Abstract
Objectives
Investigations of pediatric critical illness typically focus on inpatient cohorts drawn from wide referral areas and diverse healthcare systems. Cohorts amenable to investigating the full spectrum of critical illness as it develops within a community have yet to be studied in the US. Our objective was to provide the first epidemiologic report of the incidence and presentation of pediatric critical illness within a US population-based birth cohort.
Design
Retrospective cohort study
Setting and Patients
We investigated a birth cohort of children (n=9,441) born 2003–2007 within a geographically defined area (Olmsted County, MN). Medical records are linked across all health systems accessed by this population. All intensive care unit (ICU) services are provided within a single children’s hospital.
Measurements and Main Results
During the study period, there were a total of 15,277 ICU admissions to Mayo Clinic Children’s Hospital. A total of 577 birth cohort children accounted for 824 of these admissions during the 61,770 person-years of follow-up accumulated. Incidence of first-time ICU admission was 9.3 admits per 1,000 person-years. Admission rates were highest in the first year of life and then declined steadily. Respiratory problems were among the most common reasons for admission at any age and diagnoses reflect changes in health risk factors as children grow and develop over time. After 1 year of age, a majority of children admitted have pre-existing chronic comorbidities and/or prior ICU stays. In-hospital mortality occurred exclusively in children admitted prior to 5 days of age (n=4). Seven children died after hospital discharge.
Conclusions
This is the first report characterizing critical illness within a population-based birth cohort of US children. The results demonstrate the changing incidence, presentation, and healthcare requirements associated with critical illness across the developmental spectrum as a population of children ages.
Keywords: children, pediatric critical illness, PICU, population-base, epidemiology
Introduction
Pediatric critical illness can profoundly disrupt child health and development and negatively affect family function and well-being. Although pediatric intensive care unit (PICU) mortality is declining, a growing number of survivors develop deficits that persist beyond hospital discharge (1, 2). As a result, clinical focus has shifted beyond survival to focus on identifying and reducing post-ICU morbidity. Leading outcome experts have suggested that, “Maximizing long-term (health-related) quality of life may represent the most important goal of medicine in general and of intensive care in particular” (3). With few exceptions, research to date has focused almost exclusively on critical illness within referral centers that may select for the most complex cases drawn from a widespread geographical area. As a result, our understanding of the risk factors associated with the development, incidence, and outcome of critical illness within a general population of children remains limited (1). This knowledge gap hinders our ability to develop and test strategies to prevent severe illness, minimize its negative consequences, and estimate healthcare resource requirements within a broad population (4). Addressing these challenges will require accurate and representative data with sufficient detail to characterize each child’s baseline (pre-hospital) health status, ICU clinical course, and health outcomes well beyond hospital discharge (1,5). Unfortunately, until recently population-based cohorts amenable to this kind of longitudinal epidemiologic investigation had yet to be developed in the US (6).
As an initial step to address this gap, we constructed a population-based birth cohort of every child born during a 5 year period (2003–2007) within a geographically defined area (Olmsted County, MN). By identifying each child at birth, we are able to use epidemiologic methods to conduct a longitudinal investigation of pediatric critical illness as it develops within this population (7). The medical records for these children and their families are linked across all the health systems accessed by this population. This detailed information permits characterization of the prenatal/birth history, baseline health status, disease development, and health care resource utilization across the lifespan of every child within the cohort. In addition, this population-based approach can support evaluation of risk factors and outcomes for children with and w/o ICU exposure (8). Our objective was to provide the first report of the incidence and presentation of pediatric critical illness within a general population of US children as a pre-requisite step for longitudinal investigation of the impact of critical illness on child health and development over time.
Materials and Methods
Using the resources of the Rochester Epidemiology Project (9), we studied a population-based birth cohort of children born to mothers residing in Olmsted County, MN during five years (1/1/2003 – 12/31/2007). We identified all ICU hospitalizations for this birth cohort during up to 11 years of follow-up (to 12/31/2013). The Mayo Clinic institutional review board approved this study.
Study Setting
Olmsted County, MN occupies 653 square miles in southeastern Minnesota. With a population of 144,248 in 2010 (10), it is classified as a small metro county by the National Center for Health Statistics (11). In 2007, of the 16,330 children enrolled within the Olmsted County public school system, 27% were non-white (11% black, 7% Hispanic, and 9% Asian), and, like the US population, the county population continues to become more racially and ethnically diverse with time (12). The county is 86 miles from the next-closest tertiary care pediatric center (Minneapolis, MN), and 98% of medical care received by county residents is delivered by the Mayo Clinic, Olmsted Medical Center (OMC), and its affiliated hospitals (13). All children with critical illness are cared for at a single hospital, within one of three ICUs (neonatal ICU, cardiac ICU, and pediatric ICU), and managed by specialists in neonatal and pediatric critical care medicine. The hospital is a Level 1 pediatric trauma center and provides the full complement of pediatric tertiary care services which includes; congenital cardiac surgery, extracorporeal membrane oxygenation (ECMO), pediatric transport (ground, air, and ECMO transport), and solid organ and bone marrow transplantation.
Identifying the 2003–2007 population-based birth cohort
Using the Rochester Epidemiology Project database, we studied children born to mothers who were residents of Olmsted County at the time of the child’s birth (n=10,899 from 01/01/2003 to 12/31/2007). Children were excluded if their parents denied research authorization for the use of the child’s medical records (n=1,458, 13.4% of the eligible birth cohort), leaving 9,441 newborns in the studied birth cohort. The Rochester Epidemiology Project is a unique research infrastructure which links and archives the medical records of nearly all persons residing in Olmsted County, Minnesota (9). A continuously updated census generates residency timelines for each individual that includes the dates of migration into, or out of, Olmsted County, MN. These timelines were used to establish the denominators for the incidence rate calculations. For example, for the age category from 1.0 to <2.0 years, we calculated the total person-years that cohort members resided in Olmsted County between that age range. If a given cohort member resided in Olmsted County on their first birthday, but moved out of Olmsted County at the age of 1.5 they would have contributed 0.5 person-years to this denominator. This approach for calculating incidence rate estimates accounts for migration into and out of the birth cohort.
Data abstraction and management
All ICU admissions to the Mayo Eugenia Litta Children’s Hospital within this birth cohort from January 1, 2003 through December 31, 2013 were reviewed. Inpatient and outpatient medical records were manually reviewed to identify baseline demographics, pre-ICU health status, chronic comorbidities, pre-admission referral source, admitting diagnosis, ICU clinical data, and vital status. Reviews were performed by 3 research assistants and directly supervised by a board-certified pediatric critical care specialist to ensure accuracy and consistency in abstraction procedures. Multiple ICU admissions during the same hospital stay were considered part of the same critical care episode and counted only once. A primary admitting diagnosis was identified for each critical care episode and categorized within organ systems. Within each organ system, diagnoses were categorized as operative or non-operative. A diagnosis was considered operative only if the patient was admitted to the ICU directly from the OR (e.g., following spinal fusion). Children admitted with a medical diagnosis who required an operation later during their ICU course were classified as non-operative admissions (e.g., traumatic brain injury with external ventricular drain placement on ICU day 2). Two additional categories were included to characterize the indication for admission. Admissions related to premature birth (<37 weeks gestational age) were assigned a primary admission diagnosis of prematurity. Admissions following a traumatic injury were classified as trauma; regardless of the organ system involved (e.g. traumatic brain injury was classified as trauma rather than neurologic) to support future investigations into trauma related epidemiology and outcomes within this cohort. Chronic comorbidities pre-dating ICU admission were identified from outpatient and inpatient clinical notes and grouped into the categories of the Pediatric Complex Chronic Conditions (PCCC) (19). The Pediatric Risk of Mortality score (PRISM III) and Pediatric Logistic Organ Dysfunction (PELOD) score were calculated to characterize severity and course of illness. Dates of death and last follow-up were obtained using Rochester Epidemiology Project resources (9).
Statistical Analysis
We calculated the incidences of 1) first lifetime admission to the ICU and 2) total ICU admissions during the study period. The denominator was the cumulative age-group specific person-years of residency in Olmsted County from January 1, 2003 to December 31, 2013 for the birth cohort. This approach takes into account the varying ages at last follow-up and the fact that some cohort members moved out of Olmsted County during the follow-up period. We calculated standard errors and 95% confidence intervals (CI) for the incidence rates based on the Poisson error distribution. We categorized age at ICU admission as 0 to 4 days, 5 to 29 days, 30 days to < 1 year, 1 to 3 years, and 4 to 10 years (No cohort member had reached their 11th birthday by the end of the study period). After determining the “all cause” incidence of critical illness, children with a primary admission diagnosis of prematurity were excluded from the remaining analyses.
Unless otherwise noted, results are presented as median (interquartile range [IQR]) for continuous variables and frequency (percentage) for categorical variables. We compared categorical variables using the chi-square or Fisher’s exact test and continuous variables using the Kruskal-Wallis test. Survival at 3 and 5 years following the first ICU admission was estimated for hospital survivors using the Kaplan-Meier method. In all cases, two-tailed p-values are reported with p-values ≤ 0.05 used to denote statistical significance unless otherwise noted.
Results
Population-based incidence of “all cause” critical illness (includes ICU admissions for prematurity)
During the study period, there were a total of 15,277 ICU admissions to Mayo Eugenia Litta Children’s Hospital. A total of 577 birth cohort children accounted for 824 of these admissions during the 61,770 person years of follow-up accumulated during the study period. Incidence was highest during the first month of life and declined with age (Figure 1). Neonates 0 to 4 days of age (n=343, of which 195 were admitted to the NICU for prematurity) had the highest ICU admission rate, at 2,649 per 1,000 person-years (95% CI: 2,382 to 2,947). ICU admission rates for the 5 to 29 day olds dropped precipitously but remained much higher than children in the older age groups. Of the 824 total ICU birth cohort admissions, 490 children had a single ICU admission, 48 had 2 admissions, 22 had 3–4 admissions, 9 had 5–9 admissions, and 8 had 10 or more ICU admissions.
Figure 1.
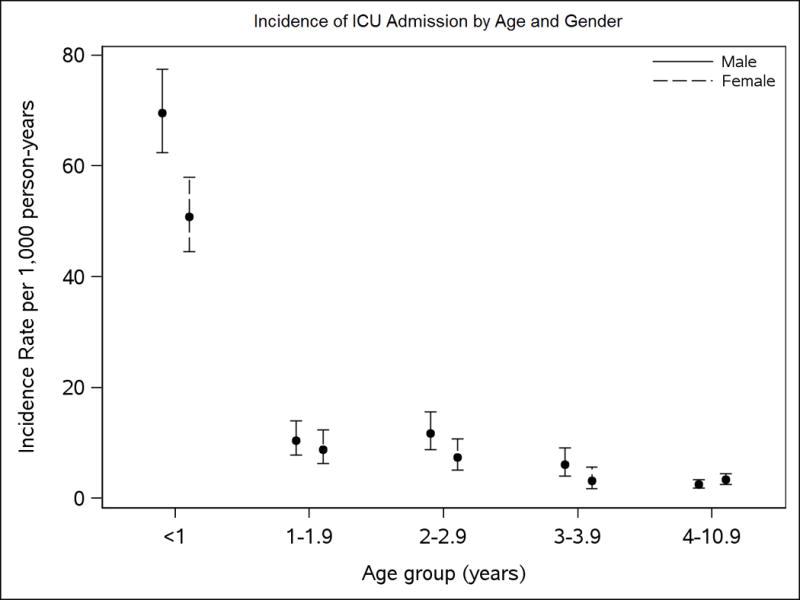
Comparison of ICU admission incidence by age and gender
ICU Admissions Excluding Premature Newborns
The incidence of ICU admission was highest during the first year of life and declined thereafter. The demographics and admission characteristics for the first ICU hospitalization of the 382 birth cohort children born at term or later are summarized in Table 1. The majority of patients were male in all age groups. Operative admissions accounted for 30% of the first time admits. Respiratory-related diagnoses were among the top 3 reasons for ICU admission across all age ranges. Admissions for gastrointestinal (GI) disease were the most frequent in children < 30 days old. Admission rates for trauma were low until age 4 when trauma became the leading indication for ICU admission (27%). The proportion of children presenting to the ICU with chronic medical conditions increased substantially after the first month of life to nearly two-thirds by one year of age.
Table 1.
Demographics and admission characteristics, excluding children admitted for prematurity*
| 0 to 4 days (N=147) |
5 to 29 days (N=65) |
30 days to 0.9 years (N=63) |
1.0 to 3.9 years (N=76) |
4.0 to 10.9 years (N=30) |
P | |
|---|---|---|---|---|---|---|
| Age | 1d (0, 2) | 7d (5, 12) | 4.7m (2.5, 8.3) | 2.3y (1.5, 2.8) | 5.5y (4.6, 6.7) | – |
| Male | 87 (59%) | 37 (57%) | 32 (51%) | 40 (53%) | 17 (57%) | 0.796 |
| Race, white + | 110 (76%) | 54 (84%) | 42 (67%) | 51 (67%) | 17 (59%) | 0.035 |
| Number of chronic comorbidities§ | <.001 | |||||
| None | 102 (69%) | 52 (80%) | 27 (43%) | 24 (32%) | 11 (37%) | |
| 1 | 36 (25%) | 9 (14%) | 22 (35%) | 31 (41%) | 9 (30%) | |
| 2 or more | 9 (6%) | 4 (6%) | 14 (22%) | 21 (28%) | 10 (33%) | |
| Admission source | <.001 | |||||
| Emergency department | 12 (8%) | 24 (37%) | 30 (48%) | 26 (34%) | 17 (57%) | |
| Ward | 1 (1%) | 1 (2%) | 2 (3%) | 3 (4%) | 2 (7%) | |
| Clinic | 17 (12%) | 30 (46%) | 2 (3%) | 2 (3%) | 0 (0%) | |
| Newborn/Level II nursery | 84 (57%) | 3 (5%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Operating room/PACU | 2 (1%) | 4 (6%) | 28 (44%) | 45 (59%) | 9 (30%) | |
| Outside hospital | 31 (21%) | 3 (5%) | 1 (2%) | 0 (0%) | 2 (7%) | |
| Primary admission diagnosis | <.001 | |||||
| Cardiovascular | 22 (15%) | 3 (5%) | 10 (16%) | 6 (8%) | 1 (3%) | |
| Respiratory | 59 (40%) | 10 (15%) | 18 (29%) | 11 (14%) | 6 (20%) | |
| GI | 34 (23%) | 38 (58%) | 2 (3%) | 3 (4%) | 1 (3%) | |
| GU | 2 (1%) | 4 (6%) | 0 (0%) | 1 (1%) | 2 (7%) | |
| Hematologic | 4 (3%) | 1 (2%) | 0 (0%) | 0 (0%) | 1 (3%) | |
| Musculoskeletal | 0 (0%) | 0 (0%) | 1 (2%) | 0 (0%) | 0 (0%) | |
| Trauma | 0 (0%) | 0 (0%) | 1 (2%) | 3 (4%) | 8 (27%) | |
| Endocrine-metabolic | 5 (3%) | 0 (0%) | 1 (2%) | 0 (0%) | 0 (0%) | |
| Neurologic | 17 (12%) | 5 (8%) | 15 (24%) | 16 (21%) | 5 (17%) | |
| ENT | 3 (2%) | 3 (5%) | 15 (24%) | 36 (47%) | 5 (17%) | |
| Other | 1 (1%) | 1 (2%) | 0 (0%) | 0 (0%) | 1 (3%) | |
| Operative | 23 (16%) | 5 (8%) | 29 (46%) | 45 (59%) | 9 (30%) | <.001 |
Values are n (%) for categorical variables and median (Q1, Q3) (min–max) for continuous variables. Due to rounding, percentages will not always sum to 100%.
Race data was unavailable for 4 subjects
Characteristics were compared across groups using the chi-square test unless specified otherwise.
Number of chronic comorbidities based on Pediatric Complex Chronic Conditions Categories (12). P value is from the Kruskal-Wallis test.
Details of the ICU course, resource use, and mortality are outlined in Table 2. The 0–4 day old group had the greatest severity of illness based on a number of factors. They had the highest use of noninvasive (n=26, 18%, p=.001) and invasive (n=55, 37%, p<.001) mechanical ventilation and vasopresor support (n=26, 18%, p= .002). PRISM III (p<.001) and PELOD (p<.001) scores were highest in this age group, as were rates of respiratory failure, shock, and acute kidney injury (p<.001). ICU and hospital lengths of stay were also significantly longer (p<.001). In-hospital mortality, after excluding infants admitted for prematurity, was low. All deaths occurred among those admitted prior to 5 days of age (n=4). In patients discharged alive from the hospital, the Kaplan-Meier estimate (95% CI) for mortality at 1 and 5 years following hospital discharge was 1.4% (0.2% to 2.6%) and 2.0% (0.5% to 3.5%) respectively (Figure 2). Characteristics of the seven children that died after hospital discharge are summarized in Table 3.
Table 2.
Characteristics of critical illness and ICU course among children born at term or later*
| 0 to 4 days (N=147) |
5 to 29 days (N=65) |
30 days to 0.9 years (N=63) |
1.0 to 3.9 years (N=76) |
4.0 to 10.9 years (N=30) |
P value | |
|---|---|---|---|---|---|---|
| PRISM III score | 3 (0, 4) | 0 (0, 3) | 0 (0, 2) | 0 (0, 3) | 0 (0, 3) | <.001 |
| (0–30) | (0–10) | (0–12) | (0–19) | (0–11) | ||
| Initial PELOD score | 10 (10, 11) | 10 (10, 10) | 2 (0, 10) | 1 (0, 10) | 0.5 (0, 10) | <.001 |
| (0–41) | (0–20) | (0–31) | (0–21) | (0–20) | ||
| Maximum PELOD score | 10 (10, 11) | 10 (10, 10) | 10 (0, 10) | 1 (0, 10) | 1.5 (0, 10) | <.001 |
| (0–42) | (0–21) | (0–31) | (0–21) | (0–32) | ||
| Invasive mechanical ventilation | 54 (37%) | 7 (11%) | 15 (24%) | 15 (20%) | 4 (13%) | <.001 |
| Duration mechanical ventilation (d, n=96) | 2.6 (0.8, 5.9) | 0.9 (0.9, 1.2) | 0.8 (0.7, 2.8) | 0.5 (0.3, 0.7) | 1.8 (0.5, 63.5) | 0.043 |
| (0.0–94.6) | (0.8–20.3) | (0.2–7.7) | (0.1–6.6) | (0.0–124.4) | ||
| Noninvasive mechanical ventilation | 26 (18%) | 2 (3%) | 7 (11%) | 2 (3%) | 2 (7%) | 0.001 |
| Sedatives/Narcotics | 51 (35%) | 11 (17%) | 33 (52%) | 35 (46%) | 13 (43%) | <.001 |
| Vasopressors/Inotropes | 25 (17%) | 1 (2%) | 5 (8%) | 5 (7%) | 1 (3%) | 0.003 |
| Total ICU length of stay (d) | 4.2 (1.6, 9.0) | 1.7 (1.0, 2.7) | 0.9 (0.6, 2.8) | 0.8 (0.6, 1.0) | 0.9 (0.8, 2.0) | <.001 |
| (0.1–119.9) | (0.3–40.1) | (0.1–12.7) | (0.2–8.6) | (0.3–215.2) | ||
| Hospital length of stay (d) | 4.5 (1.8, 9.7) | 2.0 (1.3, 3.3) | 2.8 (1.3, 5.2) | 1.3 (1.0, 3.9) | 2.7 (1.2, 6.8) | <.001 |
| (0.1–120.3) | (0.7–40.8) | (0.3–16.7) | (0.4–10.5) | (0.4–358.0) | ||
| Hospital mortality | 4 (3%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0.305 |
Values are n (%) for categorical variables and median (Q1, Q3) (min–max) for continuous variables. Due to rounding, percentages will not always sum to 100%.
Characteristics are compared across age groups using Fisher’s exact tests for categorical variables and the Kruskal-Wallis test for continuous variables
Acute kidney injury is defined as risk, injury, or failure.
Figure 2.
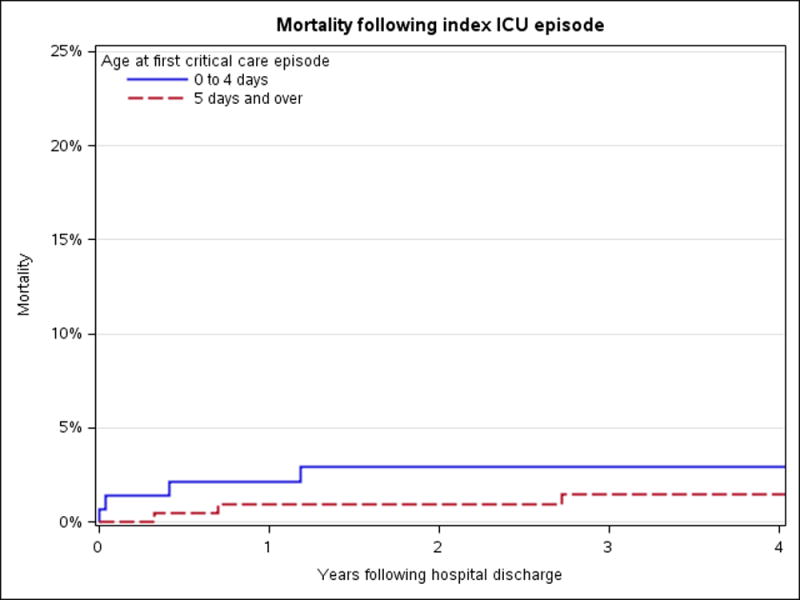
Mortality following ICU discharge.
Table 3.
Characteristics of children who died after ICU discharge
| Age at First Lifetime ICU Admission | Primary Admitting Diagnosis | Chronic Conditions | Multiple ICU Admissions | Age at Death | Cause of Death |
|---|---|---|---|---|---|
| 1 day | Neurologic-nonoperative (Seizure) | None | Yes | 17 months | Acute Respiratory Failure -Pneumonia |
| 1 day | Cardiovascular-operative (CHD) | Yes | Yes | 6 months | Respiratory Arrest |
| 1 day | Neurologic-nonoperative (Seizure) | Yes | No | 2 months | Status epilepticus-withdrawal of support |
| 3 years | Gastrointestinal-operative (Malignancy) | Yes | Yes | 6 years | Brain herniation-metastatic disease |
| 1 day | Cardiovascular-operative (CHD) | Yes | No | 10 days | Respiratory Arrest |
| 11 months | Gastrointestinal-operative (Nissen/G-tube) | Yes | Yes | 20 months | Acute Respiratory Failure – Pneumonia |
| 4 years | Respiratory-operative (Malignancy) | Yes | No | 5 years | Progressive Disease/Hospice |
CHD Congenital Heart Disease
Discussion
This is the first report characterizing the incidence, clinical course, and mortality rate for all-cause pediatric critical care hospitalization within a US population-based birth cohort. We found that critical illness is a rare event after the neonatal period within a general, non-urban population of children. ICU admission rates are highest within the first month of life and then decline steadily. Children admitted within 4 days of birth have the greatest severity of illness, ICU resource use, and mortality even after excluding those admitted for prematurity. Respiratory problems are among the most common reasons for admission at any age, and shock is rare. The indication for ICU admission changes as the population ages and reflects the shifting risk profiles associated with pediatric health and development over time. After 1 year of age, most children requiring ICU care have pre-existing chronic comorbidities and/or prior ICU stays.
Our study is the first to our knowledge to characterize pediatric critical illness within a geographically defined population so that every ICU admission is captured and incidence can be quantified. This work leverages the unique and comprehensive data resources of the Rochester Epidemiology Project to access medical records linked across all the health systems accessed by this population. Abstracting data directly from the patient medical record addresses many of the limitations associated with the administrative datasets typically employed for this type of epidemiologic investigation. These code-based datasets are prone to coding bias, temporal changes in coding practices, and often contain insufficient clinical detail to rigorously evaluate potential confounding variables (13, 14). In contrast, the medical records were manually reviewed for each subject in our cohort to identify baseline demographics, pre-ICU health status, and characteristics of the hospital course.
ICU mortality in our population was low (3%) and isolated to admissions during the first 4 days of life. This observation fell within the lower range of PICU mortality rates (1.9 % to 6%) reported in the literature (15–17). This likely reflects the population-based nature of our cohort (vs. studies based exclusively on ICU inpatient admissions that include referral patients). For most tertiary center PICU populations, referral patients include those children transferred in from smaller PICUs in outside health systems when disease severity exceeds the local resources. In 13,017 emergent PICU admissions across 20 PICUs, patients transferred from outside healthcare systems had greater severity of illness and required more ICU related resources compared with emergent admissions from within the institution (18). Adult studies consistently observe longer ICU stays, duration of mechanical ventilation, higher ICU resource utilization, and greater mortality risk in referral patients compared to admissions from the community or inter-hospital system (19–21). This “referral bias” leads to over-representation of severe disease within an inpatient study population and hinders evaluation of prevention and early recognition strategies relevant to milder disease. Indeed, recent evidence demonstrates that all types of PICU patients are at risk for acquired morbidity, not just those with the greatest severity of illness (17). The population-based setting and single PICU system accessed by our birth cohort permits capturing the full spectrum of illness severity as it develops within the community and leads to the initiation of critical care services.
A growing body of evidence suggests that children with chronic illness represent a distinct group within the PICU population (22). More than two thirds of children admitted after 1 year of age in our cohort had pre-existing chronic health conditions (Figure 3). Chronic illness was present for 52.1% of PICU admits according to the VPS database (15), and approximately 60% within the Kids Inpatient Database (KID) and Pediatric Health Information System (PHIS) administrative databases (23). Chronic comorbidity is associated with higher healthcare resource requirements and increased risk for new morbidity and mortality during a critical illness event (2, 24–26). Little is known regarding the specific challenges and risk factors surrounding critical illness that predict poor outcome within this chronically ill subset. Longitudinal follow-up of our birth cohort will provide unique opportunities to evaluate risk factors and explore strategies to mitigate the negative consequences of critical illness on the health and development of these children.
Figure 3.
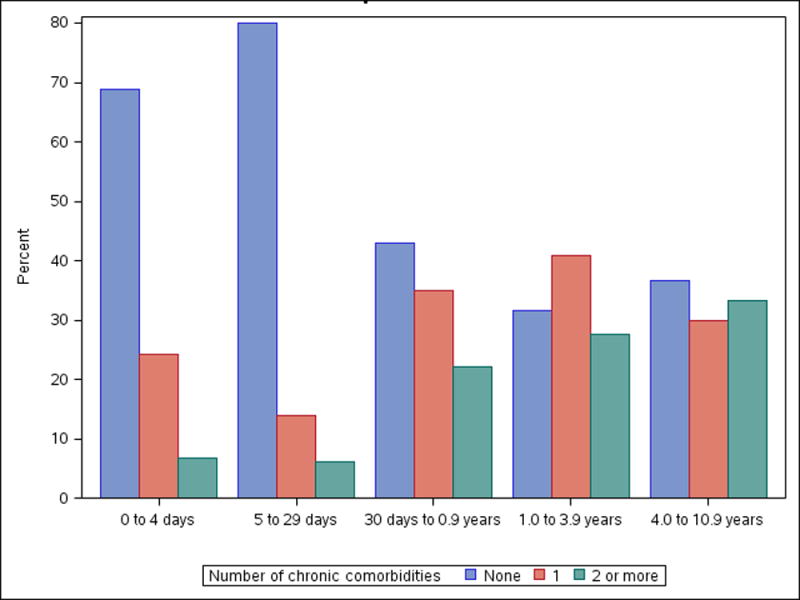
Number of chronic conditions present at ICU admission by age
With few exceptions, research to date has focused on disease within PICU populations where accessible data elements are often restricted to the hospital course. Reducing critical illness morbidity requires documenting pre-illness health status and post-discharge outcomes in addition to describing features of the ICU course (3, 27). Longitudinal follow-up of our birth cohort provides access to data generated at or before birth and continues indefinitely as the child grows to adulthood. Historically, knowledge gained from birth cohort investigations has identified health disparities (e.g. higher infant mortality in low income families), characterized changes in population demographics (e.g. maternal age at childbirth), and linked environmental exposures to disease development (e.g. smoking and lung cancer, maternal smoking and infant mortality) (28). Observational population-based studies have been used to identify risk factors for disease, develop risk-prediction algorithms, and discover significant biologic/environmental interactions (29, 30). These studies rely on existing data generated through “real-life” medical encounters that represent delivery of care within routine practice. This approach has been used to evaluate; 1) toxicity and efficacy of therapeutic interventions (e.g. vaccines, thalidomide), 2) rare exposures, 3) policy changes (e.g. seatbelts and motor vehicle accident mortality), and 4) changing burden of disease (e.g. back to sleep campaign and Sudden Infant Death Syndrome) (30–32).
Observing the implications of PICU admission within a population-based birth cohort provides an opportunity to simultaneously evaluate multiple effects from this single exposure (33). Physical health, emotional and behavioral disturbances, and the financial burden of a PICU admission can be longitudinally examined and contrasted between children/families with and without a critical illness event (32). Data generated can estimate sample size and patient recruitment timelines to inform randomized clinical trial (RCT) design (30, 32). Birth cohort children are now 9 to 13 years old and follow-up data is immediately available to investigate significant associations between critical illness and morbidity to guide prospective studies in selecting high yield assessments to optimize the impact of these resource intense initiatives (34). Finally, this population provides a platform for investigating hypotheses not readily amenable to RCT design (e.g. children requiring mechanical ventilation cannot be randomized to sedation versus placebo to investigate neurotoxicity). This work is directly in line with the NICHD’s directives for research, “Before designing controlled experiments comparing one intervention with another, we need quality epidemiologic and descriptive studies to guide trial design. For many, if not most common conditions in the PICU, these studies are lacking.” (5).
The unique setting of this study is both a strength and a limitation. First, our results are based on receipt of ICU care, which may not equate with presence of critical illness per se. Nonetheless, receipt of care in an ICU generally represents a need for care to treat or monitor conditions that carry a high risk of substantial morbidity. The Mayo Clinic healthcare system may not be representative of all ICUs treating children (or even pediatric tertiary academic ICUs) and our admission and practice patterns may differ. However, many similarities exist. According to the 2000 U.S. census data, the Olmsted County population is comparable to the overall U.S. population with respect to median age (35.0 y vs. 35.3 y) and male gender (49.1% vs. 49.1%). The ethnic characteristics of this population (87.6% white in Olmsted County, MN) are similar to other upper Midwest states (90.2% white in North and South Dakota, Iowa, and Wisconsin) (35). Compared to the entire US population, whites are more prevalent (87.6%vs. 74.3% US) (10). Despite these limitations, advocates for population-based research suggest there may be important advantages to this type of geographically isolated population. These “microclimates” can be valuable for achieving a more comprehensive analysis of the full spectrum of biological, environmental, and societal influences on health within these populations (36). Smaller well-defined populations can often be characterized in a level of detail that would be outside the scope of larger resource intense multicenter investigations. Geographically defined cohorts enhance recruitment and retention and permit accessing a patient’s entire support system due to the local proximity of the community (36). The population-based birth cohort described in this study permits the first opportunity to longitudinally evaluate the full spectrum of critical illness as it develops within a general non-hospital based population of children.
Conclusions
We provide the first report characterizing critical illness within a population-based birth cohort of children. The results demonstrate the changing incidence, presentation, and healthcare requirements associated with critical illness as a general population of children ages. Additional studies of this cohort will permit longitudinal evaluation of the factors across each child’s lifespan that impact the development, clinical course, and outcome of pediatric critical illness. This knowledge promises to inform health care delivery and identify opportunities for prevention, early recognition, intervention, and follow-up to reduce the risk for critical illness related morbidity during childhood.
Supplementary Material
Acknowledgments
This work was performed at the Mayo Clinic in Rochester, MN
Financial support for this study:
Dr. Crow’s work was supported by the National Institute of Child Health and Human Development, Pediatric Critical Care and Trauma Scientist Development Program-K12 (K12 HD 47349).
Critical Care Research Subcommittee, Mayo Clinic, Rochester, MN
Center for Clinical and Translational Science (CCaTS), Mayo Clinic, Rochester, MN
Contributor Information
Sheri S. Crow, Division of Pediatric Critical Care, Department of Pediatrics and Adolescent Medicine, Department of Health Services Research, Mayo Clinic College of Medicine, Rochester, MN.
Chaitanya Undavalli, Division of Pediatric Critical Care, Department of Pediatrics and Adolescent Medicine, Mayo Clinic College of Medicine, Rochester, MN.
David O. Warner, Department of Anesthesiology, Mayo Clinic College of Medicine, Rochester, MN.
Slavica K. Katusic, Department of Epidemiology and Pediatrics and Adolescent Medicine, Mayo Clinic College of Medicine, Rochester, MN.
Pujan Kandel, Division of Gastroenterology, Department of Internal Medicine, Mayo Clinic College of Medicine, Rochester, MN.
Sinead L. Murphy, Mayo Medical School, Mayo Clinic College of Medicine, Rochester, MN
Darrell R. Schroeder, Department of Biomedical Statistics and Informatics, Mayo Clinic College of Medicine, Rochester, MN
R. Scott Watson, Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Washington School of Medicine, Center for Child Health, Behavior, and Development; Seattle Children’s Research Institute, Seattle, WA.
References
- 1.Namachivayam P, Shann F, Shekerdemian L, et al. Three decades of pediatric intensive care: Who was admitted, what happened in intensive care, and what happened afterward. Pediatr Crit Care Med. 2010;11:549–555. doi: 10.1097/PCC.0b013e3181ce7427. [DOI] [PubMed] [Google Scholar]
- 2.Goodman DM, Hall M, Levin A, et al. Adults with chronic health conditions originating in childhood: Inpatient experience in children’s hospitals. Pediatrics. 2011;128:5–13. doi: 10.1542/peds.2010-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aspesberro F, Mangione-Smith R, Zimmerman JJ. Health-related quality of life following pediatric critical illness. Intensive Care Med. 2015;41:1235–1246. doi: 10.1007/s00134-015-3780-7. [DOI] [PubMed] [Google Scholar]
- 4.Nicholson CE, Gans BM, Chang AC, et al. Pediatric critical care medicine: Planning for our research future. Pediatr Crit Care Med. 2003;4:196–202. doi: 10.1097/01.PCC.0000059728.63798.DA. [DOI] [PubMed] [Google Scholar]
- 5.Williams TA, Dobb GJ, Finn JC, et al. Determinants of long-term survival after intensive care. Crit Care Med. 2008;36:1523–1530. doi: 10.1097/CCM.0b013e318170a405. [DOI] [PubMed] [Google Scholar]
- 6.Zimmerman JJ, Anand KJS, Meert KL, et al. Research as a standard of care in PICU. Pediatr Crit Care Med. 2016;17(1):e13–21. doi: 10.1097/PCC.0000000000000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gail MH, Benichou J. In: Encyclopedia of Epidemiological Methods. Armitage Peter, Colton Theodore., editors. John Wiley & Sons, Inc; New York, NY, USA: 2000. [Google Scholar]
- 8.Kremers HM, Myasoedova E, Crowson CS, Savova G, Gabriel SE, Matteson EL. The Rochester Epidemiology Project: exploiting the capabilities for population base research in rheumatic diseases. Rheumatology. 2011;50(1):6–15. doi: 10.1093/rheumatology/keq199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.St Sauver JL, Grossardt BR, Yawn BP, Melton LI, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester Epidemiology Project. Am J Epidemiol. 2011;173(9):1059–1068. doi: 10.1093/aje/kwq482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olmsted County MN population estimates for 2010- US census data. Available online at: http://quickfacts.census.gov/qfd/states/27/27109.html. Accessed February 16, 2016.
- 11.Ingram DD, Franco SJ. NCHS urban–rural classification scheme for counties. National Center for Health Statistics. Vital Health Stat. 2013;2(166):2014. [PubMed] [Google Scholar]
- 12.Minnesota Department of Education. Website available online at: http://w20.education.state.mn.us/MDEAnalytics/Summary.jsp. Accessed February 13, 2016.
- 13.Cooke CR, Iwashyna TJ. Using existing data to address important clinical questions in critical care. Crit Care Med. 2013;41(3):886–896. doi: 10.1097/CCM.0b013e31827bfc3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gorelick MG, Knight S, Alessandrini EA, et al. Lack of agreement in pediatric emergency department discharge diagnosis from clinical and administrative data sources. Acad Emerg Med. 2007;14(7):646–652. doi: 10.1197/j.aem.2007.03.1357. [DOI] [PubMed] [Google Scholar]
- 15.Typpo KV, Petersen NJ, Petersen LA, Mariscalco MM. Children with chronic illness return to their baseline functional status after organ dysfunction on the first day of admission in the pediatric intensive care unit. J Pediatr. 2010;157:108–13. doi: 10.1016/j.jpeds.2009.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leteurtre S, Duhamel A, Salleron J, et al. PELOD-2: An Update of the Pediatric Logistic Organ Dysfunction Score. Crit Care Med. 2013;41:1761–1773. doi: 10.1097/CCM.0b013e31828a2bbd. [DOI] [PubMed] [Google Scholar]
- 17.Pollack MM, Holubkov R, Tomohiko F, Clark A. Pediatric intensive care outcomes: Development of new morbidities during pediatric critical care. Pediatr Crit Care Med. 2014;15(9):821–827. doi: 10.1097/PCC.0000000000000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gregory CJ, Nasrollahzadeh F, Dharmar M, et al. Comparison of critically ill injured children transferred from referring hospitals versus in-house admissions. Pediatrics. 2008;121(4):e906–e911. doi: 10.1542/peds.2007-2089. [DOI] [PubMed] [Google Scholar]
- 19.Combes A, Luyt CE, Trouillet JL, et al. Adverse effect on a referral intensive care unit’s performance of accepting patients transferred from another intensive care unit. Crit Care Med. 2005;33(4):705–710. doi: 10.1097/01.ccm.0000158518.32730.c5. [DOI] [PubMed] [Google Scholar]
- 20.Golestanian E, Scruggs JE, Gangnon RE, et al. Effect of interhospital transfer on resource utilization and outcomes at a tertiary care referral center. Crit Care Med. 2007;35(6):1470–1476. doi: 10.1097/01.CCM.0000265741.16192.D9. [DOI] [PubMed] [Google Scholar]
- 21.Seferian EG, Afessa B, Gajic O, Keegan MT, Hubmayr RD. Comparison of community and referral intensive care unit patients in a tertiary medical center: Evidence for referral bias in the critically ill. Crit Care Med. 2008;35:2779–2786. doi: 10.1097/ccm.0b013e318186ab1b. [DOI] [PubMed] [Google Scholar]
- 22.Rennick JE, Childerhose JE. Redefining success in the PICU: New patient populations shift targets of care. Pediatrics. 2015;135:e289. doi: 10.1542/peds.2014-2174. [DOI] [PubMed] [Google Scholar]
- 23.Bennyworth BD, Bennett WE, Carroll AE. Cross-sectional comparison of critically ill pediatric patients across hospitals with various levels of pediatric care. BMC Research Notes. 2015;8:693. doi: 10.1186/s13104-015-1550-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dosa NP, Boeing NM, Ms N, et al. Excess risk of severe acute illness in children with chronic health conditions. Pediatrics. 2001;107:499–504. doi: 10.1542/peds.107.3.499. [DOI] [PubMed] [Google Scholar]
- 25.Edwards JD, Houtrow AJ, Vasilevskis EE, et al. Chronic conditions among children admitted to US pediatric intensive care units: Their prevalence and impact on risk for mortality and prolonged length of stay. Crit Care Med. 2012;40:2196–2203. doi: 10.1097/CCM.0b013e31824e68cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mestrovic J, Kardum G, Polic B, et al. The influence of chronic health conditions on susceptibility to severe acute illness of children treated in PICU. Eur J Pediatr. 2006;165:526–529. doi: 10.1007/s00431-006-0114-3. [DOI] [PubMed] [Google Scholar]
- 27.Needham DM, Davidson J, Cohen H, et al. Improving long-term outcomes after discharge from intensive care unit: Report from a stakeholders’ conference. Crit Care Med. 2012;40:502–509. doi: 10.1097/CCM.0b013e318232da75. [DOI] [PubMed] [Google Scholar]
- 28.Pearson H. The Life Project: Britain’s birth-cohort studies are the envy of the scientific world, but will they survive the 21st century? The Life Project Soft Skull Press. 2016 [Google Scholar]
- 29.Thygesen LC, Eroboll AK. When the entire population is the sample: Strengths and limitations in register-based epidemiology. Eur J Epidemiol. 2014;29(8):551–558. doi: 10.1007/s10654-013-9873-0. [DOI] [PubMed] [Google Scholar]
- 30.Szklo M. Population-based cohort studies. Epidemiol Rev. 1998;20:81–90. doi: 10.1093/oxfordjournals.epirev.a017974. [DOI] [PubMed] [Google Scholar]
- 31.Booth CM, Tannock IF. Randomized controlled trials and population-based observational research: Partners in the evolution of medical evidence. British Journal of Cancer. 2014;110:551–555. doi: 10.1038/bjc.2013.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simon GE, Unutzer J, Young BE, Pincus HA. Large medical databases, population based research and patient confidentiality. Am J Psychiatry. 2000;157:1731–1737. doi: 10.1176/appi.ajp.157.11.1731. [DOI] [PubMed] [Google Scholar]
- 33.LaMorte WW. Boston University School of Public Health. 2016 doi: 10.1016/S0140-6736(16)00214-2. www.sphweb.bumc.bu.edu/otit/MPH-modules, Accessed on 10/27/2016. [DOI] [PubMed]
- 34.St Sauver JL, Grossardt BR, Leibson CL, et al. Generalizability of epidemiological findings and public health decisions: An illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87(2):151–160. doi: 10.1016/j.mayocp.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Townsend ML, Riepsamen A, Georgiou C, et al. Longitudinal intergenerational birth cohort designs: A systematic review of Australian and New Zealand studies. PLoS ONE. 2016;11(3):e0150491. doi: 10.1371/journal.pone.0150491. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


