Abstract
The physicochemical, functional, antioxidant and anticancer properties of protein isolates from the seeds of Soybean (SP), Black soybean (BSP), Adzuki bean (ABP), and Mung bean (MBP) were comparatively characterized. The difference was found in the protein composition and physicochemical properties of the four types of legume proteins, which affected the functional properties and bioactivities. BSP and SP had six predominant proteins with a molecular weight (MW) range of 20–95 kDa, whereas ABP and MBP showed the most intense bands of 48 kDa. ABP with higher essential amino acids content and the highest solubility exhibited the highest antioxidant activities among the four types of proteins. While BSP with higher content of acidic amino acids, low content of the hydrophobic amino acids and higher WHC, may have potential nutraceutical uses.
Keywords: Legume seed proteins, Physicochemical characterization, Functional properties, Antioxidant activities, Anticancer activities
Introduction
Legume seeds have been recognized as a valuable source of high quality protein for a long time, and they are applied in food systems to improve nutrition and functionality, which are attributed to the high-quality proteins, fats, flavonoids, saponins, vitamins and minerals, etc. (Yu et al. 2016). Previous studies have revealed the active components are associated with various beneficial activities, including antioxidant, immunomodulatory (Garcia-Mora et al. 2015), anticancer (Chan et al. 2016) and other biological effects. There are many species of legumes such as soybean (Yu et al. 2016), pinto bean (Garcia-Mora et al. 2015), and kidney bean (Shevkani et al. 2015), etc. Among them, Soybean (Glycine max (L.) Merr.), Black soybean (Glycine max (L.) Merr.), Adzuki bean (Vignaangularis (Willd.) Ohwiet Ohashi) and Mung bean (Vignaangularis (Linn.) Wilczek) are widely cultivated and consumed in the world, which have high content of protein, e.g. soybean about 40% of protein (Yu et al. 2016), and mung bean contain more than 25% of protein (Li et al. 2010). The wide application of legume proteins depends on their physicochemical properties such as solubility, foaming and emulsifying capacities, water and oil holding capacities and other beneficial properties, which are dependent on molecular size, structure, and charge distribution of the protein molecular and processing conditions (Tang and Sun 2011).
Recently, there has been rising public awareness about research on unconventional proteins from legumes sources. Functional and nutritional characteristics of proteins isolated from some varieties of legume seed sources have been studied including soybean (Tang and Sun 2011), pea bean (Stone et al. 2015) and kidney bean (Wani et al. 2015). However, there is no study on comparative evaluations of physicochemical, functional properties and bioactivities of the four kinds of protein. Thus, detailed comparison studies about these four protein isolates are required in order to identify their suitable food applications.
The purpose of this study was to characterize protein separated from Soybean, Black soybean, Adzuki bean and Mung bean for amino acid composition, structure, thermal and their functional properties. The antioxidant and in vitro anticancer activities of the four proteins were also investigated.
Materials and methods
Materials and chemicals
Soybean, Black soybean, Adzuki bean, and Mung bean seeds were purchased from local market of Zhaozhuang (Shandong province, China). The 1-diphenyl-2-picrylhydrazyl (DPPH) was purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). All the other reagents used in the study were of analytical grade.
Total protein preparation
The proteins were prepared by the isoelectric precipitation method (Rayaprolu et al. 2013). Briefly, legume seeds (soybean, black soybean, adzuki bean, and mung bean seeds) were ground in a Christy Laboratory Mill (Cheff Food Processor, Japan) and sieved (250 μm) to obtain uniform particle size. Total protein content (nitrogen × 6.25) in the bean flours were determined by the Kjeldahl method (AOAC Intl 1995). The flours were defatted by using the petroleum ether (flour: petroleum ether, 1:10; w/v) at room temperature for 2 h for three times. Then the defatted flours were suspended in distilled water (1:15; w/v), adjusted to pH 9.5 with 0.5 N NaOH, stirred at room temperature for 60 min. The isolate protein was obtained using isoelectric precipitation method at pH 4.5 and then lyophilized (FD-1-50, Beijing Boyikang Experimental Instrument Co., Ltd, Beijing, China). The four kinds of proteins from Soybean, Black soybean, Adzuki bean, and Mung bean were named as SP, BSP, ABP, and MBP, respectively.
Amino acid analysis
An HPLC system was used to determine the amino acid profiles as described by Misurcova et al. (2014) with some modification. Briefly, protein samples (50 mg) were hydrolyzed with 2 mL of 6 N HCl (containing 0.1% phenol) in an oven at 110 °C in a sealed glass tubes for 24 h. For the determination of sulfur amino acids (Met and Cys), samples were submitted to 16 h oxidation with a mixture of 30% (v/v) hydrogen peroxide and 98% (v/v) formic acid (1:9 v/v) and were subsequently hydrolyzed in the way mentioned above. After the hydrolysis, excess hydrochloric acid was evaporated and the derivatization reagent including phenyl isothiocyanate (PITC), acetonitrile and triethylamine were added. PITC-amino acid derivatives were filtered through a 0.45 μm filter and ready for HPLC analysis on ODS C18 column (10 mm × 250 mm, 5 μm, YMC Co., Ltd. Japan). The flow rate was 1.0 mL/min, column temperature was 36 °C, injection volume was 20 μL and the elution was monitored at 254 nm. The analysis was performed by using a gradient elution with acetate buffer solution (0.1 mol/L, pH 6.5)-acetonitrile (97:3, v/v) as mobile phase A and acetonitrile–water (4:1, v/v) as mobile phase B. The elution was performed with following mobile phase B: 0–39 min, 5–48%; 40–45 min, 100%; 46–60 min, 5%. Mixed standard amino acids were analyzed before sampling. The amino acids of the protein samples were identified and quantified by comparing peak profiles of the protein samples with standard amino acid profiles.
SDS-PAGE profiles
The sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) was performed by the method described by Tan et al. (2014). The final acrylamide concentration of the separating gel and stacking gel were 15.0, 5.0%, respectively. 10 μL samples (16 μg protein isolates) were heated at 95 °C for 5 min prior loading. Electrophoresis was carried out at constant voltage at 120 V until the dye migrated to the bottom of the gel. The gels were stained with Brilliant Blue G-250 (0.1% w/v) in methanol-acetic acid–water (40:10:50 v/v/v) for 2 h and destained in a solution containing methanol-acetic acid–water (40:10:50 v/v/v) for 3 h. The calibration kit (17–180 kDa) was used as the molecular weight marker.
Functional properties assay
Protein solubility in the pH value range of 1.0–11.0 was evaluated according to our previous study (Zhang et al. 2015). Water and oil holding capacity (WHC and OHC) were evaluated according to the method described by Stone et al. (2015). Emulsifying capacity (EC) and foaming capacity (FC) of samples were determined following the method described by Wani et al. (2015). And the thermal behavior was examined using a differential scanning calorimeter (model DSC-60 Plus, Shimadzu, Japan). Samples were placed into coated aluminium pans and were heated from 30 to 250 °C at a rate of 5 °C min−1.
Antioxidant activities assay
Scavenging activity of proteins on DPPH free radicals were determined according to our previous study (Zhang et al. 2015). Briefly, 100 μL protein aqueous solutions at different concentration (6.0–30.0 mg/ml) were mixed with 2.9 mL DPPH solution (120 μM). Then the mixture was incubated in the dark at 37 °C for 30 min and the absorbance of the mixture was read by a UV detector (UV-9200, Shimadzu, Japan) at 517 nm. The DPPH radical-scavenging activity was assessed as follows:
| 1 |
where, A blank is the absorbance of the control, A sample is the absorbance of the test sample.
The ferric reducing power (FRP) of proteins was measured according to the method of Zhou et al. (2012). In brief, 100 μL of different concentrations of protein samples (dissolved in de-ionized water at the concentration of 6.0–30.0 mg/ml) were mixed with 0.7 mL of PBS (0.2 M, pH 6.6) and 2.0 mL of a 30 mM potassium ferricyanide aqueous solution. Reaction mixtures were incubated at 50 °C for 20 min. After incubation, 2.0 mL aliquots of 10% trichloroacetic acid was added and centrifuged at 4000×g for 10 min. Then 1.0 mL upper layer of the solution was mixed well with 3.0 mL of 1.7 mM ferric chloride (FeCl3). Finally, the absorbance was measured at 700 nm using a UV–Vis spectrophotometer (UV-9200, Shimadzu, Japan). Increased absorbance of the reaction mixture indicated higher reducing power.
The hydroxyl radical assay was measured by the method of Ghiselli et al. (1998). Briefly, protein samples were dissolved in de-ionized water at the concentration of 6.0–30.0 mg/ml. Then 0.1 ml deoxyribose (60 mM) was added in the sample solution (0.1 ml). The mixture was mixed with 0.4 ml of reaction buffer [50 mM phosphate buffer (pH 7.4)] and followed by adding 0.1 ml Ferric chloride (2.0 mM)and 0.1 ml EDTA (2.0 mM). 0.1 ml of 10 mM H2O2 and 0.1 ml of 2.0 mM Vc then were added to the reaction solution. The reaction solution was incubated at 37 °C for 60 min and the reaction was stopped by the addition of 1 ml of 25% hydrochloric acid (HCl) and 1 ml of 1% thiobarbituric acid (TBA). After homogenization, the mixture was boiled for 15 min and cooled to room temperature. Subsequently, the absorbance of the mixture at 532 nm was measured. The ability to scavenge hydroxyl radical was assessed as follows:
| 2 |
where A blank is the absorbance of mixture solution without sample; A sample is the absorbance of the sample.
Anticancer activity
Cell culture
Ovarian cancer cell line (SKOV3) and hepatocellular carcinoma cells (SMMC-7721) were obtained from Chinese Academy of Sciences Committee on Type Culture Collection Cell Bank (Shanghai, China). Cells were cultured in DMEM with 10% FBS, 100 U/mL penicillin, 100 g/mL streptomycin and incubated at 37 °C under 5% CO2 atmosphere.
MTT assay
The cancer cell proliferation activity was evaluated by using the MTT assay. Briefly, Cancer cells were seeded into 96-well plates at a density of 5 × 104 cells/mL and then treated with 100 μL samples in DMEM (containing 2.5% DMSO) at different concentration (0.2, 0.4, 0.6, 0.8 and 1.0 mg/mL). After incubation for 72 h, MTT solution (50 μL, 2 mg/mL) was added and incubated for 4 h at 37 °C. Then, 150 μL of dimethyl sulfoxide (DMSO) was added, and the optical density of each well was measured at 492 nm by Multiskan Spectrum (DNM-9602, Perlong Scientific, China).
Statistical analysis
All measurements were run in triplicate and results are expressed as mean ± standard deviation (SD). Analysis was evaluated using the Student’s t test. Statistical significance was defined as P < 0.05.
Results and discussion
Protein extraction from the four legume seeds
Table 1 showed that the protein content of flours from soybean, black soybean, adzuki bean and mung bean were 39.07, 46.94, 26.78, 27.97%, respectively, which were in accordance with the previous reports (Yu et al. 2016; Li et al. 2010; Barac et al. 2015). The black soybean was found to have the highest content of protein among the four legume seeds. The protein extraction from SP, BSP, ABP and MBP were 82.08, 84.61, 78.75, 84.66%, respectively. These differences might be due to the varying content of proteins present in raw materials.
Table 1.
Total protein content and protein extraction yield from different legume
| Flour | Soybean | Black soybean | Adzuki bean | Mung bean |
|---|---|---|---|---|
| Total protein content (%) | 39.07 ± 2.19b | 46.94 ± 0.88a | 26.78 ± 1.75c | 27.97 ± 2.20c |
| Extraction yield (%) | 82.08 ± 1.74a | 84.61 ± 1.58b | 78.75 ± 1.62a | 84.66 ± 3.44a |
All data were expressed by mean values of triplicates ± standard deviation. Means within the same horizontal column not followed by the same letter are significantly different at P < 0.05 level of significance, according to Tukey’s test
Amino acid composition
Amino acid composition differed among the four protein isolates (Table 2). Total amino acid contents of SP, BSP, ABP, and MBP were 883.56, 905.89, 902.61, 923.12 mg g−1, respectively. For all proteins, the most abundant amino acids were found to be glutamic acid (121.73–139.61 mg/g), alanine acid (120.67–129.72 mg/g), and lysine (64.59–100.75 mg/g). The ratio of essential to total amino acid (E/TN) of MBP was obviously higher (0.41) than those of ABP (0.38), SP (0.37), BSP (0.35), which was also higher than the minimum E/TN ratio (0.36) suggested by FAO/WHO/UNU (1985). Among the essential amino acids, lysine and leucine were predominant. The E/TN ratio observed was a little higher than reported for soybean protein (0.36) by Tan et al. (2014), but lower than that of protein from mung bean (0.44) by Kudre et al. (2013) which might be due to the difference of source and extraction methods. All proteins are rich in aspartic acid and glutamic acid especially BSP, which suggested that these proteins possesses acidic characteristic. The contents of hydrophobic amino acids in MBP (45.90%) were the highest, followed by ABP (45.20%), SP (45.18%) and BSP (44.22%), respectively. The hydrophobic amino acids are were reported to be more thermally stable (Kudre et al. 2013). The results demonstrated MBP with higher ratio of essential to total amino acids, especially in content of lysine, leucine, isoleucine, and phenylalanine, which might be a good candidate for functional foods.
Table 2.
Amino acid composition of protein isolates from four common legumes
| Amino acid | SP (mg g−1) | BSP (mg g−1) | ABP (mg g−1) | MBP (mg g−1) |
|---|---|---|---|---|
| Nonessential amino acid | ||||
| Asp | 63.83 ± 0.74 | 72.75 ± 0.22 | 63.67 ± 0.14 | 48.38 ± 0.11 |
| Glu | 125.14 ± 2.4 | 139.61 ± 0.44 | 121.73 ± 4.4 | 126.57 ± 2.2 |
| Ser | 30.45 ± 0.52 | 37.77 ± 1.07 | 35.29 ± 0.71 | 38.53 ± 0.38 |
| Gly | 42.94 ± 2.26 | 43.39 ± 0.43 | 47.08 ± 0.11 | 41.10 ± 0.51 |
| His | 33.40 ± 0.26 | 34.34 ± 0.11 | 32.46 ± 0.16 | 32.32 ± 0.34 |
| Arg | 43.47 ± 1.9 | 40.30 ± 0.090 | 43.51 ± 0.85 | 43.92 ± 0.36 |
| Ala | 127.46 ± 3.5 | 129.72 ± 0.61 | 122.65 ± 1.1 | 120.67 ± 1.3 |
| Pro | 52.44 ± 1.6 | 55.66 ± 0.12 | 51.29 ± 0.31 | 52.91 ± 0.14 |
| Tyr | 38.98 ± 0.21 | 38.96 ± 0.24 | 37.69 ± 0.29 | 40.22 ± 0.25 |
| Essential amino acid | ||||
| Lys | 72.27 ± 1.4 | 64.59 ± 0.17 | 85.01 ± 0.76 | 100.75 ± 0.40 |
| Phe | 35.14 ± 3.2 | 42.70 ± 0.21 | 59.81 ± 0.62 | 69.16 ± 0.14 |
| Leu | 73.76 ± 1.6 | 79.85 ± 0.90 | 73.02 ± 0.21 | 70.03 ± 0.28 |
| Ile | 41.14 ± 1.4 | 34.48 ± 0.87 | 46.96 ± 0.38 | 54.85 ± 0.32 |
| Thr | 33.85 ± 2.5 | 33.55 ± 0.17 | 28.13 ± 0.35 | 27.61 ± 0.12 |
| Met | 24.67 ± 0.71 | 16.34 ± 0.058 | 12.70 ± 0.10 | 12.76 ± 0.38 |
| Val | 44.61 ± 0.23 | 41.88 ± 0.76 | 41.62 ± 0.14 | 43.35 ± 0.11 |
| Total essential amino acids (N) | 325.45 | 313.38 | 347.25 | 378.51 |
| Total nonessential amino acids (N) | 558.12 | 592.51 | 555.36 | 544.62 |
| E/TN ratio | 0.37 | 0.35 | 0.38 | 0.41 |
| Percentage of amino acid with different characteristics | ||||
| Acidica | 188.97 | 212.36 | 185.39 | 174.95 |
| Basicb | 149.14 | 139.24 | 160.98 | 176.99 |
| Hydrophobicc | 399.23 | 400.63 | 408.05 | 423.73 |
aAcidic: aspartic acid, glutamic acid
bBasic: lysine, arginine, histidine
cHydrophobic: alanine, isoleucine, leucine, methionine, phenylalanine, proline, valine
Gel electrophoresis
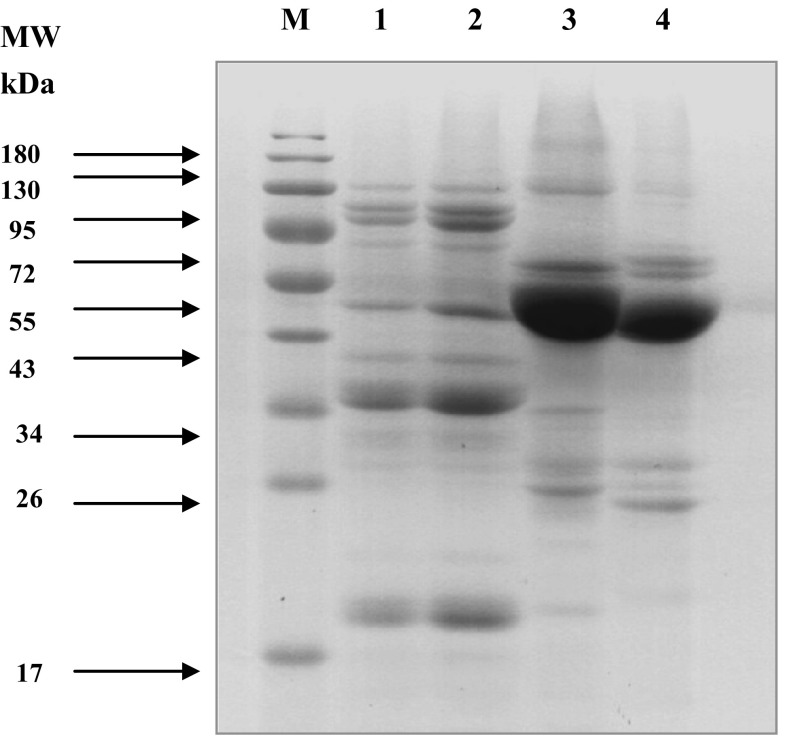
There were some noticeable differences in subunit compositions of the four proteins (Fig. 1). SP and BSP (Lanes 1 and 2, respectively) had proteins with the molecular weight (MW) of 84, 77, 49, 40, 36, and 20 kDa as the dominant subunits, which were considered as 7S globulins (Rayaprolu et al. 2013) and 11S globulins. However, BSP showed bands with a slightly higher content of 7S and 11S globulins than SP. In addition, extra bands with MW of 95 and 67 kDa were also observed in SP and BSP, but the content was low.
Fig. 1.
SDS-PAGE profile of SP, BSP, ABP, and MBP at non-reducing conditions. Lane M standard protein marker; lane 1 SP; lane 2 BSP; lane 3 ABP and lane 4 MBP
ABP and MBP (Lanes 3 and 4, respectively) showed the unique profiles compared to SP and BSP, which had the most intense bands at around 48 kDa and indicated that their major storage protein were 7S and 8S globulins, respectively. (Tjahjadi et al. 1988; Liu et al. 2015). Besides, the acidic and the basic 11S subunits of ABP and MBP were nearly absent compared to those of SP and BSP. Some noticeable differences were found between ABP and MBP. ABP had intense bands at 59, 46, 28, and 25 kDa, while MBP showed major bands with MW of 62, 57, 46, 27, 24 kDa. In addition, the bands with MW of 95 kDa was present in ABP while absence in MBP. The bands with MW of 28 and 25 kDa for ABP and 27 and 24 kDa for MBP might be due to the presence of 11S globulin (Barac et al. 2015). The results suggested that protein compositions of different protein isolates varied in type and size.
Functional properties assay
pH-dependent protein solubility
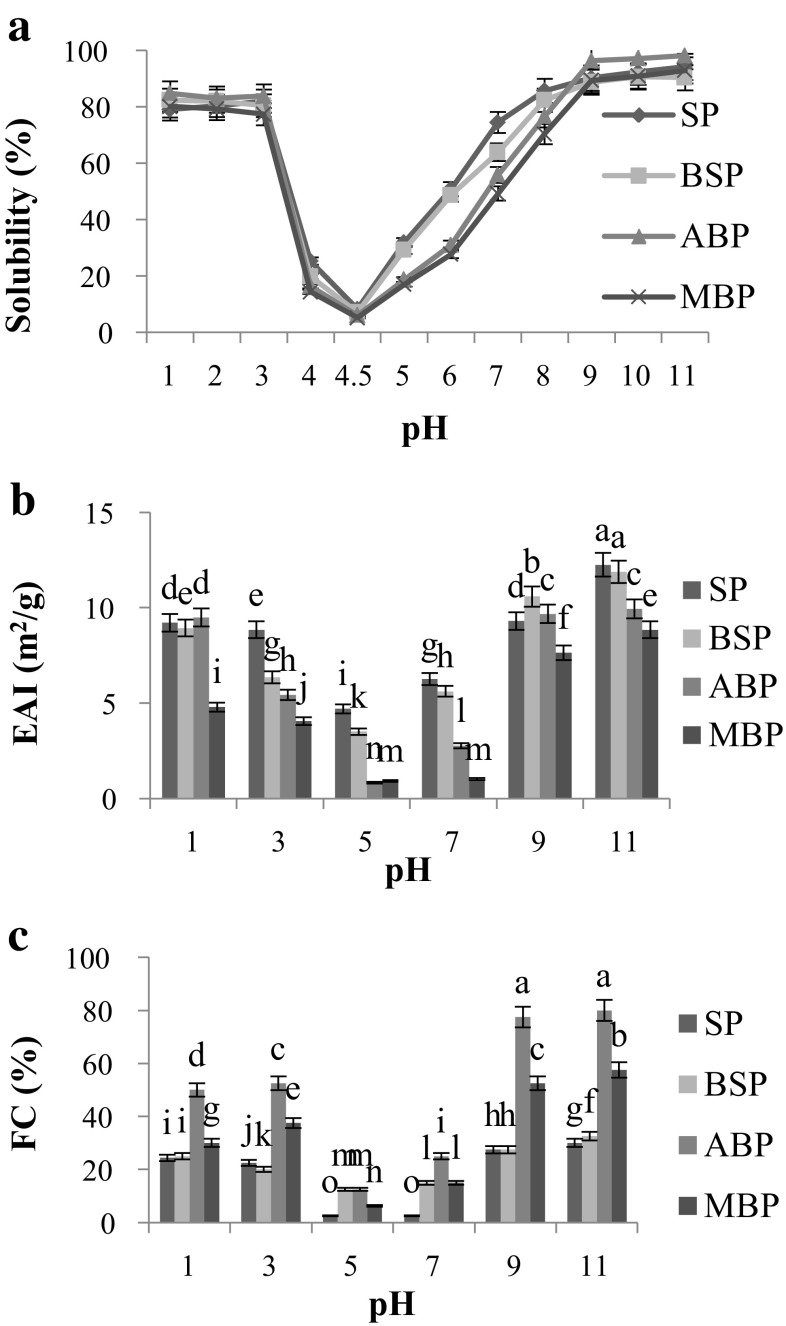
The solubility profile of the four proteins in the pH value range of 1.0–11.0 showed typical U-shaped curves (Fig. 2a). The solubility of the four proteins was the weakest at pH 4.5, while it was increased gradually when the pH values were below 4.5 or above 4.5. This might be attributed to no net charge on the protein surface induced at pH 4.5 leading to precipitation of proteins. Similar observations had been reported for kidney bean protein isolate (Wani et al. 2015) and oat bran protein (Guan et al. 2007). ABP had the highest solubility (98.12%) at pH 11.0. The higher solubility of ABP might be attributed to its high content of 7S golublin fraction (Fig. 1, Lane 3). These results were in agreement with the reports of Kudre et al. (2013) that the differences in solubility of protein isolates might be owing to difference in protein type, amino acid composition. In addition, solubility of the protein would affect other functionalities, such as emulsification and foaming (Guan et al. 2007). Therefore, ABP with the high solubility may have promising functional food applications.
Fig. 2.
Solubility (a), Emulsifying capacity (b), Foaming capacity (c) of SP, BSP, ABP, and MBP. Different letters indicate significant differences between groups (P < 0.05)
Water and oil holding capacity
BSP showed the highest water holding capacity (WHC) (4.27 g/g) followed by MBP (4.09 g/g), SP (3.76 g/g) and ABP (3.75 g/g) (Table 3). There were significant differences between the WHC of BSP, MBP, and SP (P < 0.05), while SP (3.76 g/g) and ABP (3.75 g/g) showed that no significant (P > 0.05) difference in WHC. The values obtained here were little higher than pea protein isolates (0.3–3.6 g/g) reported earlier (Stone et al. 2015) and oat bran protein (1.94–2.27 g/g) (Guan et al. 2007), which might be due to the different sources and extraction methods. MBP exhibited the highest oil holding capacity (OHC) (4.83 g/g), while ABP showed the lowest value (4.00 g/g) (Table 3). There were significant differences between the OHC of these four proteins (P < 0.05). OHC in this study were higher than that of pea protein isolates (3.5–3.8 g/g) (Stone et al. 2015). High WHC and OHC are needed in some foods, thus BSP and MBP might be good candidates for such food application.
Table 3.
Comparisons of functional properties of the four protein isolates
| Properties | SP | BSP | ABP | MBP |
|---|---|---|---|---|
| Denaturation temperature, Td (ºC) | 70.12 ± 0.63c | 71.16 ± 0.69b | 69.99 ± 2.14c | 73.75 ± 0.93a |
| Thermal enthalpy, △H (J/g) | 66.04 ± 5.11a | 51.45 ± 3.83c | 57.72 ± 2.91b | 59.70 ± 3.98b |
| Water holding capacity (g/g) | 3.76 ± 0.21c | 4.27 ± 0.14a | 3.75 ± 0.09c | 4.09 ± 0.05b |
| Oil holding capacity (g/g) | 4.53 ± 0.20b | 4.23 ± 0.21c | 4.00 ± 0.10d | 4.83 ± 0.34a |
All data were expressed by mean values of triplicates ± standard deviation. Means within the same horizontal column not followed by the same letter are significantly different at P < 0.05 level of significance, according to Tukey’s test
Emulsifying capacity (EC)
Emulsifying activity index (EAI) values were used to evaluate the emulsifying capacity (EC). The EAI of SP, BSP, ABP and MBP were the lowest (4.70, 3.50, 0.83 and 0.92 m2 g−1, respectively) at pH 5.0, while the highest values were observed at pH 11.0 (12.25, 11.88, 9.95 and 8.84 m2 g−1, respectively) (Fig. 2b). Significant difference was observed among the EAI values of SP, ABP and MBP at all the detected pH values (P < 0.05).The EC of legume proteins were greatly affected by surface hydrophobicity, solubility, molecular size, steric hindrance, interaction between solubility and surface charge (Ghribi et al. 2015). The lowest EAI values for the four proteins were observed at pH 5, the reason might be that the net charge of the proteins was little or zero at the isoelectric point (pI) and the solubility was the lowest, so the protein adsorbed on the surface of oil–water was the least. The EAI values of SP, BSP, and ABP were higher than those of cowpea bean protein (7.7–8.9 m2 g−1) (Shevkani et al. 2015), but lower than those of kidney bean protein (17.46 m2 g−1) (Wani et al. 2015). This might be owing to the different sources, and emulsion formation conditions (oil volume, sample concentration and speed of homogenization) used.
Foaming capacity(FC)
Foaming capacity was greatly influenced by pH as shown in Fig. 2c. The foaming capacity (FC) of SP, BSP, ABP and MBP were the lowest (2.5, 12.5, 12.5, 6.25%, respectively) at pH 5.0, while the highest FC of SP (30.0%), BSP (32.5%), ABP (80.0%) and MBP (57.5%) were shown at pH 11.0. There were significant differences between the FC values of ABP and the other three proteins (P < 0.01). Solubility was positively correlated with FC indicating that as more protein can migrate to the air–water interface, more foam will be formed (Stone et al. 2015). In addition, some other factors like extraction methods, protein molecular may have affected the FC (Wani et al. 2015). Foaming capacities found in this study were higher than reported for two commercial tea protein isolates in our previous studies (Zhang et al. 2015), but little lower than that of pea protein (81.1%) (Stone et al. 2015). Foaming properties are the functional properties, where aeration and overrun are required e.g. whipped toppings, baked foods and ice-cream mixes. Hence, ABP might be used as a potent foaming agent in food industry.
Thermal transition
The thermal transitions of the four proteins were studied by differential scanning calorimetry (DSC). T d values for SP, BSP, ABP, and MBP were 70.12, 71.16, 69.99 and 73.75 °C, respectively (Table 3), thus, MBP exhibited slightly higher thermal stability than the other three types of proteins, which might be due to the higher proportion of hydrophobic amino acid (Table 3) and related secondary protein structure (Kudre et al. 2013). SP showed the highest △H value (66.04 J/g) than MBP (59.70 J/g), ABP (57.72 J/g), and BSP (51.45 J/g) (P < 0.05), which represented there higher ordered secondary structure in SP (Ghribi et al. 2015). These differences in thermal stability for all proteins may be attributed to the different ratios of specific proteins as suggested by the electrophoresis results (Fig. 1). The T d values for all protein isolates in this study were little lower than those of Phaseolus vulgaris legume (80.8–91.4 °C) (Tang and Sun 2011), which might be due to the different pretreatment method and resources. In addition, processing and environmental effects (e.g. pH, salts, etc.) can alter the molecular structure of proteins, thereby changing their thermal stability (Barbana and Boye 2013).
Antioxidant activity assays
DPPH radical-scavenging activity
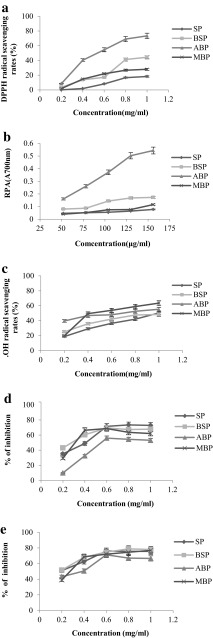
Figure 3a shows the DPPH radical-scavenging activity of all proteins changed in a dose-dependent manner with the concentration ranging from 0.2 to 1.0 mg/mL. The four proteins SP, BSP, ABP and MBP showed linear correlations between concentration and scavenging ability with the coefficient R 2 of 0.9438, 0.9268, 0.9168 and 0.8950, respectively. The half inhibition concentration (IC50) of SP, BSP, ABP and MBP were 2.11, 1.04, 0.61 and 1.47 mg/mL, respectively. There were significant differences among the scavenging DPPH radical ability of SP, BSP, ABP and MBP (P < 0.05). The results clearly indicated that ABP had the highest scavenging DPPH radical capacity, which was higher than that of the abalone viscera hydrolysate (IC50 = 4.0 mg/mL) (Zhou et al. 2012). The higher hydrophobic amino acid residues of ABP might can contribute to the higher DPPH radical scavenging activity (Zhu et al. 2008). The results suggested that adzuki bean was a good plant source antioxidants.
Fig. 3.
Antioxidant and anticancer activities of SP, BSP, ABP, and MBP. a Scavenging effects (%) on 1.1-diphenyl-2-picrylhydrazyl (DPPH); b Reducing power (absorbance at 700 nm); c Scavenging effects on hydroxyl radicals; d Inhibition effects on SKOV3; e Inhibition effects on SMMC-7721
Ferric reducing power (FRP) assay
Figure 3b showed the reducing power capacities of the four proteins changed in a dose-dependent manner (52–156 μg/mL). SP, BSP, ABP and MBP showed linear correlations between concentration and reducing power with the coefficient R 2 of 0.9937, 0.9437, 0.9533 and 0.9470, respectively. There were significant differences between the FRP of SP, BSP, ABP and MBP (P < 0.05). ABP showed the highest FRP potentials among all proteins, and the maximum activity was found at 0.54 ± 0.0090 OD in the concentration of 156 μg/mL. The reducing power of the four protein samples were in the order of ABP > BSP > MBP > SP, which was in accordance with the results of scavenging DPPH radicals earlier.
Effects on scavenging hydroxyl radicals
The scavenging effects of proteins on hydroxyl radicals were increased with increase in sample concentration (0.2–1.0 mg/mL) (Fig. 3c). The coefficient R 2 of concentration and scavenging activity for SP, BSP, ABP and MBP were 0.9896, 0.9139, 0.9464 and 0.7945 respectively. The IC50 values of SP, BSP, ABP and MBP were 0.98, 0.92, 0.68 and 0.61 mg/mL, respectively, which indicated that MBP exhibited the strongest scavenging activity against hydroxyl radicals and followed by ABP. There was a slight difference in DPPH radical scavenging activity and reducing power ability for the four proteins. The reason might be that antioxidant experiment models have different mechanisms. Earlier researchers suggested both the different mechanisms might be responsible for the hydroxyl radical scavenging abilities (Wang et al. 2008).
Anticancer activity
The inhibitory rates of the proteins against cancer cells (from 200 to 1000 µg/mL) were observed especially in the range of 200–600 µg/mL (Fig. 3d, e). The IC50 values of SP, BSP, ABP, and MBP against SKOV3 were 431.0, 327.9, 720.4, 505.1 µg/mL, respectively, while the IC50 of the samples against SMMC-7721 were 143.5, 88.9, 391.0, 323.6 µg/mL, respectively. There were significant differences among the inhibition effects of the four proteins on cancer cell proliferation (P < 0.05) and the inhibitory activity was in the order of BSP > SP > MBP > ABP. BSP showed the inhibition of 69% on SKOV3 cancer cells at the concentration of 600 µg/mL, while inhibition of 79% on the SMMC-7721cancer cells at 800 µg/mL was observed. The values in this study were higher than that reported for LBP-a4, a fraction of Lyciumbarbarum polysaccharide, which had the best inhibition activity with the highest inhibition of 36.5 ± 2.6% against on the SMMC-7721 cells at the dose of 400 mg/L (Zhang et al. 2013). In addition, the results were better than those of the soybean protein fractions with molecular weight less than 5 kDa, which showed inhibition of 64% on liver cancer cells (HepG-2) at the concentration of 800 µg/mL (Rayaprolu et al. 2013). BSP had higher inhibitory activity against both SKOV3 and SMMC-7721cancer cells, which might be due to the special protein composition and physicochemical properties of BSP including the wide distribution of the protein, higher content of acidic acids, low content of the hydrophobic amino acids and higher WHC. Previous studies demonstrated that the lectins, carbohydrate-binding proteins with molecular mass around 33 kDa could attenuate the viability of several tumor cell lines including HONE1 cells, HepG2 cells and MCF7 cells (Pan and Ng 2015; Chan et al. 2016). In this study, the proteins with molecular weight of 36 kDa in BSP showed the most intense bands than those of SP, MBP and ABP (Fig. 1), which might be lectins and might own the inhibitory effects on SKOV3 and SMMC-7721 cells. The related mechanism need further study.
Conclusion
The physicochemical, structural and functional properties as well as the antioxidant and anticancer activities of SP, BSP, ABP, and MBP were evaluated. MBP had the highest amount of essential amino acids and further exerted significantly higher thermal stability compared to those of BSP, ABP and SP. ABP with higher essential amino acids and the highest solubility, exhibited the highest antioxidant activities among the four types of proteins. While BSP with unique molecular weight distribution, higher content of acidic amino acids, low content of the hydrophobic amino acids and higher WHC, may have potential nutraceutical use against ovarian and liver cancers. Therefore, there were obvious difference found in the protein composition and physicochemical properties of the four types of legume proteins, which may have could affected the functional properties and bioactivities. Thus, the four kinds of protein isolates may be used as ingredients in food systems for different purposes.
Acknowledgements
This work was supported by the grant from National High Technology Research and Development Program (“863” Program) of China (Grant No. SS2013AA100207) and the National Natural Science Foundation of China (NSFC 31371879).
References
- AOAC Intl . Official methods of analysis of AOAC international. Arlington, Va: AOAC Intl (p. v. (loose-leaf)); 1995. [Google Scholar]
- Barac MB, Pesic MB, Stanojevic SP, Kostic AZ, Bivolarevic V. Comparative study of the functional properties of three legume seed isolates: adzuki, pea and soy bean. J Food Sci Technol. 2015;52:2779–2787. doi: 10.1007/s13197-014-1298-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbana C, Boye JI. In vitro protein digestibility and physico-chemical properties of flours and protein concentrates from two varieties of lentil (Lens culinaris) Food Funct. 2013;4:310–321. doi: 10.1039/C2FO30204G. [DOI] [PubMed] [Google Scholar]
- Chan YS, Xia L, Ng TB. White kidney bean lectin exerts anti-proliferative and apoptotic effects on cancer cells. Int J Biol Macromol. 2016;85:335–345. doi: 10.1016/j.ijbiomac.2015.12.094. [DOI] [PubMed] [Google Scholar]
- Garcia-Mora P, Frias J, Peñas E, Zieliński H, Giménez-Bastida JA, Wiczkowski W, Zielińskac D, Martínez-Villaluenga C. Simultaneous release of peptides and phenolics with antioxidant, ACE-inhibitory and anti-inflammatory activities from pinto bean (Phaseolus vulgaris L. var. pinto) proteins by subtilisins. J Funct Foods. 2015;18:319–332. doi: 10.1016/j.jff.2015.07.010. [DOI] [Google Scholar]
- Ghiselli A, Nardini M, Baldi A, Scaccini C. Antioxidant activity of different phenolic fractions separated from an Italian red wine. J Agric Food Chem. 1998;46:361–367. doi: 10.1021/jf970486b. [DOI] [PubMed] [Google Scholar]
- Ghribi AM, Gafsi IM, Sila A, Blecker C, Danthine S, Attia H, Bougatef A, Besbes S. Effects of enzymatic hydrolysis on conformational and functional properties of chickpea protein isolate. Food Chem. 2015;187:322–330. doi: 10.1016/j.foodchem.2015.04.109. [DOI] [PubMed] [Google Scholar]
- Guan X, Yao H, Chen Z, Shan L, Zhang M. Some functional properties of oat bran protein concentrate modified by trypsin. Food Chem. 2007;101:163–170. doi: 10.1016/j.foodchem.2006.01.011. [DOI] [Google Scholar]
- Kudre TG, Benjakul S, Kishimura H. Comparative study on chemical compositions and properties of protein isolates from mung bean, black bean and bambara groundnut. J Sci Food Agric. 2013;93:2429–2436. doi: 10.1002/jsfa.6052. [DOI] [PubMed] [Google Scholar]
- Li W, Shu C, Yan S, Shen Q. Characteristics of sixteen mung bean cultivars and their protein isolates. Int J Food Sci Technol. 2010;45:1205–1211. doi: 10.1111/j.1365-2621.2010.02259.x. [DOI] [Google Scholar]
- Liu H, Liu H, YanL ChengX, Kang Y. Functional properties of 8S globulin fractions from 15 mung bean (Vigna radiata(L.) Wilczek) cultivars. Int J Food Sci Technol. 2015;50:1206–1214. doi: 10.1111/ijfs.12761. [DOI] [Google Scholar]
- Misurcova L, Bunka F, Ambrozova JV, Machu L, Samek D, Kracmar S. Amino acid composition of algal products and its contribution to RDI. Food Chem. 2014;151:120–125. doi: 10.1016/j.foodchem.2013.11.040. [DOI] [PubMed] [Google Scholar]
- Pan WL, Ng TB. A dimeric Phaseolus coccineus lectin with anti-oxidative, anti-proliferative and cytokine-inducing activities. Int J Biol Macromol. 2015;81:960–966. doi: 10.1016/j.ijbiomac.2015.09.034. [DOI] [PubMed] [Google Scholar]
- Rayaprolu SJ, Hettiarachchy NS, Chen P, Kannan A, Mauromostakos A. Peptides derived from high oleic acid soybean meals inhibit colon, liver and lung cancer cell growth. Food Res Int. 2013;50:282–288. doi: 10.1016/j.foodres.2012.10.021. [DOI] [Google Scholar]
- Shevkani K, Kaur A, Kumar S, Singh N. Cowpea protein isolates: functional properties and application in gluten-free rice muffins. LWT Food Sci Technol. 2015;63:927–933. doi: 10.1016/j.lwt.2015.04.058. [DOI] [Google Scholar]
- Stone AK, Karalash A, Tyler RT, Warkentin TD, Nickerson MT. Functional attributes of pea protein isolates prepared using different extraction methods and cultivars. Food Res Int. 2015;76:31–38. doi: 10.1016/j.foodres.2014.11.017. [DOI] [Google Scholar]
- Tan ES, Ngoh YY, Gan CY. A comparative study of physicochemical characteristics and functionalities of pinto bean protein isolate (PBPI) against the soybean protein isolate (SP) after the extraction optimisation. Food Chem. 2014;152:447–455. doi: 10.1016/j.foodchem.2013.12.008. [DOI] [PubMed] [Google Scholar]
- Tang CH, Sun X. A comparative study of physicochemical and conformational properties in three vicilins from Phaseolus legumes: implications for the structure–function relationship. Food Hydrocolloids. 2011;25:315–324. doi: 10.1016/j.foodhyd.2010.06.009. [DOI] [Google Scholar]
- Tjahjadi C, Lin S, Breene WM. Isolation and characterization of Adzuki bean (Vigna angularis cv Takara) proteins. J Food Sci Technol. 1988;53:1438–1443. [Google Scholar]
- Wang J, Zhang Q, Zhang Z, Li Z. Antioxidant activity of sulfated polysaccharide fractions extracted from Laminaria japonica. Int J Biol Macromol. 2008;42:127–132. doi: 10.1016/j.ijbiomac.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Wani IA, Sogi DS, Shivhare US, Gill BS. Physico-chemical and functional properties of native and hydrolyzed kidney bean (Phaseolus vulgaris L.) protein isolates. Food Res Int. 2015;76:11–18. doi: 10.1016/j.foodres.2014.08.027. [DOI] [Google Scholar]
- WHO (1985) Energy and protein requirements. Report of a joint FAO/WHO/UNU expert consultation. Geneva, World Health Organization, WHO Technical Report Series, No. 724 [PubMed]
- Yu X, Yuan F, Fu X, Zhu D. Profiling and relationship of water-soluble sugar and protein compositions in soybean seeds. Food Chem. 2016;196:776–782. doi: 10.1016/j.foodchem.2015.09.092. [DOI] [PubMed] [Google Scholar]
- Zhang M, Tang X, Wang F, Zhang Q, Zhang Z. Characterization of Lycium barbarum polysaccharide and its effect on human hepatoma cells. Int J Biol Macromol. 2013;61:270–275. doi: 10.1016/j.ijbiomac.2013.06.031. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chen HX, Zhang N, Ma LS. Antioxidant and functional properties of tea protein as affected by the different tea processing methods. J FoodSci Technol. 2015;52:742–752. doi: 10.1007/s13197-013-1094-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou DY, Zhu BW, Qiao L, Wu HT, Li DM, Yang JF, Murata Y. In vitro antioxidant activity of enzymatic hydrolysates prepared from abalone (Haliotis discus hannai Ino) viscera. Food Bioprod Process. 2012;90:148–154. doi: 10.1016/j.fbp.2011.02.002. [DOI] [Google Scholar]
- Zhu LJ, Chen J, Tang XY, Xiong YL. Reducing, radical scavenging, and chelation properties of in vitro digests of alcalase-treated zein hydrolysate. J Agric Food Chem. 2008;56:2714–2721. doi: 10.1021/jf703697e. [DOI] [PubMed] [Google Scholar]