Abstract
High mobility group AT-hook 2 (HMGA2) is a transcriptional modulator that mediates motility and self-renewal in cancer stem cells. Gastric cancer (GC) is the third leading cause of cancer-related deaths worldwide. GC contains a population of stem-like cells that promote tumor invasion and resistance to therapy. In the current study, we investigated the expression of HMGA2 and the cancer stem cell marker CD44 in 200 GC samples and found that HMGA2 and CD44 were significantly associated with distant metastasis, histological differentiation and poor prognosis in GC patients. Positive clinical correlations of HMGA2 with CD44 were also observed in tissue sections. In vitro, overexpression of HMGA2 promoted GC sphere formation and migration in MKN74/MKN28 cells, whereas downregulation of HMGA2 decreased GC sphere formation and migration in MKN45/MGC803 cells. In addition, western blot and immunofluorescent analyses showed that HMGA2 increased the expression of the stem cell markers CD44, ALDH1, Sox2, and Oct4 and the EMT-related factors Snail and β-catenin. In a xenograft mouse model, overexpression of HMGA2 promoted tumor growth. Further immunohistochemical (IHC) analysis showed that HMGA2 increased the expression of CD44 and β-catenin, resulting in the promotion of tumor growth. Taken together, our findings indicate that HMGA2 promotes GC cancer stem cell induction and cell motility by regulating the expression of CD44. Therefore, targeting HMGA2 in GC may be therapeutically beneficial.
Keywords: Cell migration, cancer stem cell, gastric cancer, HMGA2 protein, CD44 protein
Introduction
Gastric cancer (GC) is one of the most common malignancies worldwide. There were more than 700,000 deaths due to GC in 2014, making this disease the third most common cause of cancer death globally [1,2]. Recent advances in surgical techniques, chemical therapies, radiotherapies and molecular-targeted therapies for cancers have improved the prognosis of GC [3], but the long-term outcomes of patients with GC remain dismal, especially for advanced GC, which has a 5-year overall survival rate of 30% or less [4]. GC tumors are heterogeneous and contain a hierarchy of cell types, a small percentage of which are GC stem-like cells that are highly invasive and resistant to current therapies [5,6]. Many cancer stem cell markers have been reported, such as CD44 [7]. The failure of current GC therapeutics warrants the identification of novel targets and pathways regulating stemness, invasion and tumor formation.
HMGA2 (also called HMGI-C) is a DNA binding protein that acts as an architectural transcription regulator that can assemble and maintain enhanceosomes [8]. HMGA2 binds to the AT-rich minor grooves of DNA through three conserved sequences called AT-hooks [9]. HMGA2 can regulate the expression of both oncogenes and tumor suppressors [10]. HMGA2 is important during embryonic morphogenesis [11] and is aberrantly expressed in cancer [8,11-20]. High levels of HMGA2 in cancer are associated with increased invasiveness, stemness and poor prognosis [17-19,21,22]. However, the functional importance of HMGA2 in regulating stemness and migration in GC is poorly understood.
In the present study, we observed that HMGA2 was significantly associated with distant metastasis and poor prognosis in GC patients and positively correlated with CD44 expression. In addition, we demonstrated that HMGA2 induced tumor sphere formation, increased the clonogenicity, proliferation and invasion of GC cells, and promoted tumorigenicity in vitro and vivo. Furthermore, HMGA2 increased the expression of the stem cell markers CD44, ALDH1, Sox2, and Oct4 and the EMT-related factors Snail and β-catenin. Collectively, our results suggest that HMGA2 promotes tumorigenesis by increasing GC cell motility and sphere formation.
Materials and methods
Patients and samples
Two hundred human GC tissue specimens were collected from the Tumor Tissue Bank of the Tianjin Cancer Hospital (Tianjin, China). The specimens were excised from patients with GC who underwent surgical resection at the Tianjin Medical University Cancer Institute and Hospital in China between March 2004 and December 2012. The histopathological diagnosis was confirmed by trained pathologists. Detailed pathologic and clinical data were recorded, including each patient’s age, gender, tumor size, histological differentiation, TNM stage, metastasis, recurrence, and survival time. The use of these tissue samples was approved by the Ethics Committee of Tianjin Cancer Hospital.
Immunohistochemical staining and scoring
Sections were microwaved, blocked, and incubated using a series of antibodies (Table S1). The PicTure PV6000 staining system (Zhongshan Chemical Co., Beijing, China) and Elivision Plus (Zhongshan Chemical Co., Beijing, China) were used. All sections were counterstained with hematoxylin, dehydrated, and mounted. For the negative controls, phosphate-buffered saline was used in place of the primary antibodies. The evaluation of sections was performed by two independent pathologists. The sections were semi-quantitatively assessed for both the percentage of positive neoplastic cells and the immunostaining intensity of individual tumor cells (extension + intensity) [23]. For statistical analysis, a total score of 0-3 was considered negative expression, while scores of 4-6 were considered positive expression.
Cell culture
The cell lines used in this study were MGC803, MKN45, MKN28, MKN74, and 293T. MKN28 and MKN45 cells were cultured at 37°C in 5% CO2 and saturated humidity in RPMI-1640 medium containing 10% fetal bovine serum (Invitrogen, USA). MKN74, MGC803 and 293T cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum (Invitrogen, USA). MGC803 and MKN45 were obtained from the Institute of Basic Medical Sciences Chinese Academy of Medical Sciences in 2014. The MKN28 cell line was obtained from KeyGEN BioTECH Co. (Nanjing, China) in 2014. The MKN74 cell line was obtained from Jenniobio Biotechnology Co. (Guangzhou, China) in 2015. The 293T cell line was obtained from Zhongshan Hospital Affiliated to Fudan University (Shanghai, China) in 2014.
Lentiviral constructs and cell infection
Full-length HMGA2 complementary DNA (cDNA) (catalog no.: EX-B0278-Lv201; GeneCopoeia, Rockville, MD, USA) was used to overexpress HMGA2 in MKN74 and MKN28 cells; an OmicsLink short hairpin RNA (shRNA) Expression Clones plasmid (target sequence: CTCCTAGGTTCTTAAGGATAA; catalog no.: HSH019812-LVRU6GP GeneCopoeia, Rockville, MD, USA) and the respective empty vector plasmids (target sequence: TGGCTGCCATGCTATGTTGA; catalog no.: CSHCTR001-LVRU6GP; GeneCopoeia, Rockville, MD, USA) were used for HMGA2 silencing in MGC803 and MKN45 cells. Lentiviruses were produced via transient transfection of 293T cells with specific or negative control lentiviral vectors using the Lenti-Pac HIV packaging kit (catalog no. HPK-LvTR-20; GeneCopoeia, Rockville, MD, USA) according to the manufacturer’s instructions, and the viral suspension was used to infect the target cells.
Cell maintenance of tumor spheres and colony formation
Mammosphere culture [24] and the colony formation [25] assay were performed as previously described.
Scrape assays
In the scrape assays, cell motility was assessed by measuring the migration of cells into a scrape. The speed of wound closure was monitored after 24 and 48 h by measuring the ratio of the size of the wound relative to that at hour 0. Each experiment was performed in triplicate.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide (MTT)
Human GC cells (8000 cells/well) were placed in 96-well plates and continually cultured for different periods of time (1, 2, 3, 4, 5, or 6 days). Subsequently, 10 μl of 0.5 mg/ml MTT was added to each well. The cells were incubated at 37°C for another 4 h, the medium was removed, and the precipitated formazan was dissolved in 100 μl of DMSO. After the solution was shaken for 10 min using an Eppendorf Mix Mate (Eppendorf, GRE), the absorbance was detected at 490 nm (A490) on a Bio Tek ELx800 (Bio Tek, USA).
Immunofluorescent staining
The cells were plated onto coverslips and fixed with cold methanol on ice for 10 min. The cells were blocked with 1% BSA and incubated with primary antibodies against HMGA2 (1:200 dilution), CD44 (1:100 dilution) and ALDH1 (1:100 dilution) overnight at 4°C. Then, FITC-conjugated secondary antibodies were added, and the cells were incubated at 37°C for 1 h. The sections were counterstained with DAPI and observed using a fluorescence microscope at ×200 magnification (80i; Nikon, Shinagawa, Tokyo, Japan).
Western blotting
The cell lysates were resolved by SDS-PAGE and transferred onto PVDF membranes (Millipore, Billerica, MA, USA). The blots were blocked and incubated with primary antibodies overnight at 4°C. The membranes were then incubated with the secondary antibody at 37°C for 2 h. The enhanced chemiluminescence method was used to measure protein expression, and GAPDH served as the internal control. Bands were imaged and analyzed using a C-Digit Blotting Scanner (Gene Company, Beijing, China). Details of the antibodies used are provided in Table S1.
Luciferase reporter assay
The c-Myc promoter was purchased from GeneCopoeia. 293T cells were transfected with c-Myc promoter plasmids or the control plasmid and the HMGA2 plasmid. Forty-eight hours following transfection, luciferase activity was analyzed using the Secrete-PairTM Dual Luminescence Assay Kit (GeneCopoeia, Rockville, MD, USA). The results were obtained from three independent experiments performed in duplicate.
Animal experiments
Four-week-old male BALB/c nude mice were injected subcutaneously with a suspension of 1×107 cells in the upper right flank region. After 4 weeks, the mice were sacrificed, and the xenograft tumors were weighed and fixed for histology and immunohistochemistry (IHC) analyses. All studies were performed in accordance with the American Association for the Accreditation of Laboratory Animal Care guidelines for the humane treatment of animals and adhered to national and international standards.
Statistical analysis
Data are presented as the means ± SD. SPSS 22.0 software (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Graphical representations were produced using GraphPad Prism 6 (San Diego, CA, USA) software. For clinicopathological analysis, the chi-square test or Fisher’s exact test was performed. The survival calculations were illustrated with Kaplan-Meier curves, and differences between survival curves were tested by the log-rank test. Differences between two groups were compared with the 2-tailed Student’s t-test, chi-square test, or Fisher’s exact test, as appropriate. P values (two-sided) less than 0.05 were considered statistically significant.
Results
Expression of HMGA2 is significantly upregulated in GC and clinically related to the expression of CD44
A total of 200 primary human GC specimens were collected to detect HMGA2 expression by immunohistochemical analysis and analyze associations with clinicopathological characteristics. As shown in Figure 1A and Table 1, in tumor tissues, positive HMGA2 expression was observed in the cell nucleus. Significantly, overexpression of HMGA2 was observed in poorly differentiated GC tissues.
Figure 1.
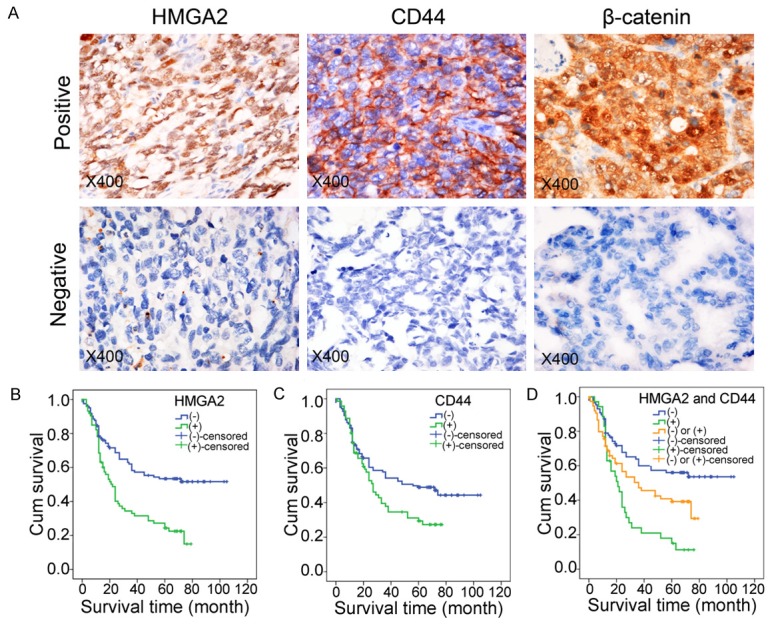
Immunohistochemical expression of HMGA2 and the CSC marker CD44. A. Immunohistochemical staining of HMGA2, CD44 and β-catenin in human GC samples. (magnification, ×400). B. Kaplan-Meier analysis of the correlation between HMGA2 expression and overall survival in GC patients. C. Kaplan-Meier analysis of the correlation between CD44 expression and overall survival in GC patients. D. Kaplan-Meier analysis of overall survival in the HMGA2+/CD44+ group and HMGA2-/CD44- group of GC patients.
Table 1.
The correlation of HMGA2 with the clinicopathological parameters of gastric cancer
| Clinicopathological feature | HMGA2 | χ2 | P Value | |
|---|---|---|---|---|
|
| ||||
| Positive | Negative | |||
| Age (years) | ||||
| <60 | 29 | 64 | 2.96 | 0.085 |
| ≥60 | 46 | 61 | ||
| Sex | ||||
| Male | 54 | 81 | 1.108 | 0.293 |
| Female | 21 | 44 | ||
| Tumor size (cm3) | ||||
| <3 | 25 | 60 | 4.126 | 0.042* |
| ≥3 | 50 | 65 | ||
| Histological differentiation | ||||
| I/II | 30 | 71 | 5.293 | 0.021* |
| III/IV | 45 | 54 | ||
| TNM satge | ||||
| I/II | 22 | 55 | 4.259 | 0.039* |
| III/IV | 53 | 70 | ||
| Lauren type | ||||
| Intestinal type | 39 | 69 | 0.193 | 0.660 |
| Diffuse type | 36 | 56 | ||
| Lymphatic metastasis | ||||
| Positive | 53 | 76 | 1.193 | 0.158 |
| Negative | 22 | 49 | ||
| Distant metastasis | ||||
| Positive | 40 | 33 | 14.671 | ** |
| Negative | 35 | 92 | ||
| Recurrence | ||||
| Positive | 16 | 23 | 0.257 | 0.612 |
| Negative | 59 | 102 | ||
| Metastasis and recurrence | ||||
| Positive | 47 | 51 | 8.969 | 0.037* |
| Negative | 28 | 74 | ||
P<0.05;
P<0.001.
The correlations between HMGA2 expression levels and clinicopathological characteristics are summarized in Table 1. HMGA2 expression was significantly correlated with tumor size (P=0.042), histological differentiation (P=0.021), TNM stage (P=0.039) and distant metastasis (P<0.001). As shown in Figure 1A and Table 2, the cancer stem cell marker CD44 was predominantly observed in the membrane of cancer cells and was significantly correlated with histological differentiation (P<0.001), Lauren type (P<0.001) and distant metastasis (P=0.006). Furthermore, HMGA2 was positively correlated with CD44 and β-catenin expression (Table 3). Importantly, we examined the prognostic significance of HMGA2 and CD44 in GC patients. Kaplan-Meier analysis showed that overall survival was lower in the HMGA2 or CD44 high-expression groups than in the corresponding low-expression groups, (Figure 1B and 1C, HMGA2, P<0.001; CD44, P=0.029). In addition, the overall survival of patients with HMGA2+/CD44+ expression was much lower than that of patients with HMGA2-/CD44- expression (Figure 1D, P<0.001).
Table 2.
The correlation of CD44 with the clinicopathological parameters of gastric cancer
| Clinicopathological feature | CD44 | χ2 | P Value | |
|---|---|---|---|---|
|
| ||||
| Positive | Negative | |||
| Age (years) | ||||
| <60 | 30 | 63 | 1.677 | 0.195 |
| ≥60 | 44 | 63 | ||
| Sex | ||||
| Male | 53 | 82 | 0.91 | 0.340 |
| Female | 21 | 44 | ||
| Tumor size (cm3) | ||||
| <3 | 36 | 49 | 1.817 | 0.178 |
| ≥3 | 38 | 77 | ||
| Histological differentiation | ||||
| I/II | 25 | 76 | 13.13 | ** |
| III/IV | 49 | 50 | ||
| TNM satge | ||||
| I/II | 22 | 55 | 3.816 | 0.051 |
| III/IV | 52 | 71 | ||
| Lauren type | ||||
| Intestinal type | 27 | 81 | 14.504 | ** |
| Diffuse type | 47 | 45 | ||
| Lymphatic metastasis | ||||
| Positive | 53 | 76 | 2.602 | 0.107 |
| Negative | 21 | 50 | ||
| Distant metastasis | ||||
| Positive | 36 | 37 | 7.48 | 0.006* |
| Negative | 38 | 89 | ||
| Recurrence | ||||
| Positive | 13 | 26 | 0.279 | 0.597 |
| Negative | 61 | 100 | ||
| Metastasis and recurrence | ||||
| Positive | 43 | 55 | 3.899 | 0.048* |
| Negative | 31 | 71 | ||
P<0.05;
P<0.001.
Table 3.
Relationship between HMGA2 and CD44 or β-catenin expression
| Variant | HMGA2 | χ2 | P-Value | ||
|---|---|---|---|---|---|
|
| |||||
| Negative | Positive | ||||
| CD44 | Negative | 88 | 38 | 7.831 | 0.005* |
| Positive | 37 | 37 | |||
| β-catenin | Negative | 74 | 33 | 4.353 | 0.037* |
| Positive | 51 | 42 | |||
P<0.05.
HMGA2 induces sphere formation and promotes the expression of cancer stem cell markers in vitro
Cultured cancer stem cells (CSCs) are believed to be able to form spheres with properties very similar to those of endogenous CSCs isolated from human tumor tissues [26,27]. To investigate whether HMGA2-promoted tumorigenesis is due to the induction of CSCs, we determined the effects of HMGA2 on stem sphere formation. Sphere formation ability was investigated in four different GC cell lines, and two GC cell lines formed tumor spheres (Figure 2B). Additionally, we detected the endogenous expression of HMGA2 in these cell lines (Figure 2A). Taken together, these results indicated that HMGA2 was highly expressed in GC cell lines that formed spheres.
Figure 2.
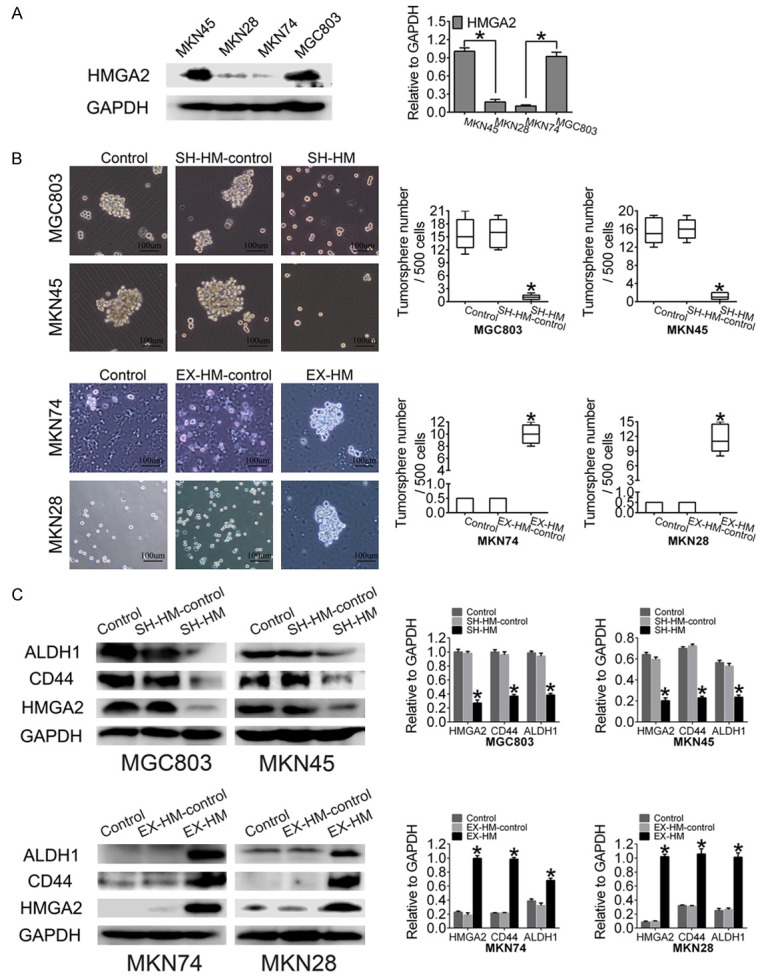
HMGA2 induces sphere formation in GC cells. A. Western blot showing HMGA2 expression in GC cell lines. B. Overexpression of HMGA2 promoted sphere formation by MKN28 and MKN74 cells, whereas knockdown of HMGA2 expression decreased sphere formation by MKN45 and MGC803 cells (scale bar represents 100 µm). C. HMGA2 upregulated the expression of CD44 and ALDH1 in MKN74 and MKN28 cells transfected with the HMGA2 expression plasmid; in contrast, CD44 and ALDH1 were decreased by sh-HMGA2. All experiments were repeated three times; *indicates P<0.05.
To determine the effects of HMGA2 on sphere formation, we transfected the pEZ-Lv201 vector expression clone HMGA2 into MKN74 and MKN28 cells, which display low endogenous expression of HMGA2. Sphere formation efficiency, a measure of self-renewal, was significantly increased in MKN74 and MKN28 cells overexpressing HMGA2 (Figure 2B). To verify the findings in a gain-of-function model, loss-of-function experiments were performed using the HMGA2 inhibitor expression clone in MGC803 and MKN45 cells; treatment with this inhibitor clearly decreased the endogenous HMGA2 levels in MGC803 and MKN45 cells. When HMGA2 was downregulated, the sphere formation capacity was reduced in MGC803 and MKN45 GC cells. Finally, western blot analysis was performed to detect the expression levels of CSC markers. The levels of both CD44 and ALDH1 were increased in HMGA2-overexpressing GCSCs relative to control cells (P<0.001) (Figure 2C), while the protein levels of the above markers were decreased in human GC cells when HMGA2 was knocked down. Similarly, the immunofluorescence results revealed upregulated CD44 and ALDH1 expression (Figure 3). Taken together, these data indicated that HMGA2 promoted the self-renewal of GCSCs.
Figure 3.
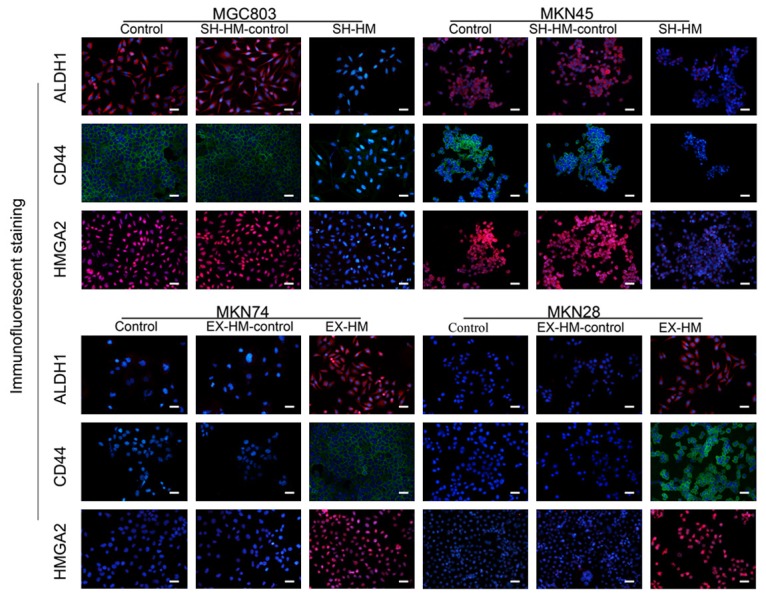
Overexpression of HMGA2 increases CD44 and ALDH1 expression in GC cell lines. Immunofluorescence staining showed that HMGA2 overexpression increased the expression of CD44 and ALDH1, whereas knockdown of HMGA2 decreased the protein expression of CD44 and ALDH1 (magnification, ×200; scale bar represents 50 µm; all experiments were repeated three times).
HMGA2 increases the clonogenicity and proliferation of GC cells
To test the hypothesis that HMGA2 promotes clonogenicity in GC, we performed clone formation assays in human cell lines. First, we tested the clonogenicity of MKN74/MKN28 cells transfected with HMGA2, and observed that the number of colonies formed was increased compared with the control groups (Figure 4A). Second, the effect of HMGA2 on the proliferation of GC cells was first examined using the MTT assay. MKN74 and MKN28 cells transfected with HMGA2 displayed a much higher ability to promote proliferation than those transfected with empty vector or non-transfected cells (Figure 4B). In addition, loss-of-function analysis was performed using shHMGA2, which strikingly decreased endogenous HMGA2 levels in MGC803 and MKN45 cells. HMGA2 knockdown decreased the clone formation activity and proliferation of GC cells (Figure 4B). Third, western blot analysis was performed to detect the expression levels of induced-pluripotent stem (i-PS) cell factors such as Sox2, Oct4 and c-Myc. As expected, enhanced HMGA2 expression led to significant elevation of Sox2, Oct-4 and c-Myc levels, while HMGA2 knockdown reduced the expression of Sox2, Oct4 and c-Myc compared with control cells (Figure 4C). Furthermore, to elucidate the molecular mechanisms by which HMGA2 regulates stemness in GC, we used dual-luciferase reporter analysis. Expression of HMGA2 significantly promoted luciferase activity controlled by the promoter of c-Myc (Figure 4D), indicating that HMGA2 may promote gene expression through its binding sequence at the promoter of c-Myc.
Figure 4.
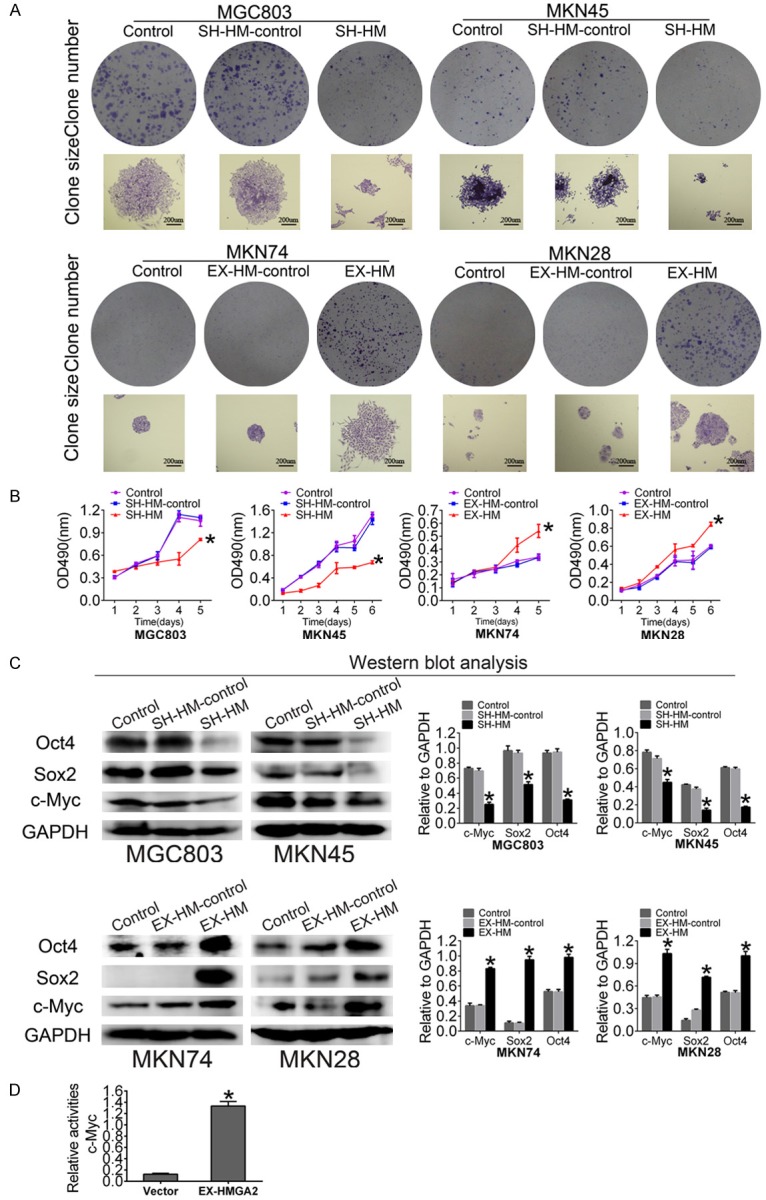
HMGA2 increases the clonogenicity and proliferation of GC cells. A. A colony formation assay was performed to analyze colony formation ability. The scale bar represents 200 µm. B. MTT assays of HMGA2-upregulated MKN74 and MKN28 cells and HMGA2-downregulated MGC803 and MKN45 cells. C. Western blot showing that HMGA2 overexpression increased the expression of c-Myc, Sox2 and Oct4, whereas HMGA2 knockdown decreased the expression of c-Myc, Sox2 and Oct4. D. Luciferase reporter assays showed that HMGA2 promotes c-Myc gene expression. All experiments were repeated three times; *indicates P<0.05.
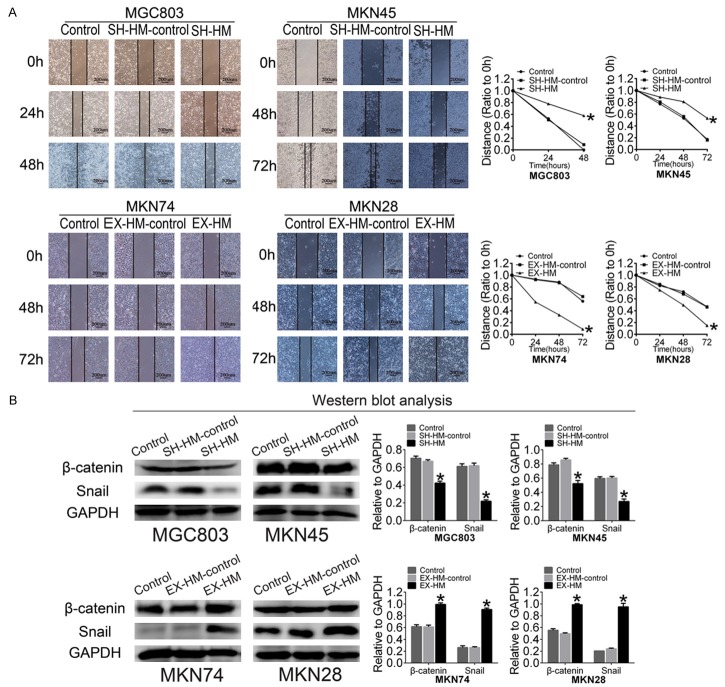
HMGA2 regulates the migratory capacity of GC cells and increases the expression of EMT-related markers in GC cells
Accumulating evidence has demonstrated that CSCs not only play an important role in tumorigenicity but also promote metastasis [28,29]; thus, we examined the role of HMGA2 in the migration of GC cells using scrape assays. As shown in Figure 5A and 5B, cellular migration was enhanced when we transfected MKN74/MKN28 cells with the HMGA2 plasmid, while knocking down cellular HMGA2 reduced the motility of GC cells.
Figure 5.
HMGA2 regulates the migratory capacity of GC cells and increases the expression of EMT-related markers in GC cells. A. Wound healing assay (the scale bar represents 200 µm). Quantitative analysis showed a significant difference at 48 h for MGC803 cells and a significant difference at 72 h for MKN45, MKN28 and MKN74 cells. B. Western blot showing that HMGA2 overexpression increased the expression of Snail and β-catenin, while HMGA2 knockdown decreased the expression of Snail and β-catenin. All experiments were repeated three times; *indicates P<0.05.
Recent studies have suggested that EMT is closely related to the CSC-like phenotype in prostate cancer [30], breast cancer [31], and GC [32,33]. To determine the molecular mechanisms underlying stemness, we analyzed the correlation between HMGA2 and EMT markers such as Snail and β-catenin in GC cells. There was a significant difference in the protein expression levels of snail and β-catenin between the negative control and HMGA2-transfected MKN74 and MKN28 cells (Figure 5C). HMGA2 decreased the expression of Snail and β-catenin in GC cells. These results indicated that HMGA2 may promote EMT.
HMGA2 promotes stemness and tumorigenicity in vivo
To validate the function of HMGA2 in vivo, MKN74/MKN28 cells stably transfected with HMGA2 and MGC803/MKN45 cells stably transfected with the HMGA2 inhibitor were subcutaneously injected into BALB/c-nu/nu mice. The animals xenografted with HMGA2-transduced cells exhibited larger tumors than the control group by 4 weeks post-injection (Figure 6A), and the weight of tumors with high HMGA2 expression was higher than that of tumors with low HMGA2 expression (P<0.05). Consistent with our previous observation, IHC staining showed that upregulation of HMGA2 significantly enhanced the expression of CD44 and β-catenin in MKN74/MKN28 cells, whereas downregulation of HMGA2 resulted in low expression of CD44 and β-catenin in MGC803/MKN45 cells (Figure 6B). These results suggested that HMGA2 promotes stemness and tumorigenicity in vivo.
Figure 6.
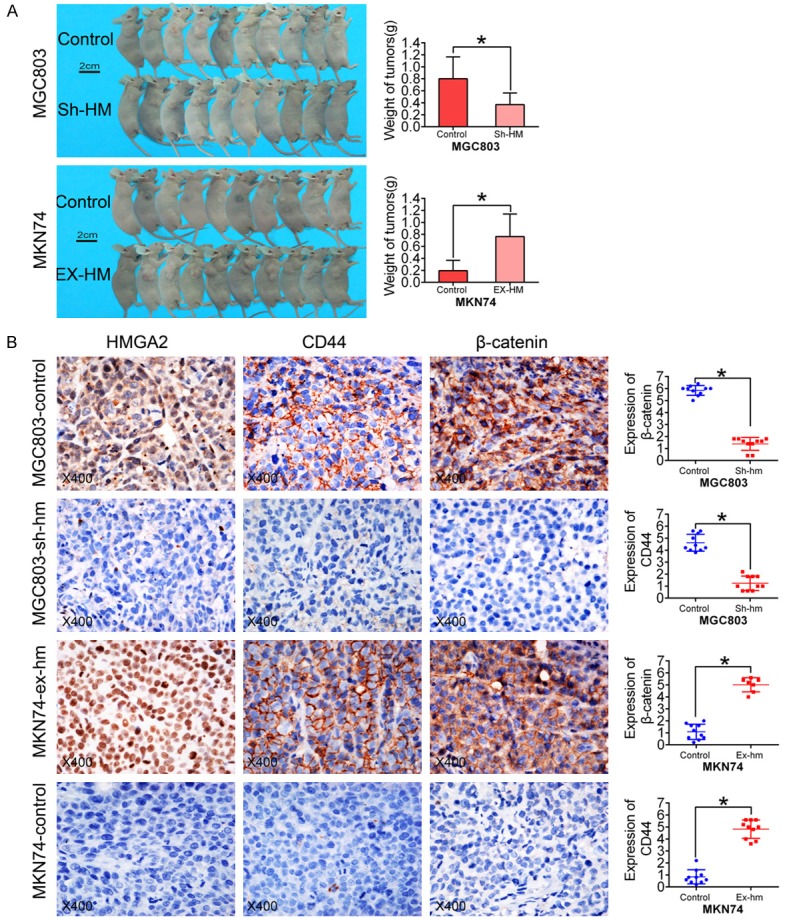
HMGA2 promotes stemness and tumorigenicity in vivo. A. Analysis of tumor weight showed that high HMGA2 expression promoted tumor growth (the scale bar represents 2 cm). B. An in vivo xenograft study showed that tumors with higher HMGA2 expression had elevated CD44 and β-catenin expression. (Magnification, ×400; *indicates P<0.05.)
Discussion
As an oncofetal protein, the expression of HMGA2 increases with the dedifferentiation of cancers in many malignant tumors, such as pancreatic adenocarcinoma [34], liposarcoma [35], and bladder cancer [36]. HMGA2 might target different down-stream genes to maintain the undifferentiated status of cells in the embryogenesis and tumorigenesis processes [37-40]. HMGA2 has been reported to promote the self-renewal of neural stem cells by negatively regulating p16Ink4a/p19Arf expression. However, whether the expression of HMGA2 is associated with the development of cancer stem-like cells in GC is not well understood. Here, we demonstrated that HMGA2 induced the formation of tumor spheres, increased the clonogenicity, proliferation and invasion of cells, and promoted tumorigenicity in vitro and vivo. In addition, HMGA2 increased the expression of the stem cell markers CD44, ALDH1, Sox2, and Oct4 and the EMT-related factors Snail and β-catenin. Furthermore, we observed that HMGA2 was significantly associated with distant metastasis and positively correlated with CD44 expression, markers that indicate poor prognosis in human GC. Our data may facilitate the addition of a therapeutically beneficial strategy to GC treatment options.
CSCs have been defined as a small subpopulation of cells that can give rise to tumor masses [41]. CSCs can be viewed as the result of mis-differentiation and possess self-renewal and differentiation potential. Recent studies have demonstrated that CSCs may be responsible for tumor initiation, invasion, distant metastasis, and chemo-resistance; thus, the development of therapies that target CSCs is increasingly appealing [42]. CSCs have been found in many types of solid tumors, such as breast cancer [43], glioblastoma [44], colon cancer [45], and GC. Thus, it is necessary to analyze the relationship between HMGA2 and CSCs. We found that HMGA2 induced sphere formation in a serum-free and growth factor- containing medium that has been used to enrich CSCs from several tumors. In addition, we found that HMGA2 enhanced the colony formation and proliferation of these cells in vitro.
Previous studies have suggested that several stem cell markers, such as CD44 or ALDH1, may be shared by CSCs in different tumor types [46-50]; in human breast cancer, prostate cancer, and colon cancer, expression of these proteins defined a subpopulation of cancer cells with high tumorigenic potential. These results indicated that the same cell marker can act as a CSC marker in multiple types of human cancer. Therefore, we detected the expression of CD44 and ALDH1, which revealed that HMGA2 increased the protein levels of CD44 and ALDH1. The four key transcription factors of the POU family (Oct4, Sox2, c-Myc, and KLF4) together may drive patient-specific induced pluripotent stem cell (i-PSC) formation [50], and these transcription factors play important roles in regulating the stemness state, self-renewal and pluripotency of stem cells [51]. We also observed that the expression of these markers increased significantly with HMGA2 upregulation, and the dual-luciferase reporter assay indicated that HMGA2 bound directly to the promoter of c-Myc and increased the protein expression levels of c-Myc. Furthermore, our study showed that HMGA2 promotes tumor formation in vivo. In addition, we found that HMGA2 was positively correlated with histological differentiation, stage, metastasis and recurrence; Kaplan-Meier curves revealed that patients co-expressing both HMGA2 and CD44 exhibited a significantly shorter survival time than patients in the ‘HMGA2- and CD44-’ group or ‘HMGA2+ or CD44+’ group. Overall, our data suggest that HMGA2 enhances the expression of CD44, which in turn induces tumor sphere formation and thus promotes tumorigenicity.
Some studies have demonstrated that CSCs may be responsible for tumor initiation, invasion, and distant metastasis, which also result in a poor prognosis for GC. Our results showed that HMGA2 promoted the mobility of tumor cells in vitro and that HMGA2 positively correlated with metastasis and recurrence in human GC. The Wnt pathway is a critical signaling axis that regulates developmental processes in the embryo and maintains the self-renewal and differentiation of stem cells [52]. Inhibition of β-catenin has been shown to decrease the sphere formation ability of GC cells. In this study, HMGA2 downregulated the expression of β-catenin in GC cells, suggesting that HMGA2 promotes the self-renewal of GC.
In conclusion, our analyses identified HMGA2 and CD44 co-expression as a negative marker of prognosis in patients with GC and indicated that HMGA2 exerts its effects partially by promoting stemness and tumorigenicity. Increased HMGA2 expression in patients with GC was correlated with poor cellular differentiation and metastasis. The upregulation of HMGA2 expression resulted in the activation of the Wnt/β-catenin signaling pathway and CD44, Which promote GC cell sphere formation and migration. Our experiments also showed that depletion of HMGA2 in GC cells reduced sphere formation, decreased clonogenicity and proliferation, and suppressed the tumor growth of GC cells in vivo, suggesting that GC stem-like cells require HMGA2 to maintain malignant properties. Therefore, targeting HMGA2 in GC may be a therapeutically beneficial strategy.
Acknowledgements
This study was supported by grants from the Key Project of the National Natural Science Foundation of China (Grant No. 81230050 to B.S.) and the National Natural Science Foundation of China (Grant No. 81572872 to X.Z.).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Plummer M, Franceschi S, Vignat J, Forman D, de Martel C. Global burden of gastric cancer attributable to Helicobacter pylori. Int J Cancer. 2015;136:487–490. doi: 10.1002/ijc.28999. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 3.Lee JH, Kim KM, Cheong JH, Noh SH. Current management and future strategies of gastric cancer. Yonsei Med J. 2012;53:248–257. doi: 10.3349/ymj.2012.53.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hartgrink HH, Jansen EP, van Grieken NC, van de Velde CJ. Gastric cancer. Lancet. 2009;374:477–490. doi: 10.1016/S0140-6736(09)60617-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh SR. Gastric cancer stem cells: a novel therapeutic target. Cancer Lett. 2013;338:110–119. doi: 10.1016/j.canlet.2013.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stojnev S, Krstic M, Ristic-Petrovic A, Stefanovic V, Hattori T. Gastric cancer stem cells: therapeutic targets. Gastric Cancer. 2014;17:13–25. doi: 10.1007/s10120-013-0254-x. [DOI] [PubMed] [Google Scholar]
- 7.Takaishi S, Okumura T, Tu S, Wang SS, Shibata W, Vigneshwaran R, Gordon SA, Shimada Y, Wang TC. Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells. 2009;27:1006–1020. doi: 10.1002/stem.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fedele M, Fusco A. HMGA and cancer. Biochim Biophys Acta. 2010;1799:48–54. doi: 10.1016/j.bbagrm.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Ozturk N, Singh I, Mehta A, Braun T, Barreto G. HMGA proteins as modulators of chromatin structure during transcriptional activation. Front Cell Dev Biol. 2014;2:5. doi: 10.3389/fcell.2014.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Narita M, Narita M, Krizhanovsky V, Nunez S, Chicas A, Hearn SA, Myers MP, Lowe SW. A novel role for high-mobility group a proteins in cellular senescence and heterochromatin formation. Cell. 2006;126:503–514. doi: 10.1016/j.cell.2006.05.052. [DOI] [PubMed] [Google Scholar]
- 11.Pfannkuche K, Summer H, Li O, Hescheler J, Droge P. The high mobility group protein HMGA2: a co-regulator of chromatin structure and pluripotency in stem cells? Stem Cell Rev. 2009;5:224–230. doi: 10.1007/s12015-009-9078-9. [DOI] [PubMed] [Google Scholar]
- 12.Di Cello F, Hillion J, Hristov A, Wood LJ, Mukherjee M, Schuldenfrei A, Kowalski J, Bhattacharya R, Ashfaq R, Resar LM. HMGA2 participates in transformation in human lung cancer. Mol Cancer Res. 2008;6:743–750. doi: 10.1158/1541-7786.MCR-07-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malek A, Bakhidze E, Noske A, Sers C, Aigner A, Schafer R, Tchernitsa O. HMGA2 gene is a promising target for ovarian cancer silencing therapy. Int J Cancer. 2008;123:348–356. doi: 10.1002/ijc.23491. [DOI] [PubMed] [Google Scholar]
- 14.Morishita A, Zaidi MR, Mitoro A, Sankarasharma D, Szabolcs M, Okada Y, D’Armiento J, Chada K. HMGA2 is a driver of tumor metastasis. Cancer Res. 2013;73:4289–4299. doi: 10.1158/0008-5472.CAN-12-3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morshedi A, Ren Z, Li J, Droge P. Probing into the biological processes infl uenced by ESC factor and oncoprotein HMGA2 using iPSCs. Stem Cell Rev. 2013;9:514–522. doi: 10.1007/s12015-012-9373-8. [DOI] [PubMed] [Google Scholar]
- 16.Zhang K GH, Wu X, Wang J, Zhou W, Sun G, Wang J, Wang Y, Mu B, Kim C, Chu P, Ho DM, Ann DK, Wong TT, Yen Y. Frequent over expression of HMGA2 in human atypical teratoid_rhabdoid tumor and its correlation withlet-7a3_let-7b mi RNA. Clin Cancer Res. 2014;20:1179–1189. doi: 10.1158/1078-0432.CCR-13-1452. [DOI] [PubMed] [Google Scholar]
- 17.Cai J, Shen G, Liu S, Meng Q. Downregulation of HMGA2 inhibits cellular proliferation and invasion, improves cellular apoptosis in prostate cancer. Tumour Biol. 2016;37:699–707. doi: 10.1007/s13277-015-3853-9. [DOI] [PubMed] [Google Scholar]
- 18.Motoyama K, Inoue H, Nakamura Y, Uetake H, Sugihara K, Mori M. Clinical significance of high mobility group A2 in human gastric cancer and its relationship to let-7 microRNA family. Clin Cancer Res. 2008;14:2334–2340. doi: 10.1158/1078-0432.CCR-07-4667. [DOI] [PubMed] [Google Scholar]
- 19.Lee CT, Wu TT, Lohse CM, Zhang L. High-mobility group AT-hook 2: an independent marker of poor prognosis in intrahepatic cholangiocarcinoma. Hum Pathol. 2014;45:2334–2340. doi: 10.1016/j.humpath.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 20.Wei L, Liu X, Zhang W, Wei Y, Li Y, Zhang Q, Dong R, Kwon JS, Liu Z, Zheng W, Kong B. Overexpression and oncogenic function of HMGA2 in endometrial serous carcinogenesis. Am J Cancer Res. 2016;6:249–259. [PMC free article] [PubMed] [Google Scholar]
- 21.Fedele M, Visone R, De Martino I, Troncone G, Palmieri D, Battista S, Ciarmiello A, Pallante P, Arra C, Melillo RM, Helin K, Croce CM, Fusco A. HMGA2 induces pituitary tumorigenesis by enhancing E2F1 activity. Cancer Cell. 2006;9:459–471. doi: 10.1016/j.ccr.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 22.Watanabe S, Ueda Y, Akaboshi S, Hino Y, Sekita Y, Nakao M. HMGA2 maintains oncogenic RAS-induced epithelial-mesenchymal transition in human pancreatic cancer cells. Am J Pathol. 2009;174:854–868. doi: 10.2353/ajpath.2009.080523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng AL, Huang WG, Chen ZC, Peng F, Zhang PF, Li MY, Li F, Li JL, Li C, Yi H, Yi B, Xiao ZQ. Identification of novel nasopharyngeal carcinoma biomarkers by laser capture microdissection and proteomic analysis. Clin Cancer Res. 2008;14:435–445. doi: 10.1158/1078-0432.CCR-07-1215. [DOI] [PubMed] [Google Scholar]
- 24.Yuhas JM, Li AP, Martinez AO, Ladman AJ. A simplified method for production and growth of multicellular tumor spheroids. Cancer Res. 1977:3639–3643. [PubMed] [Google Scholar]
- 25.Sun T, Sun BC, Zhao XL, Zhao N, Dong XY, Che N, Yao Z, Ma YM, Gu Q, Zong WK, Liu ZY. Promotion of tumor cell metastasis and vasculogenic mimicry by way of transcription coactivation by Bcl-2 and Twist1: a study of hepatocellular carcinoma. Hepatology. 2011;54:1690–1706. doi: 10.1002/hep.24543. [DOI] [PubMed] [Google Scholar]
- 26.Jinesh GG, Choi W, Shah JB, Lee EK, Willis DL, Kamat AM. Blebbishields, the emergency program for cancer stem cells: sphere formation and tumorigenesis after apoptosis. Cell Death Differ. 2013;20:382–395. doi: 10.1038/cdd.2012.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qiu X, Wang Z, Li Y, Miao Y, Ren Y, Luan Y. Characterization of sphere-forming cells with stem-like properties from the small cell lung cancer cell line H446. Cancer Lett. 2012;323:161–170. doi: 10.1016/j.canlet.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Dalerba P, Cho RW, Clarke MF. Cancer stem cells: models and concepts. Annu Rev Med. 2007;58:267–284. doi: 10.1146/annurev.med.58.062105.204854. [DOI] [PubMed] [Google Scholar]
- 29.Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755–768. doi: 10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- 30.Giannoni E, Bianchini F, Masieri L, Serni S, Torre E, Calorini L, Chiarugi P. Reciprocal activation of prostate cancer cells and cancer-associated fibroblasts stimulates epithelial-mesenchymal transition and cancer stemness. Cancer Res. 2010;70:6945–6956. doi: 10.1158/0008-5472.CAN-10-0785. [DOI] [PubMed] [Google Scholar]
- 31.Bhat-Nakshatri P, Appaiah H, Ballas C, Pick-Franke P, Goulet R Jr, Badve S, Srour EF, Nakshatri H. SLUG/SNAI2 and tumor necrosis factor generate breast cells with CD44+/CD24- phenotype. BMC Cancer. 2010;10:411. doi: 10.1186/1471-2407-10-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holland JD, Klaus A, Garratt AN, Birchmeier W. Wnt signaling in stem and cancer stem cells. Curr Opin Cell Biol. 2013;25:254–264. doi: 10.1016/j.ceb.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 33.White BD, Chien AJ, Dawson DW. Dysregulation of Wnt/beta-catenin signaling in gastrointestinal cancers. Gastroenterology. 2012;142:219–232. doi: 10.1053/j.gastro.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piscuoglio S, Zlobec I, Pallante P, Sepe R, Esposito F, Zimmermann A, Diamantis I, Terracciano L, Fusco A, Karamitopoulou E. HMGA1 and HMGA2 protein expression correlates with advanced tumour grade and lymph node metastasis in pancreatic adenocarcinoma. Histopathology. 2012;60:397–404. doi: 10.1111/j.1365-2559.2011.04121.x. [DOI] [PubMed] [Google Scholar]
- 35.Saada-Bouzid E, Burel-Vandenbos F, Ranchere-Vince D, Birtwisle-Peyrottes I, Chetaille B, Bouvier C, Chateau MC, Peoc’h M, Battistella M, Bazin A, Gal J, Michiels JF, Coindre JM, Pedeutour F, Bianchini L. Prognostic value of HMGA2, CDK4, and JUN amplification in well-differentiated and dedifferentiated liposarcomas. Mod Pathol. 2015;28:1404–1414. doi: 10.1038/modpathol.2015.96. [DOI] [PubMed] [Google Scholar]
- 36.Ding X, Wang Y, Ma X, Guo H, Yan X, Chi Q, Li J, Hou Y, Wang C. Expression of HMGA2 in bladder cancer and its association with epithelial-to-mesenchymal transition. Cell Prolif. 2014;47:146–151. doi: 10.1111/cpr.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Madison BB, Jeganathan AN, Mizuno R, Winslow MM, Castells A, Cuatrecasas M, Rustgi AK. Let-7 Represses Carcinogenesis and a Stem Cell Phenotype in the Intestine via Regulation of Hmga2. PLoS Genet. 2015;11:e1005408. doi: 10.1371/journal.pgen.1005408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu KR, Shin JH, Kim JJ, Koog MG, Lee JY, Choi SW, Kim HS, Seo Y, Lee S, Shin TH, Jee MK, Kim DW, Jung SJ, Shin S, Han DW, Kang KS. Rapid and efficient direct conversion of human adult somatic cells into neural stem cells by HMGA2/let-7b. Cell Rep. 2015 doi: 10.1016/j.celrep.2014.12.038. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 39.Alam M, Ahmad R, Rajabi H, Kufe D. MUC1-C Induces the LIN28B-->LET-7->HMGA2 Axis to Regulate Self-Renewal in NSCLC. Mol Cancer Res. 2015;13:449–460. doi: 10.1158/1541-7786.MCR-14-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parameswaran S, Xia X, Hegde G, Ahmad I. Hmga2 regulates self-renewal of retinal progenitors. Development. 2014;141:4087–4097. doi: 10.1242/dev.107326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vermeulen L, Sprick MR, Kemper K, Stassi G, Medema JP. Cancer stem cells-old concepts, new insights. Cell Death Differ. 2008;15:947–958. doi: 10.1038/cdd.2008.20. [DOI] [PubMed] [Google Scholar]
- 42.O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 43.Korkaya H, Wicha MS. Breast cancer stem cells: we’ve got them surrounded. Clin Cancer Res. 2013;19:511–513. doi: 10.1158/1078-0432.CCR-12-3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clark PA, Iida M, Treisman DM, Kalluri H, Ezhilan S, Zorniak M, Wheeler DL, Kuo JS. Activation of multiple ERBB family receptors mediates glioblastoma cancer stem-like cell resistance to EGFR-targeted inhibition. Neoplasia. 2012;14:420–IN413. doi: 10.1596/neo.12432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ohata H, Ishiguro T, Aihara Y, Sato A, Sakai H, Sekine S, Taniguchi H, Akasu T, Fujita S, Nakagama H, Okamoto K. Induction of the stem-like cell regulator CD44 by Rho kinase inhibition contributes to the maintenance of colon cancer-initiating cells. Cancer Res. 2012;72:5101–5110. doi: 10.1158/0008-5472.CAN-11-3812. [DOI] [PubMed] [Google Scholar]
- 46.Chaffer CL, Marjanovic ND, Lee T, Bell G, Kleer CG, Reinhardt F, D’Alessio AC, Young RA, Weinberg RA. Poised chromatin at the ZEB1 promoter enables breast cancer cell plasticity and enhances tumorigenicity. Cell. 2013;154:61–74. doi: 10.1016/j.cell.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu C, Kelnar K, Liu B, Chen X, Calhoun-Davis T, Li H, Patrawala L, Yan H, Jeter C, Honorio S, Wiggins JF, Bader AG, Fagin R, Brown D, Tang DG. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat Med. 2011;17:211–215. doi: 10.1038/nm.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Todaro M, Gaggianesi M, Catalano V, Benfante A, Iovino F, Biffoni M, Apuzzo T, Sperduti I, Volpe S, Cocorullo G, Gulotta G, Dieli F, De Maria R, Stassi G. CD44v6 is a marker of constitutive and reprogrammed cancer stem cells driving colon cancer metastasis. Cell Stem Cell. 2014;14:342–356. doi: 10.1016/j.stem.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 49.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S, Schott A, Hayes D, Birnbaum D, Wicha MS, Dontu G. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Flesken-Nikitin A, Hwang CI, Cheng CY, Michurina TV, Enikolopov G, Nikitin AY. Ovarian surface epithelium at the junction area contains a cancer-prone stem cell niche. Nature. 2013;495:241–245. doi: 10.1038/nature11979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chiou SH, Wang ML, Chou YT, Chen CJ, Hong CF, Hsieh WJ, Chang HT, Chen YS, Lin TW, Hsu HS, Wu CW. Coexpression of Oct4 and Nanog enhances malignancy in lung adenocarcinoma by inducing cancer stem cell-like properties and epithelial-mesenchymal transdifferentiation. Cancer Res. 2010;70:10433–10444. doi: 10.1158/0008-5472.CAN-10-2638. [DOI] [PubMed] [Google Scholar]
- 52.Van Camp JK, Beckers S, Zegers D, Van Hul W. Wnt signaling and the control of human stem cell fate. Stem Cell Rev. 2014;10:207–229. doi: 10.1007/s12015-013-9486-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.