Abstract
Rationale
Direct conversion or reprogramming of human postnatal cells into endothelial cells (ECs), bypassing stem or progenitor cell status, is crucial for regenerative medicine, cell therapy, and pathophysiological investigation but has remained largely unexplored.
Objective
We sought to directly reprogram human postnatal dermal fibroblasts (HDFs) to ECs with vasculogenic and endothelial transcription factors (TFs) and determine their vascularizing and therapeutic potential.
Methods and Results
We utilized various combinations of seven EC TFs to transduce HDFs and found that ER71/ETV2 alone best induced endothelial features. KDR+ cells sorted at day 7 from ER71/ETV2-transduced HDFs showed less mature but enriched endothelial characteristics and thus were referred to as early reprogrammed ECs (rECs), and did not undergo maturation by further culture. After a period of several weeks’ transgene-free culture followed by transient re-induction of ER71/ETV2, early rECs matured during three months of culture and showed reduced ETV2 expression, reaching a mature phenotype similar to postnatal human ECs. These were termed late rECs. While early rECs exhibited an immature phenotype, their implantation into ischemic hindlimbs induced enhanced recovery from ischemia. These two rECs showed clear capacity for contributing to new vessel formation through direct vascular incorporation in vivo. Paracrine or pro-angiogenic effects of implanted early rECs played a significant role in repairing hindlimb ischemia.
Conclusions
This study for the first time demonstrates that ER71/ETV2 alone can directly reprogram human postnatal cells to functional, mature ECs after an intervening transgene free period. These rECs could be valuable for cell therapy, personalized disease investigation, and exploration of the reprogramming process.
Keywords: Direct reprogramming, ER71/ETV2, endothelial cells, ets transcription factors, transdifferentiation, angiogenesis
INTRODUCTION
Endothelial cells (ECs) are a key element of vasculature and are indispensable for repairing injured or ischemic tissues. Over the years, many approaches have been developed to generate ECs for use in cell therapy. Despite early enthusiasm, adult stem or progenitor cells were found to have minimal endothelial transdifferentiation potential1–3. Embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) emerged as promising alternatives; however, problems such as tumorigenic potential or inefficient cell production have limited their clinical application4–6. Recently, a new approach has been developed for generating target cells through direct lineage conversion or reprogramming with cell-type specific transcription factors (TFs)7–12. This approach has many potential advantages such as procedural simplicity and avoidance of uncontrolled differentiation and tumorigenicity compared to ESCs and iPSCs.
In an attempt to generate ECs directly from somatic cells, one recent study demonstrated direct conversion of lineage committed cells into ECs via lentiviral overexpression of a combination of ETV2 (also known as ER71), ERG, and FLI1 with inhibition of TGFβ signaling7. This method, however, utilized amniotic fluid-derived c-KIT-negative cells as source cells, and reportedly did not work for postnatal cells. It is also unclear whether the source cells were fully differentiated, since their origin was amniotic tissues13 and potential contamination with stem or progenitor cells cannot be entirely excluded. Due to cell heterogeneity, clonal variability of reprogrammed cells was noted as well. Practically, this approach needs invasive amniocentesis and cannot be applied for autologous cell therapy. By employing five TFs, namely FOXO1, ETV2, KLF2, TAL1, and LMO2, which are crucial for vessel development, Han et al directly converted mouse adult skin fibroblasts into ECs14. Skeletal muscles can also be converted into ECs upon overexpression of er71 in zebrafish15. Other approaches employed pluripotency factors, but not vasculogenic/endothelial TFs, to initially induce an intermediate state, and then applied angiogenic factors to produce progenitor-stage endothelial lineage cells9, 10. These results suggest the feasibility of direct reprogramming of non-ECs into ECs, but novel methods for the direct reprogramming need to be designed for potential clinical application.
To date, no studies have clearly shown direct reprogramming of human postnatal cells into mature ECs with vasculogenic/endothelial TF(s). Since the major use for reprogrammed or induced ECs is for cell therapy or disease investigation, it would be more appropriate to use autologous cells as source cells and lineage-specific TFs for reprogramming agents. This approach would enable autologous cell therapy and personalized diseased investigation and avoid or minimize adverse effects. However, no studies have demonstrated such potential. In addition, to reduce the load of external genes in reprogramming, it would be preferable to minimize the number of TFs used. This will also facilitate investigation of yet unknown mechanisms of direct reprogramming.
Accordingly, we sought to directly reprogram human postnatal cells to ECs with TFs critical for EC specification and function. We selected the following seven factors for screening through literature search: ETV2, FOXC2, MEF2C, SOX17/SOX18, SMAD1, HEY1/HEY2, and NANOG16–24. We used various combinations of these factors and found that ETV2 alone was best to reprogram fibroblasts into ECs. Previously, we have demonstrated that ETV2, a member of the ETS TF family, plays an indispensable role in vessel development as evidenced by lack of vasculature in Etv2 deficient mouse embryos21, 25. ETV2 directly binds promoters of Flk1, VE-cadherin and Pecam121, 25, 26, and enforced expression of ETV2 in mouse ESCs increased expression of these endothelial genes and generation of FLK1+ cells21, 27. Here we report that ETV2 transduction to human postnatal fibroblasts can generate two stages of rECs which are distinguishable by expression levels of ETV2 and more mature EC proteins and duration of culture. We further demonstrate importance of the ETV2 transgene-free period followed by reactivation of ETV2 for maturation of rECs. rECs were also shown to be effective for repairing limb ischemia when transplanted in vivo.
METHODS
Generation of tetracycline-inducible lentiviral vectors expressing transcription factors and production of lentiviral particles
Lentiviral particles of the transcription factors were generated using the FUW-tetO lentiviral system, which was re-engineered from FUW-tetO-hOCT4 (Addgene plasmid 20726)28. Each transcription factor was cloned into the FUW-tetO lentiviral vector. The resulting constructs were co-transfected with pVSVG and pMDL for packaging into 293FT cells (Invitrogen) and the supernatant of the 293FT culture was collected at days 2 and 3 post-transfection, followed by PEG6000-mediated concentration29–31.
Lentiviral transduction
For EC reprogramming, human dermal fibroblasts (HDFs) were incubated with constitutively active lentiviral particles expressing the reverse tetracycline transactivator FUW-M2rtTA (Addgene plasmid 20342), along with doxycycline (DOX)-inducible lentiviral particles containing the EC transcription factors in the presence of polybrene for 18~24 hours, with virus of MOI 4. After washing, the cells were treated with DOX (2 µg/ml, Clontech) containing DMEM supplemented with 10% fetal bovine serum (FBS). The medium, replenished with DOX, was changed every other day. After 7 days, the medium was replaced with EGM-2 medium (Lonza) containing DOX and the cells were further cultured on collagen-coated plates. The medium was changed every 2–3 days for the duration of the culture period.
Induction of hindlimb ischemia and skin wound
All animal experiments were approved by Emory University IACUC. Hindlimb ischemia32 and skin wound33 were performed on male athymic nude mice as we described previously. For the hindlimb ischemia studies, the estimated minimal number for having a meaningful difference at 4 weeks is 5, so we included 5 to 10 animals in each group (n = 6 for PBS-, 10 for HUVEC-, 5 for rEC-injected groups). Before sacrifice for tissue harvest, the mice were perfused with fluorescein-labeled Griffonia (Bandeiraea) Simplicifolia Lectin I (BSL1, Vector Laboratory Inc.) by direct cardiac injection to stain functional endothelial cells in blood vessels. The tissue sections were processed for confocal imaging with a Zeiss LSM 510 Meta confocal laser scanning microscope and LSM 510 Image software (Carl Zeiss).
Details on the materials and methods, including the following items, can be found in the online-only Data Supplement: Flow cytometry32; Acetylated-LDL uptake and UEA1 lectin staining32; In vitro tube formation assay32; Immunohistochemistry and immunocytochemistry32; Real-time RT-PCR (Table 1 in the online-only Data Supplement)32; Microarray; Heat map and clustering analysis34,35; RNA-seq analysis; Statistical analysis.
RESULTS
Overexpression of endothelial TFs can convert human postnatal fibroblasts into the EC lineage
First, we generated doxycycline (DOX) inducible lentiviral constructs containing the open reading frame of each gene (Online Figure I). After transduction into human dermal fibroblasts (HDFs), expression of each TF in response to DOX treatment was confirmed by quantitative RT-PCR (qRT-PCR) (Online Figure IB). To determine whether these TFs could induce expression of EC genes in HDFs, we infected HDFs with a mixture of six of the TFs (ETV2, FOXC2, MEF2C, SOX17, SMAD1, HEY1), treated with DOX for 6 or 12 days, and conducted qRT-PCR. mRNA expression of EC genes CDH5, KDR, PECAM1, CD34, and TEK, but not VWF, were markedly increased compared to the uninfected HDFs (Online Figure IC). When NANOG, a direct upstream regulator of KDR in HUVECs18, was substituted for SMAD1, no difference was observed (data not shown). Next, we modified the protocol: after infecting with the six TFs (with NANOG replacing SMAD1), the cells were cultured in DMEM for 7 days and then in endothelial cell culture media until D20 with continuous DOX treatment (Figure 1A). From D16, some of the transduced HDFs exhibited a cobblestone morphology, a classic feature of ECs (Figure 1B). mRNA expression of endothelial genes was gradually increased up to D15, but declined at D20 (Figure 1C). We stopped DOX treatment at D20 and cultured these cells up to D39. This change induced rebound of endothelial gene expression. In detail, compared to untransduced HDFs at D0, mRNA expression of CDH5 at D15 was ~10,000-fold higher, was reduced at D20, but was still ~3,500-fold higher at D39. KDR was increased by ~500-fold at D15 and ~1000-fold at D39 compared to the control. Expression of TEK, PECAM1 and CD34 showed patterns similar to KDR but with less elevated levels at D39: TEK ~5-fold, PECAM1 ~40-fold and CD34 ~20-fold. Flow cytometry confirmed expression of endothelial proteins at D39, showing that approximately 12–15% of the cells expressed KDR or CDH5 (Figure 1D). A small portion of the cells took up acetylated (Ac)-LDL and formed tube-like structures on Matrigel in vitro (Figure 1E). Expression of the six TFs (except HEY1) peaked at D7 and declined from D10 (Online Figure II). Taken together, these data indicate that overexpression of these six endothelial TFs was able to induce endothelial characteristics in human postnatal fibroblasts.
Figure 1. Reprogramming of HDFs to ECs with six EC transcription factors.
(A) A schematic of the reprogramming protocol. (B–E) Endothelial characteristics of HDFs infected with lentiviral particles of six TFs (ETV2, FOXC2, MEF2C, SOX17, NANOG, HEY1). The infected HDFs exhibited cobblestone appearance (B), expressed endothelial genes and proteins measured by qRT-PCR (C) and flow cytometry (D), and were able to take up Ac-LDL (red) and form tubular structures (E). Scale bars: (B) 400 µm, (E) 200 µm (yellow) and 1 mm (black). All data are presented as mean ± s.e.m.; three independent experiments, each with technical triplicates.
Overexpression of ETV2 alone best induces expression of EC markers in HDFs
In order to determine the minimal essential TFs for EC reprogramming, we first started with combinations of 5 factors, omitting one factor from the above six factors. qRT-PCR analyses demonstrated that five-factor combinations minus ETV2 showed the most significant reduction in major endothelial gene expression such as CDH5 and KDR compared to those which included ETV2, suggesting an indispensable role for ETV2 in endothelial reprogramming (Figure 2A and 2B). We next designed two series of experiments: one with combinations of three or four factors without ETV2 and the other with combinations of ETV2 with one or two other factors [ETV2, ETV2+FOXC226, and ETV2+FOXC2+NANOG18]. In the former group, no combinations showed higher expression of endothelial genes than the ETV2 only group (data not shown). In the latter group, we chose those combinations because reports showed potent function of FOXC2 and NANOG in endothelial gene expression (Figure 2C)18, 26. Interestingly, only the ETV2 single factor-treated condition induced the highest expression levels of CDH5, KDR, PECAM1, TEK and CD34 (Figure 2C). Inclusion of FOXC2 into ETV2 or ETV2+NANOG did not show any synergistic or additive effects on endothelial gene expression compared to ETV2 alone. Rather, the expression of endothelial genes decreased upon co-transduction of FOCX2 with other factors, suggesting that overexpression of FOXC2 is dispensable for direct reprogramming of HDFs into ECs. Collectively, these data strongly argue that ETV2 alone may be sufficient to convert human postnatal fibroblasts into ECs.
Figure 2. ETV2 is essential for reprogramming of HDFs to endothelial cells.
(A and B) qRT-PCR results of HDFs infected with combinations of five TFs for CDH5 (A) and KDR (B) at day (D) 6, 12, and 18. (C) qRT-PCR results of HDFs infected with one to three TFs including ETV2 for various EC genes at D0, 4, and 7. All data are presented as mean ± s.e.m.; three independent experiments, each with technical triplicates.
HDFs transduced with ETV2 and cultured short-term show immature EC characteristics and have the capability for vessel formation
We then optimized infection conditions of ETV2 and found that multiplicity of infection (MOI) 4 induced the highest infection efficiency and the lowest cell death (Online Figure III and part of data not shown). HDFs infected with only ETV2 at MOI 4 demonstrated a cobblestone appearance as early as D2 (Figure 3A) and expressed CDH5 at ~50% and KDR at ~39% by flow cytometric analyses at D7 (Figure 3B). Around ~67% of the KDR+ cells also expressed CDH5. During the 7 days culture, 2 × 105 of HDFs used for the infection at D0 gave rise to 1.5 × 106 at D7. Thus, it was calculated that one HDF generates approximately 3.7 CDH5+ cells, 3 KDR+ cells, and 2 CDH5+KDR+ cells, respectively, upon ETV2 transduction. Neither KDR nor CDH5 was expressed in control virus-infected HDFs regardless of DOX treatment nor in ETV2-infected HDFs without DOX treatment (Online Figure IV). Infection of HDFs with other single factors at MOI 4 did not induce expression of EC markers (data not shown). Compared to HDFs at D0, mRNA expression levels of endothelial genes were consistently higher in ETV2-transduced HDFs during the first 7 days, with a peak at day 7 except for CD34 and VWF: CDH5 (~10,000 fold), KDR (~7,000 fold), TEK (~50 fold), PECAM1 (~50 fold), CD34 (~5 fold), and VWF (~10 fold) (Figure 3C). These results showed higher levels of EC gene expression compared to those of the six factor-infected HDFs at D15 (Figure 1C).
Figure 3. Endothelial characteristics of the single factor ETV2-transduced, short-term cultured HDFs.
(A) Morphologic changes showing emergence of cobblestone appearance in HDFs as early as D2 after transduction with ETV2. (B) Flow cytometric analyses of transduced HDFs at D7 showing expression of CDH5 and KDR. It also shows that about 67% of KDR+ cells expressed CDH5. All data are presented as mean ± s.e.m.; three independent experiments, each with technical triplicates. ***P < 0.001 vs. isotype control, standard unpaired student t test. (C) qRT-PCR analyses demonstrated induction of various endothelial genes in ETV2-transduced HDFs. At D7, the results of unsorted cells and the sorted KDR+ and KDR− cells are shown. KDR+ cells showed significantly higher endothelial gene expression (except VWF) compared to unsorted (D7) and KDR− cells. All data are presented as mean ± s.e.m.; three independent experiments, each with technical triplicates. ***P < 0.001, **P < 0.01, *P < 0.05, standard unpaired Student’s t test. (D) Immunohistochemistry showed expression of CDH5 and KDR (upper panel), and VWF and PECAM1 (lower panel) in transduced HDFs at D7. (E) The sorted KDR+ cells at D7 formed tubes on Matrigel, also showing Ac-LDL uptake (red) and UEA1 lectin (green) binding. (F) Contribution of reprogrammed HDFs to vessel formation. The transduced HDFs at D7 were labeled with CM-Dil (red), and injected into the wounded skin of mice. Three weeks later, the mice were perfused with BS1 lectin (FITC-BSL1), and tissues were processed for confocal microscopic imaging. Injected cells (red) were either incorporated into the blood vessels as shown by colocalization with BSL1 (green) (arrows) or localized in close proximity to the vessels, indicating contribution to vessel formation. DAPI (blue). Scale bars: (A, E) 400 µm, (D) 100 µm, (G) 25 µm.
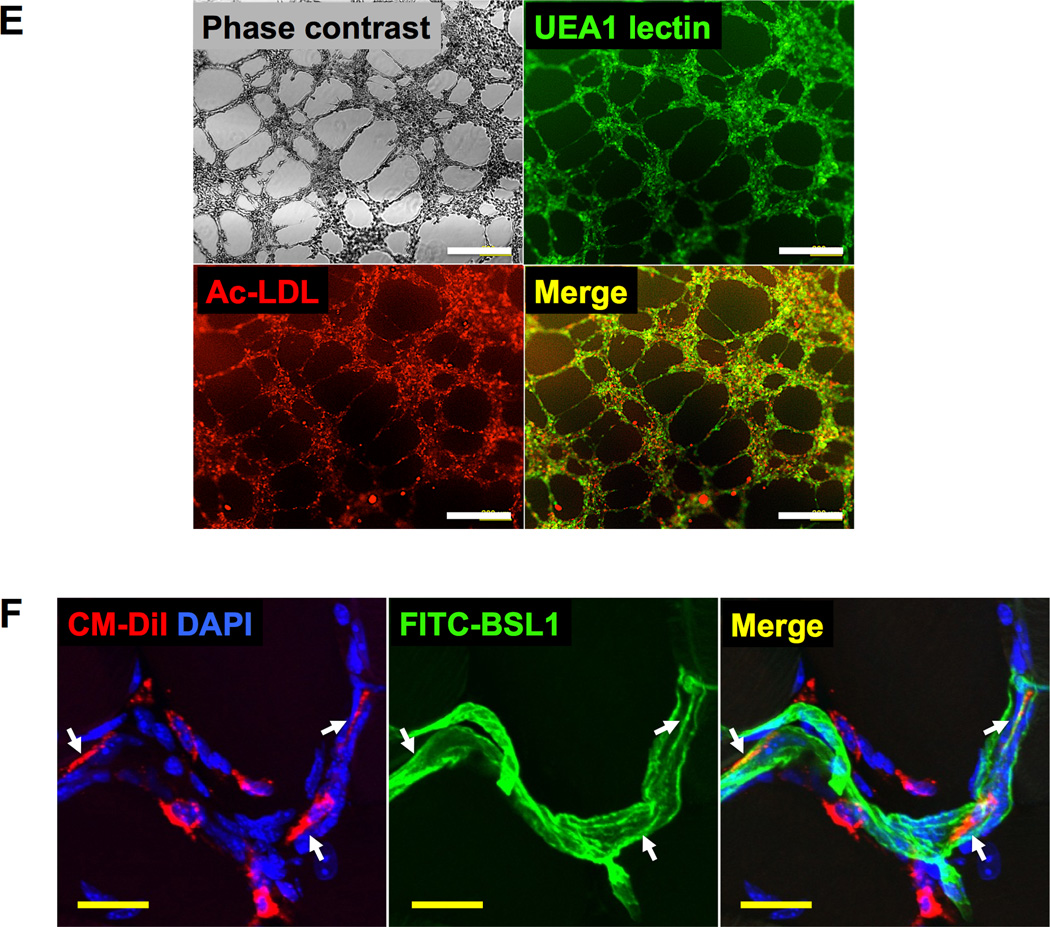
We then FACS sorted the heterogeneous cells using KDR, a comprehensive marker for endothelial-lineage cells, at D7 to enrich endothelial-lineage cells36. The KDR+ cells showed substantially higher mRNA expression of endothelial genes compared to the KDR− cells (Figure 3C). Compared to HUVECs, KDR+ cells showed similar or higher expression of CDH5, KDR and TEK but lower expression of PECAM1 and VWF, which are known markers of mature ECs. Western blot analysis (Online Figure V) further confirmed expression of all four EC markers, CDH5, KDR, PECAM1, and VWF. Consistent with the qRT-PCR results shown in Figure 3C, PECAM1 and VWF levels were weaker in KDR+ cells compared to HUVECs. Thus we referred to these cells as early reprogrammed ECs (rECs). Expression of S100A4, a fibroblast marker, was significantly reduced in the KDR+ cells compared to the KDR− cells or control HDFs suggesting suppression of fibroblast nature in the reprogrammed cells (Online Figure VIA). Transduction of HDFs with ETV2 did not induce expression of pluripotency genes POU5F1 and NANOG (Online Figure VIB and VIC). Immunocytochemistry also confirmed that ETV2-infected HDFs at D7 expressed CDH5, KDR, VWF and PECAM1 (Figure 3D and Online Figure VII). Approximately 30% of these unsorted cells took up Ac-LDL and stained for UEA1 lectin (Online Figure VID–VIF). KDR+ cells sorted at D7 by magnetic bead-based cell sorting (MACS®) readily formed tubular structures, took up Ac-LDL, and stained for UEA1 lectin indicating their functional endothelial capability (Figure 3E).
To further evaluate vessel-forming capability in vivo, ETV2-infected HDFs (D7) were injected into a mouse skin wound model after being labeled with a red fluorescent dye, CM-Dil37. Three weeks later, the animals were perfused with BS-1 lectin (BSL1) and the skin tissue was prepared for histologic analysis. Confocal microscopic examination of the skin demonstrated that the injected cells were either incorporated into vessels and stained for BSL1 or localized in close proximity to the vessels, indicating contribution of rECs to vessel formation in vivo (Figure 3F). Together, these results show that overexpression of ETV2 was able to convert HDFs to functional endothelial cells through direct reprogramming.
Maturation of early rECs to late rECs via booster transduction of ETV2
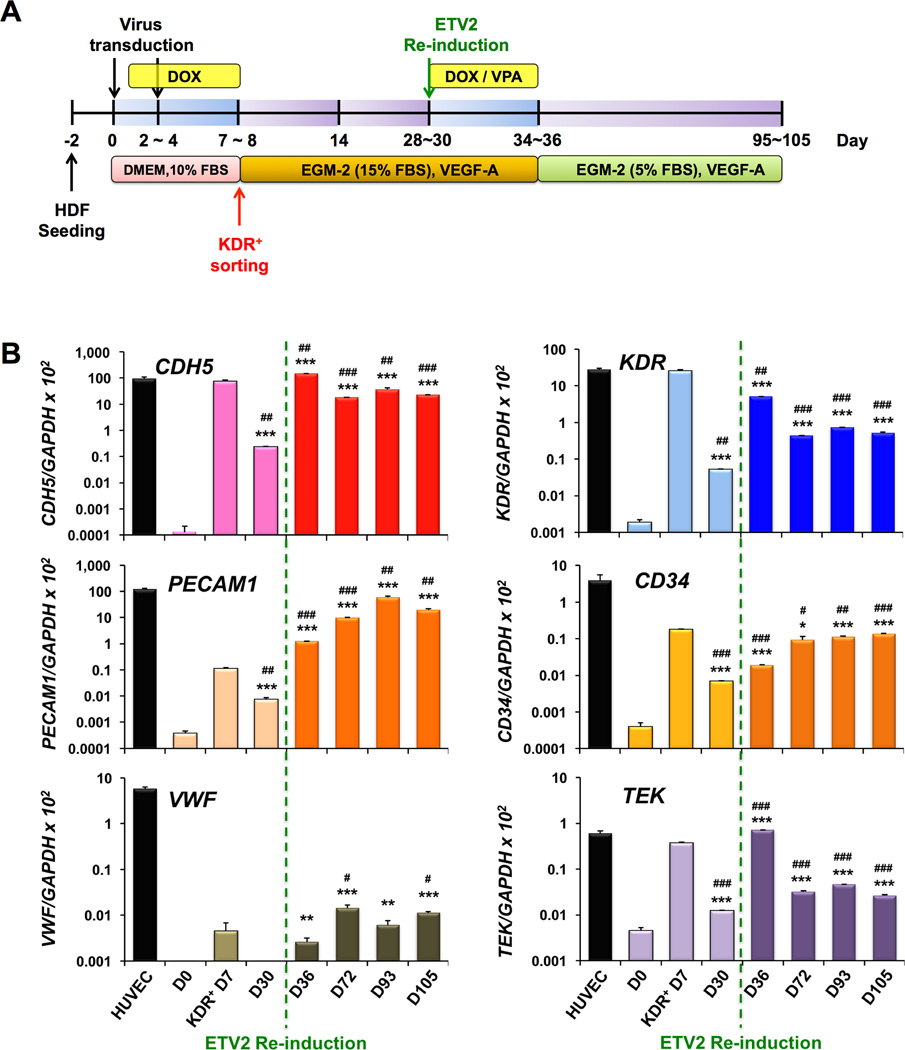
The low expression of PECAM1 in these early rECs (Figure 3C) indicated a need for further reprogramming into a mature EC stage. Thus we attempted a number of protocols by varying coating substrates (i.e. collagen, gelatin, Matrigel, OP9 cells), serum percentages, culture media, culture duration, and small molecular epigenetic modifiers, and identified efficient working conditions. The 7 day-sorted KDR+ cells, i.e. early rECs, were cultured for another 20 days in EGM-2 medium supplemented with 15% FBS and 20 ng/ml of VEGF-A (Figure 4A). However, the endothelial gene expression was significantly reduced at D30 (Figure 4B). We therefore treated these cells with DOX, together with valproic acid (VPA) for another 6 days to reactivate ETV2, expecting enhanced reprogramming38 (Figure 4A), and then further cultivated them in EGM-2 medium supplemented with 5% FBS and 20 ng/ml of VEGFA until D105 (Online Figure VIIIA). Immediately after reactivation of ETV2 (D36), the endothelial mRNA expression increased again to the levels of early rECs. After termination of DOX treatment, levels were maintained or increased over the course with CDH5 and PECAM1 expression reaching the level of HUVECs at D93 (Figure 4B). Immunocytochemistry showed that these cells stained positive for EC markers such as CHD5, KDR, PECAM1/CD31 and VWF (Online Figure VII). Flow cytometry analyses further confirmed gene expression analyses and immunocytochemistry results, showing a gradual increase in both CDH5+ cells and PECAM1+ cells, reaching ~83% and 60% at D93, respectively (Figure 4C and 4D). During the long-term culture over 86 days through 12~13 passages, 2 × 105 of early rECs (KDR+ cells at D7) were culture-expanded to 2 × 109 at D93. Thus, it was estimated that one HDF at D0 generated approximately 24,900 CDH5+ cells, and 18,000 PECAM1+ cells, respectively, over 93 days. Endothelial morphologies were maintained throughout the process (Online Figure VIIIB). They formed tubular structures in a Matrigel assay with LDL uptake and binding of UEA1 lectin (Figure 4E). Some of these cells also stained positive for 4-amino-5-methylamino-2′,7′-difluorofluorescein (DAF-FM), a membrane NO-specific fluorescence indicator (Online Figure VIIIC), suggesting that these long-term cultured cells are capable of producing nitric oxide. qRT-PCR analysis demonstrated that ETV2 expression was 16,000-fold higher in early rECs compared to HDFs at D0 (basal level), but in these long-term cultured cells it was similar to basal levers (Online Figure IX and Figure 4B). This low expression of ETV2 together with high expression of mature EC markers, particularly PECAM1, in these long-term cultured cells suggest their postnatal EC nature and they were referred to as late rECs. Our finding is consistent with the previous report7, which showed that suppression of ETV2 expression is critical for EC maturation during the direct reprogramming process. The expression of EC markers was not induced in HDFs transduced with control viral particles with or without DOX, or HDFs transduced with lentiviral ETV2 without DOX at D93 (Online Figure IV). We again evaluated direct vessel-incorporating capability of late rECs in vivo. CM-DiI-labeled late rECs were injected into mouse hindlimb ischemia (Figure 4F) and skin wound models (Online Figure X) and the animals were perfused with BSL1 and sacrificed for histologic analysis at two weeks or three months, respectively. Confocal microscopic examination demonstrated that the injected cells were heavily localized around vessels and incorporated into vessels as ECs, clearly indicating contribution of late rECs to vessel formation in vivo (Figure 4F and Online Figure X). Collectively, these results suggest that transient re-induction of ETV2 together with an epigenetic modifier can mature early rECs into late rECs, which are also functionally competent for vessel formation.
Figure 4. Endothelial characterization of long-term cultured KDR+ cells isolated from ETV2-tranduced HDFs, late rECs.
(A) A schematic of the culture protocol for long-term cultured ETV2-transduced HDFs, late rECs. (B–D) Endothelial characteristics of the long-term cultured KDR+ cells in vitro which were FACS-sorted from ETV2-tranduced HDFs at D7. qRT-PCR analysis (Y axis shown in log scale) (B) and flow cytometric analysis (C, D) demonstrated increased expression and maintenance of EC genes and proteins to D93. The green lines or arrows indicate the time point of reinduction of ETV2 expression via DOX. Note that ~60% of the cells are positive for PECAM1 at D93 (D). All data are presented as mean ± s.e.m.; three independent experiments, technical triplicates/experiment for qRT-PCR analysis, single/experiment for flow cytometric analysis. ***P < 0.001, **P < 0.01, *P < 0.05, vs. D0;###P < 0.001,##P < 0.01,#P < 0.05, vs. D7, standard unpaired Student’s t test. The cells at D93 were able to form tubular structures and stained positive for Ac-LDL and UEA1-lectin (E). (F) Cells prepared at D93 were labeled with CM-Dil and injected into mice in a hindlimb ischemia model. The mice were perfused with FITC-BSL1 at 3 months, and subjected to immunohistochemistry for confocal microscope imaging. Injected cells (red) were either incorporated into the blood vessels and expressed BSL1 (green) (arrows) or localized close to the vessels, indicating contribution to vessel formation. DAPI (blue). Scale bars: (E) 200 µm, (F) 25 µm.
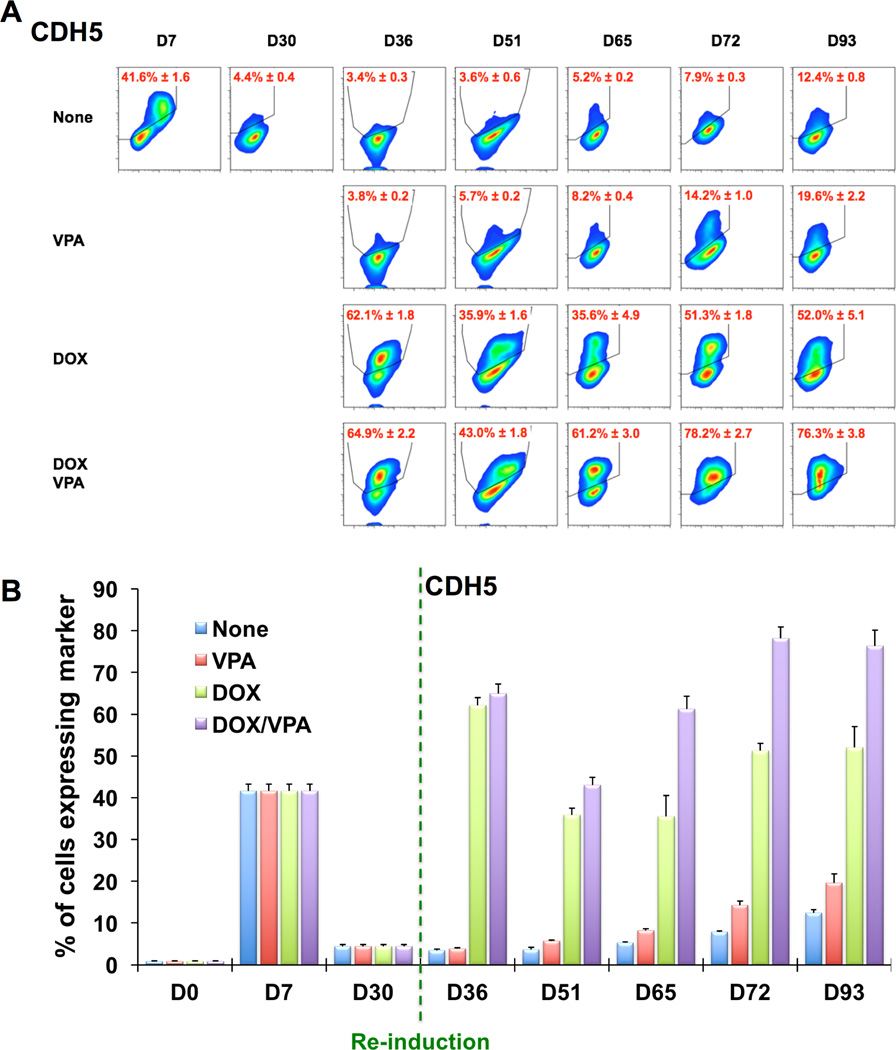
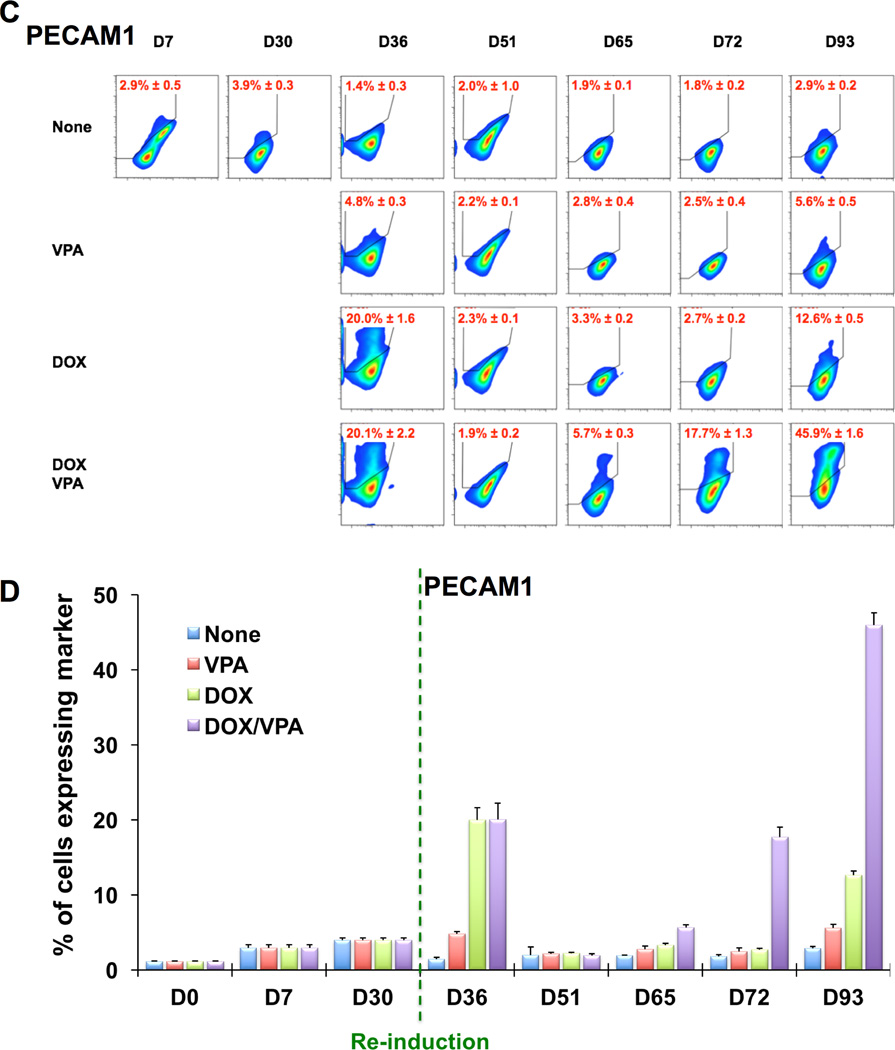
To further determine to what extent ETV2 re-induction or VPA contributed to the re-expression of EC genes at day 36, we performed experiments with the following combinations: 1) none: no re-induction of ETV2 (no DOX) and no VPA, 2) VPA: VPA without re-induction of ETV2 (no DOX), 3) DOX: re-induction of ETV2 (+DOX) without VPA, 4) DOX/VPA: re-induction of ETV2 (+DOX) with VPA (Figure 5). These treatments were applied between D30 and D36. When analyzing the expression of CDH5, we found that only groups having re-induction of ETV2 regardless of VPA treatment (DOX and DOX/VPA groups) had significantly increased numbers of CDH5+ cells (Figure 5A and 5B). After removing DOX and VPA from D36, both groups maintained the number of CDH5+ cells throughout the culture up to D93, with the numbers always being higher in the DOX/VPA group compared to the DOX alone group. During this period, the VPA-only treated group showed a slight increase in the number of CDH5+ cells. Overall, a similar trend was observed for PECAM1+ cells which showed a more drastic decrease in its numbers between D51 and D65 and a later catch-up phenomenon in the DOX and DOX/VPA groups (Figure 5C and 5D). The VPA-only treated group showed a marginal increase in PECAM1+ cells only at D93. These results suggest that ETV2 is the major factor for the reprogramming and that VPA plays an additive role.
Figure 5. Flow cytometric analysis of rECs after re-induction of ETV2 by DOX and treatment with VPA.
Flow cytometric analyses of CDH5 (A and B) and PECAM1 (C and D) in rECs after the following treatments. None (blue): no re-induction of ETV2 (no DOX) and no VPA, VPA (red): VPA without re-induction of ETV2 (no DOX), DOX (green): re-induction of ETV2 (+DOX) without VPA, DOX/VPA (purple): re-induction of ETV2 (+DOX) with VPA. Shown are the representative plots of flow cytometry (A and C) and their quantification results (B and D). All data are presented as mean ± s.e.m.; three independent experiments.
Long-term cultured rECs display a transcriptome profile similar to HUVECs
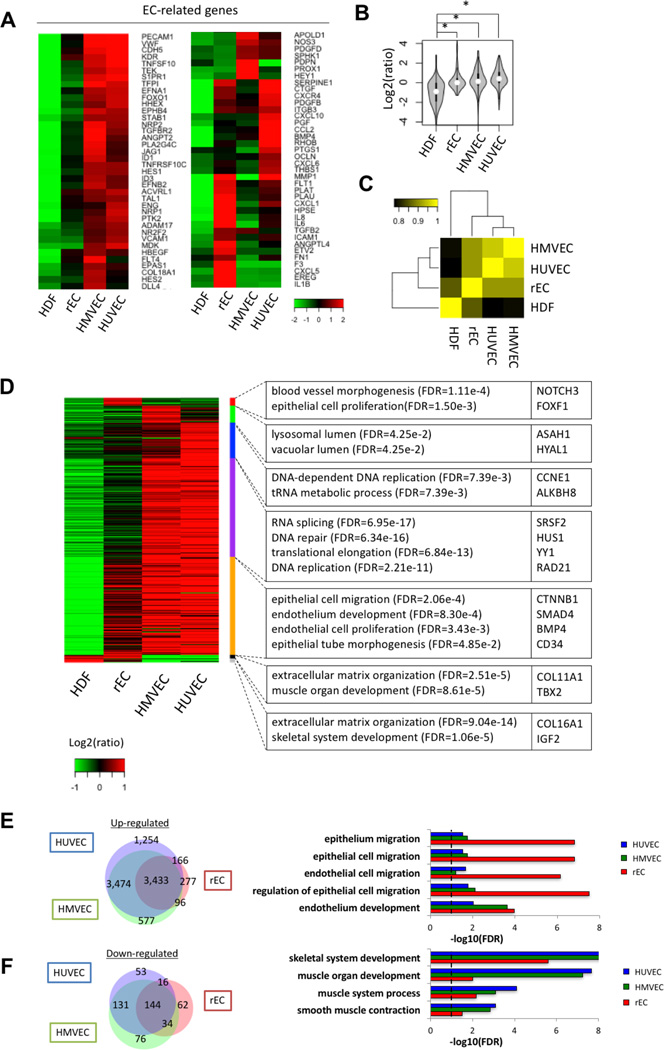
We then compared transcriptome profiles of early (Online Figure XIA and XIC) and late rECs (Online Figure XIB and XID) with HDFs and HUVECs. Heat map analyses demonstrated that both early- and late rECs showed significantly enriched EC gene expression and that the pattern of EC gene expression was closer to HUVECs than to HDFs, with late rECs being more similar to HUVECs (Online Figure XIA and XIB). In addition, the patterns of fibroblast genes were more analogous between rECs and HUVECs, with late rECs sharing a closer signature with HUVECs and significant turnoff of many fibroblastic genes (Online Figure XIC and XID). To gain more detailed insight into the transcriptome profile during the conversion process, we performed genome-wide RNA sequencing analysis on late rECs together with HDFs, human microvascular endothelial cells (HMVECs), and HUVECs (Figure 6). We selected 174 EC-related genes (Online Table II) and found that late rECs displayed significant induction of EC-related genes, which was more similar to that of HMVECs and HUVECs than HDFs (Figure 6A and 6B, p < 2.2e-16). Moreover, Pearson correlation analysis of expression of all genes showed that late rECs possess a global transcriptional pattern similar to that of HMVECs and HUVECs (Figure 6C, Correlation coefficient=0.915 and 0.916, respectively). Through Gene Ontology (GO) analysis with 9,290 differentially-expressed genes (Online Table III), we found that genes specifically expressed in late rECs were related to blood vessel morphogenesis and muscle organ development. Compared to HDFs, late rECs were mainly enriched with GO terms associated with endothelium development, endothelial cell proliferation and epithelial tube morphogenesis (Figure 6D–6F). Comparison of expression profiles (Venn Diagram) of late rECs with those of HMVECs and HUVECs showed that 93% of up-regulated genes in late rECs were also up-regulated in either HMVECs or HUVECs and 75% of down-regulated genes in late rECs were also down-regulated in either HMVECs or HUVECs or (Figure 6E and 6F). Together, these gene expression analyses demonstrated that rECs, particularly late rECs, closely resemble mature ECs.
Figure 6. Transcriptome analysis on late rECs via RNA sequencing.
(A) Heatmap representing EC-related genes with 2-fold difference. Expression values relative to the average expression values across all samples were represented by colors from green to red (log2 scale). (B) Comparison of EC-related genes among four samples. *P < 0.05 by two-sided Student’s t test. (C) Global gene expression similarity among HDFs, rECs, HMVECs and HUVECs. The color from black to yellow represents Pearson correlation. (D) Heatmap showing differentially-expressed genes in rECs, HMVECs and HUVECs compared to HDFs. Relative expression levels to median value with log2 scale were shown by green to red colors. Representative GO terms and gene symbols were also shown in right panel. (E) Overlap of highly-expressed genes (> 2 fold) among rECs, HMVECs and HUVECs. The right panel represents significant GO terms of up-regulated genes. (F) Overlap of lowly-expressed genes (> 2 fold) among rECs, HMVECs and HUVECs. The right panel represents significant GO terms of down-regulated genes.
Early rEC transplantation enhances recovery from limb ischemia and increases neovascularization
Despite immature EC features, early rECs exhibited functional EC characteristics in both in vitro and in vivo assays as described above. Thus, we investigated the therapeutic effects of early rECs on repair of tissue ischemia. To this end, we intramuscularly injected early rECs into nude mice in a hindlimb ischemia model (Figure 7). Laser Doppler perfusion imaging revealed significantly enhanced blood perfusion in the early rEC-injected limbs compared to the HUVEC-, HDF (transduced with the empty vector + DOX)- or phosphate-buffered saline (PBS)-injected limbs at 1, 2, 3, and 4 weeks (Figure 7A and 7B). Mice receiving the early rECs showed lower limb loss scores at day 28 compared to the HUVEC-, HDF- or PBS-injected mice, indicating better tissue repair (Figure 7C and Online Figure XIIA). The capillary density in the hindlimb muscle was significantly higher in the early rEC-injected mice than in the HUVEC, HDF-, or PBS-injected mice at day 28 (Figure 7D and Online Figure XIIB). Again, confocal microscopic examination demonstrated incorporation of the rECs labeled with CM-Dil into the vasculature of the ischemic hindlimbs (Figure 7E). Next, to further validate these observations and quantify the incorporated rECs into the vasculature of the hindlimbs, we first transduced HDFs with lentiviral ETV2 as well as eGFP (to avoid any potential problems with CM-Dil), and sorted KDR+eGFP+ cells at D7 for injection into the ischemic hindlimbs. Twenty-eight days after the injection, the tissues were harvested and imaged. Three dimensional confocal microscopic analysis revealed that eGFP+ cells were clearly co-localized with BSL-Rhodamine-labeled blood vessels (Figure 7F and Online Figure XIIIA and XIIIB). Quantitatively, we found that approximately 7% of the injected rECs were incorporated into or anastomosed to the blood vessels in the hindlimb tissue (Online Figure XIIIC). Concordantly, detailed gene expression analysis revealed that early rECs exhibited higher expression levels of representative angiogenic factors such as VEGFA, FGF2, ANGPT1, and MMPs compared to HUVECs (Figure 7G). Together these data suggest that early rECs can efficiently enhance recovery of hindlimb ischemia and promote postnatal neovascularization through both direct vascular incorporation and angiogenesis.
Figure 7. Enhanced blood flow recovery and neovascularization by rECs in ischemic hindlimbs.
Early rECs were intramuscularly injected into ischemic himdlimbs of nude mice. (A, B) Laser Doppler perfusion images (A) and quantitative analysis of blood flow (B) showed improved limb perfusion in the rEC- compared to the HUVEC-, HDF- or PBS-group. All data are presented as mean ± s.e.m.; n = 5 for rEC-, 10 for HUVEC-, 5 for HDF-, or 6 for PBS-group. ***P < 0.001, **P < 0.01, Repeated Measures ANOVA followed by multiple comparisons with Bonferroni’s method. (C) The rEC-injected group showed lower limb loss score compared to the HUVEC-, HDF-, or PBS-groups at day 28, suggesting better limb protection. All data are presented mean ± s.e.m.; n = 5 for rEC-, 10 for HUVEC-, 5 for HDF-, or 6 for PBS-group. **P < 0.01, *P < 0.05, standard unpaired Student’s t test. (D) The mice were perfused with FITC-BSL1 at 4 weeks and the frozen-sections of the muscle were examined under confocal microscope imaging. Quantitative analysis of vascular density is shown. Note that the vascular density was significantly increased in the rEC-, compared to the HDF-, HUVEC- or PBS-groups. All data are presented as mean ± s.e.m.; n = 5 for rEC-, 10 for HUVEC-, 5 for HDF-, or 6 for PBS-group. ***P < 0.001, **P < 0.01, standard unpaired Student’s t test. (E) Injected rECs (red) were incorporated into the blood vessels (green) as indicated by arrows, suggesting contribution of the rECs to vessel formation. DAPI (blue). Scale bars: 25 µm. (F) Early rECs transduced with lentiviral-eGFP (green) were injected into ischemic hindlimbs. The mice were perfused with Rhodamine-BSL1 (red) at 28 days and the frozen-sections of the hindlimb muscle were subjected to confocal microscope imaging. The co-localization of GFP-rEC (green) and BSL1-labeled blood vessel (red) is indicated by arrows and confirmed by orthogonal image. Scale bars: 50 µm. (G) Increased expression levels of angiogenic genes in early rECs compared to HUVECs. Red and green indicate decreased and increased levels of gene expression, respectively.
DISCUSSION
Recent endothelial reprogramming approaches utilized pluripotency factors, multiple vascular TFs, or embryonic cells, which limit their application in cell therapy or disease investigation7–10. In our study, several innovations were made in endothelial reprogramming strategies. First, this study demonstrated that human postnatal cells can be directly converted to ECs, overcoming the major obstacle to using autologous cells for reprogramming. Second, we found that ETV2 was able to induce reprogramming of HDFs to ECs. This study uncovered a novel role of ETV2 in fate changes of differentiated cells into ECs. Third, we also identified two different stages of reprogrammed ECs: early and late rECs. Finally, we further demonstrated the therapeutic utility of ETV2-induced rECs in repairing tissue ischemia and enhancing neovascularization in vivo.
We found that there were two different stages of reprogrammed ECs. Early rECs, which appeared within a week after transduction of ETV2, showed characteristics of immature ECs, with mixed signatures of ECs and fibroblasts. The expression of PECAM1 and VWF was low while other endothelial genes or proteins were highly expressed. However, early rECs were capable of taking up Ac-LDL, formed tubular structures, and contributed to vessel formation in animal models, suggesting functional competency as ECs in vitro and in vivo. These cells were expandable in culture, enabling their use for cell therapy. Although some fibroblast features remained, one could argue that this may be beneficial, as the paracrine pro-angiogenic effects of fibroblasts can augment vessel formation39, 40. The early rECs were more enriched with angiogenic factors such as VEGFA, FGF2, ANGPT1, and MMPs than HUVECs, and after direct injection into ischemic hindlimbs, early rECs demonstrated robust reparative effects for tissue ischemia and promoted vessel formation in vivo. Regarding the mechanisms underlying therapeutic and vessel-forming effects of early rECs in the hindlimb ischemia model, given the effects of rECs on rapid perfusion recovery and vessel formation, the observed effects appear to be attributed more to non-cell autonomous effects such as secretion of angiogenic factors than to direct incorporation of early rECs into the vessels. With future clinical application in mind, for this study, we selected early rECs for testing therapeutic effects on tissue ischemia. The shorter duration of culture and fewer transductions of ETV2 required for early rEC generation are advantages for clinical use.
Late rECs, which appeared after the second round of ETV2 transduction, showed features of more mature stage ECs. We made a number of attempts to increase mature EC genes in early rECs. Our initial approach of continuous overexpression of ETV2 did not work, down-regulating its own expression and most endothelial genes. After a 20-day window without transgene overexpression, early rECs responded to 6-day overexpression of ETV2. After this booster transduction, no evident dip was noted in the expression of CDH5, KDR, CD34 or VWF. We used VPA for enhancing reprogramming as reported for other cell types38. While VPA did not affect reprogramming toward early rECs (data not shown), it played an additive role for induction to late rECs. Late rECs showed dramatically increased PECAM1 expression from ~2% to ~60% by flow cytometric analyses, suggesting a transition to a more mature endothelial phenotype over long-term culture. The mature nature of late rECs was supported by global gene expression analyses that showed repression of fibroblast-specific genes, more robust expression of mature EC genes, minimal expression of ETV2, and production of NO, mimicking the phenotype of HUVECs and HMVECs. Late rECs also had the capability to contribute to vessel formation in vivo, and persisted in vessels as ECs at least 3 months post-injection, indicating long-term durability in vivo. Due to their mature nature, late rECs could be more optimal for the purpose of pathophysiological investigation of vascular disease or drug discovery.
Han et al. showed that a combination of five transcription factors (FOXO1, ETV2, KLF2, TAL1, and LMO2) was able to directly convert mouse fibroblasts into ECs14. Although the four factors without ETV2 resulted in reduced generation of TIE2+ cells as a readout of ECs from the fibroblasts, the authors observed that ETV2 was incapable of converting fibroblasts into ECs. However, our study demonstrated that ETV2 alone could reprogram human fibroblasts into ECs. Similar to our finding, Morita et. al. reported that the single factor ETV2 was able to directly convert HDFs to ECs, referred to as ETVECs41. However, there are major differences between the two studies. First, the authors claimed that transient induction of ETV2 at the initial stage of the process was sufficient for the conversion into and the maintenance of ETVECs. However, this study demonstrated continued overexpression of ETV2 in their ETVECs. ETVECs are PECAM1high cells selectively sorted at 15 days after ETV2 overexpression and expressed ETV2 at very high levels during the culture period of more than 50 days. It is well known that ETV2 is not or minimally expressed in any mammalian postnatal ECs42–44. Indeed, sustained expression of ETV2 in vascular endothelial cells during embryogenesis leads to vascular abnormality as evidenced by dilated vessels in the extraembryonic yolk sac45, suggesting that a transient expression of ETV2 is critical for ensuring proper development of the vascular system. In agreement with this report, a study showed an inverse relationship between the expression of ETV2 and PECAM1 during the direct conversion of non-ECs to ECs7. Further, they found that short-term, but not continued, expression of ETV2 in the early phase of the reprogramming process is critical for the generation of induced ECs with a mature phenotype. Thus, we argue that ETVECs are not reprogrammed or induced ECs but rather selected cells that show the ectopic expression of EC markers and some characteristics of ECs due to overexpression of ETV2. Since key EC genes including CDH5 and PECAM1 are direct targets of ETV246, 47, extreme overexpression of ETV2 leads to forced expression of such EC genes in selected cell colonies, as shown in their study. This also explains why ETVECs highly express PECAM1, a direct downstream target of ETV2, from the beginning and throughout, and why no maturation process was shown or observed in the study throughout the duration of culture. In contrast, our study found that PECAM1, which represents a more mature marker, showed low expression at an early stage of reprogramming when ETV2 was highly expressed but PECAM1 expression at both the mRNA and protein levels was progressively and concordantly increased over maturation (Figure 5). Most importantly, our late rECs, had minimal expression of transduced ETV2, which is an obligatory characteristic of genuine postnatal human ECs. Moreover, late rECs, but not ETVECs, produced nitric oxide, which provides additional evidence of mature ECs. Second, our study demonstrated that there is discontinuity in the reprogramming process toward rECs and continuous expression of ETV2 cannot sustain a phenotype of rECs and mature rECs. Only after an ETV2-free period followed by temporary re-induction of ETV2 could the phenotype of early rECs be surpassed, entering into the maturation process. Third, Morita’s study reported an important role for FOXC2 in the induction of reprogramming by ETV2. However, we found that overexpression of FOXC2 was dispensable in EC reprogramming. Fourth, there is much critical information missing in their study41. For example, there is no information regarding the reprogramming efficiency or the role of ETVECs in in vivo studies. Also, the vascular incorporation capability is unconvincing.
Elucidating mechanisms of direct endothelial conversion is crucial for understanding the reprogramming process and enhancing the efficiency of generating reprogrammed ECs. It remains to be determined how ETV2 activates EC genes while suppressing fibroblast genes during the reprogramming process. Although ETV2 is a potent vasculogenic TF upregulating endothelial genes in development21, 26, 48, 49, as yet no studies have demonstrated the mechanisms by which ETV2 directly reprograms somatic cells to ECs. We have previously shown that ETV2 can induce de novo formation of KDR+ cells through direct binding to the promoter of the gene21. These results have been supported by other studies26, 50–52. In a subsequent study, using a genome wide ChIP-sequencing approach, we have clearly demonstrated a significant induction of genes critical for endothelial cell generation and function by ETV246. Together with our results showing rapid and significant induction of endothelial genes upon ETV2 transduction into HDFs, it is likely that the reprogramming process can also be achieved in part by direct action of ETV2 on vasculo-angiogenic genes. Moreover, since overexpression of ETV2 in fibroblasts induced global changes in gene expression, ETV2 might have directly modulated epigenetic targets. In fact, ETV2 was shown to interact with Jmjd1a53, 54, a histone demethylase. Thus, it is tempting to speculate that overexpressed ETV2 in HDFs not only activates endothelial genes directly, but also induces epigenetic changes, probably through interactions with histone and/or DNA modifying molecules. As intriguing are the mechanisms underlying maturation or transition of early rECs to late rECs, because in adult endothelial cells, ETV2 is not, or is very minimally, expressed42–44, 55. Moreover, it remains to be determined why VPA has additive effects in promoting reprogramming into late rECs. Further studies are necessary to address the mechanisms of ETV2-mediated EC reprogramming.
Taken together, the successful demonstration of direct reprogramming of human postnatal somatic cells into ECs via a single TF, ETV2, in this study will facilitate the understanding of direct reprogramming of fibroblasts to ECs and its utilization in biomedical science. Specifically, this novel reprogramming approach and the resultant two stages of rECs will enable autologous cell therapy, individualized drug testing, and personalized disease investigation for various cardiovascular diseases. The next step would be to employ non-integrating episomal vectors or substitute a non-genetic method for lentiviral vectors, as were recently developed for iPSC generation56–60.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What is Known?
Vascular Endothelial cells (ECs) plays key roles in vessel formation and ischemic tissue repair.
ETV2 is an indispensable transcription factor for endothelial lineage specification during development.
Direct lineage conversion or reprogramming is an emerging technology to generate target cells via overexpression of cell-type-specific transcription factors, bypassing the pluripotent stem cell stage.
What New Information Does This Article Contribute?
ETV2 alone is sufficient to induce direct conversion of human dermal fibroblasts into ECs, referred to as reprogrammed ECs (rECs).
Implantation of rECs promote neovascularization and ischemic tissue repair.
We found at least two different stages of rECs, early and late rECs, which respectively demonstrate immature and mature EC characteristics.
Direct reprogramming with cell-type-specific TFs emerged as a promising strategy to generate target cells. Although studies demonstrated the feasibility of direct conversion of non-ECs into ECs, no studies have shown direct reprogramming of human postnatal cells into mature ECs with endothelial TFs. Among various combinations of seven EC TFs, we found ETV2 alone was sufficient to reprogram human fibroblasts into ECs. We further found that direct reprogramming by ETV2 generated at least two distinct stages of rECs: early and late rECs. Early rECs exhibited characteristics of immature ECs, but had functional and therapeutic potential for repairing ischemic disease and promoting vessel formation. Late rECs, which appeared after further cultivation of early rECs with a second round of ETV2 transduction showed features of more mature ECs and the ability to contribute to vessel formation. These new types of ECs generated by direct reprogramming provide novel opportunities for autologous cell therapy for cardiovascular diseases, drug discovery, disease modeling, and precision medicine. Moreover, this reprogramming protocol as well as the two types of rECs can allow investigation of yet unknown mechanisms of direct endothelial reprogramming from fully differentiated somatic cells.
Acknowledgments
SOURCES OF FUNDING
This work was supported by grants from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (No HI15C2782), the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MSIP) (No 2015M3A9C6031514), NIDDK (DP3-DK094346, DP3-DK108245) and NHLBI (R01HL127759, R01HL129511), and Pilot grant of Emory-Georgia Tech Regenerative Medicine (National Center for Advancing Translational Sciences of the NIH, UL1TR000454) (to Y-s. Y); AHA, 11SDG7390074, NIH HL119291 (to C. P).
Nonstandard Abbreviations and Acronyms
- rECs
Reprogrammed Endothelial Cells
- TFs
Transcription Factors
- iPSCs
induced Pluripotent Stem Cells
- ESCs
Embryonic Stem Cells
- DOX
Doxycycline
Footnotes
DISCLOSURES
None.
REFERNCES
- 1.Fadini GP, Losordo D, Dimmeler S. Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circ Res. 2012;110:624–637. doi: 10.1161/CIRCRESAHA.111.243386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Neill TJt, Wamhoff BR, Owens GK, Skalak TC. Mobilization of bone marrow-derived cells enhances the angiogenic response to hypoxia without transdifferentiation into endothelial cells. Circ Res. 2005;97:1027–1035. doi: 10.1161/01.RES.0000189259.69645.25. [DOI] [PubMed] [Google Scholar]
- 3.Ziegelhoeffer T, Fernandez B, Kostin S, Heil M, Voswinckel R, Helisch A, Schaper W. Bone marrow-derived cells do not incorporate into the adult growing vasculature. Circ Res. 2004;94:230–238. doi: 10.1161/01.RES.0000110419.50982.1C. [DOI] [PubMed] [Google Scholar]
- 4.Cohen DE, Melton D. Turning straw into gold: directing cell fate for regenerative medicine. Nat Rev Genet. 2011;12:243–252. doi: 10.1038/nrg2938. [DOI] [PubMed] [Google Scholar]
- 5.Knoepfler PS. Deconstructing stem cell tumorigenicity: a roadmap to safe regenerative medicine. Stem Cells. 2009;27:1050–1056. doi: 10.1002/stem.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamanaka S. A fresh look at iPS cells. Cell. 2009;137:13–17. doi: 10.1016/j.cell.2009.03.034. [DOI] [PubMed] [Google Scholar]
- 7.Ginsberg M, James D, Ding BS, Nolan D, Geng F, Butler JM, Schachterle W, Pulijaal VR, Mathew S, Chasen ST, Xiang J, Rosenwaks Z, Shido K, Elemento O, Rabbany SY, Rafii S. Efficient direct reprogramming of mature amniotic cells into endothelial cells by ETS factors and TGFbeta suppression. Cell. 2012;151:559–575. doi: 10.1016/j.cell.2012.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, Srivastava D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurian L, Sancho-Martinez I, Nivet E, Aguirre A, Moon K, Pendaries C, Volle-Challier C, Bono F, Herbert JM, Pulecio J, Xia Y, Li M, Montserrat N, Ruiz S, Dubova I, Rodriguez C, Denli AM, Boscolo FS, Thiagarajan RD, Gage FH, Loring JF, Laurent LC, Izpisua Belmonte JC. Conversion of human fibroblasts to angioblast-like progenitor cells. Nat Methods. 2013;10:77–83. doi: 10.1038/nmeth.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Margariti A, Winkler B, Karamariti E, Zampetaki A, Tsai TN, Baban D, Ragoussis J, Huang Y, Han JD, Zeng L, Hu Y, Xu Q. Direct reprogramming of fibroblasts into endothelial cells capable of angiogenesis and reendothelialization in tissue-engineered vessels. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:13793–13798. doi: 10.1073/pnas.1205526109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sekiya S, Suzuki A. Direct conversion of mouse fibroblasts to hepatocyte-like cells by defined factors. Nature. 2011;475:390–393. doi: 10.1038/nature10263. [DOI] [PubMed] [Google Scholar]
- 12.Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bossolasco P, Montemurro T, Cova L, Zangrossi S, Calzarossa C, Buiatiotis S, Soligo D, Bosari S, Silani V, Deliliers GL, Rebulla P, Lazzari L. Molecular and phenotypic characterization of human amniotic fluid cells and their differentiation potential. Cell Res. 2006;16:329–336. doi: 10.1038/sj.cr.7310043. [DOI] [PubMed] [Google Scholar]
- 14.Han JK, Chang SH, Cho HJ, Choi SB, Ahn HS, Lee J, Jeong H, Youn SW, Lee HJ, Kwon YW, Cho HJ, Oh BH, Oettgen P, Park YB, Kim HS. Direct conversion of adult skin fibroblasts to endothelial cells by defined factors. Circulation. 2014;130:1168–1178. doi: 10.1161/CIRCULATIONAHA.113.007727. [DOI] [PubMed] [Google Scholar]
- 15.Veldman MB, Zhao C, Gomez GA, Lindgren AG, Huang H, Yang H, Yao S, Martin BL, Kimelman D, Lin S. Transdifferentiation of fast skeletal muscle into functional endothelium in vivo by transcription factor Etv2. PLoS Biol. 2013;11:e1001590. doi: 10.1371/journal.pbio.1001590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fischer A, Schumacher N, Maier M, Sendtner M, Gessler M. The Notch target genes Hey1 and Hey2 are required for embryonic vascular development. Genes Dev. 2004;18:901–911. doi: 10.1101/gad.291004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Francois M, Caprini A, Hosking B, Orsenigo F, Wilhelm D, Browne C, Paavonen K, Karnezis T, Shayan R, Downes M, Davidson T, Tutt D, Cheah KS, Stacker SA, Muscat GE, Achen MG, Dejana E, Koopman P. Sox18 induces development of the lymphatic vasculature in mice. Nature. 2008;456:643–647. doi: 10.1038/nature07391. [DOI] [PubMed] [Google Scholar]
- 18.Kohler EE, Cowan CE, Chatterjee I, Malik AB, Wary KK. NANOG induction of fetal liver kinase-1 (FLK1) transcription regulates endothelial cell proliferation and angiogenesis. Blood. 2011;117:1761–1769. doi: 10.1182/blood-2010-07-295261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kume T, Jiang H, Topczewska JM, Hogan BL. The murine winged helix transcription factors, Foxc1 and Foxc2, are both required for cardiovascular development and somitogenesis. Genes Dev. 2001;15:2470–2482. doi: 10.1101/gad.907301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lechleider RJ, Ryan JL, Garrett L, Eng C, Deng C, Wynshaw-Boris A, Roberts AB. Targeted mutagenesis of Smad1 reveals an essential role in chorioallantoic fusion. Dev Biol. 2001;240:157–167. doi: 10.1006/dbio.2001.0469. [DOI] [PubMed] [Google Scholar]
- 21.Lee D, Park C, Lee H, Lugus JJ, Kim SH, Arentson E, Chung YS, Gomez G, Kyba M, Lin S, Janknecht R, Lim DS, Choi K. ER71 acts downstream of BMP, Notch, and Wnt signaling in blood and vessel progenitor specification. Cell stem cell. 2008;2:497–507. doi: 10.1016/j.stem.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin Q, Lu J, Yanagisawa H, Webb R, Lyons GE, Richardson JA, Olson EN. Requirement of the MADS-box transcription factor MEF2C for vascular development. Development. 1998;125:4565–4574. doi: 10.1242/dev.125.22.4565. [DOI] [PubMed] [Google Scholar]
- 23.Sakamoto Y, Hara K, Kanai-Azuma M, Matsui T, Miura Y, Tsunekawa N, Kurohmaru M, Saijoh Y, Koopman P, Kanai Y. Redundant roles of Sox17 and Sox18 in early cardiovascular development of mouse embryos. Biochem Biophys Res Commun. 2007;360:539–544. doi: 10.1016/j.bbrc.2007.06.093. [DOI] [PubMed] [Google Scholar]
- 24.Seo S, Fujita H, Nakano A, Kang M, Duarte A, Kume T. The forkhead transcription factors, Foxc1 and Foxc2, are required for arterial specification and lymphatic sprouting during vascular development. Dev Biol. 2006;294:458–470. doi: 10.1016/j.ydbio.2006.03.035. [DOI] [PubMed] [Google Scholar]
- 25.Liu F, Kang I, Park C, Chang LW, Wang W, Lee D, Lim DS, Vittet D, Nerbonne JM, Choi K. ER71 specifies Flk-1+ hemangiogenic mesoderm by inhibiting cardiac mesoderm and Wnt signaling. Blood. 2012;119:3295–3305. doi: 10.1182/blood-2012-01-403766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Val S, Chi NC, Meadows SM, Minovitsky S, Anderson JP, Harris IS, Ehlers ML, Agarwal P, Visel A, Xu SM, Pennacchio LA, Dubchak I, Krieg PA, Stainier DY, Black BL. Combinatorial regulation of endothelial gene expression by ets and forkhead transcription factors. Cell. 2008;135:1053–1064. doi: 10.1016/j.cell.2008.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim JY, Lee RH, Kim TM, Kim DW, Jeon YJ, Huh SH, Oh SY, Kyba M, Kataoka H, Choi K, Ornitz DM, Chae JI, Park C. OVOL2 is a critical regulator of ER71/ETV2 in generating FLK1+, hematopoietic, and endothelial cells from embryonic stem cells. Blood. 2014;124:2948–2952. doi: 10.1182/blood-2014-03-556332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hockemeyer D, Soldner F, Cook EG, Gao Q, Mitalipova M, Jaenisch R. A drug-inducible system for direct reprogramming of human somatic cells to pluripotency. Cell Stem Cell. 2008;3:346–353. doi: 10.1016/j.stem.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kingston RE, Chen CA, Okayama H. Calcium phosphate transfection. Curr Protoc Cell Biol. 2003;Chapter 20(Unit 20 3) doi: 10.1002/0471143030.cb2003s19. [DOI] [PubMed] [Google Scholar]
- 30.Kutner RH, Zhang XY, Reiser J. Production, concentration and titration of pseudotyped HIV-1-based lentiviral vectors. Nat Protoc. 2009;4:495–505. doi: 10.1038/nprot.2009.22. [DOI] [PubMed] [Google Scholar]
- 31.Wernig M, Lengner CJ, Hanna J, Lodato MA, Steine E, Foreman R, Staerk J, Markoulaki S, Jaenisch R. A drug-inducible transgenic system for direct reprogramming of multiple somatic cell types. Nat Biotechnol. 2008;26:916–924. doi: 10.1038/nbt1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim H, Cho HJ, Kim SW, Liu B, Choi YJ, Lee J, Sohn YD, Lee MY, Houge MA, Yoon YS. CD31+ cells represent highly angiogenic and vasculogenic cells in bone marrow: novel role of nonendothelial CD31+ cells in neovascularization and their therapeutic effects on ischemic vascular disease. Circ Res. 2010;107:602–614. doi: 10.1161/CIRCRESAHA.110.218396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee JY, Park C, Cho YP, Lee E, Kim H, Kim P, Yun SH, Yoon YS. Podoplanin-expressing cells derived from bone marrow play a crucial role in postnatal lymphatic neovascularization. Circulation. 2010;122:1413–1425. doi: 10.1161/CIRCULATIONAHA.110.941468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 2003;31:e15. doi: 10.1093/nar/gng015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, Hornik K, Hothorn T, Huber W, Iacus S, Irizarry R, Leisch F, Li C, Maechler M, Rossini AJ, Sawitzki G, Smith C, Smyth G, Tierney L, Yang JY, Zhang J. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 2004;5:R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamaguchi TP, Dumont DJ, Conlon RA, Breitman ML, Rossant J. flk-1, an flt-related receptor tyrosine kinase is an early marker for endothelial cell precursors. Development. 1993;118:489–498. doi: 10.1242/dev.118.2.489. [DOI] [PubMed] [Google Scholar]
- 37.Yoon YS, Wecker A, Heyd L, Park JS, Tkebuchava T, Kusano K, Hanley A, Scadova H, Qin G, Cha DH, Johnson KL, Aikawa R, Asahara T, Losordo DW. Clonally expanded novel multipotent stem cells from human bone marrow regenerate myocardium after myocardial infarction. J Clin Invest. 2005;115:326–338. doi: 10.1172/JCI22326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huangfu D, Maehr R, Guo W, Eijkelenboom A, Snitow M, Chen AE, Melton DA. Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nat Biotechnol. 2008;26:795–797. doi: 10.1038/nbt1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fukumura D, Xavier R, Sugiura T, Chen Y, Park EC, Lu N, Selig M, Nielsen G, Taksir T, Jain RK, Seed B. Tumor induction of VEGF promoter activity in stromal cells. Cell. 1998;94:715–725. doi: 10.1016/s0092-8674(00)81731-6. [DOI] [PubMed] [Google Scholar]
- 40.Tulloch NL, Muskheli V, Razumova MV, Korte FS, Regnier M, Hauch KD, Pabon L, Reinecke H, Murry CE. Growth of engineered human myocardium with mechanical loading and vascular coculture. Circ Res. 2011;109:47–59. doi: 10.1161/CIRCRESAHA.110.237206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morita R, Suzuki M, Kasahara H, Shimizu N, Shichita T, Sekiya T, Kimura A, Sasaki K, Yasukawa H, Yoshimura A. ETS transcription factor ETV2 directly converts human fibroblasts into functional endothelial cells. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:160–165. doi: 10.1073/pnas.1413234112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hollenhorst PC, Jones DA, Graves BJ. Expression profiles frame the promoter specificity dilemma of the ETS family of transcription factors. Nucleic Acids Res. 2004;32:5693–5702. doi: 10.1093/nar/gkh906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kataoka H, Hayashi M, Nakagawa R, Tanaka Y, Izumi N, Nishikawa S, Jakt ML, Tarui H. Etv2/ER71 induces vascular mesoderm from Flk1+PDGFRalpha+ primitive mesoderm. Blood. 2011;118:6975–6986. doi: 10.1182/blood-2011-05-352658. [DOI] [PubMed] [Google Scholar]
- 44.Park C, Lee TJ, Bhang SH, Liu F, Nakamura R, Oladipupo SS, Pitha-Rowe I, Capoccia B, Choi HS, Kim TM, Urao N, Ushio-Fukai M, Lee D, Miyoshi H, Kim BS, Lim DS, Apte RS, Ornitz DM, Choi K. Injury-Mediated Vascular Regeneration Requires Endothelial ER71/ETV2. Arteriosclerosis, thrombosis, and vascular biology. 2016;36:86–96. doi: 10.1161/ATVBAHA.115.306430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hayashi M, Pluchinotta M, Momiyama A, Tanaka Y, Nishikawa S, Kataoka H. Endothelialization and altered hematopoiesis by persistent Etv2 expression in mice. Exp Hematol. 2012;40:738–750. e11. doi: 10.1016/j.exphem.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 46.Liu F, Li D, Yu YY, Kang I, Cha MJ, Kim JY, Park C, Watson DK, Wang T, Choi K. Induction of hematopoietic and endothelial cell program orchestrated by ETS transcription factor ER71/ETV2. EMBO Rep. 2015;16:654–669. doi: 10.15252/embr.201439939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kohler EE, Wary KK, Li F, Chatterjee I, Urao N, Toth PT, Ushio-Fukai M, Rehman J, Park C, Malik AB. Flk1+ and VE-cadherin+ endothelial cells derived from iPSCs recapitulates vascular development during differentiation and display similar angiogenic potential as ESC-derived cells. PloS one. 2013;8:e85549. doi: 10.1371/journal.pone.0085549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oh SY, Kim JY, Park C. The ETS Factor, ETV2: a Master Regulator for Vascular Endothelial Cell Development. Mol Cells. 2015;38:1029–1036. doi: 10.14348/molcells.2015.0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sumanas S, Lin S. Ets1-related protein is a key regulator of vasculogenesis in zebrafish. PLoS Biol. 2006;4:e10. doi: 10.1371/journal.pbio.0040010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gomez GA, Veldman MB, Zhao Y, Burgess S, Lin S. Discovery and characterization of novel vascular and hematopoietic genes downstream of etsrp in zebrafish. PloS one. 2009;4:e4994. doi: 10.1371/journal.pone.0004994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rasmussen TL, Kweon J, Diekmann MA, Belema-Bedada F, Song Q, Bowlin K, Shi X, Ferdous A, Li T, Kyba M, Metzger JM, Koyano-Nakagawa N, Garry DJ. ER71 directs mesodermal fate decisions during embryogenesis. Development. 2011;138:4801–4812. doi: 10.1242/dev.070912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wong KS, Proulx K, Rost MS, Sumanas S. Identification of vasculature-specific genes by microarray analysis of Etsrp/Etv2 overexpressing zebrafish embryos. Dev Dyn. 2009;238:1836–1850. doi: 10.1002/dvdy.21990. [DOI] [PubMed] [Google Scholar]
- 53.Knebel J, De Haro L, Janknecht R. Repression of transcription by TSGA/Jmjd1a, a novel interaction partner of the ETS protein ER71. J Cell Biochem. 2006;99:319–329. doi: 10.1002/jcb.20945. [DOI] [PubMed] [Google Scholar]
- 54.Loh YH, Zhang W, Chen X, George J, Ng HH. Jmjd1a and Jmjd2c histone H3 Lys 9 demethylases regulate self-renewal in embryonic stem cells. Genes Dev. 2007;21:2545–2557. doi: 10.1101/gad.1588207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hollenhorst PC, Shah AA, Hopkins C, Graves BJ. Genome-wide analyses reveal properties of redundant and specific promoter occupancy within the ETS gene family. Genes Dev. 2007;21:1882–1894. doi: 10.1101/gad.1561707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim D, Kim CH, Moon JI, Chung YG, Chang MY, Han BS, Ko S, Yang E, Cha KY, Lanza R, Kim KS. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell stem cell. 2009;4:472–476. doi: 10.1016/j.stem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Warren L, Manos PD, Ahfeldt T, Loh YH, Li H, Lau F, Ebina W, Mandal PK, Smith ZD, Meissner A, Daley GQ, Brack AS, Collins JJ, Cowan C, Schlaeger TM, Rossi DJ. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell stem cell. 2010;7:618–630. doi: 10.1016/j.stem.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou H, Wu S, Joo JY, Zhu S, Han DW, Lin T, Trauger S, Bien G, Yao S, Zhu Y, Siuzdak G, Scholer HR, Duan L, Ding S. Generation of induced pluripotent stem cells using recombinant proteins. Cell stem cell. 2009;4:381–384. doi: 10.1016/j.stem.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jia F, Wilson KD, Sun N, Gupta DM, Huang M, Li Z, Panetta NJ, Chen ZY, Robbins RC, Kay MA, Longaker MT, Wu JC. A nonviral minicircle vector for deriving human iPS cells. Nat Methods. 2010;7:197–199. doi: 10.1038/nmeth.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322:949–953. doi: 10.1126/science.1164270. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.