Abstract
Objectives
Little is known about the ongoing mortality risk and healthcare utilization among U.S. children after discharge from a hospitalization involving ICU care. We sought to understand risks for hospital readmission and trends in mortality during the year following ICU discharge.
Design
We performed a retrospective observational cohort study using administrative claims data from the years 2006–2013 obtained from the Truven Health Analytics MarketScan® Database.
Subjects
We included all children in the dataset admitted to a U.S. ICU ≤18 years old.
Interventions
The primary outcome was non-elective readmission in the year following discharge. Risk of rehospitalization was determined using a Cox proportional hazards model.
Measurements and Main Results
We identified 109,130 children with at least one ICU admission in the dataset. Over three-quarters of the index ICU admissions (78.6%) had an ICU length of stay ≤ 3 days and the overall index hospitalization mortality rate was 1.4%. In multivariate analysis, risk of non-elective readmission for children without cancer was higher with longer index ICU admission LOS, younger age, and several chronic and acute conditions. By the end of the one year observation period, 36.0% of children with an index ICU LOS ≥ 14 days had been readmitted, compared to only 13.9% of children who had an index ICU LOS = 1 day. Mortality in the year after ICU discharge was low overall (106 deaths per 10,000 person-years of observation), but was highest among children with an initial index ICU admission LOS ≥ 14 days (599 deaths per 10,000 person-years).
Conclusions
Readmission after ICU care is common. Further research is needed to investigate the potentially modifiable factors affecting likelihood of readmissions after discharge from the ICU. While late mortality was relatively uncommon overall, it was ten-fold higher in the year after ICU discharge than in the general U.S. pediatric population.
Keywords: pediatrics, pediatric critical care, pediatric intensive care, outcomes, readmission, late mortality
Scientific advances over the last two decades have led to improved survival after critical illness in childhood.1–3 Of the nearly 300,000 children admitted to US pediatric intensive care units (PICUs) every year, more than 97% survive and return home to their families, schools and communities.3–5 Despite improved mortality, little is known about ongoing mortality risk and healthcare utilization after critical illness in childhood. Small cohort studies of selected populations demonstrate lasting neurological, developmental, and multisystem medical sequelae in 10–70% of PICU survivors.6–10 In some cases, medical and psychological problems extend several years after hospital discharge.11–13 While some studies suggest that age,14 severity of illness at presentation,15 and PICU length of stay6 predispose children to worse outcome, there are conflicting data.16 And while a 2013 systematic review found that hospital readmission was common in adults in the year following an ICU admission,17 no such study has been conducted in children.
Therefore, we conducted this study to understand the risk factors for non-elective readmission and the trends in mortality among U.S. children in the year following their discharge from a hospitalization that included ICU care. We used a large, multistate private health insurance claims dataset to identify demographic and patient-level characteristics of children with an ICU admission and the risk factors associated with non-elective hospital readmission in the year following an index ICU admission. Our secondary outcome was mortality rate for hospital survivors in the year after hospital discharge.
MATERIALS AND METHODS
Data sources
We performed a retrospective observational cohort study using administrative claims data from the years 2006–2013 in the Truven Health Analytics MarketScan® Commercial Claims and Encounters Database. The MarketScan database includes claims data from approximately 100 different employer-sponsored insurance plans covering active employees, Consolidated Omnibus Budget Reconciliation Act (COBRA) continuees and their dependents. It includes information from paid insurance claims for inpatient and outpatient medical care, home care equipment and pharmacy claims. It also includes de-identified demographic data about the enrollees, their medical providers, and healthcare facilities. An encrypted person identifier allows longitudinal follow-up of enrollees over time. Because the MarketScan dataset contains no patient identifiers, the Washington University Human Research Protection Office gave this study exempt approval as a non-human subject study.
Case selection and definitions
We defined children as any person 0–18 years of age and categorized age into four groups: < 1, 1–4, 5–9 and 10–18 years of age. Because we wished to follow subjects up to one year after discharge from their index ICU admission, we included all children with at least one ICU stay between July 1, 2006 and December 31, 2012 in our study. We identified ICU admissions using ICU revenue codes 0200-0203 (general, medical-surgical, and pediatric intensive care) and 0207-0209 (burn, trauma, and other intensive care) during a hospitalization. The first ICU admission during the study period was defined as the ‘index’ ICU admission, with all others defined as ‘subsequent’ ICU admissions.
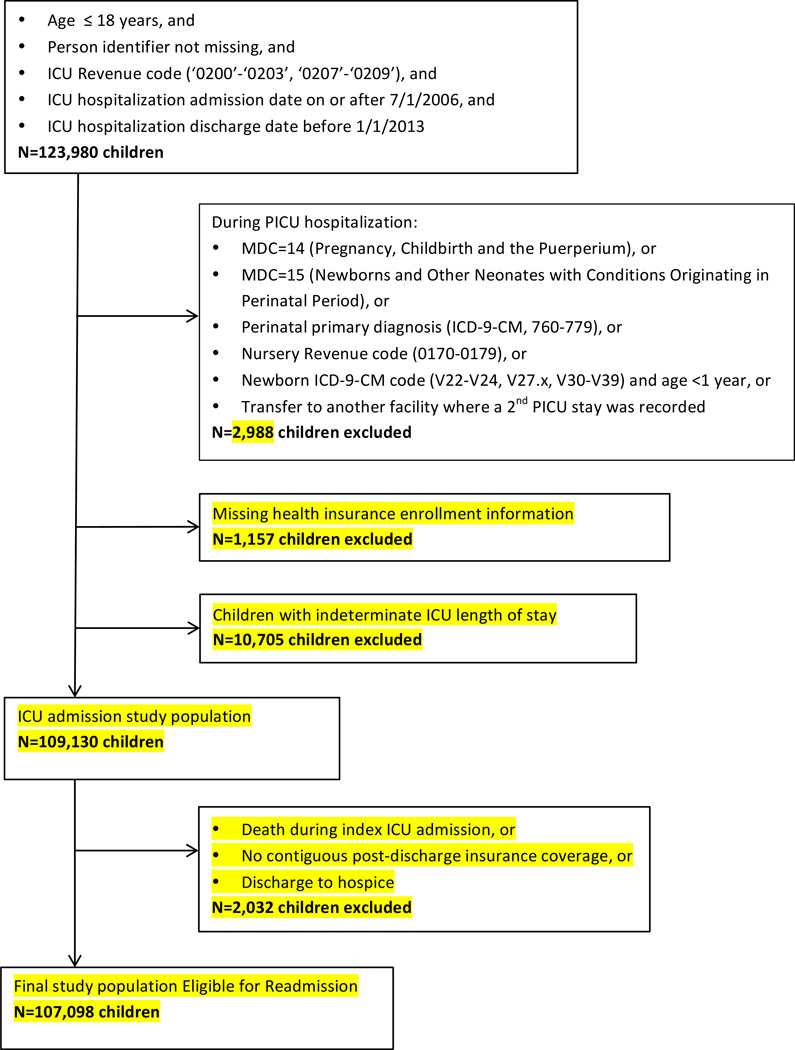
Our target cohort was all non-neonatal children admitted to a U.S. ICU for medical or surgical critical care. Therefore, we excluded all children whose index ICU admission was in major diagnostic category 14 or 15 (delivering a baby or being born), whose hospitalization included a perinatal diagnosis (ICD-9-CM codes 760.x-779.x), whose admission ocurred in a newborn nursery (UB-04 revenue codes 0170-0179), or whose index ICU admission was in a neonatal ICU (NICU). We further excluded all children missing a person identifier or if the ICU length of stay (LOS) was unknown (Figure 1). To ensure that ICU length of stay was not affected by inter-hospital transfer, we also excluded all patients with inter-hospital transfers during the index ICU admission (n=1,727) (Figure 1). Follow-up after discharge from the index ICU hospitalization continued until death, end of health insurance coverage, or the end of one year, whichever came first.
Figure 1. Case selection for analysis.
The MarketScan dataset included 35,165,676 children during our study period, of which 109,130 had at least one ICU admission and met our eligibility criteria.
Explanatory variables
We used the Pediatric Medical Complexity Algorithm to identify underlying co-morbid illness during the index hospitalization.18 Hospitalizations were defined as primary medical or surgical admissions on the basis of their diagnosis related group (DRG) code designation at discharge.19 We categorized primary diagnosis ICD-9-CM diagnosis codes into broader categories of ‘reason for hospital admission’ based on previously used schema and clinical judgement, including: hematology/oncology, cardiovascular, congenital anomalies, endocrine, gastrointestinal, infection, injury/poisoning, neurology, respiratory and other (Appendix 1).20–22 We identified all additional conditions using ICD-9-CM diagnosis codes.
We calculated ICU and ward LOS by first using the quantity of services variable (denoting number of days) and second using the difference between the “date service incurred” and “date service ending” variables. The method that resulted in a sum matching the total hospitalization LOS (± 1 day) was considered the most accurate sum, and the ICU LOS from the matching method was used as the ICU LOS. We divided the study population into 5 groups based on the index ICU LOS: 1, 2–3, 4–7, 8–13 and ≥ 14 ICU days.
Outcomes
The primary outcome of interest was non-elective re-hospitalization in the year following discharge from the index ICU admission. We defined a non-elective admission as any hospitalization with an admission date on the same day as an emergency department (revenue codes: 0450-0459, or emergency department place of service) or outpatient clinic encounter (CPT-4 codes: 99201-99205, 99211-99215, 99241-99245, 99381-99385, 99391-99395). Our secondary outcome was death in the year following discharge from the index ICU admission. Death was defined in 4 ways: 1) discharge status of death during an inpatient hospitalization, 2) sudden death (ICD-9-CM diagnosis codes 798.1, 798.2, or 798.9) or 3) cardiac arrest (≥ 2 records with ICD-9-CM diagnosis code 427.5, at least one of them a facility claim) followed by cessation of records within 14 days, or 4) hospice claims (≥ 2 records with a place of service of hospice facility or provider type of hospice facility provider, with the date of the last record considered the date of death). The presence of 2 or more non-durable medical equipment, non-radiology, and/or non-laboratory records > 30 days (60 days for hospice) following an assumed date of death was considered evidence death did not occur.
Statistical analyses
We used counts with proportions to describe univariate and bivariate distributions. Children who died during the index ICU hospitalization (n=1,502), lacked contiguous post-discharge insurance coverage (n=163), or were discharged home under hospice care (n=367) were excluded from readmission analyses (final number for readmission analysis = 107,098, 98.1% of initial population). Kaplan Meier curves were used to plot time to non-elective readmission by ICU LOS group. Standarized differences were used in univariable analysis to compare comorbidities and acute conditions during the index hospitalization for children with and without a non-elective readmission within one year.23 Covariates with standardized differences ≥ 0.05, ICU LOS group, gender, age group and hospitalization type were entered into a multivariable Cox proportional hazards model to estimate risk factors for one-year non-elective readmission. We separately performed this analysis for children with and without cancer, since children with cancer are a unique population with frequent readmissions. We used chi-square and Fishers exact tests to make all categorical comparisons. All statistical associations were tested at a significance level of 0.05. We used SAS Enterprise Guide, version 5.1 (SAS Institute Inc., Cary, NC, USA) for all data management and analyses.
RESULTS
Characteristics of the cohort
The MarketScan dataset included 35,165,676 children from July 1, 2006 – December 31, 2012, of which 109,130 had at least one ICU admission and met eligibility criteria (Figure 1). More index ICU admissions were in the southern region of the United States than other regions, consistent with the characteristics of the MarketScan dataset as a whole (Appendix 2). The majority of the 109,130 children with index ICU admissions (67.0%) were in a pediatric-specific ICU (PICU), and the remaining third were in general medical-surgical ICUs (Table 1). Overall, children admitted to PICUs were younger than children admitted to general ICUs (median age of 7 years [IQR 1,13] vs 15 years [IQR 6,17], p<0.001) and more likely to have a comorbidity (71.7% of children admitted to PICUs vs 60.6% of children admitted to general ICUs, p<0.001). The children admitted to PICUs were more likely to have a congenital condition (14.3% vs. 5.6%, p < .001), and less likely to be admitted due to injury (10.8% vs. 28.6%, p < .001) or poisoning (4.5% vs. 11.21%, p < .001) compared to children admitted to general ICUs. Despite this, there was no clinically significant difference between the median ICU LOS (2 days [IQR 1,3] for both) or the index ICU admission mortality rate of patients in PICUs (1.3 vs. 1.5%, p=0.002) compared to those in general ICUs.
Table 1.
Characteristics of the ICU study cohort
| Variable | ICU LOS 1 day |
ICU LOS 2–3 days |
ICU LOS 4–7 days |
ICU LOS 8–13 days |
ICU LOS 14 or more days |
Pediatric- specific ICU (PICU) cohort |
Entire ICU cohort |
|---|---|---|---|---|---|---|---|
| Column total, n | 42,878 | 42,859 | 14,892 | 4,841 | 3,660 | 73,108 | 109,130 |
| Female, n (%) | 19,282 (45.0) | 18,924 (44.2) | 6,442 (43.3) | 2,095 (43.3) | 1,639 (44.8) | 33,063 (45.2) | 48,382 (44.3) |
| Age group, n (%) | |||||||
| Age < 1 year | 4,866 (11.3) | 6,209 (14.5) | 3,136 (21.1) | 1,234 (25.5) | 940 (25.7) | 13,412 (18.3) | 16,385 (15.0) |
| Age 1–4 years | 9,002 (21.0) | 9,031 (21.1) | 2,820 (18.9) | 906 (18.7) | 683 (18.7) | 17,399 (23.8) | 22,442 (20.6) |
| Age 5–12 years | 10,893 (25.4) | 11,127 (26.0) | 3,704 (24.9) | 1,084 (22.4) | 791 (21.6) | 21,256 (29.1) | 27,599 (25.3) |
| Age 13–18 years | 18,117 (42.3) | 16,492 (38.5) | 5,232 (35.1) | 1,617 (33.4) | 1,246 (34.0) | 21,041 (28.8) | 42,704 (39.1) |
| Underlying comorbidity | |||||||
| Pulmonary/respiratory | 5,106 (11.9) | 6,113 (14.3) | 2,191 (14.7) | 830 (17.1) | 890 (24.3) | 11,109 (15.2) | 15,130 (13.9) |
| Neurological | 6,878 (16.0) | 7,643 (17.8) | 3,371 (22.6) | 1,509 (31.2) | 1,499 (41.0) | 15,377 (21.0) | 20,900 (19.2) |
| Gastrointestinal | 1,192 (2.8) | 1,387 (3.2) | 778 (5.2) | 367 (7.6) | 523 (14.3) | 3,135 (4.3) | 4,247 (3.9) |
| Immunological | 740 (1.7) | 882 (2.1) | 539 (3.6) | 246 (5.1) | 350 (9.6) | 2,070 (2.8) | 2,757 (2.5) |
| Malignancy | 1,664 (3.9) | 1,885 (4.4) | 1,071 (7.2) | 428 (8.8) | 472 (12.9) | 4,155 (5.7) | 5,520 (5.1) |
| Endocrinologic | 4,396 (10.3) | 4,449 (10.4) | 815 (5.5) | 289 (6.0) | 328 (9.0) | 6,767 (9.3) | 10,277 (9.4) |
| Metabolic | 583 (1.4) | 794 (1.9) | 455 (3.1) | 251 (5.2) | 306 (8.4) | 1,727 (2.4) | 2,389 (2.2) |
| Hematological | 1,122 (2.6) | 1,552 (3.6) | 902 (6.1) | 462 (9.5) | 684 (18.7) | 3,480 (4.8) | 4,722 (4.3) |
| Mental disease | 5,338 (12.4) | 4,316 (10.1) | 1,240 (8.3) | 450 (9.3) | 450 (12.3) | 6,175 (8.4) | 11,794 (10.8) |
| Musculoskeletal | 4,458 (10.4) | 3,857 (9.0) | 1,430 (9.6) | 449 (9.3) | 521 (14.2) | 8,176 (11.2) | 10,715 (9.8) |
| Ophthalmological | 592 (1.4) | 718 (1.7) | 401 (2.7) | 268 (5.5) | 264 (7.2) | 1,669 (2.3) | 2,243 (2.1) |
| Otologic | 234 (0.5) | 232 (0.5) | 70 (0.5) | 31 (0.6) | 24 (0.7) | 383 (0.5) | 591 (0.5) |
| Cardiac | 4,737 (11.0) | 6,386 (14.9) | 3,959 (26.6) | 1,621 (33.5) | 1,605 (43.9) | 14,014 (19.2) | 18,308 (16.8) |
| Renal | 561 (1.3) | 854 (2.0) | 534 (3.6) | 264 (5.5) | 351 (9.6) | 1,889 (2.6) | 2,564 (2.3) |
| Genitourinary | 306 (0.7) | 396 (0.9) | 181 (1.2) | 67 (1.4) | 73 (2.0) | 813 (1.1) | 1,023 (0.9) |
| Dermatological | 87 (0.2) | 108 (0.3) | 96 (0.6) | 83 (1.7) | 152 (4.2) | 320 (0.4) | 526 (0.5) |
| Craniofacial | 395 (0.9) | 358 (0.8) | 74 (0.5) | 22 (0.5) | 22 (0.6) | 729 (1.0) | 871 (0.8) |
| Genetic | 1,110 (2.6) | 1,382 (3.2) | 819 (5.5) | 332 (6.9) | 349 (9.5) | 3,231 (4.4) | 3,992 (3.7) |
| Any comorbidity | 27,578 (64.3) | 28,596 (66.7) | 10,893 (73.1) | 3,879 (80.1) | 3,282 (89.7) | 52,389 (71.7) | 74,228 (68.0) |
|
Medical or Surgical admission, n (%) |
|||||||
| Medical | 28,742 (67.0) | 28,017 (65.4) | 7,790 (52.3) | 2,179 (45.0) | 1,188 (32.5) | 43,940 (60.1) | 67,916 (62.2) |
| Surgical | 14,136 (33.0) | 14,842 (34.6) | 7,102 (47.7) | 2,662 (55.0) | 2,472 (67.5) | 29,168 (39.9) | 41,214 (37.8) |
|
Primary admitting diagnosis category, n (%) |
|||||||
| Hematology/oncology | 2,069 (4.8) | 2,293 (5.4) | 1,110 (7.5) | 357 (7.4) | 429 (11.7) | 4,525 (6.2) | 6,258 (5.7) |
| Cardiovascular | 1,450 (3.4) | 1,633 (3.8) | 797 (5.4) | 321 (6.6) | 223 (6.1) | 3,304 (4.5) | 4,424 (4.1) |
| Congenital | 4,034 (9.4) | 4,922 (11.5) | 2,447 (16.4) | 623 (12.9) | 420 (11.5) | 10,419 (14.3) | 12,446 (11.4) |
| Endocrine | 3,786 (8.8) | 3,774 (8.8) | 420 (2.8) | 94 (1.9) | 83 (2.3) | 5,419 (7.4) | 8,157 (7.5) |
| Gastrointestinal | 1,815 (4.2) | 1,868 (4.4) | 678 (4.6) | 234 (4.8) | 181 (4.9) | 3,155 (4.3) | 4,776 (4.4) |
| Infection | 1,221 (2.8) | 1,646 (3.8) | 816 (5.5) | 337 (7.0) | 294 (8.0) | 3,007 (4.1) | 4,314 (4.0) |
| Injury | 7,145 (16.7) | 6,917 (16.1) | 2,412 (16.2) | 969 (20.0) | 771 (21.1) | 7,897 (10.8) | 18,214 (16.7) |
| Neurology | 4,259 (9.9) | 4,355 (10.2) | 1,240 (8.3) | 318 (6.6) | 222 (6.1) | 7,921 (10.8) | 10,394 (9.5) |
| Poisoning | 4,573 (10.7) | 2,394 (5.6) | 280 (1.9) | 35 (0.7) | 20 (0.5) | 3,264 (4.5) | 7,302 (6.7) |
| Respiratory | 6,313 (14.7) | 8,788 (20.5) | 3,185 (21.4) | 1,106 (22.8) | 726 (19.8) | 15,336 (21.0) | 20,118 (18.4) |
| Other | 6,213 (14.5) | 4,269 (10.0) | 1,507 (10.1) | 447 (9.2) | 291 (8.0) | 8,861 (12.1) | 12,727 (11.7) |
| In-hospital death, n(%) | 518 (1.2) | 353 (0.8) | 279 (1.9) | 127 (2.6) | 225 (6.1) | 949 (1.3) | 1,502 (1.4) |
ICU = intensive care unit; LOS = length of stay
Index ICU Admission Characteristics
Over three-quarters of all index ICU admissions for U.S. children in our study period (78.6%) had an ICU LOS ≤ 3 days (Table 1). These short-stay admissions were nearly evenly distributed between those 1 day long (n=42,878) and 2–3 days long (n=42,859). Children with a short ICU LOS (≤ 3 days) had a median age of 10 (IQR 2,15) years, and about two-thirds (62.2%) were admitted for medical (as compared to surgical) conditions. Overall mortality during the index ICU admission for children with an ICU LOS = 1 day was low (1.2%), with the majority of deaths occurring among children admitted either for injury (243 of 518 deaths, 46.9% of deaths during index hospitalizations with one day of ICU stay) or cardiovascular diagnoses (16.2% of deaths in index hospitalizations with one day of ICU stay). With increasing ICU LOS, in-hospital mortality dropped slightly (0.8% for ICU LOS = 2–3 days) and then climbed steadily up to 6.1% among children with an ICU LOS ≥ 14 days (Table 1). Only 3.4% of all index ICU admissions had an ICU LOS ≥ 14 days, and children with these longer ICU admissions were much more likely to have an underlying chronic comorbidity (89.7% vs. 64.3% of children with ICU LOS = 1 day, p<0.001) and surgical diagnosis (67.5% vs. 33.0% of children with ICU LOS = 1 day, p<0.001) (Table 1).
Overall, the primary diagnoses during the index ICU admissions were widely distributed among the 10 diagnostic categories (Table 1). However, injury/poisoning accounted for nearly a quarter of all admissions (23.4%), followed by respiratory conditions (18.4%) and admissions for congenital anomalies (11.4% of all index ICU admissions). Children admitted to PICUs had a higher proportion of admissions related to congenital anomalies (14.3% vs 5.6%, p<0.001) and a lower proportion related to injury/poisoning (15.3% vs 39.9%, p<0.001) than children admitted to general medical-surgical ICUs. Admitting diagnosis of hematology/oncology was increasingly common as the duration of ICU length of stay increased (4.8% for all admissions with LOS = 1 day vs 11.7% of all admitting diagnoses for ICU LOS ≥ 14 days, p<0.001). In contrast, admissions for endocrinologic indications accounted for 8.8% of all ICU admissions with a LOS = 1 day, but accounted for only 2.3% of admissions with a LOS ≥ 14 days (p<0.001) (Table 1). Children with an underlying comorbidity had a slightly higher in-hospital mortality rate during the index ICU admission compared to children without a comorbidity (1.5% versus 1.1%, p<0.001). In-hospital mortality was also slightly higher for boys compared to girls (1.5% versus 1.2%, p<0.001).
Non-elective readmission in the year following ICU discharge
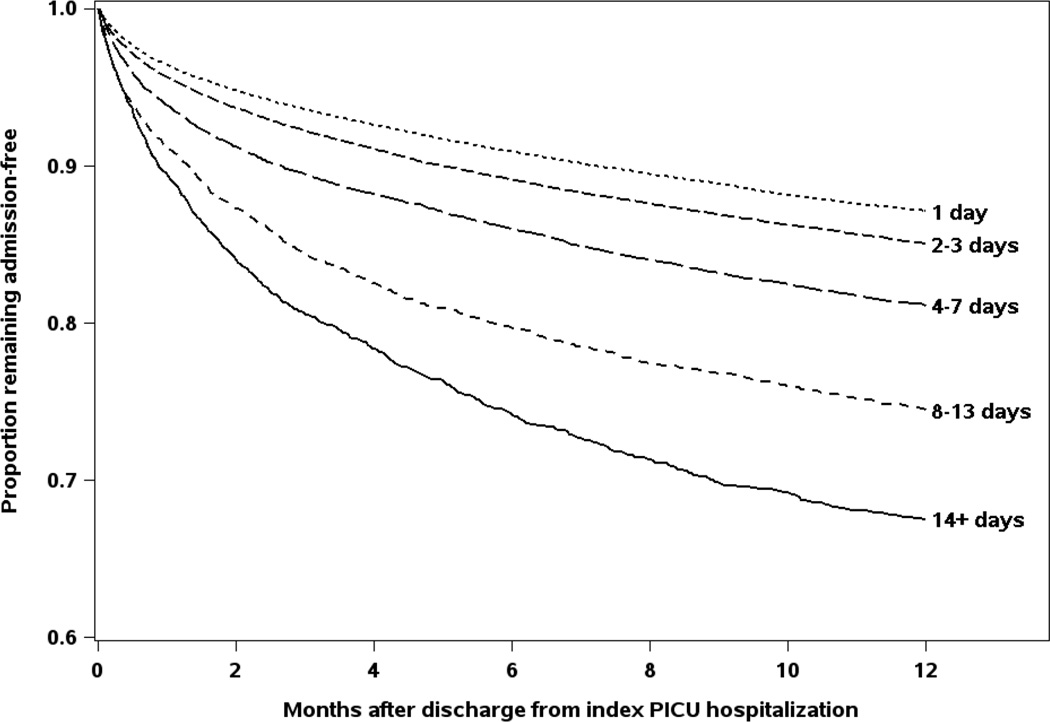
There were 54,159 total readmissions among 24,328 children (22.7% of the 107,098 children eligible for readmission) in the year following the index ICU hospitalization discharge (rate of 62 readmissions per 100 person-years of observation [PYO]). Fifty-two percent of the total readmissions were non-elective (n = 28,127), associated with either a same-day emergency department or clinic encounter. These non-elective readmissions in the year following the index ICU hospitalization occurred in 16,298 children (32 non-elective readmissions per 100 PYO). Nearly a third of these non-elective readmissions included stay in an ICU (8,839 readmissions among 6,329 children, 10 non-elective ICU readmissions per 100 PYO). The median time to non-elective readmission was 63 days (IQR 18,163). The rate of non-elective readmission for children with an index ICU LOS ≥ 14 days was three times higher than for children whose index ICU LOS = 1 day (78 vs 25 admissions per 100 PYO, p<0.001). By the end of the one year observation period, 36.0% of children with an index ICU LOS ≥ 14 days had at least one non-elective readmission, compared to only 13.9% of children who had an an index ICU LOS = 1 day (Figure 2).
Figure 2. Readmission in the year after hospital discharge, by index intensive care unit (ICU) admission length of stay (LOS).
By the end of the one year observation period, 36.0% of children with an index ICU LOS ≥ 14 days had been readmitted, compared to only 13.9% of children who had an an index ICU LOS = 1 day.
In multivariable analysis, risk of non-elective readmission for children without cancer was higher with longer index ICU admission LOS, younger age, and several chronic and acute conditions (Table 2). For example, while an index ICU admission for injury or poisoning was associated with a lower risk of readmission in the following year (HR=0.53 and 0.78, respectively), children admitted for a complication of sickle cell disease had a very high risk of non-elective readmission (HR=3.9, Table 2). Having any of the identified comorbidities was associated with a slightly increased risk of readmission in the following year, with GI and neurologic comorbidities having the strongest relationship with readmission. In addition, several acute conditions typically thought of as self-limited, including sepsis and pneumonia, were associated with a slightly increased risk for readmission in the following year (Table 2).
Table 2.
Variables associated with non-elective readmission in the year after intensive care unit (ICU) discharge for children without cancer
| Variable | Hazard Ratio |
95% Confidence Interval |
p - value |
|---|---|---|---|
| Reference group: ICU LOS = 1 day | 1.00 | ||
| ICU LOS 2–3 days | 1.11 | 1.07–1.15 | <0.001 |
| ICU LOS 4–7 days | 1.34 | 1.28–1.42 | <0.001 |
| ICU LOS 8–13 days | 1.64 | 1.53–1.77 | <0.001 |
| ICU LOS ≥ 14 day | 1.59 | 1.46–1.74 | <0.001 |
| Female | 1.08 | 1.05–1.12 | <0.001 |
| Age < 1 year | 1.45 | 1.38–1.53 | <0.001 |
| Age 1–4 years | 1.03 | 0.98–1.08 | 0.205 |
| Age 5–12 years | 0.76 | 0.73–0.80 | <0.001 |
| Reference group: Age 13–18 years | 1.00 | ||
| Surgical index ICU admission | 0.73 | 0.70–0.76 | <0.001 |
| Underlying comorbidities | |||
| Pulmonary/respiratory | 1.34 | 1.29–1.41 | <0.001 |
| Neurological | 1.72 | 1.65–1.80 | <0.001 |
| Gastrointestinal | 1.89 | 1.77–2.01 | <0.001 |
| Immunological | 1.32 | 1.21–1.43 | <0.001 |
| Endocrinological | 1.25 | 1.15–1.36 | <0.001 |
| Metabolic | 1.38 | 1.27–1.51 | <0.001 |
| Hematological | 1.16 | 1.07–1.26 | <0.001 |
| Mental disease | 1.45 | 1.37–1.54 | <0.001 |
| Cardiac | 1.16 | 1.10–1.21 | <0.001 |
| Renal | 1.53 | 1.40–1.68 | <0.001 |
| Genitourinary | 1.39 | 1.22–1.58 | <0.001 |
| Genetic | 1.39 | 1.30–1.49 | <0.001 |
|
Acute conditions during index ICU admission |
|||
| Poisoning | 0.78 | 0.72–0.85 | <0.001 |
| Injury | 0.53 | 0.50–0.56 | <0.001 |
| Septicemia | 1.09 | 1.01–1.17 | 0.030 |
| Diabetes with ketoacidosis | 1.45 | 1.31–1.61 | <0.001 |
| Diabetes insipidis | 1.32 | 1.05–1.66 | 0.016 |
| Sickle cell with crisis | 3.93 | 3.30–4.68 | <0.001 |
| Status epilepticus | 1.19 | 1.09–1.30 | <0.001 |
| Anoxic brain damage | 1.22 | 1.04–1.43 | 0.017 |
| Conduction disorders | 1.08 | 1.02–1.14 | 0.005 |
| Pneumonia (bacterial & viral) | 1.11 | 1.06–1.17 | <0.001 |
| Acute and chronic respiratory failure | 1.35 | 1.18–1.55 | <0.001 |
| Tracheostomy complications | 1.47 | 1.23–1.76 | <0.001 |
| Gastrostomy tube complications | 1.74 | 1.45–2.08 | <0.001 |
| Acute renal failure | 0.89 | 0.80–0.98 | 0.025 |
| Device complications | 1.59 | 1.48–1.71 | <0.001 |
| Convulsions | 1.15 | 1.08–1.21 | <0.001 |
| Feeding difficulties | 1.31 | 1.21–1.41 | <0.001 |
| Complications of transplanted organ | 2.16 | 1.85–2.53 | <0.001 |
ICU = intensive care unit; LOS = length of stay
| n | 101,762 |
| events | 14,144 |
| LR test statistic | 6436.6 |
| C-statistic | 0.70 |
For children with cancer, there were far fewer conditions associated with an increased likelihood of non-elective readmission, and younger age and longer index ICU LOS were not significantly associated with increased risk of readmission in this population (Table 3). While acute renal failure during the index ICU admission was not associated with increased risk of readmission for children without cancer, both acute renal failure and weight loss were associated with an increased risk of readmission for the children with cancer. Index ICU admissions for surgical conditions, as opposed to medical conditions, were associated with a lower risk of readmission the following year in both groups.
Table 3.
Variables associated with non-elective readmission in the year after intensive care unit (ICU) discharge for children with cancer
| Variable | Hazard Ratio |
95% Confidence Interval |
p-value |
|---|---|---|---|
| Reference: ICU LOS = 1 day | 1.00 | ||
| ICU LOS 2–3 days | 1.10 | 0.98–1.22 | 0.094 |
| ICU LOS 4–7 days | 1.17 | 1.04–1.33 | 0.011 |
| ICU LOS 8–13 days | 1.38 | 1.17–1.62 | <0.001 |
| ICU LOS 14 days | 1.02 | 0.85–1.23 | 0.814 |
| Age Group | |||
| Age < 1 year | 1.18 | 0.97–1.44 | 0.098 |
| Age 1–4 years | 1.45 | 1.30–1.63 | <0.001 |
| Age 5–12 years | 1.17 | 1.06–1.30 | 0.003 |
| Reference: Age 13–18 years | 1.00 | ||
| Surgical index ICU admission | 0.62 | 0.56–0.67 | <0.001 |
| Underlying comorbidity | |||
| Gastrointestinal | 1.22 | 1.04–1.43 | 0.013 |
| Endocrinologic | 0.84 | 0.71–0.99 | 0.033 |
| Hematological | 1.21 | 1.08–1.36 | <0.001 |
| Musculoskeletal | 0.76 | 0.60–0.96 | 0.022 |
| Index ICU admission acute condition | |||
| Conduction disorders | 1.28 | 1.12–1.47 | <0.001 |
| Acute renal failure | 1.69 | 1.42–2.02 | <0.001 |
| Device complications | 1.39 | 1.20–1.61 | <0.001 |
| Abnormal weight loss | 1.47 | 1.05–2.05 | 0.025 |
| Feeding difficulties | 1.27 | 1.03–1.57 | 0.024 |
ICU = intensive care unit; LOS = length of stay
| n | 5336 |
| events | 2154 |
| LR test statistic | 377.5 |
| C-statistic | 0.83 |
Mortality in the year following hospital discharge
Mortality in the year following hospital discharge was low, with a total of 931 observed deaths in the year after index ICU discharge (0.9% of hospital survivors, 106 deaths per 10,000 person-years of observation [PYO], Table 4). Longer LOS during the index ICU admission was associated with higher mortality rates in the subsequent year (62 deaths per 10,000 PYO for children with an index ICU LOS = 1 day, vs 599 deaths per 10,000 PYO for those with an index ICU admission ≥ 14 days, p<0.001). Index ICU primary diagnoses of hematology/oncology, infection, and cardiovascular conditions were associated with the highest mortality rates in the year following hospital discharge (Table 4). Death in the year after discharge was also strongly associated with presence of a comorbidity; children with any underlying comorbidity had a mortality rate five times higher than children without a comorbidity (142 vs 28 deaths per 10,000 PYO, p<0.001). Just over a third of deaths in the year following discharge from the index ICU admission (37.3%, n=347) were among children with a previous diagnosis of cancer. The post-discharge mortality rate for children with cancer was over 11 times higher than among children without cancer, 804 vs 70 deaths per 10,000 PYO, p<0.001). Mortality was also higher among children who had been admitted to PICUs vs those who had been cared for in general medical-surgical ICUs (120 vs 77 deaths per 10,000 PYO, p<0.001).
Table 4.
Mortality in the year after ICU hospitalization discharge
| Variable | Deaths (n) | Mortality rate (per 10,000 person-years) |
|---|---|---|
| Index ICU LOS | ||
| 1 day | 216 | 62 |
| 2–3 days | 268 | 77 |
| 4–7 days | 177 | 148 |
| 8–13 days | 114 | 302 |
| ≥ 14 days | 156 | 599 |
| Primary diagnosis during index ICU admission | ||
| Hematology/oncology | 254 | 502 |
| Cardiovascular | 60 | 174 |
| Congenital | 63 | 62 |
| Endocrine | 40 | 60 |
| Gastrointestinal | 44 | 113 |
| Infection | 70 | 208 |
| Injury | 58 | 40 |
| Neurology | 88 | 103 |
| Poisoning | 17 | 29 |
| Respiratory | 149 | 93 |
| Other | 88 | 84 |
| Underlying comorbidity | ||
| Any comorbidity | 851 | 142 |
| No comorbidity | 80 | 28 |
| Underlying malignancy | ||
| No malignancy | 584 | 70 |
| Malignancy | 347 | 804 |
| Type of ICU | ||
| General medical-surgical ICUs | 221 | 77 |
| Pediatric-specific ICUs | 710 | 120 |
ICU = intensive care unit; LOS = length of stay
DISCUSSION
Using a large insurance dataset, we identified 109,130 ICU admissions for U.S. children over a seven year period. The overwhelming majority of these ICU stays were short (78.6% had an ICU LOS ≤ 3 days) and the in-hospital mortality rate was very low, at 1.4%. Although mortality in the year after discharge from the index hospitalization was rare, even the post-discharge mortality rate for children who were admitted to the ICU for only 1 day (62 per 10,000 PYO) was still two and a half times times higher than the baseline pediatric mortality rate for US children 1–4 years old (26 per 10,000 PYO) and nearly five times higher than the mortality rate for US children 5–14 years old (13 per 10,000 PYO).24
After discharge, 15.2% of ICU survivors had at least one non-elective hospital readmission in the following year, and nearly a third of observed non-elective readmissions included ICU care. This readmission rate is more than two times higher than the 30-day unplanned readmission rate for U.S. children described by Berry et al., highlighting the increased risk of readmission for children whose hospitalization included ICU care and even more importantly, prolonged ICU care.25 Our study differed in that we assessed readmission up to one year after hospital discharge, which may additionally account for our higher readmission rate. Rates of hospital readmission in our population were also higher than the hospital admission rate for U.S. children in general (3 per 100 children per year from 2006 to 2013),26 presumably due to the difference in overall health of an ICU survivor population compared to the U.S. pediatric population as a whole. Even when controlling for other factors, children without cancer with longer ICU LOS were over 1.5 times more likely to be readmitted in the year after discharge than children admitted to the ICU for only one day.
In this cohort, we found that ICU care for children is generally well regionalized to hospitals with designated services for children, as just over two-thirds of all admissions were in units that self identified as pediatric-specific ICUs (PICUs). Those children who were admitted to general medical-surgical ICUs were older, healthier and more likely to have traumatic injuries than those in PICUs. These findings may indicate that ICU care for children with medical complexity is generally well regionalized to children’s hospitals in the U.S., and that local community hospitals continue to provide vital ICU services for high acuity traumatic injuries and more straightforward medical problems in children. Children whose index ICU admission was in a PICU also had higher in-hospital mortality rates than children admitted to general medical surgical ICUs; likely due to the regionalization of cancer care and care for children with chronic medical complexity within children’s hospitals.
This study is strengthened by its large size, geographic diversity and the generalizeable nature of the patient population (over 100 million working American adults and their families). The MarketScan database provides both tremendous statistical power to study rare diseases and has the added benefit of a patient identifier that permits longitudinal analysis. It has been used previously to study readmission in adults with a number of different conditions.27,28 However, this is the first study of pediatric critical illness with both a large and diverse patient population, supplemented by rich longitudinal health care utilization data over long periods of time.
This study does have several limitations, however. First, detailed information about in-hospital care is not always reliably captured within the MarketScan dataset. Therefore, physiologic and severity of illness indicators, including vital signs, laboratory data, presence/absence and duration of mechanical ventilation, and use of other extracorporeal support was not available. Inclusion of these details as part of the description of the index ICU admission would have provided a richer understanding of the patient population and its similarity to that at any given hospital. However, our study cohort was large, distributed across the entire U.S., primarily cared for in pediatric-specific ICUs, and had an overall mortality rate comparable to tertiary and quaternary U.S. PICUs.19,20,29 We believe our cohort is likely representative of high-acuity U.S. PICUs. This study was also subject to the known limitations of diagnostic coding in administrative datasets. However, we used a highly reliable code (ICU admission) as the criteria for inclusion in this study, and grouped less reliable codes (primary diagnosis) into general categories solely for general description purposes.
In addition, the MarketScan dataset is not linked to U.S. vital records, and as such, deaths outside the hospital were difficult to identify. Within the MarketScan data, cessation of enrollment is not accompanied by an explanation, and therefore an out of hospital death can appear identical to a sudden termination of employment and loss of benefits. Because the overall pediatric mortality rate is very low, and very few children die outside the medical setting, we believe the risk of having missed deaths in our study cohort is quite low. However, it is possible that we have underestimated post-hospital mortality.
Lastly, while the MarketScan dataset encompasses the geographic majority of the U.S. and a large number of payers, it is not inclusive of the entire U.S. population. We acknowledge that this dataset does not include families covered by Medicaid or other forms of public insurance and therefore does not truly represent the entire U.S. population. Given that our final population was quite large, extended across the entire U.S. and over time, and included working families at a variety of income levels, we believe our results are generalizeable to a large proportion of American children. This analysis informs on the risk of late mortality and readmission for U.S. children with commercial health insurance.
In conclusion, this is the largest observational cohort study of critically ill children in the U.S. to date, which uniquely provides a glimpse into hospital admission patterns for children with critical illness. It is also the first cohort of this size to be followed longitudinally after discharge, identifying risk factors for post-ICU, non-elective hospital readmission and trends in post-ICU mortality. Children with longer hospital stays and pre-existing medical complexity appear to be at highest risk for both of these outcomes, and therefore might be a target population for interventions to reduce inpatient care and overall health care costs. In addition, children with medical complexity have frequent healthcare exposure, and the interplay of underlying co-morbidity with critical illness is still poorly understood. As more centers develop comprehensive programs for children with medical complexity, analyses that explore the role of new or recurring ICU care on underlying health status and healthcare utilization in this population would be invaluable. Claims datasets such as this, that include inpatient, outpatient and pharmacy data provide a unique opportunity to explore this relationship.
These data not only help us to understand the broader picture of ICU care for US children, but should also help to frame future studies aimed at the potentially modifiable factors for hospital readmission and mortality in the year after hospital discharge.
What’s known on this subject
Psychological sequelae have been studied in survivors of critical illness in childhood, and other long-term outcomes have been studied in survivors of severe sepsis.
What this adds to the field
This large, population-based study describes the epidemiology of ICU care for US children, risk of readmission and mortality trends in the year following their ICU discharge.
Acknowledgments
Dr. Olsen and Dr. Saeed are both members of the Washington University Center for Administrative Data Research (CADR). Their contributions to this work was funded in part by the Washington University Institute of Clinical and Translational Sciences grant UL1 TR000448 from the National Center for Advancing Translational Sciences (NIH) and by grant number R24 HS19455 (PI: V. Fraser) from the Agency for Healthcare Research and Quality (AHRQ). The authors also wish to acknowledge Ms. Harini Subramaniam for her initial work with data programming.
Abbreviations
- PICU
pediatric intensive care unit
- NICU
neonatal intensive care unit
- CICU
cardiac intensive care unit
- LOS
length of stay
- PCCC
Pediatric Complex Chronic Conditions
- ADHD
Attention deficit/Hyperactivity disorder
- DRG
diagnosis related group
- IQR
interquartile range
Appendix 1
Diagnosis codes for index ICU admission primary diagnosis categories
| Diagnosis Category | ICD-9-CM Diagnosis Codes |
|---|---|
| Injury | 800-904, 925-959, 990-994 |
| Poisoning | 960-989 |
| Respiratory | 460-519, 997.31 |
| Cardiovascular | 390-459 |
| Hematology/Oncology | 140-239, 280-289, V58.11, V58.12 |
| Endocrine | 240-279 |
| Neurology | 046-049, 062-064, 320-359, 780.3x, 996.2, |
| Congenital anomalies | 740-759 |
| Gastrointestinal | 070.x, 520-579, 787.x, 789.x, |
| Infection | 001.0-045.9, 050.0-061, 065.0-069, 071-139.8, 590.x, 597.x, 599.0x, 614.0-616.0, 616.10, 616.3, 616.4, 634.0x, 635.0x, 636.0x, 637.0x, 638.0, 639.0, 646.6x, 647.0x, 647.1x, 647.2x, 647.8x, 658.4x, 659.3x, 670.x, 680-686, 771.82, 790.7, 771.81, 771.83, 780.6x, 991.3x, 998.5x, 996.6x |
Appendix 2
General Characteristics of Total MarketScan Population
| Variable | n (%) |
|---|---|
| Year | |
| 2006 | 6,947 (6.4)* |
| 2007 | 13,675 (12.5) |
| 2008 | 18,065 (16.6) |
| 2009 | 18,431 (16.9) |
| 2010 | 15,399 (14.1) |
| 2011 | 18,694 (17.1) |
| 2012 | 17,919 (16.4) |
| Region | |
| Northeast | 16,424 (15.0) |
| North Central | 29,450 (27.0) |
| South | 42,925 (39.3) |
| West | 17,528 (16.1) |
| Unknown | 2,803 (2.6) |
Dataset begins on July 1, 2006
Footnotes
Financial disclosure The authors have no financial disclosures
Conflict of interest The authors have no conflict of interest to report
Contributors’ Statement
Dr. Hartman Conceptualized the study, performed data analysis, drafted the initial manuscript and approved the final manuscript as submitted
Dr. Saeed Performed the final data analysis, had primary authorship of the methods, secondary authorship of results, and approved the final version of the manuscript
Dr. Bennett Had primary authorship of the discussion, critically reviewed and approved the final version of the manuscript
Dr. Typpo Primary author of introduction, critically reviewed and approved the final version of the manuscript
Dr. Matos Secondary authorship of the discussion, critical reviewer of entire manuscript
Dr. Olsen Supervision of data analysis and senior reviewer of the manuscript. Dr. Olsen made multiple, substantive and detailed recommendations regarding the content and format of results. She critically reviewed and approved the final version of the manuscript.
REFERENCES
- 1.Mangia CM, Kissoon N, Branchini OA, Andrade MC, Kopelman BI, Carcillo J. Bacterial sepsis in Brazilian children: a trend analysis from 1992 to 2006. PloS one. 2011;6(6):e14817. doi: 10.1371/journal.pone.0014817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Oliveira CF, de Oliveira DS, Gottschald AF, et al. ACCM/PALS haemodynamic support guidelines for paediatric septic shock: an outcomes comparison with and without monitoring central venous oxygen saturation. Intensive Care Med. 2008;34(6):1065–1075. doi: 10.1007/s00134-008-1085-9. [DOI] [PubMed] [Google Scholar]
- 3.Tilford JM, Roberson PK, Lensing S, Fiser DH. Improvement in pediatric critical care outcomes. Crit Care Med. 2000;28(2):601–603. doi: 10.1097/00003246-200002000-00072. [DOI] [PubMed] [Google Scholar]
- 4.Alkandari O, Eddington KA, Hyder A, et al. Acute kidney injury is an independent risk factor for pediatric intensive care unit mortality, longer length of stay and prolonged mechanical ventilation in critically ill children: a two-center retrospective cohort study. Crit Care. 2011;15(3):R146. doi: 10.1186/cc10269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tilford JM, Simpson PM, Green JW, Lensing S, Fiser DH. Volume-outcome relationships in pediatric intensive care units. Pediatrics. 2000;106(2 Pt 1):289–294. doi: 10.1542/peds.106.2.289. [DOI] [PubMed] [Google Scholar]
- 6.Namachivayam P, Taylor A, Montague T, et al. Long-stay children in intensive care: long-term functional outcome and quality of life from a 20-yr institutional study. Pediatr Crit Care Med. 2012;13(5):520–528. doi: 10.1097/PCC.0b013e31824fb989. [DOI] [PubMed] [Google Scholar]
- 7.Bronner MB, Knoester H, Bos AP, Last BF, Grootenhuis MA. Posttraumatic stress disorder (PTSD) in children after paediatric intensive care treatment compared to children who survived a major fire disaster. Child Adolesc Psychiatry Ment Health. 2008;2(1):9. doi: 10.1186/1753-2000-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bronner MB, Knoester H, Sol JJ, Bos AP, Heymans HS, Grootenhuis MA. An explorative study on quality of life and psychological and cognitive function in pediatric survivors of septic shock. Pediatr Crit Care Med. 2009;10(6):636–642. doi: 10.1097/PCC.0b013e3181ae5c1a. [DOI] [PubMed] [Google Scholar]
- 9.Davydow DS, Richardson LP, Zatzick DF, Katon WJ. Psychiatric morbidity in pediatric critical illness survivors: a comprehensive review of the literature. Arch Pediatr Adolesc Med. 2010;164(4):377–385. doi: 10.1001/archpediatrics.2010.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morrison AL, Gillis J, O'Connell AJ, Schell DN, Dossetor DR, Mellis C. Quality of life of survivors of pediatric intensive care. Pediatr Crit Care Med. 2002;3(1):1–5. doi: 10.1097/00130478-200201000-00001. [DOI] [PubMed] [Google Scholar]
- 11.de Graaf J, van Lingen RA, Simons SH, et al. Long-term effects of routine morphine infusion in mechanically ventilated neonates on children's functioning: five-year follow-up of a randomized controlled trial. Pain. 2011;152(6):1391–1397. doi: 10.1016/j.pain.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 12.Hanekamp MN, Mazer P, van der Cammen-van Zijp MH, et al. Follow-up of newborns treated with extracorporeal membrane oxygenation: a nationwide evaluation at 5 years of age. Crit Care. 2006;10(5):R127. doi: 10.1186/cc5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nijhuis-van der Sanden MW, van der Cammen-van Zijp MH, Janssen AJ, et al. Motor performance in five-year-old extracorporeal membrane oxygenation survivors: a population-based study. Critical Care (London, England) 2009;13(2):R47. doi: 10.1186/cc7770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buysse CM, Raat H, Hazelzet JA, et al. Long-term health-related quality of life in survivors of meningococcal septic shock in childhood and their parents. Qual Life Res. 2007;16(10):1567–1576. doi: 10.1007/s11136-007-9271-8. [DOI] [PubMed] [Google Scholar]
- 15.Rennick JE, Morin I, Kim D, Johnston CC, Dougherty G, Platt R. Identifying children at high risk for psychological sequelae after pediatric intensive care unit hospitalization. Pediatr Crit Care Med. 2004;5(4):358–363. doi: 10.1097/01.pcc.0000128603.20501.0d. [DOI] [PubMed] [Google Scholar]
- 16.Bronner MB, Knoester H, Bos AP, Last BF, Grootenhuis MA. Follow-up after paediatric intensive care treatment: parental posttraumatic stress. Acta Paediatr. 2008;97(2):181–186. doi: 10.1111/j.1651-2227.2007.00600.x. [DOI] [PubMed] [Google Scholar]
- 17.Lone NI, Seretny M, Wild SH, Rowan KM, Murray GD, Walsh TS. Surviving intensive care: a systematic review of healthcare resource use after hospital discharge*. Crit Care Med. 2013;41(8):1832–1843. doi: 10.1097/CCM.0b013e31828a409c. [DOI] [PubMed] [Google Scholar]
- 18.Simon TD, Cawthon ML, Stanford S, et al. Pediatric medical complexity algorithm: a new method to stratify children by medical complexity. Pediatrics. 2014;133(6):e1647–e1654. doi: 10.1542/peds.2013-3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Czaja AS, Zimmerman JJ, Nathens AB, Czaja AS, Zimmerman JJ, Nathens AB. Readmission and late mortality after pediatric severe sepsis. Pediatrics. 2009;123(3):849–857. doi: 10.1542/peds.2008-0856. [DOI] [PubMed] [Google Scholar]
- 20.Bernard AM, Czaja AS. Unplanned pediatric intensive care unit readmissions: a single-center experience. Journal of critical care. 2013;28(5):625–633. doi: 10.1016/j.jcrc.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 21.Christensen EW, Payne NR. Pediatric Inpatient Readmissions in an Accountable Care Organization. J Pediatr. 2016;170:113–119. doi: 10.1016/j.jpeds.2015.11.022. [DOI] [PubMed] [Google Scholar]
- 22.Krmpotic K, Lobos AT. Clinical profile of children requiring early unplanned admission to the PICU. Hosp Pediatr. 2013;3(3):212–218. doi: 10.1542/hpeds.2012-0081. [DOI] [PubMed] [Google Scholar]
- 23.Mamdani M, Sykora K, Li P, et al. Reader's guide to critical appraisal of cohort studies: 2. Assessing potential for confounding. Bmj. 2005;330(7497):960–962. doi: 10.1136/bmj.330.7497.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.U.S. CDC. 2015 http://www.cdc.gov/nchs/fastats/child-health.htm.
- 25.Berry JG, Toomey SL, Zaslavsky AM, et al. Pediatric readmission prevalence and variability across hospitals. Jama. 2013;309(4):372–380. doi: 10.1001/jama.2012.188351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. [Accessed July 21, 2016]; hcupnet.ahrq.gov.
- 27.Miletic KG, Taylor TN, Martin ET, Vaidya R, Kaye KS. Readmissions after diagnosis of surgical site infection following knee and hip arthroplasty. Infect Control Hosp Epidemiol. 2014;35(2):152–157. doi: 10.1086/674854. [DOI] [PubMed] [Google Scholar]
- 28.Allen LA, Smoyer Tomic KE, Smith DM, Wilson KL, Agodoa I. Rates and predictors of 30-day readmission among commercially insured and Medicaid-enrolled patients hospitalized with systolic heart failure. Circulation Heart failure. 2012;5(6):672–679. doi: 10.1161/CIRCHEARTFAILURE.112.967356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Odetola FO, Gebremariam A, Freed GL, Odetola FO, Gebremariam A, Freed GL. Patient and hospital correlates of clinical outcomes and resource utilization in severe pediatric sepsis. Pediatrics. 2007;119(3):487–494. doi: 10.1542/peds.2006-2353. [DOI] [PubMed] [Google Scholar]