Abstract
Mitochondrial reactive oxygen species (mtROS) are signaling molecules, which drive inflammatory cytokine production and T cell activation. In addition, cardiovascular diseases, cancers, and autoimmune diseases all share common feature of increased mtROS level. Both mtROS and ATP are produced as a result of electron transport chain activity, but it remains enigmatic whether mtROS could be generated independently from ATP synthesis. A recent study shed light to this important question and found that during endothelial cell (EC) activation, mtROS could be upregulated in a proton leak-coupled, but ATP synthesis-uncoupled manner. As a result, EC could upregulate mtROS production for physiological EC activation without compromising mitochondrial membrane potential and ATP generation, and consequently without causing mitochondrial damage and EC death. Thus, a novel pathophysiological role of proton leak in driving mtROS production was uncovered for low grade physiological EC activation, patrolling immunosurveillance cell trans-endothelial migration and other signaling events without compromising cellular survival. This new working model explains how mtROS could be increasingly generated independently from ATP synthesis and endothelial damage/death. Mapping the connections between mitochondrial metabolism, physiological EC activation, patrolling cell migration and pathological inflammation is significant towards the development of novel therapies for inflammatory diseases and cancers.
Keywords: mitochondria, ROS, cardiovascular disease, cancer, vascular inflammation
Mitochondrial reactive oxygen species, induced by newly classified conditional danger signals, orchestrate inflammatory responses
Historically considered as merely cellular “powerhouses” that manufacture ATP and other metabolites, mitochondria are increasingly being recognized as “metabolic hub” that are involved in the crosstalk and signal integration of cell proliferation, death, differentiation, inflammation, and repair pathways. Remarkably, it is now increasingly appreciated that mitochondria serve as “sentinel” organelles that are not only capable of detecting cellular insults but also orchestrating inflammatory responses1. One of the most well characterized mechanisms of such involves mitochondria-derived reactive oxygen species (ROS), which are often induced in response to cellular homeostasis disruptions including infections, sterile damage, and metabolic disturbances. Reciprocally, mitochondrial ROS (mtROS), as signaling molecules, convey these cellular insult signals to the rest of the cell by engaging in a variety of cellular pathways, such as inflammasome activation, proinflammatory transcription factor activation, apoptosis, autophagy, and DNA-based neutrophil extracellular trap formation2, 3. The key cellular receptors that recognize the “threat” signals include Toll-like receptors (TLRs) and cytosolic sensing NLRs [NOD (nucleotide binding and oligomerization domain)-like receptors] that recognize a variety of pathogen-associated molecular patterns (PAMPs) and endogenous metabolites-related danger-associated molecular patterns (DAMPs)4. Together, 4 TLRs work synergistically with NLRs to recognize PAMPs and DAMPs, particularly in inflammation privileged tissues where inflammasome component genes for activating pyrotopsis (inflammatory cell death) are not constitutively expressed5–11. Moreover, four additional DAMP receptors including transmembrane C-type lectin receptors, retinoid acid inducible gene I (RIG-I), AIM2 (absent in melanoma 2), receptor for advanced glycation end products (RAGE, also a receptor for high mobility group box 1(HMGB1)) have been characterized12. Herein, these 6 categories of DAMP receptors were referred as classical DAMP receptors13. However, we are still far from uncovering the identities of DAMP receptors that are responsible for recognizing all the elevated endogenous metabolites-related DAMPs. In fact, there are as many as 41,993 different entries in current human Metabolome Database (http://www.hmdb.ca/). In order to have a sufficient binding affinities14, additional DAMP receptors must exist in order to transduce specific and effective signals from diverse human metabolite-related DAMPs13.
A series of research articles published from our laboratory recently addressed this important issue by using the endogenous lysophospholipid (LPL) family members as the prototype. LPLs are bioactive, lipid-derived metabolites that act through G-protein coupled receptors (GPCRs) and they are generated by regiospecific phospholipases on substrates such as membrane phospholipids and sphingolipids15. Some of the LPLs that have been identified include lysophosphatidic acid (LPA), lysoyphosphatidylcholine (LPC), lysophosphatidylethanolamine (LPE), lipophosphoglycan (LPG), lysophosphatidylinositol (LPI), and lysophosphatidylserine (LysoPS)16. The prominent roles of LPLs in chronic inflammatory disorders such as coronary artery disease (CAD), hypertension, atherosclerosis and severe vascular diseases are well established 17–20. 18, 19In our recent report, we proposed a conceptually innovative paradigm that G-protein-coupled receptors (GPCRs) of LPLs can be classified as novel conditional DAMP receptors for the following reasons13: (1) LPLs are elevated during cellular stress or under stimulation by cardiovascular risk factors21; (2) Basal level of LPLs mediate normal cellular functions while elevated LPLs initiate signaling cascade to either activate or dampen innate immune responses via LPL-GPCRs; (3) The expression of anti-inflammatory LPLs and their receptor are also elevated in response to inflammation and may play a role in resolution of inflammation; and we designate such LPL receptors as homeostasis-associated molecular patterns (HAMPs); and (4) LPL receptors and classically DAMP receptors reciprocally regulate the expression of each other, which is responsible for either progression or resolution of inflammation13. Thus, LPL receptors serve as conditional DAMP receptors by modulating physiological activities and housekeeping functions under normal conditions, while contributing to DAMP-mediated signaling pathways during cellular stress. We consolidated this working model by demonstrating that LPLs “transform” resting endothelial cells (ECs) to conditional innate immune cells22 via caspase-1 activating-sirtuin 1 inhibiting pathway23. In addition, we found that in addition to post-translationally activating inflammasome, long-term low dose cardiovascular disease risk factors such as LPLs are required for transcriptionally upregulating low levels of or missing inflammasome components in order to elicit inflammatory responses6, presumably via histone modifications24 and noncoding RNAs regulation25, 26.
Despite these significant advances, it was unclear how distinct GPCR in response to LPL stimulation could converge to activate the inflammasome and in turn activate ECs. Previous studies investigated the role of cytosolic ROS-generating systems such as nicotinamide adenine dinucleotide phosphate (NADPH) oxidase in this process27–29. It has been shown that LPL could induce nicotinamide adenine dinucleotide oxidase (mitochondrial)/ NADPH oxidase (cytosolic)-related ROS in ECs30. In addition, LPC-induced ROS production and inflammasome activation depends on sodium influx, which might contribute to the production of NADPH oxidase31. Furthermore, it is well proven that alteration NADH/NAD+ ratio, by ischemia, mutation, dysfunction of cytochrome c or even low levels of ATP, can induce mtROS production (mainly superoxide and hydrogen peroxide) at the complex 1 of mitochondrial electron transport chain. Meanwhile, reverse electron transport due to alterations in redox potential also causes the generation of mtROS from complex 1. These alterations could be sensed by NADPH oxidase components gp91PHOX and p22PHOX to assemble inflammasome and regulate cell signaling32, 33. Nevertheless, key NADPH oxidase components gp91PHOX and p22PHOX are found to be dispensable for inflammatory cytokine production, whereas blockade of mtROS reduces inflammatory cytokine secretion instead3. Moreover, mtROS downstream of T cell receptor signaling are also required for antigen-specific T cell activation34, suggesting that membrane receptor signal-linked mtROS promote T cell cytokine generation35, T cell proliferation36 and T cell/regulatory T cell (Treg)37–40 metabolism41. In agreement with previous studies, we found that LPC could induce both mitochondrial ROS and cytosolic ROS in a similar manner in human aortic ECs21. Importantly, LPC-induced mtROS are found to be increased independent of NADPH oxidase function, but rather as a result of Ca2+ entry from the cytosol to the mitochondria. MtROS production is determined by the rates of both mtROS production and disposal. We found that LPL do not significantly regulate the expressions of mitochondrial antioxidant superoxide dismutase 2 (SOD2) and uncoupling molecule uncoupling protein 3 (UCP3), although there is a slight expression increase of adenine nucleotide translocator (ANT), another mitochondrial uncoupling molecule, after LPL stimulation. Of note, there are multiple different species of mtROS, including superoxide and hydrogen peroxide, both of which seem to be upregulated by LPL in ECs42. Collectively, these results indicated that LPL triggers mtROS and subsequent transformation of EC to immune-like cells through means of stimulating cytosolic Ca2+ entry into the mitochondria. Given the fact that LPL-induced EC activation has been implicated as an initiation step of atherogenesis and tumor angiogenesis19, 20, therapeutic agents that target mitochondrial calcium uniporter and mtROS might serve as novel therapeutic targets against atherosclerosis and cancer. However, two fundamental questions remain to be answered before these applications could be used are that, how mitochondria, in response to the stimulation of conditional DAMPs such as LPC, generate mtROS in non-damaged ECs without compromising cellular energy supply; and how non-damaged ECs recruit patrolling monocytes and other innate immune cells in physiological conditions to perform their immune surveillance duty43. More specifically, it remains unclear how mitochondria could produce mtROS specifically for cellular signaling purpose independently from ATP synthesis, despite the fact that both ROS and ATP production are coupled to the mitochondrial electron transport chain activity44 (Figure 1).
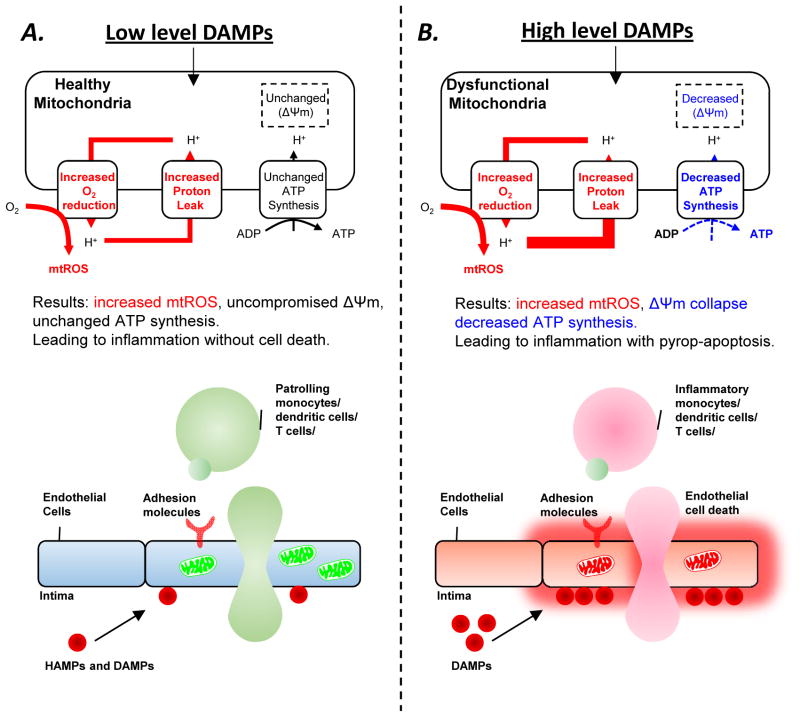
Figure 1. A new working model of physiological and pathological endothelial activation.
Mitochondrial reactive oxygen species (mtROS), un-coupled from ATP synthesis, control endothelial cell activation in response to different levels of danger signals associated molecular pattern (DAMPs) stimulation. The levels of proton leak control the scales of mtROS generation in healthy mitochondria or dysfunctional mitochondria. A. MtROS, generated in modest levels of increased proton leak and uncompromised ATP synthesis and mitochondrial membrane potential (Δψm), control physiological EC activation and patrolling immune cell recruitment in response to low levels of DAMPs and our newly proposed anti-inflammatory homeostasis associated molecular patterns (HAMPs). B. MtROS, generated in high levels of increased proton leak, compromised ATP synthesis, and decreased Δψm, control pathological EC activation, EC pyroptosis (inflammatory cell death) and apoptosis, and increased proinflammatory cell recruitment in response to high levels of DAMPs.
Reciprocal regulation of mtROS and mitochondrial proton leak drives EC activation
Our recent study shed light into the important question outlined above and uncovered a previously unrecognized role of mitochondrial proton leak in driving ATP-synthesis-uncoupled mtROS for normal physiological immune surveillance purpose21. Jensen first observed the formation of ROS in the respiration chain45, followed by the pioneer works by Chance and colleagues on the mitochondrial production of hydrogen peroxide46, 47. As explained in our recent review1, generation of mtROS mainly takes place at the electron transport chain located on the inner mitochondrial membrane during the process of oxidative phosphorylation44. During mitochondrial respiration, the electrons originated from the Krebs cycle pass along the electron transport chain, which ultimately lead to the generation of H2O or ROS due to complete or partial mitochondrial O2 reduction, respectively. Meanwhile, protons (H+) are being pumped from the mitochondrial matrix to the intermembrane space, thus creating mitochondrial membrane potential across the inner mitochondrial membrane. This proton gradient was used to synthesize ATP when protons re-enter the mitochondrial matrix through ATP synthase (also termed complex V). Thus, the production of mtROS via electron leak48 and ATP is intimately linked. Nevertheless, mtROS production is incompletely coupled to ATP synthesis, since mtROS is also coupled to the proton leak process mediated by uncoupling proteins49, through which protons in the intermembrane space leak across the inner membrane independently of ATP synthase (Figure 1). Thus, one would argue that, in addition to its physiological and pathological importance for energy generation and metabolic rate, adaptive non-shivering thermogenesis (in brown adipose tissue)50, body mass regulation48, 49 and increased heat for acute inflammation 49, 51, proton leak might play a role in the mitochondria’s decision to induce mtROS or produce ATP. In fact, a recent study suggested that mtROS is induced during proton leak in vivo52, and that mtROS are also required for the activation of proton leak. However, the role of proton leak in mtROS production was not addressed in this study. In our recent report21, we also demonstrated that during EC activation process induced by endogenous proatherogenic stimuli LPC, mitochondrial proton leak and mtROS are both induced. In addition, we found that LPC induce the expression of ANT, which mediates both basal and inducible proton conductance. Importantly, we were able to demonstrate that proton leak induction is used by ECs to induce ATP-synthesis-uncoupled mtROS production (Figure 1)21. Thus, it appears that a positive regulatory loop between proton leak and mtROS exists, which might be needed to induce high amount of ATP-synthesis-uncoupled mtROS during physiological EC activation and pathological inflammatory responses (Figure 1).
MtROS level dictates endothelial response and “activation scale”
Another important observation from our reports is that mtROS level dictates EC response21. By using mouse aortic microarray analysis, mouse plasma and aortic metabolomic analysis, the Seahorse Mitochondrial Stress test, flow cytometry mtROS detection, mtROS inhibitor mitoTEMPO, electron spin resonance, confocal microscopy, and human aortic endothelial cells, we found that lower dosage (10μM) of LPC induced ATP-synthesis-uncoupled mtROS, which does not lead to cell death but is required for low grade physiological EC activation. In contrast, higher dosage (>20μM) of LPC that presumably mimic pathological conditions, however, induces higher level of mtROS, which are associated with significant EC death21. 10μM LPC is effective in inducing mtROS and elicit physiological EC activation. It was found that this response is due to mitochondrial Ca2+ entry from the cytosol, as LPC-induced mtROS is completely blocked by Ruthenium Red, the general inhibitor for transient receptor potential vanilloid molecules (TRPVs) on the plasma membrane53, 54 and presumably also inhibitors of mitochondrial calcium uniporter on the mitochondrial membrane55, 56. Our work have demonstrated for the first time that low dose endogenous LPC stimulated endothelial cells can achieve a physiological activation status without endothelial injury and damage, as judged by decreased ATP generation. The mtROS generation, coupled with increased proton leak but uncoupled from ATP synthesis, is the hallmark of this type of physiological endothelial cell activation, which is conceptually innovative21. Of note, high levels of mtROS drive pathological endothelial cell activation, which is in agreement with our previous publications. Our findings argue prominent roles of mtROS in driving both physiological EC activation and inflammation responses as well as initiating cell death in pathological conditions10, 23. We speculate that during inflammation, the amount of conditional DAMPs or PAMPs in the cellular environment could be sensed by endothelial mitochondria57, which reciprocally signal through various levels of mtROS to dictate the ultimate endothelial activation scales in both physiological and pathological conditions. Such mechanisms are not only important in recruiting and regulating normal physiological “patrolling” immune functions58 and immune surveillance59 for anti-microbiota and anti-tumor immunity60, but also seems to be easily deranged during chronic inflammatory diseases, such as atherosclerosis (Figure 1).
Endothelial mtROS physiologically control patrolling cell immunosurveillance duty, which are “hijacked” during early atherogenesis to recruit inflammatory cells
It has been widely recognized that Ly6Chigh inflammatory monocyte recruitment into aorta in response to the stimulation of metabolic cardiovascular disease risk factors including hyperlipidemia61, hyperglycemia62, disturbed blood flow63, hyperglycemia64, and hyperhomocysteinemia64–67 plays significant roles in accelerating vascular inflammation and atherogenesis68. Pathological endothelial activation and dysfunction69 via generating a strong chemokine gradient are critical for recruiting inflammatory monocytes62. In our report, using monocyte adhesion assay, intravital microscopy, flow cytometry, and other biochemical assays as well as atherogenic apolipoprotein E deficient (ApoE−/−) mouse model, we found that LPC-induced mtROS then contribute to aortic EC activation by regulating nuclear binding of activator protein-1 (AP-1) and inducing intercellular adhesion molecule-1 (ICAM-1) gene expression in vitro. Furthermore, mtROS inhibitor MitoTEMPO decreases aortic endothelial activation and aortic monocyte recruitment in atherogenic mice during early atherosclerosis in vivo21. In addition, along the same line, it has been shown that deficiency of superoxide dismutase 2 gene in ApoE−/− mice, which encodes a mitochondrial antioxidant protein, accelerates atherogenesis70. Moreover, overexpression of thioredoxin 2 in ECs, which is another mitochondrial antioxidant enzyme, improves EC function and suppresses atherosclerosis development in ApoE−/− mice71. Furthermore, scavenging mtROS specifically in macrophages also decreases lesion formation in atherosclerotic mice72. Taken together, these studies suggested that in addition to promoting low scale physiological EC activation, mtROS promote atherosclerosis development by pathologically recruiting inflammatory cells to the lesions21.
Until recently, the important issue regarding how patrolling monocytes and other innate immune cells73 cross endothelium and patrol the vessels and tissues under physiological homeostatic conditions remained much less understood74. Although it has been reported that orphan nuclear receptor NR4A1 controls the differentiation of patrolling Ly6C- monocytes75; and that lymphocyte function-associated antigen 1 (LFA1) mediates patrolling monocyte recruitment at homeostatic conditions76, the molecular mechanisms underlying how ECs at non-dysfunctional/damaged conditions become activated and recruit patrolling monocytes are still unknown. Our study provided a framework which explains how mtROS could be increasingly generated independently from ATP synthesis and endothelial damage/death21. We speculate that mtROS may be utilized by ECs to recruit patrolling monocyte for detecting and clearing harmful pathogens and malignant cells under normal physiological conditions, but this pathway is “hijacked” during pathological conditions such as atherosclerosis and autoimmune diseases, which mediates continuous inflammatory cell recruitment into arteries and tissues (Figure 1).
A new working model of physiological and pathological endothelial activation
In summary (Figure 1), we proposed a new working model of EC activation: Under normal physiological conditions, low levels of DAMPs and our newly proposed HAMPs are important for mediating the patrolling immune cell adhesion and migration77 by driving ATP-synthesis-uncoupled, but proton leak-coupled mtROS. As a result, mtROS are specifically induced for EC activation purpose without causing EC cell death. However, during pathological conditions such as atherosclerosis, higher levels of DAMPs lead to mtROS production that is coupled with decreased ATP synthesis and mitochondrial membrane potential (Δψm) collapse as a result of mitochondrial dysfunction. This leads to pathological EC activation with EC cell death, including apoptosis, pyroptosis23 and pyrop-apoptosis (a hybrid form of pyroptosis and apoptosis) that we recently reported10. Consequently, this leads to pathological recruitment of Ly6Chigh inflammatory monocytes64–66, 68, dendritic cells77, 78, B cells79 and T cells80. We propose that one of the major physiological functions of mitochondrial proton leak in ECs maybe fine-tuning mtROS generation for cell signaling purpose, but such mechanism is easily compromised in pathological conditions, which might lead to uncontrolled inflammation and cell death leading to the development of inflammatory diseases, such as atherosclerosis.
Concluding remarks and future directions
Further studies that map the connections between mitochondria and inflammation in ECs are of great interest, as endothelial mtROS seem to control patrolling cell immunosurveillance process under normal physiological conditions, which is important for controlling cancer cells and detecting infected pathogens. However, endothelial mtROS are “hijacked” during atherosclerosis development, which mediate harmful inflammatory cell migration to the lesions, contributing to early atherogenesis. Targeting mitochondrial metabolism thus may serve as novel therapies for cancers and inflammatory diseases including atherosclerosis. However, in order to translate this knowledge from benchtop to bedside, several imminent questions remain to be addressed: 1) Despite the importance of oxidative stress in the development of cardiovascular disease, antioxidant therapies such as Vitamin C and Vitamin E fail to demonstrate their therapeutic efficacy. This may be related to our incomplete understanding of the physiological and pathological roles of different ROS species originated from different subcellular organelles. In fact, only a small portion of known naturally occurring antioxidants in vivo are located in the mitochondria1. Future studies that investigate the effects of mitochondria-targeting antioxidant in cardiovascular diseases are warranted. 2) It is still unknown how mitochondria are “hijacked” during pathological conditions and additional mitochondrial factors that contribute to diseases remain to be identified. 3) Potential side effects of mitochondria-targeting drugs need to be studied since mtROS also play significant housekeeping roles under normal physiological conditions. We hope that our review will encourage investigators to enter this important field and accelerate the pace of translational medicine.
Acknowledgments
This work was partially supported by the National Institutes of Health Grants to XFY and HW.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Author’s contribution
XL carried out the primary literature search and drafted the manuscript. PF, WY, KC, ML, KX, TG, and HW provided material input and helped critically reading the manuscript. XFY designed the study, provided field expertise and revised the manuscript. All authors read and approved the final manuscript.
References
- 1.Li X, Fang P, Mai J, Choi ET, Wang H, Yang XF. Targeting mitochondrial reactive oxygen species as novel therapy for inflammatory diseases and cancers. Journal of hematology & oncology. 2013;6:19. doi: 10.1186/1756-8722-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in nlrp3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 3.Bulua AC, Simon A, Maddipati R, Pelletier M, Park H, Kim KY, Sack MN, Kastner DL, Siegel RM. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in tnfr1-associated periodic syndrome (traps) The Journal of experimental medicine. 2011;208:519–533. doi: 10.1084/jem.20102049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang XF, Yin Y, Wang H. Vascular inflammation and atherogenesis are activated via receptors for pamps and suppressed by regulatory t cells. Drug Discov Today Ther Strateg. 2008;5:125–142. doi: 10.1016/j.ddstr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yin Y, Pastrana JL, Li X, Huang X, Mallilankaraman K, Choi ET, Madesh M, Wang H, Yang XF. Inflammasomes: Sensors of metabolic stresses for vascular inflammation. Front Biosci (Landmark Ed) 2013;18:638–649. doi: 10.2741/4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yin Y, Yan Y, Jiang X, Mai J, Chen NC, Wang H, Yang XF. Inflammasomes are differentially expressed in cardiovascular and other tissues. Int J Immunopathol Pharmacol. 2009;22:311–322. doi: 10.1177/039463200902200208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li YF, Huang X, Li X, Gong R, Yin Y, Nelson J, Gao E, Zhang H, Hoffman NE, Houser SR, Madesh M, Tilley DG, Choi ET, Jiang X, Huang CX, Wang H, Yang XF. Caspase-1 mediates hyperlipidemia-weakened progenitor cell vessel repair. Front Biosci (Landmark Ed) 2016;21:178–191. doi: 10.2741/4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lopez-Pastrana J, Ferrer LM, Li YF, Xiong X, Xi H, Cueto R, Nelson J, Sha X, Li X, Cannella AL, Imoukhuede PI, Qin X, Choi ET, Wang H, Yang XF. Inhibition of caspase-1 activation in endothelial cells improves angiogenesis: A novel therapeutic potential for ischemia. The Journal of biological chemistry. 2015;290:17485–17494. doi: 10.1074/jbc.M115.641191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen J, Yin Y, Mai J, Xiong X, Pansuria M, Liu J, Maley E, Saqib NU, Wang H, Yang XF. Caspase-1 recognizes extended cleavage sites in its natural substrates. Atherosclerosis. 2010;210:422–429. doi: 10.1016/j.atherosclerosis.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xi H, Zhang Y, Xu Y, Yang WY, Jiang X, Sha X, Cheng X, Wang J, Qin X, Yu J, Ji Y, Yang X, Wang H. Caspase-1 inflammasome activation mediates homocysteine-induced pyrop-apoptosis in endothelial cells. Circulation research. 2016;118:1525–1539. doi: 10.1161/CIRCRESAHA.116.308501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrer LM, Monroy AM, Lopez-Pastrana J, Nanayakkara G, Cueto R, Li YF, Li X, Wang H, Yang XF, Choi ET. Caspase-1 plays a critical role in accelerating chronic kidney disease-promoted neointimal hyperplasia in the carotid artery. Journal of cardiovascular translational research. 2016 doi: 10.1007/s12265-016-9683-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Venereau E, Ceriotti C, Bianchi ME. Damps from cell death to new life. Frontiers in immunology. 2015;6:422. doi: 10.3389/fimmu.2015.00422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X, Li YF, Nanayakkara G, Shao Y, Liang B, Cole L, Yang WY, Li X, Cueto R, Yu J, Wang H, Yang XF. Lysophospholipid receptors, as novel conditional danger receptors and homeostatic receptors modulate inflammation-novel paradigm and therapeutic potential. Journal of cardiovascular translational research. 2016;9:343–359. doi: 10.1007/s12265-016-9700-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kastritis PL, Bonvin AM. On the binding affinity of macromolecular interactions: Daring to ask why proteins interact. Journal of the Royal Society, Interface / the Royal Society. 2013;10:20120835. doi: 10.1098/rsif.2012.0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li YF, Li RS, Samuel SB, Cueto R, Li XY, Wang H, Yang XF. Lysophospholipids and their g protein-coupled receptors in atherosclerosis. Front Biosci (Landmark Ed) 2016;21:70–88. doi: 10.2741/4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y-f, Li Rong-shan, Samuel Sonia B, Cueto Ramon, Li Xinyuan, Wang Hong, Yang Xiao-feng. Lysophospholipids and their g protein-coupled receptors in atherosclerosis. Frontiers in Bioscience (Landmark Edition) 2016;21:70–88. doi: 10.2741/4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smyth SS, Mueller P, Yang F, Brandon JA, Morris AJ. Arguing the case for the autotaxin-lysophosphatidic acid-lipid phosphate phosphatase 3-signaling nexus in the development and complications of atherosclerosis. Arterioscler Thromb Vasc Biol. 2014;34:479–486. doi: 10.1161/ATVBAHA.113.302737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdel-Latif A, Heron PM, Morris AJ, Smyth SS. Lysophospholipids in coronary artery and chronic ischemic heart disease. Curr Opin Lipidol. 2015 doi: 10.1097/MOL.0000000000000226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan M, Hao F, Xu X, Chisolm GM, Cui MZ. Lysophosphatidylcholine activates a novel pkd2-mediated signaling pathway that controls monocyte migration. Arterioscler Thromb Vasc Biol. 2009;29:1376–1382. doi: 10.1161/ATVBAHA.109.191585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurano M, Suzuki A, Inoue A, Tokuhara Y, Kano K, Matsumoto H, Igarashi K, Ohkawa R, Nakamura K, Dohi T, Miyauchi K, Daida H, Tsukamoto K, Ikeda H, Aoki J, Yatomi Y. Possible involvement of minor lysophospholipids in the increase in plasma lysophosphatidic acid in acute coronary syndrome. Arterioscler Thromb Vasc Biol. 2015;35:463–470. doi: 10.1161/ATVBAHA.114.304748. [DOI] [PubMed] [Google Scholar]
- 21.Li X, Fang P, Li Y, Kuo YM, Andrews AJ, Nanayakkara G, Johnson C, Fu H, Shan H, Du F, Hoffman NE, Yu D, Eguchi S, Madesh M, Koch WJ, Sun J, Jiang X, Wang H, Yang X. Mitochondrial reactive oxygen species mediate lysophosphatidylcholine-induced endothelial cell activation. Arteriosclerosis, thrombosis, and vascular biology. 2016;36:1090–1100. doi: 10.1161/ATVBAHA.115.306964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mai J, Virtue A, Shen J, Wang H, Yang XF. An evolving new paradigm: Endothelial cells--conditional innate immune cells. Journal of hematology & oncology. 2013;6:61. doi: 10.1186/1756-8722-6-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yin Y, Li X, Sha X, Xi H, Li YF, Shao Y, Mai J, Virtue A, Lopez-Pastrana J, Meng S, Tilley DG, Monroy MA, Choi ET, Thomas CJ, Jiang X, Wang H, Yang XF. Early hyperlipidemia promotes endothelial activation via a caspase-1-sirtuin 1 pathway. Arteriosclerosis, thrombosis, and vascular biology. 2015;35:804–816. doi: 10.1161/ATVBAHA.115.305282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shao Y, Chernaya V, Johnson C, Yang WY, Cueto R, Sha X, Zhang Y, Qin X, Sun J, Choi ET, Wang H, Yang XF. Metabolic diseases downregulate the majority of histone modification enzymes, making a few upregulated enzymes novel therapeutic targets-“sand out and gold stays”. J Cardiovasc Transl Res. 2016;9:49–66. doi: 10.1007/s12265-015-9664-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mai J, Virtue A, Maley E, Tran T, Yin Y, Meng S, Pansuria M, Jiang X, Wang H, Yang XF. Micrornas and other mechanisms regulate interleukin-17 cytokines and receptors. Front Biosci (Elite Ed) 2012;4:1478–1495. doi: 10.2741/474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Virtue A, Wang H, Yang XF. Micrornas and toll-like receptor/interleukin-1 receptor signaling. Journal of hematology & oncology. 2012;5:66. doi: 10.1186/1756-8722-5-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holmstrom KM, Finkel T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nature reviews. Molecular cell biology. 2014;15:411–421. doi: 10.1038/nrm3801. [DOI] [PubMed] [Google Scholar]
- 28.Schieber M, Chandel NS. Ros function in redox signaling and oxidative stress. Current biology : CB. 2014;24:R453–462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Panday A, Sahoo MK, Osorio D, Batra S. Nadph oxidases: An overview from structure to innate immunity-associated pathologies. Cellular & molecular immunology. 2015;12:5–23. doi: 10.1038/cmi.2014.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takeshita S, Inoue N, Gao D, Rikitake Y, Kawashima S, Tawa R, Sakurai H, Yokoyama M. Lysophosphatidylcholine enhances superoxide anions production via endothelial nadh/nadph oxidase. Journal of atherosclerosis and thrombosis. 2000;7:238–246. doi: 10.5551/jat1994.7.238. [DOI] [PubMed] [Google Scholar]
- 31.Schilling T, Eder C. Sodium dependence of lysophosphatidylcholine-induced caspase-1 activity and reactive oxygen species generation. Immunobiology. 2011;216:118–125. doi: 10.1016/j.imbio.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 32.Abais JM, Xia M, Zhang Y, Boini KM, Li PL. Redox regulation of nlrp3 inflammasomes: Ros as trigger or effector? Antioxidants & redox signaling. 2015;22:1111–1129. doi: 10.1089/ars.2014.5994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doughan AK, Harrison DG, Dikalov SI. Molecular mechanisms of angiotensin ii-mediated mitochondrial dysfunction: Linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circulation research. 2008;102:488–496. doi: 10.1161/CIRCRESAHA.107.162800. [DOI] [PubMed] [Google Scholar]
- 34.Sena LA, Li S, Jairaman A, Prakriya M, Ezponda T, Hildeman DA, Wang CR, Schumacker PT, Licht JD, Perlman H, Bryce PJ, Chandel NS. Mitochondria are required for antigen-specific t cell activation through reactive oxygen species signaling. Immunity. 2013;38:225–236. doi: 10.1016/j.immuni.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li X, Mai J, Virtue A, Yin Y, Gong R, Sha X, Gutchigian S, Frisch A, Hodge I, Jiang X, Wang H, Yang XF. Il-35 is a novel responsive anti-inflammatory cytokine--a new system of categorizing anti-inflammatory cytokines. PloS one. 2012;7:e33628. doi: 10.1371/journal.pone.0033628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang XF, Weber GF, Cantor H. A novel bcl-x isoform connected to the t cell receptor regulates apoptosis in t cells. Immunity. 1997;7:629–639. doi: 10.1016/s1074-7613(00)80384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mo H, Shi Y, Han X, Zhou S, He X, Liu P, Yang J, Zhang C, Yang S, Qin Y, Gui L, Yao J, Zhao L, Zhang S. Absolute monocyte count is a prognostic indicator in a patient with diffuse large b-cell lymphoma after autologous peripheral blood stem cell transplant. Leukemia & lymphoma. 2015;56:515–517. doi: 10.3109/10428194.2014.920504. [DOI] [PubMed] [Google Scholar]
- 38.Lopez-Pastrana J, Shao Y, Chernaya V, Wang H, Yang XF. Epigenetic enzymes are the therapeutic targets for cd4(+)cd25(+/high)foxp3(+) regulatory t cells. Translational research : the journal of laboratory and clinical medicine. 2015;165:221–240. doi: 10.1016/j.trsl.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiong Z, Song J, Yan Y, Huang Y, Cowan A, Wang H, Yang XF. Higher expression of bax in regulatory t cells increases vascular inflammation. Front Biosci. 2008;13:7143–7155. doi: 10.2741/3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xiong Z, Yan Y, Song J, Fang P, Yin Y, Yang Y, Cowan A, Wang H, Yang XF. Expression of tctp antisense in cd25(high) regulatory t cells aggravates cuff-injured vascular inflammation. Atherosclerosis. 2009;203:401–408. doi: 10.1016/j.atherosclerosis.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buck MD, O’Sullivan D, Pearce EL. T cell metabolism drives immunity. The Journal of experimental medicine. 2015;212:1345–1360. doi: 10.1084/jem.20151159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watanabe N, Zmijewski JW, Takabe W, Umezu-Goto M, Le Goffe C, Sekine A, Landar A, Watanabe A, Aoki J, Arai H, Kodama T, Murphy MP, Kalyanaraman R, Darley-Usmar VM, Noguchi N. Activation of mitogen-activated protein kinases by lysophosphatidylcholine-induced mitochondrial reactive oxygen species generation in endothelial cells. The American journal of pathology. 2006;168:1737–1748. doi: 10.2353/ajpath.2006.050648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berraondo P, Teijeira A, Melero I. Cancer immunosurveillance caught in the act. Immunity. 2016;44:525–526. doi: 10.1016/j.immuni.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 44.Murphy MP. How mitochondria produce reactive oxygen species. The Biochemical journal. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jensen PK. Antimycin-insensitive oxidation of succinate and reduced nicotinamide-adenine dinucleotide in electron-transport particles. I. Ph dependency and hydrogen peroxide formation. Biochimica et biophysica acta. 1966;122:157–166. doi: 10.1016/0926-6593(66)90057-9. [DOI] [PubMed] [Google Scholar]
- 46.Loschen G, Flohe L, Chance B. Respiratory chain linked h(2)o(2) production in pigeon heart mitochondria. FEBS letters. 1971;18:261–264. doi: 10.1016/0014-5793(71)80459-3. [DOI] [PubMed] [Google Scholar]
- 47.Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. The Biochemical journal. 1973;134:707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jastroch M, Divakaruni AS, Mookerjee S, Treberg JR, Brand MD. Mitochondrial proton and electron leaks. Essays in biochemistry. 2010;47:53–67. doi: 10.1042/bse0470053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Busiello RA, Savarese S, Lombardi A. Mitochondrial uncoupling proteins and energy metabolism. Frontiers in physiology. 2015;6:36. doi: 10.3389/fphys.2015.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zha L, Li F, Wu R, Artinian L, Rehder V, Yu L, Liang H, Xue B, Shi H. The histone demethylase utx promotes brown adipocyte thermogenic program via coordinated regulation of h3k27 demethylation and acetylation. The Journal of biological chemistry. 2015;290:25151–25163. doi: 10.1074/jbc.M115.662650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chandrasoma PT, CR . Acute inflammatory response. In: Chandrasoma PT, CR, editors. Concise pathology. 3. Four Stamford Plaza, PO Box 120041, Stamford, Connecticut 06912-0041: Appleton & Lange, A Simon & Shuster Company; 1998. [Google Scholar]
- 52.Chouchani ET, Kazak L, Jedrychowski MP, Lu GZ, Erickson BK, Szpyt J, Pierce KA, Laznik-Bogoslavski D, Vetrivelan R, Clish CB, Robinson AJ, Gygi SP, Spiegelman BM. Mitochondrial ros regulate thermogenic energy expenditure and sulfenylation of ucp1. Nature. 2016;532:112–116. doi: 10.1038/nature17399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kohler R, Heyken WT, Heinau P, Schubert R, Si H, Kacik M, Busch C, Grgic I, Maier T, Hoyer J. Evidence for a functional role of endothelial transient receptor potential v4 in shear stress-induced vasodilatation. Arterioscler Thromb Vasc Biol. 2006;26:1495–1502. doi: 10.1161/01.ATV.0000225698.36212.6a. [DOI] [PubMed] [Google Scholar]
- 54.Zhang L, Papadopoulos P, Hamel E. Endothelial trpv4 channels mediate dilation of cerebral arteries: Impairment and recovery in cerebrovascular pathologies related to alzheimer’s disease. British journal of pharmacology. 2013;170:661–670. doi: 10.1111/bph.12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu S, Chisholm AD. C. Elegans epidermal wounding induces a mitochondrial ros burst that promotes wound repair. Dev Cell. 2014;31:48–60. doi: 10.1016/j.devcel.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gorlach A, Bertram K, Hudecova S, Krizanova O. Calcium and ros: A mutual interplay. Redox Biol. 2015;6:260–271. doi: 10.1016/j.redox.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Harbauer AB, Zahedi RP, Sickmann A, Pfanner N, Meisinger C. The protein import machinery of mitochondria-a regulatory hub in metabolism, stress, and disease. Cell metabolism. 2014;19:357–372. doi: 10.1016/j.cmet.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 58.Thomas G, Tacke R, Hedrick CC, Hanna RN. Nonclassical patrolling monocyte function in the vasculature. Arteriosclerosis, thrombosis, and vascular biology. 2015;35:1306–1316. doi: 10.1161/ATVBAHA.114.304650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Colaco HG, Moita LF. Initiation of innate immune responses by surveillance of homeostasis perturbations. The FEBS journal. 2016;283:2448–2457. doi: 10.1111/febs.13730. [DOI] [PubMed] [Google Scholar]
- 60.Hanna RN, Cekic C, Sag D, Tacke R, Thomas GD, Nowyhed H, Herrley E, Rasquinha N, McArdle S, Wu R, Peluso E, Metzger D, Ichinose H, Shaked I, Chodaczek G, Biswas SK, Hedrick CC. Patrolling monocytes control tumor metastasis to the lung. Science. 2015;350:985–990. doi: 10.1126/science.aac9407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. The Journal of clinical investigation. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sena CM, Pereira AM, Seica R. Endothelial dysfunction - a major mediator of diabetic vascular disease. Biochimica et biophysica acta. 2013;1832:2216–2231. doi: 10.1016/j.bbadis.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 63.Nam D, Ni CW, Rezvan A, Suo J, Budzyn K, Llanos A, Harrison DG, Giddens DP, Jo H. A model of disturbed flow-induced atherosclerosis in mouse carotid artery by partial ligation and a simple method of rna isolation from carotid endothelium. Journal of visualized experiments : JoVE. 2010 doi: 10.3791/1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fang P, Zhang D, Cheng Z, Yan C, Jiang X, Kruger WD, Meng S, Arning E, Bottiglieri T, Choi ET, Han Y, Yang XF, Wang H. Hyperhomocysteinemia potentiates hyperglycemia-induced inflammatory monocyte differentiation and atherosclerosis. Diabetes. 2014;63:4275–4290. doi: 10.2337/db14-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang D, Jiang X, Fang P, Yan Y, Song J, Gupta S, Schafer AI, Durante W, Kruger WD, Yang X, Wang H. Hyperhomocysteinemia promotes inflammatory monocyte generation and accelerates atherosclerosis in transgenic cystathionine beta-synthase-deficient mice. Circulation. 2009;120:1893–1902. doi: 10.1161/CIRCULATIONAHA.109.866889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang D, Fang P, Jiang X, Nelson J, Moore JK, Kruger WD, Berretta RM, Houser SR, Yang X, Wang H. Severe hyperhomocysteinemia promotes bone marrow-derived and resident inflammatory monocyte differentiation and atherosclerosis in ldlr/cbs-deficient mice. Circulation research. 2012;111:37–49. doi: 10.1161/CIRCRESAHA.112.269472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu S, Xiong X, Zhao X, Yang X, Wang H. F-bar family proteins, emerging regulators for cell membrane dynamic changes-from structure to human diseases. Journal of hematology & oncology. 2015;8:47. doi: 10.1186/s13045-015-0144-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang J, Zhang L, Yu C, Yang XF, Wang H. Monocyte and macrophage differentiation: Circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomarker research. 2014;2:1. doi: 10.1186/2050-7771-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shao Y, Cheng Z, Li X, Chernaya V, Wang H, Yang XF. Immunosuppressive/anti-inflammatory cytokines directly and indirectly inhibit endothelial dysfunction--a novel mechanism for maintaining vascular function. Journal of hematology & oncology. 2014;7:80. doi: 10.1186/s13045-014-0080-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ballinger SW, Patterson C, Knight-Lozano CA, Burow DL, Conklin CA, Hu Z, Reuf J, Horaist C, Lebovitz R, Hunter GC, McIntyre K, Runge MS. Mitochondrial integrity and function in atherogenesis. Circulation. 2002;106:544–549. doi: 10.1161/01.cir.0000023921.93743.89. [DOI] [PubMed] [Google Scholar]
- 71.Zhang H, Luo Y, Zhang W, He Y, Dai S, Zhang R, Huang Y, Bernatchez P, Giordano FJ, Shadel G, Sessa WC, Min W. Endothelial-specific expression of mitochondrial thioredoxin improves endothelial cell function and reduces atherosclerotic lesions. The American journal of pathology. 2007;170:1108–1120. doi: 10.2353/ajpath.2007.060960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang Y, Wang GZ, Rabinovitch PS, Tabas I. Macrophage mitochondrial oxidative stress promotes atherosclerosis and nuclear factor-kappab-mediated inflammation in macrophages. Circulation research. 2014;114:421–433. doi: 10.1161/CIRCRESAHA.114.302153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang W, Fan C, Chen L, Cui Z, Bai Y, Lan F. Pathological effects of the fmr1 cgg-repeat polymorphism (5–55 repeat numbers): Systematic review and meta-analysis. The Tohoku journal of experimental medicine. 2016;239:57–66. doi: 10.1620/tjem.239.57. [DOI] [PubMed] [Google Scholar]
- 74.Ginhoux F, Jung S. Monocytes and macrophages: Developmental pathways and tissue homeostasis. Nature reviews. Immunology. 2014;14:392–404. doi: 10.1038/nri3671. [DOI] [PubMed] [Google Scholar]
- 75.Hanna RN, Carlin LM, Hubbeling HG, Nackiewicz D, Green AM, Punt JA, Geissmann F, Hedrick CC. The transcription factor nr4a1 (nur77) controls bone marrow differentiation and the survival of ly6c- monocytes. Nature immunology. 2011;12:778–785. doi: 10.1038/ni.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nature reviews. Immunology. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ait-Oufella H, Sage AP, Mallat Z, Tedgui A. Adaptive (t and b cells) immunity and control by dendritic cells in atherosclerosis. Circulation research. 2014;114:1640–1660. doi: 10.1161/CIRCRESAHA.114.302761. [DOI] [PubMed] [Google Scholar]
- 78.Biessen EA, Christ A. Plasmacytoid dendritic cells in atherosclerosis: Knocking at t-cell’s door. Circulation. 2014;130:1340–1342. doi: 10.1161/CIRCULATIONAHA.114.012641. [DOI] [PubMed] [Google Scholar]
- 79.Perry HM, Bender TP, McNamara CA. B cell subsets in atherosclerosis. Frontiers in immunology. 2012;3:373. doi: 10.3389/fimmu.2012.00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tse K, Tse H, Sidney J, Sette A, Ley K. T cells in atherosclerosis. International immunology. 2013;25:615–622. doi: 10.1093/intimm/dxt043. [DOI] [PMC free article] [PubMed] [Google Scholar]