Abstract
Pancreatic cancer (PC) is usually diagnosed at advanced stage. Our aim was to investigate the risk of malignant and premalignant pancreatic lesions in individuals with family history of PC. Individuals at risk of PC were enrolled prospectively in a screening program in Taiwan. All risk individuals received genetic testing of cationic trypsinogen (PRSS1) gene and the serine protease inhibitor Kazal type 1 (SPINK1) gene. They were stratified into three risk groups (high, moderate, and low) based on the family history and genetic testing. Magnetic resonance imaging (MRI) with magnetic resonance cholangiopancreatogram (MRCP) were performed in all screened individuals. A total of three hundred and three risk individuals in 165 families were enrolled with the mean age of 51.1 years, 38.3% of whom were male. A total of 24 of 303 (7.9%) screened individuals had the PRSS1 mutation, and 7/234 (0.3%) had the SPINK1 mutation. Nineteen (6.3%) risk individuals had pancreatic pathology including seven with pancreatic cancer, and four with pancreatic mucinous neoplasms. The earliest age of onset of PC in affected members was an independent factor associated with risk of developing PC in all risk groups. DM was associated with much-increased risk of developing PC in low and moderate risk groups (OR45.8. 95% CI. 13.82-151.64, P=0.001). Combined family history of non-PC malignancy in the family in the low-risk individual was associated with abnormal findings on MRI (OR8.4, 95% CI 3.29-21.88, P < 0.0001). There was no any complication of screening. In summary, pancreatic cancer screening may benefit in risk individuals with family history of pancreatic cancer in our population. The diagnostic yield is similar to prior studies. MRCP as initial screening modality is safe and effective. Future study will be needed to tailor PC screening strategy in different risk populations.
Keywords: Pancreatic cancer, cancer screening, risk individual, diabetes, cohort
Introduction
Pancreatic cancer is one of the most lethal human cancers and predicted to be the second leading cause of cancer death by the year 2030 [1,2]. The number of the newly diagnosed pancreatic cancer has increased significantly in recent years in the World and Taiwan [3]. The most common histologic type of pancreatic cancer is adenocarcinoma [4]. Pancreatic cancer carries the worst prognosis of any cancer with median survival 6 months and 5-year survival around 5%. Surgical resection, the only potentially curative measure, is possible in 15 to 20% of cases and of these patients, median 5-year survival is less than 10% [5,6]. The advanced incurable stage at which most patients with pancreatic cancer present clinically and delayed diagnosis are the primary reasons for the poor prognosis associated with pancreatic cancer [7]. In the majority of the risk individual, pancreatic cancer has progressed before clinical manifestation and hardly to be detected in an early resectable stage. There is an urgent need to detect small asymptomatic cancers or precursor lesions, which are potentially curable for the most devastating disease.
A recently published study suggested that there may be a large window with an excellent opportunity for detecting pancreatic cancer in earliest resectable stage [8]. Therefore, identification of high-risk populations and early detection through screening become essential to reduce the mortality rate of pancreatic cancer. Some distinct clinical and/or genetic features are thought to increase the risk of developing pancreatic cancer [9]. It has been estimated that about 10-15% of pancreatic cancer has a familial basis [10,11]. Hereditary pancreatic cancer implied inherited cancer syndromes (with a known germline mutation associated with an increased risk of pancreatic cancer, including familial breast cancer (BRCA2), hereditary genetic pancreatitis (cationic trypsinogen, PRSS1 and serine protease inhibitor Kazal type 1, SPINK1) et al. [10]. Familial Pancreatic cancer (FPC) has defined as at least two first-degree relatives with pancreatic cancer with not yet identified a genetic abnormality. The National Familial Pancreas Tumor Registry (Johns Hopkins University) showed different increased risk of pancreatic cancer as number of PC in familial pancreatic cancer family (increased the risk of 4.6-, 6.4-, and 32-folds of pancreatic cancer in individuals with 1, 2, and ≥3 affected first-degree relatives, respectively) [4]. An international consortium held to discuss pancreatic cancer screening recommended screening pancreatic cancer in high-risk populations, including individuals with the lifetime risk of pancreatic cancer over 5% or/and increased relative risk over five times for early detection to improve the prognosis of pancreatic cancer [12]. There is no standard protocol for pancreatic cancer screening. A trend of screening programs by the combination of imaging modalities was suggested [7,8]. Consensus practice recommendations, based on expert opinion, suggest selecting individuals with a threshold of a >10-fold increased risk of developing pancreatic for screening [7,9]. However, the efficacy and benefit of screening individuals with moderate risk (5- to 10-fold increased) and conventional low risk (3-5 fold increased) were not well-understood. In Taiwan, the incidence of pancreatic cancer is increasing as other western countries, with the incidence of 10-11/100,000 persons. The increase of the incidence is marked especially in the female population with a 25% increase within past years. We did not know whether the phenomenon related to predisposition genetic factors or the environmental factor. Familiar pancreatic cancer is regarded to be less common in our population compared to western countries, and there is no formal report to address this issue in the literature. Whether the pancreatic cancer screening is only beneficial in high-risk individuals or also in moderate or conventional low-risk groups has never been compared.
We started a pancreatic cancer screening program at our multidisciplinary pancreas center since the year of 2003 and we enrolled all risk individuals with a family history of pancreatic cancer who were interested in screening. All risk individuals were risk-stratified into high (estimated over 10 times risk), moderate (estimated 5-10 times risk), and conventional low-risk groups (below 5 times risk) based on family history and genetic testing for PRSS1 and SPINK1, and screened with imaging. We report our initial finding for pancreatic cancer screening.
Patients and methods
Study design
We enrolled risk individuals with a family history of pancreatic cancer and who were interested in their risk of disease in a prospective cohort starting from the year of 2003 in National Taiwan University Hospital, the largest tertiary referred center for pancreatic cancer in Taiwan. The screening program included a detailed history and physical examination, collection of family history, personal and family health history, results of all imaging and blood testing, and storage of frozen serum and any surgically resected tissue if obtained. Blood was processed for DNA extraction. This study was approved by our Institutional Review Board in the National Taiwan University Hospital and all participants provided informed consent.
Screening program
In initial visit, risk individuals were assessed for their level of risk according to the number of pancreatic cancer in family members, the age of onset of pancreatic cancer in family members (including the earliest age of onset), family history of non-pancreatic cancer (PC) malignancies, body mass index (BMI) at initial screening and genetic testing of PRSS1 and SPINK1 (Figure 1). Risk of pancreatic cancer were classified into three categories: High(H) risk with over 10 times of risk of PC than normal population; moderate (M) risk with about 5-10 times of risk of PC than normal population; and low(L) risk with risk of PC below 5 times. In H groups were further classified into H1, H2, H3 and H4 subgroups. Risk individuals with a family history and genetic testing consistent with one of the genetic cancer syndromes (BRCA2, PRSS1, and SPINK1) were classified as the H1 group. Risk individuals with any three affected relatives not belonging H1 group were classified as the H2 group. Risk individuals with two first-degree relatives and not belonging H1 group with pancreatic cancer were classified to H3 group. Risk individuals with one first-degree and at least one second-degree relatives, one with onset at age < 55 were classified as the H4 group. The M groups were further classified into M1 and M2 subgroups. Individuals were classified as moderate risk including M1 and M2 subgroups. Individuals with over 2 family history of PC in any degree who did not fit high risk criteria were classified as MR group. Individuals with family history of one first-degree family member with the onset of disease below 55 years of age but did not meet the criteria for high-risk classification were classified into MR group. Risk individuals were classified as conventional low risk (L group) if they had only one family member affected at age >55 years. These low risk (L group) individuals were conventionally not recommended for screening. All of the screened individuals received MRI or/and EUS of the pancreas as well as serum cancer antigen 19-9 (CA 19-9) and lipase examination. Annual MRI was arranged if any abnormality on initial MRI imaging. For risk individuals without any abnormal findings in initial screening (including blood testing, MRI with MRCP, genetic test), follow up MRI/MRP was arranged every 2-3 years.
Figure 1.
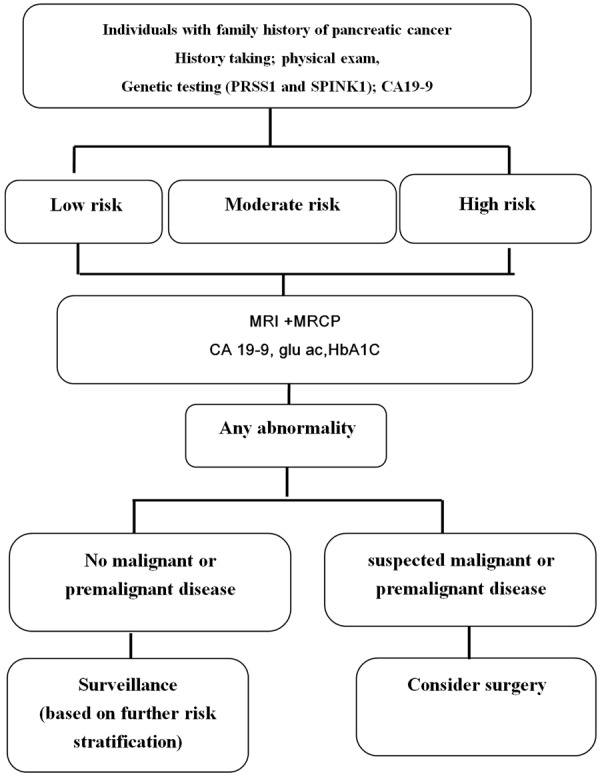
Pancreatic cancer (PC) screening algorithm used in our cohort.
Genetic testing
Genetic counseling was provided by a clinician with particular expertise in pancreatic cancer genetics before and after the test with signed informed consent obtained. Mutations of PRSS1 and SPINK1 gene were analyzed by direct sequencing for all screened risk individuals as previously described [13]. Individuals with family history of both breast cancer and pancreatic cancer were offered BRCA1 and BRCA2 testing by next generation sequencing (Siva Genomics, Taipei, Taiwan). In the case of documented BRCA2 mutations, prophylactic mastectomy and enhanced screening were discussed.
Pancreatic imaging
MRI and MRCP
MRI images were obtained at 1.5 Tesla (GE Signa HDxt; Siemens Sonata) and 3.0 Tesla (Siemens TrioTim, Verio and Biograph mMR) using a body array coil for reception according to corresponding machine. All patients had fasted for at least 4 hours. The fast spin-echo for T1-weighted and T2-weighted with fat suppression were performed through the liver, pancreas and kidney in axial plane. The double-echo IP/OP sequence was sequentially performed with the same coverage. The optional single-shot echo-planar DWI was performed in 3.0 Tesla MRI machine. The biliary tree and pancreatic duct were imaged on a 4-mm-thin slice half-Fourier acquisition single-shot turbo spin-echo in coronal and axial planes and on a 50-mm thick slice rapid acquisition with relaxation enhancement in 8 different orientations. Finally, a dynamic (arterial, portal and equilibrium phases) three-dimensional T1 gradient echo sequences (liver accelerated volume acquisition for GE machine and volumetric interpolated breath-hold examination for Siemens machine) were obtained and following injection of 0.1 mmol/kg of gadodiamide, gadobutrol or gadoteric acid contrast in individuals with glomerular filtration rate >30 mL/minute as estimated from serum creatinine. Images were reviewed by experienced radiologists (C.H.W. B.B.C., and P.C.L.), but blinded to the pancreatic cancer risk factors of risk individuals.
Endoscopic ultrasound (EUS) and fine needle aspiration (FNA)
Endoscopic ultrasound (EUS) was a complementary examination in our screening program. All EUS procedures were performed by an experienced endosonographer (Y.T.C) with a curvilinear echoendoscope (Olympus UCT 240 or UCT 260, Olympus Corporation). Abnormalities of interest included mass lesions, IPMNs, cysts, and chronic pancreatitis-like parenchymal changes. Fine needle aspiration (FNA) under EUS guidance was done with a linear array echoendoscope when mass lesions, cysts, or suspicious lymph nodes were encountered. In general, FNA specimens were evaluated for abnormal cells by an onsite cytopathologist, and the cystic fluid aspirated was sent for carcinoembryonic antigen (CEA), CA19-9, amylase and lipase.
Follow-up
After examination of the first series of genetic, radiologic, and blood testing, risk individuals were evaluated by gastroenterologists, radiologists, surgeons, and oncologists as clinically indicated. The possibility of surgical intervention and the procedure were discussed. Findings including resectable solid mass lesions, a high suspicion for main-duct IPMN (IPMN-M), or abnormal cytology on EUS-FNA were among those considered for surgery. Risk individuals without surgical attempt were again risk-stratified based upon history and testing, and entered surveillance. High risk individual who received pancreatectomies was followed by MRI and/or EUS. Risk individual at moderate risk underwent annual imaging, and those at conventional low risk returned for annual visits and further testing if they developed symptoms or new onset diabetes mellitus.
Statistical analysis
The data are expressed as mean or median and/or range as appropriate. The quantitative variables were compared using Student’s t-test and the qualitative variables using the χ2 test or Fisher’s exact test as appropriate. We estimated strength of association by calculating the odds ratio (OR). All tests were 2-tailed, with statistical significance set at P < 0.05. All analyses were performed with the SPSS software package version 17 (SPSS, Chicago, IL, USA).
Results
Risk individuals -risk stratification by genetic testing and numbers of PC in their family
Three hundred and three potentially risk individuals, enrolled from 2003 to 2015, from 165 unrelated families received initial screening. The mean age at screening was 51.1 years (range, 24-88). The mean follow up time in our program was 6.5 years (ranging 1-12 years). There were 116 (38.3%) male persons, 62 (20.5%) had a previous history of smoking, and 18 (5.9%) had a history of alcohol use (Table 1). They were 119 high risk individuals, 32 moderate risk individuals and 152 low risk individuals. There were 24 (7.9%) of risk individuals with Diabetes mellitus (DM) when initial screening. There were all Han Chinese. There were three risk individuals (0.99%) had a personal history of cancer, including one gastric cancer, one breast cancer, and one thyroid papillary cancer.
Table 1.
Characteristics of risk individuals
| Number of risk individuals | 303 |
| Number of unique families | 165 |
| Mean age (SD), years | 51.1±13.9 |
| Male gender (%) | 116 (38.3%) |
| Ever smoked (%) | 62 (20.5%) |
| Alcohol use (%) | 18 (5.9%) |
| Type 2 diabetes mellitus (%) | 24 (7.9%) |
| Number of patients with a personal history of nonpancreatic cancer (%) | 3 |
| Gastric adenocarcinoma | 1 |
| Breast adenocarcinoma | 1 |
| Thryoid papillary ca | 1 |
PRSS1 and SPINKI mutation
All screened risk individuals had received genetic testing for PRSS1 and SPINK1 mutations. Of all the risk individuals tested, there were 24 (7.9%) persons with mutations of PRSS1 gene and 7 (0.3%) with SPINK1 mutations (Table 2).
Table 2.
Genetic testing of risk individuals
| Overall (n=303) | |
|---|---|
| PRSS1 mutation individual | 24/303 (7.9%) |
| PRSS1 family members | 47/303 (15.5%) |
| SPINK1 mutation individual | 7/303 (0.3%) |
| SPINK1 family members | 17/303 (5.6%) |
| BRCA1/2 mutation individual | 1 (0.3%) |
Family history of pancreatic cancer
Forty-seven (15.5%) risk individuals had at least two first-degree relatives with pancreatic cancer. Sixty-eight (22.4%) risk individuals had at least two first-, second-, or third-degree relatives with pancreatic cancer (Table 3).
Table 3.
Family cancer risk profiles for the risk individuals in our cohort
| Median youngest age (range) of PC onset in family, years | 63.0 (24-88) |
| Risk category, number (%) | |
| High risk | 119 (39.3%) |
| H1 | 65 |
| H2 | 23 |
| H3 | 25 |
| H4 | 6 |
| Moderate risk | 32 (10.6%) |
| M1 | 1 |
| M2 | 31 |
| Low risk | 152 (50.2%) |
| Number of risk individual with family history suggestive of | |
| Familial pancreatic cancer | 66 |
| BRCA1/2 | 1 |
| Hereditary pancreatitis, PRSS1, family | 47 |
| Hereditary pancreatitis, S: PINK1, family | 17 |
| Number of risk individual with | |
| ≥3 first-degree relatives with PC | 9 |
| 2 first-degree relatives with PC | 38 |
| 1 first-degree relative with PC | 223 |
| 0 first-degree relatives with PC | 33 |
| Number of risk individual with | |
| ≥4 first-, second-, and third-degree relatives with PC | 25 |
| ≥3 first-, second-, and third-degree relatives with PC | 2 |
| 2 first-, second-, and third-degree relatives with PC | 41 |
| 1 first-, second-, and third-degree relative with PC | 235 |
| Number of risk individual with | |
| 2 first-, second-, and third-degree relatives with non-PC | 5 |
| 1 first-, second-, and third-degree relative with non-PC | 88 |
| 0 first-, second-, and third-degree relatives with non-PC | 210 |
| Number of risk individual with first- or second-degree relatives with | |
| Colon cancer | 65 |
| Gastric cancer | 22 |
| Breast cancer | 35 |
| Cervical cancer | 4 |
| Thyroid papillary cancer | 4 |
| Lung cancer | 7 |
| Renal cell carcinoma | 1 |
| Hepatocellular carcinoma | 1 |
Abbreviation: PC: pancreatic cancer; PRSS1, cationic trypsinogen gene [protease, serine, 1 (trypsin 1) (PRSS1)]; SPINK1: and the pancreatic secretory trypsin inhibitor gene [serine peptidase inhibitor, Kazal type 1.
Family history of non-pancreatic cancer malignancy and cancer syndrome
The family history of non-pancreatic cancer malignancy was collected. There were 17 (7.3%) risk individuals had two or more first-, second-, or third-degree relatives with non-pancreatic cancer. There were 93 (30.7%) risk individuals had one or more first-, second-, or third-degree relatives with non-pancreatic cancer. Colon cancer was the most common extra-pancreatic cancer (21.4% of all risk individuals), followed by breast cancer (11.6%), gastric cancer (7.2%), lung cancer (2.3%), cervical cancer (1.3%), thyroid papillary cancer (1.3%), renal cell carcinoma (0.3%), and hepatocellular carcinoma (0.3%) (Table 2). In the families with both pancreatic cancer and colon cancer, the age of onset was all above 45 years old. All these family did not fit the criteria for Lynch syndrome [14].
There were 26 risk individuals, belonging to 9 families, having a family history of both pancreatic cancer and breast cancer. There were 23 risk individuals, belonging to 8 families, received the genetic test for BRCA1 and BRCA2 genes. There was only one individual had mutated BRCA2 gene. The risk individual’s mother had metachronous breast cancer and pancreatic cancer. The risk individual received consultation and detailed examination in gynecological and breast field and did not reveal any abnormality.
Risk stratification
All evaluated risk individuals were stratified into conventional low risk, moderate risk, or high risk group based on the criteria in this cohort (Figure 1). One hundred and nine (39.2%) risk individuals were classified as high risk, 32 (10.6%) as moderate risk, and 152 (50.1%) as “conventional low” risk (Table 3). There were 65 risk individuals with genetic syndrome with PC, defined by H1 risk group. They included 1 BRCA2 syndrome, 47 risk individuals (in 16 unrelated families) belonging to PRSS1 families, 17 risk individuals (in 5 unrelated families) belonging to SPINK1 families. There were 23 risk individuals (without genetic syndrome) with at least three first-, second-, or third-degree with PC, defined as the H2 risk group. There were 25 risk individuals (without genetic syndrome) with 2 first-degree with PC, defined H3 risk group. There was 6 risk individuals (without genetic syndrome) with one first and 1 second-degree with PC; 1 at < 55 years old, defined H4 risk group. In the moderate risk group, there were one risk individuals with at least two first-, second-, or third-degree with PC who did not meet any criteria for the high-risk category, defined as the M1 risk group. There were 31 risk individuals with at one first-degree with PC < 55 years old who did not meet any criteria for the high-risk category, defined as the M2 risk group. There were 152 low- risk individuals had one first- degree relative with PC who did not meet any criteria for high or moderate risk category (Tables 2, 3).
Serum CA19-9 and lipase
Of the enrolled 303 risk individuals, there were 85 (28.1%) risk individuals with at least once elevation of CA19-9. There were 142 of 234 (46.8%) risk individuals with the elevation of lipase for over six months.
MRI with MRCP screening
Of the enrolled 303 risk individuals, all the individuals received MRI/MRCP examination (Table 4). There were 128 (42.2%) risk individuals had any abnormalities on MRI and 97 (32.0%) risk individuals with focal lesions (Table 4). There were 47 (15.51%) risk individuals with the solid mass lesion and 54 (17.8%) with cystic lesions. Of the 54 persons with cystic lesions, there were 11 people with multiple cystic lesions. There were 47 (15.5%) cystic lesions fulfilled the imaging criteria of IPMN. There were 68 (22.4%) risk individuals had MRI/MRCP findings suggestive chronic pancreatitis. The most common non-focal abnormal findings on MRI/MRCP were irregularities of the main pancreatic duct, parenchymal changes. Ten of the 54 initially screened cystic lesions resolved in the follow-up MRI/MRCP examination.
Table 4.
Diagnostic findings in the pancreas for risk individuals: low, moderate and high risk groups
| Overall (n=303) | High (n=119) | Moderate (n=32) | Low (n=152) | |
|---|---|---|---|---|
| MRI/MRCP | ||||
| Any pancreatic lesion | 128 (42.2%) | 56 (47.1%) | 11 (34.4%) | 61 (40.1%) |
| Any focal lesion | 97 (32.0%) | 44 (37.0%) | 5 (15.6%) | 48 (31.6%) |
| Solid mass lesion | 47 (15.5%) | 23 (19.3%) | 5 (15.6%) | 19 (12.5%) |
| Cystic lesion* | 54 (17.8%) | 22 (18.5%) | 0 (0.0%) | 32 (21.1%) |
| IPMN* | 47 (15.5%) | 20 (16.8%) | 0 (0.0%) | 27 (17.8%) |
| Others cystic | 6 (1.9%) | 1 (0.8%) | 0 (0.0%) | 5 (3.3%) |
| Chronic pancreatitis change with PD irregularity | 68 (22.4%) | 31 (26.1%) | 8 (25.0%) | 29 (19.1%) |
| EUS | 18 | 8 | 0 | 10 |
| FNA | 11 | 5 | 0 | 6 |
| Of mass or parenchyma | 6 | 2 | 0 | 4 |
| Of cysts or IPMN lesions | 6 | 2 | 0 | 4 |
| Pancreas surgery | 19 | 6 | 2 | 11 |
| Pathology | ||||
| Chronic pancreatitis | 3 | 1 | 1 | 1 |
| Neuroendocrine tumor | 1 | 0 | 0 | 1 |
| IPMN | 3 | 3 | 0 | 0 |
| Ductal adenocarcinoma | 7 | 2 | 1 | 4 |
| SPEN | 1 | 0 | 0 | 1 |
| Mucinous cystic tumor | 3 | 0 | 0 | 3 |
| Serous cystadenoma | 1 | 0 | 0 | 1 |
P < 0.05 between 3 groups.
Abbreviation: PD, pancreatic duct; EUS: endoscopic ultrasound; FNA: fine needle aspiration; IPMN, intraductal papillary mucinous neoplasm.
There were 18 (5.9%) risk individual underwent EUS after MRI examination because of suspected or uncertain abnormality, or MRI/MRCP findings indicating surgery but they hesitated about surgery at that time point. There were 11 of 18 risk individuals received fine needle aspiration (FNA). Among them, there were six persons received EUS-FNA for solid lesions and 6 for cystic lesions. There were 3 of 6 aspirated solid lesions showed cellular atypia. All these three risk individuals received surgery, and one had stage 1 pancreatic adenocarcinoma (0.7 cm, pT1N0M0), two had chronic pancreatitis with pseudotumor formation. The other three aspirated solid lesions revealed benign acinar and ductal cells and these three risk individuals lived well in the follow up. There were three risk individuals received EUS-FNA for screened asymptomatic cystic lesions in MRI with MRCP. The pre-EUS imaging diagnosis of these three risk individuals was MCN. The diagnosis of EUS-FNA was two MCNs and one pseudocyst. The two risk individuals having the diagnosis of MCN received surgery with the same pathological diagnosis. There was no any complication developed in screening process.
Pathologic diagnosis
A total of 18 risk individuals had pancreas pathology, including 17 surgical specimens from pancreatectomy and one from CT-guided biopsy (Table 4). There were including seven ductal adenocarcinomas (Table 4). Some of the surgically treated risk individuals refused preoperatively EUS or EUS-FNA because they insisted on receiving surgery for fear of having pancreatic malignancy like their families. Among the seven risk individuals with ductal adenocarcinoma, two had received EUS-FNA before surgery. One 61-year-old woman had cytology showed cellular atypia and the other one 55-year-old man had cytology of adenocarcinoma. The other five risk individuals favored receiving pancreatectomy after abnormal MRI/MRCP findings occurred. All the seven risk individuals were resectable pancreatic cancer when main pancreatic duct abnormality or mass was detected. Among the seven risk individuals, there were two belonging to the high-risk group, one belonging to moderate risk group and four belonging to “conventional low risk group”. The median follow-up period and age starting screening were not different between the three groups. The age of screened pancreatic cancer in the conventional low-risk group was 50.1, 65.2, 83.0 and 61.0 years old with their family having pancreatic cancer diagnosed at 61, 62, 72 and 70 years old. The risk individual with pancreatic cancer, belonging to moderate risk, was a 37-year-old man. He had screened for three years. His mother was diagnosed to have inoperable pancreatic cancer at her 51 years old. One of the two screened risk individuals belonging to the high-risk group was SPINK1 family. The other one screened risk individuals had two first degree relatives diagnosed pancreatic cancer. The other 11 risk individuals received pancreatic surgery with surgical specimens obtained. They included one neuroendocrine tumor, 3 IPMN, one solid and papillary epithelial neoplasm, three mucinous cystic neoplasms and one serous cystadenoma combined with a pseudocyst, and three chronic pancreatitis with pseudotumor formation or pseudocyst (Table 5). One screened risk individuals having main duct type IPMN on initial screening MRI had received Whipple surgery. The pathology disclosed main IPMN with mild dysplasia. The patient developed multiple IPMN on the follow-up MRI with MRCP in the remnant pancreas in the follow-up periods. She had four family members with pancreatic cancer, including three first-degree relatives with early onset pancreatic cancer (age at their forties) and one second-degree relative with age at 72. She refused to receive further pancreatectomy and remained asymptomatic. The other one female patient had suspected branched type IPMN on initial screening MRI and MRCP. The surgical pathology revealed low-grade dysplasia. Follow up MRI/MRCP after surgery showed finding suggestive chronic pancreatitis and did not show any focal lesions.
Table 5.
Predictors of screened pancreatic cancer in risk individuals
| High risk | Low and moderate risk | |||
|---|---|---|---|---|
|
|
||||
| Predictors | P value | OR (95% CI) | P value | OR (95% CI) |
| Earliest Age*,† | 0.004 | 0.90 (0.84-0.97) | 0.003 | 0.86 (0.78-0.95) |
| Gender | 0.717 | 1.74 (0.08-34.26) | 0.405 | 0.34 (0.03-4.36) |
| Smoking | 0.869 | 0.78 (0.04-14.26) | 0.149 | 8.47 (0.46-154.63) |
| BMI ≥25 | 0.620 | 2.05 (0.12-34.52) | 0.944 | 1.09 (0.11-10.75) |
| FH of Non PC cancer | 0.216 | 0.72 (0.32-161.93) | 0.116 | 30.72 (0.43-2189.6) |
| Diabetes† | 0.170 | 8.86 (0.39-20.07) | 0.001 | 45.8 (13.82-151.64) |
Earliest age indicated earliest age of pancreatic cancer in family; BMI: body mass index at early adulthood ≥25;
P < 0.05 in multivariate analysis of predictors in moderately and high risk;
P < 0.05 in multivariate analysis of predictors in low risk.
In summary, a total of 15 of the 18 risk individuals with pathological diagnosis in this cohort were disclosed to have malignant (7 ductal adenocarcinoma, one neuroendocrine tumor, one solid and papillary epithelial neoplasm) or possibly premalignant lesions (3 IPMNs and 3 MCNs). Among these 15 risk individuals, nine individuals belonged to the conventional low-risk group, 5 were in high risk, and 1 was in moderate risk group. They included 9 of 152 (5.9%) low-risk individuals, 5 of 119 (4.2%) high-risk individuals, and 1 of 32 (3.1%) moderate-risk individuals in this pancreatic cancer screening program. The frequencies of histologically confirmed malignancy or pre-malignancy were not statistically different in between H1/H2/H3/H4 subgroups or between M1/M2 subgroups.
Predictors of screened pancreatic cancer
Factors to be reported to be related increased risk of pancreatic cancer were analyzed in univariate and multivariate analysis, including age (starting screening), gender, history of smoking, alcohol, presence of DM, follow-up periods, the earliest age of PC in their families, number of FH of non-pancreatic cancer in their families, and body mass index. DM was the only one risk factor predicting pancreatic cancer in all risk individuals or stratified risk groups in the univariate and multivariate analysis (Table 5). In the high risk group, earliest age of pancreatic cancer in their family was associated with risk of developing pancreatic cancer (odds ratio 0.90, 95% CI: 0.84-0.97, P=0.004). In the high risk group, earliest age of pancreatic cancer in their family and DM were associated with risk of developing pancreatic cancer (earlier age of PC in their family odds ratio (OR): 0.86, 95% CI. 0.78-0.95, P=0.003; DM, OR: 45.8, 95% CI. 13.8-151.6, P=0.001, Table 5).
Predictors of screened any abnormality in pancreas on MRI/MRCP
Factors including age (starting screening), gender, history of smoking, alcohol, presence of DM, follow-up periods, the earliest age of PC in their families, the number of FH of non-pancreatic cancer in their families, and body mass index were analyzed in univariate and multivariate analysis (Table 6). In the low and moderate risk group, positive family history of non-PC cancers had increased risk of having abnormal findings on screening MRI/MRCP (odds ratio 8.48, 95% CI 3.29-21.88, P < 0.0001) in multivariate analysis. In the high risk group, positive family history of non-PC cancers did not increase the risk of having abnormal findings on screening MRI/MRCP (odds ratio 1.77, 95% CI 0.68-4.64, P=0.243) in multivariate analysis in our cohort (Table 6).
Table 6.
Predictors of any lesion in MRI screening in risk individuals
| High risk | Low and moderate risk | |||
|---|---|---|---|---|
|
|
||||
| Predictors | P value | OR (95% CI) | P value | OR (95% CI) |
| Earliest Age | 0.618 | 0.99 (0.97-1.02) | 0.064 | 0.98 (0.96-1.001) |
| Gender | 0.597 | 0.78 (0.31-1.97) | 0.305 | 0.65 (0.29-1.47) |
| Smoking | 0.091 | 2.58 (0.86-7.76) | 0.389 | 0.64 (0.23-1.79) |
| BMI ≥25 | 0.142 | 0.44 (0.15-1.32) | 0.175 | 0.54 (0.23-1.31) |
| FH of Non PC cancer† | 0.243 | 1.77 (0.68-4.64) | < 0.0001 | 8.48 (3.29-21.88) |
| DM | 0.503 | 0.62 (0.15-2.51) | 0.734 | 1.28 (0.31-5.24) |
Earliest age indicated earliest age of pancreatic cancer in family; BMI: body mass index at early adulthood ≥25; *P < 0.05 in multivariate analysis of predictors in high risk;
P < 0.05 in multivariate analysis of predictors in low and moderately risk.
Discussion
We describe the results of the pancreatic cancer screening program in a prospective cohort of risk individuals with a family history of pancreatic cancer, stratified by conventional low (< 5 times risk), moderate (5-10 times risk) and high risk (>10 times risk) in Taiwan. Our protocol recruited some conventional “low” risk individuals compared to other reported screening programs in the literature [15,16]. We enrolled risk individuals more broadly than other criteria used before, especially the siblings who were stratified as conventional “low” risk group before. Our strategy was to stratify the risk of each risk individual into low, moderate, and high risk group according to and modified from the consensus conference [8]. Overall, there were 15 of 303 (4.9%) of risk individuals with pathologically diagnosed with a malignant or premalignant disease of the pancreas. There were 128 of 303 (42.2%) risk individuals with any abnormality on MRI/MRCP imaging. The frequency of tissue proved premalignant or malignant pancreatic lesions in high, moderate and (conventional) low-risk group was not statistically different. The results were unexpected because of there was no difference between these high and conventional “low” risk groups. There is consensus for screening of pancreatic cancer in high risk population [11,15,17], especially those inherited pancreatic cancer syndromes with known germline mutations [15,16]. Familial pancreatic cancer (FPC) is a syndrome with undetermined genetic susceptibility. Most of the studies recommend to screening PC in FPC [15,17-23]. One recent study demonstrated that the benefit of surveillance in families with FPC is less evident than germline genetic pancreatic cancer syndromes [15]. In the past, there was little report focusing on conventional low-risk group (risk below five times of normal population). Verna et al. had reported a screening program with different risk individuals including five average risk individuals and 45 moderate or high individuals [17]. Among the five average risk individuals, there were no any premalignant or malignant pathology could be identified [15]. There were 10% high-risk individuals and 14% moderate risk individuals with premalignant or malignant pancreatic pathology [15]. Our cohort had enrolled 152 low-risk individuals. There were 4 pancreatic adenocarcinoma and 3 mucinous cystic neoplasms in the “low-risk” group. In our study, the frequency of premalignant and malignant pathology was not statistically different from the high-risk group. The finding of screening outcome in our stratified three groups arose our awareness of screening of pancreatic cancer for “conventional low” risk group in our population. If we took a look at the frequencies of pancreatic lesions detected by MRI, there was no difference between low, moderate and high-risk groups (Table 4). Are those conventional “low” risk individuals truly low risk and are not worthy being screened need further investigation.
Patient with pancreatic cancer is diagnosed at the advanced stage if symptoms occur. Screening for pancreatic cancer is an option for high-risk individuals to allow early detection and treatment of curable pancreatic neoplasms at a pre-invasive stage [7]. Screening is suggested in high-risk populations, including individuals with the lifetime risk of pancreatic cancer over 5% or/and increased relative risk over five times [15,16,18-23]. In moderate and high-risk individuals, our cohort confirms the results that malignant and premalignant lesions can be identified through screening with similar findings.
The best modality to screen pancreatic cancer in risk individuals is under debate. We did not compare the differences and diagnostic yield of screening imaging modalities as previous study [11]. In our protocol, all the screened risk individuals received MRI with MRCP at the initial visit. EUS or EUS-FNA were suggested and performed for those with uncertain findings on MRI or who requested EUS-FNA as a complementary examination. In Taiwan, both the fee for anesthesia for endoscopic examination and the needle used in FNA were not covered by our insurance. Furthermore, many of risk individuals hesitated about an invasive procedure. These were the reasons we used non-invasive MRI with MRCP as the mainstream of screening tool. A recently published result of a multi-center prospective screening program for high-risk individuals with both MRI and EUS demonstrated that both these two imaging techniques were complementary [24]. In their report, EUS and/or MRI detected clinically relevant pancreatic lesions in 6% of high-risk individuals, the same frequency in our cohort mainly based on MRI.
Currently, it is still not known what kind of factors could predict the higher diagnostic yield within high-risk groups. In high risk group, we had two individuals had screened pancreatic cancer. Multivariate analysis demonstrated that the earliest age of developing pancreatic cancer in the family was the only independent predictor for screened pancreatic cancer (Table 5). In low and moderate risk groups, the earliest age of developing pancreatic cancer in family and DM were both independent predictors for screened pancreatic cancer in our cohort (Table 5). In the high risk group, 56 of 119 (47.1%) individuals had any pancreatic lesions on MRI/MRCP imaging. Risk individuals with family history of non-PC cancers were associated with higher risk developing any pancreatic lesions disclosed by MRI/MRCP imaging, in “moderate or low” risk groups (Table 6). Risk individuals, whatever the risk it fell, combined with a family history of non-PC predicting a higher risk of any pancreatic abnormalities on MRI examination. It means that we might consider encouraging individuals at risk to receive screening, including the traditional moderate and low-risk individuals in addition to the traditional high risk individuals.
In our cohort, we examined both PRSS1 and SPINIk1 mutations by direct sequencing for all screened individuals in a screening program. The mutation rate of PRSS1 and SPINK1 gene in all screened individuals were 24/303 (7.6%) and 7/303 (0.3%) respectively. The presence of PRSS1 or SPINK1 mutation was not associated with higher risk of disclosing any pancreatic lesion or pancreatic cancer. In the current study, we also investigated the role of BMI in predicting pancreatic cancer or any abnormalities of pancreas on imaging. In multivariate analysis, we did not demonstrate that the initial BMI was associated with risk of pancreatic cancer or pancreatic abnormalities on MRI. The role of BMI in the risk of developing pancreatic cancer in different risk groups needs further study.
The significance of focal lesions on imaging finding and the natural history of these pathologic lesions (such as IPMN) are not known. Therefore, there exists a potential risk of over-diagnosis and overtreatment in people who receive pancreatic cancer screening. In reported series, many risk individuals received pancreatic surgery had a benign pathological diagnosis. In our cohort, none of the risk individuals in our cohort who received surgery had high-grade dysplasia of IPMNs. Pancreatic surgery did carry some significant risk of morbidity and mortality for screened individuals, especially they were asymptomatic. Although it is accepted and suggested that focal solid mass lesions found in risk individuals at high risk should be resected, the optimal management modified by other predictors is still unknown. The natural history of IPMN remains poorly predicted, even in the high-risk group. In our cohort, one female high-risk individual had received Whipple surgery for the main IPMN when she was 52 years old. She had a family history of 3 first-degree relatives and one second-degree relative with pancreatic cancer. Multifocal metachronous branched IPMNs were diagnosed by EUS-FNA when she was 58 years old. She is now asymptomatic ten years after Whipple surgery. In those risk individuals who received surgery, it is possible that multifocal PanIN lesions disclosed on pathology might have prognostic implications for the remnant pancreas, and these risk individuals might need longer surveillance.
Pancreatic cancer is a challenging disease, both in prevention and treatment. Although premalignant lesions such as IPMN and PanIN have been disclosed in screening program, optimal screening and resection to avoid overtreatment are very important. Pancreatic cancer screening might benefit risk individuals with family history of pancreatic cancer. Our cohort had firstly demonstrated that individuals with the conventional low “risk” of pancreatic cancer had similar frequencies to have screened pancreatic cancer. Low risk individuals with one family having pancreatic cancer has much-increased risk of PC if they had diabetes mellitus. Conventional “low” risk individual combined with family history of one non-PC pancreatic cancer might predict a higher risk of any pancreatic abnormalities disclosed by MRI/MRCP. All these low-risk individuals had abnormal MRI/MRCP findings continued surveillance in our cohort. Whether these results turned out to be high-grade premalignant lesions need to be followed in the future. A comprehensive, multidisciplinary approach that combines imaging, family history of both pancreatic cancer and non-PC should be taken into consideration in pancreatic cancer screening in our population.
In summary, pancreatic cancer screening may benefit in risk individuals with family history of pancreatic cancer in our population. The diagnostic yield is similar to prior studies. MRCP as initial screening modality is safe and effective. Future study will be needed to tailor PC screening strategy in different risk populations.
Acknowledgements
Ministry of Health and Welfare (MOHW): MOHW103-TD-B-111-04, MOHW104-TDU-B-211-124-002 and MOHW105-TDU-B-211-134005; Ministry of Science and Technology (MOST): MOST 102-2321-B-002-083-, MOST 103-2321-B-002-048-, and MOST 104-2321-B-002 -009-; Taiwan Pancreas Foundation. This work was ever not presented or published before.
Disclosure of conflict of interest
None.
References
- 1.Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet. 2016;388:73–85. doi: 10.1016/S0140-6736(16)00141-0. [DOI] [PubMed] [Google Scholar]
- 2.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 3.Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144:1252–1261. doi: 10.1053/j.gastro.2013.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klein AP, Brune KA, Petersen GM, Goggins M, Tersmette AC, Offerhaus GJ, Griffin C, Cameron JL, Yeo CJ, Kern S, Hruban RH. Prospective risk of pancreatic cancer in familial pancreatic cancer kindreds. Cancer Res. 2004;64:2634–2638. doi: 10.1158/0008-5472.can-03-3823. [DOI] [PubMed] [Google Scholar]
- 5.Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371:1039–1049. doi: 10.1056/NEJMra1404198. [DOI] [PubMed] [Google Scholar]
- 6.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 7.Lennon AM, Wolfgang CL, Canto MI, Klein AP, Herman JM, Goggins M, Fishman EK, Kamel I, Weiss MJ, Diaz LA, Papadopoulos N, Kinzler KW, Vogelstein B, Hruban RH. The early detection of pancreatic cancer: what will it take to diagnose and treat curable pancreatic neoplasia? Cancer Res. 2014;74:3381–3389. doi: 10.1158/0008-5472.CAN-14-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yachida S, Jones S, Bozic I, Antal T, Leary R, Fu B, Kamiyama M, Hruban RH, Eshleman JR, Nowak MA, Velculescu VE, Kinzler KW, Vogelstein B, Iacobuzio-Donahue CA. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467:1114–1117. doi: 10.1038/nature09515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Overbeek KA, Cahen DL, Canto MI, Bruno MJ. Surveillance for neoplasia in the pancreas. Best Pract Res Clin Gastroenterol. 2016;30:971–986. doi: 10.1016/j.bpg.2016.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whitcomb DC, Shelton C, Brand RE. Genetics and genetic testing in pancreatic cancer. Gastroenterology. 2015;149:1252–1264. e4. doi: 10.1053/j.gastro.2015.07.057. [DOI] [PubMed] [Google Scholar]
- 11.Chang MC, Wong JM, Chang YT. Screening and early detection of pancreatic cancer in high risk population. World J Gastroenterol. 2014;20:2358–2364. doi: 10.3748/wjg.v20.i9.2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brand RE, Lerch MM, Rubinstein WS, Neoptolemos JP, Whitcomb DC, Hruban RH, Brentnall TA, Lynch HT, Canto MI. Advances in counselling and surveillance of patients at risk for pancreatic cancer. Gut. 2007;56:1460–1469. doi: 10.1136/gut.2006.108456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang YT, Wei SC, L PC, Tien YW, Jan IS, Su YN, Wong JM, Chang MC. Association and differential role of PRSS1 and SPINK1 mutation in early-onset and late-onset idiopathic chronic pancreatitis in Chinese subjects. Gut. 2009;58:885. doi: 10.1136/gut.2007.129916. [DOI] [PubMed] [Google Scholar]
- 14.Jasperson K, Burt RW. The Genetics of Colorectal Cancer. Surg Oncol Clin N Am. 2015;24:683–703. doi: 10.1016/j.soc.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Vasen H, Ibrahim I, Ponce CG, Slater EP, Matthai E, Carrato A, Earl J, Robbers K, van Mil AM, Potjer T, Bonsing BA, de Vos Tot Nederveen Cappel WH, Bergman W, Wasser M, Morreau H, Kloppel G, Schicker C, Steinkamp M, Figiel J, Esposito I, Mocci E, Vazquez-Sequeiros E, Sanjuanbenito A, Munoz-Beltran M, Montans J, Langer P, Fendrich V, Bartsch DK. Benefit of surveillance for pancreatic cancer in high-risk individuals: outcome of long-term prospective follow-up studies from three european expert centers. J. Clin. Oncol. 2016;34:2010–2019. doi: 10.1200/JCO.2015.64.0730. [DOI] [PubMed] [Google Scholar]
- 16.Yu J, Blackford AL, Dal Molin M, Wolfgang CL, Goggins M. Time to progression of pancreatic ductal adenocarcinoma from low-to-high tumour stages. Gut. 2015;64:1783–9. doi: 10.1136/gutjnl-2014-308653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verna EC, Hwang C, Stevens PD, Rotterdam H, Stavropoulos SN, Sy CD, Prince MA, Chung WK, Fine RL, Chabot JA, Frucht H. Pancreatic cancer screening in a prospective cohort of high-risk patients: a comprehensive strategy of imaging and genetics. Clin Cancer Res. 2010;16:5028–5037. doi: 10.1158/1078-0432.CCR-09-3209. [DOI] [PubMed] [Google Scholar]
- 18.Rulyak SJ, Brentnall TA. Inherited pancreatic cancer: surveillance and treatment strategies for affected families. Pancreatology. 2001;1:477–485. doi: 10.1159/000055851. [DOI] [PubMed] [Google Scholar]
- 19.Poley JW, Kluijt I, Gouma DJ, Harinck F, Wagner A, Aalfs C, van Eijck CH, Cats A, Kuipers EJ, Nio Y, Fockens P, Bruno MJ. The yield of first-time endoscopic ultrasonography in screening individuals at a high risk of developing pancreatic cancer. Am J Gastroenterol. 2009;104:2175–2181. doi: 10.1038/ajg.2009.276. [DOI] [PubMed] [Google Scholar]
- 20.Langer P, Kann PH, Fendrich V, Habbe N, Schneider M, Sina M, Slater EP, Heverhagen JT, Gress TM, Rothmund M, Bartsch DK. Five years of prospective screening of high-risk individuals from families with familial pancreatic cancer. Gut. 2009;58:1410–1418. doi: 10.1136/gut.2008.171611. [DOI] [PubMed] [Google Scholar]
- 21.Ludwig E, Olson SH, Bayuga S, Simon J, Schattner MA, Gerdes H, Allen PJ, Jarnagin WR, Kurtz RC. Feasibility and yield of screening in relatives from familial pancreatic cancer families. Am J Gastroenterol. 2011;106:946–954. doi: 10.1038/ajg.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Sukhni W, Borgida A, Rothenmund H, Holter S, Semotiuk K, Grant R, Wilson S, Moore M, Narod S, Jhaveri K, Haider MA, Gallinger S. Screening for pancreatic cancer in a high-risk cohort: an eight-year experience. J Gastrointest Surg. 2012;16:771–783. doi: 10.1007/s11605-011-1781-6. [DOI] [PubMed] [Google Scholar]
- 23.Canto MI, Hruban RH, Fishman EK, Kamel IR, Schulick R, Zhang Z, Topazian M, Takahashi N, Fletcher J, Petersen G, Klein AP, Axilbund J, Griffin C, Syngal S, Saltzman JR, Mortele KJ, Lee J, Tamm E, Vikram R, Bhosale P, Margolis D, Farrell J, Goggins M American Cancer of the Pancreas Screening (CAPS) Consortium. Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology. 2012;142:796–804. doi: 10.1053/j.gastro.2012.01.005. quiz e714-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harinck F, Konings IC, Kluijt I, Poley JW, van Hooft JE, van Dullemen HM, Nio CY, Krak NC, Hermans JJ, Aalfs CM, Wagner A, Sijmons RH, Biermann K, van Eijck CH, Gouma DJ, Dijkgraaf MG, Fockens P, Bruno MJ. A multicentre comparative prospective blinded analysis of EUS and MRI for screening of pancreatic cancer in high-risk individuals. Gut. 2016;65:1505–13. doi: 10.1136/gutjnl-2014-308008. [DOI] [PubMed] [Google Scholar]


