Abstract
Pregnant women are exposed to various chemicals, including endocrine-disrupting chemicals (EDCs) such as phthalates and bisphenols. Increasing evidence suggests that early life exposures to phthalates and bisphenols may contribute to cardiometabolic risks. The aim of this narrative review was to summarize current knowledge of the effects of fetal and childhood exposure to phthalates and bisphenols on child growth and child cardiometabolic outcomes and the effects on maternal outcomes. In total, 54 studies were identified and included. The majority of studies found effects of phthalates and bisphenols on maternal, child growth, and cardiometabolic outcomes. Currently results suggest that early life exposure to phthalates and bisphenols may have a substantial influence on perinatal and postnatal cardiometabolic programming. In a large part of the investigated outcomes studies show contradictory results. However, the majority of the existing evidence is based on non-cohort studies with single samples neglecting time-variant effects and complicating conclusions regarding causal inference. More studies are needed investigating the mechanisms and its potential interactions.
Keywords: Phthalates, bisphenols, prenatal and childhood exposure, growth, cardiometabolic effects, review
Introduction
Pregnant women are exposed to a variety of chemicals,[1, 2] including endocrine-disrupting chemicals (EDCs) such as phthalates and bisphenols.[3-6] Increasing evidence suggests that early life exposures to phthalates and bisphenols may contribute to the burden of cardiovascular and metabolic disease in western countries. Recent work suggests that these exposures may be costly. Health care costs of obesity and diabetes attributable to adult phthalate and bisphenol exposure in Europe is in the order of €17 billion annually.[7] Insofar as prenatal and childhood exposures may even be more impactful, the costs of cardiometabolic conditions due to these exposures may be higher.
In this narrative review, we summarize current knowledge of the effects of fetal and childhood exposure to phthalates and bisphenols on child growth and child cardiometabolic outcomes. Additionally, we summarize the effects of phthalate and bisphenol exposure on maternal outcomes.
Phthalates and Bisphenols
Phthalates are synthetic chemical esters of phthalic acid that are widely used in a variety of consumer products to impart flexibility, pliability and elasticity to plastics and therefore known as “plasticizers”.[8] Phthalates can be classified in two groups. Low molecular weight (LMW) phthalates (e.g. di-methyl phthalate (DMP), di-ethyl phthalate (DEP), di-n-butyl phthalate (DBP)) are frequently added to personal care products as aerosol delivery agents, emollients, to impart flexibility in nail polishes, and to retain scent.[9] High molecular weight (HMW) phthalates (e.g. di-2-ethylhexylphthalate (DEHP), di-isononylphthalate (DiNP), di-isodecylphthalate (DiDP), di-n-octylphthalate (DnOP), butylbenzyl phthalate (BBzP)) are used as plasticizers to impart flexibility in vinyl plastics (e.g. polyvinyl chloride plastics (PVC)) for diverse applications including flooring, medical devices and food packaging.[10] In the category of HMW phthalates, di-2-ethylhexylphthalate (DEHP) is of particular interest, considering many food packaging methods include the use of plastics containing DEHP.[11] However, the last few years DiNP and DiDP have replaced DEHP to a great extent, mainly due to governmental embargoes.[12]
Bisphenol A (BPA) is used to produce polycarbonate plastics and epoxy resins used in various consumer products, including the lining of metal cans, toys and water pipes.[13] The last few years, bisphenol A has been substituted by synthetic bisphenol analogues like bisphenol F (BPF) and bisphenol S (BPS), which has been determined in various food items[14]. BPS has been found as well in paper and paper products, including currency bills.[15]
Routes of Exposure and Metabolism
Phthalates are non-covalently bound to many plastics, creating a large risk for release into the environment over time.[9] Phthalates are generally lipophilic[16] and have short biological half-lives (less than 24h), undergoing hydrolysis and sometimes oxidation before glucuronidation or sulfation before excretion into urine of feces, but it can be measured as well in blood and breast milk.[9] A portion of the unconjugated (free) monoester and/or its secondary metabolites may also be directly excreted in urine.[17] The primary routes of exposure to phthalates are ingestion, salivary absorption, inhalation, intravenous, and transdermal. Depending on the route of exposure, the chemical is distributed into various body parts based on vascular blood supply and affinity, which in turn may lead to a difference in bioavailability. Ingested chemicals often undergo a first-pass effect, entering the liver through the hepatic portal system for metabolization, which reduces bioavailability. Following inhalation, salivary absorption, intravenous, and transdermal exposure this first-pass effect is initially bypassed, provoking a higher bioavailability.[18]
Population based studies often use urine as a measurement for exposure to phthalates because it is noninvasive and notwithstanding the short biological half-life it may reasonably reflect the exposure in the last several weeks or even months.[18, 19] The majority of the population based studies using urinary phthalate concentrations measured the concentration of the free plus glucuronidated species of phthalate metabolites, together being the total concentration. However, the free metabolite concentrations are less stable over time than the total metabolite concentration, suggesting free metabolite concentrations are not a useful indicator of metabolic susceptibility. Time of collection is an important factor that must be taken into account, since concentrations of metabolites vary during the day as a result of timing of exposure.[17]
Various products containing polycarbonate plastics and epoxy resins have been studied to obtain more knowledge on bisphenol leaching. Regarding polycarbonate plastics, different results have been obtained on the effects of washing and heating on BPA leaching, although all studies found leaching. Several studies have been performed that found that heating temperature had a significant effect on BPA leaching from metallic coated food cans.[13]
Studies investigating the metabolism of BPS and BPF are lacking. Concerning BPA it is known that after ingestion BPA undergoes a first-pass metabolism in the gastrointestinal tract and liver consisting of glucuronidation and, to a lesser extent, sulfation metabolizing BPA to bisphenol A monoglucuronide (BPAG) and bisphenol A sulphate (BPAS) for approximately 98%. In plasma, more than 90% of BPA is bound, depending on the route of exposure. Exposure through inhalation and skin absorption have been reported as important routes of exposure, as unconjugated bisphenols might circulate longer in the plasma, while ingested bisphenols undergo the first-pass metabolism.[20, 21] However, it has been reported that UDP-glucuronosyltransferase (UGT) enzymes found in the airways exhibit a high activity towards bisphenols.[21] Both BPAG and BPAS are excreted in urine within 5-7 hours after oral administration.[20, 22] BPA penetrates and accumulates in the human placenta, with higher levels of BPA in the placenta compared to maternal and fetal plasma.[23] In a rat-study, BPF residues have been detected in the uterus, placenta, amniotic fluid, and fetuses, with comparable higher levels of BPF in the (intra)uterine compartment compared to maternal blood.[24]
Biomonitoring studies have observed high plasma concentrations not consistent with the observation of an extensive first-pass metabolism of oral BPA. However, concentrations of urinary BPA tend to be much higher than serum concentrations. It has been hypothesized that these relatively high concentrations both in plasma and urine could be explained by sublingual absorption, bypassing the first-pass metabolism.[25] While another study has suggested that this hypothesis does not hold, the contradictory study is beset by critical differences in site of blood collection and volume of urinary output that actually support sublingual absorption as a substantial contributor to exposure.[22, 25]
A potentially important role in metabolism with a large effect on bioavailability is reserved for the human microbiome. The microbiome comprises the residential microbes humans are colonized by and there is a broad interindividual variation. Several bacterial species possess β-glucuronidases and β-glucuronides, enzymes involved in deconjugation and conjugation. Depending on the composition of the microbiome, phthalates and bisphenols could be conjugated or deconjugated after enterohepatic circulation, resulting in a smaller or larger exposure to unconjugated chemicals, respectively.[26, 27]
Potential Mechanisms of Effect
Hydrolysed phthalate metabolites have been shown to penetrate the human placenta.[28] In vitro studies demonstrated that several commercially used phthalates may bind to estrogen receptor alpha (ERα), having a weak estrogenic activity,[29, 30] and to androgen receptors (ARs), having a strong anti-androgen activity.[31, 32]
Another potential mechanism is by activation of nuclear transcription factor peroxisome proliferator-activated receptors (PPARs). PPAR-gamma (PPARγ), expressed predominantly in adipose tissue and to a lesser extent the macrophage and liver, acts as regulator for adipocyte differentiation, lipid metabolism and reduces inflammation resulting in improved insulin sensitization.[33, 34] However, despite potential benefits, PPARγ agonists have been shown to cause adverse effects regarding increased lipid accumulation and release of adipocyte-related hormones leading to an increased susceptibility for the development of obesity.[35] Several phthalates have been shown to be PPARγ activators, causing obesogenic effects.[34-37] PPARs form heterodimers with the retinoid X receptors (RXRs), binding together on the target DNA and thereby activating the expression of downstream genes. Therefore, RXRs have the same targets as PPARs. Many common phthalates have been shown to bind to RXRs.[37] Likewise, oxidative stress is a potential mechanism for phthalate effects. In a prospective cohort study of pregnant women all urinary phthalates were associated with increased oxidative stress markers.[38]
The potential mechanisms of action from bisphenols resemble those from phthalates to a great extent. Studies regarding the mechanism of action from BPF and BPS are scarce. Bisphenols are weak xenoestrogens binding to estrogen receptors (ER) and the G-protein-coupled receptor 30 (GPR30) in its unconjugated form, with greater binding affinity to ERβ compared to ERα, considering a 100 to 10.000-fold lower relative binding affinity of bisphenols to ERs compared to estradiol (E2).[20, 39, 40] However, findings suggest that BPA is equally potent as E2 and it is suggested that this results from actions through non-genomic pathways and disruption of steroidogenesis.[13, 20, 41] BPF and BPA have been reported to increase the level of 17β-estradiol. BPF appears to be even more potent than BPA, given the higher concentrations of 17β-estradiol after exposure of H295R human adrenocortical carcinoma cell line to BPF.
Like phthalates, bisphenols have anti-androgen capacities, binding to ARs.[31, 39] A decrease in free testosterone level is reported when exposed to bisphenols, in the order BPF > BPS > BPA. Since the binding affinity of BPS to the AR is low, the testosterone effect of BPS seems to be androgen receptor independent.[39, 42] Both BPS and BPF increased progesterone levels, BPA and BPS decreased cortisol levels, and BPF increased cortisol levels. [42]
Similar to phthalates, BPA appears to induce an activation of PPARα and possibly to a smaller extent a weak activation of PPARγ, causing obesogenic effects.[34, 35, 37] Besides the binding to PPARs, also BPA binds to RXRs with more affinity to RXRs.[37] Likewise, oxidative stress is a potential mechanism for BPA effects. The same Puerto Rican prospective cohort study describing the association between phthalates during pregnancy and oxidative stress markers found a significant association between urinary BPA and oxidative stress markers in pregnant women.[43] An in vitro study showed oxidative stress effects from BPS and BPF.[42] Furthermore, BPA has been reported to have nitrosative stressor effects and effects on free fatty acids (FFAs). Oxidative stress and imbalances in FFAs are known mediators of tissue specific insulin resistance and inflammation.[44]
Adverse metabolic outcomes could also be hypothesized by interaction of phthalates and bisphenols with the adipocytokines leptin and adiponectin. Adiponectin and leptin are adipocyte-produced hormones having a role in metabolic regulation and function. In adults, leptin inhibits appetite, stimulates thermogenesis, enhances fatty acid oxidation, decreases glucose, and reduces body weight and fat. Paradoxically, leptin levels increase in obesity.[45] Meanwhile, in adults low adiponectin levels have been associated with insulin resistance, dyslipidemia, atherosclerosis, and metabolic syndrome.[45, 46] An overview of the potential mechanisms of effect is shown in Figure 1.
Figure 1. Potential mechanisms of effect.
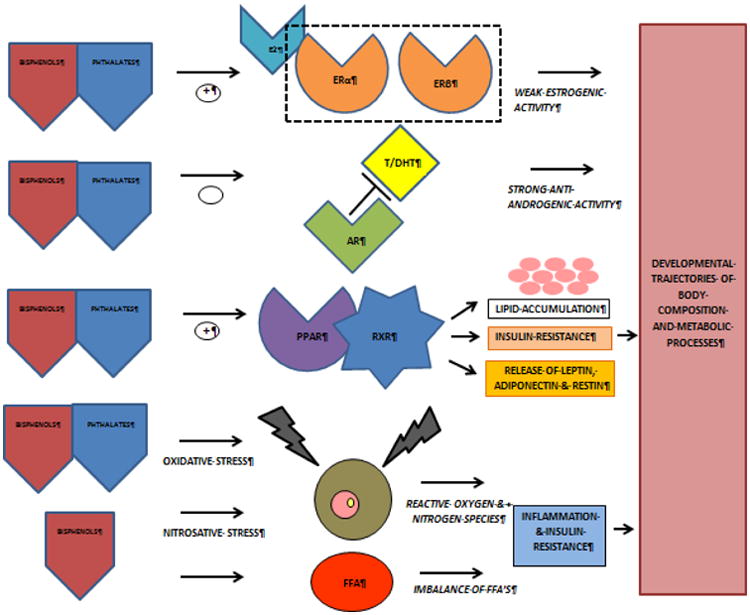
ERα: estrogen receptor alpha ; ERβ: estrogen receptor beta ; E2: estradiol ; AR : androgen receptor ; T/DHT: testosterone / dihydrotestosterone ; PPAR: peroxisome proliferator-activated receptors ; RXR: retinoid X receptors ; FFA: free fatty acids.
Maternal Pregnancy Outcomes
Two studies investigated the effect of phthalates and BPA on the time to pregnancy. A Canadian pregnancy cohort found no association between first trimester phthalates or BPA and recalled time to pregnancy.[47] Meanwhile, a preconceptional cohort study exploring the effects of phthalates and BPA on fertility in the both men and women in the United States found several phthalates to be associated with longer time to pregnancy in males, while other phthalates were associated with shorter time to pregnancy in females. Neither male nor female BPA concentration was associated with time to pregnancy.[48] Two human studies have pointed out that a higher level of BPA was associated with (recurrent) aneuploidy and euploid miscarriages.[49, 50]
During pregnancy maternal glucose levels increase to provide adequate nutrition for fetal growth and development. Two studies investigated the effect of phthalates and BPA on maternal glucose levels and gestational diabetes mellitus (GMD).[51, 52] Neither phthalates nor BPA was associated with increased maternal glucose levels or GMD. However, a small cohort study of pregnant women found that women in the highest tertile of urinary MiBP and MBzP had lower blood glucose levels at time of the routine GMD screening compared to women in the lowest concentration tertile.[52] A Japanese study investigated the effect of DEHP on maternal triglycerides and fatty acid levels, detecting an inverse association between maternal serum MEHP concentrations and triglyceride and fatty acid levels during pregnancy. These findings could have implications for fetal health. The growth and development of the fetus and its organs depend on a sufficient supply of nutrients including fatty acids and lipids crossing the placenta, determining birth outcomes. However, in this particular study no effect on fetal growth was observed.[53]
Only one study explored the association with preeclampsia, investigating differences in the distribution of BPA between maternal, placental and fetal compartments. This small case-control study reported that preeclamptic women have significantly higher concentrations of BPA in placental tissue compared to normotensive pregnant women, while concentrations of BPA in maternal serum and cord blood did not differ significantly. Accordingly, BPA was equally distributed between maternal, placental and fetal compartments in the normotensive group, while preeclamptic women showed an unequal distribution with a high level of BPA in the placental compartment.[54]
Overall, the amount of studies for the separate outcome measures is limited. However, the presented outcomes suggest potential adverse effects of phthalates and bisphenols on fecundity, miscarriages, and preeclampsia. There are no studies reporting on pregnancy induced hypertension or HELLP syndrome. An overview of the included studies including outcomes, strengths and weaknesses is given in Table 1. Study characteristics and results of all included studies separately are shown in Supplementary Table 1.
Table 1. Overview of the included studies per outcome.
| Outcome | Phthalates and/or bisphenols | Sample size | Time of exposure | Associations | Outcome | Strenghts (S)/Weaknesses (W) |
|---|---|---|---|---|---|---|
| MATERNAL PREGNANCY OUTCOMES | ||||||
| Time to Pregnancy[47 -48] | Phthalates | 2001[47] 501[48] | First trimester[47] Preconceptional inclusion[48] | Positive / negative / null | One pregnancy cohort found no association between first trimester phthalates and recalled time to pregnancy.[47] A preconceptional cohort found several phthalates to be associated with longer time to pregnancy in males, while other phthalates were associated with shorter time to pregnancy in females.[48] | S: 1 preconceptional cohort study, couples with infertility/sterility excluded, urinary samples adjusted for urinary dilution, the preconceptional study explored both males and females W: 1 retrospective analysis in a cohort study based on recalled data. |
| BPA | 2001[47] 501[48] | First trimester[47] Preconceptional inclusion[48] | Null | One pregnancy cohort found no association between first trimester BPA and recalled time to pregnancy.[47] A preconceptional cohort found no associations between BPA and time to pregnancy.[48] |
S: 1 preconceptional cohort study, couples with infertility/sterility excluded, urinary samples adjusted for urinary dilution, the preconceptional study explored both males and females W: 1 retrospective analysis in a cohort study based on recalled data |
|
| Miscarriage risk[49-50] | BPA | 115[49] 50[50] | First trimester[49-50] | Positive | Both studies found a positive association between the level of BPA and (recurrent) aneuploidy and euploid miscarriages.[49,50] | S: 1 cohort study, only unknown etiology of miscarriages included W: 1 case-control study, blood samples instead of urinary samples |
| Maternal blood glucose, triglycerides and fatty acid levels[51-53] | Phthalates | 72[52] 318[53] | First trimester[52] Second trimester[53] | Negative | Women in the highest tertile of urinary MiBP and MBzP had lower blood glucose levels at time of routine GMD screening compared to women in the lowest tertile.[52] Maternal serum MEHP concentrations were associated with lower triglyceride and fatty acid levels during pregnancy.[53] | S: 2 cohort studies, quite accurate about the time of sampling W: Only one sample, 1 study with blood samples, regarding triglycerides and fatty acids only DEHP and MEHP measured |
| BPA | 94[51] | Third trimester[51] | Null | No association between BPA and gestational diabetes mellitus was found.[51] | S: Accurate about time of sampling W: Case-control study, only one urinary sample |
|
| Preeclampsia [54] | BPA | 58 | Before delivery and umbilical cord blood | Positive | Preeclamptic women have significantly higher concentrations of BPA in placental tissue compared to normotensive pregnant women, while concentrations in serum and cord blood did not differ significantly.[54] | S: Placental biopsies W: Case-control study, blood samples instead of urinary samples, only BPA measured |
| FETAL OUTCOMES | ||||||
| Gestational age at birth[55-65] | Phthalates | 283[55] 404[56] 86[57] 207[58] 482[59] 482[60] 84[61] 60[62] 72[63] | First trimester[55] Third trimester[56,62] Shortly postpartum or cord blood[57,58,61] Four samples over pregnancy[59-60] Term not specified[63] | Mainly negative | Six studies found a significant association between phthalate exposure and gestational age reduction or preterm delivery.[58-63] One study found no association [58] and two studies found a positive correlation between phthalate exposure and gestational age at delivery.[55, 56] | S: All (subsets from) cohort studies, mainly urinary samples used all corrected for dilution, the majority of studies reported information on gestational age estimation W: Only 2 studies multiple sampling, sampling often taken over broad time, sampling time in one study not specified, various outcome measures used |
| BPA | 404[56] 72[63] 567[64] 60[65] | Third trimester[56,65] Term not specified[63] Before delivery[64] | Mainly negative | Three studies found a significant association between BPA and gestational age reduction [63-65] of which in one this association only remained in male infants after stratification for gender.[63] One study found no associations.[56] | S: All cohort studies or subsets from cohort studies, mainly urinary samples used all corrected for dilution, the majority of studies reported information on gestational age estimation W: No multiple sampling, sample often taken over a broad period of time, sampling time in one study not specified, various outcome measures used |
|
| Body size measures at birth[53,56-58,64,66-73] | Phthalates | 318[53] 404[56] 86[57] 207[58] 126[66] 119[67] 201[68] | Second trimester[53] Third trimester[56,66,67] Shortly postpartum or cord blood[57,58,68] | Mainly negative | Four studies found a negative effect of phthalates on body size measures at birth.[58,66-68] One study found no associations.[53] Two studies found positive associations between LMW phthalates and head circumference at birth.[56,57] | S: The majority of studies were cohort studies or subsets from cohort studies, based on urinary samples corrected for dilution W: No multiple sampling, sample often taken over a broad period of time |
| BPA | 404[56] 567[64] 520[69] 550[70] 97[71] 219[72] 737[73] | Second trimester[69,70] Third trimester[56, 73] Before delivery[64, 71] Cord blood[71] Three samples over pregnancy[72] | Mainly null, study with repeated measurements negative | Four studies found no associations.[56,64,69,70] Two studies, including a study with up to 3 measurements during pregnancy, found a negative association between BPA exposure and body size measures at birth.[71,72] One study found a positive association between BPA and birth weight in male neonates and ponderal index in female neonates.[73] | S: The majority of studies were cohort studies or subsets from cohort studies, based on urinary samples corrected for dilution. One study collected up to three samples W: The majority of studies collected only one sample, which was often taken over a broad period of time. |
|
| CHILDHOOD OUTCOMES | ||||||
| Childhood growth, prenatal exposure[6,69, 74-78] | Phthalates | 89[74] 234[75] 391[76] | Cord blood[74] Third trimester[75] First & third trimester[76] | Boys: negative Girls: positive | All three studies found prenatal exposure to phthalates to be associated with reduced BMI in boys. Additionally, one of the studies found also a reduced fat mass and waist circumference in boys, not girls.[75] In one of the studies prenatal exposure to phthalates was associated with a higher BMI in girls.[76] | S: All studies were cohort studies, 1 study with repeated sampling, one of the studies collected additionally multiple childhood samples, urinary samples corrected for dilution W: Study with multiple urinary samples did not analyse the measurements separately but averaged the results, 1 smaller study with cord blood |
| BPA | 402[6] 520[69] 402[77] 297[78] | First & second trimester[6] Second trimester[69,78] First & third trimester[77] | Positive / negative | Two studies showed that prenatal BPA exposure had a negative effect on BMI in girls, not boys.[6, 78] After stratification for puberty stage in one of these studies, only in prepubertal girls prenatal BPA exposure remained negatively associated with BMI and waist circumference.[6] Two studies, of which one consisted only of boys, reported a positive effect of prenatal BPA exposure on several growth parameters, including height adjusted weight (not significant), waist circumference and BMI.[69,77] | S: All studies were cohort studies with urinary samples, 3 studies collected two pregnancy samples, two of the studies collected additionally multiple childhood samples, all except for one study corrected for urinary dilution, one study performed sensitivity analyses for BPA measurements by trimester W: Studies with multiple urinary samples did not analyse the measurements separately but averaged the results |
|
| Childhood growth, childhood exposure [6,75,78-93] | Phthalates | 234[75] Not specified [79] 493[80] 259[81] 387[82] 2884[83] 845[84] 90[85] 76[86] | Toddlers till adolescence[86] Preschoolers[75] Preschool till mid childhood[84] Mid Childhood[80,82,85] Mid childhood till adolescence[79,81,83] | Mainly positive, most for boys | Five studies found a positive effect of childhood exposure to phthalates on childhood growth parameters, including BMI, subscapular skinfold thickness, hip-and waist circumference, predominantly found in boys.[79-83] Two studies found no associations.[85,86] Two studies found a negative association between childhood exposure to phthalates and mid-childhood growth, including, BMI, BSA, weight and height.[75,84] | S: All included studies collected urinary samples of which 1 study with multiple childhood samples, all samples were corrected for dilution |
| BPA BPS | 402[6] 297[78] 90[85] 76[86] 39[87] 80[88] 259[89] 2838[90] 3370[91] 2200[92] 1326[93] | Toddlers till adolescence[86] Preschool till mid childhood[87,93] Mid childhood[85,88] Mid childhood till adolescence[89-92] | Mainly positive, not enough evidence to draw conclusions for BPS | Six studies found a positive effect of childhood exposure to BPA on childhood growth parameters measured from preschool till adolescence, including BMI, waist-to-height ratio and hip circumference.[6,89-93] One study found that early childhood exposure was associated with a lower BMI at the age of two, but BMI slopes increased more rapidly between 2 and 5 years of age.[78] No clear difference between the sexes. Two studies found no associations.[87,88] Two studies found a negative association between childhood exposure to BPA and BMI.[85,86] One of these studies also investigated BPS, but no associations were found.[86] | S: All included studies collected urinary samples of which 2 studies with multiple childhood samples, all samples were corrected for dilution, one study also included the newer bisphenol BPS | |
| Cardiovascul ar risk, prenatal exposure, outcome measured during childhood[76] | Phthalates | 391[76] | First & third trimester | Negative | In girls the molar sum of both HMW and LMW phthalates were significantly associated with lower systolic blood pressure z-score. No studies have been performed on bisphenols. | S: Cohort study with two maternal urinary samples, reasonable follow-up, samples corrected for urinary dilution W: Urinary samples were averaged in the analysis, only phthalates measured, only blood pressure as a cardiovascular risk outcome |
| Cardiovascul ar risk, childhood exposure and outcome[87,94-96] | Phthalates | 2838[94] 667[96] | Mid childhood till adolescence[94,96] | Positive | Both studies reported a positive link between phthalate exposure during childhood and adverse cardiovascular outcomes, including systolic blood pressure z-score and increased albuminuria.[94,96] | S: Childhood urinary samples corrected for urinary dilution, 1 study on blood pressure, 1 on albuminuria W: 2 cross-sectional studies, no studies with multiple sampling |
| BPA | 39[87] 770[95] | Preschool till mid childhood[87] Mid childhood till adolescence[95] | Positive | Both studies showed also a positive association between childhood BPA exposure and adverse cardiovascular outcomes, including increased albuminuria and in boys an increased diastolic blood pressure.[87,95] | S: Childhood urinary samples corrected for urinary dilution, 1 study on blood pressure, 1 on albuminuria. W: 1 cross-sectional and one small case study, no studies with multiple sampling, one study measured also lipid levels but did not report on that outcome. |
|
| Metabolic risk, childhood exposure and outcome[87-88,91,97-99] | Phthalates | 766[97] 356[98] | Adolescence[97,98] | Positive | Both studies found a positive association between childhood HMW phthalate exposure, mainly DEHP, and the newer DINP and insulin resistance.[97,98] | S: Both studies based on urinary samples corrected for urinary dilution W: No multiple sampling, both cross-sectional studies |
| BPA | 39[87] 80[88] 3370[91] 188[99] | Preschool till mid childhood[87] Mid childhood[88,99] Mid childhood till adolescence[91] | Mainly null | One study showed a negative association between childhood BPA exposure and fasting insulin and insulin resistance, unfortunately not adjusted for urinary dilution.[87] Three studies did not find any associations between BPA and childhood metabolic outcomes.[88,91,99] | S: All studies collected urinary samples corrected for urinary dilution, except for one all studies used insulin resistance as an outcome measure W: No multiple sampling |
|
Fetal Outcomes
In addition, the same mechanisms that affect maternal outcomes may also affect fetal outcomes. Phthalates have been investigated more thoroughly for their effects on prematurity compared to bisphenols. Nine studies explored the relationship between prenatal phthalate exposure and prematurity. In contrast to our hypothesis, two of these studies found a positive association between phthalate exposure and gestational age at delivery, reporting an increase of only one or two days to the gestational age at birth due to higher levels of phthalates. Both studies measured phthalates in urinary samples in the beginning of the third trimester.[55, 56] A French small prospective cohort study found no association of dibutylphthalate and its main metabolite monobutylphthalate in cord blood and breast milk with length of gestation.[57] The remaining six studies found a significant association between a higher exposure to phthalates and reduced gestational age at time of birth.[58-63] Two of these studies performed the measurements of exposure in cord blood samples.[58, 61] Of the remaining four studies with phthalate measurements performed in urinary samples, one found a significant reduction in gestational age which only remained in male infants after analysis was stratified for gender. Unfortunately, the timing of urinary sample collection was not specified.[63] Two included studies consist of the same birth cohort with up to four urinary samples during pregnancy investigating the overall relationship with prematurity and windows of vulnerability for spontaneous and placental preterm birth, respectively. Both studies report that the odds of preterm birth compared to term birth are 1.3-1.5 times higher for children prenatally exposed to high levels of phthalates compared to children exposed to lower levels of phthalates.[59, 60] One of these studies revealed that spontaneous and placental preterm birth, defined as delivery with presentation of preeclampsia or intrauterine growth restriction, had different sensitivity windows of exposure to phthalates during pregnancy. Spontaneous preterm birth, defined as delivery with presentation of spontaneous labor of preterm premature rupture of membranes, showed to be significantly associated with higher phthalate metabolite concentrations measured at the beginning of the third trimester, while placental preterm birth was associated with higher phthalate metabolite concentrations measured during the first trimester.[60]
Regarding bisphenols, all four studies presented in this review examined only BPA and its relationship with prematurity.[56, 63-65] Three of these studies associated higher urinary BPA levels, mainly measured during third trimester, with a significant reduction in gestational age at time of birth of one to four days.[63-65] As for phthalates the gestational age reduction remained only significant in male infants in one of these pregnancy cohorts.[63] The remaining study on bisphenols found no relationship between BPA with gestational age at time of birth.[56]
The association of phthalates and bisphenols with fetal growth is explored with 7 studies reporting on phthalates and 7 studies on bisphenols, including the already presented results from the Japanese study investigating also fetal nutrients during pregnancy displaying no association between DEHP and fetal growth.[53] With respect to phthalates, of the remaining 6 studies, 4 all Chinese studies found that a higher exposure to phthalates was significantly related with reduced body size measures at birth.[58, 66-68] A cohort study among 207 women reported a reduction in birth weight of 15-139 grams per natural log increase in phthalate concentrations.[58] One of these studies also displayed a negative association between phthalates and DNA methylation in the human placenta, suggesting placental methylation as a possible mediator of the relationship between phthalates and birth measurements.[67] Contrasting with our hypothesis, two studies found positive associations between phthalates and fetal head circumference at time of birth.[56, 57]
Of the studies on BPA, 4 studies found no association between BPA and body size measures at birth.[56, 64, 69, 70] Two studies found higher BPA levels to be associated with decreased body size measures at birth.[71, 72] In a subset of the Dutch Generation R study fetal weight and head circumference were significantly decreased per unit increase in creatinine corrected BPA among women with three urinary BPA measurements during pregnancy. Children born to women in second highest exposure group had an average decrease of 683 grams birth weight. This relationship progressively attenuated among women with fewer BPA measurements.[72] On the contrary, a Korean multi-center birth cohort study found a higher creatinine corrected urinary BPA levels during third trimester to be associated with and increased birth weight in male neonates and increased ponderal index in female neonates.[73]
Hence, the majority of studies show mostly negative effects of phthalates and negative or no effects of BPA on gestational age and body size measures at birth. No studies have explored the other bisphenols besides BPA. An overview of the included studies is given in Table 1. Details of all described studies are represented in Supplementary Table 1.
Childhood Outcomes
Phthalates and bisphenols have been reported to have an influence on childhood growth. All three studies exploring the relationship between prenatal phthalates and childhood growth discovered different results in males and females, predominantly resulting in negative associations with growth in males.[74-76] An extensive Spanish birth cohort reported that the molar sum of first and third trimester urinary HMW phthalates was significantly associated with reduced weight gain z-score in the first 6 months in boys and nonsignificantly with lower BMI z-scores in boys at any age (measured until 7 years of age). Meanwhile, in girls the molar sum of first and third trimester HMW phthalates was nonsignificantly associated with higher BMI z-scores.[76]
Two out of 4 studies reported that a higher level of prenatal BPA exposure was associated with an increase in several childhood growth parameters in preschoolers, including BMI z-score, weight and waist circumference.[69, 77] The remaining two studies showed prenatal BPA exposure to be related to a lower postnatal BMI in preschool till mid-childhood aged girls, not boys.[6, 78] After subdivision in prepubertal and pubertal girls, only in prepubertal girls prenatal BPA exposure remained negatively associated with BMI z-score and waist circumference.[6]
In total, 18 studies investigated the influence of exposure to phthalates and bisphenols during childhood on childhood growth. Five out of nine studies found childhood exposure to phthalates to be associated with increased growth parameters in mid-childhood till adolescence, including obesity indices consisting of subscapular skinfold thickness, BMI, hip- and waist circumference.[79-83] Four of these studies reported different effects in males and females, suggesting sex to be an effect modifier.[79-82] Also ethnicity is a potential modifier of effects. A cross-sectional analysis of the 2003-2008 NHANES data showed an increase in overweight and obesity among non-Hispanic blacks related to childhood phthalate exposure, while among other ethnic groups no significant associations have been found.[83] Two studies found childhood phthalate exposure to be associated with reduced mid-childhood growth measures, including BMI, fat mass, waist circumference, BSA, weight and height.[75, 84] The two remaining studies found no relationship between childhood phthalate exposure and preschool till adolescent growth, defined as a BMI >85th and >95th percentile, respectively.[85, 86]
Eleven studies examined the relationship between levels of BPA in childhood and growth, of which 2 studies did not find any relationship between BPA and preschool till mid-childhood growth measures, including weight, height, waist circumference and BMI.[87, 88] The earlier mentioned HOME study found two-sided outcomes: exposure early in childhood to higher levels of BPA was associated with a lower BMI at the age of two. However, their BMI slopes increased more rapidly between 2 and 5 years of age.[78] Six studies showed that higher BPA levels in childhood were associated with increased growth parameters, including BMI, waist circumference, waist-to-height ratio, hip circumference and body fat.[6, 89-93] Measurements are obtained over the whole age spectrum of childhood and adolescence. Three of these studies were based on subsamples from the National Health and Nutrition Examination Survey (NHANES) in the United States. Samples were not identical as a result of heterogeneous inclusion criteria and outcome measures differed to a certain extent. However, all examined the relationship with BMI.[90-92] Both sex and ethnicity were shown to be potential effect modifiers of the relation between childhood exposure to BPA and childhood growth parameters. In the NHANES data, childhood urinary BPA concentrations were significantly associated with increased risks for obesity and increased waist-to-height ratios among non-Hispanic white boys.[90-92] Contrastingly, a study of Chinese schoolchildren found a significant association between childhood BPA exposure and increased BMI and hip circumference in girls, but not in boys. However, it must be noted that BPA concentrations were not adjusted for urinary dilution.[93] Two earlier mentioned studies found childhood BPA exposure to be associated with reduced overweight and obesity, respectively.[85, 86] One of these studies is the only study included in this review investigating not only BPA but also BPS. However, no associations have been found with BPS regarding growth measures.[86]
The aforementioned Spanish study showed that in girls the molar sum of prenatal exposure to both HMW and LMW phthalates was significantly associated with lower systolic blood pressure z-scores at 4 and 7 years of age. For both sexes there was a negative association with diastolic blood pressure, but none of the associations reached the level of significance.[76]
Four studies have examined childhood exposure to phthalates and bisphenols with respect to cardiovascular outcomes. All studies, including 2 studies published on phthalates and 2 studies on BPA, confirmed the hypothesis that childhood exposure to phthalates and bisphenols is associated with adverse cardiovascular outcomes in terms of increased blood pressure and low-grade albuminuria in preschool till adolescent age.[87, 94-96] Details are shown in Supplementary Table 1.
The relationship between childhood exposure to phthalates and bisphenols and childhood metabolic outcomes has been explored in six studies. The majority of studies used the homeostatic model assessment of insulin resistance (HOMA-IR) to assess insulin resistance in which a HOMA-IR ≥ 4.39 was defined as insulin resistance.[87, 88, 91, 97, 98] The analysis of the 2003-2008 NHANES data showed HMW phthalate metabolites to be related to a higher HOMA-IR in adolescents, which was mainly DEHP dependent. Adding BPA to the model did not change the association.[97] A recent follow-up analysis of the 2009-2012 NHANES data confirmed this association and explored whether the newer DINP, a replacement for DEHP, was also associated with increased insulin resistance. Also DINP showed to be associated with an increased insulin resistance.[98] Further evidence on the association with phthalates is lacking.
Three studies of BPA failed to find an association with childhood metabolic outcomes in mid-childhood till adolescent age, including adipokines, insulin resistance, blood lipids, insulin and glucose.[88, 91, 99] The earlier mentioned case study of obese children found a significant mainly negative non-monotonic exposure-response relationship between BPA and fasting insulin and HOMA-IR in a case study of obese or overweight children aged 3-8 years. Unfortunately, BPA was not adjusted for urinary dilution in this analysis, which made it difficult to estimate its adjusted effect.[87]
To conclude, the greater part of studies examined the association of phthalates and bisphenols with growth, giving contradictory results. Sex, stage of puberty and ethnicity are proposed as potential effect modifiers. An overview of the included studies is given in Table 1. Details of all described studies are represented in Supplementary Table 1.
Discussion
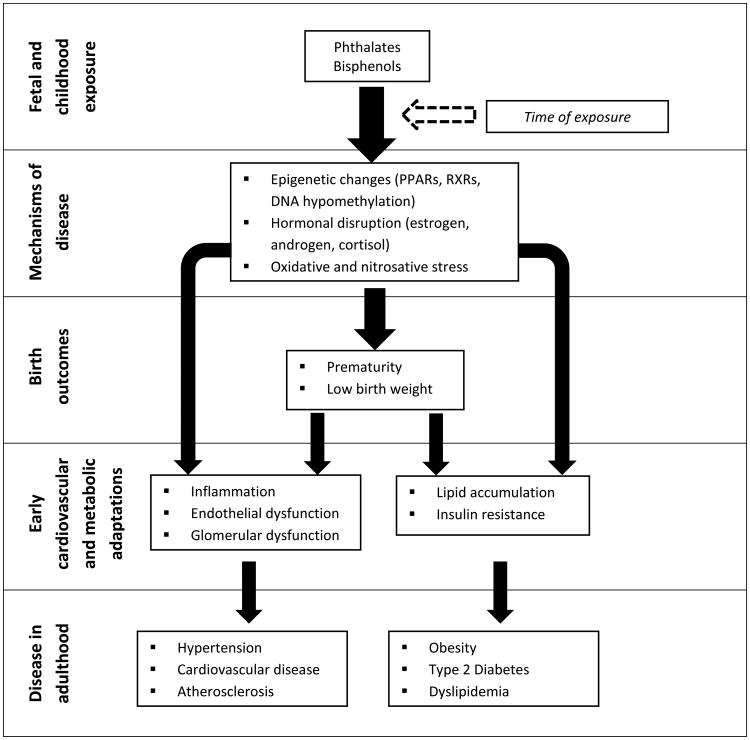
We identified many studies investigating potential effects of prenatal and childhood phthalate and bisphenol exposure on growth and cardiometabolic risks. As presented throughout the paragraphs, exposure to phthalates and bisphenols is believed to induce pathways towards several adverse health effects. This conceptual model of mechanisms is shown in Figure 2. In vitro studies showed that exposure to phthalates and bisphenols causes hormonal disruption, epigenetic changes, oxidative and nitrosative stress. The effects could be affected by the time of exposure, both during fetal life and childhood. Exposure during fetal life may induce prematurity and low birth weight. Aside from the many disadvantageous outcomes from premature birth, both premature birth and low birth weight have been associated with cardiometabolic dysfunction, including overweight in adolescence and adulthood, dyslipidemia, insulin resistance, endothelial and glomerular dysfunction.[100-103] As shown before, exposure during childhood is associated with aberrant growth patterns inducing overweight and obesity and early signs of cardiovascular and metabolic dysfunction, including glomerular and endothelial dysfunction, increased blood pressure and increased insulin resistance. Together with the earlier mentioned cellular changes both fetal and childhood exposure to phthalates and bisphenols may give rise to an increased risk for cardiovascular and metabolic disease in adulthood. As shown in the earlier paragraphs, effects could be affected by the time of exposure, both during fetal life and childhood, as well as by the sex and race of the child.
Figure 2.
Exposure to phthalates and bisphenols is believed to induce pathways towards several adverse health effects, including epigenetic changes, hormonal disruption, oxidative and nitrosative stress. Effects could be affected by the time of exposure, both during fetal life and childhood. Fetal exposure has been linked with prematurity and low birth weight. Childhood exposure has been associated with early signs of cardiovascular and metabolic dysfunction. Both prematurity and low birth weight have also been associated with cardiometabolic dysfunction. Therefore, both fetal and childhood exposure may give rise to an increased risk of cardiovascular and metabolic disease in adulthood.
Little is known about the epigenetic changes phthalates and bisphenols can cause and what mechanism lies behind it. The epigenetic effects of fetal BPA exposure were explored in mouse models. This study showed that BPA induces DNA hypomethylation, while maternal nutritional supplementation of methyl donors like folate negated the BPA-induced DNA hypomethylation in the offspring.[104] This could be a major point of engagement, yet has not been examined in humans. An in vitro study of human adipocytes from prepubertal non-obese children investigated the effect of BPA on gene expression in adipocytes, disclosing an increase in pro-inflammatory cytokines involved in the lipid metabolism and a decrease in a gene involved in insulin production.[105] As mentioned in the mechanisms paragraph phthalates and bisphenols are reported to interfere with PPARs and RXRs, potentially causing epigenetic changes. RXRs form heterodimers using its nuclear receptor with all members of the class 1 nuclear receptors, including PPARs, but also the vitamin D3 receptor and the thyroid hormone receptor. Several drugs and mechanisms are claimed to interfere with the nuclear receptor from RXRs.[106, 107] However, little is known about the interaction mechanisms. Overall, very few studies have been investigating the mechanisms of phthalates and bisphenols combined with clinical outcomes in humans. More studies are needed investigating mechanisms and potential.
This review shows several limitations in the present literature, especially regarding time of exposure. One of the main limitations is the inconsistent exposure-response relationship in almost all studies performed so far, causing non-differential misclassification of exposure leading to underestimation of the effect. Only one study controlled for puberty, while the degree of puberty could be an important modifier of effects. Only one study described in this review investigated the effects of exposure to BPS, while all other studies only examined BPA. Exposure to BPF has not been studied at all. As shown in the paragraph on maternal pregnancy outcomes, studies on maternal pregnancy outcomes are scarce, in particular studies investigating the effect of phthalates. Studies investigating prenatal exposure to phthalates and bisphenols and metabolic outcomes are lacking, just as studies investigating prenatal exposure to bisphenols and childhood cardiovascular outcomes. The cardiovascular effects of prenatal phthalates have only been examined in one study; therefore it is difficult to draw conclusions. Adult studies on delayed effects of prenatal and childhood exposure to phthalates and bisphenols are lacking. Due to the long effect latency this might be harder to investigate.
In total, 54 studies are included in this review, of which the majority uses urinary samples to estimate phthalate or bisphenol exposure. Eight cohort studies collected more than one urine sample during pregnancy,[6, 59, 60, 72, 76-78, 99] of which 3 studies collected additionally childhood urine samples.[6, 78, 99] Only three of these multi-sample studies during pregnancy did not take the average of measured concentrations, but analyzed the samples separately, trying to find windows of vulnerability.[6, 60, 99] An overview of the included studies and their strengths and weaknesses is presented in Table 1.
Concluding, the human evidence for effects of these chemicals is suggested but limited by methodological difficulties that complicate interpretation. However, an underestimation of effect is most likely. Future studies should focus on also the newer phthalates and bisphenols investigated in a distinct range of time with preferably multiple urine samples to reveal windows of sensitivity for the various biomarkers of effect. When windows of sensitivity during pregnancy would be uncovered, future parents could be informed through preconceptional consults or in early pregnancy. Moreover, to gain a better understanding in the effects, more studies are needed investigating the mechanisms and its potential interactions preferably combined with clinical outcomes. Furthermore, to provide more evidence on prolonged health effects epigenetics should be included in future studies in order to guide evidence-based prevention of harmful exposures.
Supplementary Material
Highlights.
Early exposure to bisphenols and phthalates may have a great influence on health
In a large part of the investigated outcomes studies show contradictory results
Most evidence is based on single sampling studies neglecting time-variant effects
Acknowledgments
Sources of support and funding: This study was supported by grant ES022972 from the National Institutes of Health. The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health.
Abbreviations
- AR
androgen receptor
- BBzP
butylbenzyl phthalate
- BMI
body mass index
- BPA
bisphenol A
- BPAG
bisphenol A monoglucuronide
- BPAS
bisphenol A sulphate
- BPF
bisphenol F
- BPS
bisphenol S
- DBP
di-n-butyl phthalate
- DEHP
di-2-ethylhexylphthalate
- DEP
di-ethyl phthalate
- DiDP
di-isodecylphthalate
- DiNP
di-isononylphthalate
- DnOP
di-n-octylphthalate
- DMP
di-methyl phthalate
- DOHaD
Developmental Origins of Health and Disease
- E2
estradiol
- EDC
endocrine disrupting chemical
- ER
estrogen receptor
- FFA
free fatty acid
- GMD
gestational diabetes mellitus
- HDL
high-density lipoprotein
- HMW
high molecular weight
- HOMA-IR
homeostatic model assessment of insulin resistance
- IGF-1
insulin-like growth factor 1
- LMW
low molecular weight
- MiBP
mono-isobutyl phthalate
- MBzP
monobenzyl phthalate
- MECPP
mono(2-ethyl-5-carboxypentyl) phthalate
- MEHHP
mono-(2-ethyl-5-hydroxyhexyl) phthalate
- MEOHP
mono-(2-ethyl-5-oxohexyl) phthalate
- NHANES
National Health and Nutrition Examination Survey
- PPAR
peroxisome proliterator-activated receptor
- PVC
polyvinyl chloride plastics
- RXR
retinoid X receptor
- UGT
UDP-glucuronosyltransferase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Woodruff TJ, Zota AR, Schwartz JM. Environmental chemicals in pregnant women in the United States: NHANES 2003-2004. Environ Health Perspect. 2011;119(6):878–85. doi: 10.1289/ehp.1002727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ye X, Pierik FH, Hauser R, Duty S, Angerer J, Park MM, et al. Urinary metabolite concentrations of organophosphorous pesticides, bisphenol A, and phthalates among pregnant women in Rotterdam, the Netherlands: the Generation R study. Environ Res. 2008;108(2):260–7. doi: 10.1016/j.envres.2008.07.014. doi:S0013-9351(08)00155-2[pii]10.1016/j.envres.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arbuckle TE, Davis K, Marro L, Fisher M, Legrand M, LeBlanc A, et al. Phthalate and bisphenol A exposure among pregnant women in Canada--results from the MIREC study. Environ Int. 2014;68:55–65. doi: 10.1016/j.envint.2014.02.010. doi:S0160-4120(14)00060-9[pii]10.1016/j.envint.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Casas L, Fernandez MF, Llop S, Guxens M, Ballester F, Olea N, et al. Urinary concentrations of phthalates and phenols in a population of Spanish pregnant women and children. Environ Int. 2011;37(5):858–66. doi: 10.1016/j.envint.2011.02.012. doi:S0160-4120(11)00043-2[pii]10.1016/j.envint.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 5.Casas M, Valvi D, Luque N, Ballesteros-Gomez A, Carsin AE, Fernandez MF, et al. Dietary and sociodemographic determinants of bisphenol A urine concentrations in pregnant women and children. Environ Int. 2013;56:10–8. doi: 10.1016/j.envint.2013.02.014. doi:S0160-4120(13)00062-7[pii]10.1016/j.envint.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 6.Harley KG, Aguilar Schall R, Chevrier J, Tyler K, Aguirre H, Bradman A, et al. Prenatal and postnatal bisphenol A exposure and body mass index in childhood in the CHAMACOS cohort. Environ Health Perspect. 2013;121(4):514–20. doi: 10.1289/ehp.1205548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Legler J, Fletcher T, Govarts E, Porta M, Blumberg B, Heindel JJ, et al. Obesity, diabetes, and associated costs of exposure to endocrine-disrupting chemicals in the European Union. J Clin Endocrinol Metab. 2015;100(4):1278–88. doi: 10.1210/jc.2014-4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sathyanarayana S. Phthalates and children's health. Curr Probl Pediatr Adolesc Health Care. 2008;38(2):34–49. doi: 10.1016/j.cppeds.2007.11.001. doi:S1538-5442(07)00102-2[pii]10.1016/j.cppeds.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Braun JM, Sathyanarayana S, Hauser R. Phthalate exposure and children's health. Curr Opin Pediatr. 2013;25(2):247–54. doi: 10.1097/MOP.0b013e32835e1eb6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Serrano SE, Braun J, Trasande L, Dills R, Sathyanarayana S. Phthalates and diet: a review of the food monitoring and epidemiology data. Environ Health. 2014;13(1):43. doi: 10.1186/1476-069X-13-43. doi:1476-069X-13-43[pii]10.1186/1476-069X-13-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fromme H, Gruber L, Schlummer M, Wolz G, Bohmer S, Angerer J, et al. Intake of phthalates and di(2-ethylhexyl)adipate: results of the Integrated Exposure Assessment Survey based on duplicate diet samples and biomonitoring data. Environ Int. 2007;33(8):1012–20. doi: 10.1016/j.envint.2007.05.006. doi:S0160-4120(07)00102-X[pii]10.1016/j.envint.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Zota AR, Calafat AM, Woodruff TJ. Temporal trends in phthalate exposures: findings from the National Health and Nutrition Examination Survey, 2001-2010. Environ Health Perspect. 2014;122(3):235–41. doi: 10.1289/ehp.1306681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA) Reprod Toxicol. 2007;24(2):139–77. doi: 10.1016/j.reprotox.2007.07.010. doi:S0890-6238(07)00237-7[pii]10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 14.Liao C, Kannan K. A survey of bisphenol A and other bisphenol analogues in foodstuffs from nine cities in China. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2014;31(2):319–29. doi: 10.1080/19440049.2013.868611. [DOI] [PubMed] [Google Scholar]
- 15.Liao C, Liu F, Kannan K. Bisphenol s, a new bisphenol analogue, in paper products and currency bills and its association with bisphenol a residues. Environ Sci Technol. 2012;46(12):6515–22. doi: 10.1021/es300876n. [DOI] [PubMed] [Google Scholar]
- 16.Schettler T. Human exposure to phthalates via consumer products. Int J Androl. 2006;29(1):134–9. doi: 10.1111/j.1365-2605.2005.00567.x. discussion 81-5. doi:IJA567[pii]10.1111/j.1365-2605.2005.00567.x. [DOI] [PubMed] [Google Scholar]
- 17.Meeker JD, Calafat AM, Hauser R. Urinary phthalate metabolites and their biotransformation products: predictors and temporal variability among men and women. J Expo Sci Environ Epidemiol. 2012;22(4):376–85. doi: 10.1038/jes.2012.7. doi:jes20127[pii]10.1038/jes.2012.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Needham LL, Barr DB, Calafat AM. Characterizing children's exposures: beyond NHANES. Neurotoxicology. 2005;26(4):547–53. doi: 10.1016/j.neuro.2004.09.006. doi:S0161-813X(04)00134-2[pii]10.1016/j.neuro.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 19.Hauser R, Meeker JD, Park S, Silva MJ, Calafat AM. Temporal variability of urinary phthalate metabolite levels in men of reproductive age. Environ Health Perspect. 2004;112(17):1734–40. doi: 10.1289/ehp.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mattison DR, Karyakina N, Goodman M, LaKind JS. Pharmaco- and toxicokinetics of selected exogenous and endogenous estrogens: a review of the data and identification of knowledge gaps. Crit Rev Toxicol. 2014;44(8):696–724. doi: 10.3109/10408444.2014.930813. [DOI] [PubMed] [Google Scholar]
- 21.Gramec Skledar D, Troberg J, Lavdas J, Peterlin Masic L, Finel M. Differences in the glucuronidation of bisphenols F and S between two homologous human UGT enzymes, 1A9 and 1A10. Xenobiotica. 2014:1–9. doi: 10.3109/00498254.2014.999140. [DOI] [PubMed] [Google Scholar]
- 22.Teeguarden JG, Twaddle N, Churchwell MI, Yang X, Fisher JW, Seryak LM, et al. 24-hour human urine and serum profiles of bisphenol A: Evidence against sublingual absorption following ingestion in soup. Toxicol Appl Pharmacol. 2015 doi: 10.1016/j.taap.2015.01.009. doi:S0041-008X(15)00019-8[pii]10.1016/j.taap.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 23.Schonfelder G, Wittfoht W, Hopp H, Talsness CE, Paul M, Chahoud I. Parent bisphenol A accumulation in the human maternal-fetal-placental unit. Environ Health Perspect. 2002;110(11):A703–7. doi: 10.1289/ehp.110-1241091. doi:sc271_5_1835[pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cabaton N, Chagnon MC, Lhuguenot JC, Cravedi JP, Zalko D. Disposition and metabolic profiling of bisphenol F in pregnant and nonpregnant rats. J Agric Food Chem. 2006;54(26):10307–14. doi: 10.1021/jf062250q. [DOI] [PubMed] [Google Scholar]
- 25.Gayrard V, Lacroix MZ, Collet SH, Viguie C, Bousquet-Melou A, Toutain PL, et al. High bioavailability of bisphenol A from sublingual exposure. Environ Health Perspect. 2013;121(8):951–6. doi: 10.1289/ehp.1206339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dabek M, McCrae SI, Stevens VJ, Duncan SH, Louis P. Distribution of beta-glucosidase and beta-glucuronidase activity and of beta-glucuronidase gene gus in human colonic bacteria. FEMS Microbiol Ecol. 2008;66(3):487–95. doi: 10.1111/j.1574-6941.2008.00520.x. doi:FEM520[pii]10.1111/j.1574-6941.2008.00520.x. [DOI] [PubMed] [Google Scholar]
- 27.Plottel CS, Blaser MJ. Microbiome and malignancy. Cell Host Microbe. 2011;10(4):324–35. doi: 10.1016/j.chom.2011.10.003. doi:S1931-3128(11)00296-4[pii]10.1016/j.chom.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mose T, Mortensen GK, Hedegaard M, Knudsen LE. Phthalate monoesters in perfusate from a dual placenta perfusion system, the placenta tissue and umbilical cord blood. Reprod Toxicol. 2007;23(1):83–91. doi: 10.1016/j.reprotox.2006.08.006. doi:S0890-6238(06)00204-8[pii]10.1016/j.reprotox.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 29.Jobling S, Reynolds T, White R, Parker MG, Sumpter JP. A variety of environmentally persistent chemicals, including some phthalate plasticizers, are weakly estrogenic. Environ Health Perspect. 1995;103(6):582–7. doi: 10.1289/ehp.95103582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harris CA, Henttu P, Parker MG, Sumpter JP. The estrogenic activity of phthalate esters in vitro. Environ Health Perspect. 1997;105(8):802–11. doi: 10.1289/ehp.97105802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sohoni P, Sumpter JP. Several environmental oestrogens are also anti-androgens. J Endocrinol. 1998;158(3):327–39. doi: 10.1677/joe.0.1580327. [DOI] [PubMed] [Google Scholar]
- 32.Christen V, Crettaz P, Oberli-Schrammli A, Fent K. Some flame retardants and the antimicrobials triclosan and triclocarban enhance the androgenic activity in vitro. Chemosphere. 2010;81(10):1245–52. doi: 10.1016/j.chemosphere.2010.09.031. doi:S0045-6535(10)01046-5[pii]10.1016/j.chemosphere.2010.09.031. [DOI] [PubMed] [Google Scholar]
- 33.Millar JS. Novel benefits of peroxisome proliferator-activated receptors on cardiovascular risk. Curr Opin Lipidol. 2013;24(3):233–8. doi: 10.1097/MOL.0b013e3283613a7d. [DOI] [PubMed] [Google Scholar]
- 34.Pereira-Fernandes A, Demaegdt H, Vandermeiren K, Hectors TL, Jorens PG, Blust R, et al. Evaluation of a screening system for obesogenic compounds: screening of endocrine disrupting compounds and evaluation of the PPAR dependency of the effect. PLoS One. 2013;8(10):e77481. doi: 10.1371/journal.pone.0077481PONE-D-13-25127[pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taxvig C, Dreisig K, Boberg J, Nellemann C, Schelde AB, Pedersen D, et al. Differential effects of environmental chemicals and food contaminants on adipogenesis, biomarker release and PPARgamma activation. Mol Cell Endocrinol. 2012;361(1-2):106–15. doi: 10.1016/j.mce.2012.03.021. doi:S0303-7207(12)00215-8[pii]10.1016/j.mce.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 36.Hurst CH, Waxman DJ. Activation of PPARalpha and PPARgamma by environmental phthalate monoesters. Toxicol Sci. 2003;74(2):297–308. doi: 10.1093/toxsci/kfg145kfg145[pii]. [DOI] [PubMed] [Google Scholar]
- 37.Sarath Josh MK, Pradeep S, Vijayalekshmi Amma KS, Balachandran S, Abdul Jaleel UC, Doble M, et al. Phthalates efficiently bind to human peroxisome proliferator activated receptor and retinoid X receptor alpha, beta, gamma subtypes: an in silico approach. J Appl Toxicol. 2014;34(7):754–65. doi: 10.1002/jat.2902. [DOI] [PubMed] [Google Scholar]
- 38.Ferguson KK, Cantonwine DE, Rivera-Gonzalez LO, Loch-Caruso R, Mukherjee B, Anzalota Del Toro LV, et al. Urinary phthalate metabolite associations with biomarkers of inflammation and oxidative stress across pregnancy in Puerto Rico. Environ Sci Technol. 2014;48(12):7018–25. doi: 10.1021/es502076j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goldinger DM, Demierre AL, Zoller O, Rupp H, Reinhard H, Magnin R, et al. Endocrine activity of alternatives to BPA found in thermal paper in Switzerland. Regul Toxicol Pharmacol. 2015;71(3):453–62. doi: 10.1016/j.yrtph.2015.01.002. doi:S0273-2300(15)00003-3[pii]10.1016/j.yrtph.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 40.Gutendorf B, Westendorf J. Comparison of an array of in vitro assays for the assessment of the estrogenic potential of natural and synthetic estrogens, phytoestrogens and xenoestrogens. Toxicology. 2001;166(1-2):79–89. doi: 10.1016/s0300-483x(01)00437-1. doi:S0300-483X(01)00437-1[pii] [DOI] [PubMed] [Google Scholar]
- 41.Zhang X, Chang H, Wiseman S, He Y, Higley E, Jones P, et al. Bisphenol A disrupts steroidogenesis in human H295R cells. Toxicol Sci. 2011;121(2):320–7. doi: 10.1093/toxsci/kfr061. doi:kfr061[pii]10.1093/toxsci/kfr061. [DOI] [PubMed] [Google Scholar]
- 42.Rosenmai AK, Dybdahl M, Pedersen M, Alice van Vugt-Lussenburg BM, Wedebye EB, Taxvig C, et al. Are structural analogues to bisphenol a safe alternatives? Toxicol Sci. 2014;139(1):35–47. doi: 10.1093/toxsci/kfu030. doi:kfu030[pii]10.1093/toxsci/kfu030. [DOI] [PubMed] [Google Scholar]
- 43.Watkins DJ, Ferguson KK, Anzalota Del Toro LV, Alshawabkeh AN, Cordero JF, Meeker JD. Associations between urinary phenol and paraben concentrations and markers of oxidative stress and inflammation among pregnant women in Puerto Rico. Int J Hyg Environ Health. 2015;218(2):212–9. doi: 10.1016/j.ijheh.2014.11.001. doi:S1438-4639(14)00114-X[pii]10.1016/j.ijheh.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Veiga-Lopez A, Pennathur S, Kannan K, Patisaul HB, Dolinoy DC, Zeng L, et al. Impact of gestational bisphenol a on oxidative stress and free Fatty acids: human association and interspecies animal testing studies. Endocrinology. 2015;156(3):911–22. doi: 10.1210/en.2014-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yadav A, Kataria MA, Saini V, Yadav A. Role of leptin and adiponectin in insulin resistance. Clin Chim Acta. 2013;417:80–4. doi: 10.1016/j.cca.2012.12.007. doi:S0009-8981(12)00577-3[pii]10.1016/j.cca.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 46.Ashley-Martin J, Dodds L, Arbuckle TE, Ettinger AS, Shapiro GD, Fisher M, et al. A birth cohort study to investigate the association between prenatal phthalate and bisphenol A exposures and fetal markers of metabolic dysfunction. Environ Health. 2014;13:84. doi: 10.1186/1476-069X-13-84. doi:1476-069X-13-84[pii]10.1186/1476-069X-13-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Velez MP, Arbuckle TE, Fraser WD. Female exposure to phenols and phthalates and time to pregnancy: the Maternal-Infant Research on Environmental Chemicals (MIREC) Study. Fertil Steril. 2015;103(4):1011–20 e2. doi: 10.1016/j.fertnstert.2015.01.005. doi:S0015-0282(15)00040-0[pii]10.1016/j.fertnstert.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 48.Buck Louis GM, Sundaram R, Sweeney AM, Schisterman EF, Maisog J, Kannan K. Urinary bisphenol A, phthalates, and couple fecundity: the Longitudinal Investigation of Fertility and the Environment (LIFE) Study. Fertil Steril. 2014;101(5):1359–66. doi: 10.1016/j.fertnstert.2014.01.022. doi:S0015-0282(14)00067-3[pii]10.1016/j.fertnstert.2014.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lathi RB, Liebert CA, Brookfield KF, Taylor JA, vom Saal FS, Fujimoto VY, et al. Conjugated bisphenol A in maternal serum in relation to miscarriage risk. Fertil Steril. 2014;102(1):123–8. doi: 10.1016/j.fertnstert.2014.03.024. doi:S0015-0282(14)00265-9[pii]10.1016/j.fertnstert.2014.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sugiura-Ogasawara M, Ozaki Y, Sonta S, Makino T, Suzumori K. Exposure to bisphenol A is associated with recurrent miscarriage. Hum Reprod. 2005;20(8):2325–9. doi: 10.1093/humrep/deh888. doi:deh888[pii]10.1093/humrep/deh888. [DOI] [PubMed] [Google Scholar]
- 51.Robledo C, Peck JD, Stoner JA, Carabin H, Cowan L, Koch HM, et al. Is bisphenol-A exposure during pregnancy associated with blood glucose levels or diagnosis of gestational diabetes? J Toxicol Environ Health A. 2013;76(14):865–73. doi: 10.1080/15287394.2013.824395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Robledo CA, Peck JD, Stoner J, Calafat AM, Carabin H, Cowan L, et al. Urinary phthalate metabolite concentrations and blood glucose levels during pregnancy. Int J Hyg Environ Health. 2015;218(3):324–30. doi: 10.1016/j.ijheh.2015.01.005. doi:S1438-4639(15)00007-3[pii]10.1016/j.ijheh.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jia X, Harada Y, Tagawa M, Naito H, Hayashi Y, Yetti H, et al. Prenatal maternal blood triglyceride and fatty acid levels in relation to exposure to di(2-ethylhexyl)phthalate: a cross-sectional study. Environ Health Prev Med. 2015;20(3):168–78. doi: 10.1007/s12199-014-0440-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leclerc F, Dubois MF, Aris A. Maternal, placental and fetal exposure to bisphenol A in women with and without preeclampsia. Hypertens Pregnancy. 2014;33(3):341–8. doi: 10.3109/10641955.2014.892607. [DOI] [PubMed] [Google Scholar]
- 55.Adibi JJ, Hauser R, Williams PL, Whyatt RM, Calafat AM, Nelson H, et al. Maternal urinary metabolites of Di-(2-Ethylhexyl) phthalate in relation to the timing of labor in a US multicenter pregnancy cohort study. Am J Epidemiol. 2009;169(8):1015–24. doi: 10.1093/aje/kwp001. doi:kwp001[pii]10.1093/aje/kwp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wolff MS, Engel SM, Berkowitz GS, Ye X, Silva MJ, Zhu C, et al. Prenatal phenol and phthalate exposures and birth outcomes. Environ Health Perspect. 2008;116(8):1092–7. doi: 10.1289/ehp.11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brucker-Davis F, Wagner-Mahler K, Bornebusch L, Delattre I, Ferrari P, Gal J, et al. Exposure to selected endocrine disruptors and neonatal outcome of 86 healthy boys from Nice area (France) Chemosphere. 2010;81(2):169–76. doi: 10.1016/j.chemosphere.2010.06.068. doi:S0045-6535(10)00742-3[pii]10.1016/j.chemosphere.2010.06.068. [DOI] [PubMed] [Google Scholar]
- 58.Huang Y, Li J, Garcia JM, Lin H, Wang Y, Yan P, et al. Phthalate levels in cord blood are associated with preterm delivery and fetal growth parameters in Chinese women. PLoS One. 2014;9(2):e87430. doi: 10.1371/journal.pone.0087430PONE-D-13-36064[pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ferguson KK, McElrath TF, Meeker JD. Environmental phthalate exposure and preterm birth. JAMA Pediatr. 2014;168(1):61–7. doi: 10.1001/jamapediatrics.2013.3699. doi:1769137[pii]10.1001/jamapediatrics.2013.3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ferguson KK, McElrath TF, Ko YA, Mukherjee B, Meeker JD. Variability in urinary phthalate metabolite levels across pregnancy and sensitive windows of exposure for the risk of preterm birth. Environ Int. 2014;70:118–24. doi: 10.1016/j.envint.2014.05.016. doi:S0160-4120(14)00166-4[pii]10.1016/j.envint.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Latini G, De Felice C, Presta G, Del Vecchio A, Paris I, Ruggieri F, et al. In utero exposure to di-(2-ethylhexyl)phthalate and duration of human pregnancy. Environ Health Perspect. 2003;111(14):1783–5. doi: 10.1289/ehp.6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meeker JD, Hu H, Cantonwine DE, Lamadrid-Figueroa H, Calafat AM, Ettinger AS, et al. Urinary phthalate metabolites in relation to preterm birth in Mexico city. Environ Health Perspect. 2009;117(10):1587–92. doi: 10.1289/ehp.0800522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weinberger B, Vetrano AM, Archer FE, Marcella SW, Buckley B, Wartenberg D, et al. Effects of maternal exposure to phthalates and bisphenol A during pregnancy on gestational age. J Matern Fetal Neonatal Med. 2014;27(4):323–7. doi: 10.3109/14767058.2013.815718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tang R, Chen MJ, Ding GD, Chen XJ, Han XM, Zhou K, et al. Associations of prenatal exposure to phenols with birth outcomes. Environ Pollut. 2013;178:115–20. doi: 10.1016/j.envpol.2013.03.023. doi:S0269-7491(13)00142-5[pii]10.1016/j.envpol.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 65.Cantonwine D, Meeker JD, Hu H, Sanchez BN, Lamadrid-Figueroa H, Mercado-Garcia A, et al. Bisphenol a exposure in Mexico City and risk of prematurity: a pilot nested case control study. Environ Health. 2010;9:62. doi: 10.1186/1476-069X-9-62. doi:1476-069X-9-62[pii]10.1186/1476-069X-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao Y, Chen L, Li LX, Xie CM, Li D, Shi HJ, et al. Gender-specific relationship between prenatal exposure to phthalates and intrauterine growth restriction. Pediatr Res. 2014;76(4):401–8. doi: 10.1038/pr.2014.103. doi:pr2014103[pii]10.1038/pr.2014.103. [DOI] [PubMed] [Google Scholar]
- 67.Zhao Y, Shi HJ, Xie CM, Chen J, Laue H, Zhang YH. Prenatal phthalate exposure, infant growth, and global DNA methylation of human placenta. Environ Mol Mutagen. 2015;56(3):286–92. doi: 10.1002/em.21916. [DOI] [PubMed] [Google Scholar]
- 68.Zhang Y, Lin L, Cao Y, Chen B, Zheng L, Ge RS. Phthalate levels and low birth weight: a nested case-control study of Chinese newborns. J Pediatr. 2009;155(4):500–4. doi: 10.1016/j.jpeds.2009.04.007. doi:S0022-3476(09)00367-9[pii]10.1016/j.jpeds.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Philippat C, Botton J, Calafat AM, Ye X, Charles MA, Slama R, et al. Prenatal exposure to phenols and growth in boys. Epidemiology. 2014;25(5):625–35. doi: 10.1097/EDE.0000000000000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Burstyn I, Martin JW, Beesoon S, Bamforth F, Li Q, Yasui Y, et al. Maternal exposure to bisphenol-A and fetal growth restriction: a case-referent study. Int J Environ Res Public Health. 2013;10(12):7001–14. doi: 10.3390/ijerph10127001. doi:ijerph10127001[pii]10.3390/ijerph10127001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chou WC, Chen JL, Lin CF, Chen YC, Shih FC, Chuang CY. Biomonitoring of bisphenol A concentrations in maternal and umbilical cord blood in regard to birth outcomes and adipokine expression: a birth cohort study in Taiwan. Environ Health. 2011;10:94. doi: 10.1186/1476-069X-10-94. doi:1476-069X-10-94[pii]10.1186/1476-069X-10-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Snijder CA, Heederik D, Pierik FH, Hofman A, Jaddoe VW, Koch HM, et al. Fetal growth and prenatal exposure to bisphenol A: the generation R study. Environ Health Perspect. 2013;121(3):393–8. doi: 10.1289/ehp.1205296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee BE, Park H, Hong YC, Ha M, Kim Y, Chang N, et al. Prenatal bisphenol A and birth outcomes: MOCEH (Mothers and Children's Environmental Health) study. Int J Hyg Environ Health. 2014;217(2-3):328–34. doi: 10.1016/j.ijheh.2013.07.005. doi:S1438-4639(13)00099-0[pii]10.1016/j.ijheh.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 74.de Cock M, de Boer MR, Lamoree M, Legler J, van de Bor M. First year growth in relation to prenatal exposure to endocrine disruptors - a Dutch prospective cohort study. Int J Environ Res Public Health. 2014;11(7):7001–21. doi: 10.3390/ijerph110707001. doi:ijerph110707001[pii]10.3390/ijerph110707001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maresca MM, Hoepner LA, Hassoun A, Oberfield SE, Mooney SJ, Calafat AM, et al. Prenatal Exposure to Phthalates and Childhood Body Size in an Urban Cohort. Environ Health Perspect. 2015 doi: 10.1289/ehp.1408750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Valvi D, Casas M, Romaguera D, Monfort N, Ventura R, Martinez D, et al. Prenatal Phthalate Exposure and Childhood Growth and Blood Pressure: Evidence from the Spanish INMA-Sabadell Birth Cohort Study. Environ Health Perspect. 2015 doi: 10.1289/ehp.1408887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Valvi D, Casas M, Mendez MA, Ballesteros-Gomez A, Luque N, Rubio S, et al. Prenatal bisphenol a urine concentrations and early rapid growth and overweight risk in the offspring. Epidemiology. 2013;24(6):791–9. doi: 10.1097/EDE.0b013e3182a67822. [DOI] [PubMed] [Google Scholar]
- 78.Braun JM, Lanphear BP, Calafat AM, Deria S, Khoury J, Howe CJ, et al. Early-life bisphenol a exposure and child body mass index: a prospective cohort study. Environ Health Perspect. 2014;122(11):1239–45. doi: 10.1289/ehp.1408258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Buser MC, Murray HE, Scinicariello F. Age and sex differences in childhood and adulthood obesity association with phthalates: analyses of NHANES 2007-2010. Int J Hyg Environ Health. 2014;217(6):687–94. doi: 10.1016/j.ijheh.2014.02.005. doi:S1438-4639(14)00017-0[pii]10.1016/j.ijheh.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang Y, Meng X, Chen L, Li D, Zhao L, Zhao Y, et al. Age and sex-specific relationships between phthalate exposures and obesity in Chinese children at puberty. PLoS One. 2014;9(8):e104852. doi: 10.1371/journal.pone.0104852PONE-D-14-00549[pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang H, Zhou Y, Tang C, He Y, Wu J, Chen Y, et al. Urinary phthalate metabolites are associated with body mass index and waist circumference in Chinese school children. PLoS One. 2013;8(2):e56800. doi: 10.1371/journal.pone.0056800PONE-D-12-22796[pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Teitelbaum SL, Mervish N, Moshier EL, Vangeepuram N, Galvez MP, Calafat AM, et al. Associations between phthalate metabolite urinary concentrations and body size measures in New York City children. Environ Res. 2012;112:186–93. doi: 10.1016/j.envres.2011.12.006. doi:S0013-9351(11)00311-2[pii]10.1016/j.envres.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Trasande L, Attina TM, Sathyanarayana S, Spanier AJ, Blustein J. Race/ethnicity-specific associations of urinary phthalates with childhood body mass in a nationally representative sample. Environ Health Perspect. 2013;121(4):501–6. doi: 10.1289/ehp.1205526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Boas M, Frederiksen H, Feldt-Rasmussen U, Skakkebaek NE, Hegedus L, Hilsted L, et al. Childhood exposure to phthalates: associations with thyroid function, insulin-like growth factor I, and growth. Environ Health Perspect. 2010;118(10):1458–64. doi: 10.1289/ehp.0901331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wolff MS, Teitelbaum SL, Windham G, Pinney SM, Britton JA, Chelimo C, et al. Pilot study of urinary biomarkers of phytoestrogens, phthalates, and phenols in girls. Environ Health Perspect. 2007;115(1):116–21. doi: 10.1289/ehp.9488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xue J, Wu Q, Sakthivel S, Pavithran PV, Vasukutty JR, Kannan K. Urinary levels of endocrine-disrupting chemicals, including bisphenols, bisphenol A diglycidyl ethers, benzophenones, parabens, and triclosan in obese and non-obese Indian children. Environ Res. 2015;137:120–8. doi: 10.1016/j.envres.2014.12.007. doi:S0013-9351(14)00456-3[pii]10.1016/j.envres.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 87.Khalil N, Ebert JR, Wang L, Belcher S, Lee M, Czerwinski SA, et al. Bisphenol A and cardiometabolic risk factors in obese children. Sci Total Environ. 2014;470-471:726–32. doi: 10.1016/j.scitotenv.2013.09.088. doi:S0048-9697(13)01126-1[pii]10.1016/j.scitotenv.2013.09.088. [DOI] [PubMed] [Google Scholar]
- 88.Lee HA, Kim YJ, Lee H, Gwak HS, Park EA, Cho SJ, et al. Effect of urinary bisphenolA on androgenic hormones and insulin resistance in preadolescent girls: a pilot study from the Ewha Birth & Growth Cohort. Int J Environ Res Public Health. 2013;10(11):5737–49. doi: 10.3390/ijerph10115737. doi:ijerph10115737[pii]10.3390/ijerph10115737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang HX, Zhou Y, Tang CX, Wu JG, Chen Y, Jiang QW. Association between bisphenol A exposure and body mass index in Chinese school children: a cross-sectional study. Environ Health. 2012;11:79. doi: 10.1186/1476-069X-11-79. doi:1476-069X-11-79[pii]10.1186/1476-069X-11-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Trasande L, Attina TM, Blustein J. Association between urinary bisphenol A concentration and obesity prevalence in children and adolescents. JAMA. 2012;308(11):1113–21. doi: 10.1001/2012.jama.11461. doi:1360865[pii]10.1001/2012.jama.11461. [DOI] [PubMed] [Google Scholar]
- 91.Eng DS, Lee JM, Gebremariam A, Meeker JD, Peterson K, Padmanabhan V. Bisphenol A and chronic disease risk factors in US children. Pediatrics. 2013;132(3):e637–45. doi: 10.1542/peds.2013-0106. doi:peds.2013-0106[pii]10.1542/peds.2013-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bhandari R, Xiao J, Shankar A. Urinary bisphenol A and obesity in U.S. children. Am J Epidemiol. 2013;177(11):1263–70. doi: 10.1093/aje/kws391. doi:kws391[pii]10.1093/aje/kws391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li DK, Miao M, Zhou Z, Wu C, Shi H, Liu X, et al. Urine bisphenol-A level in relation to obesity and overweight in school-age children. PLoS One. 2013;8(6):e65399. doi: 10.1371/journal.pone.0065399PONE-D-12-32379[pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Trasande L, Sathyanarayana S, Spanier AJ, Trachtman H, Attina TM, Urbina EM. Urinary phthalates are associated with higher blood pressure in childhood. J Pediatr. 2013;163(3):747–53 e1. doi: 10.1016/j.jpeds.2013.03.072. doi:S0022-3476(13)00391-0[pii]10.1016/j.jpeds.2013.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Trasande L, Attina TM, Trachtman H. Bisphenol A exposure is associated with low-grade urinary albumin excretion in children of the United States. Kidney Int. 2013;83(4):741–8. doi: 10.1038/ki.2012.422. doi:ki2012422[pii]10.1038/ki.2012.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Trasande L, Sathyanarayana S, Trachtman H. Dietary phthalates and low-grade albuminuria in US children and adolescents. Clin J Am Soc Nephrol. 2014;9(1):100–9. doi: 10.2215/CJN.04570413. doi:CJN.04570413[pii]10.2215/CJN.04570413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Trasande L, Spanier AJ, Sathyanarayana S, Attina TM, Blustein J. Urinary phthalates and increased insulin resistance in adolescents. Pediatrics. 2013;132(3):e646–55. doi: 10.1542/peds.2012-4022. doi:peds.2012-4022[pii]10.1542/peds.2012-4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Trasande L, Attina TM. Association of Exposure to Di-2-Ethylhexylphthalate Replacements With Increased Insulin Resistance in Adolescents From NHANES 2009-2012. J Clin Endocrinol Metab. 2015;100(7):2640–50. doi: 10.1210/jc.2015-1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Volberg V, Harley K, Calafat AM, Dave V, McFadden J, Eskenazi B, et al. Maternal bisphenol a exposure during pregnancy and its association with adipokines in Mexican-American children. Environ Mol Mutagen. 2013;54(8):621–8. doi: 10.1002/em.21803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sipola-Leppanen M, Vaarasmaki M, Tikanmaki M, Matinolli HM, Miettola S, Hovi P, et al. Cardiometabolic risk factors in young adults who were born preterm. Am J Epidemiol. 2015;181(11):861–73. doi: 10.1093/aje/kwu443. doi:kwu443[pii]10.1093/aje/kwu443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lewandowski AJ, Davis EF, Yu G, Digby JE, Boardman H, Whitworth P, et al. Elevated blood pressure in preterm-born offspring associates with a distinct antiangiogenic state and microvascular abnormalities in adult life. Hypertension. 2015;65(3):607–14. doi: 10.1161/HYPERTENSIONAHA.114.04662. doi:HYPERTENSIONAHA.114.04662[pii]10.1161/HYPERTENSIONAHA.114.04662. [DOI] [PubMed] [Google Scholar]
- 102.Juonala M, Cheung MM, Sabin MA, Burgner D, Skilton MR, Kahonen M, et al. Effect of birth weight on life-course blood pressure levels among children born premature: the Cardiovascular Risk in Young Finns Study. J Hypertens. 2015;33(8):1542–8. doi: 10.1097/HJH.000000000000061200004872-201508000-00010[pii]. [DOI] [PubMed] [Google Scholar]
- 103.Hussain SM, Kahonen M, Raitakari OT, Skilton MR, Witt N, Chaturvedi N, et al. Impact of fetal growth and preterm birth on the retinal microvasculature in mid-adulthood. Microcirculation. 2015;22(4):285–93. doi: 10.1111/micc.12197. [DOI] [PubMed] [Google Scholar]
- 104.Dolinoy DC, Huang D, Jirtle RL. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc Natl Acad Sci U S A. 2007;104(32):13056–61. doi: 10.1073/pnas.0703739104. doi:0703739104[pii]10.1073/pnas.0703739104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Menale C, Piccolo MT, Cirillo G, Calogero RA, Papparella A, Mita L, et al. Bisphenol A effects on gene expression in adipocytes from children: association with metabolic disorders. J Mol Endocrinol. 2015;54(3):289–303. doi: 10.1530/JME-14-0282. doi:JME-14-0282[pii]10.1530/JME-14-0282. [DOI] [PubMed] [Google Scholar]
- 106.Gronemeyer H, Gustafsson JA, Laudet V. Principles for modulation of the nuclear receptor superfamily. Nat Rev Drug Discov. 2004;3(11):950–64. doi: 10.1038/nrd1551. doi:nrd1551[pii]10.1038/nrd1551. [DOI] [PubMed] [Google Scholar]
- 107.Ghosh JC, Yang X, Zhang A, Lambert MH, Li H, Xu HE, et al. Interactions that determine the assembly of a retinoid X receptor/corepressor complex. Proc Natl Acad Sci U S A. 2002;99(9):5842–7. doi: 10.1073/pnas.092043399092043399[pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.