Abstract
The hemiketal (HK) eicosanoids HKE2 and HKD2 are the major products resulting from the biosynthetic cross-over of the 5-lipoxygenase and cyclooxygenase-2 pathways. They are formed by activated human leukocytes ex vivo, and, therefore, may be involved in regulation of the inflammatory response as autocrine or paracrine mediators. HKE2 and HKD2 are not commercially available and, so far, no method for their total chemical synthesis has been reported. The limited availability has impeded the characterization of their biological effects. Here, we describe a method for biomimetic preparation of HKE2 and HKD2 by reaction of recombinant human cyclooxygenase-2 with chemically synthesized 5S-HETE. We found that HKE2 did not induce or inhibit the release of TNFα and IL-1β by human THP-1 monocytes and phorbol ester treatment-derived macrophages.
Keywords: lipoxygenase, cyclooxygenase, hemiketal, di-endoperoxide, macrophage, cytokine
Introduction
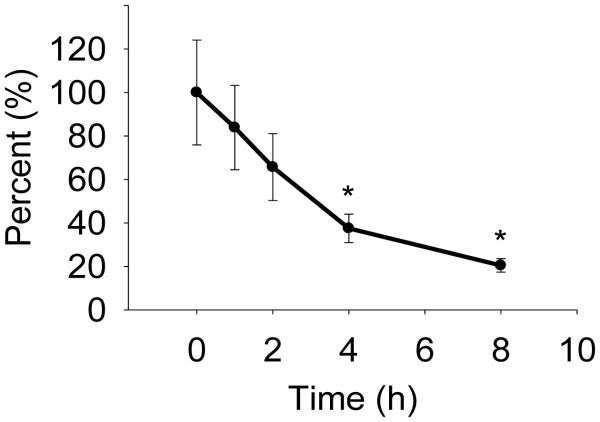
The 5-LOX product, 5S-HETE, is an efficient substrate for COX-2 thereby linking the two enzymes in a converging biosynthetic pathway (1). A di-endoperoxide is the major product resulting from this cross-over pathway (Fig. 1) (1). The reaction also yields two by-products, 5S,11R-diHETE and 5S,15R,S-diHETE that are the 5-hydroxy analogs to the 11R- and 15R,S-HETE by-products in the reaction of COX-2 with arachidonic acid (2-4). The di-endoperoxide is functionally equivalent to the prostaglandin endoperoxide PGH2 formed in the COX reaction with arachidonic acid (2). Like PGH2 the di-endoperoxide is an unstable intermediate that can undergo further enzymatic and non-enzymatic transformations (5). Treatment with redox active metal ions or hematin results in cleavage of the peroxide moieties, yielding the aldehydes 8-oxo-5-hydroxy-6E-octenoic acid, malondialdehyde, and 4-hydroxy-2E-nonenal (6). In the absence of a redox catalyst the di-endoperoxide rearranges by opening of the peroxide moieties into keto/hydroxy groups that engage in intramolecular acetal formation yielding the hemiketals HKE2 and HKD2 (7).
Fig. 1.
Consecutive oxygenation of arachidonic acid by 5-LOX and COX-2 gives a diendoperoxide as the major and 5,11- and 5,15-diHETE as by-products. Further enzymatic or non-enzymatic transformation of the di-endoperoxide gives HKD2 and HKE2. The diendoperoxide can be cleaved by FeCl2 or hematin into three aldehydes. COX-2 reacts 5S-HETE with 3 molecules of oxygen, and their incorporation into the di-endoperoxide and subsequent fate in the HKs and other products is marked by the different colors.
The di-endoperoxide is a substrate for the hematopoietic type of PGD synthase forming HKD2. Reaction with the lipocalin-type PGDS may result in HKE2 (7). Activated human leukocytes stimulated with LPS and calcium ionophore A23187 ex vivo release HKE2 and HKD2 but it is not known whether these are enzymatic or non-enzymatic rearrangement products of the COX-2 derived di-endoperoxide (7). Stimulation of human leukocytes also gives 5,11- and 5,15-diHETE as products of the 5-LOX/COX-2 biosynthetic cross-over pathway (8).
Little is known about the biological role of the 5-LOX/COX-2 cross-over pathway and its products. One of the reasons for this lack in knowledge is that the HKs are not readily available. They are not sold commercially, and their chemical synthesis has not been reported. Due to the lack of methods for the synthesis of HKs, biochemical synthesis is an adequate method to obtain HKs for biological testing. In this report we provide a detailed description of the procedure we have developed for their preparation.
The HKs have been shown to stimulate tubulogenesis in microvascular endothelial cells isolated from mouse lungs (7). The endothelial cells also migrated in response to HK treatment. Because HKs are formed by mixtures of human leukocytes in response to stimulation by LPS and calcium ionophore, we decided to test whether they can regulate cytokine release by human THP-1 monocytes and THP-1 derived macrophages.
Materials and Methods
Materials
Arachidonic acid (90%+) was obtained from NuChek Prep. (Elysian, MN). Recombinant human COX-2 was expressed and purified as described (9). THP-1 cells were from ATCC (Manassas, VA).
Synthesis of 5S-HETE
A modified procedure for synthesis and chiral resolution of 5S-HETE has been described in detail previously (6,10).
Enzymatic synthesis of HKE2 and HKD2
Recombinant human COX-2 (0.2 μM) was added to 2 ml of 100 mM Tris-HCl pH 8 containing 1 μM hematin and 0.5 mM phenol. The solution was warmed to 37°C, and 30 μg 5S-HETE were added. The reaction was kept at 37°C for 5 min followed by incubation at room temperature for 45 min. To the reaction were added 16 μl glacial acetic acid and 50 μl methanol before extraction using a preconditioned 30 mg Waters HLB cartridge. The cartridge was preconditioned by washing with 2 ml of methanol followed by 2 ml of water. Samples were loaded, washed with water, and eluted with 0.5 ml of ethyl acetate followed by 0.5 ml of methanol into a conical vial. The water bubble in the bottom of the vial after ethyl acetate elution was removed with a pointy glass syringe prior to elution of the cartridge with methanol. The solvents were evaporated under a stream of N2, and the sample was reconstituted in 100 μl of methanol for HPLC analysis. For preparative isolation of HKs 10 or 20 2 ml-reactions were conducted in parallel, and two reactions each were extracted using one HLB cartridge.
HPLC isolation of HKE2 and HKD2
RP-HPLC was performed using a Waters Symmetry C18 5 μm-column (4.6 × 250 mm) eluted with a gradient starting with acetonitrile/water/acetic acid 20/80/0.01 (v/v) to 70/30/0.01 (v/v) in 10 min at a flow rate of 0.4 ml/min. From 10 to 20 min the flow rate was held at 0.4 ml/min and then switched to 1.0 ml/min for the next 10 min before returning to the starting solvent. Elution was monitored using an Agilent 1200 diode array system programmed to record 4 wavelengths simultaneously: 205, 220, 235, and 270 nm. Products collected from RP-HPLC were evaporated under a stream of N2 to remove organic solvent, diluted to 1 ml using 0.1% acetic acid and extracted using a 30 mg Waters HLB cartridge as described above.
SP-HPLC was performed using an Agilent Zorbax RX-SIL 5 μm-column (4.6 × 250 mm) eluted with hexane/isopropanol/acetic acid 80/20/0.1 (v/v) at 1.0 ml/min flow rate and UV detection at 4 wavelengths as described above. HKE2 used for biological testing was purified by SP-HPLC prior to use in the assays.
Quantification of HKE2
HKE2 was dissolved in 150 μl acetonitrile-d3 and transferred to a 3 mm NMR tube. The sample was spiked with 0.25 μmoles CH2Cl2 dissolved in 10 μl CCl4. The sample was analyzed using standard pulse frequencies with the delay time (D1) set to 30 s to ensure complete relaxation and correct integration of the signals. NMR spectra were recorded using a Bruker DRX 600-MHz spectrometer equipped with a cryoprobe at 298 K.
Stability of HKE2
HKE2 (1 μM) was dissolved in 2.7 ml of RPMI and placed in a cell culture incubator at 37°C. At 0, 1, 2, 4, and 8 an aliquot of 250 μl was removed and extracted using a preconditioned 30 mg Waters HLB cartridge. The cartridge was eluted with methanol and evaporated. The sample was dissolved in 40 μl of acetonitrile/water (1:1, by vol.) and analyzed by LC-ESI-MS in the negative ion mode.
THP-1 monocyte treatment and analysis
THP-1 cells were maintained in RPMI 1640 medium supplemented with 10% FBS, 2 mM glutamine, 100 U/ml penicillin, and 100 mg/ml streptomycin, and grown at 37°C in 5% CO2 at constant humidity. The cells were maintained between 300,000 – 800,000 cells/ml, and subcultured every 2-3 days. Passages 5-10 and population doubling level from 10-30 were used for all the experiments. Cells were seeded in 96-well plates at a concentration of 500,000 cells/ml in complete medium (10% FBS) or deprived-medium (0.1% FBS), incubated for 24 h, and treated with 100 ng/ml LPS in the presence or absence of 1 μM of HKE2 for 5 h. Cells treated with vehicle (ethanol) were used as control. After treatment, media from 3 different wells corresponding to each treatment were pooled and kept at −80ºC until analysis. TNF-α and IL-1β were analyzed in culture media using an ELISA kit from Peprotech (Rocky Hill, NJ).
THP-1 derived macrophage treatment and analysis
Differentiation of THP-1 monocytes into macrophages was performed as previously described (11). 500,000 monocytes/ml were cultured in 96-well plates and treated with 100 ng/ml of phorbol-12-myristate-13-acetate (PMA) for 72 h. The medium was removed, and the cells were washed 3 times with PBS. The macrophages were incubated with complete or FBS-deprived medium for 24 h. The cells were treated with 1 μM of HKE2 in the presence or absence of LPS (100 ng/ml) for 5 h, and TNF-α and IL-1β were analyzed as described for monocytes.
Results and Discussion
Synthesis of 5S-HETE
5S-HETE is readily synthesized following a procedure originally developed by Corey and Hashimoto (10). The procedure uses iodolactonization of arachidonic acid to a δ-lactone for the introduction of oxygen at the 5-carbon. Base-catalyzed removal of the iodine as HI yields the 6,7-trans double bond. The lactone is opened using triethylamine in methanol to form racemic 5-HETE methyl ester. The synthesis can be performed starting from arachidonic acid of low purity (>90%). The reaction steps do not require specialized equipment and can be carried out in a standard laboratory.
We routinely use a semi-preparative Chiralpak AD column (1 cm inner diameter) operated in straight phase mode for the resolution of milligram amounts of the enantiomers of 5-HETE methyl ester per chromatographic run (12). The R- elutes before the S-enantiomer (13).
Hydrolysis of the collected 5S-HETE methyl ester is achieved by dissolving the collected enantiomer in ethanol and adding an equal volume of 15% KOH. If large enough volumes of solvents are used (ca. 1 ml each for 1 mg of 5-HETE methyl ester) the solvent mixture results in a single phase with 5-HETE methyl ester dissolved without micelle formation. This is required for efficient hydrolysis. If no micelles are formed, the hydrolysis can be complete in as fast as 1 min at room temperature. If micelles form, these may be dissolved by adding 10 or 20 vol.-% dichloromethane. Success of hydrolysis can be assessed by direct injection of a small aliquot of the reaction mixture on RP-HPLC before termination of the reaction. If hydrolysis is complete, the solution is acidified using 1 N HCl to pH = 3, evaporated from ethanol, and extracted twice using dichloromethane. It is advisable to wash the combined dichloromethane phase 2-3 times with water to remove acid in order to reduce the chance for lactonization of 5-HETE during evaporation and storage. On the other hand, the more complete the acid is removed from the dichloromethane solution the greater is the tendency of 5-HETE to act as a detergent and form an emulsion during washing. Persistent emulsions are best dealt with by centrifugation.
5-HETE is quantified by UV spectroscopy using an ε value of 25,000 M−1cm−1 at 238 nm (14). An alcoholic solution of 10 μg/ml of 5-HETE gives an absorbance of 0.75 AU at 238 nm. Acetonitrile is a good solvent for preparation and storage of 5-HETE stock solutions. It reduces the chance of esterification to the methyl or ethyl ester derivatives that can occur when stock solutions are prepared in the corresponding alcohols.
Preparation of HKs
It is advisable to use enantiomerically pure 5S-HETE because the R-enantiomer will react to some degree with COX-2 (1) and form a diastereomeric product that will be difficult to resolve from the 5S-derived products. The recombinant COX-2 enzyme can be obtained commercially. We use recombinant human COX-2 expressed in Sf9 insect cells that is purified by ease of a His6-tag that has been encoded into the cDNA following the N-terminal signal peptide cleavage site (9,15).
Small amounts of standards of HKE2 and HKD2 for MS or HPLC analyses can be prepared using a 100 μl reaction scale and containing 2 μg 5S-HETE and 200 nM COX-2. All reactions require phenol (0.5 mM) as a co-substrate in the peroxidase active site and hematin (1 μM, freshly prepared) to replenish heme in the POX active site that may have been lost during purification of the enzyme. The hemiketals can be analyzed by their molecular ion using LC-MS in the negative ion mode (m/z 399) or by characteristic fragment ions in SRM analyses (HKE2: m/z 399 -> 151; HKD2: m/z 399 -> 183) (7).
For isolation of HKs we scale up by using 2 ml reactions with 30 μg 5S-HETE substrate. We test the enzyme activity by performing pilot reactions with different amounts of enzyme to optimize conversion of 5S-HETE. We usually perform 10 or 20 parallel 2 ml reactions since increasing the volume beyond 2 ml and scaling reagents accordingly has not resulted in an increase in the absolute yield of HKs. We warm the buffer with enzyme, hematin, and phenol to 37°C and add 5S-HETE to start the reaction which is held at 37°C for 5 min (during the enzymatic reaction) and then incubated at room temperature for 45 min to allow for the rearrangement of the di-endoperoxide into the HKs (7).
HPLC isolation
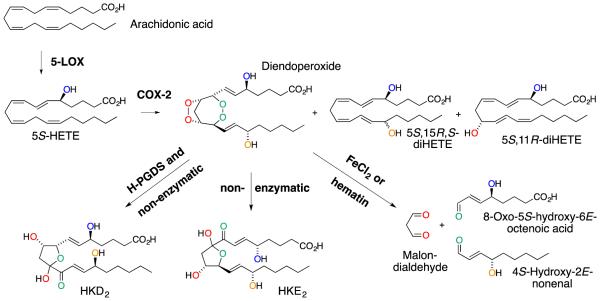
The extracted reaction mixture is first analyzed using RP-HPLC. A typical chromatogram shows elution of HKE2 and HKD2 as well as 5,11- and 5,15-diHETE ahead of the unreacted 5S-HETE substrate (Fig. 2A). We modified the flow rate for the gradient elution in order to achieve resolution of the HKs from phenol which tended to elute between HKE2 and HKD2 when using more generic conditions. The starting material and products can be differentiated by their UV spectra (Fig. 2B). HKE2 eluted as a sharp peak whereas HKD2 consistently eluted as a broad peak, and at times appeared to have several maxima. Inspection of the UV spectra showed that the HK chromophore (λmax 236 nm) was present throughout the entire peak of HKD2. The unfavorable peak shape may be related to the opening and closing of the hemiketal ring during the chromatographic run that appears to be more prevalent with HKD2 than HKE2.
Fig. 2.
RP-HPLC/diode array analysis of the reaction of 5S-HETE with COX-2. (A) RP-HPLC analysis showing the elution of HKE2, HKD2, 5,15- and 5,11-diHETE, as well as unreacted substrate, 5-HETE. The single (*) and double (**) asterisk indicate the elution of detergent from the protein solution and of phenol, respectively. (B) UV spectra for products and substrate obtained in the HPLC analysis.
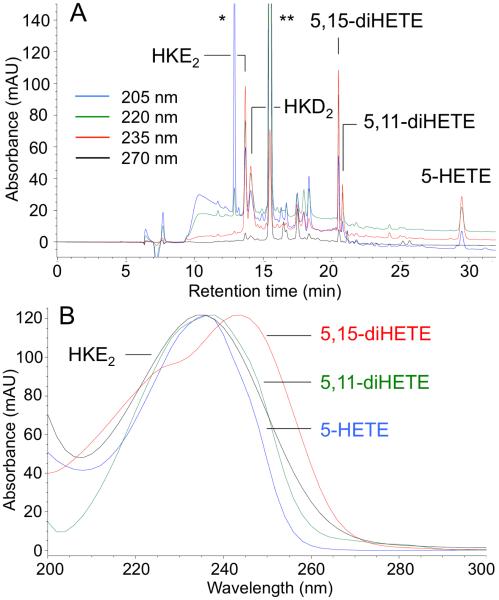
The collected products are extracted from RP-HPLC solvent and further purified to remove the co-eluting isomers using SP-HPLC (Fig. 3). The peaks collected from SP-HPLC are evaporated directly and prepared for quantification.
Fig. 3.
SP-HPLC analysis of HKE2 (A) and HKD2 (B) collected off RP-HPLC. The products were analyzed at different days which resulted in a slight discrepancy of the retention times between the chromatograms in (A) and (B).
The di-endoperoxide precursor of the HKs can readily be isolated when the reaction times with COX-2 are kept short and the ensuing extraction and HPLC analyses are performed at cold temperature (1,7). We have noticed, however, that the di-endoperoxide can break down during RP-HPLC if a “wrong” column was used. Breakdown of the diendoperoxide is apparent from an early eluting peak of 8-oxo,5S-hydroxy-6E-octenoic acid and a later peak of 4-hydroxy-2E-nonenal which elutes closely to the diendoperoxide; both aldehydes can be recognized by their typical enone chromophore with λmax around 220 nm (6,16). We are uncertain as to what factors render a column unfit for preparation of di-endoperoxide but they may include old columns that have accumulated redox-active metal ions or other reactive material.
Quantification of HKE2
HKE2 collected from SP-HPLC was quantified by 1H NMR by adding a defined amount of CH2Cl2 dissolved in CCl4 as internal standard. We chose CH2Cl2 and CCl4 because both can be readily removed from the sample by evaporation. In the NMR solvent used (acetonitrile-d3), HKE2 exists as two isomers of about similar abundance. For the signal chosen for quantification (H-7) the integrated areas of the dd signals at 6.69 and 6.82 ppm were added together. Comparison of the areas of the methylene signal of CH2Cl2 (5.42 ppm) to the signals for H-7 allowed the amount of HKE2 to be calculated. The quantified sample was then evaporated and analyzed by UV spectroscopy to determine the molar extinction coefficient ε. The procedure was performed twice using two different samples resulting in ε236 (MeOH) = 11,000 ± 1,000 M−1cm−1 for HKE2. This value should be considered preliminary since only microgram amounts of HKE2 were available for quantification. The ε of HKD2 is assumed to be similar although this was not confirmed experimentally.
Despite many trials to improve the yield of the procedure we were unable to prepare more than ca. 20 μg each of isolated and highly purified HKE2 and HKD2 starting from 500 μg 5S-HETE. This is less than 5% overall yield, and a major factor is likely the need to perform both RP- and SP-HPLC purification steps. Attempts to use only SP-HPLC as a single purification step were unsuccessful.
Stability of HKE2
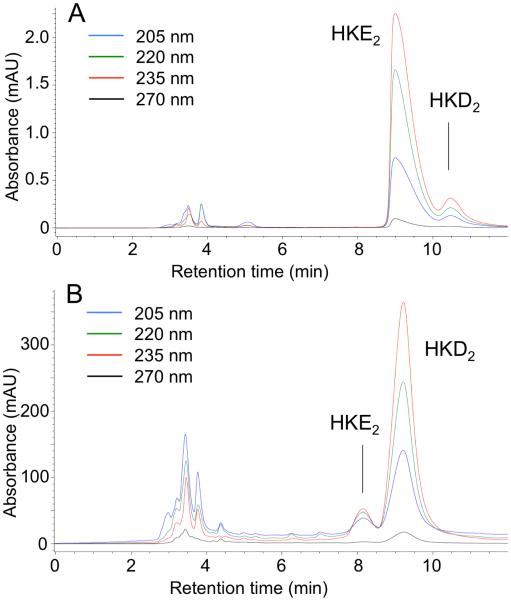
We analyzed the stability of HKE2 in RPMI cell culture medium at 37°C (Fig. 4). Aliquots were removed from the incubation at 0, 1, 2, 4, and 8 h and extracted. Remaining HKE2 was quantified using LC-MS. After 4 h there was 38% of the starting amount remaining and about 20% after 8 h. Although HKE2 undergoes transformation in buffer it is sufficiently stable for use in bioassays that require less than 4 h incubation time. For long term storage, stock solutions of HKE2 in ethanol or methanol are best kept at −80°C.
Fig. 4.
Stability of HKE2 in cell culture medium. HKE2 (1 μM) was dissolved in RPMI and incubated at 37°C. At the times indicated an aliquot was removed, extracted, and quantified by LC-MS analysis. The asterisk indicates significant difference in ANOVA analysis (p<0.05) from the starting conditions at t=0 h (n = 3 independent experiments).
Effect of HKE2 on cytokine release by THP-1 monocytes and macrophages
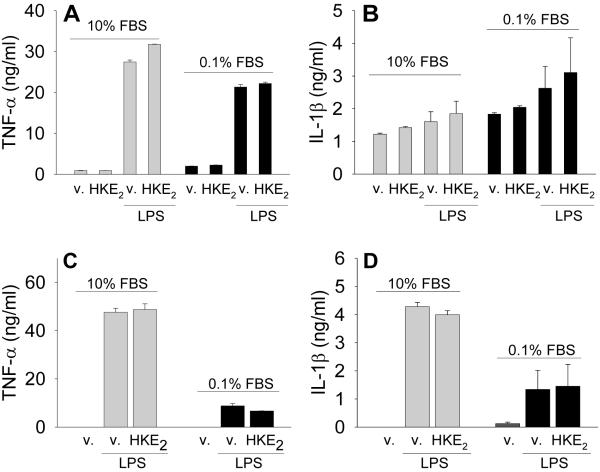
Assuming that HKs, like many other eicosanoids, could function as autocrine or paracrine mediators in inflammation (17-19), we tested whether HKE2 can regulate the release of TNF-α and IL-1β from activated monocytes and macrophages. THP-1 cells were used as monocytes or differentiated into macrophages by culturing in the presence of PMA for 72 h. We also tested the effect of FBS supplementation of the culture medium, using either 10% or 0.1% FBS. LPS stimulation resulted in the release of TNF-α into the culture medium by the macrophages whereas it had much less, if any, effect on IL-1β (Figure 5A, B). Treatment of the macrophages with 1 μM HKE2 in the absence or presence of LPS did not have an effect on TNF-α or IL-1β release (Figure 5A, B). In the monocytes HKE2 was likewise without an effect on LPS-induced release of TNF-α or IL-1β (Figure 5C, D). The effect of HKE2 in the absence of LPS could not be determined as the levels of TNF-α and IL-1β were below the limit of detection of the analysis. These data indicate that HKE2 may not function as a simple regulator of cytokine release from monocyte/macrophages and may affect other target cells or be acting in a more subtle or more complex manner than what was tested here.
Fig. 5.
Effect of HKE2 on the release of TNF-α and IL-1β by THP-1 monocytes and phorbol ester-treatment derived macrophages. Macrophages (A, B) and monocytes (C, D) were treated with or without LPS (100 ng/ml) in the absence or presence of HKE2 (1 μM) for 5 h before collection of the cell culture media for ELISA. Cells were supplemented with either 10% or 0.1% FBS during treatment. Data are expressed as mean ± SD of two (A,C) or three (B,D) independent experiments.
Conclusion
Preparation of HKE2 and HKD2 on a small scale as reference compounds in MS analyses can be achieved using all commercial reagents. Both 5S-HETE and COX-2 are available from various suppliers, and the enzymatic reaction is a simple incubation in buffer. Together with co-workers at the Chemistry Department at Vanderbilt University we have begun to develop a process for the total chemical synthesis of HKE2 and HKD2 (Boer, R.E., Schneider, C., Sulikowski, G.A. et al., unpublished). The synthesis is a multi-step process and entails reactions that could be difficult to perform in a molecular biology lab. In the absence of a straightforward chemical synthesis for preparation of HKE2 and HKD2 enzymatic synthesis with recombinant COX-2 starting from 5S-HETE is a feasible approach for preparation of small amounts of HKs for biological testing or as a standard in LC-MS analyses.
Highlights.
HKE2 and HKD2 are formed by cross-over of the 5-LOX and COX-2 pathways.
A simple method for the preparation of HKE2 and HKD2 is described.
HKE2 and HKD2 do not regulate release of TNFα and IL-1β from THP-1 cells.
Acknowledgement
This work was supported by the National Institute of General Medical Sciences of the National Institutes of Health through award R01GM076592 to CS. JAG-B is supported by a postdoctoral fellowship award by the American Heart Association (16POST30690001).
Abbreviations
- COX
cyclooxygenase
- (di)HETE
(di)hydroxyeicosatetraenoic acid
- HK
hemiketal
- LOX
lipoxygenase
- PG
prostaglandin
- PMA
phorbol-12-myristate-13-acetate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schneider C, Boeglin WE, Yin H, Stec DF, Voehler M. Convergent oxygenation of arachidonic acid by 5-lipoxygenase and cyclooxygenase-2. J. Am. Chem. Soc. 2006;128:720–72. doi: 10.1021/ja056517y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamberg M, Samuelsson B. Oxygenation of unsaturated fatty acids by the vesicular gland of sheep. J. Biol. Chem. 1967;242:5344–5354. [PubMed] [Google Scholar]
- 3.Schneider C, Boeglin WE, Prusakiewicz JJ, Rowlinson SW, Marnett LJ, Samel N, Brash AR. Control of prostaglandin stereochemistry at the 15-carbon by cyclooxygenases-1 and 2. A critical role for serine 530 and valine 349. J. Biol. Chem. 2002;277:478–485. doi: 10.1074/jbc.M107471200. [DOI] [PubMed] [Google Scholar]
- 4.Mulugeta S, Suzuki T, Tejera Hernandez N, Griesser M, Boeglin WE, Schneider C. Identification and absolute configuration of dihydroxy-arachidonic acids formed by oxygenation of 5S-HETE by native and aspirin-acetylated COX-2. J. Lipid Res. 2010;51:575–585. doi: 10.1194/jlr.M001719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nugteren DH, Hazelhof E. Isolation and properties of intermediates in prostaglandin biosynthesis. Biochim. Biophys. Acta. 1973;326:448–461. doi: 10.1016/0005-2760(73)90145-8. [DOI] [PubMed] [Google Scholar]
- 6.Griesser M, Boeglin WE, Suzuki T, Schneider C. Convergence of the 5-LOX and COX-2 pathways. Heme-catalyzed cleavage of the 5S-HETE-derived di-endoperoxide into aldehyde fragments. J. Lipid Res. 2009;50:2455–2462. doi: 10.1194/jlr.M900181-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Griesser M, Suzuki T, Tejera N, Mont S, Boeglin WE, Pozzi A, Schneider C. Biosynthesis of hemiketal eicosanoids by cross-over of the 5-lipoxygenase and cyclooxygenase-2 pathways. Proc. Natl. Acad. Sci. U.S.A. 2011;108:6945–6950. doi: 10.1073/pnas.1019473108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tejera N, Boeglin WE, Suzuki T, Schneider C. COX-2-dependent and -independent biosynthesis of dihydroxy-arachidonic acids in activated human leukocytes. J. Lipid Res. 2012;53:87–94. doi: 10.1194/jlr.M017822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schneider C, Boeglin WE, Brash AR. Identification of two cyclooxygenase active site residues, leucine-384 and glycine-526, that control carbon ring cyclization in prostaglandin biosynthesis. J. Biol. Chem. 2004;279:4404–4414. doi: 10.1074/jbc.M307431200. [DOI] [PubMed] [Google Scholar]
- 10.Corey EJ, Hashimoto S. A practical process for large-scale synthesis of (S)-5-hydroxy-6-trans-8,11,14,cis-eicosatetraenoic acid (5-HETE) Tetrahed. Lett. 1981;22:299–302. [Google Scholar]
- 11.Tsuchiya S, Kobayashi Y, Goto Y, Okumura H, Nakae S, Konno T, Tada K. Induction of maturation in cultured human monocytic leukemia cells by a phorbol diester. Cancer Res. 1982;42:1530–1536. [PubMed] [Google Scholar]
- 12.Schneider C, Yu Z, Boeglin WE, Zheng Y, Brash AR. Enantiomeric separation of hydroxy and hydroperoxy eicosanoids by chiral column chromatography. Methods Enzymol. 2007;433:145–157. doi: 10.1016/S0076-6879(07)33008-5. [DOI] [PubMed] [Google Scholar]
- 13.Schneider C, Boeglin WE, Brash AR. Enantiomeric separation of hydroxy-eicosanoids by chiral column chromatography: effect of the alcohol modifier. Anal. Biochem. 2000;287:186–189. doi: 10.1006/abio.2000.4847. [DOI] [PubMed] [Google Scholar]
- 14.Gibian MJ, Vandenberg P. Product yield in oxygenation of linoleate by soybean lipoxygenase: the value of the molar extinction coefficient in the spectrophotometric assay. Anal. Biochem. 1987;163:343–349. doi: 10.1016/0003-2697(87)90234-x. [DOI] [PubMed] [Google Scholar]
- 15.Smith T, Leipprandt J, DeWitt D. Purification and characterization of the human recombinant histidine-tagged prostaglandin endoperoxide H synthases-1 and -2. Arch. Biochem. Biophys. 2000;375:195–200. doi: 10.1006/abbi.1999.1659. [DOI] [PubMed] [Google Scholar]
- 16.Schneider C, Tallman KA, Porter NA, Brash AR. Two distinct pathways of formation of 4-hydroxynonenal. Mechanisms of nonenzymatic transformation of the 9- and 13-hydroperoxides of linoleic acid to 4-hydroxyalkenals. J. Biol. Chem. 2001;276:20831–20838. doi: 10.1074/jbc.M101821200. [DOI] [PubMed] [Google Scholar]
- 17.Smyth EM, Grosser T, Wang M, Yu Y, FitzGerald GA. Prostanoids in health and disease. J. Lipid Res. 2009;50(Suppl):S423–428. doi: 10.1194/jlr.R800094-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aoki T, Narumiya S. Prostaglandins and chronic inflammation. Trends Pharmacol. Sci. 2012;33:304–311. doi: 10.1016/j.tips.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 19.Buckley CD, Gilroy DW, Serhan CN. Proresolving lipid mediators and mechanisms in the resolution of acute inflammation. Immunity. 2014;40:315–327. doi: 10.1016/j.immuni.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]