16.70a
(+7.67) |
19.71b
(+27.08) |
3.36a | 4.80a
(42.86) |
2.37b,c
(−29.46) |
1.39b
(−58.63) |
23.17b,c | 27.96b,c
(20.67) |
14.93c
(−35.56) |
20.73a
(−10.53) |
| VL 514 | 21.39c | 20.90b
(−2.29) |
20.42b,c
(−4.53) |
21.56b
(+0.79) |
18.24a,b | 22.30a,b
(+22.26) |
16.54a
(−9.32) |
18.62b
(+2.08) |
2.58b | 3.41b
(42.86) |
2.28b,c
(−11.63) |
1.31b
(−49.22) |
25.20b | 30.23a,b
(19.96) |
22.05b
(−12.50) |
17.38b
(−31.03) |
| Average | 22.47A | 22.02A | 21.63A | 21.72A | 17.73B | 22.16A | 17.41B | 20.63A | 3.00A,B | 3.88A | 2.51B | 1.72C | 24.77b | 30.55a | 20.04c | 17.76c |
Values in parentheses indicate % increase or % decrease (negative sign) over raw values
a, b, c and d superscript are significantly (p < 0.05) different cultivar within a column
A, B, C and D superscript are significantly (p < 0.05) different treatment within a row
R raw, DH dehulled, G germinated, C cooked
Total lipid in lentil was affected by dehulling, germination and cooking (Table 1). Lipid content increased significantly (p < 0.05) after dehulling whereas, it decreased significantly (p < 0.05) after germination and cooking. This could be due to total solid loss during soaking prior to germination or use of fat as an energy source in sprouting process (Wang et al. 1997). Cooking significantly decreased fat content for all lentil varieties (1.31–2.07 %) as compared to the raw seeds (2.58–3.58 %). The total lipids in germinated samples varied from 2.50 (VL Masoor 133) to 3.23 (VL 142) percent of dry matter, whereas after dehulling, lowest (3.27 %) was in the VL Masoor 125 and the highest (4.80 %) VL Masoor 507. Total lipid levels improved significantly after dehulling due to removal of hull portion and concentration of endosperm. The results were comparable with findings of Ghavidel and Prakash (2007) for lentils.
Starch content increased significantly (p < 0.05) after dehulling (Table 1) whereas it decreased after germination and cooking. Starch content in raw samples ranged from 21.53 g/100 g for VL 142 to 29.01 g/100 g for VL Masoor 125. Dehulling resulted in 15.25–42.64 % increase in starch content whereas germination and cooking caused 9.93–35.56 and 10.53–44.16 % decrease. Starch level improved significantly after dehulling which could be due to removal of hull portion that have less content of starch confirming results reported by Wang (2008). A previous study in lentil showed a significant decrease in starch content after germination (Frias et al. 1998).
Effect of dehulling and germination on anti-nutritive properties
The results on antinutritional quantitative analysis for the raw, dehulled, germinated and cooked lentil samples are presented in Table 2. The concentration of tannins in raw, dehulled, germinated and cooked samples ranged from 5.13 to 9.38, 0.39 to 0.87, 1.19 to 3.1 and 1.01 to 2.25 g/kg, respectively. Significant differences among all treatments viz., control, dehulled, germinated and cooked seeds were observed. Germination and dehulling significantly (p < 0.05) reduced tannin content of lentil as previously observed by Ghavidel and Prakash (2007) in cowpea, chickpea, green gram and lentil. Germination resulted in a decrease of 58.57–66.52 % tannins whereas cooking caused a 68.57–84.63 % reduction in tannin content for the five lentil varieties. After dehulling, there was a little tannin detectable in cotyledons, indicating that most of the tannin are present in seed coat. Pal et al. (2016) also reported similar results for horse gram.
Table 2.
Effect of dehulling, germination and cooking on tannins, phytic acid and trypsin inhibitor activity (TIA) contents of lentil flours (on dry weight basis)
| Variety | Tannins (g/kg dry matter) | Phytic acid (%) | TIA (mg/g dry matter) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R | DH | G | C | R | DH | G | C | R | DH | G | C | |
| VL 125 | 9.38a | 0.87a
(−90.72) |
3.14a
(−66.52) |
2.25a
(−76.01) |
0.25d | 0.11b
(−56.00) |
0.15a,b
(−40.00) |
0.17b,c
(−32.00) |
2.36b | 1.33d
(−43.64) |
0.90b
(−61.86) |
0.46a,b
(−80.51) |
| VL 133 | 6.83b,c | 0.72b
(−89.46) |
2.83a,b
(−58.57) |
1.05c
(−84.63) |
0.32b | 0.13b
(−59.38) |
0.13b
(−59.38) |
0.18a,b,c
(−43.75) |
3.62a | 2.13b
(−41.16) |
1.09a
(−69.89) |
0.53a,b
(−85.36) |
| VL 142 | 5.95c,d | 0.44c
(−92.61) |
2.46b
(−58.56) |
1.87b
(−68.57) |
0.38a | 0.18a
(−52.63) |
0.17a
(−55.26) |
0.21a
(−44.74) |
2.39b | 1.64c
(−31.38) |
0.88b
(−63.18) |
0.39b
(−83.68) |
| VL 507 | 7.28b | 0.51c
(−92.99) |
2.84a,b
(−60.99) |
2.07a,b
(−71.57) |
0.30b,c | 0.12b
(−60.00) |
0.13b
(−56.67) |
0.19a,b
(−36.67) |
4.25a | 2.55a
(−40.00) |
1.22a
(−71.29) |
0.62a
(−85.41) |
| VL 514 | 5.13d | 0.39c
(−92.40) |
1.91c
(−62.77) |
1.01c
(−80.31) |
0.27c,d | 0.12b
(−55.56) |
0.15a,b
(−44.44) |
0.16c
(−40.74) |
2.20b | 1.17d
(−46.82) |
0.83b
(−62.27) |
0.41b
(−81.36) |
| Average | 6.91A | 0.59D | 2.64B | 1.65C | 0.30A | 0.13C | 0.15C | 0.18B | 2.96A | 1.76B | 0.98C | 0.48D |
Values in parentheses indicate % increase or % decrease (negative sign) over raw values
a, b, c and d superscript are significantly (p < 0.05) different cultivar within a column
A, B, C and D superscript are significantly (p < 0.05) different treatment within a row
R raw, DH dehulled, G germinated, C cooked
The amount of phytic acid in raw sample was found significantly higher than dehulled, germinated and cooked samples (Table 2). In raw samples, phytic acid varied from 0.25 (VL Masoor 125) to 0.38 (VL 142) mg/100 mg whereas in dehulled samples it varied from 0.11 (VL Masoor 125) to 0.18 (VL 142) mg/100 mg. This result correlates well with an earlier report on wheat, that dehulling to get refined flours considerably reduced phytate content (Ghavidel and Prakash 2007). In germinated samples, phytic acid varied from 0.13 (VL Masoor 133, VL Masoor 507) to 0.17 (VL 142) mg/100 mg. Dehulling resulted in a decrease of 52.63–56.00 % of phytic acid content whereas germination caused a 40.00–59.38 % reduction in phytic acid content in all lentil varieties used in the study. In cooked samples, VL 142 contained the highest (0.21 mg/100 mg) whereas, VL Masoor 514 contained the lowest (0.16 mg/100 mg) amount of phytic acid. Decrease in phytic acid content during germination could be due to increase in phytase activity as reported in horse gram (Borhade et al. 1984). Previous report showed that phytate phosphorus significantly decreased with germination and it accounted for only 20 % in horse gram and 26 % in moth bean, of the total phosphorus in the germinating seeds after 48 h (Borhade et al. 1984). High level of phytic acid is of nutritional significance as not only is the phytate phosphorus unavailable to humans, but it also lowers the availability of many other essential minerals (Ghavidel and Prakash 2007). Trypsin inhibitors from lentil seeds in the raw, dehulled, germinated and cooked samples had an average content of 2.96, 1.76, 0.98 and 0.48 mg/g dry matter; raw samples contained significantly higher amount of trypsin inhibitors than all other processed samples. In raw samples, trypsin inhibitor varied from 2.20 (VL Masoor 514) to 4.25 (VL Masoor 507) mg/g, whereas in dehulled samples it varied from 1.17 (VL Masoor 514) to 4.25 (VL Masoor 507) mg/g. Amongst germinated and cooked samples, VL Masoor 507 contained the highest (1.22 and 0.62 mg/g, respectively) whereas, VL Masoor 514 in germinated and VL Masoor 142 in cooked samples contained the lowest amount (0.83 and 0.39 mg/g, respectively) of trypsin inhibitors. Dehulling, germination and cooking resulted in 31.38–46.82, 61.86–71.29, 81.36–85.41 % reduction, respectively, in trypsin inhibitors content for the five lentil varieties. Wang et al. (1997) reported that trypsin inhibitors decreased during germination and increased slightly as the length of germination increased. Sangronis and Machado (2007) found a significant decrease of trypsin inhibitor activity (TIA) in pigeon pea, white beans and black beans after 5 days of germination. Cooking has been reported to be effective in inactivating protease inhibitors in pulses (Wang 2008). Data from this study indicated that cooking reduced TIA more effectively than dehulling, confirming the finding of Wang et al. (2009).
Effect of dehulling and germination on antioxidative metabolites
Concentrations of the individual phenolic compounds in raw and cooked lentils are summarized in Table 3. Results showed that dehulling, germination and cooking significantly (p < 0.05) decreased the gallic acid, catechins di-hydrate, and quercetin contents in lentil flours. Gallic acid in raw samples varied from 195.59 µg/g (VL 142) to 236.99 µg/g (VL Masoor 125) whereas after dehulling, VL Masoor 507 recorded the highest (149.89 µg/g) and VL Masoor 125 recorded the lowest (117.84 µg/g) gallic acid. Dehulling resulted in a decrease of 25.11–50.28 % dry matter in dehulled seeds. This indicated that gallic acid is present mainly in seed coats, which was in agreement with the results reported by Xu and Chang (2008). The mean gallic acid content in germinated seeds flour was 160.07 µg/g dry matter (Table 3), which was in the range reported by Xu and Chang (2008). Germination significantly (p < 0.05) reduced gallic acid content in the lentil varieties (Table 3). The results are in agreement with that reported in other pulses (Rao and Prabhavathi 1982).
Table 3.
Effect of dehulling, germination and cooking on gallic acid, catechins di-hydrate, and quercetin contents of lentil flours (on dry weight basis/g)
| Variety | Gallic acid (µg/g) | Catechins di-hydrate (µg/g) | Quercetin (µg/g) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R | DH | G | C | R | DH | G | C | R | DH | G | C | |
| VL 125 | 236.99a | 117.84b
(−50.28) |
154.83b,c
(−34.67) |
197.80a
(−16.54) |
166.63a | 54.80b
(−67.11) |
77.06a
(−53.75) |
111.30a
(−33.21) |
100.09a | 31.99d
(−68.04) |
55.63c
(−44.42) |
54.24b
(−45.81) |
| VL 133 | 211.07b | 131.73a,b
(−37.59) |
182.22a
(−13.67) |
149.65b
(−29.10) |
136.01c | 61.13b
(−55.05) |
56.69b
(−58.32) |
74.75c
(−45.04) |
67.50d | 29.58d
(−56.18) |
65.36b
(−3.17) |
39.23d
(−41.88) |
| VL 142 | 195.59b | 132.66a,b
(−32.17) |
155.06b,c
(−20.72) |
190.34a
(−2.68) |
149.80b | 60.90b
(−59.35) |
72.03a
(−51.92) |
95.20b
(−36.45) |
75.68c | 36.45c
(−51.84) |
36.51d
(−51.76) |
46.59c
(−38.44) |
| VL 507 | 200.14b | 149.89a
(−25.11) |
172.11a,b
(−14.01) |
200.04a
(−0.05) |
126.56d | 55.07b
(−56.49) |
77.21a
(−38.99) |
98.16a,b
(−22.44) |
63.35d | 60.38a
(−4.69) |
55.98c
(−11.63) |
60.88a
(−3.90) |
| VL 514 | 228.44a | 142.35a
(−37.69) |
136.12c
(−40.41) |
191.39a
(−16.22) |
143.82b | 91.43a
(−36.43) |
71.03a
(−50.61) |
100.35a,b
(−30.23) |
87.30b | 53.71b
(−38.48) |
82.91a
(−5.03) |
45.12c
(−48.32) |
| Average | 214.45A | 134.89D | 160.07C | 185.84B | 144.56A | 64.67C | 70.80C | 95.95B | 78.78A | 42.42C | 59.28B | 49.21B,C |
Values in parentheses indicate % increase or % decrease (negative sign) over raw values
a, b, c and d superscript are significantly (p < 0.05) different cultivar within a column
A, B, C and D superscript are significantly (p < 0.05) different treatment within a row
R raw, DH dehulled, G germinated, C cooked
Gallic acid content in the cooked samples were varied from 149.65 (VL Masoor 133) to 200.04 (VL Masoor 507) µg/g, which were significantly lower than in the raw but higher than germinated seeds. The reduction may be due to heat-induced breakdown of cellular constituents and cell walls which can lead to enhanced release of certain food bioactives (Randhir et al. 2008). Dehulling, germination and cooking resulted in 25.11–50.28, 13.67–40.41 and 0.05–29.10 % reduction respectively in gallic acid content in the five lentil varieties.
Catechins di-hydrate (CDH) content in raw samples varied from 126.56 (VL Masoor 507) to 166.63 (VL Masoor 125) µg/g in lentil varieties with a mean of 144.56 µg/g dry matter (Table 3), which was comparable with that reported by Randhir et al. (2008). Dehulling resulted in a decrease of 36.43–67.11 % CDH in dehulled seeds whereas germination caused a significant reduction (38.99–58.32 %) in CDH content. Similar result was reported by Randhir et al. (2008) in lentil. CDH content in the cooked samples ranged from 74.75 (VL Masoor 133) to 111.30 (VL Masoor 125) µg/g which showed is a significant reduction (22.44–45.04 %) from raw samples. Quercetin (Q) content in raw samples varied from 63.35 (VL Masoor 507) to 100.09 (VL Masoor 125) µg/g in lentil varieties with a mean of 78.78 µg/g dry matter (Table 3), which was comparable with that earlier report by Wang and Daun (2004). Dehulling resulted in a decrease of 4.69–68.04 %, Q content in dehulled seeds whereas germination caused a significant reduction (3.17–51.76 %) in Q content. Quercetin content in the cooked samples were from 39.23 (VL Masoor 133) to 60.88 (VL Masoor 507) µg/g which showed significant reduction (3.90–48.32 %) from raw samples. Xu and Chang (2008) observed that about 30–40 % of phenolics could be removed from common beans by cooking and discarding the cooking water. In the present study, it was found that on an average about 33.63–55.27 % of catechins di-hydrate and 24.76–46.15 % quercetin, the major flavonoids were reduced in lentil due to processing, and 13.13–37.10 % of gallic acid, a major polyphenol in lentil were reduced due to different processing methods.
Effect of dehulling and germination on antioxidant activities
As shown in Table 4, the total antioxidant activity (TA) by phosphomolybdate method of lentil was affected by the dehulling, germination and cooking. The phosphomolybdenum method usually detects antioxidants such as ascorbic acid, some phenolics, α-tocopherol, and carotenoids (Prieto et al. 1999). The results showed that the overall, TA decreased significantly (p < 0.05) after dehulling and cooking whereas the total antioxidant activity decreased non significantly by germination. Total antioxidant activity in raw samples varied from 7.12 (VL Masoor 507) to 8.19 µM TE/g DW (VL Masoor 125) whereas after dehulling, a significant decrease (10.70–38.95 %) in amount of total antioxidant activity was found and the lowest was recorded (5.00 µM TE/g DW) in VL Masoor 125 and the highest in VL Masoor 514 (6.93 µM TE/g DW). In cooked samples, the highest TA (6.94 µM TE/g DW) was found in VL Masoor 514 whereas the lowest (4.39 µM TE/g DW) was recorded in VL Masoor 125 which showed a significant reduction in TA (7.58–46.40 %) from raw samples. Ferric reducing antioxidant power (FRAP) assay is a colorimetric method based on the reduction of a ferrictripyridyltriazine (TPTZ) complex to its ferrous form. This reduction creates an intense blue complex with an absorption maximum at 593 nm (Benzie and Strain 1996). FRAP value of the studied lentil samples were affected by processing (dehulling, germination and cooking). The results showed that the FRAP value decreased significantly (p < 0.05) after dehulling, germination and cooking. Dehulled samples showed the lowest FRAP value among all treatments. In raw samples the FRAP value varied from 24.72 (VL Masoor 125) to 37.64 µM TE/g DW (VL Masoor 507) whereas after dehulling, a significant decrease (50.29–62.08 %) in amount of FRAP value was recorded and it was the lowest in VL 142 (11.16 µM TE/g DW) and the highest in VL Masoor 507 (18.71 µM TE/g DW). In germinated samples, the highest content of FRAP value with 15.33 % decrease from raw (20.93 µM TE/g DW) was found in VL Masoor 125 whereas the lowest content of FRAP value with 61.01 % decrease from raw was recorded in VL Masoor 514 (13.51 µM TE/g DW). FRAP value in the cooked samples varied from 14.39 (VL Masoor 125) to 31.63 µM TE/g DW (VL Masoor 507) which showed is significant reduction (8.68–41.79 %) from raw samples. The high reducing power exhibited by the extracts might be indicative of the hydrogen donating ability of the active species present in the extracts. The results showed that the reducing power decreased significantly (p < 0.05) after dehulling, germination and cooking. Dehulled samples showed the lowest reducing power among all treatments. In raw samples the reducing power varied from 0.14 (VL Masoor 507) to 0.23 Abs unit at 700 nm (VL Masoor 514) whereas after dehulling, a significant decrease (57.89–85.71 %) in the reducing power was recorded which lowest was (0.02) in VL Masoor 507 and the highest (0.09) in VL Masoor 514. In germinated samples, the highest reducing power with 30.43 % decrease over raw sample was found in VL Masoor 514 (0.16) whereas the lowest amount of reducing power with 50.0 % decrease over raw was recorded in VL Masoor 507 (0.07). The results are in agreement with the study conducted in horse gram by Pal et al. (2016). Reducing power in the cooked samples varied from 0.05 (VL Masoor 507) to 0.11 (VL Masoor 133) Abs unit at 700 nm which showed a significant reduction (42.11–64.29 %) over raw samples.
Table 4.
Effect of dehulling, germination and cooking on total antioxidant activity, FRAP value, and reducing power of different varieties lentil
| Variety | Total antioxidant activity (µM TE/g DW) | FRAP value (µM TE/g DW) | Reducing power (Abs 700 nm) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R | DH | G | C | R | DH | G | C | R | DH | G | C | |
| VL 125 | 8.19a | 5.00c
(−38.95) |
8.57a
(4.64) |
4.39d
(−46.40) |
24.72b | 11.58c
(−53.16) |
20.93a
(−15.33) |
14.39c
(−41.79) |
0.20a,b | 0.06b
(−70.00) |
0.10b
(−50.00) |
0.08b
(−60.00) |
| VL 133 | 7.48b,c | 5.89b
(−21.26) |
7.77b
(3.88) |
5.94c
(−20.59) |
29.39b | 12.82b,c
(−56.38) |
14.71a,b
(−49.95) |
26.84a,b
(−8.68) |
0.19b | 0.08a
(−57.89) |
0.12b
(−36.84) |
0.11a
(−42.11) |
| VL 142 | 7.80a,b | 5.69b,c
(−27.05) |
6.85d
(−12.18) |
6.05c
(−22.44) |
29.43b | 11.16c
(−62.08) |
13.83a,b
(−53.01) |
26.16b
(−11.11) |
0.20a,b | 0.04c
(−80.00) |
0.12b
(−40.00) |
0.09b
(−55.00) |
| VL 507 | 7.12c | 6.34a,b
(−10.96) |
7.66b,c
(7.58) |
6.58b
(−7.58) |
37.64a | 18.71a
(−50.29) |
16.15a,b
(−57.09) |
31.63a
(−15.97) |
0.14c | 0.02c
(−85.71) |
0.07c
(−50.00) |
0.05c
(−64.29) |
| VL 514 | 7.76b | 6.93a
(−10.70) |
7.22c
(−6.96) |
6.94a
(−10.57) |
34.65a | 15.30b
(−55.84) |
13.51b
(−61.01) |
31.57a
(−8.89) |
0.23a | 0.09a
(−60.87) |
0.16a
(−30.43) |
0.13a
(−43.48) |
| Average | 7.67A | 5.97B | 7.61A | 5.98B | 31.17A | 13.91C | 15.83C | 26.12B | 0.19A | 0.06B | 0.11A,B | 0.09B |
Values in parentheses indicate % increase or % decrease (negative sign) over raw values
a, b, c and d superscript are significantly (p < 0.05) different cultivar within a column
A, B, C and D superscript are significantly (p < 0.05) different treatment within a row
R raw, DH dehulled, G germinated, C cooked
Effect of dehulling and germination on fatty acid profile
Although lentils contain only 2.58–3.58 % oil, its fatty acid composition had a very favorable omega-3 to omega-6 ratio (1:4) (Zhang et al. 2014). The effect of dehulling, germination and cooking on the fatty acid composition of lentils was investigated, and the results are summarized in Table 5. In all lentil cultivars, linoleic acid was the dominant fatty acid ranging from 32.35 to 39.19 %, followed by oleic acid (27.00–33.85 %), palmitic acid (15.48–17.15 %) and linolenic acid (10.34–11.51 %). These results are in agreement with those reported by Zhang et al. (2014), in their study on 20 Canadian lentil cultivars. The high levels of unsaturated fatty acids make lentil a suitable legume for nutritional applications. The potential benefits of PUFA include prevention of cardiovascular diseases and other health problems (Ajayi and Ajayi 2009). Myristic acid content in raw samples varied from 0.30 (VL Masoor 514) to 0.80 % (VL Masoor 507) whereas after dehulling, a significant decrease (10.00–34.48 %) in amount of myristic acid was found and was recorded the lowest (0.27 mg/100 mg) in VL Masoor 514 and the highest (0.38 mg/100 mg) in VL Masoor 133 and VL 142. It was found that in general, germination and cooking significantly (p < 0.05) reduced the myristic acid over raw samples; which was in line with an earlier report on lentil (Zhang et al. 2014). Dehulling caused a significant decrease (6.59–10.82 %) in amount of palmitic acid whereas cooking did not have any significant effect on the palmitic acid content. Stearic acid content was significantly decreased by dehulling and cooking whereas germination did not have significant effect on the stearic acid content.
Table 5.
Effect of dehulling, germination and cooking on fatty acids (% dry weight basis) of different blentils varieties
| Variety | Myristic acid (14:0) | Palmitic acid (16:0) | Stearic acid (18:0) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R | DH | G | C | R | DH | G | C | R | DH | G | C | |
| VL 125 | 0.53c | 0.37a
(−30.19) |
0.36b
(−32.08) |
0.35c
(−33.96) |
16.70a | 15.41a
(−7.72) |
16.29a
(−2.46) |
16.33a
(−2.22) |
3.54b | 2.07c
(−41.53) |
3.16b,c
(−10.73) |
2.40a,b
(−32.20) |
| VL 133 | 0.58b | 0.38a
(−34.48) |
0.49a
(−15.52) |
0.38b
(−34.48) |
17.15a | 15.42a
(−10.09) |
16.70a
(−2.62) |
16.35a
(−4.66) |
3.43b,c | 2.15c
(−37.32) |
3.26b
(−4.96) |
2.18b
(−36.44) |
| VL 142 | 0.46d | 0.38a
(−17.39) |
0.38b
(−17.39) |
0.43a
(−6.52) |
16.44a | 15.29a
(−7.00) |
15.34a
(−6.69) |
16.76a
(1.95) |
4.90a | 3.28a
(−33.06) |
4.75a
(−3.06) |
2.24b
(−54.2)9 |
| VL 507 | 0.80a | 0.36a
(−55.00) |
0.52a
(−35.00) |
0.37b
(−53.75) |
15.48a | 14.46a
(−6.59) |
15.40a
(−0.52) |
15.50a
(0.13) |
3.25b,c | 2.65b
(−18.46) |
2.64c
(−18.77) |
1.74c
(−46.46) |
| VL 514 | 0.30e | 0.27b
(−10.00) |
0.27c
(−10.00) |
0.29c
(−3.33) |
16.55a | 14.76a
(−10.82) |
15.48a
(−6.47) |
16.28a
(−1.63) |
3.01c | 2.58b
(−14.29) |
2.95b,c
(−1.99) |
2.64a
(−12.29) |
| Average | 0.53A | 0.35B | 0.40B | 0.37B | 16.46A | 15.07B | 15.84A,B | 16.24A | 3.63A | 2.55B | 3.35A | 2.24B |
| Variety | Oleic acid (18:1) | Linoleic acid (18:2) | Linolenic acid (18:3) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VL 125 | 28.04b | 25.59b
(−8.74) |
35.20a
(25.53) |
28.87b
(2.96) |
39.19a | 40.58a,b
(3.55) |
33.19b
(−15.31) |
37.66a
(−3.90) |
10.34a | 11.33a
(9.57) |
8.91a
(−13.83) |
10.71a
(3.58) |
| VL 133 | 28.05b | 26.63b
(−5.06) |
30.51b
(8.77) |
30.59b
(9.06) |
35.65a,b | 40.67a
(14.08) |
31.28c
(−12.26) |
30.81b
(−13.58) |
11.51a | 12.05a
(4.69) |
9.67a
(−15.99) |
10.56a
(8.25) |
| VL 142 | 27.00b | 26.53b
(−1.74) |
29.72b
(10.07) |
27.67b
(2.48) |
38.20a | 41.77a
(9.32) |
32.80b
(−14.16) |
37.88a
(−0.86) |
11.03a | 11.69a
(5.98) |
9.15a
(−17.04) |
11.67a
(5.80) |
| VL 507 | 33.85a | 33.48a
(−1.09) |
35.38a
(4.52) |
36.35a
(7.39) |
32.35b | 34.48b
(6.58) |
31.26c
(−3.37) |
31.43b
(−2.84) |
10.70a | 11.59a
(8.32) |
9.02a
(−15.70) |
10.53a
(−1.59) |
| VL 514 | 30.39a,b | 28.28b
(−6.94) |
30.50b
(0.36) |
31.01b
(2.04) |
35.93a,b | 39.64a
(10.33) |
35.92a
(−0.03) |
34.33a,b
(−4.45) |
10.90a | 12.04a
(10.46) |
9.67a
(−11.28) |
11.34a
(4.04) |
| Average | 29.47A | 28.10A | 32.26A | 30.90A | 36.27B | 39.43A | 32.89B | 34.42A,B | 10.90A | 11.74A | 9.28B | 10.96A |
Values in parentheses indicate % increase or % decrease (negative sign) over raw values
a, b, c and d superscript are significantly (p < 0.05) different cultivar within a column
A, B, C and D superscript are significantly (p < 0.05) different treatment within a row
R raw, DH dehulled, G germinated, C cooked
The oleic acid content in raw samples varied from 27.00 (VL 142) to 33.85 % (VL Masoor 507). Dehulling caused no significant decrease (1.09–8.74 %) in the amount of oleic acid which was the lowest (25.59 %) in VL Masoor 125 and the highest (33.48 %) in the VL Masoor 507. Germinated samples showed increase (0.36–25.53 %) in the oleic acid content over the raw samples. In germinated samples, the highest content of oleic acid (35.38 %) was found for VL Masoor 507 whereas the lowest (29.72 %) was recorded for VL 142. Cooked samples showed increase (2.04–9.06 %) in the oleic acid content over the raw samples. Decline in oleic acid may be due to presence of this fatty acid in hull portion. The results are in the accordance with an earlier report in pearl millet (Zhang et al. 2014).
The linoleic acid content varied from 32.35 to 39.19 mg/100 mg in the different raw samples of lentil. Overall, linoleic acid content increased significantly after the dehulling whereas, germination and cooking, non-significantly decreased the content based on mean of varieties. Dehulled samples showed increase (3.55–10.33 %) whereas germinated (0.03–15.31 %) and cooked samples (0.86–13.58 %) showed decrease in the content of linoleic acid content over the raw samples. The linolenic acid content varied from 10.34 to 11.51 % in the different raw samples of lentil. No significant difference among varieties in all treatments were found. Overall, linoleic acid content decreased significantly after germination while after dehulling and cooking, non-significant increase was seen. The decrease in fatty acid could be due to hydrolysis during germination and used to produce the necessary energy for the biochemical and physicochemical modifications, which occurred in the seed (Moongngarm and Saetung 2010).
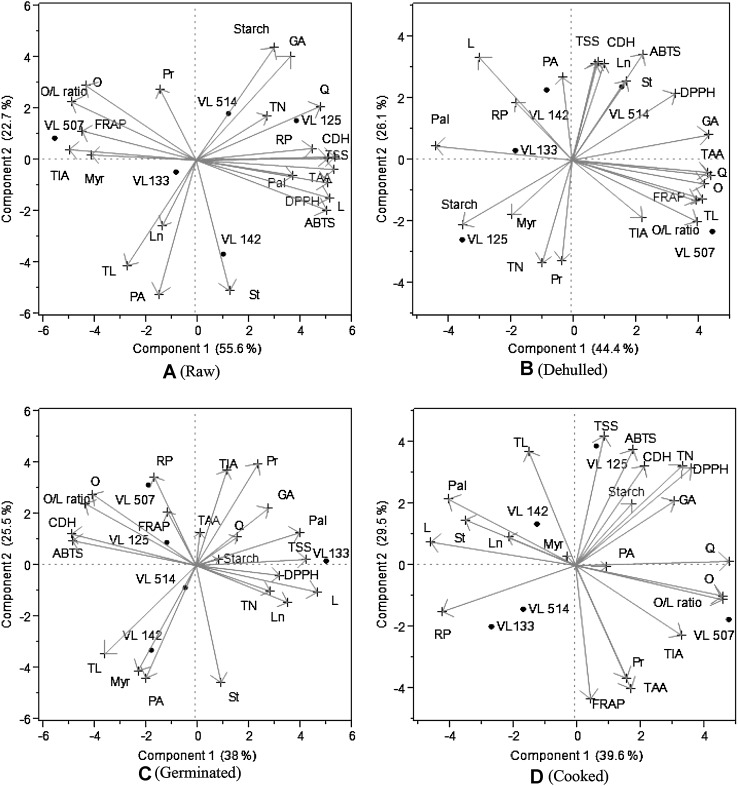
Principal component analysis (PCA)
Principal component analysis (PCA) is a useful statistical technique, which has found applications in finding out interrelationships between the different variables (Mishra et al. 2013). The projections of varieties and traits are shown in PC1 and PC2 biplot (Fig. 1a–d). In PCA the length, direction and the angles between the lines indicate correlation between the variables or between variables and principal component axes (e.g., α = 0° and/or 180° and r = 1; α = 90° and r = 0). The longer the line, the higher is the variance. The cosine of the angle between the lines approximates the correlation between the variables they represent. The closer the angle is to 90° or 270°, the smaller the correlation. An angle of 0° or 180° reflects a correlation of 1 or −1, respectively (Lopez et al. 2006).
Fig. 1.
Multifactorial comparison and correlation matrix of studied parameters (TN-starch, GA-gallic acid, TN-tannins, Q-quarcitin, TL-total lipid, PA-phytic acid, TIA-trypsin inhibitor activity, C-catechins, Q-quercetin, Myr-myristic acid, RP-reducing power, CDH-catechins di-hydrate, TSS-total soluble sugars, TAA-total antioxidant activity, Pal-palmitic acid, FRAP- ferric reducing antioxidant power, ST-stearic acid, Pal-palmitic acid, O-oleic acid, L-linoleic acid, Ln-linolenic acid) obtained from lentil varieties using principal component analysis (PCA) in raw (A), dehulled (B), germinated (C) and cooked lentils (D)
In present study, multifactorial comparisons using principal component analysis clearly indicated correlation between various nutritive, anti-nutritive, fatty acids, antioxidant parameters and their relationship under raw, dehulled, germinated and cooked lentil samples. The principal component analysis (PCA) and their correlation are shown in raw, dehulled, germinated and cooked samples (Fig. 1a–d). Among the data, first component 1 represented 55.60 % of variability, whereas the component 2 represented 22.70 % of variability in case of raw samples whereas in case of dehulled, germinated and cooked samples component 1 represented 44.40, 38.00 and 39.60 % of variability and the component 2 represented 26.10, 25.50 and 29.50 % variability, respectively. In case of raw samples, most of the parameters (TN—starch, GA—gallic acid, TN—tannins, Q—quarcitin, RP—reducing power, CDH—catechins di-hydrate, TSS—total soluble sugars, TAA—total antioxidant activity, Pal—palmitic acid, DPPH radical inhibition, ABTS radical inhibition, ST—stearic acid) were occupied on the right side of the biplot. This suggested that these parameters had positive correlation among themselves and these parameters have negative correlation with the parameters occupying on the left side of the biplot. In case of dehulled, germinated and cooked samples, some of the parameters showed change in their positions from one bi-plot to another bi-plot. Based on this mathematical rule, uncorrelated variables occur at right angles to one another because the cosine of the angle between them is cosine 90° = 0, or not correlated. Similarly, the cosine of 0 is 1, which denotes a positive correlation between the variables (Lopez et al. 2006).
Conclusion
The variety and processing (cooking and dehulling) affected the composition, minerals and anti-nutritional factors in lentils. Significant differences in proximate composition, antioxidant metabolites, antioxidant activities, trypsin inhibitor activity (TIA), phytic acid and tannin content were found among different treatments based on average of studied varieties. The antioxidant compound (GA, CDH and Q), antioxidant capacities (total antioxidant activity and ferric reducing antioxidant power) and free radical scavenging activity against DPPH and ABTS were significantly decreased after dehulling and germination. Present study also suggest that germination and cooking would be useful in formulation of foods where lentil oil is an important consideration.
Acknowledgments
The authors are grateful to Indian Council of Agricultural Research (ICAR), for financial support to carry out this work at Vivekanand Parvatiya Krishi Anusandhan Sansthan (VPKAS), Almora (Uttarakhand) 263601.
Compliance with ethical standards
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- Adsule RN, Kadam SS. Proteins. In: Salunkhe DK, Kadam SS, editors. Handbook of world food legumes 1. Florida: CRC Press; 1989. pp. 76–91. [Google Scholar]
- Aguilera Y, Dueñas M, Estrella I, Hernández T, Benitez V, Esteban RM, Martín-Cabrejas MA. Evaluation of phenolic profile and antioxidant properties of Pardina lentil as affected by industrial dehydration. J Agric Food Chem. 2010;58:10101–10108. doi: 10.1021/jf102222t. [DOI] [PubMed] [Google Scholar]
- Ajayi OB, Ajayi DD. Effect of oilseed diets on plasma lipid profile in albino rats. Pak J Nutr. 2009;8:116–118. [Google Scholar]
- AOAC . Official methods of analysis. 18. Washington, DC: Association of Official Analytical Chemists; 2005. [Google Scholar]
- Benzie I, Strain J. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Phys. 1959;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Borhade VP, Kadam SS, Salunke DK. Solubilization and functional properties of mothbean (Vigna aconitifolia marechal) and horse gram (Macrotyloma uniflorum L. Verdc.) J Food Biochem. 1984;8:229–235. doi: 10.1111/j.1745-4514.1984.tb00326.x. [DOI] [Google Scholar]
- Frias J, Fornal J, Ring SG, Vidal-Valverde C. Effect of germination on physico-chemical properties of lentil starch and its components. LWT Food sci Technol. 1998;31(3):228–236. doi: 10.1006/fstl.1997.0340. [DOI] [Google Scholar]
- Ghadge SV, Raheman H. Biodiesel production from mahua (Madhuca indica) oil having high free fatty acids. Biomass Bioenergy. 2005;28(6):601–605. doi: 10.1016/j.biombioe.2004.11.009. [DOI] [Google Scholar]
- Ghavidel RA, Prakash J. The impact of germination and dehulling on nutrients, antinutrients, in vitro iron and calcium bioavailability and in vitro starch and protein digestibility of some legume seeds. LWT Food Sci Technol. 2007;40:1292–1299. doi: 10.1016/j.lwt.2006.08.002. [DOI] [Google Scholar]
- Ghumman A, Kaur A, Sing N (2016) Impact of germination on flour, protein and starch characteristics of lentil (Lens culinaris) and horsegram (Macrotyloma uniflorum L.) lines. LWT Food Sci Technol 65:137–144
- Haug W, Lantzsch HJ. Sensitive method for the rapid determination of phytate in cereals and cereal products. J Sci Food Agr. 1983;34:1423–1426. doi: 10.1002/jsfa.2740341217. [DOI] [Google Scholar]
- Hedge JE, Hofreiter BT (1962) Determination of reducing sugars and carbohydrate. In: Whistler RL, BeMiller JN (eds) Methods in carbohydrate chemistry, vol 17. Academic Press, New York, p 420
- Huda-Faujan N, Noriham A, Norrakiah AS, Babji AS. Antioxidant activity in plants methanol extract containing phenolic compounds. Afr J Biotechnol. 2009;8:484–489. [Google Scholar]
- Kaur M, Sandhu KS. Functional, thermal and pasting characteristics of fl ours from different lentil (Lens culinaris) cultivars. J Food Sci Technol. 2010;47(3):273–278. doi: 10.1007/s13197-010-0042-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez A, Montano A, Garcia P, Garrido A. Fatty acid profile of table olives and its multivariate characterization using unsupervised (PCA) and supervised (DA) chemometrics. J Agric Food Chem. 2006;54:6747–6753. doi: 10.1021/jf0612474. [DOI] [PubMed] [Google Scholar]
- Mishra KK, Pal RS, Arun KR, Chandrashekara C, Jain SK, Bhatt JC. Antioxidant properties of different edible mushroom species and increased bioconversion efficiency of Pleurotus eryngii using locally available casing materials. Food Chem. 2013;138:1557–1563. doi: 10.1016/j.foodchem.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Moongngarm A, Saetung N. Comparison of chemical compositions and bioactive compounds of germinated rough rice and brown rice. Food Chem. 2010;122(3):782–788. doi: 10.1016/j.foodchem.2010.03.053. [DOI] [Google Scholar]
- Pal RS, Bhartiya A, ArunKumar R, Kant L, Aditya JP, Bisht JK. Impact of dehulling and germination on nutrients, antinutrients, and antioxidant properties in horsegram. J Food Sci Technol. 2016;53(1):337–347. doi: 10.1007/s13197-015-2037-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem. 1999;269:337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- Randhir R, Kwon YI, Shetty K. Effect of thermal processing on phenolics, antioxidant activity and health-relevant functionality of select grain sprouts and seedlings. Innov Food Sci Emerg Technol. 2008;9:355–364. doi: 10.1016/j.ifset.2007.10.004. [DOI] [Google Scholar]
- Rao BSN, Prabhavathi T. Tannin content of foods commonly consumed in India and its influence on ionisable iron. J Sci Food Agr. 1982;33:89–96. doi: 10.1002/jsfa.2740330116. [DOI] [PubMed] [Google Scholar]
- Reddy NR, Pierson MD, Sathe SK, Salunkhe DK. Chemical, nutritional and physiological aspects of dry bean carbohydrates: a review. Food Chem. 1984;13:25–68. doi: 10.1016/0308-8146(84)90026-8. [DOI] [Google Scholar]
- Sangronis E, Machado CJ. Influence of germination on the nutritional quality of Phaseolus vulgaris and Cajanus cajan. J Food Sci Agri Technol. 2007;40(1):116–120. [Google Scholar]
- Smith C, Megen WV, Twaalfhoven L, Hitchcock C. The determination of trypsin inhibitor levels in foodstuffs. J Sci Food Agric. 1980;31:321–350. doi: 10.1002/jsfa.2740310319. [DOI] [PubMed] [Google Scholar]
- Torres A, Frias J, Granito M, Vidal-Valverde C. Germinated Cajanus cajan seeds as ingredients in pasta products: chemical, biological and sensory evaluation. Food Chem. 2007;101(1):202–211. doi: 10.1016/j.foodchem.2006.01.018. [DOI] [Google Scholar]
- Vasishtha H, Srivastava RP, Verma P. Effect of dehusking and cooking on protein and dietary fibre of different genotypes of desi, kabuli and green type chickpeas (Cicer arietinum) J Food Sci Technol. 2014;51(12):4090–4095. doi: 10.1007/s13197-012-0909-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal-Valverde C, Frias J, Sierra I, Blazquez I, Lambien F, Kuo YH. New functional legume food by germination: effect on the nutritive value of beans, lentils and peas. Eur Food Res Technol. 2002;215:472–476. doi: 10.1007/s00217-002-0602-2. [DOI] [Google Scholar]
- Wang N. Effect of variety and crude protein content on dehulling quality and on the resulting chemical composition of red lentil (Lens culinaris) J Sci Food Agric. 2008;88:885–890. doi: 10.1002/jsfa.3165. [DOI] [Google Scholar]
- Wang N, Daun JK. Eff ect of variety and crude protein content on nutrients and certain anti-nutrients in field pea (Pisum sativum) J Sci Food Agric. 2004;84:1021–1029. doi: 10.1002/jsfa.1742. [DOI] [Google Scholar]
- Wang N, Lewis MJ, Brennan JG, Westby A. Effect of processing methods on nutrients and antinutritional factors in cowpea. Food Chem. 1997;58:59–68. doi: 10.1016/S0308-8146(96)00212-9. [DOI] [Google Scholar]
- Wang N, Hatcher DW, Toews R, Gawalko EJ (2009) Influence of cooking and dehulling on nutritional composition of several varieties of lentils (Lens culinaris). LWT Food Sci Technol 42:842–848
- Xu B, Chang SK. Effect of soaking, boiling, and steaming on total phenolic content and antioxidant activities of cool season food legumes. Food Chem. 2008;110:1–13. doi: 10.1016/j.foodchem.2008.01.045. [DOI] [PubMed] [Google Scholar]
- Zhang B, Deng Z, Tang Y, Chen P, Liu R, Ramdath DD, Liu Q, Hernandez M, Tsao R. Fatty acid, carotenoid and tocopherol compositions of 20 Canadian lentil cultivars and synergistic contribution to antioxidant activities. Food Chem. 2014;161:296–304. doi: 10.1016/j.foodchem.2014.04.014. [DOI] [PubMed] [Google Scholar]