Abstract
The current study was designed to explore the beneficial properties of chickpeas consumption on suppressing appetite, excessive blood glucose excursions, and energy intake (EI) from a subsequent meal. Two caloric preloaded foods, chickpeas, and white bread were compared to water control, fed to healthy female subjects at equal energy density, volume, and available carbohydrate content in two experiments spanning over 60 and 120 min. Blood glucose was measured by a portable glucometer and satiety by using a visual analogue scale questionnaire at baseline and every 15 up to 60 min in both experiments and then every 30 until 120 min in Experiment 2 after the preloads ingestion. A test meal was served at the end of both experiments to calculate EI and percent energy compensation (%EC). The results suggest a reduction of 29–36% in blood glucose concentration, and 83–98% EC after the chickpeas in Experiments 1 and 2 respectively compared to white bread. The average appetite showed a positive association with EI. We conclude that the consumption of chickpeas is beneficial on glycemic control and may help in body weight management through suppressing appetite and energy intake.
Keywords: Chickpeas, Appetite, Glycemic control, Energy compensation
Introduction
After the industrial revolution and especially with the development of food industry, processed foods have become more available at a lower price causing people to move from the consumption of their traditional, unprocessed foods to highly refined and caloric-dense meals. The imbalance between the excess caloric ingestion than its expenditure resulting in weight gain is well established. Obesity poses a major risk factor for diet-related chronic diseases such as type 2 diabetes, cardiovascular heart diseases, and hypertension, etc. According to the Centre for Disease Control (CDC) above 29 million people in the United States alone have type 2 diabetes (Boyle et al. 2010). The prevalence of diabetes has increased 120% in the past 25 years and is projected to increase from affecting 382 million adults in 2013 to 592 million by 2035 worldwide (Guariguata et al. 2014).
This epidemic level of obesity, and consequently type 2 diabetes prevalence are becoming a challenge for health professionals who are struggling to explore a breakthrough in preventive strategies for weight control. Restrained eating and dieting practices have a notorious rate of failure (Ohsiek and Williams 2011). Reduced energy consumption could become possible through improved satiating and satiety ability of the diets. Legumes are a major source of carbohydrate, quality protein, dietary fibers, resistant starch and valuable bioactive compounds that may become beneficial in suppressing appetite (McCrory et al. 2010). Health authorities such as Dietary Guidelines for Americans (USDA 2010) and Canada’s Food Guide recommend consumption of legumes regularly to prevent the diet-induced risk of chronic diseases (Health Canada 2007). Many observational studies and controlled trials have associated legume’s consumption with weight loss and reduced chronic diseases in populations (Bazzano et al. 2001; Jenkins et al. 2012; Abete et al. 2009, Mollard et al. 2012a, b). However, the role of legumes ingestion on food intake regulation needs further understanding.
Acute studies have reported controversial results in the literature on the consumption of beans on satiety and subsequent energy intake. Canned navy beans fed to young men suppressed EI more than a glucose control drink when administered in a volume of 50 g available carbohydrate, yet they failed to decrease EI when compared to white bread as a control consumed in an either 50 g available carbohydrate or isocaloric amount (Wong et al. 2009). Chickpea flour supplemented white wheat bread failed to suppress EI or reduce glycemic effect when consumed at a portion of 50 g available carbohydrate (Johnson et al. 2005), but not when pulses were eaten ad libitum 4 h before the test meal (Mollard et al. 2012a, b). Subjects were feeling hungrier when chickpea flour supplemented bread was fed compared to white bread after 90 min (Zafar et al. 2015). These discrepancies manifest differences in the effect of legumes on glycemic response, satiety and EI from a subsequent meal.
The standard practice of testing carbohydrate foods in an amount of 50 g available carbohydrate is necessary for controlling the confounding effect of variation in absorbable glucose on the glycemic response of the test food and consequently, the impact on satiety or EI for a given period. However, it is important to note that this practice may not hold when legumes are assessed on satiety and EI, because legumes are consumed as cooked food holding a lot of liquid that when given in equal portion of 50 g available carbohydrates are bulkier compared to control such as bread that holds less moisture. For example, canned beans in 50 g available carbohydrate portion weighed 350–450 g whereas white bread weighed around 110 g per 3–4 slices (Wong et al. 2009). The recommended pulses consumption is a 3/4th cup that weighs around 150 g (Guenther et al. 2013) but administering the required amount of cooked beans to achieve 50 g of available carbohydrate muddles their effect on satiety and EI, especially if the interval between preload and test meal is short.
The objective of the present study was, therefore, to explore the effect on glycemic response, subjective appetite, and EI from a test meal offered at 60 and 120 min, by giving realistic portion sized preloads of canned chickpeas and white bread equalized in available carbohydrates, energy density, and total volume. Canned chickpeas and white toast bread as 1 cup (200 g) and two slices (50 g), respectively, provided 18 g of available carbohydrate and 218 kcal each. The study was conducted using young healthy female volunteers. In the recruiting interview, it was learned that two bread slices were a usual portion size as their regular breakfast. Obesity is overwhelming women more than men, it was, therefore, intended to explore the satiety cues and EI in this population group (Badr et al. 2012).
Material and methods
Subjects
Volunteers (n = 12) selected from a convenient sample of healthy female students participated in both experiments. The girls were within the body mass index (BMI in kg/m2) range of 20–25 and age 17–30 years. They were recruited through flyers and by word of mouth from the College of Life Sciences. Excluded were those with high fasting blood glucose, taking any medication, or who customarily skipped breakfast or were restrained eaters. Those who scored ≥11 on the Eating Habits Questionnaire were identified as restrained eaters (Polivy et al. 1978). The study was conducted in the Human Nutrition laboratories of the Department of Food Science and Nutrition. Subjective appetite and blood glucose measurements were recorded in a room separate from those where test foods and the meal were consumed. A consent form was signed by all subjects prior to the study approved by the Human Subjects’ Review Committee, Ethics Review Office of the University.
Test foods
The test foods—white bread (Kuwait Flour Mills & Bakeries Co., Kuwait), canned chickpeas (Giant Chickpeas with Chilies, Al-Danah brand, Al-Wazzan Foods Co., Kuwait), and bottled water (Aquafina)—were purchased from the local market. Weighed amounts corresponding to 18 g available carbohydrate (total carbohydrate–dietary fiber) of the white wheat bread (50 g, ~2 slices) and canned chickpeas (200 g, 1 cup) were used in both experiments. White bread was served immediately after toasting for 30 s, and canned chickpeas were heated for 90 s in a microwave oven before serving. Ten gram f butter was served with the bread to match the calories with those of the chickpeas. The water control was served at refrigerator temperature. Additional water was served with the test foods to equalize their volume in the stomach and to facilitate swallowing. The composition of the test foods is shown in Table 1.
Table 1.
Nutrition Composition of the Test Preloads
| Nutrient information | Water | White Bread | Canned chickpeas |
|---|---|---|---|
| Energy (kcal) | 0 | 218 | 218 |
| Available carbohydrate (g)* | 0 | 18 | 18 |
| Total fat (g) | 0 | 12.8** | 2.3 |
| Protein (g) | 0 | 8.2 | 12.3 |
| Dietary fiber (g) | 0 | 0.7 | 10.6 |
| Weight (g) | 500 | 50 | 200 |
| Water served (ml) | 500 | 450 | 300 |
| Total volume (ml) | 500 | 500 | 500 |
| Energy density (kcal/g) | 0 | 0.436 | 0.436 |
* Available carbohydrate = Total carbohydrate − dietary fiber
** Total fat for bread preload includes fat from 2 T butter to match the caloric content of the chickpeas preload
Experimental procedures
Both the experiments used a repeated-measures design whereby each subject consumed each preload treatment and served as her own control in both experiments with the test foods and control administered randomly to each subject. Blood glucose was measured in a finger prick sample and subjective appetite by a Visual Analogue Scale (VAS) questionnaire at baseline (fasting) and at 15 min intervals for 1 h after the preloads were administered for both experiments and then at 30 min intervals in the second hour of Experiment 2. At the end of each test, a meal was served with a bottle of water and subjects were asked to eat and drink until comfortably full.
Subjects came for the study sessions between 8.00 and 10.00 am (2 h after waking), after an overnight fast of 10–12 h and arrived at this time for each test session. Water was permitted up to 1 h before the scheduled start time. All subjects were instructed to maintain a regular pattern of food intake and physical activity throughout the study. The finger prick blood sampling used a Monojector Lancet Device and a portable blood glucose monitoring system (One Touch Ultra, Life Scan Inc and Johnson & Johnson Company, USA) whereby a drop of blood was placed on a test strip and glucose concentration recorded as is given elsewhere (Zafar et al. 2015). Test session was not given during the menstrual cycle to prevent any possible hormonal effect on blood glucose or appetite (Escalante and Alpizar 1999).
Food intake
After completion of blood glucose and subjective appetite measurements, subjects were served with a pizza meal and a bottle of water (1.0 L). The pizzas were 5-inch round containing about 200 kcal and available in two varieties (Four Cheeses and Deep N Delicious Veggie Pizza; McCain Foods Ltd). These pizzas were chosen for their uniformity of crust and filling ensuring uniformity of energy intake. They were prepared and served as described in our earlier studies (Wong et al. 2009). Briefly, the pizzas were cut into four pieces and served in consecutive trays within 6–7 min of each other.
Food intake was assessed by weighing the cooked pizza before and after serving each test. The caloric consumption was calculated from the information provided by the manufacturer.
Data analysis
The area under the blood glucose response curves (AUC), ignoring any area below fasting, was determined for each food for each subject. For analysis of VAS appetite responses, an average appetite score was calculated for each time point using the formula as given earlier (Flint et al. 2000):
The %EC at the second meal for the test food calories was calculated by the following formula (Anderson et al. 2015):
Statistical analysis was conducted using SPSS (statistical package for social sciences). The repeated measures analysis of variance (ANOVA) was used to assess the effect of treatments on outcome variables, including changes from baseline in blood glucose concentrations, average appetite scores at each time point, incremental area under the curve (AUC) for these changes, EI and % EC at meals. Two-way repeated measures ANOVA was conducted on changes in appetite and blood glucose concentration scores at each test period to determine time and treatment effects and for a time by the treatment interaction.
Tukey’s posthoc tests were performed when treatment effects were statistically significant (p < 0.05). All results presented are mean ± standard error of the mean (SEM). Correlation analyses were conducted using the Pearson Correlation coefficient.
Results and discussion
Subjects
The subjects participated in the study had a mean age of 23.67 ± 1.87 years. and a body mass index of 22.39 ± 1.32 kgm2. The participants were students with undergraduate courses in the College of Life Sciences and fulfilled the inclusion criteria set for the study.
Blood glucose
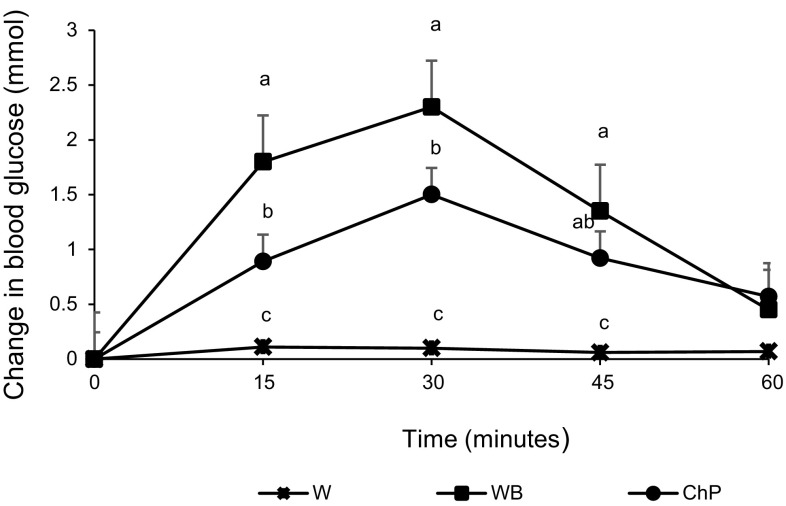
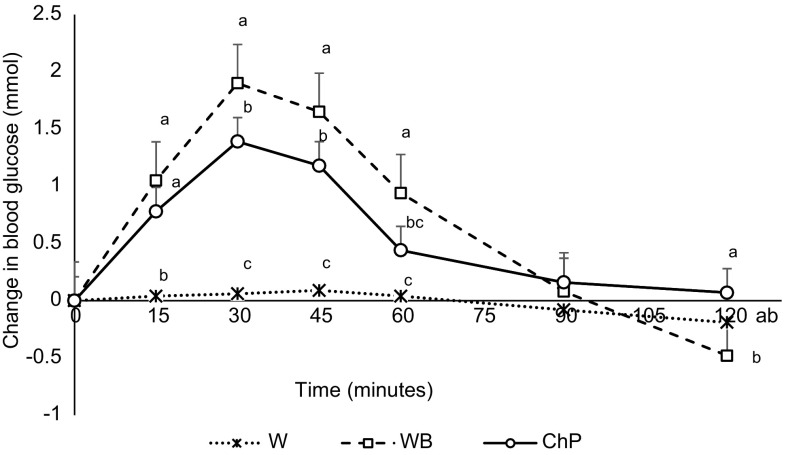
No significant differences were observed in baseline blood glucose concentrations among the preload treatments in either experiment (p > 0.05). Blood glucose changes were affected by both treatment (p < 0.001) and time (p < 0.001), with a time by treatment interaction (p < 0.001). Peaks in blood glucose level occurred at 30 min after both energy preload treatments followed by declines. In both experiments, white bread resulted in the most rapid increase in blood glucose level but then declined to levels not significantly different from water or chickpeas at 60 min in Experiment 1 (Fig. 1), and at 90 min in Experiment 2. However, by 120 min the levels had fallen below the baseline after bread and water but not after chickpeas. Chickpeas reached a significantly lower rise at 30 min and maintained the glucose levels above the baseline at 120 min (Fig. 2). Before the meal, blood glucose concentration was not different among the three treatments at 60 min, however, at 120 min, it was significantly lower after white bread compared to the chickpeas; water control remained non-significantly different.
Fig. 1.
Effect of treatments on change from blood glucose concentration from baseline to 60 min in Experiment 1 in healthy female subjects. Data presented is as Mean ± SEM, n = 12 in Experiment 1; Different superscript letters denote a significant difference at p < 0.05 in the same row (Tukey’s posthoc test). W water, WB white bread, ChP = chickpeas
Fig. 2.
Effect of treatments on change from blood glucose concentration from baseline to 120 min in Experiment 2 in healthy female subjects. Data presented is as Mean ± SEM, n = 12 in Experiment 2; Different superscript letters denote a significant difference at p < 0.05 in the same row (Tukey’s posthoc test). W water, WB white bread, ChP chickpeas
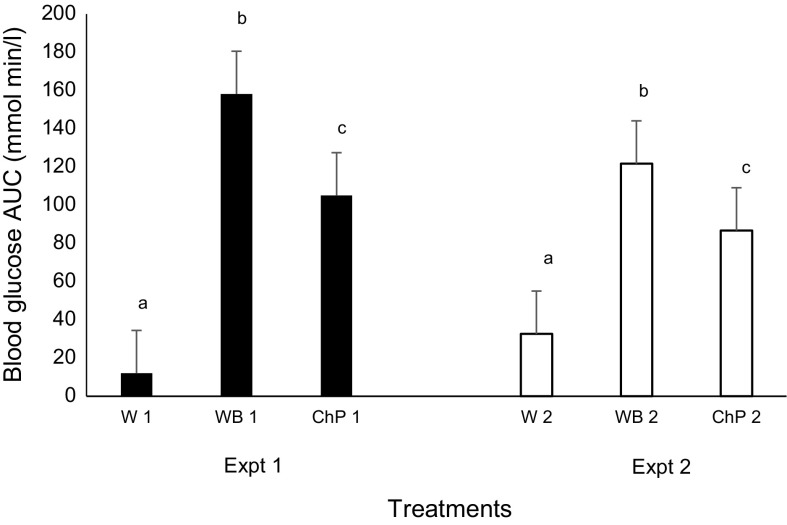
The calculated area under the curve (AUC) for blood glucose change was significantly higher for white bread followed by chickpeas and water both at over 60 and 120 min (Fig. 3). This reduction in the AUC of blood glucose concentration after the chickpea treatment calculated was 29 and 36% in Experiment 1 and 2, respectively, compared to bread treatment. These results are supported by others who found reduction in blood glucose response and in AUC after consumption of chickpeas compared to white bread in healthy (Zafar et al. 2011; Wong et al. 2009; Mollard et al. 2011, 2012a, b; Hall et al. 2005; Keogh et al. 2011); or diabetic subjects (Jenkins et al. 2012; Sievenpiper et al. 2009). However, when fed as chickpea flour or extruded flour supplemented to white bread, no difference was observed for the glycemic effect or subjective appetite compared to the white bread (Johnson et al. 2005). This inconsistency might be explained by the fact that the amount of chickpea flour used in bread supplementing might not be enough to overcome the glycemic starch content of the white flour when comparisons were made at 50 g available carbohydrate.
Fig. 3.
Effect of treatments on the blood glucose area under the curve 0–60 min in Experiment 1 and 0–120 min in Experiment 2 in healthy female subjects. Data presented is as Mean ± SEM, n = 12 in Experiments 1 and 2; Different superscript letters denote a significant difference at p < 0.05 within the same Expt (Tukey’s posthoc test). Expt experiment; W water, WB white bread, ChP Chickpeas; “1” denotes Expt 1 and “2” Expt 2
Food intake
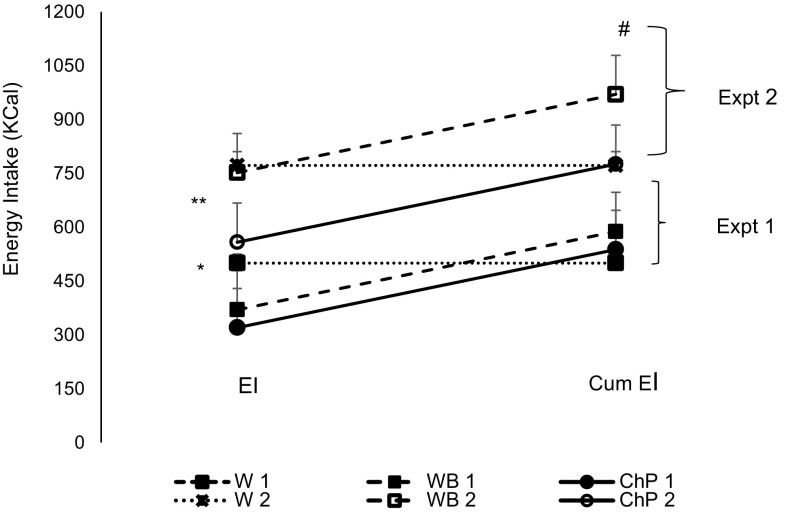
In Experiment 1, Kcal intake in both experiments was different among the three treatments (p < 0.05). Subjects on the control treatment in Experiment 1 had consumed 130 and 180 kcal more than the bread and chickpeas treatment, respectively. The cumulative energy intake was significantly higher after both white bread and chickpeas compared to control, resulting in partial EC of 60% after bread and 83% after chickpeas. The difference of EI expanded when the time gap between the preload ingestion and the test meal was increased to 120 min in Experiment 2. Subjects on the chickpeas treatment had a significantly lower EI compared to either bread or water (p < 0.001), while bread and water were not different from each other. The cumulative energy consumption, therefore, was significantly different between the water and bread but not between water and chickpeas (Fig. 4). EC calculated was 98% after the chickpeas and only 9% after the bread treatment.
Fig. 4.
Effect of treatments on energy intake and cumulative energy intake in Experiments 1 and 2 in healthy females. Data presented is as Mean ± SEM, n = 12; difference at p < 0.05 by Tukey’s posthoc test. Expt experiment; W water, WB white bread, ChP Chickpeas; “1” denotes Expt 1 and “2” Expt 2; EI energy intake; Cum EI cumulative energy intake. EI is the number of calories calculated from the ingested pizza meal at the end of each Expt; Cum EI the total calories from the pizza meal plus the calories from the preload treatments. *EI in Expt 1 is significant lower after ChP1 and WB1 compared to W1 treatment at 60 min **EI in Expt 2 is significantly lower after ChP2 compared to WB2 and W2 at 120 min #represents significantly higher Cum EI after WB2 compared to ChP2 and W2
These results are supported by the previous studies where chickpeas had a similar pattern of caloric suppression from a test meal consumed 120 min after the preload (Zafar et al. 2011) or food intake delayed at the test meal when calories were consumed ad libitum (Ball et al. 2003). A few conflicting results are, however, reported by others. Chickpeas, fed at 50 g available carbohydrates, compared to glucose drink as a control, had suppressed EI in healthy men at 120 min, but not when it was compared to white bread (Wong et al. 2009); or when fed in bread as chickpea flour or extruded chickpea flour supplemented at 50 g available carbohydrate (Johnson et al. 2005). Also, there was no effect of pulses reported by the meta-analysis of acute feeding studies on the suppression of EI (Li et al. 2014).
Average appetite and associations among the outcomes
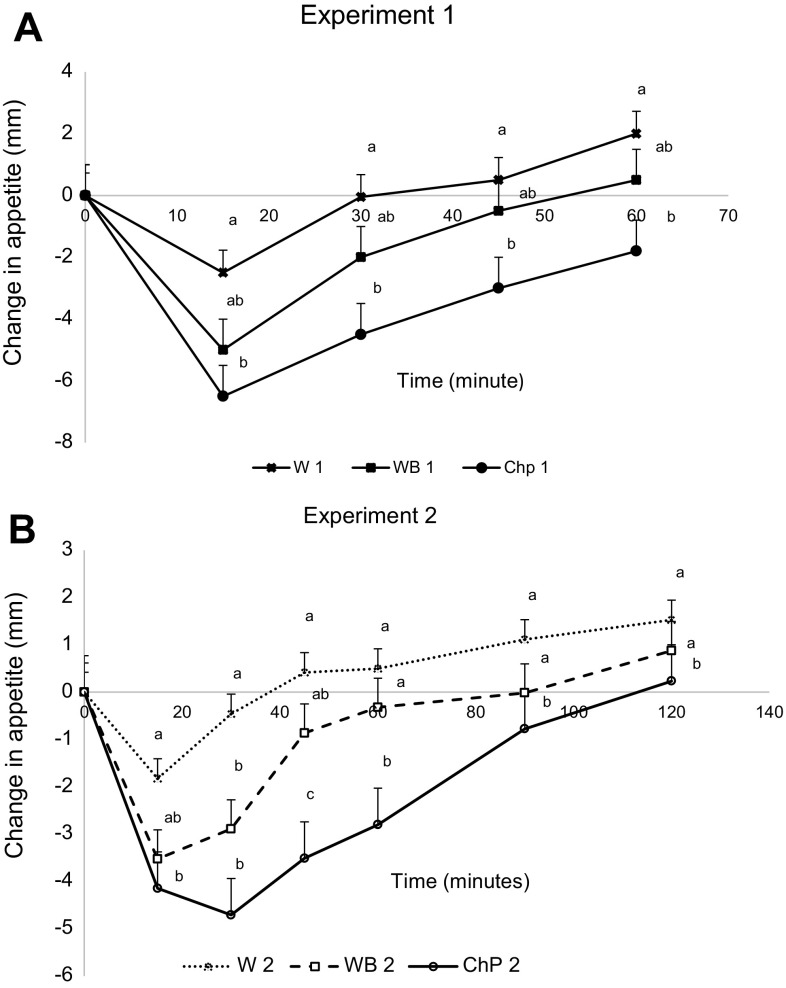
There was no difference at baseline for average appetite scores among all the treatments in either experiment (p > 0.05). The average change in appetite was affected by treatment (p < 0.05), time (p < 0.05), and treatment by time interaction was observed (p < 0.05) in both experiments. The lowest values for average appetite score change were observed between 15 and 60 min in both experiments after the chickpeas compared to water preloads. Whereas bread was not significantly different from both water and chickpeas at any time point in Experiment 1 (Fig. 5A), yet 45 and 60 min appetite after bread was significantly higher compared to chickpeas in Experiment 2 (Fig. 5B). AUCs for appetite score changes among the treatments, however, did not differ by the test treatments in both experiments (data not shown).
Fig. 5.
Effect of treatments on change in appetite from baseline to 60 min in Experiment 1 and to 120 min in Experiment 2 in healthy female subjects. Data presented is as Mean ± SEM, n = 12; Different superscript letters denote a significant difference at p < 0.05 in the same row (Tukey’s posthoc test). W water, WB white bread, ChP chickpeas; 1 Experiment 1, 2 Experiment 2
Despite that the average change in appetite AUC was not significantly different among the treatments, a positive correlation was observed with Kcal intake as (r = 0.552, p < 0.05) and (r = 0.430, p < 0.01) in Experiments 1 and 2, respectively. This is supporting a decrease in food intake with a cumulative change in appetite, meaning a reduction in appetite, as would be expected. On the other hand, the cumulative change in blood glucose AUC over 60 min or 120 min in Experiments 1 and 2 was not correlated with the food intake or with the average change in appetite AUC. The correlation between the final blood glucose before the meal and calories intake from the meal was time dependent. For example, there was a negative correlation at 120 min (r = −0.332, p < 0.05) and no correlation at 60 min (r = 0.145, p > 0.05) in Experiments 1 and 2, respectively.
Postprandial blood glucose excursions are as important to regulate as is the fasting blood glucose concentration to prevent hyperglycemia. Frequent elevations in blood glucose levels may lead to an increased level of glycated hemoglobin (HbA1c), which in turn puts the individual at a greater risk for diabetes and its related complications (Litwak, et al. 2013). Our results support the low glycemic and high satiety properties of chickpeas. A reduction of 29–36% in the blood glucose concentration AUCs had occurred 0–60 and 0–120 min, respectively. An earlier research documented the consumption of one cup of beans by type-2 diabetics for three months had significantly decreased HbA1c level, a measure of the control of blood glucose concentration, by 0.5% (Jenkins et al. 2012). The FDA recommendation of 0.3–0.6% reduction in HbA1c is a reasonable control of the blood glucose concentrations by diabetics (U. S. Department of Health and Human Services, 2008).
Many factors have been suggested contributing to the low glycemic effect of legumes. Besides that they are a good source of quality protein and dietary fiber, the most important is the quality and quantity of their starch. Legumes’ low digestible and high resistant starch, amylose, and fiber content impart less glucose into the circulation, hence is low glycemic (McCrory et al. 2010). Chickpeas compared to wheat starch contain 51 versus 64% digestible starch, 30–40 versus 20% amylose, and 16.4 versus 3% dietary fiber (Idriss et al. 2012). The explanation through which the nutritional composition of chickpeas influences the glycemic and satiety impact may include several possible mechanisms. The various dietary soluble viscous fiber, i.e., galactomannans (polysaccharide), with good gelling properties decreases gastric emptying, increases gastric distention, and satiety (Dilwari et al. 1981); decreases digestive process and lowers glucose release. 2) Some of the high amylose component may escape digestion and convert to resistant starch, thus rendering a low glycemic effect. 3) Additionally, high protein and resistant starch are documented to stimulate gut hormones such as such GLP-1, GIP and PYY. GLP-1 and GIP regulate postprandial blood glucose concentration through insulin secretion (MacNeil et al. 2013; Zhou et al. 2008; Pratley, 2009) and PYY is a satiety hormone that increases the feeling of fullness (Zhou et al. 2008).
It is well established that low GI foods are slowly digested and are steadily absorbed, thus sustain blood glucose concentration within the normal range with exerting less demand on insulin secretion by the pancreas. On the contrary, high GI simple carbohydrate foods are quickly digested and abruptly absorbed with an insulin surge causing excessive blood glucose disposal that leads to a reactive hypoglycemia and possibly hunger (Ludwig 2000). According to the Glucostatic theory, blood glucose concentration regulates food intake via hypothalamic mechanisms. A transient decline in blood glucose causes reduced glucose utilization by various organs that signals hunger and initiation of feeding. Whereas increased blood glucose concentration after meals signals satiety and termination of feeding (Mayer 1996). A meta-analysis of 39 trials showed a reduction in the fasting blood glucose, insulin or the markers of glycemic control when pulses were consumed either alone, as a part of high fiber diets or as a low glycemic diet (Sievenpiper et al. 2009).
The results of the current study demonstrated suppression of appetite and therefore, reduction in EI at the subsequent meal. This effect on appetite was more pronounced by the chickpeas when the period was extended to 2 h resulting in 98% EC for the preload calories. The effect of chickpeas on the blood glucose concentration before the meal might also be a factor in its reduced EI compared to the bread treatment, as reflected by the negative correlation of blood glucose level before meal and EI. The lower blood glucose at 120 min after the high glycemic bread treatment might have caused more hunger and thus more EI (Ludwig 2007). These results are supported by others where subjects with a 43 to 46% reduction in the glucose AUC after a low GI-whole meal diet and a low GI-replacement diet postponed their request for the test meal compared to high GI-replacement meal by 3.9 versus 3.1 h, respectively (Ball et al. 2003).
Although, a meta-analysis study of acute research trials calculated a 31% increase in satiety AUC following legume consumption; nevertheless, this did not show any effect on the EI from the second meal (Li et al. 2014). In fact, a range of factors can implicate the appetite responses and thus the subsequent food ingestion; for example, energy density, nutritional composition, the total volume of the preload, available carbohydrate and the inter-meal interval. In the current study, after controlling the preload treatments for factors, including energy density, total volume, available carbohydrate from a more usual portion sized quantity preloads, we had been able to observe a reduction in appetite and EI after the chickpeas preloads in both experiments, and more so with the bigger inter-meal interval. The EI was only 50 kcal less at 60 min in Experiment 1 but amounted to 194 kcal less at 120 min in Experiment 2. If this much deficit in calories intake occurs on a daily basis, it may result in a loss of more than a pound of body fat in 20 days; that would be equivalent to about 20 lbs. a year. Although, this calculated success in weight loss, which needs further exploration, may happen by the fact that chickpeas and other pulses suppress EI is, in part, due to the increased satiety or sustained blood glucose concentration but not to the restrained eating. Losing body weight through extreme dieting causes psychological disparities and an increased desire for food. One of the suggested reasons for the failure in a long-term adherence to weight loss dieting programs includes psychological hunger and food cravings (Wing et al. 2008), which cause relapses and weight regain (Ohsiek and Williams 2011; Tate et al. 2007).
The limitation of our study was the small sample size, which was calculated for the 80% power to differentiate the treatment effect at the p value of ≤0.05 on EI but not on the subjective appetite. This might be the reason our results did not demonstrate the effect of treatment on the average change in appetite. Other researchers were able to claim successful weight loss by legumes consumption through appetite and glycemic control (Abete et al. 2009; Crujeiras et al. 2007, McCrory et al. 2010; Ball et al. 2003; Jenkins et al. 2012). It is thus proposed that weight loss, if achieved, through appetite control may have enduring results. Furthermore, not only chickpeas but the consumption of other pulses and whole foods more frequently, may prove beneficial in the dietary management of metabolic syndrome and to improve the general health.
Our results, however, need affirmation as our study population was only healthy subjects of college- going females. Further studies are warranted in both males and females of various age groups as well as in overweight and diabetic individuals before any generalization could be made.
Conclusion
Dietary pulses including beans, lentils, dried peas and chickpeas are considered low glycemic index foods that are presumably beneficial in weight management because of their chemical composition such as high dietary fiber, protein, resistant starch and other functional ingredients that enhance satiety and reduce food intake. However, due to variation in research study design, conflicting results are reported in the literature. Focusing only on one legume, we reported that chickpeas consumption improved postprandial blood glucose control, suppressed appetite, and caused a reduction in energy intake from a subsequent meal in the healthy female subjects. We may propose that the impact of cooked dietary pulses on satiety or EC compared to a reference is better assessed at a similar Kcal, energy density, and available carbohydrate level consumed in a physiologically comfortable volume. It is, therefore, recommended to encourage weight loss practices through appetite management that might be achieved through frequent consumption of dietary pulses and other high fiber whole foods with a potential of suppressing appetite.
Acknowledgements
This research was conducted with the support of a Grant approved by the Kuwait University Research Sector, Grant # WF04/07.
Compliance with ethical standards
Conflict of interest
Authors declare no conflict of interest.
References
- Abete I, Parra D, Martinez JA. Legume-, fish-, or high-protein-based hypocaloric diets: effects on weight loss and mitochondrial oxidation in obese men. J Med Food. 2009;12:100–108. doi: 10.1089/jmf.2007.0700. [DOI] [PubMed] [Google Scholar]
- Anderson GH, Khodabandeh S, Patel B, Luhovyy BL, Bellissino N, Mollard RC. Mealtime exposure to food advertisement while watching television increases food intake in overweight and obese girls but has a paradoxical effect in boys. Appl Physiol Nutr Meta. 2015;40(2):162–167. doi: 10.1139/apnm-2014-0249. [DOI] [PubMed] [Google Scholar]
- Badr HE, Shah NM, Shah MA. Obesity among Kuwaitis aged 50 years or older: prevalence, correlates and comorbidities. The Gerontologist. 2012;53(4):555–566. doi: 10.1093/geront/gns108. [DOI] [PubMed] [Google Scholar]
- Ball SD, Keller KR, Moyer-Mileur LJ, Ding YW, Donaldson D, Jackson WD. Prolongation of satiety after low versus moderately high glycemic index meals in obese adolescents. Pediatrics. 2003;111(3):488–494. doi: 10.1542/peds.111.3.488. [DOI] [PubMed] [Google Scholar]
- Bazzano LA, He J, Ogden LG, et al. Legume consumption and risk of coronary heart disease in US men and women: NHANES I epidemiologic follow-up study. Arch Intern Med. 2001;161:2573–2578. doi: 10.1001/archinte.161.21.2573. [DOI] [PubMed] [Google Scholar]
- Boyle JP, Honeycutt AA, Narayan KMV, Hoerger TJ, Geiss LS, Chen H, Thompson TJ. Projection of Diabetes Burden through 2050: impact of changing demography and disease in the US. Diabetes Care. 2010;24:1936–1940. doi: 10.2337/diacare.24.11.1936. [DOI] [PubMed] [Google Scholar]
- Crujeiras AB, Parra D, Abete I, Martinez JA. A hypocaloric diet enriched in legumes specifically mitigates lipid peroxidation in obese subjects. Free Radic Res. 2007;41:498–506. doi: 10.1080/10715760601131935. [DOI] [PubMed] [Google Scholar]
- Dilwari JB, Kamath PS, Batta RP. Reduction of postprandial plasma glucose by Bengal gram dal (Cicer arietinum) and Rajmah (Phaeolus vulgaris) Am J Clin Nutr. 1981;34:2450–2453. doi: 10.1093/ajcn/34.11.2450. [DOI] [PubMed] [Google Scholar]
- Escalante PJM, Alpizar SM. Change in insulin sensitivity, secretion and glucose effectiveness during menstrual cycle. Arch Med Res. 1999;30:19–22. doi: 10.1016/S0188-0128(98)00008-6. [DOI] [PubMed] [Google Scholar]
- Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Intern J Obes. 2000;24(1):38–48. doi: 10.1038/sj.ijo.0801083. [DOI] [PubMed] [Google Scholar]
- Guariguata L, Whiting DR, Hambleton C, Beagley J, Linnenkemp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035: IDF Diabetes Atlas. Diabetes Res Clin Pract. 2014;103:137–149. doi: 10.1016/j.diabres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Guenther PM, Casavale KO, Reedy J, et al. Update of the healthy eating index: HEI-2010. J Acad Nutr Dietetics. 2013;113:569–580. doi: 10.1016/j.jand.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall RS, Thomas SJ, Johnson SK. Australian sweet lupin flour addition reduces the glycemic index of a white bread breakfast without affecting palatability in healthy human volunteers. Asia Pacific J Clin Nutr. 2005;14:91–97. [PubMed] [Google Scholar]
- Health Canada (2007) Eating well with Canada’s food guide. Publication H39e166/1990E. Ottawa, ON: Health Products and Food Branch, Office of Nutrition and Promotion
- Idriss M, Ahmed AR, Senge B. Dough rheology and bread quality of wheat–chickpea flour blends. J Food Sci Technol. 2012;36:196–202. [Google Scholar]
- Jenkins DA, Kendall CWC, Augustin LSA, et al. Effect of legumes as part of a low glycaemic index diet on glycaemic control and cardiovascular risk factors in type 2 diabetes mellitus. Arch Intern Med. 2012;172:1653–1660. doi: 10.1001/2013.jamainternmed.70. [DOI] [PubMed] [Google Scholar]
- Johnson SK, Thomas SJ, Hall RS. Palatability and glucose, insulin and satiety responses of chickpea flour and extruded chickpea flour bread eaten as part of a breakfast. Eur J Clin Nutr. 2005;59:169–176. doi: 10.1038/sj.ejcn.1602054. [DOI] [PubMed] [Google Scholar]
- Keogh J, Atkinson F, Eisenhauer B, Inamdar A, Brand-Miller J. Food intake, postprandial glucose, insulin and subjective satiety responses to three different bread-based test meals. Appetite. 2011;57:707–710. doi: 10.1016/j.appet.2011.08.015. [DOI] [PubMed] [Google Scholar]
- Li SS, Kendall CWC, de Souza RJ, Jayalath VH, et al. Dietary pulses, satiety and food intake: a systemic review and meta-analysis of acute feeding trials. Obesity. 2014;22(8):1773–1780. doi: 10.1002/oby.20782. [DOI] [PubMed] [Google Scholar]
- Litwak L, Goh SY, Hussein Z, Malek R, Prusty V, Khamseh ME. Prevalence of diabetes complications in people with type 2 diabetes mellitus and its association with baseline characteristics in multinational A 1 chieve study. Diabetol Metab Syndr. 2013;5(1):57. doi: 10.1186/1758-5996-5-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig DS. Clinical update: the low-glycemic-index diet. The Lancet. 2007;369:891–892. doi: 10.1016/S0140-6736(07)60427-9. [DOI] [PubMed] [Google Scholar]
- MacNeil S, Rebry RM, Tetlow IJ, Emes MJ, McKeown B, Graham TE. Resistant starch intakes at breakfast affects postprandial responses in type 2 diabetics and enhances the glucose-dependent insulintropic polypetide—insulin relationship following a second meal. Appl Physiol Nutr Metab. 2013;38:1187–1195. doi: 10.1139/apnm-2013-0023. [DOI] [PubMed] [Google Scholar]
- Mayer J. Glucostatic mechanism of regulation of food intake. 1953. Obes Res. 1996;4:493–496. doi: 10.1002/j.1550-8528.1996.tb00260.x. [DOI] [PubMed] [Google Scholar]
- McCrory MA, Hamaker BR, Lovejoy JC, Eichelsdoerfer PE. Pulse consumption, satiety, and weight management. Adv Nutr. 2010;1:17–30. doi: 10.3945/an.110.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollard RC, Wong CL, Luhovyy BL, Anderson GH. First and second meal effects of pulses on blood glucose, appetite, and food intake at a later meal. Appl Physiol Nutr Metab. 2011;36:634–642. doi: 10.1139/h11-071. [DOI] [PubMed] [Google Scholar]
- Mollard RC, Luhovyy BL, Panahi S, Nunez M, Hanley A, Anderson H. Regular consumption of pulses for 8 weeks reduces metabolic syndrome risk factors in overweight and obese adults. Br J Nutr. 2012;108:111S–122S. doi: 10.1017/S0007114512000712. [DOI] [PubMed] [Google Scholar]
- Mollard RC, Zykus A, Luhovyy BL, Nunez MF, Wong CL, Anderson GH. The acute effects of a pulse-containing meal on glycaemic responses and measures of satiety and satiation within and at a later meal. Br J Nutr. 2012;108(3):509–517. doi: 10.1017/S0007114511005836. [DOI] [PubMed] [Google Scholar]
- Ohsiek S, Williams M. Psychological factors influencing weight loss maintenance: an integrative literature review. J Am Acad Nurse Pract. 2011;23:592–601. doi: 10.1111/j.1745-7599.2011.00647.x. [DOI] [PubMed] [Google Scholar]
- Polivy J, Herman CP, Warsh S, Villaume C, Beck B, Rohr R, Poitel JP, Debry G. Internal and external components of emotionally restrained and unrestrained eaters. J Abnorm Psych. 1978;87:497–504. doi: 10.1037/0021-843X.87.5.497. [DOI] [PubMed] [Google Scholar]
- Pratley RE. Expanding treatment options for type 2 diabetes: the old and the new. Diabetes Educ. 2009;35:4S–11S. doi: 10.1177/0145721709331522. [DOI] [PubMed] [Google Scholar]
- Sievenpiper JL, Kendall CWC, Esfahani A, et al. Effect of non-oil-seed pulses on glycaemic control: a systematic review and meta-analysis of randomized controlled experimental trials in people with and without diabetes. Diabetologia. 2009;52:1479–1495. doi: 10.1007/s00125-009-1395-7. [DOI] [PubMed] [Google Scholar]
- Tate DF, Jeffery RW, Sherwood NE, Wing RR. Long-term weight losses associated with prescription of higher physical activity goals. Are higher levels of physical activity protective against weight regain? Am J Clin Nutr. 2007;85(4):954–959. doi: 10.1093/ajcn/85.4.954. [DOI] [PubMed] [Google Scholar]
- U. S. Department of Health and Human Services (2008) Food and drug administration. Center for Drug Evaluation and Research. Guidance for Industry Diabetes Mellitus: Developing drugs and therapeutics biologics for treatments and prevention. (http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformat). Accessed 10 Aug 2016
- U.S. Department of Agriculture. (2010) U.S. Department of Health and Human Services: Report of the Dietary Guidelines Advisory Committee on the Dietary Guidelines for Americans 2010, Washington, DC. U.S. Government Printing Office
- Wing RR, Papandonatos G, Lava JL, et al. Maintaining large weight losses. The role of behavioral and psychological factors. J Consult Clin Physiol. 2008;76:1015–1021. doi: 10.1037/a0014159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CL, Mollard RC, Zafar TA, Luhovyy BL, Anderson GH. Food intake and satiety following a serving of pulses in young men: effect of processing, recipe, and pulse variety. J Am Coll Nutr. 2009;28:543–552. doi: 10.1080/07315724.2009.10719786. [DOI] [PubMed] [Google Scholar]
- Zafar TA, Kabir Y, Ghazaii C. Low glycemic index foods suppress glycemic response, appetite and food intake in young Kuwaiti females. Kuwait J Sci Eng. 2011;38(1A):111–123. [Google Scholar]
- Zafar TA, Al-Hassawi F, Al-Khulaifi F, Ghanima Al-Rayyes, Waslien C, Huffman FG. Organoleptic and glycemic properties of chickpea-wheat composite breads. J Food Sci Technol. 2015;52(4):2256–2263. doi: 10.1007/s13197-013-1192-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Martin RJ, Tully RT, Raggio AM, McCutcheon KL, Shen L, Danna SC, Tripathy S, Hegsted M, Keenana MJ. Dietary resistant starch regulates total GLP-1 and PYY in a sustained day-long manner through fermentation in rodents. Am J Physiol Endocrinol Metab. 2008;295:E1160–E1166. doi: 10.1152/ajpendo.90637.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]