Abstract
Lung cancer is the foremost cause of cancer-related deaths world-wide. Both, the major forms of lung cancer, Non-small cell lung cancer (NSCLC) and Small cell lung cancers (SCLC), have responded effectively to chemo-, radiation and adjuvant-therapies. Tumor removal through surgery also appeared as a good therapeutic strategy. However, these therapies demonstrated unfavourable side-effects, and hence novel drugs targeting lung cancer emerged essential. Activation of epidermal growth factor receptor (EGFR)-tyrosine kinases is a key reason for lung cancer progression. Two important strategies that have attenuated lung cancers were through treatments with EGFR-tyrosine kinase-inhibitors, erlotinib and gefitinib, or EGFR-neutralizing antibodies, cetuximab and bevacizumab. A major advantage with erlotinib and gefitinib was their role in second and third-line treatments following chemotherapies. Phase II/III clinical trials showed that combinatorial treatment of tyrosine kinase (TK)-inhibitors with chemotherapeutics, such as docetaxel and pemetrexed, caused significant improvements in progression-free survival and overall survival.Phase I and II clinical studies also revealed that combination of tyrosine kinase-inhibitors with the EGFR-targeted antibodies was an effective approach for treating lung cancer. However, patients having T790M-mutations within EGFR gene were resistant to erlotinib and gefitinib. Alternatively, another second-generation EGFR-tyrosine kinase-inhibitor, afatinib, that could circumvent the problem of drug resistance has been developed as lung cancer therapy. The current review focuses on the role of EGFR in lung cancer progression and apprises about the EGFR-targeted therapies. The review also informs on the adverse side-effects of these therapies and enlightens the need for safer therapeutic regimens to eradicate this dreaded disease.
Keywords: Erlotinib, gefitinib, cetuximab, bevacizumab, therapy, lung malignancy
Introduction
Lung cancer is a frequent form of malignancy with thousands of death reported in the Western and Eastern countries world-wide [1]. Cigarette smoking has been identified as one of the major reasons for lung cancer, which affects elderly men and women as well as the young and adolescents [2]. The non-smoking categories of lung malignancy are Non-small cell lung cancer (NSCLC) and small cell lung cancers (SCLC), characterized by three important histopathological classes, viz. squamous cell, large cell and adeno carcinoma [3]. Although NSCLC and SCLC together account for 85% of all reported lung cancer cases, around 45%, 35% and 10% of lung cancers come within the category of squamous cell, large cell and adeno carcinoma respectively [3].
It has been observed that 80% of the lung cancer patients suffer from NSCLC, with a span of three months to one-year survival post-diagnosis, depending on stages, I-IV [4]. Removal of malignant tumors through surgery has been considered as the best treatment option for NSCLC, which promoted long-term response and survival for about 30% of the patients [5]. However, despite surgery, symptoms of recurring tumor growth and progression with severely poor prognosis have also been reported [6]. Hardly any second or third lines of therapies are available post-relapse for NSCLC, resulting in an incurable situation that culminates in inevitable mortality [3].
SCLC manifests quick tumor growth and metastasis, where surgical intervention appears difficult [7]. Radiation therapy and chemotherapy emerge as alternative therapeutic choices [8]. However, the two therapies trigger unbearable adverse side-effects and often result in limited survival [9]. Alternatively, adjuvant chemotherapy developed as a good therapeutic procedure for both SCLC and NSCLC, with 5% increase in patient survival compared to radiation therapy and chemotherapies [10]. Nonetheless, adjuvant therapy also proved toxic in elderly patients with history of chronic obstructive pulmonary disease [11]. Supportively, clinical trials in NSCLC and SCLC patients using chemotherapy, radiotherapy and adjuvant therapy showed severe side effects [3]. Hence, novel therapies for treating lung cancer with minimum toxicity seemed extremely essential.
Epidermal growth factor receptor (EGFR) and lung cancer
Lung cancer progression originates from deregulated cellular proliferation, which prompts normal cells to undergo malignant transformation [12]. Research has proven EGFR group of growth factors as key molecules that promote lung cancer generation and propagation [13]. Because of their inability to penetrate cell membrane, secreted growth factors function via targeted signal transduction pathways that carry cellular information from EGFR to the inside of the lung cells [14]. These signalling events are activated through autocrine or paracrine pathways or both simultaneously [14,15]. Ultimately, the signalling cascades not only help cell growth and development, but also promote lung metastasis [13]. Particularly, EGFR-receptor tyrosine kinases (RTK) play an important role in initiating and triggering signalling events for both NSCLC and SCLC [16].
Genetic factors also prominently contribute to EGFR activation [17]. Mutations within the EGFR influence both autocrine and inducible growth factor secretion and activation in lung cancers [17]. EGFR mutations also affect diverse growth factor signalling pathways in lung neoplasm, which ultimately leads to aggressive lung carcinogenesis and metastasis [3]. EGFR mutation-induced adenocarcinoma, particularly Bronchioloalveolar cell carcinoma (BAC), has been reported to be a common form of NSCLC [18,19]. From about 120 adenocarcinoma patients in Japan, around 50% had features of non-mucinous BAC [20]. Around 80% of these BAC patients showed significant mutations in their EGFR gene [21]. This BAC category, resulting from EGFR tyrosine kinase mutation, comprises a large group of lung carcinoma patients, who could probably be treated using EGFR tyrosine kinase inhibitors [22]. Besides adenocarcinoma, other features of lung carcinoma include acinar, papillary solid, lepidic, micropapillary and papillary mucinous tumours [23-25].
In the current review, we propose to illustrate the role of EGFR group of growth factors in lung cancer progression and metastasis. We will particularly emphasize upon the impact of well-known therapeutics targeting EGFR and hence lung carcinoma.
EGFR: mechanism of action in lung cancer
A member of the transmembrane receptor family, EGFR is composed of three important regions [26] Figure 1. The extracellular ligand-binding domain binds to EGFR ligands, viz., EGF, Heparin-binding EGF-like growth factor (HB-EGF), transforming growth factor-α (TGFα), betacellulin, epiregulin and amphiregulin [3,27]. Of these EGFR ligands, EGF is most prominently up-regulated in lung cancer [3,27]. The transmembrane domain of EGFR links the ligand-binding domain to intracellular tyrosine kinase signalling domain [26]. Following ligand binding, EGFR undergoes auto-dimerization and hetero-dimerization with the other HER/erbB family of tyrosine kinases, such as HER1 (EGFR/erbB1), HER2 (neu, erbB2), HER3 (erbB3), and HER4 (erbB4) [28]. Ligand binding and dimerization are essential prerequisites that trigger EGFR signalling and targeted functions [13,29]. The dimeric form impedes the auto-inhibitory role of intracellular tyrosine kinase domain of EGFR, and promotes tyrosine phosphorylation and down-stream signalling [26,30]. The signalling mechanisms are initiated through ATP-mediated auto-phosphorylation of tyrosine, which primarily stimulates the mammalian mechanistic target of rapamycin (mTOR)-serine/threonine protein kinase pathway in lung cancer [31]. Other than the EGF ligand, up-regulated amphiregulin has also been reported to cause poor prognosis, with fewer chances of survival in NSCLC [32]. Both for the increased EGF and amphiregulin ligands, there are reports of severe lung cancer progression and metastasis that lead to bronchial lesions and secondary malignant growths [33-35].
Figure 1.
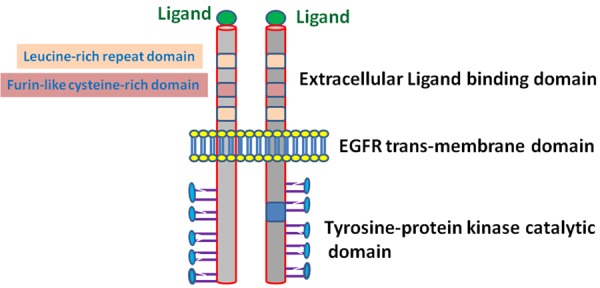
EGFR structure and ligand binding.
Growth and metastasis of lung tumors involve EGFR-dependent activations of Ras/Mitogen-activated Protein kinase cascade (MAPK) and phosphatidylinositol-3 kinase/Akt (PI3K/AKT) pathways [16]. PI3K/AKT is a pro-proliferative signalling pathway that promotes cellular multiplication and then attenuates apoptosis in SCLC and NSCLC [16]. For the metastatic process, lung cancer cells that escape the tumor growth site penetrate int lymphatic circulation [36]. Through systemic blood flow, the malignant cells reached distant sites where they multiply and proliferate into metastatic colonies [37]. For the malignant cells to get released from tumors and translocate, angiogenesis is proved to play an extremely important role [38]. During this process, activated-EGFR prompts break-down of the extracellular matrix (ECM) of lung tissues causing enhanced blood supply to the tumor blood vessels through angiogenesis [37,38]. Activated-EGFR also triggers enhanced expression of the angiogenic growth factors, particularly, vascular epidermal growth factor (VEGF), basic-fibroblast growth factor, platelet-derived endothelial cell growth factor and interleukin-8 [39].
Targeting EGFR in lung cancer
Systematic research has proven that EGFR may act as a novel target in lung cancer therapy [3]. Two major strategies that have been proposed for inhibiting EGFR functioning are (1) inactivation of intracellular TK signalling, and (2) use of neutralizing antibodies against EGFR and its ligands (Figure 2) [40,41]. The most well-studied EGFR-TK-inactivators for lung cancer include erlotinib and gefitinib [42,43]. Cetuximab and bevacizumab are monoclonal antibodies that block EGFR functioning [16,44]. These two categories of EGFR inhibitors have been found to be effective in suppressing proliferation of malignant lung cells, enhancing apoptosis and reducing lung cancer metastasis [16,45]. Particularly, these EGFR inhibitors were successful in suppressing lung cancer progression in the preclinical studies [46,47]. Both in vitro and in vivo animal models of lung cancer demonstrated that cetuximab in combination with radiotherapy and chemotherapy caused an additive or synergistic increase in the apoptosis of lung cancer cells [48]. Various Phase I and II clinical studies also reported certain relief in lung cancer patients treated with cetuximab, either alone or in combination with chemotherapy [49]. In pre-treated recurring lung cancer patients, the therapeutic impact of cetuximab was equivalent to chemotherapy [48]. However, the impact of cetuximab, co-treated with chemotherapeutic agents, was more potent [49,50]. Treatment of standard chemotherapeutic agents, cisplatin and vinorelbine, along with cetuximab showed prominent improvements and significant chances of survival in 86 lung cancer patients compared to the drugs alone [51]. The EGFR inactivators viz. erlotinib and gefitinib, were found to be more penetrable within the tumorous cells compared to cetuximab [42]. Erlotinib and gefitinib upon oral treatments provided minimum adverse side effects, to the extent of skin itches, stomach upsets, hand-foot syndrome, exhaustion, coagulation abnormalities and hemoptysis [52,53]. Clinical trials using EGFR inactivators, particularly gefitinib, demonstrated 20% cure and 40% symptomatic relief in NSCLC patients [43,54]. However, the gefitinib mono-therapy failed in Phase-III clinical trials that demonstrated very low survival [55]. In this trial, a combined gefitinib treatment with other chemotherapies was incapable of providing any advantage [55]. Erlotinib was more useful in this situation, where along with other chemotherapies, the drug showed potential benefits, even in the Phase III double-blind clinical trials on NSCLC [56].
Figure 2.
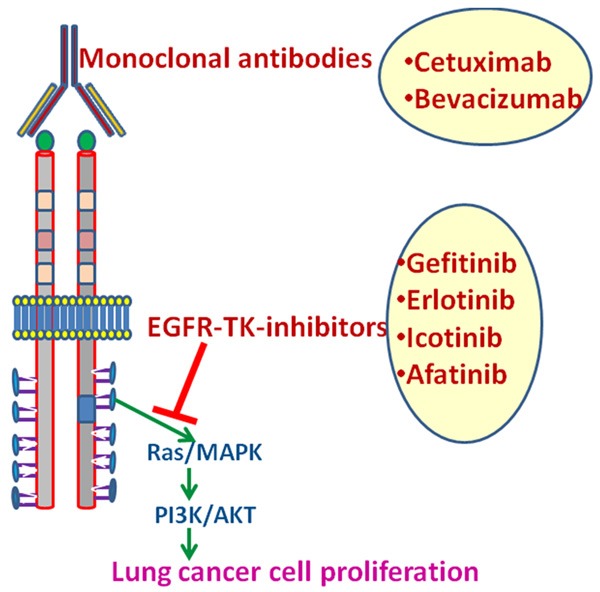
Targeting EGFR in lung cancer. Monoclonal antibodies block EGFR functioning EGFR-inhibit EGFR signaling.
Clinical trials revealed that erlotinib and gefitinib were effective in patients with EGFR mutations [22,57]. Erlotinib and gefitinib failed to target threonine to methionine mutations at codon 790 of exon 20 in EGFR gene [58,59]. However, the drugs could strongly target mis-sense and in-frame mutations within exons 18-21 at the EGFR-TK domain that significantly contribute to lung cancer progression and metastasis [22,47,57]. These EGFR-TK-inhibitors promoted survival rate and longevity of lung cancer patients having the aforesaid EGFR mutations [46,60]. The effects were stronger than standard chemotherapeutics [46,60]. Nonetheless, erlotnib and gefinib resistance proved to be a major problem in patients undergoing long-term treatment, post-recurrence [58,59]. This EGFR-TK-inhibitor resistance was typically due to a mutation at threonine to methionine of codon 790 at the exon 20 sites of EGFR, preventing the binding of these drugs [58,59]. For these patients, therapeutics that mediated EGFR inhibition with binding sites away from these codon sites seemed essential [58,59].
Targeting specific EGFR inhibitors
Erlotinib
Erlotinib is a well-established therapeutic for metastatic lung carcinoma [61], which inhibits tyrosine phosphorylation via blocking the intracellular ATP binding site of EGFR [62]. Phase II and III trials at Cancer Institutes of Canada have demonstrated around 12% diminution in lung cancer symptoms in NSCLC patients following erlotinib treatment [62]. In the phase III/IV clinical trials, 150 mg/Kg erlotinib prominently controlled rate of lung cancer metastasis and caused a progression-free survival and overall survival of three and eight months respectively [56]. Despite its significant efficacy, continued treatment with erlotinib proved difficult because of its extensive adverse side effects, particularly on skin, intestine and eyes [56]. Hence, reduced doses and interrupted erlotinib treatments had been suggested for managing these toxic effects [56]. However, patients with non-smoking history showed overall good survival that was progression-free [63].
Erlotinib was more effective in NSCLC patients who had undergone four rounds of platinum chemotherapies [61]. Supportively, clinical trials proved a specific role of erlotinib for second and third-line treatments in NSCLC and SCLC patients [61]. Hence, trials with 150 mg/Kg erlotinib in lung cancer patients, already exposed to platinum-based chemotherapy, revealed increased progression-free survival and overall survivals at a rate much higher than erlotinib alone [56]. Erlotinib co-treatment also improved survival rate of NSCLC patients compared to single first-line chemotherapy [64]. Use of erlotinib as a second-line treatment was very effective, particularly in older NSCLC patients [65,66]. The combined treatment remarkably improved the patients’ quality of life with attenuated symptoms of cough, respiratory uneasiness and chest pain and discomfort [67]. Post-treatment, the patients showed 60-70% recovery and significant improvements in physical ability, with the above three symptoms at a range of 35-45% of placebo [67]. Generally, as a second or third-line treatment, erlotinib had minimum side effects, limited to dysentery and minor skin irritations [56]. Erlotinib was also remarkably effective as a second as well as third-line treatment in combination with docetaxel and pemetrexed chemotherapeutics [68]. In fact, erlotinib emerged as the most efficient third-line therapeutic choice for patients with deteriorated performance status where chances of survival and quality of lives had prominently worsened [68]. Erlotinib as a third-line treatment not only improved the quality of lives but also the palliative symptoms of lung cancer [69,70], and the drug was well tolerated as well [71]. A notable advantage for erlotinib co-treatment was its easier affordability compared to chemotherapeutics [61].
Gefitinib
Food and Drug Administration (FDA) endorsed gefitinib in the year 2003 as a third-line treatment, based on the data obtained from two randomized phase II trials, such as Iressa Dose Evaluation in Advanced Lung Cancer-1 (IDEAL-1) and IDEAL-2 [43]. For the patients already treated with a course of chemotherapy in IDEAL-1 study, gefitinib at a 250 mg/Kg dose showed improved response rate, symptomatic recovery, better overall survival and progression-free survival [43,54]. Most importantly, side-effects of gefitinib in the IDEAL-1 study were less [54]. In the IDEAL-2 clinical trials, NSCLC patients who had already been exposed to two regimens of chemotherapy underwent gefibitinb treatment [43]. Here too, the drug prevented metastasis and showed improved response rates, even where platinum-based and docetaxel chemotherapies had failed [72]. On the contrary, in a phase III trial of around 1700 patients, 250 mg/Kg of gefitinib along with the best supportive care failed to show recovery in the overall survival [55]. These reports helped FDA to rule on the mandatory use of gefitinib in patients already pre-treated with chemotherapeutic regimens [55,61]. In a Phase III trial at USA in lung cancer patients with mild to moderate metastasis, comparison between a well-known chemotherapeutic for lung cancer, docetaxel, and gefitinib showed almost similar effects for the median overall survival and quality of life [73]. Similarly, in patients who were non-smokers, the effects of two drugs were approximately same [73]. However, patients with higher expression of EGFR gene and with a history of smoking responded more positively to gefitinib [73]. These data indicate the superiority of gefitinib in EGFR-dependent proliferation and mitosis of the lung cancer cells [73]. Notably, gefitinib was well-tolerated and its unfavorable effects were much less compared to docetaxel [73].
Although erlotinib and gefitinib were the only drugs with well-established uses as second and third-line treatments against lung cancer, these first-generation EGFR tyrosine kinase inhibitors had certain disadvantages too [74]. Most remarkable was a resistance to erlotinib or gefitinib in patients kept on continued treatment to the drugs [75-77]. Patients, who had acquired a secondary mis-sense mutation at exon 10 of EGFR-tyrosine kinase, termed as the T790M “gate-keeper mutation”, showed greater resistance to erlotinib and gefitinib [78,79]. In fact, around 50-60% of patients resistant to erlotinib and gefitinib had T790M mutation within their EGFR gene [78,79]. In addition, lung cancer owing to enhanced hepatocyte growth factor or the non-EGFR growth factors was resistant to erlotinib and gefitinib [80,81]. Hence, to evade these problems, the second-generation irreversible tyrosine kinase inhibitor, afatinib, was proposed for the treatment of lung cancer [74].
Afatinib
Afatinib performs its role as an EGFR-inhibitor by binding to cysteine-773, 805 and 803 residues of EGFR-tyrokine kinases, particularly at ErbB4 [82,83]. Afatinib also prevents dimerization of EGFR and ErbB4 and hetero-merization with HER2 [84]. Afatinib appeared effective in lung cancer patients who were resistant to erlotinib and gefitinib, and in lung cancer cell lines possessing HER2 as well as T790M mutations within EGFR gene [82,85]. Few phase I clinical studies proved the efficacy of afatinib at an oral dose of around 50 mg/day [86,87]. However, patients exposed to 50 mg/day afatinib showed signs of skin rash and stomach upsets, and hence a dose of 40 mg/day that demonstrated almost similar efficacies (as 50 mg/Kg) and with reduced adverse effects had been chosen for advanced clinical trials in lung cancer [84,88]. A co-treatment of 40 mg/day afatinib with 250 mg/m2 cetaximib in the phase I clinical trial, followed by a phase II trial in combination with 500 mg/m2 cetaximib demonstrated a 30% overall response and 75% partial response among 97 NSCLC patients [89]. The drug combinations demonstrated 30-36% partial response for both with and without T790M mutations [74]. However, around 50% stability was observed in patients with T790M secondary mutation and 30% for the ones without the mutation, indicating greater effectiveness of afatinib in patients containing the mutation [89]. Likewise, a Phase II clinical study (LUX-Lung 2 study) using afatinib demonstrated an average of around 60% objective response rate in 129 patients having the EGFR mutations [88]. Nonetheless, studies on these combination therapies of afatinib are still underway, and prophylactic dose and duration of individual drugs in the combination therapy strategy await standardization [74].
Combination therapies of afatinib with chemotherapeutics and tyrosine kinase inhibitors were found to be beneficial in Phase III LUX-Lung-5 trial [84]. For this trial, patients first underwent treatment with 40-50 mg afatinib alone for 12 weeks, and then a combination with paclitaxel [84]. The combination treatments led to enhanced progression-free survival for six months, with markedly improved overall response rate compared to single therapies [84]. For NSCLC patients, where a combination of erlotinib or gefitinib with the tyrosine kinase monoclonal antibody (cetuximab) failed, afitinib and cetuximab co-treatment were effective [82]. The erlotinib/gefitinib and cetuximab combinations were ineffective due to EGFR T790M mutation within the patients’ gene, which could be targeted by afatinib and cetuximab together [89]. Supportively, a preclinical study on EGFR T790M transgenic NSCLC mice showed remarkable recovery following afatinib and cetuximab combination treatments [82]. LUX-Lung 3 and LUX-Lung 6 phase III studies revealed that compared to platinum-based double-used chemotherapies, such as cisplatin and pemetrexed or cisplatin and gemcitabine, the progression-free survival for afatinib was four months higher [90]. Recovery with afatinib was also comparatively better in patients with deleted exon-19 or L858R mutation within EGFR gene [90]. A study showed that around 630 of 700 afatinib-treated patients had a better overall survival of three months compared to the standard chemotherapies (twenty-seven months versus 24 months respectively) [84,91]. Moreover, a Phase III clinical study on 699 squamous lung cancer patients, who had not responded to platinum-based chemotherapies, demonstrated five-month greater median progression-free survival following afatinib treatment as compared to erlotinib [91]. The study also informed that although the overall response rate was almost identical for both afatinib and erlotinib-treated patients, the disease control rate was markedly better for the former [91]. Nonetheless, despite these superior responses, the adverse impact for afatanib was at times unbearable, with reports of severe diarrhea and mouth ulcers [74].
Presently, FDA endorsed 40 mg daily oral dose of afatinib as a potent therapy for NSCLC in patients with EGFR mutation on exon 19 and 21 (L858R) [74]. Nevertheless, afatinib also bears the risk of developing resistance in lung cancer patients [74]. In vitro studies have revealed EGFR T790M alleles to be inducing afatinib resistance in lung tumors [92]. Secondly, mutations in hepatocyte growth factor receptor, MET, ErbB2, etc. failed as afatinib targets [82]. For these reasons, detailed research on this aspect of afitinib resistance is needed, both for individual as well as combinatorial treatments.
Icotinib
Concomitant with lung cancer, metastasis of the central nervous system (CNS) has become a familiar problem, both showing very poor prognoses [93]. Around 25% of NSCLC patients suffered from brain metastasis during diagnosis itself and 50% while the treatment process [93]. Patients suffering from both lung and brain metastasis survived for around six months and untreated ones for a few weeks [94]. Chemotherapeutic drugs for NSCLC failed to cross the blood-brain barrier, and erlotinib, gefitinib and afatinib could reach to the extent of extra-cranial lesions only [95]. The EGFR-TK-inhibior, icotinib, that also bears the brand-name Conmana, marketed in China, was the first drug proven to be effective for both NSCLC and brain metastasis [96]. Hence, icotinib was considered a new treatment choice for pre-treated advanced NSCLC patients [96]. A phase II clinical trial for Conmona demonstrated significant effectiveness against both NSCLC and CNS metastasis, and phase III trial (ICOGEN) verified its potency and safety as well [96]. The response rate, overall survival and progression-free survivals were around 80%, 7 and 15 months respectively [97]. These patients had no history of smoking and had not been pre-treated with chemotherapies [97]. Only a few patients underwent radiotherapy either prior to or during icotinib treatment [97]. Another phase II trial also revealed that icotinib could prevent lung and brain metastasis even in EGFR mutated patients [98,99]. The response rate and longevity for these icotinib-treated patients were higher than that for wild-type EGFR [98,99]. A phase II clinical trial at China demonstrated that icotinib together with whole-brain radiation therapy were quite potent in treating NSCLC patients having EGFR mutations and suffering from CNS metastases [97]. The median progression-free survival was much higher, twelve months compared to eight months for the co-treatment compared to icotinib only [97,100]. Other than a few reports of acneiform lesions and diarrhea, side effects of icotinib were milder and free from liver damage [100]. Patients studied for icotinib safety showed around 96% disease control rate, with an overall survival of twenty one months and progression-free survival of eleven months [100]. In general, it may be deduced that icotinib was not only potent in treating lung cancer and associated brain metastases, but was safe for advanced NSCLC patients [100]. Nonetheless, unlike gefitinib and erlotinib, laboratory and clinical trials performed for icotinib are fewer [100]. Hence, more world-wide multi-centric studies are required to provide perfect data on effectiveness and safety of icotinib [100].
Other drugs targeting EGFR
Other than erlotinib, gefitinib, afatinib and icotinib, EGFR has also been targeted by a few other drugs as a third-line of treatment [61]. The monoclonal chimeric antibody, cetuximab, has been applied in combination with other chemotherapies, such as cisplatin and vinorelbin in NSCLC pati-ents [48]. A weekly injection of cetuximab in 66 patients reduced the disease progression and enhanced overall survival by 95% [48]. Another EGFR-tyrosine kinase inhibitor, Sunitinib malate (SutentR), when treated orally in 63 patients at a dose of 60 mg/Kg for a period of four weeks daily, and then without the drug for two weeks, repeatedly for six weeks showed promising effects in patients who failed the platinum-based therapies [101]. Here too, the response rates, progression-free and overall survivals were 95% each [101]. Phase II clinical trial with a Raf/MEK/ERK inhibitor, sorafenib, at 400 mg oral dose prolonged the progression-free survival and overall survival in 52 pre-treated patients with relapsed and advanced NSCLC [102]. The toxic side effects were relatively mild for these refractory NSCLC patients [102]. Another selective MEK inhibitor, AZD6244, was effective for second and third-line treatments for lung cancer [103]. However, the success of AZD6244 could be prominently assessed when studied in ERK-mutated cases rather than EGFR mutations [61]. Inhibitors of mTOR kinases that stimulate lung metastasis have also undergone clinical trials in lung cancer patients [104]. A Phase II trial with mTOR kinase inhibitor, everolimus (RAD001), in combination with the EGFR antagonists, showed progression-free survival of 2.6-2.7 months in patients who failed two regimens of chemotherapy [104]. A serine/threonine inhibitor, enzastaurin, has also been found to be effective for persistent NSCLC [105]. An oral dose of 500 mg/day enzastaurin showed an improved overall survival of around eight months and progression-free survival of two months in patients who failed two chemotherapeutic regimens [105]. The multi-kinase inhibitor, vandetanib, when combined with docetaxel appeared effective as a second-line therapy [53]. Retinoid receptor modulators, like bexarotene, also limited lung tumor progression via targeting the nuclear receptor, RXRα, β and γ [106]. Phase II clinical trials using bexarotene in 146 patients resulted in five months overall survival for 95% patients and a survival of one year for around 25% patients [107]. Nevertheless, although these drugs have been used for laboratory experiments and clinical trials, unlike the typical EGFR inhibitors (erlotinib, gefitinib, etc.), detailed toxicity studies for the drugs are pending. Hence, these drugs await FDA endorsement.
Selective use of second and third lines of treatment
Generally, first and second-lines of therapeutic interventions are being widely used in different clinical trials for lung cancer [61]. The concept of third-line treatment is particularly applicable for critical patients suffering from severe lung metastasis [108]. However, patients who truly require the third-line of treatment are fairly difficult to identify [61]. For these treatments, EGFR mutation grade, position, patient age, cigarette smoking history, weight loss and tumor size require precise consideration [108]. A single study on the comparative role of erlotinib as a first, second and third-line treatment revealed about 27%, 45% and 28% recovery respectively [109]. Docetaxel as a therapy for second and third line treatment has been tried for 74 NSCLC clinical cases at the Princess Margaret Hospital, Toronto [68]. A comparative study among patients receiving docetaxol and tyrosine kinase inhibitors as second and third-line treatments revealed almost the same overall survival and progression-free survival [110]. Nonetheless, erlotinib appeared as a more suitable second-line and third-line therapeutic, especially in patients who failed to respond to chemotherapeutic interventions [111]. However, an open-label phase III trial (INTEREST) and another clinical trial on 477 patients administered with either erlotinib or gefitinib as second-line and third-line therapeutic demonstrated almost similar overall and progression-free survivals for the two drugs [111]. Hence, comparative data on these EGFR inhibitors as second and third-line therapies are difficult to obtain, showing comparable efficacies for both. Thus, large cohort studies that may compare the efficacies of second and third-line therapeutics in lung cancer are needed to deduce the individual treatment efficacies.
Targeting VEGF that promotes tumoregenesis and lymphangiogenesis
Angiogenic growth factors, particularly, VEGF may also be up-regulated during EGFR activation [39]. Lung cancer proliferation is stimulated during angiogenesis where VEGF has a marked contributory role promoting blood supply to the tumor site [112]. VEGF has a prominent mitogenic function in the endothelial cells that participate in inducing angiogenesis and lymphangiogenesis [112]. VEGF also triggers microvascular hyperpermeability that intricately associates or precedes angiogenesis [113]. The VEGF family has seven members, viz. VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E, VEGF-F, and PlGF, and particuarly VEGF-C promotes lung adenocarcinoma, tumor lymphangiogenesis and metastasis at the lymph nodes [114]. Transgenic mouse models revealed participation of VEGF-C in tumor lymphangiogenesis and metastasis at the lymph nodes [115]. A VEGF-D to VEGF-C ratio governs both enhanced penetration of malignant cells within lymph nodes and metastasis in lung adenocarcinoma, the [114]. VEGF-A together with VEGF-C enhance lung carcinoma progression, where VEGF-A participates more in the lymph node invasion, and VEGF-C promotes tumor enlargement close to the metastatic and angiogenic region [112]. The VEGF-A and fibrin or fibrinogenconjugates stimulate hyperpermeability via the endothelial junctions, promoting the endothelization process [112]. VEGF also participates by interfering with the functions of p53 tumor suppressor gene that inhibits angiogenesis [116]. Reduced p53 helps in up-regulating VEGF-A via bind-ing to VEGF-promoter involving the functional hypoxia inducible factors (HIF-1a) and HIF-1b [116]. Increased heterodimerization of these two isoforms of HIF-1 stimulates VEGF-A expression, promoting angiogenesis in lung tumors [116]. VEGF-A also functions via influencing the expression of the matrix metalloproteases (MMP) that disrupt the cell extracellular matrix and promote angiogenesis [117]. Via feed-back mechanism, MMPs, especially MMP-9 up-regulates VEGF-A expression as well [117]. Increased reactive oxygen species and free radical generation enhances VEGF and MMP expressions, promoting lung tumor growth [117]. VEGF suppresses apoptosis in the lung cancer cells by enhancing anti-apoptotic B-cell lymphoma-2 (BCl2) expression that further arbitrates cell proliferation [116]. BCl-2 also increases synthesis of interleukin-8 that triggers angiogenesis and contributes to enhanced proliferation of malignant lung cells [118]. Platelet aggregation and adherence to endothelia trigger the secretion of VEGF that otherwise stays dormant in the platelet granules [119]. Hence along with the aggregated platelets, VEGF promotes lung tumor cell growth and metastasis [119].
The major function of VEGF-A in enhancing endothelial permeability is via activation of the receptor TK (RTK), i.e. VEGFR1 and VEGFR2 [120]. A monoclonal antibody to VEGF, marketed as bevacizumab (Avastin; Genentech; South San Francisco, CA) is a well-known anti-angiogenic agent used for lung cancer treatment [112]. Co-treating bevacizumab with the established chemotherapies, paclitaxel and carboplatin, enhanced their responses and reduced lung metastasis phenotypes, as observed in ninety nine patients going through Phase II clinical trials [121]. However, bevacizumab treatment caused pulmonary blood loss in patients suffering from NSCLC of the squamous epithelia [121]. In addition, treatment regimens of bevacizumab, paclitaxel and carboplatin caused increased death (17 of 878 patients) reports in comparison to paclitaxel+carboplatin in phase III trials [121]. Nonetheless, because of the significant positive effects in suppressing lung metastasis, doctors continued the treatments of bevacizumab, paclitaxel and carboplatin along with other precautions and safety measures that reduced patient death rate.
Targeting SCLC
SCLC is resistant to the established chemotherapies, and new targets for its attenuation are essential [122]. An interesting aspect with SCLC is the augmented expression of neuropeptide growth factor receptors that cause increased secretion of autocrine neuropeptides [122]. The two important SCLC-induced neuropeptides are gastrin-releasing peptide (GRP; the mammalian homolog of bombesin) and arginine vasopressin (AVP) [123]. The other receptors that also undergo mild to moderate induction in lung cancer are Bombesin, Bradykinin, cholecystokinin (CCK), Endothelin-1, galanin, Neurotensin, Somatostatin, Vasopressin and VIP/PACAP [3]. The neuropeptides once bound to the receptors signal through Gq and G12/13 proteins that trigger intracellular Ca2+ release, and PKC, ERK and JNK activations [124]. The bombesin-like neuropeptides stimulates autocrine and paracrine growth of lung cancers [124]. This has been further established using bombesin antagonists and neutralizing antibodies that attenuated autocrine growth factor expressions in SCLC [124]. For CCK, the pre-form of the neuropeptides undergoes processing into the active forms, and CCK binding with CCK-A and CCK-B triggers the signalling pathways via Ca2+ mobilization in proliferative SCLC [3]. SCLC cells secrete arginine vasopressin (AVP), and increased plasma AVP levels are observed in SCLC patients [3].
The modulated expression of V2 receptor mediates AVP-induced SCLC proliferation [124]. V2 receptors are usually heterotrimeric G-protein coupled, seven transmembrane receptors that via the Gq-proteins activate phospholipase Cβ (PLCβ) [124]. The PLC pathway then activates inositol trisphosphate (IP3) that consequently causes Ca2+ channel opening [124]. This situation triggers ERK and protein kinase D or PKCμ signalling [124]. The neuropeptides also bind to G12 and G13 proteins that then activate Ras and Rho proteins [124]. These activated molecules then up-regulate JNK members of the MAP kinase family [124]. Cytoskeletal tyrosine kinase, Src and Tec/Bmx proteins, are also evident in SCLC [124]. It has been observed that Substance P derivatives of neuropeptides modulate functioning of the neuropeptide receptors in SCLC patients [122]. Substance P targets the Ca2+ mobilization mechanism, triggered by the neuropeptides AVP, CCK and GRP in SCLC cells [122]. This observation had been strongly detected in the in nude mice xenografts, where Substance P derivatives induced apoptosis in lung cancer cell lines through the G12 and G13 proteins [122].
A typical example of a direct therapy against neuroendocrine peptides and receptors is mAb 2A11 that targets GRP and prevents its receptor binding [3]. However, mAb failed in clinical trials [3]. Neutralizing antibodies to AVP and pro-vasopressin have also been recognized as therapeutic agents against SCLC mitogenesis [3]. Substance-P analogs abrogated functioning of substance P as well as GRP [3]. Studies on neuropeptide growth factors as therapeutics for SCLC are underway, where the efficacy and safety aspects are strongly considered.
Conclusion and future direction
Clinical trials in lung cancer patients have revealed that the EGFR-TK-inhibitors and monoclonal antibodies (mAbs) are effective in attenuating the progression of lung cancer. There are several reports where these therapies, individually and in combinations, have failed. Even when applied along with chemo and radiation therapies, the TK-inhibitors and mAbs have been found to be ineffective. Hence, although advances have been made using EGFR-TK-inhibitors and EGFR-targeted mAbs in treating lung cancer and metastasis, more potent therapeutic regimens are still needed. One important aspect that could solve this problem, to some extent, is via the generation of broad-spectrum growth factor inhibitors. Secondly, other than using a combination of TK inhibitors and mAbs only, inhibitors of EGFR could also be co-treated with that of VEGFR. This concept appears useful for both NSCLC and SCLC patients. However, toxicity studies on these combined treatments would ultimately decide feasibility of their usage during lung malignancy. Targeting angiogenesis and lung tumor growth using fibroblast and VEGF inhibitors could also provide a partial solution to the problem of EGFR-TK resistance. In addition, extensive research on individual patient’s tumor biology may be necessary for appropriate targeted therapy. This individual patient approach would ultimately result in improved progression-free survival and overall survival for the diverse category of lung cancer patients.
Acknowledgements
This study was supported by the grant from the National Natural Science Foundation of China (No. 81071919), Norman Bethune Program of Jilin University (No. 2012220), and the Natural Science Foundation of Jilin Province (No. 20150101151JC).
Disclosure of conflict of interest
None.
References
- 1.Ferlay J, Autier P, Boniol M, Heanue M, Colombet M, Boyle P. Estimates of the cancer incidence and mortality in europe in 2006. Ann Oncol. 2007;18:581–592. doi: 10.1093/annonc/mdl498. [DOI] [PubMed] [Google Scholar]
- 2.Boyle P. Cancer, cigarette smoking and premature death in europe: a review including the Recommendations of european cancer experts consensus meeting, helsinki, october 1996. Lung Cancer. 1997;17:1–60. doi: 10.1016/s0169-5002(97)00648-x. [DOI] [PubMed] [Google Scholar]
- 3.Hodkinson PS, Mackinnon A, Sethi T. Targeting growth factors in lung cancer. Chest. 2008;133:1209–1216. doi: 10.1378/chest.07-2680. [DOI] [PubMed] [Google Scholar]
- 4.Smythe WR American College of Chest Physicians. Treatment of stage I non-small cell lung carcinoma. Chest. 2003;123:181S–187S. [PubMed] [Google Scholar]
- 5.Carney DN, Hansen HH. Non-small-cell lung cancer--stalemate or progress? N Engl J Med. 2000;343:1261–1262. doi: 10.1056/NEJM200010263431710. [DOI] [PubMed] [Google Scholar]
- 6.Uramoto H, Tanaka F. Recurrence after surgery in patients with NSCLC. Transl Lung Cancer Res. 2014;3:242–249. doi: 10.3978/j.issn.2218-6751.2013.12.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohe Y. Chemoradiotherapy for lung cancer: current status and perspectives. Int J Clin Oncol. 2004;9:435–443. doi: 10.1007/s10147-004-0453-x. [DOI] [PubMed] [Google Scholar]
- 8.Morabito A, Carillio G, Daniele G, Piccirillo MC, Montanino A, Costanzo R, Sandomenico C, Giordano P, Normanno N, Perrone F, Rocco G, Di Maio M. Treatment of small cell lung cancer. Crit Rev Oncol Hematol. 2014;91:257–270. doi: 10.1016/j.critrevonc.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 9.De Ruysscher D, Van Meerbeeck J, Vandecasteele K, Oberije C, Pijls M, Dingemans AM, Reymen B, van Baardwijk A, Wanders R, Lammering G, Lambin P, De Neve W. Radiation-induced oesophagitis in lung cancer patients. Is susceptibility for neutropenia a risk factor? Strahlenther Onkol. 2012;188:564–567. doi: 10.1007/s00066-012-0098-z. [DOI] [PubMed] [Google Scholar]
- 10.Schmid T, Papachristofilou A, Zimmermann F. [Modern radiotherapeutic concept-stereotactic, adjuvant, palliative] . Ther Umsch. 2012;69:420–428. doi: 10.1024/0040-5930/a000309. [DOI] [PubMed] [Google Scholar]
- 11.Kim HK, Lee YJ, Han KN, Choi YH. Pulmonary function changes over 1 year after lobectomy in lung cancer. Respir Care. 2016;61:376–382. doi: 10.4187/respcare.04284. [DOI] [PubMed] [Google Scholar]
- 12.Fong KM, Sekido Y, Minna JD. Molecular pathogenesis of lung cancer. J Thorac Cardiovasc Surg. 1999;118:1136–1152. doi: 10.1016/S0022-5223(99)70121-2. [DOI] [PubMed] [Google Scholar]
- 13.Carcereny E, Moran T, Capdevila L, Cros S, Vila L, de Los Llanos Gil M, Remon J, Rosell R. The epidermal growth factor receptor (EGRF) in lung cancer. Transl Respir Med. 2015;3:1. doi: 10.1186/s40247-015-0013-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woll PJ. New perspectives in lung cancer. 2. Growth factors and lung cancer. Thorax. 1991;46:924–929. doi: 10.1136/thx.46.12.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ciardiello F, Tortora G. Interactions between the epidermal growth factor receptor and type I protein kinase a: biological significance and therapeutic implications. Clin Cancer Res. 1998;4:821–828. [PubMed] [Google Scholar]
- 16.Marmor MD, Skaria KB, Yarden Y. Signal transduction and oncogenesis by ErbB/HER receptors. Int J Radiat Oncol Biol Phys. 2004;58:903–913. doi: 10.1016/j.ijrobp.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Zandi R, Larsen AB, Andersen P, Stockhausen MT, Poulsen HS. Mechanisms for oncogenic activation of the epidermal growth factor receptor. Cell Signal. 2007;19:2013–2023. doi: 10.1016/j.cellsig.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka T, Matsuoka M, Sutani A, Gemma A, Maemondo M, Inoue A, Okinaga S, Nagashima M, Oizumi S, Uematsu K, Nagai Y, Moriyama G, Miyazawa H, Ikebuchi K, Morita S, Kobayashi K, Hagiwara K. Frequency of and variables associated with the EGFR mutation and its subtypes. Int J Cancer. 2010;126:651–655. doi: 10.1002/ijc.24746. [DOI] [PubMed] [Google Scholar]
- 19.Gahr S, Stoehr R, Geissinger E, Ficker JH, Brueckl WM, Gschwendtner A, Gattenloehner S, Fuchs FS, Schulz C, Rieker RJ, Hartmann A, Ruemmele P, Dietmaier W. EGFR mutational status in a large series of caucasian european NSCLC patients: data from daily practice. Br J Cancer. 2013;109:1821–1828. doi: 10.1038/bjc.2013.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakuma Y, Matsukuma S, Yoshihara M, Nakamura Y, Noda K, Nakayama H, Kameda Y, Tsuchiya E, Miyagi Y. Distinctive evaluation of nonmucinous and mucinous subtypes of bronchioloalveolar carcinomas in EGFR and K-ras gene-mutation analyses for Japanese lung adenocarcinomas: confirmation of the correlations with histologic subtypes and gene mutations. Am J Clin Pathol. 2007;128:100–108. doi: 10.1309/WVXFGAFLAUX48DU6. [DOI] [PubMed] [Google Scholar]
- 21.Tam IY, Chung LP, Suen WS, Wang E, Wong MC, Ho KK, Lam WK, Chiu SW, Girard L, Minna JD, Gazdar AF, Wong MP. Distinct epidermal growth factor receptor and KRAS mutation patterns in non-small cell lung cancer patients with different tobacco exposure and clinicopathologic features. Clin Cancer Res. 2006;12:1647–1653. doi: 10.1158/1078-0432.CCR-05-1981. [DOI] [PubMed] [Google Scholar]
- 22.Marchetti A, Martella C, Felicioni L, Barassi F, Salvatore S, Chella A, Camplese PP, Iarussi T, Mucilli F, Mezzetti A, Cuccurullo F, Sacco R, Buttitta F. EGFR mutations in non-small-cell lung cancer: analysis of a large series of cases and development of a rapid and sensitive method for diagnostic screening with potential implications on pharmacologic treatment. J. Clin. Oncol. 2005;23:857–865. doi: 10.1200/JCO.2005.08.043. [DOI] [PubMed] [Google Scholar]
- 23.Yoshizawa A, Sumiyoshi S, Sonobe M, Kobayashi M, Fujimoto M, Kawakami F, Tsuruyama T, Travis WD, Date H, Haga H. Validation of the IASLC/ATS/ERS lung adenocarcinoma classification for prognosis and association with EGFR and KRAS gene mutations: analysis of 440 Japanese patients. J Thorac Oncol. 2013;8:52–61. doi: 10.1097/JTO.0b013e3182769aa8. [DOI] [PubMed] [Google Scholar]
- 24.Sun PL, Seol H, Lee HJ, Yoo SB, Kim H, Xu X, Jheon S, Lee CT, Lee JS, Chung JH. High incidence of EGFR mutations in Korean men smokers with no intratumoral heterogeneity of lung adenocarcinomas: correlation with histologic subtypes, EGFR/TTF-1 expressions, and clinical features. J Thorac Oncol. 2012;7:323–330. doi: 10.1097/JTO.0b013e3182381515. [DOI] [PubMed] [Google Scholar]
- 25.Prabhakar CN. Epidermal growth factor receptor in non-small cell lung cancer. Transl Lung Cancer Res. 2015;4:110–118. doi: 10.3978/j.issn.2218-6751.2015.01.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krause DS, Van Etten RA. Tyrosine kinases as targets for cancer therapy. N Engl J Med. 2005;353:172–187. doi: 10.1056/NEJMra044389. [DOI] [PubMed] [Google Scholar]
- 27.Lee DC, Fenton SE, Berkowitz EA, Hissong MA. Transforming growth factor alpha: expression, regulation, and biological activities. Pharmacol Rev. 1995;47:51–85. [PubMed] [Google Scholar]
- 28.Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141:1117–1134. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yarden Y. The EGFR family and its ligands in human cancer. signalling mechanisms and therapeutic opportunities. Eur J Cancer. 2001;37(Suppl 4):S3–8. doi: 10.1016/s0959-8049(01)00230-1. [DOI] [PubMed] [Google Scholar]
- 30.Cohen S, Carpenter G, King L Jr. Epidermal growth factor-receptor-protein kinase interactions. Prog Clin Biol Res. 1981;66:557–567. [PubMed] [Google Scholar]
- 31.Siegelin MD, Borczuk AC. Epidermal growth factor receptor mutations in lung adenocarcinoma. Lab Invest. 2014;94:129–137. doi: 10.1038/labinvest.2013.147. [DOI] [PubMed] [Google Scholar]
- 32.Busser B, Sancey L, Josserand V, Niang C, Khochbin S, Favrot MC, Coll JL, Hurbin A. Amphiregulin promotes resistance to gefitinib in nonsmall cell lung cancer cells by regulating Ku70 acetylation. Mol Ther. 2010;18:536–543. doi: 10.1038/mt.2009.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsao MS, Zhu H, Viallet J. Autocrine growth loop of the epidermal growth factor receptor in normal and immortalized human bronchial epithelial cells. Exp Cell Res. 1996;223:268–273. doi: 10.1006/excr.1996.0081. [DOI] [PubMed] [Google Scholar]
- 34.Richardson CM, Sharma RA, Cox G, O’Byrne KJ. Epidermal growth factor receptors and cyclooxygenase-2 in the pathogenesis of non-small cell lung cancer: potential targets for chemoprevention and systemic therapy. Lung Cancer. 2003;39:1–13. doi: 10.1016/s0169-5002(02)00382-3. [DOI] [PubMed] [Google Scholar]
- 35.Shoelson SE. SH2 and PTB domain interactions in tyrosine kinase signal transduction. Curr Opin Chem Biol. 1997;1:227–234. doi: 10.1016/s1367-5931(97)80014-2. [DOI] [PubMed] [Google Scholar]
- 36.Cox G, Steward WP, O’Byrne KJ. The plasmin cascade and matrix metalloproteinases in non-small cell lung cancer. Thorax. 1999;54:169–179. doi: 10.1136/thx.54.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cox G, Walker RA, Andi A, Steward WP, O’Byrne KJ. Prognostic significance of platelet and microvessel counts in operable non-small cell lung cancer. Lung Cancer. 2000;29:169–177. doi: 10.1016/s0169-5002(00)00124-0. [DOI] [PubMed] [Google Scholar]
- 38.Giatromanolaki A, Koukourakis M, O’Byrne K, Fox S, Whitehouse R, Talbot DC, Harris AL, Gatter KC. Prognostic value of angiogenesis in operable non-small cell lung cancer. J Pathol. 1996;179:80–88. doi: 10.1002/(SICI)1096-9896(199605)179:1<80::AID-PATH547>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 39.Bruns CJ, Solorzano CC, Harbison MT, Ozawa S, Tsan R, Fan D, Abbruzzese J, Traxler P, Buchdunger E, Radinsky R, Fidler IJ. Blockade of the epidermal growth factor receptor signaling by a novel tyrosine kinase inhibitor leads to apoptosis of endothelial cells and therapy of human pancreatic carcinoma. Cancer Res. 2000;60:2926–2935. [PubMed] [Google Scholar]
- 40.Huang SM, Bock JM, Harari PM. Epidermal growth factor receptor blockade with C225 modulates proliferation, apoptosis, and radiosensitivity in squamous cell carcinomas of the head and neck. Cancer Res. 1999;59:1935–1940. [PubMed] [Google Scholar]
- 41.Perrotte P, Matsumoto T, Inoue K, Kuniyasu H, Eve BY, Hicklin DJ, Radinsky R, Dinney CP. Anti-epidermal growth factor receptor antibody C225 inhibits angiogenesis in human transitional cell carcinoma growing orthotopically in nude mice. Clin Cancer Res. 1999;5:257–265. [PubMed] [Google Scholar]
- 42.Langer CJ. Emerging role of epidermal growth factor receptor inhibition in therapy for advanced malignancy: focus on NSCLC. Int J Radiat Oncol Biol Phys. 2004;58:991–1002. doi: 10.1016/j.ijrobp.2003.09.099. [DOI] [PubMed] [Google Scholar]
- 43.Kris MG, Natale RB, Herbst RS, Lynch TJ Jr, Prager D, Belani CP, Schiller JH, Kelly K, Spiridonidis H, Sandler A, Albain KS, Cella D, Wolf MK, Averbuch SD, Ochs JJ, Kay AC. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA. 2003;290:2149–2158. doi: 10.1001/jama.290.16.2149. [DOI] [PubMed] [Google Scholar]
- 44.Johnson DH, Fehrenbacher L, Novotny WF, Herbst RS, Nemunaitis JJ, Jablons DM, Langer CJ, DeVore RF 3rd, Gaudreault J, Damico LA, Holmgren E, Kabbinavar F. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J. Clin. Oncol. 2004;22:2184–2191. doi: 10.1200/JCO.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 45.Raben D, Helfrich B, Chan DC, Ciardiello F, Zhao L, Franklin W, Baron AE, Zeng C, Johnson TK, Bunn PA Jr. The effects of cetuximab alone and in combination with radiation and/or chemotherapy in lung cancer. Clin Cancer Res. 2005;11:795–805. [PubMed] [Google Scholar]
- 46.Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, Naoki K, Sasaki H, Fujii Y, Eck MJ, Sellers WR, Johnson BE, Meyerson M. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 47.Shigematsu H, Lin L, Takahashi T, Nomura M, Suzuki M, Wistuba II, Fong KM, Lee H, Toyooka S, Shimizu N, Fujisawa T, Feng Z, Roth JA, Herz J, Minna JD, Gazdar AF. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005;97:339–346. doi: 10.1093/jnci/dji055. [DOI] [PubMed] [Google Scholar]
- 48.Hanna N, Lilenbaum R, Ansari R, Lynch T, Govindan R, Janne PA, Bonomi P. Phase II trial of cetuximab in patients with previously treated non-small-cell lung cancer. J. Clin. Oncol. 2006;24:5253–5258. doi: 10.1200/JCO.2006.08.2263. [DOI] [PubMed] [Google Scholar]
- 49.Robert F, Blumenschein G, Herbst RS, Fossella FV, Tseng J, Saleh MN, Needle M. Phase I/IIa study of cetuximab with gemcitabine plus carboplatin in patients with chemotherapy-naive advanced non-small-cell lung cancer. J. Clin. Oncol. 2005;23:9089–9096. doi: 10.1200/JCO.2004.00.1438. [DOI] [PubMed] [Google Scholar]
- 50.Thienelt CD, Bunn PA Jr, Hanna N, Rosenberg A, Needle MN, Long ME, Gustafson DL, Kelly K. Multicenter phase I/II study of cetuximab with paclitaxel and carboplatin in untreated patients with stage IV non-small-cell lung cancer. J. Clin. Oncol. 2005;23:8786–8793. doi: 10.1200/JCO.2005.03.1997. [DOI] [PubMed] [Google Scholar]
- 51.Rosell R, Robinet G, Szczesna A, Ramlau R, Constenla M, Mennecier BC, Pfeifer W, O’Byrne KJ, Welte T, Kolb R, Pirker R, Chemaissani A, Perol M, Ranson MR, Ellis PA, Pilz K, Reck M. Randomized phase II study of cetuximab plus cisplatin/vinorelbine compared with cisplatin/vinorelbine alone as first-line therapy in EGFR-expressing advanced non-small-cell lung cancer. Ann Oncol. 2008;19:362–369. doi: 10.1093/annonc/mdm474. [DOI] [PubMed] [Google Scholar]
- 52.Hidalgo M, Siu LL, Nemunaitis J, Rizzo J, Hammond LA, Takimoto C, Eckhardt SG, Tolcher A, Britten CD, Denis L, Ferrante K, Von Hoff DD, Silberman S, Rowinsky EK. Phase I and pharmacologic study of OSI-774, an epidermal growth factor receptor tyrosine kinase inhibitor, in patients with advanced solid malignancies. J. Clin. Oncol. 2001;19:3267–3279. doi: 10.1200/JCO.2001.19.13.3267. [DOI] [PubMed] [Google Scholar]
- 53.Herbst RS, Maddox AM, Rothenberg ML, Small EJ, Rubin EH, Baselga J, Rojo F, Hong WK, Swaisland H, Averbuch SD, Ochs J, LoRusso PM. Selective oral epidermal growth factor receptor tyrosine kinase inhibitor ZD1839 is generally well-tolerated and has activity in non-small-cell lung cancer and other solid tumors: results of a phase I trial. J. Clin. Oncol. 2002;20:3815–3825. doi: 10.1200/JCO.2002.03.038. [DOI] [PubMed] [Google Scholar]
- 54.Fukuoka M, Yano S, Giaccone G, Tamura T, Nakagawa K, Douillard JY, Nishiwaki Y, Vansteenkiste J, Kudoh S, Rischin D, Eek R, Horai T, Noda K, Takata I, Smit E, Averbuch S, Macleod A, Feyereislova A, Dong RP, Baselga J. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) [corrected] . J. Clin. Oncol. 2003;21:2237–2246. doi: 10.1200/JCO.2003.10.038. [DOI] [PubMed] [Google Scholar]
- 55.Thatcher N, Chang A, Parikh P, Rodrigues Pereira J, Ciuleanu T, von Pawel J, Thongprasert S, Tan EH, Pemberton K, Archer V, Carroll K. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (iressa survival evaluation in lung cancer) Lancet. 2005;366:1527–1537. doi: 10.1016/S0140-6736(05)67625-8. [DOI] [PubMed] [Google Scholar]
- 56.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S, Smylie M, Martins R, van Kooten M, Dediu M, Findlay B, Tu D, Johnston D, Bezjak A, Clark G, Santabárbara P, Seymour L National Cancer Institute of Canada Clinical Trials Group. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 57.Tokumo M, Toyooka S, Kiura K, Shigematsu H, Tomii K, Aoe M, Ichimura K, Tsuda T, Yano M, Tsukuda K, Tabata M, Ueoka H, Tanimoto M, Date H, Gazdar AF, Shimizu N. The relationship between epidermal growth factor receptor mutations and clinicopathologic features in non-small cell lung cancers. Clin Cancer Res. 2005;11:1167–1173. [PubMed] [Google Scholar]
- 58.Kobayashi S, Boggon TJ, Dayaram T, Janne PA, Kocher O, Meyerson M, Johnson BE, Eck MJ, Tenen DG, Halmos B. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352:786–792. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- 59.Kobayashi S, Ji H, Yuza Y, Meyerson M, Wong KK, Tenen DG, Halmos B. An alternative inhibitor overcomes resistance caused by a mutation of the epidermal growth factor receptor. Cancer Res. 2005;65:7096–7101. doi: 10.1158/0008-5472.CAN-05-1346. [DOI] [PubMed] [Google Scholar]
- 60.Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, Singh B, Heelan R, Rusch V, Fulton L, Mardis E, Kupfer D, Wilson R, Kris M, Varmus H. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A. 2004;101:13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Syrigos KN, Saif MW, Karapanagiotou EM, Oikonomopoulos G, De Marinis F. The need for third-line treatment in non-small cell lung cancer: an overview of new options. Anticancer Res. 2011;31:649–659. [PubMed] [Google Scholar]
- 62.Perez-Soler R, Chachoua A, Hammond LA, Rowinsky EK, Huberman M, Karp D, Rigas J, Clark GM, Santabarbara P, Bonomi P. Determinants of tumor response and survival with erlotinib in patients with non--small-cell lung cancer. J. Clin. Oncol. 2004;22:3238–3247. doi: 10.1200/JCO.2004.11.057. [DOI] [PubMed] [Google Scholar]
- 63.Clark GM, Zborowski DM, Santabarbara P, Ding K, Whitehead M, Seymour L, Shepherd FA National Cancer Institute of Canada Clinical Trials Group. Smoking history and epidermal growth factor receptor expression as predictors of survival benefit from erlotinib for patients with non-small-cell lung cancer in the national cancer institute of canada clinical trials group study BR. 21. Clin Lung Cancer. 2006;7:389–394. doi: 10.3816/clc.2006.n.022. [DOI] [PubMed] [Google Scholar]
- 64.Hensing TA, Schell MJ, Lee JH, Socinski MA. Factors associated with the likelihood of receiving second line therapy for advanced non-small cell lung cancer. Lung Cancer. 2005;47:253–259. doi: 10.1016/j.lungcan.2004.07.040. [DOI] [PubMed] [Google Scholar]
- 65.Rossi D, Dennetta D, Ugolini M, Catalano V, Alessandroni P, Giordani P, Baldelli AM, Casadei V, Graziano F, Luzi Fedeli S. Activity and safety of erlotinib as second-and third-line treatment in elderly patients with advanced non-small cell lung cancer: a phase II trial. Target Oncol. 2010;5:231–235. doi: 10.1007/s11523-010-0163-4. [DOI] [PubMed] [Google Scholar]
- 66.Lyseng-Williamson KA. Erlotinib: a pharmacoeconomic review of its use in advanced non-small cell lung cancer. Pharmacoeconomics. 2010;28:75–92. doi: 10.2165/10482880-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 67.Zhu CQ, da Cunha Santos G, Ding K, Sakurada A, Cutz JC, Liu N, Zhang T, Marrano P, Whitehead M, Squire JA, Kamel-Reid S, Seymour L, Shepherd FA, Tsao MS National Cancer Institute of Canada Clinical Trials Group Study BR.21. Symptom improvement in lung cancer patients treated with erlotinib: quality of life analysis of the national cancer institute of canada clinical trials group study BR.21. J. Clin. Oncol. 2006;24:3831–3837. doi: 10.1200/JCO.2006.05.8073. [DOI] [PubMed] [Google Scholar]
- 68.Ng R, Loreto M, Lee R, Leighl NB. Brief report: retrospective review of efficacy of erlotinib or gefitinib compared to docetaxel as subsequent line therapy in advanced non-small cell lung cancer (NSCLC) following failure of platinum-based chemotherapy. Lung Cancer. 2008;61:262–265. doi: 10.1016/j.lungcan.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 69.Dancey J, Shepherd FA, Gralla RJ, Kim YS. Quality of life assessment of second-line docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy: results of a prospective, randomized phase III trial. Lung Cancer. 2004;43:183–194. doi: 10.1016/j.lungcan.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 70.De Marinis F, Pereira JR, Fossella F, Perry MC, Reck M, Salzberg M, Jassem J, Peterson P, Liepa AM, Moore P, Gralla RJ. Lung cancer symptom scale outcomes in relation to standard efficacy measures: an analysis of the phase III study of pemetrexed versus docetaxel in advanced non-small cell lung cancer. J Thorac Oncol. 2008;3:30–36. doi: 10.1097/JTO.0b013e31815e8b48. [DOI] [PubMed] [Google Scholar]
- 71.Cappuzzo F, Ciuleanu T, Stelmakh L, Cicenas S, Szczesna A, Juhasz E, Esteban E, Molinier O, Brugger W, Melezinek I, Klingelschmitt G, Klughammer B, Giaccone G SATURN investigators. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2010;11:521–529. doi: 10.1016/S1470-2045(10)70112-1. [DOI] [PubMed] [Google Scholar]
- 72.Cohen MH, Williams GA, Sridhara R, Chen G, McGuinn WD Jr, Morse D, Abraham S, Rahman A, Liang C, Lostritto R, Baird A, Pazdur R. United states food and drug administration drug approval summary: gefitinib (ZD1839; Iressa) tablets. Clin Cancer Res. 2004;10:1212–1218. doi: 10.1158/1078-0432.ccr-03-0564. [DOI] [PubMed] [Google Scholar]
- 73.Kim ES, Hirsh V, Mok T, Socinski MA, Gervais R, Wu YL, Li LY, Watkins CL, Sellers MV, Lowe ES, Sun Y, Liao ML, Osterlind K, Reck M, Armour AA, Shepherd FA, Lippman SM, Douillard JY. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet. 2008;372:1809–1818. doi: 10.1016/S0140-6736(08)61758-4. [DOI] [PubMed] [Google Scholar]
- 74.Joshi M, Rizvi SM, Belani CP. Afatinib for the treatment of metastatic non-small cell lung cancer. Cancer Manag Res. 2015;7:75–82. doi: 10.2147/CMAR.S51808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, Nishiwaki Y, Ohe Y, Yang JJ, Chewaskulyong B, Jiang H, Duffield EL, Watkins CL, Armour AA, Fukuoka M. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 76.Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I, Fujita Y, Okinaga S, Hirano H, Yoshimori K, Harada T, Ogura T, Ando M, Miyazawa H, Tanaka T, Saijo Y, Hagiwara K, Morita S, Nukiwa T North-East Japan Study Group. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 77.Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, Asami K, Katakami N, Takada M, Yoshioka H, Shibata K, Kudoh S, Shimizu E, Saito H, Toyooka S, Nakagawa K, Fukuoka M West Japan Oncology Group. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–128. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 78.Arcila ME, Oxnard GR, Nafa K, Riely GJ, Solomon SB, Zakowski MF, Kris MG, Pao W, Miller VA, Ladanyi M. Rebiopsy of lung cancer patients with acquired resistance to EGFR inhibitors and enhanced detection of the T790M mutation using a locked nucleic acid-based assay. Clin Cancer Res. 2011;17:1169–1180. doi: 10.1158/1078-0432.CCR-10-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yu HA, Arcila ME, Rekhtman N, Sima CS, Zakowski MF, Pao W, Kris MG, Miller VA, Ladanyi M, Riely GJ. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 2013;19:2240–2247. doi: 10.1158/1078-0432.CCR-12-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, Bergethon K, Shaw AT, Gettinger S, Cosper AK, Akhavanfard S, Heist RS, Temel J, Christensen JG, Wain JC, Lynch TJ, Vernovsky K, Mark EJ, Lanuti M, Iafrate AJ, Mino-Kenudson M, Engelman JA. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3:75ra26. doi: 10.1126/scitranslmed.3002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li T, Kung HJ, Mack PC, Gandara DR. Genotyping and genomic profiling of non-small-cell lung cancer: implications for current and future therapies. J. Clin. Oncol. 2013;31:1039–1049. doi: 10.1200/JCO.2012.45.3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen X, Zhu Q, Zhu L, Pei D, Liu Y, Yin Y, Schuler M, Shu Y. Clinical perspective of afatinib in non-small cell lung cancer. Lung Cancer. 2013;81:155–161. doi: 10.1016/j.lungcan.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 83.Solca F, Dahl G, Zoephel A, Bader G, Sanderson M, Klein C, Kraemer O, Himmelsbach F, Haaksma E, Adolf GR. Target binding properties and cellular activity of afatinib (BIBW 2992), an irreversible ErbB family blocker. J Pharmacol Exp Ther. 2012;343:342–350. doi: 10.1124/jpet.112.197756. [DOI] [PubMed] [Google Scholar]
- 84.Yap TA, Popat S. Toward precision medicine with next-generation EGFR inhibitors in non-small-cell lung cancer. Pharmgenomics Pers Med. 2014;7:285–295. doi: 10.2147/PGPM.S55339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li D, Ambrogio L, Shimamura T, Kubo S, Takahashi M, Chirieac LR, Padera RF, Shapiro GI, Baum A, Himmelsbach F, Rettig WJ, Meyerson M, Solca F, Greulich H, Wong KK. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene. 2008;27:4702–4711. doi: 10.1038/onc.2008.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Murakami H, Tamura T, Takahashi T, Nokihara H, Naito T, Nakamura Y, Nishio K, Seki Y, Sarashina A, Shahidi M, Yamamoto N. Phase I study of continuous afatinib (BIBW 2992) in patients with advanced non-small cell lung cancer after prior chemotherapy/erlotinib/gefitinib (LUX-Lung 4) Cancer Chemother Pharmacol. 2012;69:891–899. doi: 10.1007/s00280-011-1738-1. [DOI] [PubMed] [Google Scholar]
- 87.Yap TA, Vidal L, Adam J, Stephens P, Spicer J, Shaw H, Ang J, Temple G, Bell S, Shahidi M, Uttenreuther-Fischer M, Stopfer P, Futreal A, Calvert H, de Bono JS, Plummer R. Phase I trial of the irreversible EGFR and HER2 kinase inhibitor BIBW 2992 in patients with advanced solid tumors. J. Clin. Oncol. 2010;28:3965–3972. doi: 10.1200/JCO.2009.26.7278. [DOI] [PubMed] [Google Scholar]
- 88.Yang JC, Wu YL, Schuler M, Sebastian M, Popat S, Yamamoto N, Zhou C, Hu CP, O’Byrne K, Feng J, Lu S, Huang Y, Geater SL, Lee KY, Tsai CM, Gorbunova V, Hirsh V, Bennouna J, Orlov S, Mok T, Boyer M, Su WC, Lee KH, Kato T, Massey D, Shahidi M, Zazulina V, Sequist LV. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol. 2015;16:141–151. doi: 10.1016/S1470-2045(14)71173-8. [DOI] [PubMed] [Google Scholar]
- 89.Ribeiro Gomes J, Cruz MR. Combination of afatinib with cetuximab in patients with EGFR-mutant non-small-cell lung cancer resistant to EGFR inhibitors. Onco Targets Ther. 2015;8:1137–1142. doi: 10.2147/OTT.S75388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sequist LV, Yang JC, Yamamoto N, O’Byrne K, Hirsh V, Mok T, Geater SL, Orlov S, Tsai CM, Boyer M, Su WC, Bennouna J, Kato T, Gorbunova V, Lee KH, Shah R, Massey D, Zazulina V, Shahidi M, Schuler M. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J. Clin. Oncol. 2013;31:3327–3334. doi: 10.1200/JCO.2012.44.2806. [DOI] [PubMed] [Google Scholar]
- 91.Melosky B. Review of EGFR TKIs in metastatic NSCLC, including ongoing trials. Front Oncol. 2014;4:244. doi: 10.3389/fonc.2014.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kim Y, Ko J, Cui Z, Abolhoda A, Ahn JS, Ou SH, Ahn MJ, Park K. The EGFR T790M mutation in acquired resistance to an irreversible second-generation EGFR inhibitor. Mol Cancer Ther. 2012;11:784–791. doi: 10.1158/1535-7163.MCT-11-0750. [DOI] [PubMed] [Google Scholar]
- 93.Villano JL, Durbin EB, Normandeau C, Thakkar JP, Moirangthem V, Davis FG. Incidence of brain metastasis at initial presentation of lung cancer. Neuro Oncol. 2015;17:122–128. doi: 10.1093/neuonc/nou099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Langer CJ, Mehta MP. Current management of brain metastases, with a focus on systemic options. J. Clin. Oncol. 2005;23:6207–6219. doi: 10.1200/JCO.2005.03.145. [DOI] [PubMed] [Google Scholar]
- 95.Fekrazad MH, Ravindranathan M, Jones DV Jr. Response of intracranial metastases to erlotinib therapy. J. Clin. Oncol. 2007;25:5024–5026. doi: 10.1200/JCO.2007.13.3751. [DOI] [PubMed] [Google Scholar]
- 96.Shi Y, Zhang L, Liu X, Zhou C, Zhang L, Zhang S, Wang D, Li Q, Qin S, Hu C, Zhang Y, Chen J, Cheng Y, Feng J, Zhang H, Song Y, Wu YL, Xu N, Zhou J, Luo R, Bai C, Jin Y, Liu W, Wei Z, Tan F, Wang Y, Ding L, Dai H, Jiao S, Wang J, Liang L, Zhang W, Sun Y. Icotinib versus gefitinib in previously treated advanced non-small-cell lung cancer (ICOGEN): a randomised, double-blind phase 3 non-inferiority trial. Lancet Oncol. 2013;14:953–961. doi: 10.1016/S1470-2045(13)70355-3. [DOI] [PubMed] [Google Scholar]
- 97.Fan Y, Huang Z, Fang L, Miao L, Gong L, Yu H, Yang H, Lei T, Mao W. A phase II study of icotinib and whole-brain radiotherapy in Chinese patients with brain metastases from non-small cell lung cancer. Cancer Chemother Pharmacol. 2015;76:517–523. doi: 10.1007/s00280-015-2760-5. [DOI] [PubMed] [Google Scholar]
- 98.Eichler AF, Kahle KT, Wang DL, Joshi VA, Willers H, Engelman JA, Lynch TJ, Sequist LV. EGFR mutation status and survival after diagnosis of brain metastasis in nonsmall cell lung cancer. Neuro Oncol. 2010;12:1193–1199. doi: 10.1093/neuonc/noq076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Porta R, Sanchez-Torres JM, Paz-Ares L, Massuti B, Reguart N, Mayo C, Lianes P, Queralt C, Guillem V, Salinas P, Catot S, Isla D, Pradas A, Gurpide A, de Castro J, Polo E, Puig T, Taron M, Colomer R, Rosell R. Brain metastases from lung cancer responding to erlotinib: the importance of EGFR mutation. Eur Respir J. 2011;37:624–631. doi: 10.1183/09031936.00195609. [DOI] [PubMed] [Google Scholar]
- 100.Xu J, Liu X, Yang S, Zhang X, Shi Y. Efficacy and safety of icotinib in patients with brain metastases from lung adenocarcinoma. Onco Targets Ther. 2016;9:2911–2917. doi: 10.2147/OTT.S102472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Socinski MA, Novello S, Brahmer JR, Rosell R, Sanchez JM, Belani CP, Govindan R, Atkins JN, Gillenwater HH, Pallares C, Tye L, Selaru P, Chao RC, Scagliotti GV. Multicenter, phase II trial of sunitinib in previously treated, advanced non-small-cell lung cancer. J. Clin. Oncol. 2008;26:650–656. doi: 10.1200/JCO.2007.13.9303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Blumenschein GR Jr, Gatzemeier U, Fossella F, Stewart DJ, Cupit L, Cihon F, O’Leary J, Reck M. Phase II, multicenter, uncontrolled trial of single-agent sorafenib in patients with relapsed or refractory, advanced non-small-cell lung cancer. J. Clin. Oncol. 2009;27:4274–4280. doi: 10.1200/JCO.2009.22.0541. [DOI] [PubMed] [Google Scholar]
- 103.Hainsworth JD, Cebotaru CL, Kanarev V, Ciuleanu TE, Damyanov D, Stella P, Ganchev H, Pover G, Morris C, Tzekova V. A phase II, open-label, randomized study to assess the efficacy and safety of AZD6244 (ARRY-142886) versus pemetrexed in patients with non-small cell lung cancer who have failed one or two prior chemotherapeutic regimens. J Thorac Oncol. 2010;5:1630–1636. doi: 10.1097/JTO.0b013e3181e8b3a3. [DOI] [PubMed] [Google Scholar]
- 104.Soria JC, Shepherd FA, Douillard JY, Wolf J, Giaccone G, Crino L, Cappuzzo F, Sharma S, Gross SH, Dimitrijevic S, Di Scala L, Gardner H, Nogova L, Papadimitrakopoulou V. Efficacy of everolimus (RAD001) in patients with advanced NSCLC previously treated with chemotherapy alone or with chemotherapy and EGFR inhibitors. Ann Oncol. 2009;20:1674–1681. doi: 10.1093/annonc/mdp060. [DOI] [PubMed] [Google Scholar]
- 105.Oh Y, Herbst RS, Burris H, Cleverly A, Musib L, Lahn M, Bepler G. Enzastaurin, an oral serine/threonine kinase inhibitor, as second-or third-line therapy of non-small-cell lung cancer. J. Clin. Oncol. 2008;26:1135–1141. doi: 10.1200/JCO.2007.14.3685. [DOI] [PubMed] [Google Scholar]
- 106.Xu XC, Sozzi G, Lee JS, Lee JJ, Pastorino U, Pilotti S, Kurie JM, Hong WK, Lotan R. Suppression of retinoic acid receptor beta in non-small-cell lung cancer in vivo: implications for lung cancer development. J Natl Cancer Inst. 1997;89:624–629. doi: 10.1093/jnci/89.9.624. [DOI] [PubMed] [Google Scholar]
- 107.Govindan R, Crowley J, Schwartzberg L, Kennedy P, Williams C, Ekstrand B, Sandler A, Jaunakais D, Bolejack V, Ghalie R. Phase II trial of bexarotene capsules in patients with advanced non-small-cell lung cancer after failure of two or more previous therapies. J. Clin. Oncol. 2006;24:4848–4854. doi: 10.1200/JCO.2006.07.7404. [DOI] [PubMed] [Google Scholar]
- 108.Girard N, Jacoulet P, Gainet M, Elleuch R, Pernet D, Depierre A, Dalphin JC, Westeel V. Third-line chemotherapy in advanced non-small cell lung cancer: identifying the candidates for routine practice. J Thorac Oncol. 2009;4:1544–1549. doi: 10.1097/JTO.0b013e3181bbf223. [DOI] [PubMed] [Google Scholar]
- 109.Ailawadhi S, Derby L, Natarajan R, Fetterly G, Reid M, Ramnath N. Erlotinib for metastatic non-small-cell lung cancer: first-, second-or third-line setting-does it matter? A single-institution experience. Oncology. 2009;76:85–90. doi: 10.1159/000187427. [DOI] [PubMed] [Google Scholar]
- 110.Popat S, Barbachano Y, Ashley S, Norton A, O’Brien M. Erlotinib, docetaxel, and gefitinib in sequential cohorts with relapsed non-small cell lung cancer. Lung Cancer. 2008;59:227–231. doi: 10.1016/j.lungcan.2007.08.027. [DOI] [PubMed] [Google Scholar]
- 111.Ramalingam S, Sandler AB. Salvage therapy for advanced non-small cell lung cancer: factors influencing treatment selection. Oncologist. 2006;11:655–665. doi: 10.1634/theoncologist.11-6-655. [DOI] [PubMed] [Google Scholar]
- 112.Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev. 2004;56:549–580. doi: 10.1124/pr.56.4.3. [DOI] [PubMed] [Google Scholar]
- 113.Nor JE, Christensen J, Liu J, Peters M, Mooney DJ, Strieter RM, Polverini PJ. Up-Regulation of Bcl-2 in microvascular endothelial cells enhances intratumoral angiogenesis and accelerates tumor growth. Cancer Res. 2001;61:2183–2188. [PubMed] [Google Scholar]
- 114.Niki T, Iba S, Tokunou M, Yamada T, Matsuno Y, Hirohashi S. Expression of vascular endothelial growth factors A, B, C, and D and their relationships to lymph node status in lung adenocarcinoma. Clin Cancer Res. 2000;6:2431–2439. [PubMed] [Google Scholar]
- 115.Mandriota SJ, Jussila L, Jeltsch M, Compagni A, Baetens D, Prevo R, Banerji S, Huarte J, Montesano R, Jackson DG, Orci L, Alitalo K, Christofori G, Pepper MS. Vascular endothelial growth factor-C-mediated lymphangiogenesis promotes tumour metastasis. EMBO J. 2001;20:672–682. doi: 10.1093/emboj/20.4.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fontanini G, Boldrini L, Vignati S, Chine S, Basolo F, Silvestri V, Lucchi M, Mussi A, Angeletti CA, Bevilacqua G. Bcl2 and p53 regulate vascular endothelial growth factor (VEGF)-mediated angiogenesis in non-small cell lung carcinoma. Eur J Cancer. 1998;34:718–723. doi: 10.1016/s0959-8049(97)10145-9. [DOI] [PubMed] [Google Scholar]
- 117.Arbiser JL, Petros J, Klafter R, Govindajaran B, McLaughlin ER, Brown LF, Cohen C, Moses M, Kilroy S, Arnold RS, Lambeth JD. Reactive oxygen generated by Nox1 triggers the angiogenic switch. Proc Natl Acad Sci U S A. 2002;99:715–720. doi: 10.1073/pnas.022630199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yuan A, Yu CJ, Luh KT, Kuo SH, Lee YC, Yang PC. Aberrant p53 expression correlates with expression of vascular endothelial growth factor mRNA and interleukin-8 mRNA and neoangiogenesis in non-small-cell lung cancer. J. Clin. Oncol. 2002;20:900–910. doi: 10.1200/JCO.2002.20.4.900. [DOI] [PubMed] [Google Scholar]
- 119.Salven P, Orpana A, Joensuu H. Leukocytes and platelets of patients with cancer contain high levels of vascular endothelial growth factor. Clin Cancer Res. 1999;5:487–491. [PubMed] [Google Scholar]
- 120.Davis-Smyth T, Chen H, Park J, Presta LG, Ferrara N. The second immunoglobulin-like domain of the VEGF tyrosine kinase receptor Flt-1 determines ligand binding and may initiate a signal transduction cascade. EMBO J. 1996;15:4919–4927. [PMC free article] [PubMed] [Google Scholar]
- 121.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 122.Langdon S, Sethi T, Ritchie A, Muir M, Smyth J, Rozengurt E. Broad spectrum neuropeptide antagonists inhibit the growth of small cell lung cancer in vivo. Cancer Res. 1992;52:4554–4557. [PubMed] [Google Scholar]
- 123.Bepler G, Rotsch M, Jaques G, Haeder M, Heymanns J, Hartogh G, Kiefer P, Havemann K. Peptides and growth factors in small cell lung cancer: production, binding sites, and growth effects. J Cancer Res Clin Oncol. 1988;114:235–244. doi: 10.1007/BF00405828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Heasley LE. Autocrine and paracrine signaling through neuropeptide receptors in human cancer. Oncogene. 2001;20:1563–1569. doi: 10.1038/sj.onc.1204183. [DOI] [PubMed] [Google Scholar]


