Abstract
Background
Lymphopenia is increasingly recognized as a consequence of acute illness and may predispose to infections. We investigated whether admission lymphopenia (AL) is associated with increased risk of infectious complications and poor outcome in patients with spontaneous intracerebral hemorrhage (ICH).
Methods
We retrospectively analyzed a prospectively collected cohort of ICH patients ascertained between 1994 and 2015. We identified subjects with lymphocyte count obtained within 24 h from onset, and AL was defined as lymphocyte count < 1000/µL. Infectious complications were assessed through retrospective chart review. Association between AL, infections, and mortality was investigated using multivariable logistic regression.
Results
Of the 2014 patients meeting inclusion criteria, 548 (27.2%) had AL and 605 (30.0%) developed an infectious complication. Case-fatality at 90 days was 36.9%. Patients with AL had larger hematoma volumes, higher frequency of intraventricular hemorrhage, and lower Glasgow Coma Scale score on presentation (all p < 0.001). AL was independently associated with increased risk of pneumonia [odds ratio (OR) 1.97, 95% confidence interval (CI) 1.50–2.58, p < 0.001] and multiple infections (OR 1.84, 95% CI 1.24–2.71, p = 0.003). AL was also an independent predictor of 90-day mortality (OR 1.55, 95% CI 1.18–2.04, p = 0.002) after adjusting for confounders.
Conclusions
AL is common in ICH patients and independently associated with increased risk of infectious complications and poor outcome. Further studies will be needed to determine whether prophylactic antibiotics in ICH patients with AL can improve outcome.
Keywords: Stroke, Cerebral hemorrhage, Cerebrovascular disorders, Lymphopenia, Infection, Pneumonia
Introduction
Intracerebral hemorrhage (ICH) is still the deadliest type of stroke, with lack of an acute phase treatment proven to reduce mortality and improve functional outcome [1]. Inflammation and lymphocyte migration into the brain appear to play a key role in secondary brain damage and clinical deterioration following acute ICH [2]. Clinical trials of fingolimod in ICH have, therefore, been guided by the theory that lymphocyte depletion may improve outcome reducing edema and inflammation-mediated tissue damage [3]. Prior studies, however, suggest that hemorrhagic stroke, like ischemic stroke or other acute severe illnesses can be accompanied by immunodepression, manifested by lymphopenia [4–6]. Lymphopenia, in turn, may predispose to the development of infections [7, 8]. The role of lymphopenia in ICH, and in particular, whether it predisposes to infectious complications (IC) during the acute course is poorly understood and may have implications for the design of future clinical trials of immunomodulation in ICH. In this study we investigated the frequency and determinants of lymphopenia in subjects with ICH and whether it was associated with increased risk of IC and poor outcome.
Methods
Study Design and Patient Selection
All aspects of the study were approved by the Institutional Review Board (IRB). Informed written or verbal consent was obtained by patients or family members or waived by the IRB.
We retrospectively analyzed an ongoing prospective cohort of patients with spontaneous ICH collected at a single academic hospital from January 1994 to April 2015 [9, 10]. The inclusion criteria for the present study were: (1) diagnosis of spontaneous ICH on non-contrast CT scan (2) complete white blood cell count obtained within 24 h from stroke onset. Subjects were excluded if there was evidence of (1) traumatic intracranial bleeding, (2) intracranial tumor, aneurysm, or other vascular malformation presumed to be the cause of the hemorrhage, (3) hemorrhagic conversion of acute brain infarction, (4) missing data on infectious complications, and (5) missing follow-up data on mortality at 90 days.
Image Acquisition and Analysis
All images were analyzed by study staff blinded to all clinical and laboratory variables. Non-contrast CT images were acquired with an axial technique and 5-mm-thickness slices, 120–140 kVp, 10–500 mA and reviewed for determination of ICH location and the presence of intraventricular extension of the hematoma (IVH). Hematoma volume was calculated with semi-automated computer-assisted technique (Analyze Direct 11.0 software).
Clinical Variables
Demographic and clinical data were systematically collected through patient and family members’ interviews and retrospective review of hospital charts. We assessed the presence of medical history of hypertension, diabetes mellitus, hypercholesterolemia, antiplatelet therapy, and oral anticoagulant treatment (OAT) as previously described in detail [10]. We also collected data on pre-stroke functional status, and functional dependence was defined as requiring assistance in at least one instrumental activity of daily living before the index ICH [11].
Because previous reports have demonstrated that the relationship between total lymphocyte count and susceptibility to infections is not linear and that the odds of experiencing an IC increase only when the lymphocyte count drops below a critical threshold [7, 12], we analyzed admission lymphopenia (AL) defined as absolute lymphocyte count < 1000/µL [7, 13]. We also collected data on admission leukopenia (absolute leukocyte count < 4000/µL), admission neutropenia (absolute neutrophil count < 1500/µL), and admission monocytopenia (absolute monocyte count < 200/µL) [14, 15].
Infectious Complications and Mortality
IC were identified through a retrospective review of hospital charts, discharge reports, laboratory, and radiological tests. The presence of an IC during the hospital stay was established according to previously published criteria [16–18] by two investigators (AM, SM), blinded to the presence of AL. In particular, we looked for evidence of the following infections during the hospital stay: pneumonia, urinary tract infections, and sepsis. The diagnosis of pneumonia was based on the combination of typical clinical presentation with confirmatory chest X-ray changes [16]. A positive urine culture was required for the diagnosis of urinary tract infection [17]. Sepsis was diagnosed in case of documented source of infection associated with evidence of acute organ dysfunction [18]. Mucocutaneous infections, gastrointestinal infections, and meningoencephalitis were grouped in the category “other infections.” The case-fatality rate at 90 days was assessed via telephone interviews and querying of the Social Security Death Index (SSDI) national database as previously described [10].
Statistical Analysis
Categorical variables were expressed as count (%) while continuous variables as median (interquartile range, IQR). Differences between patients with and without AL and with and without infections were examined using the χ2 test or Mann–Whitney U test as appropriate.
The association between AL and infectious complications was investigated with a multivariable logistic regression analysis, adjusted for predictors of infections [19, 20]. All the variables with p value <0.1 in univariate analysis were included in the regression model. The relationship between AL and 90-day mortality was investigated with a multivariable logistic regression, accounting for known predictors of outcome in ICH patients (age, ICH volume, admission Glasgow Coma Scale score, presence on IVH, and infratentorial location) [21]. p values <0.05 were considered statistically significant. All analyses were performed using the statistical package SPSS v. 21, 2012 (www.spss.com).
Results
A total of 2403 patients with ICH were screened and 2014 met the eligibility criteria for the present analysis. The frequency of AL was 27.2% and a total of 605 (30.0%) patients experienced an infection during the hospital stay. Overall mortality at three months was 36.9%. A total of 351 subjects were excluded because of lack of lymphocyte count, and 38 patients were excluded because of missing clinical or demographic data. Compared to the study population, patients excluded from the analysis were older, more likely to be on antiplatelet treatment, and less likely to have a medical history of hypertension, diabetes mellitus, and hypercholesterolemia. The remaining demographic and clinical characteristics were similar between the two groups (all p values >0.05).
Factors Associated with Admission Lymphopenia
Patients with AL were older and had larger baseline hematoma volume and higher frequency of intraventricular extension of the hemorrhage (Table 1). In addition, AL was associated with infratentorial location of the hematoma and lower admission Glasgow Coma Scale score.
Table 1.
Comparison between patients with and without admission lymphopenia (n = 2014)
| Variable | Lymphopenia | p value | |
|---|---|---|---|
| No (n = 1466) | Yes (n = 548) | ||
| Age, median (IQR), year | 74 (63–82) | 76 (66–83) | 0.003 |
| Sex, male, n (%) | 785 (53.5) | 303 (55.3) | 0.484 |
| History of hypertension, n (%) | 1144 (78.0) | 435 (79.4) | 0.377 |
| History of diabetes, n (%) | 323 (22.0) | 113 (20.6) | 0.790 |
| History of hypercholesterolemia, n (%) | 565 (38.5) | 202 (36.9) | 0.732 |
| Antiplatelet treatment, n (%) | 695 (47.4) | 261 (47.6) | 0.986 |
| Warfarin treatment, n (%) | 272 (18.6) | 151 (27.6) | <0.001 |
| Pre-stroke dependence, n (%) | 194/1332 (14.6) | 77/501 (15.4) | 0.665 |
| Baseline ICH volume, median (IQR), mL | 16 (5–40) | 23 (6–57) | <0.001 |
| Admission GCS, median (IQR) | 14 (8–15) | 12 (6–15) | <0.001 |
| Infratentorial location, n (%) | 147 (10.0) | 87 (15.9) | <0.001 |
| IVH presence, n (%) | 666 (45.4) | 331 (60.4) | <0.001 |
| Intubation, n (%) | 458 (31.2) | 251 (45.8) | <0.001 |
| Surgery, n (%) | 68 (5.9) | 45 (8.2) | 0.156 |
| Any infectious complication, n (%) | 414 (28.2) | 191 (34.9) | 0.004 |
| Pneumonia, n (%) | 233 (15.9) | 138 (25.2) | <0.001 |
| Urinary tract infection, n (%) | 223 (15.2) | 83 (15.1) | 0.971 |
| Sepsis, n (%) | 32 (2.2) | 16 (2.9) | 0.335 |
| Other infection, n (%) | 20 (1.4) | 6 (1.1) | 0.634 |
| Multiple infections, n (%) | 88 (6.0) | 49 (8.9) | 0.020 |
| Length of hospital stay, median (IQR), days | 6 (3–11) | 6 (3–12) | 0.954 |
| 90-day mortality, n (%) | 474 (32.3) | 269 (49.1) | <0.001 |
IQR interquartile range, ICH intracerebral hemorrhage, GCS Glasgow Coma Scale, IVH intraventricular hemorrhage
Infectious Complications
The presence of AL was significantly higher in patients with IC (31.6 vs 25.3%, p = 0.004) whereas the frequency of leukopenia, neutropenia, and monocytopenia was similar between the two groups (Table 2).
Table 2.
Comparison between patients with and without infectious complications (n = 2014)
| Variable | Infectious complications | p value | |
|---|---|---|---|
| No (n = 1409) | Yes (n = 605) | ||
| Age, median (IQR), year | 75 (63–82) | 74 (64–83) | 0.457 |
| Sex, male, n (%) | 768 (54.5) | 320 (52.9) | 0.505 |
| History of hypertension, n (%) | 1089 (77.3) | 490 (81.0) | 0.143 |
| History of diabetes, n (%) | 300 (21.3) | 136 (22.5) | 0.813 |
| History of hypercholesterolemia, n (%) | 541 (38.4) | 226 (37.4) | 0.854 |
| Antiplatelet treatment, n (%) | 670 (47.6) | 286 (47.3) | 0.833 |
| Warfarin treatment, n (%) | 302 (21.4) | 121 (20.0) | 0.594 |
| Pre-stroke dependence, n (%) | 168/1279 (13.9) | 103/554 (18.6) | 0.003 |
| Baseline ICH volume, median (IQR), mL | 17 (5–60) | 16 (5–40) | 0.137 |
| Admission GCS, median (IQR) | 14 (7–15) | 13 (8–15) | 0.427 |
| Infratentorial location, n (%) | 173 (12.3) | 61 (10.1) | 0.189 |
| IVH presence, n (%) | 676 (48.0) | 321 (53.1) | 0.094 |
| Intubation, n (%) | 485 (34.4) | 224 (37.0) | 0.388 |
| Surgery, n (%) | 81 (5.7) | 50 (8.3) | 0.097 |
| Leukopenia, n (%) | 13 (0.9) | 7 (1.2) | 0.627 |
| Neutropenia, n (%) | 1 (0.1) | 2 (0.3) | 0.167 |
| Monocytopenia, n (%) | 62 (4.4) | 25 (4.1) | 0.786 |
| Lymphopenia, n (%) | 357 (25.3) | 191 (31.6) | 0.004 |
| Length of hospital stay, median (IQR), days | 5 (2–8) | 11 (6–19) | <0.001 |
| 90-day mortality, n (%) | 560 (39.7) | 183 (30.2) | <0.001 |
IQR interquartile range, ICH intracerebral hemorrhage, GCS Glasgow Coma Scale, IVH intraventricular hemorrhage
After adjustment for potential confounders in multivariable regression, AL was independently associated with increased risk of pneumonia, and multiple infections, but not with urinary tract infection and sepsis (Table 3).
Table 3.
Association between lymphopenia and infectious complications
| Type of infectious complication | OR (95% CI) | p value |
|---|---|---|
| Any infection* | 1.49 (1.18–1.89) | 0.001 |
| Pneumonia | 1.97 (1.50–2.58) | <0.001 |
| Urinary tract infection | 1.11 (0.83–1.48) | 0.495 |
| Sepsis | 1.89 (0.97–3.61) | 0.055 |
| Other infection | 1.01 (0.36–2.82) | 0.987 |
| Multiple infections | 1.84 (1.24–2.71) | 0.003 |
ICH intracerebral hemorrhage, GCS Glasgow Coma Scale, IVH intraventricular hemorrhage, OR odds ratio, CI confidence interval
Adjusted for age, admission GCS, baseline ICH volume, presence of IVH, intubation, infratentorial location, warfarin treatment, and length of hospital stay
Mortality
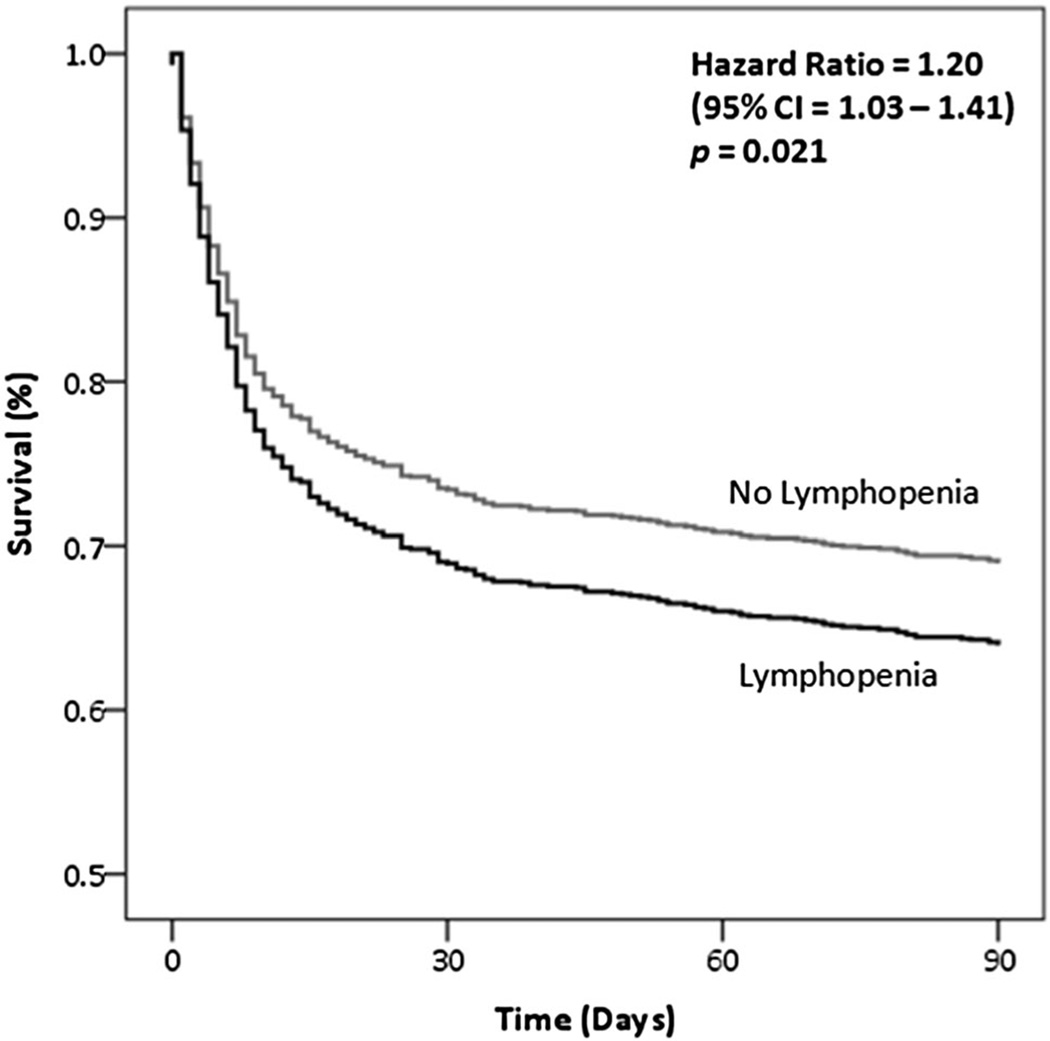
AL was independently associated with increased case-fatality at 90 days (odds ratio 1.55, 95% CI 1.18–2.04, p = 0.002) after accounting for known predictors of mortality in ICH (age, admission GCS, presence of IVH, infratentorial location of the hematoma and baseline ICH volume) [21]. Figure 1 shows the adjusted survival analysis stratified by presence of AL.
Fig. 1.
Adjusted survival curve, stratified by admission lymphopenia. Hazard Ratio (HR) was obtained with a Cox proportional hazard model, adjusted for age, admission GCS, baseline ICH volume, presence of IVH, intubation, infratentorial location. ICH intracerebral hemorrhage, GCS Glasgow Coma Scale, IVH intraventricular hemorrhage
Duration of hospitalization was longer in patients experiencing at least one IC (11 vs 5 days, p < 0.001). While patients with IC had lower mortality in univariate analysis, their mortality rate was significantly higher when the analysis was restricted to subjects surviving longer than 72 h (mortality at 90 days: 27.0 vs 21.4%, p = 0.022), consistent with the presence of survival bias underlying the unrestricted assessment of mortality [22].
All results were unchanged when pre-stroke functional status was also included in the multivariate analysis (OR for infectious complications 1.46, 95% CI 1.44–1.87, p = 0.002; OR for 90 days mortality 1.48, 95% CI 1.09–2.00, p = 0.011).
Discussion
We found that lymphopenia on admission is frequent in ICH patients and is independently associated with increased risk of IC and long-term mortality. In particular, AL was an independent predictor of pulmonary infections and multiple infections during the hospital stay.
While it remains to be determined whether the immunosuppression manifested by lymphopenia precedes the ICH or is an acute consequence of it, accumulating data suggest that brain injury can influence the physiologic interplay between the central nervous system and the immune system, leading to the onset of a systemic immunodepressive syndrome [5, 23, 24]. This immunodepressive state is characterized by the acute secretion of several stress mediators, with secondary apoptotic loss of circulating lymphocytes that appears to be proportional to the severity and extension of brain damage [5, 6, 23]. Indeed, in our study, AL was associated with ICH of higher severity. Patients with AL had larger ICH volumes, lower GCS, and higher frequency of IVH, and AL could theoretically be just a marker of ICH severity. Pre-stroke functional status may also be an important confounder and the presence of lymphopenia could simply reflect the degree of disability prior to the index ICH.
However, the association between lymphopenia, infections, and outcome remained significant after adjusting for multiple potential confounders such as pre-stroke disability and measures of ICH severity. In agreement with previous studies on stroke-associated immunodepression [4–6, 8], our results suggest that the presence of lymphopenia correlates with stroke severity but plays an independent role in predisposing to infections and unfavorable outcome.
Consistent with prior studies [13], we also demonstrated that AL is an independent predictor of poor outcome. Our results offer evidence for a mechanism through which AL may be operating. Infections are indeed a major cause of mortality in stroke patients [19, 25–27], and AL was associated with an increased risk of pneumonia and multiple infections in our study.
In this regard, we observed a higher mortality in subjects with infectious complications only restricting the analysis to patients surviving the first three days after stroke. One possible explanation is the influence of survival bias. Multiple previous studies showed that hematoma expansion, intraventricular bleeding, and limitation of care are the main determinants of early mortality after ICH [28, 29]. Conversely, infections have a negative influence on stroke outcome especially in the subacute phase, and therefore, patients that did not experience early clinical deterioration and death are at higher risk of developing a nosocomial infection [28, 29]. In line with this Katzan and Colleagues showed that stroke-associated pneumonia increased mortality after exclusion of patients dying or receiving withdrawal of care within 72 h from onset [30], again suggesting that infections have a greater influence on late rather than early mortality after stroke.
It appears, therefore, plausible that an increased susceptibility to IC may be the link between AL and worse outcome in patients hospitalized for ICH.
The association between systemic inflammation, leukocyte activation, and ICH is complex [9, 31, 32]. Our findings suggest that lymphocytes play a key role in protection against infections in ICH patients, which may have important implications for future studies. Several neuroprotective strategies targeting inflammation and leukocyte activation are currently under investigation in stroke and ICH [3, 33]. However, the results of our analysis raise the hypothesis that acute inflammation and lymphocyte activation in the acute phase of ICH may play a beneficial role, decreasing the propensity to develop an IC. This hypothesis is indirectly supported by several reports describing an increased risk of infections in patients treated with immunomodulatory drugs that reduce lymphocyte count and function [34–38].
Clinical trials targeting inflammation in stroke patients should therefore balance the possible benefits of immunomodulation against a potential increased susceptibility to infections. From a clinical standpoint, infections are a leading cause of mortality in stroke patients, and therefore, prevention of such complications is an appealing therapeutic strategy [8]. However, prophylactic antibiotic treatment to prevent infections in stroke patients did not lead to improved long-term outcome in two large randomized clinical trials [39, 40]. One possible explanation is the inclusion of patients irrespective of their probability of experiencing an IC. The presence of AL may be a useful biomarker to identify patients at increased risk of IC and, therefore, more likely to benefit from intensification of preventive measures [41] or prophylactic antibiotic administration.
Lymphocyte count is a widely available and inexpensive biomarker that may be used to stratify the risk of IC also in clinical practice. Finally, discussion of goals of care and palliative treatments is a key aspect of ICH care [1], and an accurate estimation of ICH prognosis is still an unmet need [42]. AL may provide additional value in predicting the prognosis of ICH patients.
Some limits of our study should be acknowledged. First, our results derive from a single center, retrospective analysis and should be validated in prospective studies. Second, the long recruitment period may have influenced our results since several changes in ICH management occurred in this time frame. Finally, pre-ICH lymphocyte count was not available in our dataset, and therefore, we were not able to exclude the influence of potential confounders like inflammatory, infectious, and autoimmune disorders preceding the admission for ICH.
Conclusions
AL is common in ICH patients and independently associated with increased risk of infections and mortality. This highlights a possible beneficial role of lymphocytes activation in ICH morbidity and may offer a therapeutic opportunity for prevention of infections in clinical practice or in the setting of clinical trials.
Acknowledgments
This study was supported by the following awards from the NINDS: 5R01NS073344, K23AG02872605, K23 NS086873, R01NS059727. None of the funding entities had any involvement in study design; data collection, analysis, and interpretation; writing of the manuscript; or decision to submit the study for publication.
Anand Viswanathan, Christopher D. Anderson, Jonathan Rosand, and Joshua N. Goldstein report research funding from NIH; Joshua N. Goldstein reports research funding from Boehringer Ingelheim, Pfizer, and Portola; and consulting from Bristol Myers Squibb.
Footnotes
Compliance with Ethical Standards
Ethical Approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Conflict of interest Andrea Morotti, Sandro Marini, Michael Jessel, Kristin Schwab, Christina Kourkoulis, Alison M. Ayres, M. Edip Gurol, and Steven M. Greenberg report no disclosures
References
- 1.Hemphill JC, Greenberg SM, Anderson C. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46:2032–2060. doi: 10.1161/STR.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 2.Mracsko E, Veltkamp R. Neuroinflammation after intracerebral hemorrhage. Front Cell Neurosci. 2014;8:388. doi: 10.3389/fncel.2014.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fu Y, Hao J, Zhang N, et al. Fingolimod for the treatment of intracerebral hemorrhage: a 2-arm proof-of-concept study. JAMA Neurol. 2014;71:1–10. doi: 10.1001/jamaneurol.2014.1065. [DOI] [PubMed] [Google Scholar]
- 4.Chamorro Á, Urra X, Planas AM. Infection after acute ischemic stroke: a manifestation of brain-induced immunodepression. Stroke. 2007;38:1097–1103. doi: 10.1161/01.STR.0000258346.68966.9d. [DOI] [PubMed] [Google Scholar]
- 5.Dirnagl U, Klehmet J, Braun JS, et al. Stroke-induced immunodepression: experimental evidence and clinical relevance. Stroke. 2007;38:770–773. doi: 10.1161/01.STR.0000251441.89665.bc. [DOI] [PubMed] [Google Scholar]
- 6.Meisel C, Schwab JM, Prass K, et al. Central nervous system injury-induced immune deficiency syndrome. Nat Rev Neurosci. 2005;6:775–786. doi: 10.1038/nrn1765. [DOI] [PubMed] [Google Scholar]
- 7.Merayo-Chalico J, Gómez-Martín D, Piñeirúa-Menéndez A, et al. Lymphopenia as risk factor for development of severe infections in patients with systemic lupus erythematosus: a case-control study. QJM. 2013;106:451–457. doi: 10.1093/qjmed/hct046. [DOI] [PubMed] [Google Scholar]
- 8.Klehmet J, Harms H, Richter M, et al. Stroke-induced immunodepression and post-stroke infections: lessons from the preventive antibacterial therapy in stroke trial. Neuroscience. 2009;158:1184–1193. doi: 10.1016/j.neuroscience.2008.07.044. [DOI] [PubMed] [Google Scholar]
- 9.Morotti A, Phuah C-L, Anderson CD, et al. Leukocyte count and intracerebral hemorrhage expansion. Stroke. 2016;47:1473–1478. doi: 10.1161/STROKEAHA.116.013176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biffi A, Halpin A, Towfighi A, et al. Aspirin and recurrent intracerebral hemorrhage in cerebral amyloid angiopathy. Neurology. 2010;75:693–698. doi: 10.1212/WNL.0b013e3181eee40f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katz S. Assessing self-maintenance: activities of daily living, mobility, and instrumental activities of daily living. J Am Geriatr Soc. 1983;31:721–727. doi: 10.1111/j.1532-5415.1983.tb03391.x. [DOI] [PubMed] [Google Scholar]
- 12.Hu M-H, Yu Y-B, Huang Y-C, et al. Absolute lymphocyte count and risk of short-term infection in patients with immune thrombocytopenia. Ann Hematol. 2014;93:1023–1029. doi: 10.1007/s00277-014-2014-3. [DOI] [PubMed] [Google Scholar]
- 13.Giede-Jeppe A, Bobinger T, Gerner ST, et al. Lymphocytopenia is an independent predictor of unfavorable functional outcome in spontaneous intracerebral hemorrhage. Stroke. 2016;47:1239–1246. doi: 10.1161/STROKEAHA.116.013003. [DOI] [PubMed] [Google Scholar]
- 14.Boxer LA. How to approach neutropenia. ASH Educ Progr B. 2012;2012:174–182. doi: 10.1182/asheducation-2012.1.174. [DOI] [PubMed] [Google Scholar]
- 15.Carli L, Tani C, Vagnani S, et al. Leukopenia, lymphopenia, and neutropenia in systemic lupus erythematosus: prevalence and clinical impact—a systematic literature review. Semin Arthritis Rheum. 2015;45:190–194. doi: 10.1016/j.semarthrit.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 16.Smith CJ, Kishore AK, Vail A, et al. Diagnosis of stroke-associated pneumonia: recommendations from the pneumonia in stroke consensus group. Stroke. 2015;46:2335–2340. doi: 10.1161/STROKEAHA.115.009617. [DOI] [PubMed] [Google Scholar]
- 17.Wilson ML, Gaido L. Laboratory diagnosis of urinary tract infections in adult patients. Clin Infect Dis. 2004;38:1150–1158. doi: 10.1086/383029. [DOI] [PubMed] [Google Scholar]
- 18.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lord AS, Langefeld CD, Sekar P, et al. Infection after intracerebral hemorrhage: risk factors and association with outcomes in the ethnic/racial variations of intracerebral hemorrhage study. Stroke. 2014;45:3535–3542. doi: 10.1161/STROKEAHA.114.006435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murthy SB, Moradiya Y, Shah J, et al. Nosocomial infections and outcomes after intracerebral hemorrhage: a population-based study. Neurocrit Care. 2016;25:1–7. doi: 10.1007/s12028-016-0282-6. [DOI] [PubMed] [Google Scholar]
- 21.Hemphill JC, Bonovich DC, Besmertis L, et al. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001;32:891–897. doi: 10.1161/01.str.32.4.891. [DOI] [PubMed] [Google Scholar]
- 22.Delgado-Rodriguez M. Bias. J Epidemiol Community Heal. 2004;58:635–641. doi: 10.1136/jech.2003.008466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prass K, Meisel C, Höflich C, et al. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation. J Exp Med. 2003;198:725–736. doi: 10.1084/jem.20021098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liesz A, Rüger H, Purrucker J, et al. Stress mediators and immune dysfunction in patients with acute cerebrovascular diseases. PLoS ONE. 2013;8:1–10. doi: 10.1371/journal.pone.0074839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Westendorp WF, Nederkoorn PJ, Vermeij J-D, et al. Post-stroke infection: a systematic review and meta-analysis. BMC Neurol. 2011;11:110. doi: 10.1186/1471-2377-11-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Finlayson O, Kapral M, Hall R, et al. Risk factors, inpatient care, and outcomes of pneumonia after ischemic stroke. Neurology. 2011;77:1338–1345. doi: 10.1212/WNL.0b013e31823152b1. [DOI] [PubMed] [Google Scholar]
- 27.Sellars C, Bowie L, Bagg J, et al. Risk factors for chest infection in acute stroke: a prospective cohort study. Stroke. 2007;38:2284–2291. doi: 10.1161/STROKEAHA.106.478156. [DOI] [PubMed] [Google Scholar]
- 28.Lord AS, Gilmore E, Choi HA, et al. Time course and predictors of neurological deterioration after intracerebral hemorrhage. Stroke. 2015;46:647–652. doi: 10.1161/STROKEAHA.114.007704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naidech AM, Bernstein Ra, Bassin SL, et al. How patients die after intracerebral hemorrhage. Neurocrit Care. 2009;11:45–49. doi: 10.1007/s12028-009-9186-z. [DOI] [PubMed] [Google Scholar]
- 30.Katzan IL, Cebul RD, Husak SH, et al. The effect of pneumonia on mortality among patients hospitalized for acute stroke. Neurology. 2003;60:620–625. doi: 10.1212/01.wnl.0000046586.38284.60. [DOI] [PubMed] [Google Scholar]
- 31.Xiong XY, Yang QW. Rethinking the roles of inflammation in the intracerebral hemorrhage. Transl Stroke Res. 2015;6:339–341. doi: 10.1007/s12975-015-0402-1. [DOI] [PubMed] [Google Scholar]
- 32.Boehme AK, Hays AN, Kicielinski KP, et al. Systemic inflammatory response syndrome and outcomes in intracerebral hemorrhage. Neurocrit Care. 2016;25:133–140. doi: 10.1007/s12028-016-0255-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fu Y, Liu Q, Anrather J, et al. Immune interventions in stroke. Nat Rev Neurol. 2015;11:524–535. doi: 10.1038/nrneurol.2015.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salvana EMT, Salata RA. Infectious complications associated with monoclonal antibodies and related small molecules. Clin Microbiol Rev. 2009;22:274–290. doi: 10.1128/CMR.00040-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berger JR, Houff S. Opportunistic infections and other risks with newer multiple sclerosis therapies. Ann Neurol. 2009;65:367–377. doi: 10.1002/ana.21630. [DOI] [PubMed] [Google Scholar]
- 36.Gergely P. Drug-induced lymphopenia: focus on CD4+ and CD8+ cells. Drug Saf. 1999;21:91–100. doi: 10.2165/00002018-199921020-00003. [DOI] [PubMed] [Google Scholar]
- 37.Arvin AM, Wolinsky JS, Kappos L, et al. Varicella-zoster virus infections in patients treated with fingolimod: risk assessment and consensus recommendations for management. JAMA Neurol. 2015;72:31–39. doi: 10.1001/jamaneurol.2014.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goupil R, Brachemi S, Nadeau-Fredette A-C, et al. Lymphopenia and treatment-related infectious complications in ANCA-associated vasculitis. Clin J Am Soc Nephrol. 2013;8:416–423. doi: 10.2215/CJN.07300712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kalra L, Irshad S, Hodsoll J, et al. Prophylactic antibiotics after acute stroke for reducing pneumonia in patients with dysphagia (STROKE-INF): a prospective, cluster-randomised, open-label, masked endpoint, controlled clinical trial. Lancet. 2015;386:1835–1844. doi: 10.1016/S0140-6736(15)00126-9. [DOI] [PubMed] [Google Scholar]
- 40.Westendorp WF, Vermeij JD, Zock E, et al. The preventive antibiotics in stroke study (PASS): a pragmatic randomised open-label masked endpoint clinical trial. Lancet. 2015;385:1519–1526. doi: 10.1016/S0140-6736(14)62456-9. [DOI] [PubMed] [Google Scholar]
- 41.Halperin JJ, Moran S, Prasek D, et al. Reducing hospital-acquired infections among the neurologically critically Ill. Neurocrit Care. 2016;25:170–177. doi: 10.1007/s12028-016-0286-2. [DOI] [PubMed] [Google Scholar]
- 42.Rabinstein AA. Prognosis after ICH. Neurology. 2016;86:1854–1855. doi: 10.1212/WNL.0000000000002684. [DOI] [PubMed] [Google Scholar]