Abstract
3-Methoxy-2-(methylamino)-1-(4-methylphenyl)propan-1-one (mexedrone) appeared in 2015 and was advertised by UK Internet retailers as a non-controlled mephedrone derivative (2-(methylamino)-1-(4-methylphenyl)propan-1-one), which was of particular interest to countries who operate generic drugs legislation. This study describes the synthesis and analytical characterization of mexedrone and the differentiation from its isomer, N-methoxymephedrone, which was predicted to be a suitable candidate before the identity of mexedrone was revealed. A full analytical characterization is described using various chromatographic, spectroscopic and mass spectrometric platforms and X-ray crystal structure analysis. The analytical data obtained for a vendor sample were consistent with the synthesized mexedrone reference standard and analytical differentiation between the mexedrone and N-methoxymephedrone positional isomers was achieved. Furthermore, α-chloromethylmephedrone was identified as a by-product during mexedrone synthesis. All three substances were also studied for their uptake and releasing properties at dopamine transporters (DAT), norepinephrine transporters (NET) and serotonin transporters (SERT) using in vitro monoamine transporter assays in rat brain synaptosomes and compared to mephedrone. Mexedrone was a weak non-selective uptake blocker with IC50 values in the low μM range. It was also devoid of releasing activity at DAT and NET but displayed weak releasing activity at SERT (EC50= 2.5 μM). The isomer N-methoxymephedrone was found to be a weak uptake blocker at DAT, NET and SERT, as well as a fully efficacious substrate-type releasing agent across all three transporters with EC50 values in the low micromolar range. The synthesis by-product α-chloromethylmephedrone was inactive in all assays.
Keywords: new psychoactive substances, psychostimulants, mephedrone, mexedrone, chemistry
Introduction
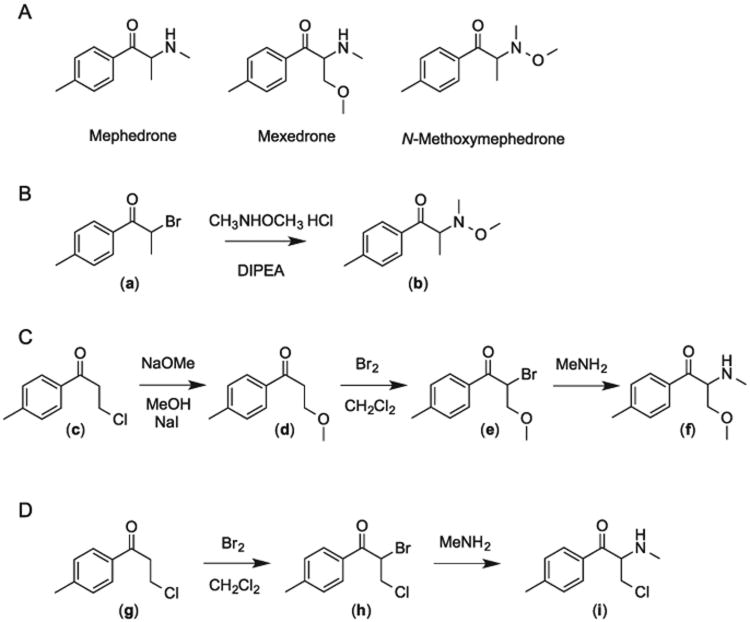
Mephedrone (4-methylmethcathinone) (Figure 1A) is a synthetic ring-substituted cathinone derivative, which was introduced onto the new psychoactive substances (NPS) drug market nearly a decade ago. It became a popular stimulant and was placed under legislative control measures across Europe in December 2010 and subsequently towards the end of 2015 it was subjected to international control measures by its addition to Schedule 2 of the Convention on Psychotropic Substances of 1971.[1,2] Although mephedrone was introduced onto the NPS drug market as a new psychostimulant, the history of mephedrone goes back to 1929 when the synthesis was first described by Saem de Burnaga Sanchez.[3] This route involved the bromination of 4-methylpropiophenone, which yielded 4-methyl-2-bromopropiophenone that was then reacted with methylamine hydrochloride and triethylamine to give 4-methylmethcathinone as a racemic mixture.[3]
Figure 1.
(A) Chemical structures of mephedrone, mexedrone and isomeric N-methoxymephedrone. (B and C) Synthesis pathways employed for mexedrone and N-methoxymephedrone. (D) Synthesis pathway employed for the route specific chlorinated by-product.
Mephedrone has been encountered as a ‘legal high’ and on the illicit drug market masquerading as cocaine (in powder form), MDMA (in tablet form) and as an adulterant.[4–7] According to the 2016 European Drugs Market Report produced by the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA), mephedrone has created a specific demand and carved its own distinct market share.[8] The drug remains a popular stimulant on the illicit drug market and is specifically sought out by some users, including chronic and marginalized drug users, possibly due to some pharmacological similarities with 3,4-methylenedioxymethamphetamine (MDMA).[8,9] Mephedrone is regarded as one of the new substances causing a wide range of serious harm throughout Europe, including acute poisonings and harm resulting from changes in the pattern of drug injection.[8,10] In Ireland, it has been observed that heroin users are now injecting mephedrone (and α-PVP) when heroin is in short supply. Furthermore, analysis of materials (e.g. precursors) seized at clandestine laboratories in Europe suggest that a range of substances, including mephedrone, are being synthesized in Europe.[8] Not surprisingly, 2014 seizure data compiled by the EMCDDA reported 1645 mephedrone seizures by 10 European countries amounting to 203kg, the majority seized by the UK and Cyprus.[11]
The pharmacological profile of mephedrone at monoamine receptors and transporters suggests that it has a high abuse liability. Several animal studies have demonstrated that mephedrone possesses psychostimulant activity and that it mimics other amphetamine-type stimulants in its ability to function as a efficacious substrate-type releaser at monoamine transporters.[12–16] Data from human-based studies have also suggested that mephedrone impairs working memory acutely, induces stimulantlike effects in users comparable to MDMA,[17,18] and disrupts cognitive function and verbal fluency.[19] It has been reported that mephedrone can be administered in a number of ways including orally, nasal insufflation, intramuscular injection, intravenous injection, rectal insertion, inhalation, or vaporization.[20,21] Fatalities associated with its use have been reported throughout the literature.[22–27]
It is well established at this stage that once a compound is placed under legislative control measures, there is a desire by NPS manufacturers to design and launch novel compounds that circumvent existing drugs legislation in order to fill the gap created in the market. Almost five years after its control, for example in the UK and Ireland, a replacement for mephedrone was eventually launched on the NPS market in August 2015. The replacement was reportedly almost two years in development and was specifically designed for markets in countries where generic cathinone bans have been enforced.[28] Interestingly, in the months leading up to its release, an intense marketing campaign by online vendors provided website users with limited details, such as the name of the new mephedrone analog, which was to be called ‘mexedrone’. During this time before its release, the authors attempted to predict the chemical structure of mexedrone. It was rationalized that the incorporation of a methoxy moiety to the terminal amine of the existing mephedrone molecular scaffold would be a suitable candidate for the NPS market, resulting in the formation of N-methoxymephedrone, 2-(methoxy(methyl)amino)-1-(4-methylphenyl)propan-1-one. This prediction agreed with a chemical structure of mexedrone that was later posted on a Polish vendor's website.[29] After its launch onto the market, however, the identity of mexedrone was revealed to be 3-methoxy-2-(methylamino)-1-(4-methylphenyl)propan-1-one. The prediction suggesting the addition of a methoxy moiety was correct although it was not incorporated into the amine as inferred, but rather attached to position 3 in the propan-1-one sequence. Currently, there are limited chemical and pharmacological data available on mexedrone. One study identified mexedrone among samples seized by police officials in China.[30] Its use as a research chemical has been discussed on different online forums and users have compared it to mephedrone, describing it as a much weaker stimulant.[31–33]
This study describes the synthesis and analytical characterization of mexedrone and the differentiation from its N-methoxy positional isomer, named N-methoxymephedrone (Figure 1A). This study was triggered by the hype surrounding the novel mephedrone analog and the purchase of a sample advertised as mexedrone from an Internet vendor based in the UK. Various chromatographic, spectroscopic and mass spectrometric platforms were employed followed by structural investigations using X-ray crystal structure analysis. In order to assess whether mexedrone and N-methoxymephedrone displayed mephedrone-like effects in vitro, their uptake and releasing properties were studied at dopamine transporters (DAT), norepinephrine transporters (NET) and serotonin transporters (SERT) using in vitro monoamine transporter assays in rat brain synaptosomes.
Experimental
Reagents and standards
All reagents and dry solvents used in the syntheses were obtained from Sigma Aldrich Ltd (Arklow, Co. Wicklow, Ireland). Liquid chromatography-mass spectrometry (LC-MS) grade solvents were obtained from Fisher Scientific (Dublin, Ireland). A vendor sample of mexedrone was obtained from an online vendor based in the UK.
Syntheses
2-(Methoxy(methyl)amino)-1-(4-methylphenyl)propan-1-one (N-methoxymephedrone)
A mixture of alpha-bromo-4-methylpropiophenone (a) (227 mg, 1mmol), N,O-dimethylhydroxylamine hydrochloride (116 mg, 1.2 mmol) and N,N-diisopropylethylamine (516 mg, 4mmol) was heated at 125–130°C for 1.5h (2 reactions; each in a Supelco micro reaction vessel, 2mL) and then allowed to cool to room temperature. The mixture was partitioned between aqueous hydrochloric acid (2M) and diethyl ether. The aqueous layer was further washed with diethyl ether and then made basic with aqueous sodium hydroxide (10 M). This was extracted with dichloromethane, the organic extract was dried (anhydrous magnesium sulfate) and the volatiles were removed under vacuum to afford a yellow oil (b) (133 mg, 0.6mmol, 63%). Formation of the hydrochloride salt using a solution (2M) of hydrogen chloride in diethyl ether, followed by trituration with tert.-butyl methyl ether, afforded a colourless solid (60 mg, 0.25 mmol, 25%). Melting point: 80–82°C; 1H NMR (d6 DMSO) δ 7.94 (d; J = 8.3 Hz; 2H; Ar-H), 7.33 (d; J = 8.3 Hz; 2H; Ar-H), 4.45 (q; J = 6.8 Hz; 1H; C(O)CHN), 3.26 (s; 3H; OCH3), 2.61 (s; 3H; NCH3), 2.38 (s; 3H; Ar-CH3) and 1.20 (d; J=6.8 Hz; 3H; CHCH3) ppm; 13C NMR (d6 DMSO) δ 198.46 (CO), 143.96 (Ar-C), 133.75 (Ar-C), 129.55 (Ar-CH), 129.30 (Ar-CH), 66.88 (CH), 59.56 (OCH3), 41.29 (NCH3), 21.63 (Ar-CH3) and 12.63 (broad, CHCH3) ppm. ESI HRMS observed m/z 208.13297 (theory [M + H]+: , m/z 208.13321, Δ =-1.15ppm).
3-Methoxy-1-(4-methylphenyl)propan-1-one
A mixture of 3-chloro-1-(4-methylphenyl)propan-1-one (c) (1.00 g, 5.47 mmol), sodium iodide (1.00g, 6.67 mmol) and sodium methoxide (1.00g, 18.52 mmol) in methanol (20 mL) was stirred overnight at room temperature. The mixture was then partitioned between dichloromethane and water. The organic layer was collected, dried (anhydrous magnesium sulfate) and the volatiles were removed to afford colourless crystals (d) (900 mg, 5mmol, 92%). Melting point: 32–34 °C; 1H NMR (d6 DMSO) δ 7.92–7.84 (m; 2H; Ar-H), 7.33–7.26 (m; 2H; Ar-H), 3.84 (t; J = 6.5 Hz; 2H; CH2O), 3.40 (s; 3H;CH3), 3.24 (t; J = 6.5 Hz;2H;COCH2)and 2.43 (s;3H;CH3) ppm.13C NMR (d6 DMSO) δ 197.95 (CO), 143.96 (Ar-C), 134.54 (Ar-C), 129.27 (Ar-CH), 128.25 (Ar-CH), 68.00 (CH2OCH3), 58.92 (CH3), 38.57 (COCH2) and 21.63 (Ar-CH3) ppm. ESI HRMS observed m/z 179.10699 (theory [M + H]+: , m/z 179.10666, Δ =1.88 ppm).
3-Methoxy-2-(methylamino)-1-(4-methylphenyl)propan-1 -one (mexedrone)
A solution of bromine (674 mg, 218 μL, 4.2mmol) in dichloromethane (7.5 mL) was added to a solution of 3-methoxy-1-(4-methylphenyl)propan-1-one (750 mg, 4.2mmol) in dichloromethane (15 mL). The mixture was stirred for 30 min at room temperature. Drying (anhydrous magnesium sulfate) and removal of the volatiles afforded a light brown oil (e). This was dissolved in acetonitrile (7.5 mL) and methanolic methylamine (1.5 mL, 8M) was added. The mixture was stirred at room temperature for 4h and the volatiles were then removed under vacuum. The residue was partitioned between dichloromethane and water. The organic layer was collected, dried (anhydrous magnesium sulfate) and the volatiles were removed to afford a brown oil (f). Formation of the hydrochloride salt using a solution (2 M) of hydrogen chloride in diethyl ether, followed by washing with acetone, afforded a colourless solid (426 mg, 1.75 mmol). This was recrystallized from ethanol to afford colourless crystals (181 mg, 0.74 mmol, 17%). Melting point: 190– 192°C; 1H NMR (d6 DMSO) δ 9.65 (s; 1H; NH), 9.21 (s; 1H; NH), 7.95 (d; J = 8.4 Hz; 2H; Ar-H), 7.43 (d; J = 8.0 Hz; 2H; Ar-H), 5.38 (m; 1H; C(O)CHN), 4.00 (dd; J = 3.2 Hz; 1H; 1H from CH2OCH3), 3.82 (dd; J= 11.3, 3.2 Hz; 1H; 1H from CH2OCH3), 3.18 (s; 3H; OCH3), 2.60 (s; 3H; NHCH3) and 2.43 (s; 3H; Ar-CH3) ppm.13C NMR (d6 DMSO) δ 193.39 (CO), 145.82 (Ar-C), 131.31 (Ar-C), 130.13 (Ar-CH), 129.26 (Ar-CH), 69.57 (CH2), 63.39 (CH), 59.17 (OCH3), 31.94 (NCH3) and 21.45 (Ar-CH3) ppm. ESI HRMS observed m/z 208.13374 (theory [M + H]+: , 208.13321, Δ =2.58 ppm).
3-Chloro-2-(methylamino)-1 -(4-methylphenyl)propan-1 -one (α-chloromethylmephedrone)
A solution of bromine (674 mg, 218 μL, 4.2 mmol) in dichloromethane (7.5 mL) was added to a solution of 3-methoxy-1-(4-methylphenyl)propan-1-one (g) (767 mg, 4.2 mmol) in dichloromethane (15 mL). The mixture was stirred for 30 min at room temperature. Drying (anhydrous magnesium sulfate) and removal of the volatiles afforded a light brown oil (h). This was dissolved in acetonitrile (7.5 mL) and methanolic methylamine (1.5 mL, 8M) was added. The mixture was stirred at room temperature for 4h and the volatiles were then removed under vacuum. The residue was partitioned between dichloromethane and water. The organic layer was collected, dried (anhydrous magnesium sulfate) and the volatiles were removed to afford a brown oil (i). Formation of the hydrochloride salt using a solution (2 M) of hydrogen chloride in diethyl ether, followed by washing with acetone, afforded a light beige powder (218 mg) which was recrystallized from ethanol to afford colourless hydrochloride salt crystals (84 mg, 0.34 mmol, 8%). Melting point: 166-168 °C; 1H NMR (d6 DMSO) δ 9.51 (s; 1H; NH), 9.35 (s; 1H; NH), 7.79 (d; J = 8.0 Hz; 2H; Ar-CH), 7.44 (d; J = 8.0 Hz; 2H; Ar-CH), 6.09 (dd; J = 8.5, 4.5 Hz; 1H, CH), 3.70–3.45 (m; 2H; CH2), 2.67 (dist. t; J = 5.0 Hz; 3H; NCH3) and 2.43 (s; 3H; Ar-CH3) ppm. 13C NMR (d6 DMSO) δ 190.83 (CO), 145.33 (Ar-C), 130.62 (Ar-C), 129.64 (Ar-C), 129.24 (Ar-C), 51.75 (CH), 49.49 (CH2), 33.05 (CH3) and 21.32 (CH3) ppm. ESI HRMS observed m/z 212.08357 (theory [M + H]+: C11H15ONCl+, 212.08367, Δ = - 0.45247 ppm).
Instrumentation
Gas chromatography-mass spectrometry (GC-MS)
Samples were prepared to give a 1 mg/mL solution in methanol and analyzed on an Agilent 6890 N GC coupled to 5975 Mass Selective Detector (Agilent, Little Island, Cork, Ireland). A HP-ULTRA 1 column(12m×0.2mm× 0.33 μm) was used with helium carrier gas at a constant flow of 1 mL/min and a split ratio of 50:1. The injector was set at 250°C and the transfer line at 280°C. The initial oven temperature was 60°C, held for 2min then ramped at 25°C/min to 295 °C with a hold time of 3 min. The mass spectra were collected after a 1.5 min solvent delay time. The ionization energy was set at 70 eV and the mass range was m/z 40-450. The total run time was 14.40 min.
Liquid chromatography-mass spectrometry (LC-MS)
LC-MS analyses were performed on an Agilent 1100 HPLC system equipped with a G13795 degasser, G1312A BinPump, a G1313A ALS and G1316A column oven (COLCOM) (Agilent, Little Island, Cork). Separation was obtained on an Allure PFP Propyl column (5μm, 50 × 2.1 mm) Restek (Bellefonte, PA, USA). Mobile phase A consisted of 0.1% formic acid in water, whereas, mobile phase B consisted of 0.1% formic acid in acetonitrile. The Agilent LC-MSD settings were as follows: positive electrospray mode, capillary voltage 3500V, drying gas (N2) 12L/min at 350°C, nebulizer gas (N2) pressure 50 psi, scan mode m/z 70-500, fragmentor voltage 50 and 110V. Samples for LC-MS analysis were dissolved in acetonitrile/water (1:1, containing 0.1% formic acid) at a concentration of 10μg/mL. The injection volume was 10.0μL, flow rate was 0.8 mL/min and the column temperature was 30°C. Total run time was 25 min. The following gradient elution program was used: 0-2 min 2% B, followed by an increase to 60% B within 15 min, followed by another increase to 80% B within 18 min before returning to 2% B within 25 min.
High-resolution electrospray ionization-mass spectrometry (HR-ESI-MS)
HR-ESI mass spectra were recorded by direct injection into a LTQ Orbitrap Discovery (Thermo Fisher Scientific, Bremen, Germany). Samples were dissolved in acetonitrile/water (1:1, containing 0.1% formic acid) and infused at a rate of 5 μL/min. Full high mass accuracy scans (30000) were performed in positive electrospray mode. Measured accurate masses were within ± 5 ppm of the theoretical masses. The following conditions were used: drying gas (N2) 10L/min, capillary temperature 310°C, spray voltage 4V, capillary voltage 22 V and tube lens 77 V The mass calibration procedure was performed in both positive and negative mode using solutions of caffeine, L-methionyl-arginyl-phenylalanyl-alanine acetate × H2O (MRFA), Ultramark 1621®, sodium docecyl sulfate and sodium taurocholate.
Nuclear magnetic resonance spectroscopy (NMR)
All analytes were prepared in deuterated dimethyl sulfoxide (DMSO-d6) at a concentration of 20mg/mL. 1H (600MHz) and 13C (150 MHz) spectra were recorded on a Bruker AV600 NMR spectrometer using a 5 mm TCI cryoprobe. 1H NMR spectra were referenced to an external TMS reference at δ = 0 ppm.
X-ray crystallography
Data for mexedrone were collected on a Bruker APEX DUO with Mo Kα radiation (λ = 0.71073 Å) using a MiTeGen micromount and at 100(2) K (Oxford Cobra Cryosystem). Bruker APEX2[34] software was used to collect and reduce data, determine the space group, solve and refine the structure. Absorption corrections were applied using SADABS.[35] All final refinements were performed with SHELXL.[36] All non-hydrogen atoms were refined anisotropically. Hydrogen atoms were assigned to calculated positions using a riding model. CCDC 1452166 contains the supplementary crystallographic data for this paper. Crystal data and structure refinement parameters for mexedrone were as follows. C12H18ClNO2, M = 243.72, T= 100(2) K, Monoclinic, P21/n, a = 7.2107(2), 7.5947(2), c = 23.6179(7) Å, (3 = 91.7912(11)°, V=1292.76(6) Å3, Z = 4, μ (Mo Kα)=0.282 mm−1, ρ = 1.252 mg/cm3, 25754 reflections collected, 3119 independent (Rint = 0.0290), aR1 = 0.0321, wR2 = 0.0800 (I>2σ(I)), S= 1.075. CCDC 1452166.aR1 =S‖Fo| − |Fc‖/Σ|Fo|, .
Monoamine transporter assays
Male Sprague-Dawley rats (250–300 g, Charles River Laboratories, Wilmington, MA, USA) were housed 2 per cage and maintained on a 12 h light-dark cycle. Food and water were provided ad libitum. Animal use procedures were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and the Animal Care and Use Committee of the Intramural Research Program of the National Institute on Drug Abuse (Baltimore, MD, USA). Rats were euthanized by CO2 narcosis and brains were processed to yield synaptosomes as previously described.[12,37] For uptake assays, synaptosomes were incubated with different concentrations of the test drugs in the presence of 5 nM [3H]dopamine, 10 nM [3H]norepinephrine or 5 nM [3H]serotonin. The uptake assays were terminated by vacuum filtration and retained radioactivity was quantified by scintillation counting. For release assays, 9 nM [3H]-1-methyl-4-phenylpyridinium ([3H]MPP+) was used as the radiolabeled substrate for dopamine transporters (DAT) and norepinephrine transporters (NET), while 5 nM [3H]5-HT was used as the radiolabeled substrate for 5-HT transporters (SERT). All buffers used in the release assay methods contained 1 μM reserpine to block vesicular uptake of substrates. The selectivity of release assays was optimized for a single transporter by including unlabeled blockers to prevent the uptake of [3H]MPP+ or [3H]5-HT by competing transporters. Synaptosomes were preloaded with radiolabeled substrate in Krebs-phosphate buffer for 1 h (steady state). Release assays were initiated by adding 850 μl of preloaded synaptosomes to 150μL of test drug. The release assays were terminated by vacuum filtration and retained radioactivity was quantified by scintillation counting.
Results and discussion
Both the analytical and pharmacological characterization of mephedrone has been well documented in the scientific literature[8–26] and studies focusing specifically on synthesis related by-products have also been conducted.[38] The cathinone molecular scaffold gives rise to a large range of biologically active compounds. Synthetic cathinones remain a popular choice amongst drug users and are the second largest family of compounds monitored by the EMCDDA Early Warning Network.[8,11] The structural diversity of the cathinone analogs present analytical challenges for forensic scientists who are trying to identify new analogs in drug seizures and/or toxicological samples. Mephedrone was subjected to European-wide control measures in 2010 and since then NPS suppliers and recreational drug users anticipated the provision of a non-controlled replacement. Almost five years later, in August 2015, the novel synthetic cathinone mexedrone, 3-methoxy-2-(methylamino)-1-(4-methylphenyl)propan-1-one, reached the NPS market. The only structural difference between mexedrone and mephedrone is the addition of a methoxy group to position 3 in the propan-1-one sequence, which means that it falls outside of the generic definitions of controlled cathinones that are operating in the UK and Ireland.[39–41]
The synthesis procedure for N-methoxymephedrone involved reacting alpha-bromo-4-methylpropiophenone (a) with N,O-dimethylhydroxylamine hydrochloride and N,N-diisopropylethylamine, which yielded the product (b) as a yellow oil (Figure 1B). Formation of the hydrochloride salt was conducted using a solution(2M)of hydrogen chlorideindiethyl ether, followed by trituration with tert.-butyl methyl ether, which afforded the N-methoxy analog as a colourless solid. The synthesis procedure employed for the preparation of. mexedrone involved reacting 3-chloro-1-(4-methylphenyl)propan-1-one (c) with sodium iodide and sodium methoxide yielding 3-methoxy-1-(4-methylphenyl) propan-1-one (d). This was then reacted with bromine yielding 2-bromo-3-methoxy-1-(4-methylphenyl)propan-1-one (e). This intermediate was dissolved in acetonitrile and methanolic methylamine was added, yielding the desired product 3-methoxy-2-(methylamino)-1-(4-methylphenyl)propan-1-one (f) as abrown oil (Figure 1C). This was then treated with hydrogen chloride in diethyl ether and washed with acetone for the formation of the hydrochloride salt, followed by recrystallization from ethanol to afford mexedrone HCl as colourless crystals. During the synthesis of mexedrone, a route specific chlorinated by-product was observed (Supporting Information). The structure of this by-product was elucidated as 3-chloro-2-(methylamino)-1-(4-methylphenyl)propan-1-one (α-chloromethylmephedrone). It is suggested that 3-chloro-1-(4-methylphenyl)propan-1-one failed to undergo complete conversion to 3-methoxy-1-(4-methylphenyl)propan-1-one. The unreacted 3-chloro-1-(4-methylphenyl)propan-1-one (g) would have then reacted with the bromine yielding 2-bromo-3-chloro-1-(4-methylphenyl)propan-1-one (h). This brominated species would then have continued through the synthesis stages, reacting with methanolic methylamine to yield 3-chloro-2-(methylamino)-1-(4-methylphenyl)propan-1-one (i), which was subsequently converted to its HCl salt form. The identification was confirmed through targeted organic synthesis of the chlorinated by-product (Figure 1D).
This present investigation reports on the synthesis and characterization of mexedrone and its N-methoxy positional isomer, N-methoxymephedrone. The analysis of the vendor sample revealed that it was consistent with mexedrone. The analysis also revealed high purity as judged by chromatographic characterizations and nuclear magnetic resonance (NMR) spectroscopy. However, traces of isopropyl alcohol were observed in the NMR proton spectrum of the vendor sample and it is surmised that this isopropyl alcohol contamination arose from the recrystallization process (Supporting Information).
In Ireland, one of the main pieces of legislation governing drugs and drug use is the Misuse of Drugs Act 1977, which was recently amended by the Misuse of Drugs (Amendment) Act 2015. In the UK, the Misuse of Drugs Act 1971 (updated by Amendment Orders) is operating. In both cases, synthetic cathinones are controlled substances by way of generic definitions (exceptions apply) based on modifications of the 2-amino-1-phenyl-1-propanone or 2-aminopropan-1-one template.[39–41] Interestingly, it was observed that these generic controls cannot be applied to mexedrone, N-methoxymephedrone or indeed the chlorinated by-product identified, as the legislation does not include the addition of a methoxy moiety or halogen atom at position 2- and 3- of the propanone side chain or at the nitrogen based terminus. This fact highlights a possible flaw in generic control legislation, which can be ambiguous and NPS manufacturers will always seek ways and means to circumvent it. However, whether these modifications lead to substances with desired psychoactive properties is another question.
Analytical features
Gas chromatography-mass spectrometry (GC-MS)
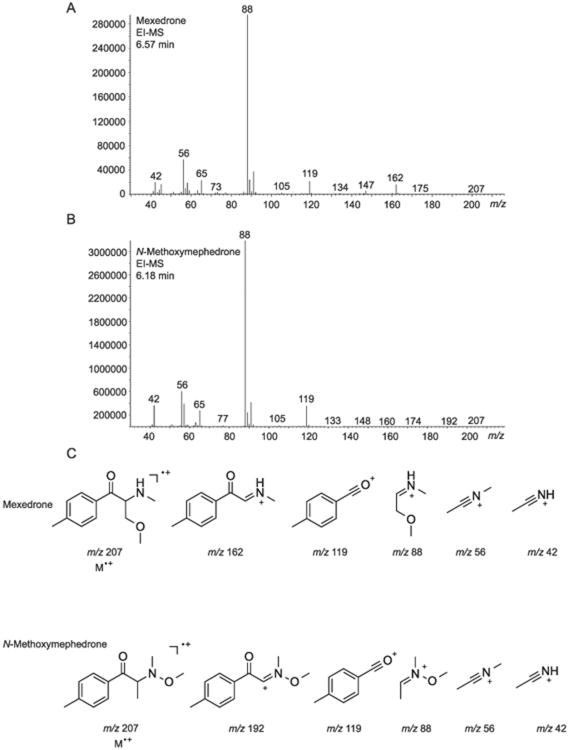
Separation of both isomers was successfully achieved using gas chromatography (GC). The retention times for mexedrone and its N-methoxy positional isomer were 6.57 and 6.18 min, respectively (Figures 2A and 2B), and a comparison with the vendor sample was in agreement with the identity of the former (Supporting Information). A comparison of both electron ionization mass spectra (EI-MS) demonstrated that both isomers shared common fragmentation patterns apart from two fragments that provided important distinguishing features (Figures 2A and 2B). In the EI-MS of mexedrone, a fragment at m/z 162 was observed and may be interpreted as the loss of methoxyethane from the parent compound, resulting in the formation of a methanaminium ion (C10H12NO+). This peak at m/z 162 was not observed in the EI-MS of N-methoxymephedrone and instead a fragment at m/z 192 was observed. This fragment could represent the formation of a hydroxylammonium ion formed by the loss of a methyl group from the parent structure. The base peak was observed at m/z 88 in both EI mass spectra. In the case of mexedrone, the m/z 88 was interpreted as the formation of another methanaminium ion (C4H10NO+) following the loss of a 4-methylbenzoyl radical from the parent structure. A loss of the same species from N-methoxymephedrone might have given rise to the formation of a hydroxylammonium ion (C4H10NO+) at m/z 88. Several common fragments were observed in the mass spectra of both isomers, including fragments at m/z 119, m/z 56 and m/z 42. The fragment at m/z 119 appeared to represent the formation of the oxonium ion (C8H7O+). The fragment at m/z 56 could be a N-ethylidynemethanaminium species and loss of a methylene group from this entity would lead to the fragment observed at m/z 42, which was thought to be an ethylidyneammonium ion. The molecular ion was detected at m/z 207 but the relative abundance was negligible. The vendor sample shared the same mass spectral characteristics compared to the synthesized mexedrone reference standard. The proposed electron ionization fragmentation patterns for both mexedrone and N-methoxymephedrone are outlined in Figure 2C.
Figure 2.
Gas chromatography mass spectrometry data. (A and B) Electron ionization mass spectra for mexedrone and N-methoxymephedrone. (C) Proposed fragments for both isomers.
Liquid chromatography-mass spectrometry (LC-MS)
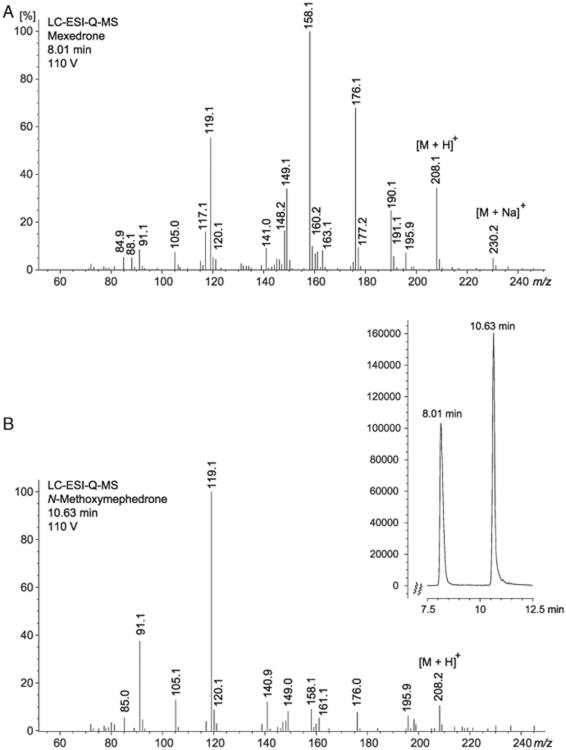
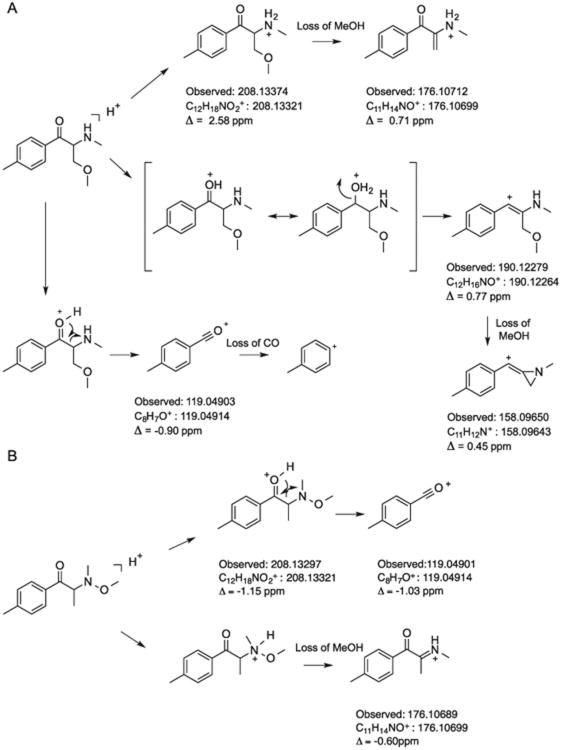
Separation of mexedrone from the N-methoxymephedrone isomer was successfully achieved using liquid chromatography (LC). The retention times obtained for mexedrone and N-methoxymephedrone were 8.01 and 10.63 min, respectively, and a comparison with the vendor sample was in agreement withtime study was set up to examine the identity of the former (Supporting Information). The electrospray ionization (ESI) single quadrupole mass spectra obtained from in-source collision-induced dissociation (CID) of the synthesized isomers (110V fragmentor voltage) shared similar fragmentation patterns but key features that allowed differentiation between the two substances were also present (Figures 3A and 3B). The suggested dissociation pathways are shown in Figures 4A and 4B. For example, the in-source CID spectrum of mexedrone displayed a sodiated adduct [M+Na]+ at m/z 230, which was not present in the in-source CID spectrum of N-methoxymephedrone. The protonated molecule [M+H]+ was present in both mass spectra at m/z 208 with a relative abundance of approximately 40% and 15% for mexedrone and N-methoxymephedrone, respectively. In the mass spectrum of mexedrone, a fragment at m/z 190 was observed and may be interpreted as the loss of water from the protonated molecule. This fragment was not present in the in-source CID spectrum of N-methoxymephedrone. The fragment observed at m/z 176 was interpreted as the formation of an aminium ion (C11H14NO+) following the loss of methanol from protonated mexedrone (Figure 4A). An m/z 176 species was also observed in the mass spectrum of N-methoxymephedrone but was considered representative of the formation of an iminium ion (C11H14NO+), again following the loss of methanol from the protonated parent structure (Figure 4B). The relative abundance of this fragment varied between isomers and was much higher in the mass spectrum of mexedrone (70%) compared to N-methoxymephedrone (10%). In the mass spectrum of mexedrone, the base peak was observed at m/z 158 and is thought to have arisen from the loss of methanol from the ion at m/z 190 to result in the formation of a methylium species (C11H12N+). In the mass spectrum of N-methoxymephedrone, the base peak was observed at m/z 119 and is represented by the formation of the oxonium species (C8H7O+). This particular ion was also detected in the mass spectrum of mexedrone but at a lower abundance (60%). Further cleavage of CO from this oxonium ion resulted in the formation the tropylium ion at m/z 91 and it was detected in the mass spectra of both isomers, although at a higher relative abundance in the mass spectrum of N-methoxymephedrone (40%). The mass spectrum of the vendor sample was consistent with that of the synthesized mexedrone reference standard (Supporting Information). Implementation of high resolution mass spectrometry provided elemental compositions with acceptable mass accuracies consistent with the proposed structures of the product ions (Figures 4A and 4B).
Figure 3.
(A and B) Product ion spectra of synthesized mexedrone and N-methoxymephedrone obtained from in-source collision induced dissociation at increased fragmentor voltage (110 V). Insert: Partial HPLC ESI single quadrupole MS trace to illustrate the separation of both isomers.
Figure 4.
The suggested dissociation pathways for mexedrone (A) and N-methoxymephedrone (B) with HR-MS providing elemental compositions with acceptable mass accuracies consistent with the proposed structures.
X-ray crystallography
The solid-state structure of mexedrone was examined using single crystal X-ray diffraction from crystals grown from a methanol solution as the HCl salt. The structure of mexedrone is shown in Figure 5. The ion pair crystallized in the monoclinic centrosymmetric space group P21/n as a racemic mixture. The cathinone skeleton in mexedrone and related congeners in the literature were similar and an overlay of the skeleton (matching enantiomer, fitting C2-C8, O9) showed a RMS deviation from fit of 0.015-0.072Å for seven examples.[42–45] The ions in mexedrone HCl were linked by N-H…Cl and CH…Cl hydrogen bonds (N11…Cl1, 3.084(1)Å; C10…Cl1#, 3.458(1)Å; symmetry transformation # = -x+3/2,y-1/2,-z+ 1/2). Strong hydrogen bonding between salts formed an extended dimeric chain that propagated parallel to the crystallographic b-axis. Weak C-H…O interactions (C7…O9*, 3.359(2)Å; *=2+x, y, z) also assisted in the structural arrangement. Information on the cell packing is provided as Supporting Information. Aromatic π- π stacking was not seen in this structure, which differed from what was observed in the structure of pentedrone HCl.[44]
Figure 5.
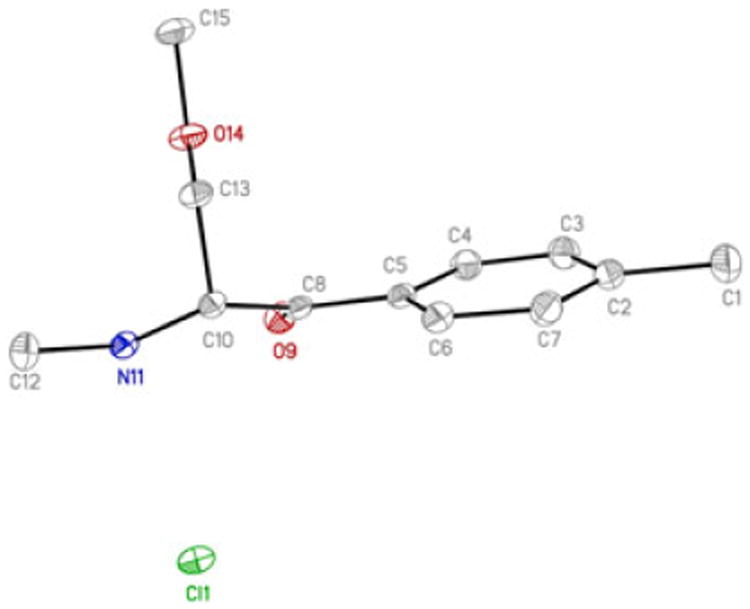
Molecular structure recorded for the mexedrone vendor sample using X-ray crystal structure analysis.
Nuclear magnetic resonance spectroscopy (NMR)
The NMR spectra associated with mexedrone and N-methoxymephedrone shared some key characteristics, such as the proton and carbon signals associated with aromatic ring, carbonyl moiety (β-carbon) and N-methyl group. However, several structural features also facilitated differentiation between the two isomers. The significant differences occurred at the chemical environments around both the nitrogen and α-carbon atom. For example, the proton signals associated with the nitrogen terminus were observed at 9.65 and 9.21 ppm in the 1H NMR spectrum of mexedrone. These signals were not detected in the 1H NMR spectrum of N-methoxymephedrone as the nitrogen atom, in this case, is connected to methyl and methoxy moieties, which were observed at 2.60 and 3.18 ppm, respectively. Further distinguishing features included the proton signals associated with the methylene group on the α-carbon atom at 4.00 and 3.82 ppm in the 1H NMR of mexedrone. These signals were not detected in the 1H NMR spectrum of N-methoxymephedrone. Instead, a proton signal affiliated with the methyl group (1.20 ppm) attached to the α-carbon atom was observed. In the 13C NMR spectrum of mexedrone, a carbon signal was observed at 63.57 ppm and is associated with the methylene group attached to the α-carbon atom. This carbon signal was absent in the 13C NMR spectrum of N-methoxymephedrone and a carbon signal was observed at 12.63 ppm instead. This signal was associated with the carbon of the methyl group attached to the α-carbon atom. The 1H and 13C NMR spectral data of the vendor product were in agreement with GC and LC data confirming the presence of mexedrone. Furthermore, the NMR spectra associated with mexedrone and the α-chloromethylmephedrone also shared mainly similar characteristics. The major distinguishing features between these two isomers were the presence of carbon and proton signals representing the methyl group of the methoxy moiety present in the mexedrone chemical structure. As expected, these signals were not detected in the NMR spectral data for α-chloromethylmephedrone.
During the characterization of N-methoxymephedrone, it was noticed that the compound had hygroscopic properties and that it was unstable in its crystalline form and over time would convert from a colourless solid to a brown solid. An additional chromatographic peak was noticed during LC-MS analysis of a second synthesized batch of N-methoxymephedrone (Supporting Information). Examination of the analytical data revealed this impurity as mephedrone. Integration using NMR proton signals confirmed that N-methoxymephedrone reference standard contained approximately 6% mephedrone (Supporting Information). A LC-MS time study was set up to examine whether the presence of mephedrone was due to a synthesis related by-product or degradation of the N-methoxymephedrone to mephedrone over time. It was considered possible that the presence of mephedrone may have been due to hydrolysis of the N-methoxy isomer to mephedrone although it seemed equally possible that the mephedrone was a synthesis by-product. After LC-MS analysis, the presence of mephedrone was considered unlikely to result from degradation as the concentration of mephedrone in the N-methoxymephedrone reference standard remained constant over 24h under the conditions of the LC mobile phase and aqueous release assay buffer. Therefore, it was concluded the mephedrone was present as a synthesis by-product.
Monoamine transporter activity
Figure 6A-C shows the effects of mexedrone, N-methoxymephedrone and α-chloromethylmephedrone on the uptake of [3H]dopamine, [3H]norepinephrine and [3H]serotonin by their respective transporters DAT, NET and SERT. The corresponding IC50 values for inhibition of uptake are provided in Table 1 and mephedrone was included for comparison. As revealed from the dose-response curves, the α-chloromethylmephedrone impurity did not display any ability to inhibit transporter uptake, whereas the remaining substances were fully efficacious uptake blockers with potency in the low micromolar range (Table 1). Mexedrone and its isomer N-methoxymephedrone showed comparable potency at DAT (IC50 =6.84 and 6.09 μM) but the latter compound was 2.6-and 1.6-fold more potent at NET and SERT, respectively. Importantly, mephedrone was more potent than all three test drugs at DAT, NET and SERT. The IC50 values for mephedrone reported here agreed with those published previously under similar experimental conditions.[46]
Figure 6.
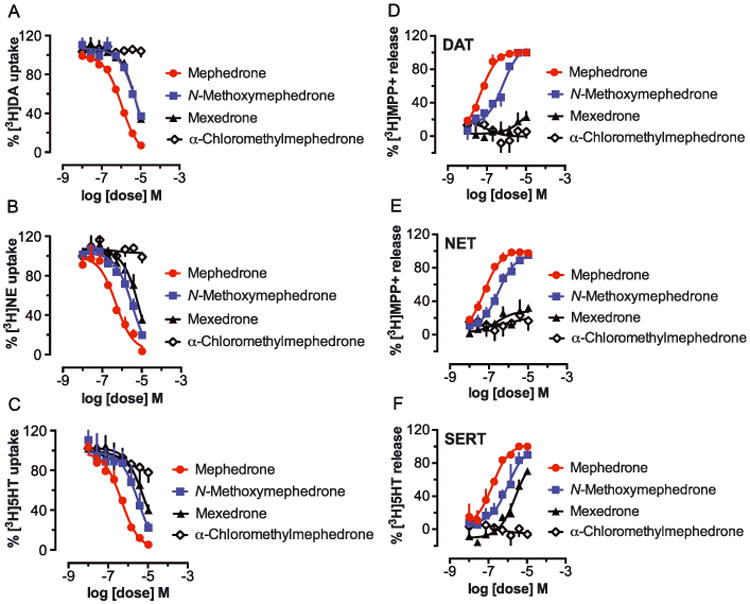
Effects of mexedrone, N-methoxymephedrone, α-chloromethylmephedrone and comparison test drug mephedrone on inhibition of uptake and stimulation of release at DAT, NET and SERT in rat brain synaptosomes. For uptake assays (A, B and C), synaptosomes were incubated with different concentrations of the test drugs in the presence of 5 nM [3H]dopamine (A, for DAT), 10 nM [3H]norepinephrine (B, for NET) or 5 nM [3H]serotonin (C, for SERT). Data are expressed as percentage of [3H]transmitter uptake (mean± SD) for n =3 experiments. For release assays (D, E and F), synaptosomes were preloaded with 9 nM [3H]MPP+ for DAT (D) and NET (E) or 5 nM [3H]serotonin for SERT (F), then incubated with different concentrations of test drugs to evoke release via reverse transport. Data are expressed as percentage of [3H]substrate release (mean ± SD) for n = 3 experiments.
Table 1. Effects of test drugs on transporter uptake inhibition from rat brain synaptosomes.
| Test drug | [3H]DA uptake via DAT IC50 (nM) | [3H]NE uptake via NET IC50 (nM) | [3H]5-HT uptake via SERT IC50 (nM) |
|---|---|---|---|
| Mephedrone | 1056 ± 85 | 494 ± 93 | 470 ± 7.6 |
| Mexedrone | 6844 ±1522 | 8869 ±3103 | 5289 ±1624 |
| N-Methoxymephedrone | 6091 ±1615 | 3457 ± 728 | 3334 ±1129 |
| α-Chloromethylmephedrone | inactive | inactive | inactive |
Data are expressed as nM concentrations (mean± SD) for n = 3 separate experiments performed in triplicate. Inactive refers to no inhibition at 10,000 nM.
Given that mephedrone has been established as a substrate-type releasing agent both in vitro and in vivo,[12,14,46–49] further investigations were carried out to assess whether mexedrone, N-methoxymephedrone and α-chloromethylmephedrone would display releasing properties. The results, also derived from rat brain synaptosomes, are displayed in Figures 6D–6F. The data demonstrate that only mephedrone and N-methoxymephedrone exhibited the expected S-shaped dose-response curve for a fully-efficacious releaser across all three transporters. The potency values are shown in Table 2, which illustrates that mephedrone, consistent with data reported before,[12,46,47] was a potent substrate for all three transporters with slightly higher selectivity for catecholamine transporters. Although less potent than mephedrone (Table 2), N-methoxymephedrone was found to be a fully efficacious substrate-type releasing agent for all three transporters with EC50 values in the low micromolar range. α-Chloromethylmephedrone was inactive in all assays. Mexedrone displayed interesting pharmacological properties in the release assays. Specifically, the drug was inactive as a releaser at DAT and NET but displayed weak releasing activity at SERT (Table 2, NFigures 6D–6F). Taken together with the uptake data, it is apparent that mexedrone displays ‘hybrid’ activity at monoamine transporters wherein the drug acts as an uptake blocker at DAT and NET but a substrate at SERT. The present and other authors have previously reported this type of activity for cathinone-related compounds such as 4-methyl--ethylcathi none.[50,51]
Table 2. Effects of test drugs on transporter-mediated release from rat brain synaptosomes.
| Test drug | [3H]MPP+ release via DAT EC50 (nM) | [3H]MPP+ release via NET EC50 (nM) | [3H]5-HT release via SERT EC50 (nM) |
|---|---|---|---|
| Mephedrone | 45 ±6 | 58 ±7 | 163 ± 30 |
| Mexedrone | inactive | inactive | 2525 ± 560 |
| N-Methoxymephedrone | 666 ±132 | 313 ±51 | 1043 ±271 |
| α-Chloromethylmephedrone | inactive | inactive | inactive |
Data are expressed as nM concentrations (mean ± SD) for n= 3 separate experiments performed in triplicate. Inactive refers to efficacy less than 33% of maximal release.
Conclusion
This report provides comprehensive analytical and pharmacological data on the NPS mexedrone, as well as data on its N-methoxy positional isomer, N-methoxymephedrone. The ability of N-methoxymephedrone to act as a fully efficacious reuptake inhibitor and substrate-type releaser could provide a stimulus for further drug developments. On the other hand, the observation that synthesis of N-methoxymephedrone could be prone to containing mephedrone as an impurity might preclude this substance from being developed as a commercially viable NPS on a larger scale. From this perspective, it is tempting to speculate that mexedrone was developed as an alternative to N-methoxymephedrone as it seems conceivable that mephedrone impurities in the latter compound would have been observed by manufacturers during the development stage. The pharmacological data suggest that N-methoxymephedrone showed a transporter-meditated releasing profile comparable to mephedrone although much lower in potency. By contrast, mexedrone was found to be a weak monoamine transporter uptake blocker and weak serotonin releasing agent, which might explain why this substance received poor reviews on user forums.
Supplementary Material
Footnotes
Supporting information: Additional supporting information may be found in the online version of this article at the publisher's web site.
References
- 1.Council of the European Union. Council Decision of 2 December 2010 on submitting 4-methylmethcathinone (mephedrone) to control measures (2010/759/EU) Off J European Union. 2010;L322:44. Available at: http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv:OJ.L_.2010.322.01.0044.01.ENG&toc=OJ:L:2010:322:TOC [18 June 2016] [Google Scholar]
- 2.United Nations Office on Drugs and Crime. Inclusion of mephedrone (4-methylmethcathinone) in Schedule II of the Convention on Psychotropic Substances of 1971. 2015 Available at: https://www.unodc.org/documents/commissions/CND/CND_Sessions/CND_58/2015_Desicions/Desicion_58_1.pdf [20 June 2016]
- 3.de Burnaga Sanchez MJS. Sur un homologue de l'éphédrine. Bull Soc Chim Fr. 1929;45:284. [Google Scholar]
- 4.McElrath K, O'Neill C. Experiences with mephedrone pre and post legislative controls, perceptions of safety and sources of supply. Int J Drug Policy. 2011;22:120. doi: 10.1016/j.drugpo.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Van Hout MC, Brennan R. Plant food for thought: A quantitative study of mephedrone in Ireland. Drugs Educ Prev Pol. 2011;18:371. [Google Scholar]
- 6.Gine CV, Espinosa IF, Vilamala MV. New psychoactive substances as adulterants of controlled drugs. A worrying phenomenon? Drug Test Anal. 2014;6:819. doi: 10.1002/dta.1610. [DOI] [PubMed] [Google Scholar]
- 7.Brunt TM, Nagy C, Bucheli A, Martins D, Ugarte M, Beduwe C, Ventura Vilamala M. Drug testing in Europe: monitoring results of the Trans European Drug Information (TEDI) project. Drug Test Anal. 2016 doi: 10.1002/dta.1954. [DOI] [PubMed] [Google Scholar]
- 8.2016 EU Drug Markets Report: In-depth Analysis EMCDDA, Europol, Lisbon. 2016 Available at: http://www.emcdda.europa.eu/system/files/publications/2373/TD0216072ENN.PDF [23 June 2016]
- 9.Public Health England. Public Health England; London: 2015. Shooting up: infections among people who inject drugs in the UK. Available at: https://www.gov.uk/government/publications/shooting-up-infections-among-people-who-inject-drugs-in-the-uk [4 August 2016] [Google Scholar]
- 10.Péterfi A, Tarján A, Horváth GC, Csesztregi T, Nyírády A. Changes in patterns of injecting drug use in Hungary: a shift to synthetic cathinones. Drug Test Anal. 2014;6:825. doi: 10.1002/dta.1625. [DOI] [PubMed] [Google Scholar]
- 11.EMCDDA. Lisbon: 2016. European Drug Report. Trends and Developments. Available at: http://www.emcdda.europa.eu/edr2016 [1 June 2016] [Google Scholar]
- 12.Baumann MH, Ayestas MA, Jr, Partilla JS, Sink JR, Shulgin AT, Daley PF, Brandt SD, Rothman RB, Ruoho AE, Cozzi NV. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology. 2012;37:1192. doi: 10.1038/npp.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martínez-Clemente J, Escubedo E, Pubill D, Camarasa J. Interaction of mephedrone with dopamine and serotonin targets in rats. Eur Neuropsychopharmacology. 2012;22:231. doi: 10.1016/j.euroneuro.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 14.Eshleman AJ, Wolfrum KM, Hatfield MG, Johnson RA, Murphy KV, Janowsky A. Substituted methcathinones differ in transporter and receptor interactions. Biochem Pharmacol. 2013;85:1083. doi: 10.1016/j.bcp.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green AR, King MV, Shortall SE, Fone KC. The preclinical pharmacology of mephedrone; not just MDMA by another name. Br J Pharmacol. 2014;171:2251. doi: 10.1111/bph.12628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hadlock GC, Webb KM, McFadden LM, Chu PW, Ellis JD, Allen SC, Andrenyak DM, Vieira-Brock PL, German CL, Conrad KM, Hoonakker AJ, Gibb JW, Wilkins DG, Hanson GR, Fleckenstein AE. 4-Methylmethcathinone (mephedrone): Neuropharmacological effects of a designer stimulant of abuse. J Pharmacol Exp Ther. 2011;339:530. doi: 10.1124/jpet.111.184119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freeman TP, Morgan CJ, Vaughn-Jones J, Hussain N, Karimi K, Curran HV. Cognitive and subjective effects of mephedrone and factors influencing use of a ‘new legal high’. Addiction. 2012;107:792. doi: 10.1111/j.1360-0443.2011.03719.x. [DOI] [PubMed] [Google Scholar]
- 18.Papaseit E, Pérez-Maná C, Mateus JA, Pujadas M, Fonseca F, Torrens M, Olesti E, de la Torre R, Farré M. Human Pharmacology of Mephedrone in Comparison with MDMA. Neuropsychopharmacology. 2016 doi: 10.1038/npp.2016.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herzig DA, Brooks R, Mohr C. Inferring about individual drug and schizotypy effects on cognitive functioning in polydrug using mephedrone users before and after clubbing. Hum Psychopharmacol. 2013;28:168. doi: 10.1002/hup.2307. [DOI] [PubMed] [Google Scholar]
- 20.EMCDDA; Lisbon: 2011. European Monitoring Centre for Drugs and Drug Addiction (EMCDDA)v Report on the risk assessment of mephedrone in the framework of the council decision on new psychoactive substances. Available at: http://www.emcdda.europa.eu/system/files/publications/571/TDAK11001ENC_WEB-OPTIMISED_FILE_280269.pdf [4 July 2016] [Google Scholar]
- 21.Kavanagh P, O'Brien J, Power JD, Talbot B, McDermott SD. ‘Smoking mephedrone’: the identification of the pyrolysis products of 4-methylmethcathinone hydrochloride. Drug Test Anal. 2013;5:291. doi: 10.1002/dta.1373. [DOI] [PubMed] [Google Scholar]
- 22.Dickson AJ, Vorce SP, Levine B, Past MR. Multiple-drug toxicity caused by the co-administration of 4-methylmethcathinone (mephedrone) and heroin. J Anal Toxicol. 2010;34:162. doi: 10.1093/jat/34.3.162. [DOI] [PubMed] [Google Scholar]
- 23.Lusthof LJ, Oosting R, Maes A, Verschraagen M, Dijkhuizwn A, Sprong AG. A case of extreme agitation and death after the use of mephedrone in The Netherlands. Forensic Sci Int. 2011;206:93. doi: 10.1016/j.forsciint.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 24.James D, Adams RD, Spears R, Cooper G, Lupton DJ, Thompson JP, Thomas SH. National Poisons Information Service. Clinical characteristics of mephedrone toxicity reported to the U.K. National Poisons Information Service. Emerg Med J. 2011;28:686. doi: 10.1136/emj.2010.096636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maskell PD, De Paoli G, Seneviratne C, Pounder DJ. Mephedrone (4-methylmethcatinone)-related deaths. J Anal Toxicol. 2011;35:188. doi: 10.1093/anatox/35.3.188. [DOI] [PubMed] [Google Scholar]
- 26.Adamowicz P, Tokarczyk B, Stanaszek R, Slopianka M. Fatal mephedrone intoxication - a case report. J Anal Toxicol. 2013;37:37. doi: 10.1093/jat/bks085. [DOI] [PubMed] [Google Scholar]
- 27.World Health Organisation. Mephedrone Critical Review Report. 2014. Expert Committee on Drug Dependence. Thirty-sixth Meeting; 16-20 June; Geneva, Switzerland. 2014. Available at: http://www.who.int/medicines/areas/quality_safety/4_12_review.pdf?ua=1 [4 July 2016] [Google Scholar]
- 28.Mexedrone.com. Thread: Mexedrone. 2015 Available at: http://mexedrone.com/about-mexedrone/ [1 September 2015]
- 29.Wolna Molekula. Mexedrone; Meofedron; 4′-methylo-N-metoksykatynon; 4-MMEOC. 2015 Available at: http://wolnamolekula.info/mexedrone-meofedron-4-metylo-n-metoksykatynon-4-mmeoc/[24 July 2015]
- 30.Qian Z, Jia W, Li T, Liu C, Hua Z. Identification and analytical characterization of four synthetic cathinone derivatives iso-4-BMC, β-TH-naphyrone, mexedrone, and 4-MDMC. Drug Test Anal. 2016 doi: 10.1002/dta.1983. [DOI] [PubMed] [Google Scholar]
- 31.Bluelight. Thread: Mexedrone. 2015 Available at: http://www.bluelight.org/vb/threads/764088-Mexedrone [1 June 2016]
- 32.UK Chemical Research. Mexedrone Trip Report. Available at https://www.ukchemicalresearch.org/Thread-Mexedrone-Trip-Report [1 June 2016]
- 33.Research Chemicals: Mexedrone. Available at: https://drugs-forum.com/forum/showthread.php?t=273743 [24 June 2016]
- 34.APEX2 v. Bruker AXS Inc.; Madison, WI, USA: 2012. 12-0. [Google Scholar]
- 35.Sheldrick GM. SADABS. Bruker AXS Inc.; Madison, Wisconsin, USA: 2012. [Google Scholar]
- 36.Sheldrick GM. A short history of SHELX. Acta Cryst. 2008;A64:112. doi: 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]
- 37.Rothman RB, Vu N, Partilla JS, Roth BL, Hufeisen SJ, Compton-Toth BA, Birkes J, Young R, Glennon RA. In vitro characterization of ephedrine-related stereoisomers at biogenic amine transporters and the receptorome reveals selective actions as norepinephrine transporter substrates. J Pharmacol Exp Ther. 2003;307:138. doi: 10.1124/jpet.103.053975. [DOI] [PubMed] [Google Scholar]
- 38.Power JD, Kavanagh PV, McLaughlin G, O'Brien J, Talbot B, Barry M, Twamley B, Dowling G, Brandt SD. Identification and characterisation of an imidazolium by-product formed during the synthesis of 4-methylmethcathinone (mephedrone) Drug Test Anal. 2015 doi: 10.1002/dta.1789. [DOI] [PubMed] [Google Scholar]
- 39.Irish Statute Book. Misuse of Drugs (Amendment) Act 2015. Available at: http://www.irishstatutebook.ie/eli/2015/act/6/enacted/en/print.html [1 March 2016]
- 40.The Misuse of Drugs Act 1971 (Amendment) Order 2010 No. 1207. Available at: http://www.legislation.gov.uk/uksi/2010/1207/article/2/made [8 August 2016]
- 41.The Misuse of Drugs Act 1971 (Amendment No. 2) Order 2010 No. 1833. Available at: http://www.legislation.gov.uk/uksi/2010/1833/pdfs/uksi_20101833_en.pdf [4 August 2016]
- 42.Nycz JE, Malecki G, Zawiazalec M, Pazdziorek T. X-ray structures and computational studies of several cathinones. J Mol Struct. 2011;1002:10. [Google Scholar]
- 43.Delori A, Maclure P, Bhardwaj RM, Johnston A, Florence AJ, Sutcliffe OB, Oswald IDH. Drug solid solutions – a method for tuning phase transformations. CrystEngComm. 2014;16:5827. [Google Scholar]
- 44.Trzybinski D, Niedzialkowski P, Ossowski T, Trynda A, Sikorski A. Single crystal x-ray diffraction analysis of designer drugs: hydrochlorides of metaphedrone and pentedrone. Forensic Sci Int. 2013;232:28. doi: 10.1016/j.forsciint.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 45.Satthaphut N, Sutcliffe OB, Oswald IDH. Putting the squeeze on mephedrone hydrogen sulfate. Z Kristallogr. 2014;229:101. [Google Scholar]
- 46.Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holy M, Rothman RB, Goldberg SR, Lupica CR, Sitte HH, Brandt SD, Srihari RT, Cozzi NV, Schindler CW. Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive “bath salts” products. Neuropsychopharmacology. 2013;38:552. doi: 10.1038/npp.2012.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kehr J, Ichinose F, Yoshitake S, Goiny M, Sievertsson T, Nyberg F, Yoshitake T. Mephedrone, compared with MDMA (ecstasy) and amphetamine, rapidly increases both dopamine and 5-HT levels in nucleus accumbens of awake rats. Br J Pharmacol. 2011;164:1949. doi: 10.1111/j.1476-5381.2011.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simmler LD, Buser TA, Donzelli M, Schramm Y, Dieu LH, Huwyler J, Chaboz S, Hoener MC, Liechti ME. Pharmacological characterization of designer cathinones in vitro. Br J Pharmacol. 2013;168:458. doi: 10.1111/j.1476-5381.2012.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suyama JA, Sakloth F, Kolanos R, Glennon RA, Lazenka MF, Negus SS, Banks ML. Abuse-related neurochemical effects of para-substituted methcathinone analogs in rats: microdialysis studies of nucleus accumbens dopamine and serotonin. J Pharmacol Exp Ther. 2016;356:182. doi: 10.1124/jpet.115.229559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blough BE, Landavazo A, Partilla JS, Baumann MH, Decker AM, Page KM, Rothman RB. Hybrid dopamine uptake blocker-serotonin releaser ligands: a new twist on transporter-focused therapeutics. ACS Med Chem Lett. 2014;5:623. doi: 10.1021/ml500113s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saha K, Partilla JS, Lehner KR, Seddik A, Stockner T, Holy M, Sandtner W, Ecker GF, Sitte HH, Baumann MH. ‘Second-generation’ mephedrone analogs, 4-MEC and 4-MePPP, differentially affect monoamine transporter function. Neuropsychopharmacology. 2015;40:1321. doi: 10.1038/npp.2014.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.