Abstract
Structural imaging studies have consistently found reduced gray matter thickness of the cerebral cortex in schizophrenia, a finding that is evident in first episode psychosis and may be progressive in some cases. Although genetic predisposition and medication effects may contribute to cortical thinning, we hypothesize that the cumulative effects of stress may represent an environmental factor impacting brain morphology in schizophrenia. We examined the relationship between allostatic load, an index of peripheral biomarkers representing the cumulative effects of stress, and cortical thickness. Allostatic load was calculated for 44 patients with schizophrenia spectrum disorders (SSD) and 33 normal controls (NC) based on 13 cardiovascular, neuroendocrine, immune, and metabolic measurements. Controlling for age, SSD had significantly elevated allostatic load as compared with NC (p=0.008). Controlling for age, whole brain average cortical thickness was lower in SSD patients compared to NC (p=0.008). However, once allostatic load was accounted for, the group difference in cortical thickness became marginal (p=0.058). Exploratory analyses on subcomponents of allostatic load suggested that elevated immune marker C-reactive protein, stress hormones, and cardiovascular indices within allostatic load were more strongly associated with reduced cortical thickness in SSD. In NC, only the association between immune marker C-reactive protein and cortical thickness was replicated. These results support the hypothesis that allostatic load may account for some of the gray matter deficits observed in schizophrenia. Among the allostatic indices, the inflammatory mechanism appears particularly relevant to cortical thickness in both schizophrenia patients and normal controls.
Keywords: Schizophrenia, cortical thickness, allostatic load, stress, C-reactive protein
1. Introduction
Loss of gray matter in schizophrenia has been consistently demonstrated in MRI studies through reduced gray matter volume, reduced cortical thickness, and increased cerebral ventricular volume measurements (Gong et al., 2016; Gupta et al., 2015). While clearly not specific to schizophrenia, loss of gray matter is arguably one of the most replicable biomarkers observed in schizophrenia. Although there is mixed evidence regarding cortical thinning prior to onset of psychosis (Jung et al., 2011; Klauser et al., 2015; Sprooten et al., 2013), widespread decreases in cortical thickness are apparent in first episode psychosis (Schultz et al., 2010; Sprooten et al., 2013), and patients with chronic schizophrenia may exhibit greater age-related cortical thinning than controls (van Haren et al., 2011). Outstanding questions regarding these brain morphological abnormalities include clarifying the relative contributions of genetic and environmental factors, and the mechanisms by which cortical gray matter thinning occurs. The high heritability of cortical thickness has led some to suggest cortical thinning could be a marker of the genetic risk for schizophrenia; however, studies do not find considerable cortical thinning in siblings of schizophrenia patients (Goldman et al., 2009) and other studies have not found a relationship between cortical thickness and polygenic risk scores for schizophrenia (Voineskos et al., 2015).
Alternatively, cortical thinning in schizophrenia may have a strong environmental (including potential environment x gene) origin. Of the myriad proposed developmental and environmental risk factors for schizophrenia, stress may be a top candidate that can negatively impact the cortical gray matter. In animal models, stress can cause reductions in dendritic spine density (Michelsen et al., 2007). Others have found that chronic stress and prolonged exposure to corticosterone reduce dendritic spine density and volume (Anderson et al., 2016; Liu and Aghajanian, 2008; Radley et al., 2013), induce selective atrophy in the apical dendritic field of pyramidal cells (Liu and Aghajanian, 2008), and initiate dendritic remodeling in the frontal cortex (Brown et al., 2005; Hamo et al., 2009). Human studies have also linked reduced gray matter volume to childhood trauma (Dannlowski et al., 2012) and urban upbringing (Haddad et al., 2015). In schizophrenia patients, gray matter reduction has been hypothesized to be caused by reduced dendritic spine density (Glausier and Lewis, 2013) although the underlying etiology is unclear. These findings raise the possibility that longitudinal exposure to stress may impact dendritic and spine structures and lead to cortical thinning in schizophrenia.
Stress, broadly defined as a homeostatic challenge to an organism, induces a complex set of physiological and psychological processes that prepare the organism for the acute resolution of the stress as well as initiating longer-term adaptation to shifting environmental conditions, a process termed ‘allostasis’ (Sterling and Eyer, 1988). Severe and chronic stressors, interacting with individual genetic and developmental variability, can push an organism beyond normal homeostatic range. The pathophysiological consequences of uncompensated allostatic processes are conceptualized as ‘allostatic load’ (McEwen, 2004; Kyrou et al., 2006), and can be operationally quantified by an index of cardiovascular, immune, stress hormones, and metabolic biomarkers. Allostatic load index provides a means to account for the systemic pathophysiological effects of cumulative stress exposure in psychiatric disorders. Previously, we found higher levels of allostatic load in patients with schizophrenia; and allostatic load was associated with worse functional capacity (Nugent et al., 2015). How elevated allostatic load may impact the brains of schizophrenia patients is unknown and is the focus on this study.
We investigated the relationship between allostatic load and cortical thickness in schizophrenia patients and healthy controls, hypothesizing that allostatic load contributes to reduced cortical thickness in schizophrenia. Furthermore, as the allostatic load concept is likely containing multiple discrete biological processes, we explored the sub-components made up the allostatic load measure and examined whether we can identify the key components most strongly contributing to cortical thinning in schizophrenia.
2. Methods
2.1 Participants
Patients (n=44, age range 18–58 years) with diagnosis of schizophrenia spectrum disorders (SSD, including schizophrenia n=34, schizoaffective disorder n=10) were recruited from the outpatient clinics at the Maryland Psychiatric Research Center and the neighboring mental health clinics. Healthy controls (n=33, age range 20–62) were recruited through media advertisements. Demographics of the sample are reported in Table 1. Diagnoses or the lack thereof were confirmed with the Structured Clinical Interview (SCID) for all participants. Exclusion criteria included neurological illnesses and history of head injury with cognitive sequelae. Patients and controls with substance dependence within the past 6 months, or current substance abuse (except nicotine) were excluded. Except for 4 medication-free participants, all SSD patients were on antipsychotic medications, including 4 taking typical antipsychotics, 34 taking atypical (including 6 taking clozapine), and 2 taking a combination of typical and atypical antipsychotics. Chlorpromazine dose equivalent (CPZ) was calculated for each patient’s medication regimen at time of study according to published equivalencies (Woods 2003). Controls had no current Axis I diagnoses and no family history of psychosis in the prior two generations. Psychosis symptoms of the patients were assessed using the psychosis subscale of the Brief Psychiatric Rating Scale (BPRS). Participants gave written informed consent approved by local IRB.
Table 1.
Demographic characteristics of participants.
| Normal controls (n=33) | Schizophrenia (n=44) | F or χ2 statistic | p-value | |||
|---|---|---|---|---|---|---|
| S.D. | S.D. | |||||
| Age (years) | 35.3 | 14.2 | 32.7 | 12.6 | 0.72 | 0.40 |
| Male/Female | 19/14 | n/a | 28/16 | n/a | 0.29 | 0.59 |
| Smoker/Non- smoker | 7/26 | n/a | 14/30 | n/a | 1.07 | 0.30 |
| Race (White/African-American/Asian) | 21/9/3 | n/a | 20/21/3 | n/a | 3.32 | 0.19 |
| Age of onset (years) | n/a | n/a | 20.1 | 5.3 | n/a | n/a |
2.2 Biomarker assessment
The measure of allostatic load was an index of 13 biomarkers, including: resting systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate; body-mass index (BMI), and waist-hip ratio; blood levels of high-density lipoprotein (HDL) cholesterol, total cholesterol, glycated hemoglobin (HbA1c), C-reactive protein (CRP), and dehydroepiandrosterone (DHEA); and 12-hour overnight levels of urine epinephrine, norepinephrine, and cortisol. To collect the urine, participants were given a plastic container, a cooler and an ice pack. Subjects were instructed to discard the first urination at 2000 h, and then to collect all subsequent urine until 0800 h the following morning, keeping the container in the cooler with the ice pack. Participants were asked to refrain from any food or drink besides water and regular medications from midnight prior to blood collection in the morning. Fasting blood samples were collected between 0900 h and 1100 h in all participants. Blood and urine samples were sent to a Clinical Laboratory Improvement Amendments (CLIA)-certified commercial laboratory for analysis. Urine cortisol, epinephrine and norepinephrine values were adjusted for creatine levels. Anthropometric measures were conducted with participants wearing only light clothing. Blood pressure and heart rate was measured after at least 10 minutes of rest. Allostatic load data for 26 patients and 17 controls in this study were reported in a previous publication (Nugent et al., 2015).
Adherent to previously reported methodology for calculation of allostatic load index (Seeman et al., 2001), we identified the 25th and 75th percentile values of each of the 13 biomarkers for the control sample distribution. Participants who had a biomarker value greater or equal to 75th percentile (or less than or equal to the 25th percentile for HDL and DHEA) received a score of 1 for that specific biomarker. Participants taking one or more hypoglycemic, antihypertensive, or lipid-lowering medication were automatically given a score of 1 for HbA1c, SBP, or total cholesterol, respectively. The sum of biomarker values was computed such that the allostatic load index score could range from 0 to 13. Urinary biomarkers were missing for 6 participants; plasma CRP was missing for 4 participants; and cholesterol measures (HDL and total cholesterol) were missing for 2 participants. For missing data points in a participant, we chose an imputation approach where we first used the number of biomarkers that were scored as 1, divided by the total number of biomarkers for which we have data; and then prorated this ratio to the 13 point scale.
2.3 Imaging
All imaging was performed at the University of Maryland Center for Brain Imaging Research using a Siemens 3T TRIO MRI (Erlangen, Germany) system equipped with a 32-channel phase array head coil. High-resolution, T1-weighted, 3D Turbo-flash sequence with an adiabatic inversion contrast pulse with the following scan parameters: TR/TI/TE=2100/785/3.04 ms, flip angle=13°, voxel size (isotropic)=0.8 mm. To obtain high resolution cortical data, each subject was scanned 5 times consecutively using the same protocol, and a single image was obtained by linearly coregistering these images and computing the average, allowing improvement over the signal-to-noise ratio and reducing motion artifacts (Kochunov et al., 2006). For quality control, we used a retrospective motion correction approach (Kochunov et al, 2006). In short, subjects were instructed not to move their head during each of the five T1 image acquisitions and informed that if they moved the image would be repeated. Subjects were notified of the beginning and end of each imaging segment. Between acquisitions subjects were allowed to slightly adjust their posture in an attempt to maintain a comfortable position for the duration of the study. Between imaging segments the scanner operator screened the image using a 3-D viewer for motion artifacts observable as “ghosting/blurring/striping” in the phase encoding directions (~20 seconds). If artifacts were present, the subject was informed and the segment repeated, though this was not necessary for any subjects in our investigation. Only patients (n=33) and controls (n=27) who completed 5 repetitions of the high-resolution, T1-weighted scans were used for cortical thickness measurement. The T1W image processing was conducted using the FreeSurfer software package. The analysis followed the procedures described by Fischl and Dale (2000). Images were corrected for magnetic field inhomogeneities, affine-registered to the Talairach–Tournoux atlas and skull-stripped. The voxels belonging to the white matter were identified based on the location and intensity. The two hemispheres were separated and the white matter voxels were grouped into a mass of connected voxels using a six-neighbor connectivity scheme. A mesh of triangular faces was tightly built around the white matter, using two triangles per exposed voxel face. The mesh was smoothed at a subvoxel resolution, using trilinear interpolation. Topological defects were corrected ensuring that the surface has the same topological properties of a sphere (Fischl and Dale, 2000). A second iteration of smoothing was applied, resulting in a realistic representation of the interface between grey and white matter producing a white matter surface. The external cortical surface, which corresponds to the grey matter, was produced by expanding the white matter surface outwards while maintaining constraints on its smoothness and on the possibility of self-intersection (Fischl and Dale, 2000). The white matter and grey matter surfaces were parcellated into smaller regions using an automated process (Fischl et al., 2004). For each subject, this was done by first homeomorphically mapping the pial surface to a spherical coordinate system, where the folding patterns were matched to an average map (Fischl and Dale, 2000). An a priori atlas of probabilities for regions of interest was used in a Bayesian approach to establish probabilities that a given vertex belongs to a certain label. In a second, iterative step, the surface was treated as an anisotropic, non-stationary Markov random field, where for each vertex, the labels assigned to its neighbors were considered. The labeling was iterated until no vertices changed their assignments (Fischl and Dale, 2000). Grey matter thickness is commonly defined as the distance from the grey matter cortical surface to the inner cortical white matter-grey matter boundary. Computationally, cortical thickness was determined by measuring the distance between grey matter and white matter polygonal meshes. The grey matter thickness was measured as the Euclidian distance from the white matter mesh vertex to corresponding vertex on the grey matter mesh. Grey matter thickness measurements were averaged for individual cortical areas for both hemispheres; the whole-brain grey matter thickness measurement was obtained by averaging grey matter thickness across left and right meshes.
2.4 Statistical analysis
Group differences on key variables were compared using ANOVA and also ANCOVA using age as covariate. A linear regression model was used to test how AL contributes to cortical thickness after account for age and diagnosis, where cortical thickness is the dependent variable and AL, age, and diagnosis were predictors. The same model was repeated for the 13 AL subcomponents using Bonferroni correction for 13 comparisons (p<0.004). Partial correlation analyses were also used to explore relationship between cortical thickness and AL and AL subcomponents after accounting for age in separate groups. All tests were two-tailed.
3. Results
3.1 Group Differences on Allostatic Load and Cortical Thickness
Controlling for age, schizophrenia patients had significantly reduced cortical thickness (F(2, 60)=7.5, p=0.008) and higher AL (F(2,76)=7.5, p=0.008). Exploring the 13 indices within AL, sub-components that showed nominally significant group differences were CRP, overnight epinephrine and norepinephrine, and resting heart rate, all of which were higher in patients (Figure 1).
Figure 1.
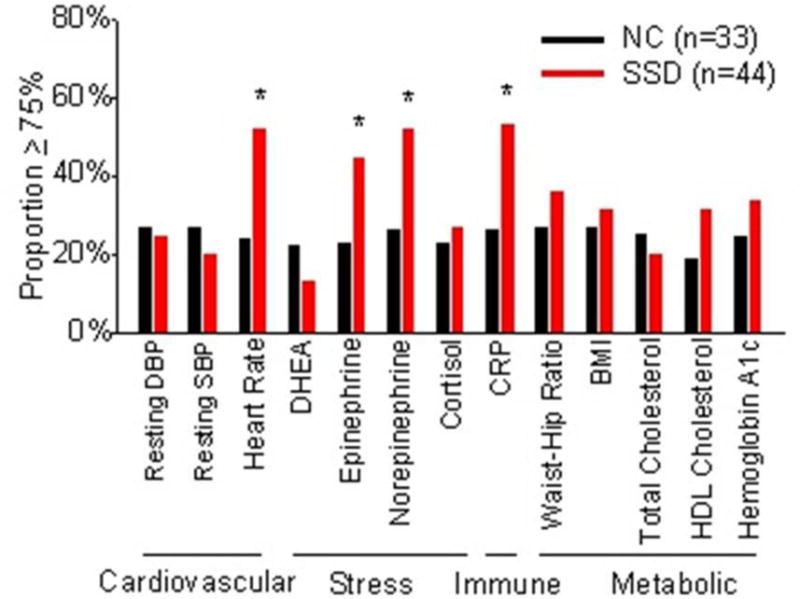
Comparisons of the 13 subcomponents of the allostatic load index between normal controls (NC) and schizophrenia spectrum disorder (SSD) patients. Participants who had a biomarker value greater or equal to 75th percentile (or less than or equal to the 25th percentile for HDL and DHEA) received a score of 1 for that specific biomarker (see Methods for more details). Y-axis represents proportion of patients had value equal to or greater than the 75% percentile. * p < 0.05.
3.2 Relationship of Allostatic Load and Cortical Thickness
The correlation between AL and cortical thickness was examined in each group first. A significant negative correlation was found in both controls (r=−0.71, p<0.001) and patients (r=−0.57, p<0.001) independently (Figure 2). The correlation coefficients were not significantly different (z=−0.88, p=0.38); therefore we combined the sample and the correlation was similar (r=−0.64, p<0.001). Because age is also known to closely relate to both AL and cortical thickness, we formally tested the above using linear regression models where effects of age and diagnosis on cortical thickness were examined with and without the effect of AL. We first tested the model of age and diagnosis effect on cortical thickness. The model was significant where both age (t=−8.0, ∆R2=51.5%, p<0.001) and diagnosis (t=−2.7, ∆R2=5.5%, p=0.008) significantly contributed to cortical thickness. This result indicated that having schizophrenia was significantly associated with reduced cortical thickness even after effect of age was accounted for. Adding AL, the model was significant where age (t=−5.7, ∆R2=51.9%, p<0.001) and AL (t=−2.8, ∆R2=8.1%, p=0.001), but not diagnosis (t=−1.9, ∆R2=2.5%, p=0.056), significantly contributed to cortical thickness, suggesting that reduced cortical thickness in schizophrenia is substantially accounted for by AL.
Figure 2.
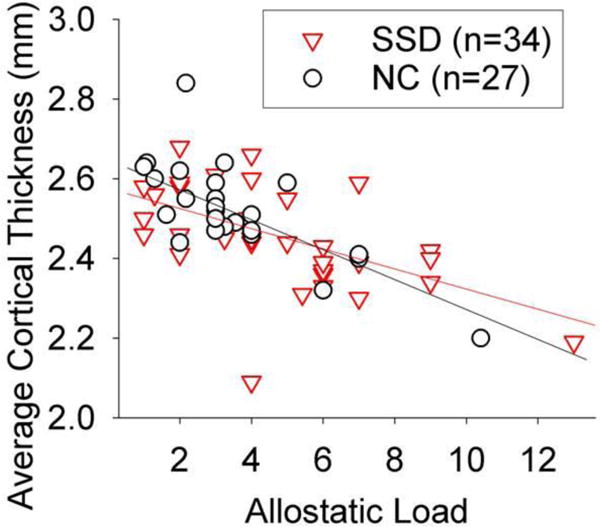
Whole brain average cortical thickness was significantly correlated with allostatic load with similar trends in normal controls (NC) and schizophrenia spectrum disorder patients (SSD) (r=−0.63, p<0.001 in the combined sample). Plot was not corrected for age (partial r=−0.40, p=0.001 after age correction).
In an exploratory analysis, we examined the correlations (corrected for age) between AL and cortical thickness by regions defined in the anatomical atlas (Supplementary Table 1). These results suggest the correlations are strongest for frontal, temporal and parietal regions in both groups, but do not suggest a different anatomical pattern associated with AL between patients and controls. Nominally significant correlations between AL and cortical thickness of two regions, fusiform cortex and inferior parietal cortex, were replicated between patient and control samples.
3.3 Inflammation Marker CRP and Cortical Thickness
To explore whether the AL index as a whole was related to cortical thickness or if this relationship was driven by particular biomarkers within the index, we repeated the same model for each of the 13 biomarkers, using Bonferroni correction for 13 comparisons. One model showed a significant biomarker effect after Bonferroni correction where CRP (t=−3.2, p=0.002) was significantly associated with cortical thickness after accounting for age (t=−9.0. p<0.001) and diagnosis (t=−2.0, p=0.048) effects. All other twelve models showed no significant effect from the AL subcomponents after Bonferroni correction. To further explore CRP effect, we repeated the analysis in controls and patients separately. CRP significantly contributed to cortical thickness in controls (t=−2.5, p=0.02) and schizophrenia patients (t=−2.4, p=0.02) independently after covarying out age effect, suggesting that inflammation as indexed by CRP may be related to cortical thickness in a manner independent of schizophrenia diagnosis.
Finally, to further explore potential patterns, we plotted the correlation of cortical thickness with each biomarker separately in controls and patients, after partial correlation to correct for age (Figure 3). Multiple AL subcomponents contributed to cortical thickness in patients, including resting diastolic blood pressure, heart rate, overnight urine norepinephrine concentration, and CRP. In NC, only CRP and BMI were significantly correlated to cortical thickness (Figure 3). The only overlapping subcomponent across the two groups was CRP.
Figure 3.
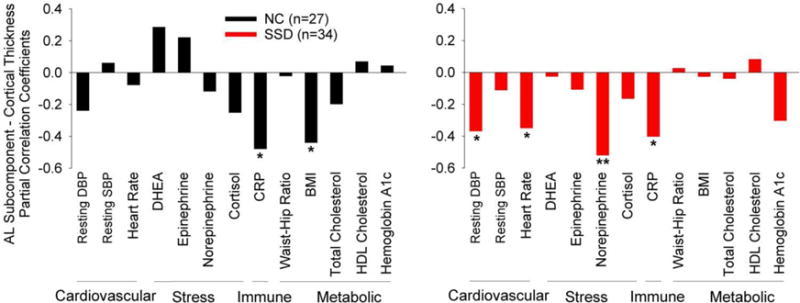
Age-corrected partial correlation coefficients between cortical thickness and individual components of the allostatic load index in normal controls (NC) and schizophrenia spectrum disorder patients (SSD). Y-axis: partial correlation coefficients. Note that DHEA and HDL are inverse measures of allostatic load (higher levels indicate lower AL). * Nominally significant at p<0.05. ** Significant after Bonferroni correction of 13 comparisons at p<0.004
3.4 Other clinical covariates (psychosis, medication, smoking, sex effects)
Among patients, psychosis symptoms were not significantly associated with AL (p=0.26) or cortical thickness (p=0.34). CPZ was also not significantly associated with AL (p=.14) or cortical thickness (p=0.96), controlling for age. Patients who smoke had marginally higher levels of AL (mean±s.d.: 5.7±3.5) than patients who were not smokers (3.8±2.1) after controlling for age (F(1,41)=3.49, p=.069); however, among controls there was no difference in AL between smokers and non-smokers (F(1,30)=1.17, p=.29). There were no significant difference in cortical thickness between smokers and non-smokers among patients (F(1,31)=1.01, p=.32) nor among controls (F(1,24)=0.03, p=.86). No gender differences were observed for AL or cortical thickness in either patients or controls (all p>.17).
4. Discussion
We found a strong inverse correlation between an index of allostatic load, thought to represent the cumulative effects of stress on key physiological parameters, and cortical thickness in both patients with schizophrenia and healthy controls. Age is well known to have a major role in both allostatic role and in cortical thickness. However, even removing age effect, allostatic load remained a significant contributor to cortical thickness, indicating a pathological process beyond normal aging. Furthermore, these results suggest the higher allostatic load observed in patients may account for some of the difference in cortical thickness between patients and controls.
Evidence suggests factors associated with inflammation may have negative influences on cortical thickness, and these effects may contribute to decline in cognitive functioning in otherwise healthy aging adults (Marsland et al., 2015). Previous studies have demonstrated a link between obesity and reduced cortical thickness, an association suspected to be mediated by low grade inflammation (Veit et al., 2014). Other evidence suggests that relative socioeconomic disadvantage is associated with alterations in cortical morphology that are mediated by greater inflammation (Gianaros et al., 2015; Krishnadas et al., 2013). Indices of allostatic load, which incorporate measures of both adiposity and inflammation, may thus have utility as an integrative marker of risk factors for changes in brain morphology in aging and stress-related illnesses. This is exemplified by recent work finding greater allostatic load to be associated with lower total brain volume and white matter volume in older adults (Booth et al., 2015). Here, we find that the allostatic load index was associated with cortical thickness in both patients and controls, even after taking into account the effects of age. Interestingly, C-reactive protein was the only individual biomarker within our index that was at least nominally significantly correlated with cortical thickness in both groups.
In the largest study (n=369) to date examining the relationship between CRP and cognition in schizophrenia, abnormal CRP (>3mg/L) was found in 28.2% of the patients and was associated with impaired general intellectual ability, working memory and a wide range of cognitive functions (Bulzacka et al., 2016). A recent meta-analysis also found that elevated CRP in schizophrenia is a reproducible finding, and is not related to antipsychotic medication use in these patients (Fernandes et al., 2016). A similar relationship of increased CRP and reduced cognition has also been observed in bipolar disorder patients (Dickerson et al., 2013). In non-psychiatric populations, higher CRP has also been linked to risk for cognitive decline over late adulthood (Laurin et al., 2009), stroke-related cognitive disorders, and dementia (Kravitz et al., 2009; Kuo et al., 2005; Mancinella et al., 2009).
The cross-sectional approach of the study limits our ability to identify the causal direction between cortical thickness and allostatic load or its subcomponents. Although it seems likely that myriad potential mechanisms can explain how metabolic, inflammatory, and hormonal abnormalities can contribute to cortical thinning, we must also consider the possibility of the opposite causal direction. For example, intact cortical thickness may contribute to resilience to stress, and thus mitigate allostatic load; or, thinner cortex due to genetic predisposition may reduce the ability to manage and regulate stress leading to abnormal metabolic, inflammatory, and hormonal parameters. Indeed, some evidence from animal models suggests that higher dendritic spine density is associated with lower susceptibility to the behavioral effects of inescapable stress (Yang et al., 2015).
Another challenge is how to determine how much of the link between AL and cortical thickness in schizophrenia is due to stressful life experiences versus the effects of chronic use of antipsychotic medications, smoking, and less exercise. Antipsychotic medications are well known to contribute to metabolic dysregulation (Patel et al., 2009) and some medications like clozapine and risperidone are also known to increase norepinephrine levels and heart rate (Breier et al., 1994; See et al, 1999). However, in the data reported here, CPZ was only marginally correlated with AL. It is also important to consider that cardiometabolic risk factors such as hypertension and increased BMI are frequent even in first-episode schizophrenia, in many cases preceding chronic exposure to anti-psychotics (Correll et al., 2014). To determine the contribution of AL to reduced cortical thickness in schizophrenia, larger studies incorporating more patients in the early stages of illness, ideally including a large sample of never medicated patients, will be necessary to more properly dissect out the confounding factor of medications. Smoking is known to have diverse negative effects on cardiovascular health, and is more prevalent in schizophrenia spectrum patients. Previous studies have demonstrated that smoking increases AL (Barboza Solís et al., 2015; Petrovic et al., 2016). Additionally, smoking was found to partially mediate the relationships between high AL and low socioeconomic status (Robertson et al., 2015) and adverse childhood experiences (Barboza Solís et al., 2015). In this study, smoking was associated with higher AL among patients, but only at a trend level. However, we did not control for socioeconomic status, which may also systematically vary between patients and controls and represent a factor contributing to unhealthy behaviors and stress, and therefore to AL.
Another important caveat to interpreting the data reported here is to recognize that cortical thickness, as calculated from MR imaging, is an indirect measure of the integrity of neural tissue that may not be simply interpreted as alterations in density of dendritic spines or any specific cellular components. Instead, the diagnosis and AL effects on cortical thickness can be confounded by a number of environmental and physiologic factors that often vary systematically between samples of healthy controls and patients (Weinberger and Radulescu, 2016). In this study, some confounds were partially addressed, for example by balancing samples on age and smoking status and by incorporating factors such as stress and BMI into the AL index. However, the number of potential confounds considered in this modest study were limited and additional research are required to comprehensively examine the multiple genetic and environmental factors contributing to cortical thickness in general and its thinning in schizophrenia.
The concept of allostatic load provides a useful framework for quantifying the cumulative physiological toll of stress and maladaptive responses to stress. This study provides initial evidence that an index of allostatic load is associated with reduced cortical thickness, and suggests that allostatic load may account for some of the loss of gray matter in schizophrenia.
Supplementary Material
Highlights.
Schizophrenia patients had significantly higher levels of an index of allostatic load compared to healthy controls; this difference partially accounted for lower cortical thickness observed in patients compared to controls
C-reactive protein was the only component of the allostatic load index inversely correlated with cortical thickness in both patients and controls independently
Acknowledgments
Funding
This work was supported by the National Institutes of Health (grant numbers U01MH108148, R01EB015611, P50MH103222, R01DA027680, R01MH085646, T32MH067533 and U54 EB020403), a State of Maryland contract (M00B6400091), and a NARSAD Young Investigator Award from the Brain and Behavior Research Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
Dr. Hong has received or is planning to receive research funding or consulting fees from Mitsubishi, Your Energy Systems LLC, Neuralstem, Taisho Pharmaceutical, Heptares, and Pfizer. All other authors declare no financial interests that could represent a conflict of interest.
References
- Anderson RM, Glanz RM, Johnson SB, Miller MM, Romig-Martin S, Radley JJ. Prolonged corticosterone exposure induces dendritic spine remodeling and attrition in the rat medial prefrontal cortex. J Comp Neurol. 2016 doi: 10.1002/cne.24027. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barboza Solís C, Kelly-Irving M, Fantin R, Darnaudéry M, Torrisani J, Lang T, Delpierre C. Adverse childhood experiences and physiological wear-and-tear in midlife: Findings from the 1958 British birth cohort. Proc Natl Acad Sci. 2015;112:738–46. doi: 10.1073/pnas.1417325112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth T, Royle NA, Corley J, Gow AJ, Valdés Hernández Mdel C, Muñoz Maniega S, Ritchie SJ, Bastin ME, Starr JM, Wardlaw JM, Deary IJ. Association of allostatic load with brain structure and cognitive ability in later life. Neurobiol Aging. 2015;36:1390–9. doi: 10.1016/j.neurobiolaging.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breier A, Buchanan RW, Waltrip RW, 2nd, Listwak S, Holmes C, Goldstein DS. The effect of clozapine on plasma norepinephrine: relationship to clinical efficacy. Neuropsychopharmacology. 1994;10:1–7. doi: 10.1038/npp.1994.1. [DOI] [PubMed] [Google Scholar]
- Brown SM, Henning S, Wellman CL. Mild, short-term stress alters dendritic morphology in rat medial prefrontal cortex. Cereb Cortex. 2005;15:1714–22. doi: 10.1093/cercor/bhi048. [DOI] [PubMed] [Google Scholar]
- Bulzacka E, Boyer L, Schürhoff F, Godin O, Berna F, Brunel L, Andrianarisoa M, Aouizerate B, Capdevielle D, Chéreau-Boudet I, Chesnoy-Servanin G, Danion JM, Dubertret C, Dubreucq J, Faget C, Gabayet F, Le Gloahec T, Llorca PM, Mallet J, Misdrahi D, Rey R, Richieri R, Passerieux C, Roux P, Yazbek H, Leboyer M, Fond G, FACE-SZ (FondaMental Academic Centers of Expertise for Schizophrenia) Group Chronic Peripheral Inflammation is Associated With Cognitive Impairment in Schizophrenia: Results From the Multicentric FACE-SZ Dataset. Schizophr Bull. 2016;42:1290–302. doi: 10.1093/schbul/sbw029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll CU, Robinson DG, Schooler NR, Brunette MF, Mueser KT, Rosenheck RA, Marcy P, Addington J, Estroff SE, Robinson J, Penn DL, Azrin S, Goldstein A, Severe J, Heinssen R, Kane JM. Cardiometabolic risk in patients with first-episode schizophrenia spectrum disorders: baseline results from the RAISE-ETP study. JAMA Psychiatry. 2014;71:1350–63. doi: 10.1001/jamapsychiatry.2014.1314. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D, Domschke K, Hohoff C, Ohrmann P, Bauer J, Lindner C, Postert C, Konrad C, Arolt V, Heindel W, Suslow T, Kugel H. Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol Psychiatry. 2012;71:286–93. doi: 10.1016/j.biopsych.2011.10.021. [DOI] [PubMed] [Google Scholar]
- Dickerson F, Stallings C, Origoni A, Vaughan C, Khushalani S, Yolken R. Elevated C-reactive protein and cognitive deficits in individuals with bipolar disorder. J Affect Disord. 2013;150:456–9. doi: 10.1016/j.jad.2013.04.039. [DOI] [PubMed] [Google Scholar]
- Fernandes BS, Steiner J, Bernstein HG, Dodd S, Pasco JA, Dean OM, Nardin P, Gonçalves CA, Berk M. C-reactive protein is increased in schizophrenia but is not altered by antipsychotics: meta-analysis and implications. Mol Psychiatry. 2016;21:554–64. doi: 10.1038/mp.2015.87. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci. 2000;97:11050–5. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Kuan DC, Marsland AL, Sheu LK, Hackman DA, Miller KG, Manuck SB. Community Socioeconomic Disadvantage in Midlife Relates to Cortical Morphology via Neuroendocrine and Cardiometabolic Pathways. Cereb Cortex. 2015 doi: 10.1093/cercor/bhv233. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glausier JR, Lewis DA. Dendritic spine pathology in schizophrenia. Neuroscience. 2013;251:90–107. doi: 10.1016/j.neuroscience.2012.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman AL, Pezawas L, Mattay VS, Fischl B, Verchinski BA, Chen Q, Weinberger DR, Meyer-Lindenberg A. Widespread reductions of cortical thickness in schizophrenia and spectrum disorders and evidence of heritability. Arch Gen Psychiatry. 2009;66:467–77. doi: 10.1001/archgenpsychiatry.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Q, Lui S, Sweeney JA. A Selective Review of Cerebral Abnormalities in Patients With First-Episode Schizophrenia Before and After Treatment. Am J Psychiatry. 2016;173:232–43. doi: 10.1176/appi.ajp.2015.15050641. [DOI] [PubMed] [Google Scholar]
- Gupta CN, Calhoun VD, Rachakonda S, Chen J, Patel V, Liu J, Segall J, Franke B, Zwiers MP, Arias-Vasquez A, Buitelaar J, Fisher SE, Fernandez G, van Erp TG, Potkin S, Ford J, Mathalon D, McEwen S, Lee HJ, Mueller BA, Greve DN, Andreassen O, Agartz I, Gollub RL, Sponheim SR, Ehrlich S, Wang L, Pearlson G, Glahn DC, Sprooten E, Mayer AR, Stephen J, Jung RE, Canive J, Bustillo J, Turner JA. Patterns of Gray Matter Abnormalities in Schizophrenia Based on an International Mega-analysis. Schizophr Bull. 2015;41:1133–42. doi: 10.1093/schbul/sbu177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad L, Schäfer A, Streit F, Lederbogen F, Grimm O, Wüst S, Deuschle M, Kirsch P, Tost H, Meyer-Lindenberg A. Brain structure correlates of urban upbringing, an environmental risk factor for schizophrenia. Schizophr Bull. 2015;41:115–22. doi: 10.1093/schbul/sbu072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung WH, Kim JS, Jang JH, Choi JS, Jung MH, Park JY, Han JY, Choi CH, Kang DH, Chung CK, Kwon JS. Cortical thickness reduction in individuals at ultra-high-risk for psychosis. Schizophr Bull. 2011;37:839–49. doi: 10.1093/schbul/sbp151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klauser P, Zhou J, Lim JK, Poh JS, Zheng H, Tng HY, Krishnan R, Lee J, Keefe RS, Adcock RA, Wood SJ, Fornito A, Chee MW. Lack of Evidence for Regional Brain Volume or Cortical Thickness Abnormalities in Youths at Clinical High Risk for Psychosis: Findings From the Longitudinal Youth at Risk Study. Schizophr Bull. 2015;41:1285–93. doi: 10.1093/schbul/sbv012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, Lancaster JL, Glahn DC, Purdy D, Laird AR, Gao F, Fox P. Retrospective motion correction protocol for high-resolution anatomical MRI. Hum Brain Mapp. 2006;27:957–962. doi: 10.1002/hbm.20235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz BA, Corrada MM, Kawas CH. Elevated C-reactive protein levels are associated with prevalent dementia in the oldest-old. Alzheimers Dement. 2009;5:318–323. doi: 10.1016/j.jalz.2009.04.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnadas R, McLean J, Batty GD, Burns H, Deans KA, Ford I, McConnachie A, McLean JS, Millar K, Sattar N, Shiels PG, Tannahill C, Velupillai YN, Packard CJ, Cavanagh J. Socioeconomic deprivation and cortical morphology: psychological, social, and biological determinants of ill health study. Psychosom Med. 2013;75:616–23. doi: 10.1097/PSY.0b013e3182a151a7. [DOI] [PubMed] [Google Scholar]
- Kuo HK, Yen CJ, Chang CH, Kuo CK, Chen JH, Sorond F. Relation of C-reactive protein to stroke, cognitive disorders, and depression in the general population: systematic review and meta-analysis. Lancet Neurol. 2005;4:371–380. doi: 10.1016/S1474-4422(05)70099-5. [DOI] [PubMed] [Google Scholar]
- Kyrou I, Chrousos GP, Tsigos C. Stress, Visceral Obesity, and Metabolic Complications. Ann NY Acad Sci. 2006;1083:77–110. doi: 10.1196/annals.1367.008. [DOI] [PubMed] [Google Scholar]
- Laurin D, Curb JD, Masaki KH, White LR, Launer LJ. Midlife C-reactive protein and risk of cognitive decline: a 31-year follow-up. Neurobiol Aging. 2009;30:1724–1727. doi: 10.1016/j.neurobiolaging.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RJ, Aghajanian GK. Stress blunts serotonin- and hypocretin-evoked EPSCs in prefrontal cortex: role of corticosterone-mediated apical dendritic atrophy. Proc Natl Acad Sci. 2008;105:359–64. doi: 10.1073/pnas.0706679105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancinella A, Mancinella M, Carpinteri G, Bellomo A, Fossati C, Gianturco V, Iori A, Ettorre E, Troisi G, Marigliano V. Is there a relationship between high C-reactive protein (CRP) levels and dementia? Arch Gerontol Geriatr. 2009;49(suppl 1):185–194. doi: 10.1016/j.archger.2009.09.028. [DOI] [PubMed] [Google Scholar]
- Marsland AL, Gianaros PJ, Kuan DC, Sheu LK, Krajina K, Manuck SB. Brain morphology links systemic inflammation to cognitive function in midlife adults. Brain Behav Immun. 2015;48:195–204. doi: 10.1016/j.bbi.2015.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Protection and damage from acute and chronic stress: Allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann NY Acad Sci. 2004;1032:1–7. doi: 10.1196/annals.1314.001. [DOI] [PubMed] [Google Scholar]
- Michelsen KA, van den Hove DL, Schmitz C, Segers O, Prickaerts J, Steinbusch HW. Prenatal stress and subsequent exposure to chronic mild stress influence dendritic spine density and morphology in the rat medial prefrontal cortex. BMC Neurosci. 2007;8:107. doi: 10.1186/1471-2202-8-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent KL, Chiappelli J, Rowland LM, Hong LE. Cumulative stress pathophysiology in schizophrenia as indexed by allostatic load. Psychoneuroendocrinology. 2015;60:120–9. doi: 10.1016/j.psyneuen.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel JK, Buckley PF, Woolson S, Hamer RM, McEvoy JP, Perkins DO, Lieberman JA, CAFE Investigators Metabolic profiles of second-generation antipsychotics in early psychosis: findings from the CAFE study. Schizophr Res. 2009;111:9–16. doi: 10.1016/j.schres.2009.03.025. [DOI] [PubMed] [Google Scholar]
- Petrovic D, Pivin E, Ponte B, Dhayat N, Pruijm M, Ehret G, Ackermann D, Guessous I, Younes SE, Pechère-Bertschi A, Vogt B, Mohaupt M, Martin PY, Paccaud F, Burnier M, Bochud M, Stringhini S. Sociodemographic, behavioral and genetic determinants of allostatic load in a Swiss population-based study. Psychoneuroendocrinology. 2016;67:76–85. doi: 10.1016/j.psyneuen.2016.02.003. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Anderson RM, Hamilton BA, Alcock JA, Romig-Martin SA. Chronic stress-induced alterations of dendritic spine subtypes predict functional decrements in an hypothalamo-pituitary-adrenal-inhibitory prefrontal circuit. J Neurosci. 2013;33:14379–91. doi: 10.1523/JNEUROSCI.0287-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson T, Benzeval M, Whitley E, Popham F. The role of material, psychosocial and behavioral factors in mediating the association between socioeconomic position and allostatic load (measured by cardiovascular, metabolic and inflammatory markers) Brain Behav Immun. 2015;45:41–9. doi: 10.1016/j.bbi.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz CC, Koch K, Wagner G, Roebel M, Schachtzabel C, Gaser C, Nenadic I, Reichenbach JR, Sauer H, Schlösser RG. Reduced cortical thickness in first episode schizophrenia. Schizophr Res. 2010;116:204–9. doi: 10.1016/j.schres.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Sprooten E, Papmeyer M, Smyth AM, Vincenz D, Honold S, Conlon GA, Moorhead TW, Job D, Whalley HC, Hall J, McIntosh AM, Owens DC, Johnstone EC, Lawrie SM. Cortical thickness in first-episode schizophrenia patients and individuals at high familial risk: a cross-sectional comparison. Schizophr Res. 2013;151:259–64. doi: 10.1016/j.schres.2013.09.024. [DOI] [PubMed] [Google Scholar]
- See RE, Fido AA, Maurice M, Ibrahim MM, Salama GM. Risperidone-induced increase of plasma norepinephrine is not correlated with symptom improvement in chronic schizophrenia. Biol Psychiatry. 1999;45:1653–6. doi: 10.1016/s0006-3223(98)00199-1. [DOI] [PubMed] [Google Scholar]
- Shansky RM, Hamo C, Hof PR, McEwen BS, Morrison JH. Stress-induced dendritic remodeling in the prefrontal cortex is circuit specific. Cereb Cortex. 2009;19:2479–84. doi: 10.1093/cercor/bhp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling P, Eyer J. Allostasis: A new paradigm to explain arousal pathology. In: Fisher S, Reason JT, editors. Handbook of life stress, cognition, and health. John Wiley & Sons; Oxford: 1988. pp. 629–649. [Google Scholar]
- Seeman TE, McEwen BS, Rowe JW, Singer BH. Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proc Natl Acad Sci. 2001;98:4770–5. doi: 10.1073/pnas.081072698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Haren NE, Schnack HG, Cahn W, van den Heuvel MP, Lepage C, Collins L, Evans AC, Hulshoff Pol HE, Kahn RS. Changes in cortical thickness during the course of illness in schizophrenia. Arch Gen Psychiatry. 2011;68:871–80. doi: 10.1001/archgenpsychiatry.2011.88. [DOI] [PubMed] [Google Scholar]
- Veit R, Kullmann S, Heni M, Machann J, Häring HU, Fritsche A, Preissl H. Reduced cortical thickness associated with visceral fat and BMI. Neuroimage Clin. 2014;6:307–11. doi: 10.1016/j.nicl.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voineskos AN, Felsky D, Wheeler AL, Rotenberg DJ, Levesque M, Patel S, Szeszko PR, Kennedy JL, Lencz T, Malhotra AK. Limited Evidence for Association of Genome-Wide Schizophrenia Risk Variants on Cortical Neuroimaging Phenotypes. Schizophr Bull. 2016;42:1027–36. doi: 10.1093/schbul/sbv180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger DR, Radulescu E. Finding the Elusive Psychiatric “Lesion” With 21st-Century Neuroanatomy: A Note of Caution. Am J Psychiatry. 2016;173:27–33. doi: 10.1176/appi.ajp.2015.15060753. [DOI] [PubMed] [Google Scholar]
- Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663–7. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- Yang C, Shirayama Y, Zhang JC, Ren Q, Hashimoto K. Regional differences in brain-derived neurotrophic factor levels and dendritic spine density confer resilience to inescapable stress. Int J Neuropsychopharmacol. 2015;18:pyu121. doi: 10.1093/ijnp/pyu121. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


