Abstract
Researchers have documented dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis in children and adolescents who experienced early life stress (ELS). The precise nature of this dysregulation, however, has been difficult to discern. In fact, both elevated and blunted patterns of diurnal cortisol regulation have been reported in children and adolescents exposed to greater ELS, including both reduced and heightened cortisol levels and change in cortisol across the day. These divergent findings may be due to developmental changes in the relation between ELS and HPA-axis functioning. The present study was designed to examine the role of puberty in the impact of the severity of ELS on the regulation of diurnal cortisol. Boys and girls (N=145) ages 9–13 years recruited from lower-risk communities completed an interview about their ELS experiences and at-home collection of diurnal cortisol. ELS experiences were objectively coded for severity, and children’s level of pubertal development was measured using Tanner Staging. Multi-level piecewise mixed-effects models tested the effects of ELS severity and pubertal stage on cortisol levels at waking, the cortisol awakening response (CAR), and the daytime cortisol slope. While we found no significant interactive effects of pubertal stage and ELS severity on cortisol levels at waking or the daytime cortisol slope, findings indicated that pubertal stage interacted with ELS severity to predict the cortisol awakening response (CAR). Specifically, in earlier puberty, higher ELS was associated with a blunted CAR compared to lower ELS; in contrast, in later puberty, higher ELS was associated with a heightened CAR compared to lower ELS. Differences in the relation between ELS severity and the CAR were uniquely determined by puberty, and not by age. By considering and examining the role of puberty, the current study provides a developmental explanation for previous divergent findings of both blunted and heightened patterns of diurnal cortisol following ELS. These results indicate that careful attention should be given to children’s pubertal status before drawing conclusions concerning the nature of diurnal cortisol dysregulation.
Keywords: diurnal cortisol, development, puberty, early life stress
1. Introduction
A growing body of research has documented adverse consequences of stressful experiences in early life for mental and physical health across the lifespan (e.g., Felitti et al., 1998; Green et al., 2010; McLaughlin et al., 2016). Although early life stress (ELS) has been associated with a wide range of negative outcomes, the mechanisms by which ELS confers risk have not been fully elucidated. Changes in the hypothalamic-pituitary-adrenal (HPA) axis, a central stress response system, may mediate the association between ELS and negative health outcomes (McCrory et al., 2010). Stress in early life may have powerful effects on the HPA axis because this system has not yet reached maturity (Gunnar & Quevedo, 2007). There is a dearth of research, however, exploring the role of potentially relevant developmental processes, such as puberty, in understanding the effects of ELS on the HPA axis.
The HPA axis governs the release of cortisol, which follows a characteristic diurnal rhythm. Specifically, on average, individuals exhibit increases in cortisol in the morning (the cortisol awakening response; CAR) followed by gradual decreases across the rest of the day (the daytime slope). Atypical activation of the HPA axis in response to persistent or overwhelming environmental stress is posited to disturb the diurnal rhythm, leading to a profile of diurnal cortisol dysregulation (McEwen et al., 2003). The precise nature of this dysregulation, however, has been difficult to discern. In fact, both elevated and blunted patterns of diurnal cortisol have been reported in youth who have been exposed to various types of ELS (Hunter et al., 2011). While some researchers have identified higher morning, afternoon, and bedtime cortisol levels in children and adolescents who have been exposed to ELS in the form of trauma (Carrion et al., 2002; Weems & Carrión, 2009), other investigators have identified attenuation of the CAR in adolescents exposed to suboptimal caregiving (Roisman et al., 2009) and lower cortisol levels 30-minutes post-waking in young children exposed to maltreatment (Bernard et al., 2010).
Although both atypically heightened and reduced production of diurnal cortisol have been posited to be problematic for neurodevelopment and risk for disease (Gunnar & Quevedo, 2007), we do not have a clear understanding of why different patterns of diurnal cortisol production are manifested following ELS. In this context, it is important to identify the factors that underlie diverse effects across the full diurnal pattern, including differences in cortisol levels at waking, the CAR, and the slope of cortisol across the day. It is noteworthy that the specific pattern of diurnal cortisol regulation exhibited by children exposed to ELS may not be permanent. Rather, the impact of ELS on diurnal cortisol may change over the course of development. In particular, the developmental process of puberty, which is defined by endocrine and physical changes, has been posited to influence HPA-axis functioning (Marceau et al., 2015). Specifically, endocrine changes that occur with adrenarche and gonadarche, including a rise in androgens and gonadotropins and concomitant growth in pubic hair, breast tissue, and external genitalia (Dorn & Biro, 2011), are initiated and regulated by the same neural and endocrine systems that are responsible for the release of cortisol (Dismukes et al., 2015).
Human and animal research suggests that there are both age- and puberty-related changes in HPA-axis functioning, including increases in cortisol reactivity to stress (Romeo, 2010; van den Bos, 2014) and normative attenuations in diurnal cortisol slopes (modeled as change in cortisol from morning to evening) at later developmental stages (Shirtcliff et al., 2013). Few investigators, however, have explicitly examined how puberty may affect the relation between ELS and diurnal cortisol. Research with children who were institutionalized in early life, however, indicates that the relation between early caregiving deprivation and diurnal functioning of the HPA axis changes over puberty. Specifically, Quevedo and colleagues (2012) found that whereas prior institutionalization was associated with a blunted CAR in pre/early puberty, by mid/late puberty institutionalization was associated with a higher CAR that was more similar to a family-reared control group. These findings support the formulation that the form of dysregulation of diurnal cortisol, specifically the manifestation of the CAR, depends on the stage of pubertal development.
By considering the role of puberty in the association between ELS and diurnal cortisol regulation, we might increase our understanding of how to define dysregulation and, consequently, of how to prevent and intervene to enhance regulation. In particular, it is important to investigate these associations in a sample of children recruited from the community. Although studies of institutionalized children have been vital for understanding the impact of early caregiving deprivation, this extreme and circumscribed form of ELS is not typical. Children developing in more normative contexts may be exposed to a broad range of ELS experiences across childhood that vary in severity. For these children, dysregulation of diurnal cortisol may persist through adolescence, but its manifestation may change.
Based on the literature reviewed above, the current study examined, for the first time, the role of pubertal stage in affecting the relation between the severity of ELS and diurnal cortisol, including the cortisol level at waking, the CAR, and the daytime slope, in a sample of community children. First, we tested the hypothesis that in a sample of community children, the impact of the severity of ELS on diurnal cortisol regulation depends on the stage of pubertal development. Second, given that some studies have reported age-related changes in diurnal cortisol regulation, we examined the unique influences of stage of pubertal development and age on the relation between the severity of ELS and diurnal cortisol regulation.
2. Methods
2.1 Participants
Participants were 145 children (57% female) ages 9–13 years (M=11.40, SD=1.07) who completed collection of diurnal cortisol as part of a longitudinal study of the psychobiological effects of ELS across the transition through puberty (descriptive statistics by child sex are presented in Table 1). Participants were recruited using a combination of media and online advertisements posted in local communities around Stanford University. Because the larger study involved a magnetic resonance imaging (MRI) session, criteria for exclusion from the study included factors that would preclude an MRI scan (e.g., metal implants, braces), as well as a history of major neurological or medical illness, severe learning disabilities that would make it difficult to comprehend the study procedures, and, for females, the onset of menses. Inclusion criteria were that children were ages 9–13 years and were proficient in spoken English. In addition, participants were recruited in order to match males and females based on pubertal stage as measured by the Tanner Staging questionnaire (described in section 2.3.2).
Table 1.
Descriptive Statistics for Males and Females
| Males | Females | t or χ2 | df | p | |
|---|---|---|---|---|---|
| Age | 11.83 (.964) | 11.08 (1.03) | −4.45 | 143 | <.001 |
| Pubertal Stage | 1.90 (.666) | 2.13 (.796) | 1.85 | 143 | .066 |
| ELS Severity | 5.25 (4.32) | 5.24 (4.35) | −.012 | 143 | .990 |
| BMI | 19.51(3.53) | 18.31(3.78) | −1.87 | 131 | .064 |
| Medication Usea | 11% | 12% | .020 | 1 | .888 |
| Race/Ethnicityb
|
.232 | 1 | .630 | ||
| White/Caucasian | 43% | 40% | |||
| Asian | 16% | 11% | |||
| Hispanic | 13% | 9% | |||
| African American | 7% | 7% | |||
| Native American | 3% | 0% | |||
| Pacific Islander | 0% | 2% | |||
| Other | 16% | 30% | |||
| No response | 2% | 1% | |||
| Family Incomec
|
.035 | 1 | .852 | ||
| <$5,000 | 0% | 1% | |||
| $5,001–10,000 | 2% | 1% | |||
| $10,001–15,000 | 0% | 1% | |||
| $15,001–25,000 | 2% | 3% | |||
| $25,001–35,000 | 1% | 1% | |||
| $35,001–50,000 | 10% | 4% | |||
| $50,001–75,000 | 8% | 8% | |||
| $75,001–100,000 | 10% | 7% | |||
| $100,001–150,000 | 22% | 25% | |||
| $150,000+ | 37% | 35% | |||
| No response | 8% | 14% | |||
| People in Home (N)d
|
.354 | 1 | .552 | ||
| 2–3 | 25% | 20% | |||
| 4–5 | 63% | 63% | |||
| 6+ | 10% | 7% | |||
| No Response | 2% | 10% | |||
| Parental Educatione
|
.263 | 1 | .608 | ||
| <High School Diploma | 0% | 1% | |||
| <4-Year College Degree | 27% | 20% | |||
| 4-Year College Degree | 32% | 45% | |||
| Graduate Degree | 39% | 24% | |||
| No Response | 2% | 10% |
Note. M (SD) or %; sex coded; ELS = Early Life Stress;
chi-square with non-concorticosteroid medication use (taking medication or medication-free),
chi-square with minority status (White/Caucasian or minority);
chi-square with median split at $100,001–150,000;
chi-square with number of people living in home of child’s primary parent (≤4 or >4 people);
chi-square with primary parent completed education level (<college degree or ≥college degree)
2.2 Procedure
The protocol for this study was approved by the Stanford University Institutional Review Board. In an initial telephone call, research staff provided information about the research protocol to families and screened participants for inclusion/exclusion criteria. Eligible families were then invited to attend a laboratory session during which research staff obtained consent from parents and assent from children. In this session, children reported their Tanner stages, and both parents and children completed interview and questionnaire measures about the child and family. At the end of the session, staff provided families with kits and instructions to collect saliva samples at home for the assessment of diurnal cortisol. Families returned the samples to the laboratory at a subsequent visit.
2.3 Measures
2.3.1 Severity of Early Life Stress
Children were interviewed about their lifetime exposure to 30 types of stressful experiences using a modified version of the Traumatic Events Screening Inventory for Children (TESI-C; Ribbe, 1996). A panel of three coders, blind to the children’s reactions and behaviors during the interview, then rated the objective severity of each type of stressor endorsed using a modified version of the UCLA Life Stress Interview coding system (Rudolph & Hammen, 1999; Rudolph et al., 2000). Specifically, a trained research assistant transcribed and delivered to the panel a description of each stressor, removing details that would reveal the child’s subjective perception of severity. Coders then made severity ratings on a scale of 0–4, increasing in half-point increments (0 = non-event or no impact; 4 = extremely severe impact; ICC=.99). Following coding, we created an index of the level of ELS severity by summing the severity ratings for each type of stressor the child endorsed. This method overcomes problems with operationalizing ELS as the number of stressors that children report (Jenness & McLaughlin, 2015). Such methods inaccurately equate children who report the same number of stressful experiences but who differ substantially in their experiences of severity. Final ELS severity scores ranged from 0–19 (M=5.24, SD=4.32). See supplement for further details.
2.3.2 Stage of Pubertal Development
Pubertal stage was assessed using the self-report Tanner Staging questionnaire (Marshall & Tanner, 1968). Tanner staging scores in the current sample ranged from 1 to 4 (M pubic hair growth=1.97, SD=.905; M breast/genitalia growth=2.08, SD=.777). Consistent with prior research (Dorn et al., 2006), we averaged Tanner scores for each participant to yield an index of average stage of pubertal development (M=2.03, SD=.749). Thirty-six percent of children had an average Tanner stage above 2—the median for the current sample.
2.3.3 Diurnal Cortisol
Within two weeks of completing the ELS interview, children completed at-home saliva collection to assess diurnal cortisol on two consecutive weekdays using SalivaBio Children’s Swabs (Salimetrics, LLC). Participants were provided with face-to-face and take-home written instructions for saliva collection. They were instructed not to eat or drink before collection of each sample at four time points: awakening (while still lying in bed); 30 minutes post-awakening; mid-afternoon (as close to 3:00pm as possible); and in the late evening (2 hours after dinner). Participants recorded collection times in a diary and placed samples in their home freezers. In previous research using this protocol in adolescents, we found that self-reported times did not significantly differ from times tracked with smart caps (LeMoult et al., 2015). See supplement for further details.
Participants returned the samples to the laboratory at a subsequent visit. Samples were stored in a −20°F freezer in the Psychology Department at Stanford University until they were assayed using a high-sensitivity (0.004 μg/dL) immunoassay kit from Immuno-Biological Laboratories Inc. (Hamburg, Germany; both intra- and inter-assay coefficients of variation (CV) for the kit ranged from 3–5%). To control for inter-assay error, samples were assayed together in large batches; all samples from the same child were assayed in duplicate. To reduce positive skew in resulting salivary cortisol data, we winsorized values greater than 2 SD above the mean to the 2-SD value following current guidelines (Stalder et al., 2016).
2.4 Data Analysis
Visual inspection indicated that cortisol levels increased from waking to 30-minutes post-waking and then gradually decreased throughout the day. Given this nonlinear pattern, we implemented multi-level piecewise mixed-effects modeling in the statistical package R (R Core Team, 2015) using the “lmer” function from the lme4 package (Bates et al., 2015). This analytic approach allowed us to separately but simultaneously estimate intra-individual change in cortisol from waking to 30-minutes post-waking (i.e., CAR) as well as from 30-minutes post-waking to the late evening (i.e., the daytime slope). Importantly, we used participant-specific time of sample collection (in hours) to predict change in diurnal cortisol in order to account for each individual’s collection profile. The random effects of subject intercepts and slopes (CAR and daytime) were included to account for individual differences in cortisol levels at waking and change over time. See supplement for further details on modeling procedures.
In the first step, a baseline (level 1) model estimated the average within-individual diurnal cortisol pattern, including the CAR, the daytime slope, and the level of cortisol upon awakening (the intercept). Before proceeding with further analyses, we compared the piecewise linear model to separate linear, quadratic, and cubic models of the diurnal cortisol pattern. The piecewise model was a superior fit for the data (piecewise AIC=−378.30, linear AIC=−343.01, quadratic AIC=−172.09, cubic=−77.76).
In the second step of the model, we added the main and interactive effects of pubertal stage and ELS severity (centered at the mean) as level 2 predictors of the baseline model coefficients. Given that growth in pubic hair and growth in breasts/genitalia reflect separate but correlated (r=.586, p<.001) pubertal processes, we first examined their effects in separate models. The separate models produced highly similar findings and identical conclusions; therefore, subsequent analyses focused on the average stage of pubertal development. In order to identify the unique significance of pubertal stage for the relation between ELS severity and diurnal cortisol, a second level 2 model estimated the interactive effects of age and ELS on baseline model coefficients. All linear mixed models were fit by Restricted Maximum Likelihood t-tests, and Satterthwaite approximations of degrees of freedom were used to compute p-values in the lmerTest package in R (Kuznetsova et al., 2016). We used simple slopes analyses to probe significant interactions (Aiken & West, 1991).
3. Results
3.1 Descriptive Statistics
Descriptive statistics by child sex are presented in Table 1. Males and females did not differ significantly from each other in pubertal stage, severity of ELS, minority status, or income (ps>.050). There was, however, a non-significant trend such that girls were more advanced in puberty than boys (p=.066). As expected because pubertal stage was matched across sexes, males were significantly older than females (p<.001). Pearson’s bivariate correlations indicated that pubertal stage was positively associated with child age (r=.309, p<.001) and ELS severity (r=.179, p=.031); child age was not significantly related to ELS (r=−.007, p=.933).
3.2 Average Diurnal Cortisol Pattern and Covariates
The baseline (level 1) model examining the pattern of diurnal cortisol across all participants indicated that children’s cortisol at waking (the model intercept) was significantly greater than zero (β=.516, SE=.016, t(145.83)=31.74, p<.001), increased significantly from waking to 30-minutes post-waking (β=.129, SE=.036, t(131.54)=3.55, p<.001), and decreased significantly across the rest of the day (β= −.040, SE=.002, t(154.82)= −23.77, p<.001). The random effect for the CAR (variance=.073, SD=.270) was larger than the random effects for cortisol level at waking (variance=.021, SD=.147) and the daytime slope (variance=.0002, SD=.015), indicating that individual variability in the CAR was greatest. We then tested whether age, sex, body mass index (BMI), non-corticosteroid medication use, income, minority status, or wake time (hours from midnight) accounted for individual differences in patterns of diurnal cortisol by adding each separately as a predictor of the baseline model coefficients. Age, sex, BMI, medication use, income, and minority status did not have significant effects on cortisol levels at waking, the CAR, or the daytime slope (ps>.050). Therefore, in order to preserve model parsimony, we did not include these variables as covariates in the final level 2 analyses (Adam, 2006; Human et al., 2015). There was, however, a significant effect of wake time on cortisol levels at waking (β= −.037, SE=.013, t(143.02)= −2.81, p=.006), such that later wake times were associated with lower levels of cortisol at waking; wake time had no effect on the CAR or the daytime slope. Therefore, we included wake time as a covariate in the level 2 analyses.
3.3 Effects of Pubertal Stage and ELS Severity on Diurnal Cortisol Pattern
We then tested the hypothesis that the impact of the severity of ELS on diurnal cortisol regulation depends on stage of pubertal development by adding these variables to a level 2 model predicting the baseline model coefficients. This analysis (presented in Table 2) yielded a significant interactive effect of pubertal stage and ELS on the CAR when controlling for wake time, indicating that the CAR depended on both level of pubertal stage and ELS. There were no significant main or interactive effects of ELS or pubertal stage on level of cortisol at waking or on the daytime slope. Given the nonsignificant trend for girls to be more advanced in puberty than boys, we conducted additional analyses to examine whether these findings were affected or explained by sex. All findings held when controlling for sex; furthermore, sex did not significantly interact with puberty or ELS to predict level of cortisol at waking, the CAR, or the daytime slope.
Table 2.
Level 2 Model: Predicting Cortisol Intercept and Slopes by ELS Severity and Stage of Pubertal Development
| Fixed Effects | β | SE | df | t | p |
|---|---|---|---|---|---|
| Waking Cortisol | .519 | .016 | 141.2 | 32.01 | <.001 |
| Wake Time | −.037 | .013 | 140.5 | −2.75 | .007 |
| Pubertal Stage | .014 | .022 | 142.4 | .637 | .525 |
| ELS | −.002 | .004 | 142.1 | −.390 | .697 |
| Pubertal Stage*ELS
|
−.006 | .005 | 143.6 | −1.26 | .208 |
| CAR | .119 | .036 | 127.9 | 3.26 | .001 |
| Wake Time | −.012 | .029 | 113.2 | −.392 | .695 |
| Pubertal Stage | −.004 | .049 | 131.7 | −.083 | .934 |
| ELS | .001 | .009 | 130.5 | .159 | .874 |
| PubertalStage*ELS
|
.026 | .010 | 141.8 | 2.47 | .015 |
| Daytime Slope | −.040 | .002 | 154.9 | −23.52 | <.001 |
| Wake Time | .001 | .001 | 172.9 | .903 | .368 |
| Pubertal Stage | .001 | .002 | 151.7 | −.207 | .836 |
| ELS | .0002 | .0004 | 155.6 | .431 | .667 |
| Pubertal Stage*ELS | −.001 | .001 | 151.4 | −1.32 | .188 |
Notes. SE = Standard Error; df = Satterthwaite approximations of degrees of freedom; pubertal stage and ELS were centered at the mean
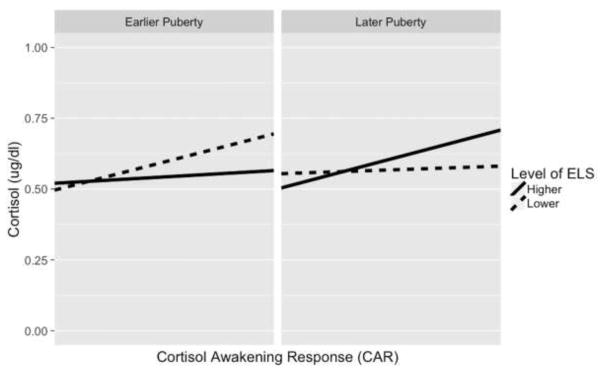
We used simple slopes analyses to examine further the significant interactive effect of ELS severity and pubertal stage on the CAR. Specifically, we estimated the CAR at the +/− 1-SD values above/below the mean of ELS and pubertal stage (Aiken & West, 1991). As depicted in Figure 1, we found that in earlier puberty (approximately Tanner stage 1), higher ELS severity was associated with a blunted CAR compared to lower ELS severity. Specifically, the CAR was significantly positive when ELS was lower (β=.199, SE= .069, t(133.2)=2.84, p=.005), but the CAR was nonsignificant when ELS was higher (β=.045, SE=.080, t(140.3)=.562, p=.575). This pattern was reversed in later puberty (approximately Tanner stage 3), such that higher ELS severity was associated with heightened CAR compared to lower ELS severity. Specifically, the CAR was nonsignificant when ELS was lower (β=.027, SE=.075, t(118.2)=.354, p=.724), but was significantly positive when ELS was higher (β=.204, SE=.063, t(141.7)=3.24, p=.001).
Figure 1.
Simple Slopes of CAR at Different Levels of Pubertal Development and ELS Severity
Notes. Simple slopes indicate the estimated CAR at the −1 SD and +1 SD values of ELS severity and average stage of pubertal development. In earlier puberty, the CAR was significantly positive at lower ELS (p = .005) and non-significant at higher ELS (p = .575). In later puberty, the CAR was nonsignificant at lower ELS (p = .724) and significantly positive at higher ELS (.001).
3.4 Age and ELS Severity
In order to clarify the unique influence of pubertal development on the relation between ELS severity and diurnal cortisol, a final level 2 model tested whether age interacted with ELS to predict diurnal cortisol when controlling for wake time. There were no significant interactive effects of age and ELS on cortisol levels at waking (β= −.004, SE=.003, t(142.4)= −1.30, p=.195), the CAR (β=.002, SE=.008, t(147.5)=.261, p=.795), or on the daytime slope (β=.0002, SE=.0003, t(155.5)=.583, p=.561).
4. Discussion
Previous research has documented that compared to their lesser- or non-exposed peers, children exposed to higher levels or extreme forms of ELS demonstrate aberrant patterns of diurnal cortisol regulation, as evidenced by differences in cortisol levels at particular times of day (e.g., morning or bedtime) and/or in change in cortisol across the day (e.g., the CAR; (Gunnar & Quevedo, 2007). The literature has been equivocal, however, in identifying the nature of diurnal cortisol regulation following ELS, with researchers reporting both blunted and heightened levels and slopes of cortisol (Hunter, 2011). Furthermore, there is limited research examining factors that underlie these divergent profiles of diurnal cortisol, especially in samples of children recruited from the community who have been exposed to ELS of varying severity. To address this issue, the present study extended previous work by elucidating the unique role of puberty in understanding the association between the severity of ELS and diurnal cortisol regulation.
While we found no significant interactive effects of pubertal stage and ELS severity on cortisol levels at waking or the daytime cortisol slope, our findings indicate that the manifestation of the CAR following ELS depends on current pubertal stage. Studying a sample of community children ages 9–13 years who were exposed to a range of ELS experiences, we found that pubertal stage interacted with the severity of ELS to predict the CAR. Specifically, in earlier puberty, higher ELS severity was associated with a blunted CAR compared to lower levels of ELS severity; in contrast, in later puberty, higher ELS severity was associated with a heightened CAR compared to lower levels of ELS severity. Importantly, differences in the manifestation of the CAR in the context of higher severity of ELS were uniquely determined by puberty; age had no influence on the relation between ELS severity and any of the indices of diurnal cortisol. While we should be cautious in making comparisons given considerable differences between samples, these results reflect findings of prior research in institutionalized children exposed to caregiving deprivation (Quevedo et al., 2012) in which institutionalization was associated with a blunted CAR only in early puberty.
Although we do not yet fully understand the mechanisms by which puberty influences the HPA axis, the biological and social changes associated with puberty may underlie a pubertal shift in the effects of ELS on the HPA axis. Indeed, research indicates that the pubertal androgen, dehydroepiandrosterone (DHEA), may modulate the effects of cortisol (Pinto et al., 2015), that higher ratios of cortisol to DHEA in adults are associated with exposure to ELS (Van Voorhees et al., 2014), and that the coupling of DHEA and cortisol becomes stronger from ages 11 to 15 years (Ruttle et al., 2013). Thus, exposure to ELS may have a pervasive impact on endocrinology, including changes in pubertal hormones that influence the production of cortisol. Furthermore, puberty is associated with significant changes in social dynamics that may affect stress response systems (Nelson et al., 2005). In particular, animal and human studies have documented changes in social buffering during adolescence, such that caregiver support ceases to serve a supplementary role in child regulation (Hostinar et al., 2014). For example, Doom and colleagues (2015) found that when caregiver support was provided during a psychosocial stressor, compared with when support was absent, children in pre/early puberty showed significantly reduced cortisol reactivity; for children in mid/later puberty, however, caregiver support did not have a significant effect on cortisol reactivity. Thus, changes in the influence of caregiver support on the HPA axis may also explain the different patterns of diurnal cortisol regulation associated with ELS severity in earlier and later puberty.
It is noteworthy that we found no significant effects of pubertal development or ELS severity on the cortisol level at waking or the daytime slope of cortisol. The CAR is a particularly relevant index in the context of ELS research because it “combines features of a reactivity index (response to awakening) with aspects tied to circadian regulation” (Stalder et al., 2016, p. 3). Previous findings indicate that although the CAR has a trait-like component, it also represents a response to the anticipated challenges of the day (Powell & Schlotz, 2012) and, in addition, is relatively distinct from cortisol levels across the rest of the day (Stalder et al., 2016). Children exposed to ELS may show an aberrant response to awakening because they anticipate engaging in an atypical environment defined by threat. The HPA axis is responsible for helping to maintain allostasis (McEwen & Wingfield, 2003, p.12); therefore, differential cortisol responses to awakening at different stages of puberty may be related to other important changes. In terms of mental health, it is well documented that specific disorders are likely to emerge and intensify during adolescence (CDC, 2016). Although hyporesponsivity of the HPA axis prior to puberty may be temporarily protective (Gunnar & Quevedo, 2007), Colich and colleagues (2015) found that both blunted cortisol responses to stress in early puberty and heightened cortisol responses to stress in later puberty foreshadow the development of depression. Adam and colleagues (2014) found that an elevated CAR in late adolescence prospectively predicted first onsets of anxiety disorders. Thus, a shift toward a heightened CAR over the course of pubertal development may be implicated in the increasing rates of disorder during adolescence.
4.1 Limitations
There are limitations of this study that highlight opportunities for future research. For example, this study was cross-sectional, which precluded examining the role of intra-individual changes in puberty on changes in the relation between ELS and diurnal cortisol. In addition, we used self-report Tanner staging to assess pubertal development to reduce participant burden. Although prior work indicates this method is reliable with the “gold standard” of physician assessment (Brooks-Gunn, et al., 1987; Chan et al., 2008; Desmangles et al., 2006) and that self-report measures of puberty reflect levels of pubertal hormones (Shirtcliff et al., 2009), future research might track changes in hormones from earlier to later puberty in order to assess their influence on diurnal cortisol. We should also note that the range of puberty was limited in this sample to Tanner stages 1 to 4. Thus, we were unable to examine potential further differences in the relation between the severity of ELS and diurnal cortisol at full pubertal maturation (Tanner stage 5).
Income and parental education levels in the current study sample were representative of the relatively low-risk communities from which we recruited children, and thus higher than national averages. ELS experiences were also heterogeneous in nature, with children exposed to various types and levels of severity. Consequently, these findings may not generalize to children who develop in higher-risk environments and/or who are more homogeneously exposed to severe stressors (e.g., children in the child welfare system). Indeed, the non-significant effects of puberty and ELS severity on cortisol levels at waking and the daytime slope might be attributed to the fact that this was a lower-risk community sample of children. Finally, exposure to ELS was assessed in this study through retrospective self-report. Although we overcame many of the limitations of self-report by objectively coding the severity of ELS, some children may have under-reported their experiences due to discomfort in discussing distressing events or amnesia for events occurring during young childhood. Although there are numerous challenges to the assessment of ELS, future research should continue to improve methods of measuring ELS.
4.2 Conclusions
This is the first study to examine the role of pubertal stage in affecting the relation between the severity of ELS and diurnal cortisol in a community sample of children. By examining the role of puberty, the current study provides a developmental explanation for previous divergent findings of both blunted and heightened patterns of diurnal cortisol following ELS. In particular, our findings suggest that researchers should consider the pubertal status of children in drawing conclusions about the nature of deviations in the CAR. Adding to a growing body of research indicating that the HPA axis is highly plastic during the pubertal period, the results of this study have important implications for the timing of interventions to improve cortisol regulation. Specifically, interventions occurring prior to a pubertal shift in HPA-axis regulation may mitigate the long-term negative health outcomes associated with ELS.
Supplementary Material
Highlights.
Boys and girls ages 9–13 years completed an ELS interview and Tanner staging.
Diurnal cortisol was collected at home on two consecutive weekdays.
Puberty interacted with ELS severity to predict the CAR.
In earlier puberty, higher ELS severity was associated with a blunted CAR.
In later puberty, higher ELS severity was associated with heightened CAR.
Acknowledgments
We thank Morgan Popolizio, Alexandria Price, Holly Pham, and Madelaine Graber for their assistance in collection and management of data for the current project. This research was supported by NIH (R01 MH101495 to IHG, K01-MH106805 to SJO, F32-MH102013 to JL, F320MH107129 to KLH), the Brain & Behavior Research Foundation (Young Investigator Awards to SJO [23582], JL [22337], and KLH [23819], the Klingenstein Third Generation Foundation (Fellowship Awards to SJO and KLH), and the National Science Foundation (Graduate Fellowship Awards to NLC and LSK)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam EK. Transactions among adolescent trait and state emotion and diurnal and momentary cortisol activity in naturalistic settings. Psychoneuroendocrinology. 2006;31(5):664–679. doi: 10.1016/j.psyneuen.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Adam EK, Vrshek-Schallhorn S, Kendall A, Mineka S, Zinbarg RE, Craske MG. Prospective associations between the cortisol awakening response and first onsets of anxiety disorders over a six-year follow-up. Psychoneuroendocrinology. 2014;44:47–59. doi: 10.1016/j.psyneuen.2014.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Newbury Park, CA: Sage; 1991. [Google Scholar]
- Bates D, Maechler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67(1):1–48. [Google Scholar]
- Bernard K, Butzin-Dozier Z, Rittenhouse J, Dozier M. Cortisol production patterns in young children living with birth parents vs children placed in foster care following involvement with child protective services. Arch Pediatr Adolesc Med. 2010;164(5):438–443. doi: 10.1001/archpediatrics.2010.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch NM, Riese H, Reijneveld SA, Bakker MP, Vehulst FC, Ormel J, Oldehinkel AJ. Timing matters: Long term effects of adversities from prenatal period up to adolescence on adolescents’ cortisol stress response. The TRAILS study. Psychoneuroendocrinology. 2012;37(9):1439–1447. doi: 10.1016/j.psyneuen.2012.01.013. [DOI] [PubMed] [Google Scholar]
- Brooks-Gunn J, Warren MP, Rosso J, Gargiulo J. Validity of self-report measures of girls ’ pubertal status. Child Dev. 1987;58(3):829–841. [PubMed] [Google Scholar]
- Carrion VG, Weems CF, Ray RD, Glaser B, Hessl D, Reiss AL. Diurnal salivary cortisol in pediatric posttraumatic stress disorder. Biol Psychiatry. 2002;51:575–582. doi: 10.1016/s0006-3223(01)01310-5. [DOI] [PubMed] [Google Scholar]
- CDC. Mental Health Surveillance Among Children — United States, 2005–2011. 2016 Retrieved from: http://www.cdc.gov/mmwr/preview/mmwrhtml/su6202a1.htm?s_cid=su6202a1_w.
- Chan NPT, Sung RYT, Kong APS, Goggins WB, So HK, Nelson EAS. Reliability of pubertal self-assessment in Hong Kong Chinese children. J Paediatr Child Health. 2008;44(6):353–358. doi: 10.1111/j.1440-1754.2008.01311.x. [DOI] [PubMed] [Google Scholar]
- Colich NL, Kircanski K, Foland-Ross LC, Gotlib IH. HPA-axis reactivity interacts with stage of pubertal development to predict the onset of depression. Psychoneuroendocrinology. 2015;55:94–101. doi: 10.1016/j.psyneuen.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmangles JC, Lappe JM, Lipaczewski G, Haynatzki G. Accuracy of pubertal Tanner staging self-reporting. J Pediatr Endocrinol. 2006;19(3):213–21. doi: 10.1515/jpem.2006.19.3.213. [DOI] [PubMed] [Google Scholar]
- Dismukes AR, Johnson MM, Vitacco MJ, Iturri F, Shirtcliff EA. Coupling of the HPA and HPG axes in the context of early life adversity in incarcerated male adolescents. Dev Psychobiol. 2015;57(6):705–718. doi: 10.1002/dev.21231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doom JR, Hostinar CE, VanZomeren-Dohm AA, Gunnar MR. The roles of puberty and age in explaining the diminished effectiveness of parental buffering of HPA reactivity and recovery in adolescence. Psychoneuroendocrinology. 2015;59:102–111. doi: 10.1016/j.psyneuen.2015.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn LD, Biro FM. Puberty and its measurement: A decade in review. J Res Adolesc. 2011;21(1):180–195. [Google Scholar]
- Dorn LD, Dahl RE, Rojahn Woodword Hermi, Biro F. Defining the boundaries of early adolescence: A user’s guide to assessing pubertal status and pubertal timing in research with adolescents. Appl Dev Sci. 2006;10(1):30–56. [Google Scholar]
- Essex MJ, Shirtcliff EA, Burk LR, Ruttle PL, Klein MH, Slatterry MJ, Kalin NH, Armstrong JM. Influence of early life stress on later hypothalamic-pituitary-adrenal axis functioning and its covariation with mental health symptoms: A study of the allostatic process from childhood into adolescence. Dev Psychopathol. 2011;23(4):1039–1058. doi: 10.1017/S0954579411000484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, … Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14(4):245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Green JG, McLaughlin KA, Berglund PA, Gruber MJ, Sampson NA, Zaslavsky AM, … Kessler RG. Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I: Associations with first onset of DSM-IV disorders. Arch Gen Psychiatry. 2010;67:113–123. doi: 10.1001/archgenpsychiatry.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar M, Quevedo K. The neurobiology of stress and development. Annu Rev Psychol. 2007;58(1):145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- Hostinar CE, Sullivan RM, Gunnar MR. Psychobiological mechanisms underlying the social buffering of the hypothalamic-pituitary-adrenocortical axis: A review of animal models and human studies across development. Psychol Bull. 2014;140(1):256–282. doi: 10.1037/a0032671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Human LJ, Whillans AV, Hoppman CA, Klumb P, Dickerson SS. Finding the middle ground: Curvilinear associations between positive affect variability and daily cortisol profiles. Emotion. 2015;15(6):705–720. doi: 10.1037/emo0000071. [DOI] [PubMed] [Google Scholar]
- Hunter AL, Minnis H, Wilson P. Altered stress responses in children exposed to early adversity: A systematic review of salivary cortisol studies. Stress. 2011;14(6):616–626. doi: 10.3109/10253890.2011.577848. [DOI] [PubMed] [Google Scholar]
- Jenness JL, McLaughlin KA. Towards a person-centered approach to the developmental psychopathology of trauma. Soc Psychiatry Psychiatr Epidemiol. 2015;50(8):1219–1221. doi: 10.1007/s00127-015-1095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsova A, Bruun PB, Christensen RBC. Tests in linear mixed effects models. 2016 Retrieved from: https://cran.r-project.org/web/packages/lmerTest/lmerTest.pdf.
- LeMoult J, Chen MC, Foland-Ross LC, Burley HW, Gotlib IH. Concordance of mother daughter diurnal cortisol production: Understanding the intergenerational transmission of risk for depression. Biol Psychol. 2015;108:98–104. doi: 10.1016/j.biopsycho.2015.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marceau K, Ruttle PL, Shirtcliff EA, Essex MJ, Susman EJ. Developmental and contextual considerations for adrenal and gonadal hormone functioning during adolescence: Implications for adolescent mental health. Dev Psychobiol. 2015;57(6):742–768. doi: 10.1002/dev.21214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM. Growth and physiological development during adolescence. Ann Rev Med. 1968;19:283–300. doi: 10.1146/annurev.me.19.020168.001435. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Horm Behav. 2003;43(1):2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- McCrory E, De Brito SA, Viding E. Research review: The neurobiology and genetics of maltreatment and adversity. J Child Psychol Psychiatry. 2010;51(1):1079–1095. doi: 10.1111/j.1469-7610.2010.02271.x. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Basu A, Walsh K, Slopen N, Sumner J, Koenen KC, Keyes KM. Childhood exposure to violence and chronic physical conditions in a national sample of US adolescents. Psychosom Med. 2016 doi: 10.1097/PSY.0000000000000366. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, McClure EB, Pine DS. The social re-orientation of adolescence: A neuroscience perspective on the process and its relation to psychopathology. Pychol Med. 2005;35:163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- Pinto A, Malacrida B, Oieni J, Serafini MM, Davin A, Galbiati V, Corsini E, Racchi M. DHEA modulates the effect of cortisol on RACK1 expression via interference with the splicing of the glucocorticoid receptor. Br J Pharmacol. 2015;172(11):2918–2927. doi: 10.1111/bph.13097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell DJ, Schlotz W. Daily life stress and the cortisol awakening response: Testing the anticipation hypothesis. PLoS ONE. 2012;7(12) doi: 10.1371/journal.pone.0052067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quevedo K, Johnson A, Loman M, Lafavor T, Gunnar M. The confluence of adverse early experience and puberty on the cortisol awakening response. Int J Behav Dev. 2012;36(1):19–28. doi: 10.1177/0165025411406860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2015. https://www.R-project.org/ [Google Scholar]
- Ribbe D. Psychometric review of Traumatic Event Screening Instrument for Children (TESI-C) In: Stamm BH, editor. Measurement of stress, trauma, and adaptation. Lutherville, MD: Sidran Press; 1996. pp. 386–387. [Google Scholar]
- Roisman GI, Susman E, Barnett-Walker K, Booth-Laforce C, Owen MT, Belsky J, … Steinberg L. Early family and child-care antecedents of awakening cortisol levels in adolescence. Child Dev. 2009;80(3):907–920. doi: 10.1111/j.1467-8624.2009.01305.x. [DOI] [PubMed] [Google Scholar]
- Romeo RD. Pubertal maturation and programming of hypothalamic-pituitary-adrenal reactivity. Front Neuroendocrinol. 2010;31(2):232–240. doi: 10.1016/j.yfrne.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Rudolph KD, Hammen C. Age and gender as determinants of stress exposure, generation, and reactions in youngsters: a transactional perspective. Child Dev. 1999;70:660–77. doi: 10.1111/1467-8624.00048. [DOI] [PubMed] [Google Scholar]
- Rudolph KD, Hammen C, Burge D, Lindberg N, Herzberg D, Daley SE. Toward an interpersonal life-stress model of depression: The developmental context of stress generation. Dev Psychopathol. 2000;12:215–234. doi: 10.1017/s0954579400002066. [DOI] [PubMed] [Google Scholar]
- Ruttle PL, Shirtcliff EA, Armstrong JM, Klein MH, Essex MJ. Neuroendocrine coupling across adolescence and the longitudinal influence of early life stress. Dev Psychobiol. 2013:1–17. doi: 10.1002/dev.21138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Allison A, Armstrong JM, Slattery MJ, Kalin NH, Essex MJ. Longitudinal stability and developmental properties of salivary cortisol levels and circadian rhythms from childhood to adolescence. Dev Psychobiol. 2013;18(9):1199–1216. doi: 10.1002/dev.20607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Dahl RE, Pollak SD. Pubertal development: Correspondence between hormonal and physical development. Child Dev. 2009;80(2):327–337. doi: 10.1111/j.1467-8624.2009.01263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalder T, Kirschbaum C, Kudielka BM, Adam EK, Pruessner JC, Wüst S, … Clow A. Assessment of the cortisol awakening response: Expert consensus guidelines. Psychoneuroendocrinology. 2016;63:414–432. doi: 10.1016/j.psyneuen.2015.10.010. [DOI] [PubMed] [Google Scholar]
- van den Bos E, de Rooij M, Miers AC, Bokhorts CL, Westenberg PM. Adolescents’ increasing stress response to social evaluation: Pubertal effects on cortisol and alpha-amylase during public speaking. Child Dev. 2014;85(1):220–236. doi: 10.1111/cdev.12118. [DOI] [PubMed] [Google Scholar]
- Van Voorhees EE, Dennis MF, Calhoun PS, Beckham JC. Association of DHEA, DHEAS, and cortisol with childhood trauma exposure and posttraumatic stress disorder. Int Clin Psychopharmacol. 2014;29(1):56–62. doi: 10.1097/YIC.0b013e328364ecd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weems CF, Carrión VG. Brief report: Diurnal salivary cortisol in youth--clarifying the nature of posttraumatic stress dysregulation. J Pediatr Psychol. 2009;34(4):389–95. doi: 10.1093/jpepsy/jsn087. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.