Abstract
Heart failure (HF) is a chronic syndrome characterized by acute exacerbations. There is significant overlap between respiratory infections and exacerbation of underlying HF. Vaccination against respiratory infections in HF patients could serve as a potential cost-effective intervention to improve patients’ quality of life and clinical outcomes. The benefits of influenza vaccination in secondary prevention of ischemic heart disease have been previously studied. However, the evidence for influenza and pneumococcal vaccination specifically in the HF population is less well established. Furthermore, questions around the optimal timing, dose, frequency, and implementation strategies are largely unanswered. This review highlights the current evidence for vaccination against influenza and pneumococcal pneumonia in HF and cardiovascular disease in general. It summarizes current understanding of the pathophysiologic mechanisms in which vaccination may provide cardioprotection. Finally, it offers opportunities for further investigation of the effects of vaccination in the HF population, including basic science, translational research, and large clinical trials.
Graphical Abstract
Introduction
HF affects approximately 5.7 million American adults, with a prevalence expected to increase over time.(1–3) Despite marked pharmacologic and device-based advances for HF with reduced ejection fraction (EF) in recent years, HF is associated with significant morbidity, mortality, and financial burden. Approximately one half of patients with chronic HF have preserved EF, with a prevalence expected to increase with aging of the population.(4) Outcomes in HF with preserved EF are similarly poor as those with HF with reduced EF. Yet, there are no current chronic therapies available improve outcome in this population.
Greater than 50% of HF patients die within five years of diagnosis. HF in the US is projected to cost $69.7 billion annually by 2030.(5) There is an unmet need for additional interventions, particularly those with a favorable cost-effectiveness profile, in HF management.
Recent data support the benefits of vaccination in patients with cardiovascular disease including those with atrial arrhythmias.(6,7) However, there are limited data regarding potential benefits specifically in patients with HF.
In the present manuscript, we aim to [1] examine the mechanisms by which vaccination may improve HF outcomes, [2] summarize the available data on influenza and pneumococcal pneumonia vaccination on HF outcomes and patients with HF, and [3] propose future research to further characterize the effect of vaccination, including optimal timing and dosing strategies, to improve quality of life and clinical outcomes in the HF population.
Methods
To identify additional relevant published data, we searched MEDLINE (via PubMed) from January 1990 to July 2016 (Supplemental Material). We used Medical Subject Headings and key words, focusing on the most relevant terms for this topic. We manually searched reference lists of pertinent studies and background data to find relevant citations that our searches might have missed. All citations were imported into an EndNote X7 database. Given the limited data on respiratory vaccination in HF, our search strategy included observational/retrospective studies and randomized control trials. We required that the primary papers include data on outcomes analyses or pragmatic interventions involving the use of pneumococcal or influenza vaccination with respect to either a HF cohort and/or HF outcomes.
Respiratory Infection, HF, and Available Vaccination
It can often be difficult to distinguish forms of respiratory distress in patients with HF. Despite this, significant overlap exists between HF and respiratory disease, with 50% of HF exacerbations being triggered by respiratory infections.(8) Large HF registry data has shown respiratory infection/pneumonia to be the leading precipitating cause of HF admission, associated with high inhospital mortality.(9) Vaccination may reduce the incidence and/or severity of respiratory infection, and thereby prevent HF exacerbations, hospitalization, excess cost and associated morbidity/mortality; however, these hypotheses have not been empirically evaluated.
Major respiratory vaccination efforts in adults have focused on influenza and pneumococcal pneumonia. Available influenza/pneumococcal vaccines in the United States are listed in Table 1.
Table 1.
Summary of Influenza/Pneumococcal Vaccines Licensed for Immunization and Distribution in the United States
| Vaccination Subtype | Trade Name | Sponsor | |
|---|---|---|---|
| Influenza Vaccine-Types A and B | Trivalent, Inactivated | Afluria | CSL Limited |
| FluLaval | ID Biomedical Corp | ||
| Fluarix | GlaxoSmithKline Biologicals | ||
| Fluvirin | Novartis Vaccines and Diagnostics | ||
| Agriflu | Novartis Vaccines and Diagnostics | ||
| Fluzone | Sanofi Pasteur, Inc | ||
| Flucelvax | Novartis Vaccines and Diagnostics | ||
| Flublok | Protein Sciences Corporation | ||
| FLUAD | Novartis Vaccines and Diagnostics | ||
| Quadrivalent, Inactivated | Fluarix | GlaxoSmithKline Biologicals | |
| Fluzone | Sanofi Pasteur, Inc | ||
| FluLaval | ID Biomedical | ||
| Trivalent, Live, Intranasal | FluMist | MedImmune, LLC | |
| Quadrivalent, Live, Intranasal | FluMist | MedImmune, LLC | |
| Pneumococcal Vaccine | Pneumococcal Vaccine, Polyvalent | Pneumovax 23 | Merck & Co, Inc |
| Pneumococcal 7-valent Conjugate Vaccine | Prevnar | Wyeth Pharmaceuticals Inc | |
| Pneumococcal 13-valent Conjugate Vaccine | Prevnar 13 | Wyeth Pharmaceuticals Inc |
Influenza infection is a common illness with substantial morbidity and mortality. Vaccination with inactivated, influenza vaccination is estimated to have prevented approximately 1.5 million cases and 65,000 hospitalizations in the 2014–2015 influenza season.(10) Inactivated influenza vaccination (IIV) formulations can differ in the amount of the glycoprotein hemagglutinin (a polysaccharide found on cell membranes) contained in the vaccine. The high dose, IIV3-HD vaccine, contains 60ug of hemagglutinin compared to 15ug in the IIV3-SD standard dose. Hemagglutinin levels correlate with immunogenicity.(11,12)
Streptococcus pneumoniae accounts for approximately 400,000 hospitalizations annually in the US with a high fatality rate.(13) There is an increased risk of pneumococcal pneumonia in the post-influenza illness state, through suspected synergistic mechanisms (14,15) Major forms of pneumococcal vaccination in the US include the pneumococcal polysaccharide 23-valent vaccine (Pneumovax or PPSV 23) and the pneumococcal 13-valent conjugate Vaccine (Prevnar or PCV13), each with different populations in which they are recommended.(16,17)
Current Respiratory Vaccination Guidelines In HF
Guideline recommendations for respiratory vaccination in a HF cohort are limited. The 2005 CDC report recommended routine yearly, inactivated influenza vaccination in adults with chronic cardiovascular disease, including HF.(18) These recommendations are supported by major cardiology societies (Class I, Level B).(19,20) The Heart Failure Society of America recommends yearly influenza vaccination specifically in HF patients without contraindications (Level B), as does the European Society of Cardiology.(21,22) Full respiratory vaccination guidelines in cardiovascular disease are listed in Table 2.
Table 2.
Current Guideline Recommendations on Respiratory Vaccination in Cardiovascular Disease
| Society | Report | Recommendation |
|---|---|---|
| Centers For Disease Control (CDC)/Advisory Committee on Immunization Practices (ACIP) | CDC Website | Yearly vaccination with inactivated influenza vaccination for “adults and children who have chronic disorders of the pulmonary or cardiovascular systems” Pneumococcal polysaccharide vaccination for all adults >65 and earlier in “high risk immunocompetent patients such as those with chronic cardiovascular disease (except hypertension)” |
| American Heart Association/American College of Cardiology Foundation (AHA/ACCF) | 2011 Secondary Prevention and Risk Reduction Therapy for Patients with Coronary and other Atherosclerotic Vascular Disease | “Patients with cardiovascular disease should have an annual influenza vaccination “(Class I, Level B) |
| 2013 Guideline for the Management of HF | “Secondary prevention interventions (e.g, lipids, smoking cessation, influenza and pneumococcal vaccines)” * | |
| Heart Failure Society of America (HFSA) | 2010 Comprehensive HF Practice Guideline | “Pneumococcal vaccine and annual influenza vaccination are recommended in all patients with HF in the absence of known contraindications “(Level B) |
| European Society of Cardiology (ESC) | European Guidelines on cardiovascular disease prevention in clinical practice (Version 2012) | “Annual influenza vaccinations are recommended for patients with established cardiovascular disease.” |
Mentioned in Table 34: Plan of Care for Patients With Chronic HF - level of evidence not included.
Mechanisms of Proposed Cardiovascular Protection
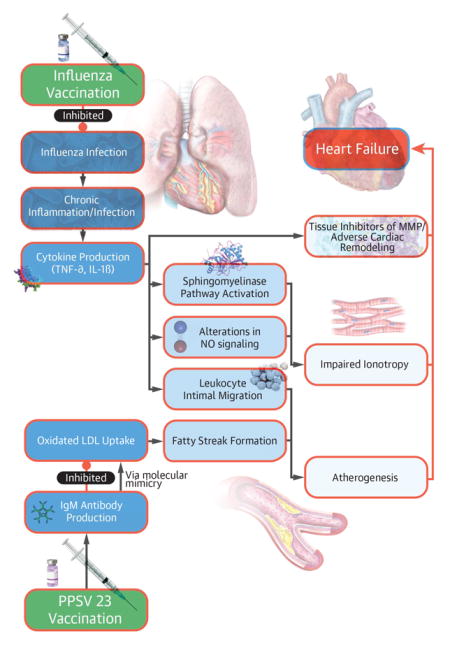
Prior research has investigated the molecular mechanisms of cardioprotection from respiratory vaccination. Major mechanisms for vaccine-induced cardioprotection are shown in the Central Illustration.
Central Illustration. Proposed Cardioprotective Mechanisms of Vaccination.
Pathophysiologic mechanisms involved in vaccination-induced cardioprotection include inhibition of oxidized low-density lipoprotein uptake by molecular mimicry from pneumococcal vaccination-induced antibodiy production and the attenuation of chronic inflammation, a pro-atherogenic process, in influenza vaccination. TNF-a = tumor necrosis factor-alpha, IL-1B = interleukin 1-beta, LDL = low-density lipoprotein, MMP = matrix metalloproteinases, NO = nitric oxide.
Respiratory infection induced inflammatory propagation may accelerate atherogenesis and impair ionotropy. Pro-inflammatory cytokines, including interleukins, tumor necrosis factor-alpha (TNF-α) and C-reactive protein up-regulate the expression of cell adhesion molecules on the endothelial surface, promoting transmigration of leukocytes into the vascular intima. This is a necessary process for lipoprotein oxidation, part of the atherogenic cascade. (23,24)
The production of TNF-α and interleukin-1-beta during acute illness can independently depress myocyte contractility.(25–29). Specifically, mechanisms include activation of a sphingomyelinase pathway and alter a nitric oxide pathway, which impairs the beta-adrenergic responsiveness of cardiac myocytes.(28,30–32) Sustained cytokine expression can lead to adverse myocardial remodeling and excess production of tissue inhibitors of matrix metalloproteinases. In murine models, inoculation of atherosclerotic apolipoprotein-E (apoE)–deficient mice with influenza A results in an influx of inflammatory cells, fibrin deposition, and thrombosis.(33) These processes have been linked to left ventricular dilatation and increases in myocardial collagen content, contributing to the HF phenotype. (28). Influenza vaccination has been theorized to prevent the adverse impact of infection/inflammation on myocardial contractility, fibrosis, and atherogenesis.(34,35)
The conjugated pneumococcal polysaccharide vaccination directly may inhibit the formation of atherogenesis via impairing low-density lipoprotein (LDL) oxidation. In murine models, pneumococcal vaccination reduced aortic root atherosclerosis by 40% at 30 weeks by a process thought to involve molecular mimicry.(36,37)
Pneumococcal vaccination leads to the production of IgM antibodies that share binding sites with naturally occurring anti-oxLDL antibodies. Specifically, both antibodies recognize the phosphorylcholine epitope on oxidized LDL. Competitive inhibition of anti-oxLDL – phosphorylcholine binding may slow the macrophage uptake of oxidized LDL, a process upstream of foam cell and plaque formation. (36,37) Data is promising but currently limited to a few studies in murine models and has not been verified in clinical research.
A direct link between vaccination-induced reduction in atherogenesis and the HF phenotype is not yet clearly established, though it would be theorized to reduce the incidence and progression of ischemic cardiomyopathy. Given these proposed mechanisms, further investigation should be conducted to understand differential responses to vaccination in those with ischemic vs. non-ischemic cardiomyopathy.
Respiratory Vaccination Rates in HF
Despite health campaigns and media attention aimed at improving vaccination rates, the rate of respiratory vaccination in patients with HF remains low.(38) A prospective analysis at Jackson Memorial Hospital in Miami, FL showed baseline influenza and pneumococcal vaccination rates to be 28.3% and 30.7% respectively in a primarily indigent population with reduced left ventricular EF. Despite enrollment in an outpatient HF disease management program, 18% of this population refused influenza vaccination.(38) The most common reason for patient refusal was fear that vaccination would cause influenza illness.
Recent evidence from the Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) trial found similar vaccination rates in a chronic HF population with reduced EF.(39) This post-hoc analysis found influenza vaccination rates of 21% in the overall study cohort of over 8,000 participants across North and South America, Asia, and Europe. Vaccination rates in the United States were 55%, significantly higher than the study average, but over 20% lower than countries such as Great Britain, Netherlands, and Belgium.(39) Predictors of vaccination included older age, white race, and interestingly, lower NYHA functional class.
Influenza Vaccination and HF Outcomes
Prior data highlight the adverse effects of pulmonary infections on patients with cardiovascular (CV) disease and suggest the potential utility of vaccination in improving outcomes. Major trials have focused primarily an acute coronary syndrome (ACS) population; HF outcomes in these trials are limited. A summary of major findings in randomized control trials in cardiovascular disease is listed in Table 3.
Table 3.
Randomized Control Efficacy Trial Evidence for Respiratory Vaccination in Cardiovascular Disease
| Study | Total n | Country | Population | Intervention | Control | Primary Outcome Variable | Results |
|---|---|---|---|---|---|---|---|
| FLUVACS (2004) | 301 | Argentina | Inpatients-ACS or planned PCI | Trivalent, inactivated influenza vaccination | No vaccination | CV death (12 months) | RR 0.34 (95% CI 0.17–0.71) P = 0.002 |
| FLUCAD (2008) | 658 | Poland | Outpatients-angiographic confirmed CAD | Trivalent, inactivated influenza vaccination | No vaccination | CV death (12 months) | HR 1.06 (95% CI: 0.15–7.56) P = 0.95 |
| Phrommintikul et al (2011) | 439 | Thailand | Inpatients-ACS within 8 weeks | Trivalent, inactivated influenza vaccination | No vaccination | Composite major CV events (death, hospitalization for ACS, HF, and stroke) (12 months) | HR 0.70 (95% CI 0.57–0.86) P = 0.004 |
| Van Erman et. Al (2013) | 28 | United States | Outpatients with HF | Double Dose influenza vaccination * | Standard dose influenza vaccination * | Antibody production by hemagglutinin inhibition assay (2&4 weeks) | 3.3 vs. 1.6 for A/H3N2, P < 0.001 1.9 vs 1.1 for A/H1N1, P = 0.009 1.7 vs 1 for B/H1N1 P = 0.02 |
Standard dose vaccination contains 15 μg hemagglutinin/strain and double dose contains 30 μg hemagglutinin/strain.
The Flu Vaccination Acute Coronary Syndromes (FLUVACS) study randomized 301 patients to receive the influenza vaccine versus no vaccination in patients admitted for myocardial infarction (MI) or planned percutaneous coronary intervention (PCI) in Argentina. Subgroup analysis did not investigate the HF population specifically. The primary endpoint of cardiovascular mortality was lower in the vaccination group compared to controls.(40) Notably, fatal and non-fatal HF events were zero in both the vaccination and control groups. Reasons for low event rates include a relatively short follow-up (12 months) and small sample size.
Similarly, the Influenza Vaccination In Secondary Prevention From Coronary Ischemic Events In Coronary Artery Disease (FLUCAD) trial showed a reduction in ischemic events in 658 Polish patients with known coronary artery disease (CAD). (41) FLUCAD excluded patient with NYHA II/IV HF. The prevalence of HF was only 12.9% and 15.9% in the vaccination and placebo groups respectively. Again, no fatal or non-fatal HF events were noted in the two groups. Limited follow-up and low incidence of influenza illness in Poland during the 2004/2005 season may have contributed to the low events rates. High rates of antigenic similarity between the trivalent vaccine used in the trial and the influenza strains isolated in the community (59% similar) may have contributed to a decreased overall influenza incidence.
Another randomized placebo-controlled trial of 439 post-ACS patients found no difference in cardiovascular death rates, though significant benefit in the vaccine group on the composite secondary outcome of all-cause mortality and hospitalization for ACS, HF, or stroke.(42) There was no difference in HF hospitalizations (1.8 vs. 4.6%, RR 0.9 (0.49–1.01), P = 0.111.) A 2013 meta-analysis of six trials that followed 6735 patients for a mean duration of 8 months supported the findings of Phrommintikul et. al. (43) Only two of the included trials had >0 events for fatal and non-fatal HF, with both failing to show significant reductions in the vaccination group.
Large epidemiological studies pooling managed care data support vaccination-induced prevention of HF hospitalization in elderly.(44–46) A study of 140,000 patients in the 1998–1999 and 1999–2000 influenza seasons found a 19% overall reduction in CV hospitalization in the vaccinated group compared to controls. There were 72 fewer hospitalizations for HF in the vaccination cohort during the 1998–1999 season (absolute risk reduction 0.3%), though this trend was not seen in the 1999–2000 season. (44) Davis et. al. found a stronger relationship in HF hospitalization prevention (OR 0.8, CI 0.7–0.9). (45) Prevention of HF admissions are estimated to have significant direct medical cost savings, up to $235 per individual vaccinated. (46)
Influenza Vaccination In a HF Population
The impact of vaccination in a HF population is incompletely studied. Most vaccination trials either have not enrolled HF patients or not assessed impact in a HF cohort sub-study.
Recent evidence from PARADIGM-HF found that influenza vaccination was associated with a reduced risk of all-cause mortality in a cohort of patients with reduced LVEF (hazard ratio: 0.81, 95% CI: 0.67–0.97).(39) In propensity adjusted models, the composite outcome of CV death and HF hospitalization did not reach statistical significance, though there was a signal toward clinical benefit in the vaccinated group. Perhaps limited long-term follow-up could explains these findings, as a cohort analysis of 1964 HF patients found no association with influenza vaccine and 1-year all cause mortality, but the relationship showed significant benefit in the vaccination group when 4-year mortality was used as the clinical endpoint.(47) A recent self-controlled case series of HF patients (regardless of LVEF) utilized complex regression to compare individuals in vaccination years to themselves in adjacent non-vaccination years.(48) Acknowledging the risk for confounding and seasonality in this analysis, the study found influenza vaccination was associated with reductions in all cause hospitalization and cardiovascular hospitalization. No randomized control trial data comparing influenza vaccination to placebo exists exclusively in a HF population. No randomized control trial data comparing influenza vaccination to placebo exists exclusively in a HF population.
What is the appropriate dose of influenza vaccine in HF?
Current CDC guidelines offer either an age-appropriate standard-dose IIV or high-dose IIV in patients 65 years or older.(49) Recent evidence has suggested a clinical benefit of high dose vaccination in this age group.(50) Patients with HF may have decreased immune responses to standard dose vaccination, suggesting the possible utility of high-dose vaccination in this population.(51) Higher immunogenicity, quantified by hemagglutinin inhibition assay titer levels, has been seen in patients receiving the high-dose vaccine. (11,12)
Given the significant morbidity associated with HF, questions remain as to whether this population would benefit from high-dose IIV. In one randomized pilot study of 28 patients with HF, individuals received either standard dose vaccination (15ug hemagglutinin) versus double dose vaccination (30ug hemagglutinin). Double dose vaccination produced significantly higher immunogenicity. The study did not assess dose response with respect to clinical outcomes, such as laboratory-confirmed influenza or HF exacerbation. (52) Unexpectedly. stratification by age did not show significant differences in immunogenicity for patients > 70 years of age. Our understanding of influenza vaccination dose variation in HF is currently limited to a single small study, though a larger, randomized, clinical trial is currently enrolling.
Pneumococcal Vaccination and Cardiovascular Outcomes
Acute bacterial pneumonia has been linked to HF and increased cardiovascular events. A small case series found that 14% of patients admitted for pneumococcal pneumonia had new or worsening HF at the time of admission. (53) As a recent meta-analysis found no suitable randomized control trial evidence on the effects of pneumococcal vaccination on cardiovascular events, our current understanding of this potential relationship is limited to observational and retrospective analyses. (54)
A large retrospective case-control study from Canada evaluated 20,000 inpatients from 1997–2003 at high risk for coronary events. High-risk status was defined as preexisting hypertension, diabetes mellitus, or dyslipidemia in men >45 years and women> 50 years.(55) Patients vaccinated >2 years prior to admission had significantly lower rates of myocardial infarction (MI). Rates of HF were not assessed between groups. Clinically important confounding variables such as smoking status, medication use, obesity, diet, and exercise were not controlled for in the analysis. In contrast, an analysis of the California Men’s Health Study found no difference in the incidence of MI and stroke in patients vaccinated with PPSV23 versus those not vaccinated.(56) Findings were controlled for dietary, lifestyle and disease state factors. The incidence of HF was actually lower in the unvaccinated group. Neither of these analyses specifically enrolled a specific HF population. The paucity of high-level evidence on the clinical outcomes associated with pneumococcal vaccination in HF presents an opportunity for further investigation.
Future Directions
While preliminary evidence suggests protective effect of vaccination in HF patients, data are limited and not systematically or consistently validated. Significant opportunities, across basic, translational and clinical research, exist for further study (Table 4).
Table 4.
Opportunities for Future Study Regarding Respiratory Vaccination in HF
| Future Clinical Studies | Description |
|---|---|
| Observational Analysis: Understanding Vaccination Rates |
|
| RCT: Vaccination and Outcomes |
|
| RCT: Dose and Timing |
|
| Observational/RCT: Implementation strategies |
|
| Basic Science and Translational Studies |
|
A deeper understanding of current vaccination practices within the HF population is necessary to guide population-level interventions aimed at improving vaccination rates. Currently, our understanding of vaccination rates in HF is limited to a small prospective analysis and trial subanalysis.(38,39) This data suggests differences in vaccination rates between different demographical groups (race, sex, socioeconomic status). Further understanding of disparities in vaccination rates should involve the use of large registry data, which would also allow for a temporal outlook. Differential rates of vaccination by cardiac and non-cardiac comorbidities (diabetes, chronic obstructive pulmonary disease) should be accessed given guidelines for vaccination in these populations.(16,17)
It may be time for a large, multicenter trial to understand the clinical outcomes of respiratory vaccination in the HF population. Previous observational analyses have focused on HF with reduced EF. The reality is that HF is a broad clinical syndrome with multiple variants, each with their own manifestations, treatment, and natural histories. The trial should enroll a broader study population, inclusive of reduced and preserved EF, ischemic and non-ischemic cardiomyopathy. Authors propose that as atherogenesis prevention is a major proposed cardioprotective mechanism of vaccination, patients with ischemic cardiomyopathy and reduced ejection fraction may derive the greatest benefit from vaccination. Primary endpoints could include a composite of cardiovascular mortality and HF hospitalizations. Secondary outcomes measures could include total number of hospitalizations, HF readmissions, and measures of cardiovascular morbidity, functional status, and quality of life.
A comparison of outcomes in HF patients randomized to receive the standard dose vs. high dose influenza vaccine is already being undertaken in scientifically rigorous ways. The VACC-HeFT feasibility trial plans to assess humoral response and secondarily all-cause hospitalization. The large scale, randomized clinical trial, INVESTED, plans to enroll 9,300 patients from North America, observed over multiple influenza seasons. The trial will randomize patients to receive standard vs. high dose influenza vaccination. Primary endpoints include all-cause mortality and cardiopulmonary hospitalizations in patients with recent MI or HF. Such rigorous designs should be employed to answer other pertinent questions, such as the optimal timing of vaccination and the need for revaccination in pneumococcal disease prevention.
Patients with HF interact with healthcare systems in multiple settings in many places. This dispersion makes implementation of vaccination campaigns a challenge, and raises questions about the optimal timing, setting and personnel needed to drive high rates of vaccination. Designing in-hospital based vaccination systems and campaigns may provide epidemiological advantages. (57) Given data that many patients seek specialists even for preventative care, heart failure disease management programs may be the optimal setting for vaccination enforcement.(58) As more evidence emerges, patient and provider-specific incentive structures established in other clinical settings should be developed and trialed in HF. (59,60) While empiric validation of these implementation strategies would have a high initial investment, there is the potential for large impacts on the way we deliver cost–effective preventative care in HF that would likely result in net value.
Conclusion
Influenza and pneumococcal pneumonia are two common infectious conditions with significant associated morbidity and mortality. There are proposed mechanisms that contribute to the HF phenotype in patients with bacterial and viral infection. There is a suggestion that influenza and pneumococcal pneumonia vaccination may have a protective role in patients with HF. Vaccination represents a low-cost intervention that may be able to preventing the significant morbidity, mortality, and system-wide cost associated with HF. Large-scale, clinical trial data are limited in determining the true risks and benefits of vaccination specifically in the HF population. There is significant opportunity for broad areas of further investigation in determining how vaccination can improve outcomes and quality of life in patients with HF.
Supplementary Material
Acknowledgments
Funding: This manuscript was prepared without additional external funding.
Footnotes
Relationships with Industry:
ASB: No relationships to disclose.
ADD: Research support from the American Heart Association, Amgen, and Novartis.
AFH: Research support from the American Heart Association (significant); Amgen (modest), NHLBI (significant), and Novartis (modest
RJM: Research support from the National Institutes of Health, Amgen, AstraZeneca, Bristol-Myers Squibb, GlaxoSmithKline, Gilead, Medtronic, Novartis, Otsuka, and ResMed; honoraria from HeartWare, Janssen, Luitpold Pharmaceuticals, Novartis, ResMed, and Thoratec/St Jude; and has served on an advisory board for Luitpold Pharmaceuticals, Inc and Boehringer Ingelheim.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933–44. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 3.Braunwald E. Heart failure. JACC Heart failure. 2013;1:1–20. doi: 10.1016/j.jchf.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Steinberg BA, Zhao X, Heidenreich PA, et al. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation. 2012;126:65–75. doi: 10.1161/CIRCULATIONAHA.111.080770. [DOI] [PubMed] [Google Scholar]
- 5.Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circulation Heart failure. 2013;6:606–19. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ajani UA, Ford ES, Mokdad AH. Examining the coverage of influenza vaccination among people with cardiovascular disease in the United States. American heart journal. 2005;149:254–9. doi: 10.1016/j.ahj.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 7.Chang TY, Chao TF, Liu CJ, et al. The association between influenza infection, vaccination, and atrial fibrillation: A nationwide case-control study. Heart Rhythm. 2016 doi: 10.1016/j.hrthm.2016.01.026. [DOI] [PubMed] [Google Scholar]
- 8.Alon D, Stein GY, Korenfeld R, Fuchs S. Predictors and outcomes of infection-related hospital admissions of heart failure patients. PloS one. 2013;8:e72476. doi: 10.1371/journal.pone.0072476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fonarow GC, Abraham WT, Albert NM, et al. Factors identified as precipitating hospital admissions for heart failure and clinical outcomes: findings from OPTIMIZE-HF. Archives of internal medicine. 2008;168:847–54. doi: 10.1001/archinte.168.8.847. [DOI] [PubMed] [Google Scholar]
- 10.Control CfD. Estimated Influenza Illnesses and Hospitalizations Averted by Vaccination — United States, 2014–15. Influenza Season. 2015 [PMC free article] [PubMed] [Google Scholar]
- 11.McElhaney JE, Herre JM, Lawson ML, Cole SK, Burke BL, Hooton JW. Effect of congestive heart failure on humoral and ex vivo cellular immune responses to influenza vaccination in older adults. Vaccine. 2004;22:681–8. doi: 10.1016/j.vaccine.2003.08.032. [DOI] [PubMed] [Google Scholar]
- 12.Vu T, Farish S, Jenkins M, Kelly H. A meta-analysis of effectiveness of influenza vaccine in persons aged 65 years and over living in the community. Vaccine. 2002;20:1831–6. doi: 10.1016/s0264-410x(02)00041-5. [DOI] [PubMed] [Google Scholar]
- 13.Epidemiology and Prevention of Vaccine-Preventable Diseases. 13. 2015. [Google Scholar]
- 14.Metersky ML, Masterton RG, Lode H, File TM, Jr, Babinchak T. Epidemiology, microbiology, and treatment considerations for bacterial pneumonia complicating influenza. International Journal of Infectious Diseases. 16:e321–e331. doi: 10.1016/j.ijid.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Shahangian A, Chow EK, Tian X, et al. Type I IFNs mediate development of postinfluenza bacterial pneumonia in mice. The Journal of clinical investigation. 2009;119:1910–20. doi: 10.1172/JCI35412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tomczyk S, Bennett NM, Stoecker C, et al. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged >/=65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Morbidity and mortality weekly report. 2014;63:822–5. [PMC free article] [PubMed] [Google Scholar]
- 17.Kobayashi M, Bennett NM, Gierke R, et al. Intervals Between PCV13 and PPSV23 Vaccines: Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Morbidity and mortality weekly report. 2015;64:944–7. doi: 10.15585/mmwr.mm6434a4. [DOI] [PubMed] [Google Scholar]
- 18.Harper SA, Fukuda K, Uyeki TM, Cox NJ, Bridges CB. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recommendations and reports : Morbidity and mortality weekly report Recommendations and reports/Centers for Disease Control. 2004;53:1–40. [PubMed] [Google Scholar]
- 19.Smith SC, Jr, Benjamin EJ, Bonow RO, et al. AHA/ACCF Secondary Prevention and Risk Reduction Therapy for Patients with Coronary and other Atherosclerotic Vascular Disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011;124:2458–73. doi: 10.1161/CIR.0b013e318235eb4d. [DOI] [PubMed] [Google Scholar]
- 20.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 21.Lindenfeld J, Albert NM, Boehmer JP, et al. HFSA 2010 Comprehensive Heart Failure Practice Guideline. Journal of cardiac failure. 2010;16:e1–194. doi: 10.1016/j.cardfail.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Perk J, De Backer G, Gohlke H, et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012): The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts) Atherosclerosis. 2012;223:1–68. doi: 10.1016/j.atherosclerosis.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 23.Libby P, Theroux P. Pathophysiology of coronary artery disease. Circulation. 2005;111:3481–8. doi: 10.1161/CIRCULATIONAHA.105.537878. [DOI] [PubMed] [Google Scholar]
- 24.Berliner JA, Subbanagounder G, Leitinger N, Watson AD, Vora D. Evidence for a Role of Phospholipid Oxidation Products in Atherogenesis. Trends in Cardiovascular Medicine. 11:142–147. doi: 10.1016/s1050-1738(01)00098-6. [DOI] [PubMed] [Google Scholar]
- 25.Danesh J, Collins R, Appleby P, Peto R. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. Jama. 1998;279:1477–82. doi: 10.1001/jama.279.18.1477. [DOI] [PubMed] [Google Scholar]
- 26.Mendall MA, Patel P, Asante M, et al. Relation of serum cytokine concentrations to cardiovascular risk factors and coronary heart disease. Heart (British Cardiac Society) 1997;78:273–7. doi: 10.1136/hrt.78.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar A, Thota V, Dee L, Olson J, Uretz E, Parrillo JE. Tumor necrosis factor alpha and interleukin 1beta are responsible for in vitro myocardial cell depression induced by human septic shock serum. The Journal of experimental medicine. 1996;183:949–58. doi: 10.1084/jem.183.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mann DL. Inflammatory mediators and the failing heart: past, present, and the foreseeable future. Circulation research. 2002;91:988–98. doi: 10.1161/01.res.0000043825.01705.1b. [DOI] [PubMed] [Google Scholar]
- 29.Levine B, Kalman J, Mayer L, Fillit HM, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. The New England journal of medicine. 1990;323:236–41. doi: 10.1056/NEJM199007263230405. [DOI] [PubMed] [Google Scholar]
- 30.Oral H, Dorn GW, Mann DL. Sphingosine Mediates the Immediate Negative Inotropic Effects of Tumor Necrosis Factor-α in the Adult Mammalian Cardiac Myocyte. Journal of Biological Chemistry. 1997;272:4836–4842. doi: 10.1074/jbc.272.8.4836. [DOI] [PubMed] [Google Scholar]
- 31.Gulick T, Chung MK, Pieper SJ, Lange LG, Schreiner GF. Interleukin 1 and tumor necrosis factor inhibit cardiac myocyte beta-adrenergic responsiveness. Proceedings of the National Academy of Sciences. 1989;86:6753–6757. doi: 10.1073/pnas.86.17.6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balligand JL, Ungureanu D, Kelly RA, et al. Abnormal contractile function due to induction of nitric oxide synthesis in rat cardiac myocytes follows exposure to activated macrophage-conditioned medium. The Journal of clinical investigation. 1993;91:2314–9. doi: 10.1172/JCI116461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Lenten BJ, Wagner AC, Nayak DP, Hama S, Navab M, Fogelman AM. High-density lipoprotein loses its anti-inflammatory properties during acute influenza a infection. Circulation. 2001;103:2283–8. doi: 10.1161/01.cir.103.18.2283. [DOI] [PubMed] [Google Scholar]
- 34.Carty CL, Heagerty P, Nakayama K, et al. Inflammatory response after influenza vaccination in men with and without carotid artery disease. Arteriosclerosis, thrombosis, and vascular biology. 2006;26:2738–44. doi: 10.1161/01.ATV.0000248534.30057.b5. [DOI] [PubMed] [Google Scholar]
- 35.Posthouwer D, Voorbij HA, Grobbee DE, Numans ME, van der Bom JG. Influenza and pneumococcal vaccination as a model to assess C-reactive protein response to mild inflammation. Vaccine. 2004;23:362–5. doi: 10.1016/j.vaccine.2004.05.035. [DOI] [PubMed] [Google Scholar]
- 36.Binder CJ, Horkko S, Dewan A, et al. Pneumococcal vaccination decreases atherosclerotic lesion formation: molecular mimicry between Streptococcus pneumoniae and oxidized LDL. Nature medicine. 2003;9:736–43. doi: 10.1038/nm876. [DOI] [PubMed] [Google Scholar]
- 37.Caligiuri G, Khallou-Laschet J, Vandaele M, et al. Phosphorylcholine-targeting immunization reduces atherosclerosis. Journal of the American College of Cardiology. 2007;50:540–6. doi: 10.1016/j.jacc.2006.11.054. [DOI] [PubMed] [Google Scholar]
- 38.Hebert K, Marzouka G, Arcement L, et al. Prevalence of vaccination rates in systolic heart failure: a prospective study of 549 patients by age, race, ethnicity, and sex in a heart failure disease management program. Congestive heart failure (Greenwich, Conn) 2010;16:278–83. doi: 10.1111/j.1751-7133.2010.00190.x. [DOI] [PubMed] [Google Scholar]
- 39.Vardeny O, Claggett B, Udell JA, et al. Influenza Vaccination in Patients With Chronic Heart Failure: The PARADIGM-HF Trial. JACC Heart failure. 2016;4:152–8. doi: 10.1016/j.jchf.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 40.Gurfinkel EP, Leon de la Fuente R, Mendiz O, Mautner B. Flu vaccination in acute coronary syndromes and planned percutaneous coronary interventions (FLUVACS) Study. European heart journal. 2004;25:25–31. doi: 10.1016/j.ehj.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 41.Ciszewski A, Bilinska ZT, Brydak LB, et al. Influenza vaccination in secondary prevention from coronary ischaemic events in coronary artery disease: FLUCAD study. European heart journal. 2008;29:1350–8. doi: 10.1093/eurheartj/ehm581. [DOI] [PubMed] [Google Scholar]
- 42.Phrommintikul A, Kuanprasert S, Wongcharoen W, Kanjanavanit R, Chaiwarith R, Sukonthasarn A. Influenza vaccination reduces cardiovascular events in patients with acute coronary syndrome. European heart journal. 2011;32:1730–1735. doi: 10.1093/eurheartj/ehr004. [DOI] [PubMed] [Google Scholar]
- 43.Udell JA, Zawi R, Bhatt DL, et al. Association between influenza vaccination and cardiovascular outcomes in high-risk patients: a meta-analysis. Jama. 2013;310:1711–20. doi: 10.1001/jama.2013.279206. [DOI] [PubMed] [Google Scholar]
- 44.Nichol KL, Nordin J, Mullooly J, Lask R, Fillbrandt K, Iwane M. Influenza vaccination and reduction in hospitalizations for cardiac disease and stroke among the elderly. The New England journal of medicine. 2003;348:1322–32. doi: 10.1056/NEJMoa025028. [DOI] [PubMed] [Google Scholar]
- 45.Davis JW, Lee E, Taira DA, Chung RS. Influenza vaccination, hospitalizations, and costs among members of a Medicare managed care plan. Medical care. 2001;39:1273–80. doi: 10.1097/00005650-200112000-00003. [DOI] [PubMed] [Google Scholar]
- 46.Nichol KL, Margolis KL, Wuorenma J, Von Sternberg T. The Efficacy and Cost Effectiveness of Vaccination against Influenza among Elderly Persons Living in the Community. New England Journal of Medicine. 1994;331:778–784. doi: 10.1056/NEJM199409223311206. [DOI] [PubMed] [Google Scholar]
- 47.Kopel E, Klempfner R, Goldenberg I. Influenza vaccine and survival in acute heart failure. European journal of heart failure. 2014;16:264–70. doi: 10.1002/ejhf.14. [DOI] [PubMed] [Google Scholar]
- 48.Mohseni H, Kiran A, Khorshidi R, Rahimi K. Influenza vaccination and risk of hospitalization in patients with heart failure: a self-controlled case series study. European Heart Journal. 2016 doi: 10.1093/eurheartj/ehw411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prevention and control of seasonal influenza with vaccines. Recommendations of the Advisory Committee on Immunization Practices--United States, 2013–2014. MMWR Recommendations and reports : Morbidity and mortality weekly report Recommendations and reports/Centers for Disease Control. 2013;62:1–43. [PubMed] [Google Scholar]
- 50.DiazGranados CA, Dunning AJ, Kimmel M, et al. Efficacy of High-Dose versus Standard-Dose Influenza Vaccine in Older Adults. New England Journal of Medicine. 2014;371:635–645. doi: 10.1056/NEJMoa1315727. [DOI] [PubMed] [Google Scholar]
- 51.Vardeny O, Sweitzer NK, Detry MA, Moran JM, Johnson MR, Hayney MS. Decreased immune responses to influenza vaccination in patients with heart failure. Journal of cardiac failure. 2009;15:368–73. doi: 10.1016/j.cardfail.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van Ermen A, Hermanson MP, Moran JM, Sweitzer NK, Johnson MR, Vardeny O. Double dose vs. standard dose influenza vaccination in patients with heart failure: a pilot study. European journal of heart failure. 2013;15:560–4. doi: 10.1093/eurjhf/hfs207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Musher DM, Rueda AM, Kaka AS, Mapara SM. The Association between Pneumococcal Pneumonia and Acute Cardiac Events. Clinical Infectious Diseases. 2007;45:158–165. doi: 10.1086/518849. [DOI] [PubMed] [Google Scholar]
- 54.Ren S, Newby D, Li SC, et al. Effect of the adult pneumococcal polysaccharide vaccine on cardiovascular disease: a systematic review and meta-analysis. Open heart. 2015;2:e000247. doi: 10.1136/openhrt-2015-000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lamontagne F, Garant MP, Carvalho JC, Lanthier L, Smieja M, Pilon D. Pneumococcal vaccination and risk of myocardial infarction. CMAJ : Canadian Medical Association journal = journal de l’Association medicale canadienne. 2008;179:773–7. doi: 10.1503/cmaj.070221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tseng HF, Slezak JM, Quinn VP, Sy LS, Van den Eeden SK, Jacobsen SJ. Pneumococcal vaccination and risk of acute myocardial infarction and stroke in men. Jama. 2010;303:1699–706. doi: 10.1001/jama.2010.529. [DOI] [PubMed] [Google Scholar]
- 57.Hospital-Based Influenza and Pneumococcal Vaccination: Sutton’s Law Applied to Prevention. Infection Control & Hospital Epidemiology. 2015;21:692–699. doi: 10.1086/501716. [DOI] [PubMed] [Google Scholar]
- 58.Kale MS, Federman AD, Ross JS. VIsits for primary care services to primary care and specialty care physicians, 1999 and 2007. Archives of internal medicine. 2012;172:1421–1423. doi: 10.1001/archinternmed.2012.3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Massoudi MS, Walsh J, Stokley S, et al. Assessing Immunization Performance of Private Practitioners in Maine: Impact of the Assessment, Feedback, Incentives, and Exchange Strategy. Pediatrics. 1999;103:1218–1223. doi: 10.1542/peds.103.6.1218. [DOI] [PubMed] [Google Scholar]
- 60.Fairbrother G, Hanson KL, Friedman S, Butts GC. The impact of physician bonuses, enhanced fees, and feedback on childhood immunization coverage rates. American Journal of Public Health. 1999;89:171–175. doi: 10.2105/ajph.89.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



