Abstract
Tumor necrosis factor alpha (TNFα)-induced angiogenesis plays important roles in the progression of various diseases, including cancer, wet age-related macular degeneration, and rheumatoid arthritis. However, the relevance and role of vascular cell adhesion molecule-1 (VCAM-1) in angiogenesis have not yet been clearly elucidated. In this study, VCAM-1 knockdown shows VCAM-1 involvement in TNFα-induced angiogenesis. Through competitive blocking experiments with VCAM-1 Ig-like domain 6 (VCAM-1-D6) protein, we identified VCAM-1-D6 as a key domain regulating TNFα-induced vascular tube formation. We demonstrated that a monoclonal antibody specific to VCAM-1-D6 suppressed TNFα-induced endothelial cell migration and tube formation and TNFα-induced vessel sprouting in rat aortas. We also found that the antibody insignificantly affected endothelial cell viability, morphology and activation. Finally, the antibody specifically blocked VCAM-1-mediated cell–cell contacts by directly inhibiting VCAM-1-D6-mediated interaction between VCAM-1 molecules. These findings suggest that VCAM-1-D6 may be a potential novel therapeutic target in TNFα-induced angiogenesis and that antibody-based modulation of VCAM-1-D6 may be an effective strategy to suppress TNFα-induced angiogenesis.
Introduction
Angiogenesis is the process by which new blood vessels form from pre-existing vessels. It is closely associated with the progression of a variety of diseases, including cancer, wet age-related macular degeneration (AMD), glaucoma, diabetic retinopathy and rheumatoid arthritis.1, 2, 3, 4 In pathological conditions, angiogenesis is tightly controlled by the coordinated actions of numerous upregulated angiogenic factors.5 Although vascular endothelial growth factor (VEGF)-dependent angiogenesis plays a role in the progression of certain diseases,6, 7 increased attention is being paid to tumor necrosis factor alpha (TNFα)-induced angiogenesis implicated in the progression of cancer, wet AMD and rheumatoid arthritis.8, 9, 10, 11, 12, 13 Bevacizumab, a humanized antibody to VEGF, ranibizumab, a fragment antigen-binding (Fab) fragment of bevacizumab and aflibercept (VEGF-Trap) are the leading biological drugs targeting VEGF, and are used in clinics to suppress VEGF-dependent abnormal angiogenesis in the progression of cancers and wet AMD.14, 15, 16, 17 However, resistance to these drugs remains a major hurdle in improving clinical outcomes.18, 19 To this end, we focused on identifying a novel therapeutic target and elucidating its functional roles and mechanisms of action in angiogenesis.
Vascular cell adhesion molecule-1 (VCAM-1) is a 90-kDa glycoprotein that is inducible and predominantly expressed in endothelial cells upon activation by any one of many extracellular stimuli, including reactive oxygen species and pro-inflammatory cytokines, such as TNFα and interleukin-1.20, 21 VCAM-1 is a type I transmembrane protein that consists of an extracellular domain containing seven homologous immunoglobulin (Ig)-like domains, a transmembrane domain and a cytosolic domain.22 During an inflammatory response, VCAM-1 acts as a cell adhesion molecule by directly interacting with α4β1 integrin expressed on leukocytes via VCAM-1's Ig-like domains 1 and 4 within the extracellular domain.23 This molecular interaction plays a key role in the recruitment and association of leukocytes with activated endothelial cells. However, despite an increasing focus on VCAM-1 in inflammatory disorders, including immune rejection and atherosclerosis,24, 25, 26, 27, 28 the functional role and molecular mechanism of VCAM-1 in TNFα-induced angiogenesis have not yet been clearly identified.
In this study, using VCAM-1 knockdown and competitive blocking experiments with VCAM-1 Ig-like domain 6 (VCAM-1-D6) protein, we obtained evidence of the role VCAM-1 plays in TNFα-induced angiogenesis and identified VCAM-1-D6 as a key domain in the regulation of the angiogenesis. With a monoclonal antibody specific to VCAM-1-D6 that we developed, we demonstrated that the antibody significantly and specifically suppressed TNFα-induced angiogenesis without affecting endothelial cytotoxicity. We propose a mechanism of action in TNFα-induced angiogenesis whereby VCAM-1-D6 plays a key role in endothelial cell–cell contact and the antibody acts as an interaction blockade directly inhibiting the VCAM-1-D6-mediated interaction between VCAM-1 molecules on adjacent endothelial cells. In summary, our findings suggest that VCAM-1-D6 is a potential novel therapeutic target in TNFα-induced angiogenesis and that antibody-based modulation of VCAM-1-D6 is an effective strategy to suppress TNFα-induced angiogenesis.
Materials and methods
Cell culture and transfection
Human umbilical vein endothelial cells (HUVECs; Lonza, Basel, Switzerland) were maintained in endothelial growth medium (EGM; Lonza) at 37 °C in a humidified incubator with 5% CO2 (Panasonic Healthcare Company, Tokyo, Japan). Human embryonic kidney 293F (HEK293F) cells were maintained in Freestyle expression medium (Invitrogen/Life Technologies, Carlsbad, CA, USA) supplemented with 1% (v/v) penicillin/streptomycin in a humidified Multitron incubation shaker (Infors HT, Basel, Switzerland) at 37 °C in 8% CO2. HUVECs were grown to 50–80% confluence and transiently transfected with ON-TARGETplus SMARTpool siRNA targeting VCAM-1 (Thermo Fisher Scientific, Waltham, MA, USA) using Lipofectamine 2000 transfection reagent (Invitrogen), according to the manufacturer's instructions.
Flow cytometry
Expression of VCAM-1 on the surface of HUVECs was evaluated by incubating 2 × 105 HUVECs in EGM in the absence or presence of 20 ng ml−1 human TNFα (hTNFα Millipore, Billerica, MA, USA) for 24 h. After harvesting and washing with phosphate-buffered saline solution (PBS), the cells were fixed in 4% paraformaldehyde (PFA) for 20 min at room temperature. After blocking with PBS containing 1% bovine serum albumin (BSA) for 1 h at room temperature, the cells were incubated first with mouse anti-VCAM-1 monoclonal antibody (1 μg ml−1; Abcam, Cambridge, UK) for 1 h at 37 °C, and then with Alexa Fluor 488-conjugated anti-mouse antibody (1:1000; Invitrogen) for 1 h at 37 °C. The effects of anti-VCAM-1-D6 IgG on endothelial cell activation were evaluated by incubating 2 × 105 HUVECs in the absence or presence of 20 ng ml−1 hTNFα (Millipore), 20 μg ml−1 control IgG or anti-VCAM-1-D6 IgG for 24 h. After blocking with PBS containing 1% BSA for 1 h at room temperature, the cells were incubated first with rabbit anti-ICAM-1 monoclonal antibody (1:500; Abcam) for 1 h at 37 °C, and then with an Alexa Fluor 488-conjugated anti-rabbit antibody (1:1000; Invitrogen) for 1 h at 37 °C. Samples were analyzed by flow cytometry using a FACSCalibur system (BD Biosciences, San Jose, CA, USA) with the aid of FlowJo software (TreeStar, Ashland, OR, USA).
Immunoblot analysis
Immunoblot analysis was performed as described previously, with minor modifications.29 Lysates of scrambled siRNA- or VCAM-1 siRNA-transfected HUVECs cultured in EGM in the presence of 20 ng ml−1 hTNFα were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto nitrocellulose membranes using a wet transfer system (GE Healthcare Life Sciences, Piscataway, NJ, USA). After blocking with Tris-buffered saline and Tween (10 mM Tris-HCl, pH 7.5, 150 mM NaCl and 0.05% (v/v) Tween 20; TBST) containing 5% (w/v) skim milk, the membranes were incubated with rabbit anti-VCAM-1 monoclonal antibody (1:1000; Abcam) or mouse anti-β-actin monoclonal antibody (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 4 °C overnight and then with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (1:5000; Santa Cruz Biotechnology) or goat anti-mouse IgG (1:5000; Santa Cruz Biotechnology). Following several washes with TBST, protein bands were visualized using SuperSignal West Pico chemiluminescent substrate (Pierce, Rockford, IL, USA) according to the manufacturer's instructions.
Preparation of anti-VCAM-1-D6 IgG and fusion protein VCAM-1-D6-Fc
Preparation of anti-VCAM-1-D6 IgG and VCAM-1-D6 crystallizable fragment (Fc) was performed as described previously, with minor modifications.30 Briefly, following production of anti-VCAM-1-D6 IgG and VCAM-1-D6-Fc in HEK293F cells using polyethylenimine (Polysciences, Washington, PA, USA), the culture medium was collected and purified by affinity chromatography on protein A Sepharose (GeneScript, Piscataway, NJ, USA). Protein concentration was determined using a NanoDrop spectrophotometer (Thermo, Wilmington, DE, USA). Samples were dialyzed against PBS and analyzed by SDS-PAGE and Coomassie Brilliant Blue staining. Aliquots of the final pooled fractions were stored at −80 °C.
Wound healing assay
The wound healing assay was performed as described previously, with minor modifications.31 To investigate the effect of VCAM-1 knockdown on HUVEC migration, 3 × 104 HUVECs transfected with scrambled siRNA or VCAM-1 siRNA were plated in each well of an ImageLock 96-well plate (Essen Bioscience, Ann Arbor, MI, USA) and maintained in EGM containing 20 ng ml−1 hTNFα for 24 h at 37 °C. A scratch was made in the monolayer using a 96-pin WoundMaker (Essen Bioscience). To investigate the effect of anti-VCAM-1-D6 IgG on HUVEC migration, 3 × 104 HUVECs were plated in each well of an ImageLock 96-well plate and maintained in EGM containing 20 ng ml−1 hTNFα for 24 h at 37 °C. Wounds were then made by a 96-pin WoundMaker. Following two washes with PBS, the wounded HUVEC cultures were incubated in the absence or presence of 20 μg ml−1 control IgG, monoclonal antibody to VCAM-1 Ig-like domain 1 (anti-VCAM-1-D1 antibody; 51-10C9, BD Biosciences) or anti-VCAM-1-D6 IgG for 10 h at 37 °C and then stained with 0.2% (w/v) crystal violet (Sigma-Aldrich, St Louis, MO, USA). For quantitative analysis, images were captured 10 h after wounding using a Leica (Wetzlar, Germany) DM IL LED light microscope, and the distance migrated was measured manually.
Tube formation assay
Tube formation assays were performed as described previously, with minor modifications.32 Briefly, 150 μl Matrigel (Corning, Tewksbury, MA, USA) was added to each well of 48-well plates and allowed to polymerize for 30 min at 37 °C. To investigate the effect of VCAM-1 knockdown on HUVEC tube formation, 1 × 105 HUVECs transfected with scrambled siRNA or VCAM-1 siRNA and maintained in EGM containing 20 ng ml−1 hTNFα for 24 h at 37 °C were seeded onto the Matrigel-coated plates. To investigate the role of VCAM-1-D6 in HUVEC tube formation, 1 × 105 HUVECs cultured in EGM containing 20 ng ml−1 hTNFα for 24 h were seeded onto the Matrigel-coated plates and incubated in the absence or presence of 5, 10, 20 or 40 μg ml−1 VCAM-1-D6-Fc fusion protein or 40 μg ml−1 Fc domain alone for 12 h at 37 °C. To determine the effect of anti-VCAM-1-D6 IgG on HUVEC tube formation, 1 × 105 HUVECs cultured in EGM containing 20 ng ml−1 hTNFα for 24 h were seeded onto the Matrigel-coated plates and incubated in the absence or presence of 20 μg ml−1 control IgG, anti-VCAM-1-D1 IgG or anti-VCAM-1-D6 IgG for 12 h at 37 °C. To assess specific inhibition of tube formation by anti-VCAM-1-D6 IgG, antigen–antibody mixture was made by pre-incubating equimolar amounts of fusion protein VCAM-1-D6-Fc and anti-VCAM-1-D6 IgG in PBS for 2 h at room temperature with gentle rotation. Then, 1 × 105 HUVECs cultured in EGM containing 20 ng ml−1 hTNFα for 24 h were seeded on Matrigel-coated plates and incubated in the absence or presence of control IgG, anti-VCAM-1-D6 IgG or antigen-antibody mixture (1:1 molar ratio) for 12 h at 37 °C. Images were obtained using a light microscope (Leica DM IL LED), and tube formation was quantified by counting the total tube branches.
Aortic ring sprouting assay
Aortic rings were prepared from 7-week-old male Sprague Dawley rats (NARA Biotech, Seoul, Korea). Rats were anesthetized and bled out, and their thoracic aortas were removed and placed in a Petri dish filled with cold sterile PBS. Following removal of the surrounding fat tissue, the aortas were cut into 1-mm segments. Individual segments were embedded in 40 μl Matrigel (Corning) in 96-well tissue culture plates. Samples were allowed to gel in a 37 °C incubator at 5% CO2 for 30 min, then an additional 40 μl Matrigel was added to each well. For the TNFα-induced aortic ring sprouting assay, the aortic segments were incubated in EGM alone, EGM containing 20 μg ml−1 control IgG or anti-VCAM-1-D6 IgG, EGM containing 20 ng ml−1 mouse TNFα (mTNFα) or EGM containing 20 ng ml−1 mTNFα together with 20 μg ml−1 control IgG or anti-VCAM-1-D6 IgG for 7 days. Images were captured using a light microscope (Leica DM IL LED), and the total numbers of vessels sprouting from each aortic ring were manually counted. To measure the lengths of the vessels, 10 fields per aortic ring were randomly selected and the vessel length was individually quantified using Image J software version 1.49j (National Institutes of Health).
Cell viability assay
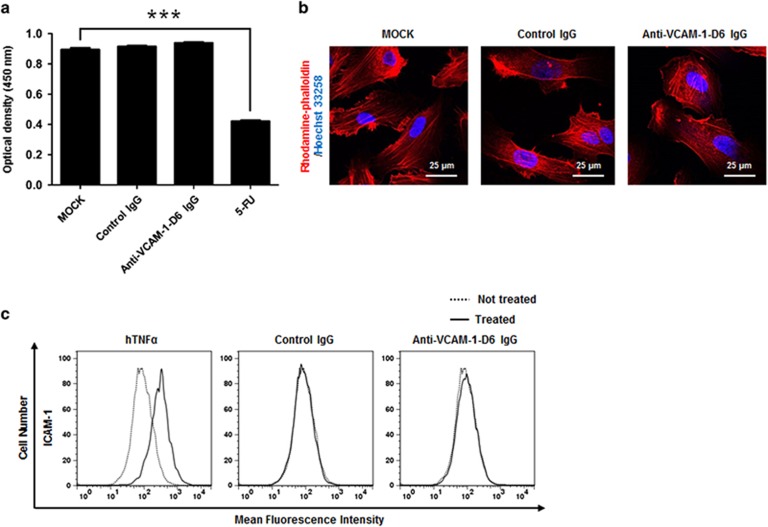
For the cell viability assay, 5 × 103 HUVECs were plated in wells of a 96-well plate and incubated in EGM in the absence or presence of 20 μg ml−1 control IgG or anti-VCAM-1-D6 IgG or 36 μg ml−1 of 5-fluorouracil (5-FU) for 48 h at 37 °C. Cell viability was determined using a Cell Counting Kit-8 (Dojindo Laboratories, Kumamoto, Japan) according to the manufacturer's instructions. The final absorbance was measured at 450 nm using a VICTOR X4 microplate reader (PerkinElmer, Waltham, MA, USA).
Immunocytochemistry
For the immunocytochemistry analysis, 5 × 104 HUVECs were cultured in EGM on 0.1% (w/v) gelatin-coated glass coverslips (Marienfeld-Superior, Lauda-Königshofen, Germany) at 37 °C overnight. The cells were incubated in the absence or presence of 20 μg ml−1 control IgG or anti-VCAM-1-D6 IgG for 24 h at 37 °C. The cells were fixed in 4% PFA, blocked by incubation in PBS containing 5% (v/w) BSA and 0.1% (v/v) Triton X-100 for 1 h at 37 °C, and then incubated with 1 unit per well rhodamine-phalloidin (Molecular Probes, Eugene, OR, USA) and 0.1 μg ml−1 Hoechst 33258 (Sigma-Aldrich) for 1 h. Images were acquired with a FluoView FV300 confocal microscope (Olympus, Tokyo, Japan).
Measurement of VCAM-1-mediated cell–cell contact
VCAM-1-mediated cell–cell contact assays were performed as described previously, with minor modifications.31 Briefly, 1.5 × 107 HEK293F cells in suspension were transfected with plasmid encoding wild-type human VCAM-1 (WT-VCAM-1), cultured in Freestyle 293 expression medium overnight, and seeded at a density of 5 × 105 cells per well in six-well plates. Cells were maintained in the absence or presence of 20 μg ml−1 control IgG or anti-VCAM-1-D6 IgG for 2 h. Cell aggregates (masses >4 cells) were counted in at least 10 fields using a light microscope (Leica DM IL LED).
Cell enzyme-linked immunosorbent assay
Cell enzyme-linked immunosorbent assays (ELISAs) were performed as described previously, with minor modifications.31 Briefly, 1 × 104 HUVECs plated in wells of a 96-well plate were cultured in EGM containing 20 ng ml−1 of hTNFα for 24 h at 37 °C. Following fixation with 4% PFA, cells were incubated with various concentrations (0–50 μg ml−1) of VCAM-1-D6-Fc-HRP or Fc-HRP for 2 h at 37 °C. For competition cell ELISA, HUVECs cultured in EGM containing 20 ng ml−1 of hTNFα were incubated with 3 μg ml−1 VCAM-1-D6-Fc-HRP in the absence or presence of increasing concentrations of anti-VCAM-1-D6 IgG for 2 h at 37 °C. After three washes with ice-cold PBS, the cells were incubated with 3,3′,5,5′-tetramethylbenzidine substrate solution (BD Biosciences). Optical density was measured at 450 nm using a VICTOR X4 plate reader.
ELISA
ELISA was performed as described previously, with minor modifications.33 Microplates (96-well) were coated with 0.1 μg recombinant human VCAM-1 extracellular domain (Sino Biological Inc, Beijing, China) in PBS overnight at 37 °C. Then, the plates were washed three times with PBS and incubated with 3% (w/v) BSA in PBS for 1 h at 37 °C. For the competition assay with anti-VCAM-1-D6 IgG, 1 μg VCAM-1-D6-Fc-HRP was incubated with recombinant human VCAM-1 extracellular domain in the absence or presence of increasing concentrations of anti-VCAM-1-D6 IgG for 2 h at room temperature. Following three washes with PBS, 100 μl of 3,3′,5,5′-tetramethylbenzidine substrate solution were added to each well. Optical density was measured at 450 nm using a VICTOR X4 microplate reader.
Statistical analysis
All data were analyzed with GraphPad Prism 5.0 (GraphPad Software, La Jolla, CA, USA) using a two-tailed Student's t-test for comparison between two groups and a one-way analysis of variance with Bonferroni's correction for multiple comparisons. All data are presented as the mean±the standard error of the mean (s.e.m.). Values of P<0.05 were considered statistically significant.
Results
VCAM-1 knockdown reduced TNFα-induced HUVEC migration and tube formation
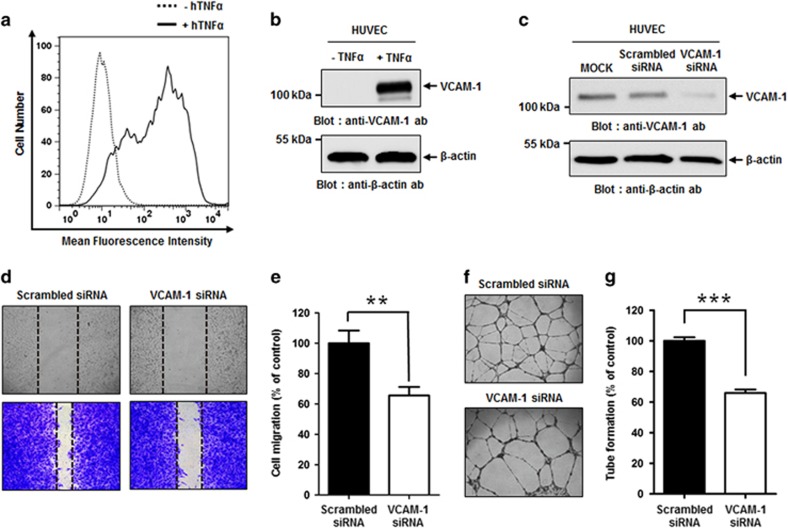
To examine the relevance of VCAM-1 in TNFα-induced angiogenesis, we first examined VCAM-1 expression on HUVECs cultured in the absence or presence of hTNFα, a strong inducer of VCAM-1, using flow cytometry and immunoblot analysis (Figures 1a and b), and found markedly increased expression of VCAM-1 on TNFα-treated HUVECs. We then carried out siRNA-mediated knockdown of VCAM-1 in HUVECs cultured in the presence of TNFα and confirmed reduced VCAM-1 expression by VCAM-1 siRNA using immunoblot analysis (Figure 1c). We also measured the effect of VCAM-1 knockdown on HUVEC migration (Figures 1d and e) and tube formation (Figures 1f and g). We found that VCAM-1 knockdown significantly decreased both HUVEC migration and tube formation. Taken together, these results suggest that VCAM-1 may play a key role in the regulation of TNFα-induced angiogenesis.
Figure 1.
Effect of VCAM-1 knockdown on HUVEC migration and tube formation. (a) HUVECs were incubated in EGM in the absence (dashed line) or presence (solid line) of 20 ng ml−1 hTNFα for 24 h. Cells were stained with anti-VCAM-1 monoclonal antibody, and VCAM-1 expression was analyzed using flow cytometry. (b) Representative immunoblot showing relative VCAM-1 expression levels on HUVECs cultured in EGM in the absence or presence of hTNFα. (c) Representative immunoblot showing relative VCAM-1 expression levels following mock transfection of HUVECs or transfection with scrambled siRNA or VCAM-1 siRNA cultured in EGM in the presence of hTNFα. β-actin was used as a loading control. (d) Light microscopy images depicting migration in wound healing assay of scrambled siRNA- or VCAM-1 siRNA-transfected HUVECs cultured in EGM in the presence of hTNFα. Images were captured at 0 h (top; unstained) and 10 h (bottom; stained with crystal violet). (e) Quantitation of migration of scrambled siRNA- or VCAM-1 siRNA-transfected HUVECs cultured in EGM in the presence of hTNFα, expressed as a percent of scrambled siRNA migration, based on the distance separating wound margins. (f) Light microscopic images depicting tube formation by scrambled siRNA- and VCAM-1 siRNA-transfected HUVECs cultured in EGM in the presence of hTNFα. (g) Quantitation of total tube branches in scrambled siRNA- or VCAM-1 siRNA-transfected HUVECs cultured in EGM in the presence of hTNFα, expressed as a percent of tube formation in the presence of scrambled siRNA. All data are presented as the mean±s.e.m. of triplicate measurements from one of the three independent experiments; **P<0.01, ***P<0.001.
VCAM-1-D6 is a key domain specifically regulating TNFα-induced HUVEC tube formation
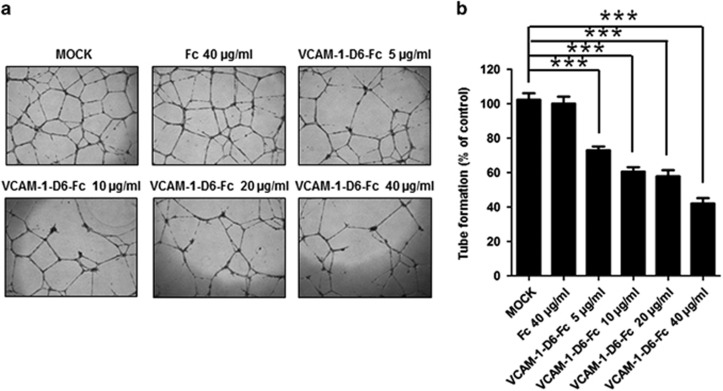
To investigate the role of VCAM-1-D6 in TNFα-induced angiogenesis, we generated a fusion protein in which the VCAM-1-D6 domain was fused to the Fc domain of an IgG molecule (VCAM-1-D6-Fc). This fusion protein, shown to be >90% pure by SDS-PAGE and Coomassie Brilliant Blue staining (Supplementary Figure S1a), was used in a competitive blocking experiment, treating HUVECs grown in the presence of TNFα with the fusion protein or the Fc domain alone as a negative control. We then determined the effect of VCAM-1-D6 on HUVEC tube formation (Figures 2a and b). Tube formation was specifically and significantly inhibited by increasing concentrations of VCAM-1-D6-Fc but not by the Fc domain alone. These data suggest that VCAM-1-D6 may be a key domain specifically regulating TNFα-induced angiogenesis.
Figure 2.
Effect of VCAM-1-D6 protein on HUVEC tube formation. (a) Light microscopic images depicting tube formation by hTNFα-treated HUVECs in the absence (MOCK) or presence of the indicated concentrations of VCAM-1-D6-Fc fusion protein or Fc domain alone. Fc served as a negative control. (b) Quantitation of total tube branches in hTNFα-treated HUVECs in presence of VCAM-1-D6-Fc or Fc, expressed as a percent of control (MOCK) tube formation. All data are presented as the mean±s.e.m. of triplicate measurements from one of the three independent experiments; ***P<0.001.
Anti-VCAM-1-D6 antibody specifically suppresses not only TNFα-induced HUVEC migration and tube formation, but also TNFα-induced vessel sprouting from rat aorta
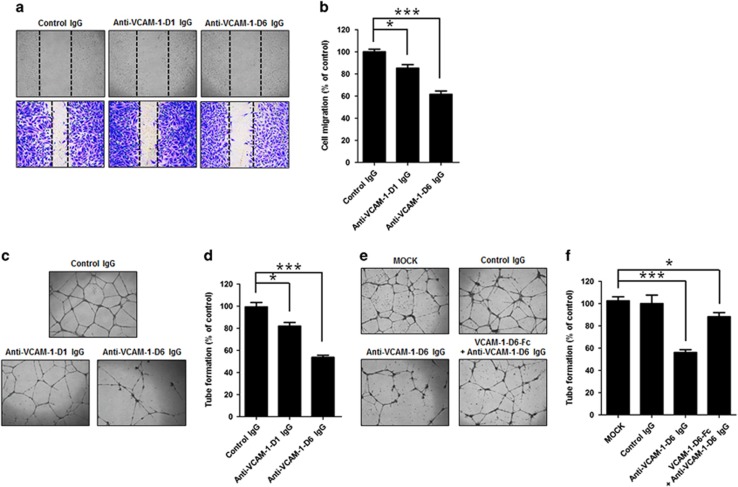
We previously reported that we generated a rabbit/human chimeric monoclonal antibody specific to VCAM-1-D6 (anti-VCAM-1-D6 IgG) using phage display technology. To elucidate the effect of anti-VCAM-1-D6 IgG on TNFα-induced angiogenesis, we treated HUVECs with control IgG, a monoclonal antibody to Ig-like domain 1 of VCAM-1 (anti-VCAM-1-D1 antibody) as a negative control, or anti-VCAM-1-D6 IgG in the presence of TNFα and then examined HUVEC migration (Figures 3a and b) and tube formation (Figures 3c and d). Anti-VCAM-1-D6 IgG was shown to be >90% pure by SDS-PAGE and Coomassie Brilliant Blue staining (Supplementary Figure S1b). We found that HUVEC migration and tube formation were significantly and strongly inhibited by anti-VCAM-1-D6 IgG but not by control IgG, whereas anti-VCAM-1-D1 had significant, but much less potent, inhibitory effects on these functions. These results demonstrated the potent inhibitory effect of anti-VCAM-1-D6 antibody on TNFα-induced angiogenesis.
Figure 3.
Effect of anti-VCAM-1-D6 IgG on HUVEC migration and tube formation. (a) Light microscopic images of hTNFα-induced HUVEC migration in the presence of 20 μg ml−1 control IgG, anti-VCAM-1-D1 IgG and anti-VCAM-1-D6 IgG. Images were captured at 0 h (top; unstained) and 10 h (bottom; stained with crystal violet). (b) Quantitation of hTNFα-induced HUVEC migration in the presence of control IgG, anti-VCAM-1-D1 IgG and anti-VCAM-1-D6 IgG, expressed as a percent of distance migrated in the presence of control IgG, based on the distance separating wound margins. (c) Light microscopic images of tubes formed by hTNFα-treated HUVECs in the presence of control IgG, anti-VCAM-1-D1 IgG and anti-VCAM-1-D6 IgG. (d) Quantitation of total tube branches from hTNFα-treated HUVECs in the presence of control IgG, anti-VCAM-1-D1 IgG and anti-VCAM-1-D6 IgG, expressed as a percent of tube formation in the presence of control IgG. (e) Light microscopic images of tubes formed by hTNFα-treated HUVECs in the absence (MOCK) or presence of 20 μg ml−1 control IgG, anti-VCAM-1-D6 IgG and complex formed by anti-VCAM-1-D6 IgG and fusion protein VCAM-1-D6-Fc. (f) Quantitation of total tube branches, expressed as a percent of control (MOCK) tube formation. All data are presented as the mean±s.e.m. of triplicate measurements from one of the three independent experiments; *P<0.05, ***P<0.001.
To confirm specific inhibition of TNFα-induced tube formation by anti-VCAM-1-D6 IgG, we performed the TNFα-induced HUVEC tube formation assays in the absence or presence of control IgG, anti-VCAM-1-D6 IgG or antibody–antigen mixture consisting of equimolar amounts of anti-VCAM-1-D6 IgG and VCAM-1-D6-Fc fusion protein (Figures 3e and f). Inhibition of tube formation by anti-VCAM-1-D6 IgG was significantly attenuated in the presence of VCAM-1-D6-Fc, supporting the hypothesis that the anti-VCAM-1-D6 antibody specifically inhibits TNFα-induced vascular tube formation.
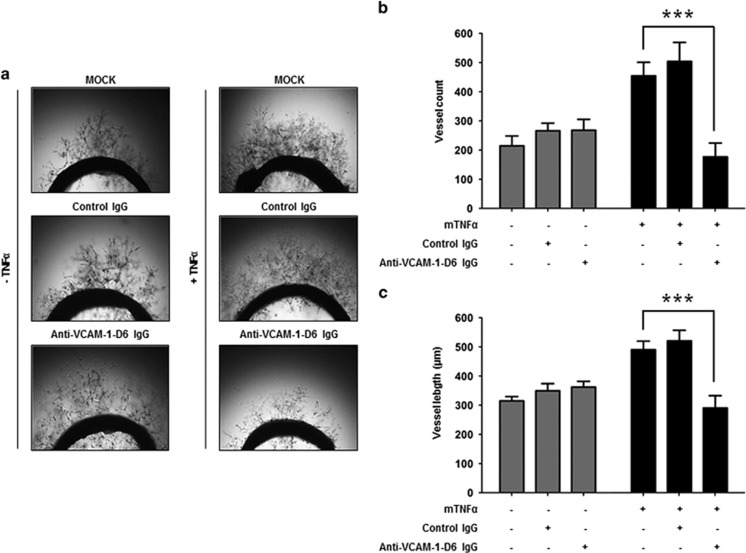
To further evaluate the effect of anti-VCAM-1-D6 IgG on TNFα-induced angiogenesis, we treated rat aortic rings with control IgG or anti-VCAM-1-D6 IgG in the absence or presence of TNFα and measured the number and length of vessels sprouting from the rat aortic rings (Figure 4a). We found that in the presence of TNFα, many vessels sprouted from the rat aortic rings. Furthermore, we observed that anti-VCAM-1-D6 IgG prominently reduced the number (Figure 4b) and length (Figure 4c) of vessels that sprouted from rat aortic rings in the presence of TNFα, whereas the antibody did not suppress the sprouting of vessels from rat aortic rings in the absence of TNFα. These results suggest that anti-VCAM-1-D6 antibody specifically inhibits TNFα-induced angiogenesis.
Figure 4.
Effect of anti-VCAM-1-D6 IgG on TNFα-induced vessel sprouting from rat aorta. (a) Rat aortic rings were incubated in EGM alone (MOCK), EGM containing 20 μg ml−1 control IgG or anti-VCAM-1-D6 IgG, EGM containing 20 ng ml−1 mTNFα or EGM containing 20 ng ml−1 mTNFα together with 20 μg ml−1 control IgG or anti-VCAM-1-D6 IgG. Vessel outgrowth was monitored by light microscopy. (b) The number of vessels sprouting from each ring in a was counted and is expressed in the bar graph. (c) The length of vessels sprouting from each ring in a was measured and is expressed in the bar graph. Data are presented as the mean±s.e.m. of nine replicate measurements from one of the two independent experiments; ***P<0.001.
Collectively, these data suggest that antibody targeting of VCAM-1-D6 may be effective for specifically suppressing TNFα-induced abnormal angiogenesis in vivo.
Anti-VCAM-1-D6 antibody does not affect HUVEC viability, morphology or activation
To evaluate the influence of anti-VCAM-1-D6 IgG on endothelial cell toxicity, we first determined the viability of HUVECs after treatment with anti-VCAM-1-D6 IgG. We found that anti-VCAM-1-D6 IgG had no cytotoxic effect on any of these cells, whereas 5-FU significantly reduced the viability of HUVECs (Figure 5a). Here, control IgG was used as a negative control. We also evaluated HUVEC morphology in the absence or presence of anti-VCAM-1-D6 IgG via immunocytochemistry. Anti-VCAM-1-D6 IgG did not alter morphology of HUVECs (Figure 5b). To investigate the effect of anti-VCAM-1-D6 IgG on endothelial cell activation, an initial inflammatory response to harmful stimuli, we treated HUVECs with anti-VCAM-1-D6 IgG and monitored HUVEC activation by measuring the expression of endothelial cell activation markers, including VCAM-1 and intercellular cell adhesion molecule-1 (ICAM-1). We used hTNFα as a positive control for endothelial cell activation. Anti-VCAM-1-D6 IgG had little effect on HUVEC activation, whereas hTNFα, as expected, induced HUVEC activation (Figure 5c). Collectively, these data suggest that the antibody may cause insignificant endothelial cytotoxicity in vivo.
Figure 5.
Effect of anti-VCAM-1-D6 IgG on HUVEC viability, morphology and activation. (a) HUVECs were incubated in the absence (MOCK) or presence of 20 μg ml−1 control IgG or anti-VCAM-1-D6 IgG or 36 μg ml−1 5-FU for 2 days. Cell viability was assessed by measuring absorbance at 450 nm. (b) HUVECs cultured in the absence (MOCK) or presence of control IgG or anti-VCAM-1-D6 IgG were stained with rhodamine-phalloidin (red) and nuclei were counterstained with Hoechst 33258 (blue), and then cells were examined by confocal microscopy. Scale bars represent 25 μm. (c) HUVECs cultured in the absence (dashed line) or presence (solid line) of hTNFα, control IgG or anti-VCAM-1-D6 IgG were stained with anti-ICAM-1 monoclonal antibody and analyzed by flow cytometry. All data are presented as the mean±s.e.m. of triplicate measurements from one of the three independent experiments; ***P<0.001.
Anti-VCAM-1-D6 antibody specifically inhibits VCAM-1-mediated cell–cell contact by directly inhibiting VCAM-1-D6-mediated interactions between VCAM-1 molecules
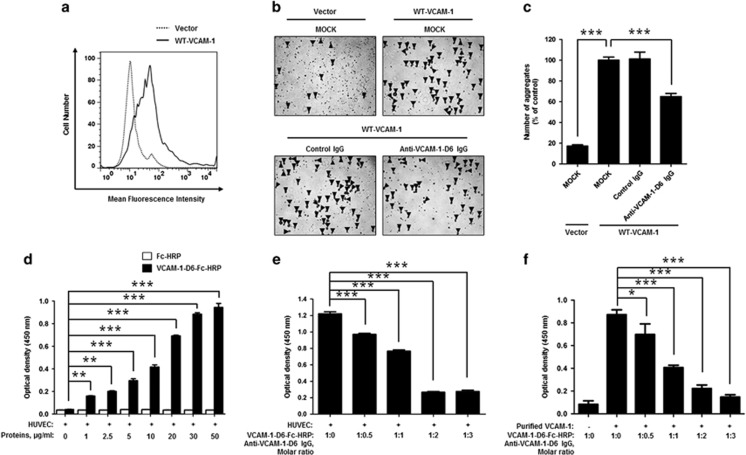
To examine the mechanism of action of anti-VCAM-1-D6 IgG in TNFα-induced angiogenesis, we overexpressed VCAM-1 in HEK293F cells, which do not express endogenous VCAM-1, and confirmed VCAM-1 expression using flow cytometry (Figure 6a). Then, we measured the number of cell aggregates, an indicator of VCAM-1-mediated cell–cell contact, in the presence of control IgG or anti-VCAM-1-D6 IgG (Figures 6b and c). We found that the number of cell aggregates was ~5-fold greater in cells overexpressing VCAM-1 than in cells transfected with vector alone and that VCAM-1-mediated cell aggregation was substantially inhibited by anti-VCAM-1-D6 IgG but not by control IgG. These results suggest that anti-VCAM-1-D6 antibody may specifically inhibit VCAM-1-mediated endothelial cell–cell contact in TNFα-induced angiogenesis.
Figure 6.
Effect of anti-VCAM-1-D6 IgG on clec14a-mediated cell–cell contact in VCAM-1-overexpressing HEK293F cells and hTNFα-treated HUVECs. (a) HEK293F cells were transfected with vector alone or expression plasmid encoding WT-VCAM-1. Cells were stained with anti-VCAM-1 monoclonal antibody and VCAM-1 expression was analyzed using flow cytometry. (b) HEK293F cells transfected with vector alone or expression plasmid encoding WT-VCAM-1 were incubated in the absence (MOCK) or presence of 20 μg ml−1 control IgG or anti-VCAM-1-D6 IgG. Cell aggregates (masses >4 cells; triangular arrowheads) were counted under a light microscope. (c) Quantitation of aggregates formed in the transfected cells in the absence (MOCK) or presence of control IgG or anti-VCAM-1-D6 IgG expressed as a percent of control (MOCK) aggregate formation. (d) ELISA results of binding of the indicated concentrations of anti-VCAM-1-D6-Fc-HRP and Fc-HRP to hTNFα-treated HUVECs. (e) ELISA results of VCAM-1-D6-Fc-HRP binding, in the absence or presence of the increasing concentrations of anti-VCAM-1-D6 IgG, to hTNFα-treated HUVECs pre-incubated with 3 μg ml−1 VCAM-1-D6-Fc-HRP. (f) One tenth of a microgram of VCAM-1-D6-Fc-HRP was pre-incubated with 1 μg recombinant human VCAM-1 extracellular domain, and binding was determined by ELISA in the absence or presence of the increasing concentrations of anti-VCAM-1-D6 IgG. All data are presented as the mean±s.e.m. of triplicate measurements from one of three independent experiments; *P<0.05, **P<0.01, ***P<0.001.
To further investigate the more detailed mode of action of anti-VCAM-1-D6 IgG at the molecular level, we first assayed the binding of VCAM-1-D6 to the surface of endothelial cells by incubating TNFα-treated HUVECs in the absence or presence of increasing concentrations of HRP-conjugated VCAM-1-D6-Fc (VCAM-1-D6-Fc-HRP) or Fc (Fc-HRP) and then performing cell ELISAs (Figure 6d). The results showed that VCAM-1-D6-Fc-HRP, but not Fc-HRP, specifically and dose-dependently bound to TNFα-treated HUVECs, suggesting the importance of VCAM-1-D6 on endothelial cell–cell contact. To investigate the blocking effect of anti-VCAM-1-D6 IgG on the interaction between VCAM-1-D6 and activated endothelial cells, we incubated TNFα-treated HUVECs with VCAM-1-D6-Fc-HRP in the absence or presence of increasing concentrations of anti-VCAM-1-D6 IgG and then performed cell ELISAs (Figure 6e). Anti-VCAM-1-D6 IgG significantly blocked the interactions between VCAM-1-D6-Fc-HRP and TNFα-treated HUVECs. To further confirm the blocking effect of anti-VCAM-1-D6 IgG on the interaction between VCAM-1-D6 and VCAM-1, ELISA plates were coated with recombinant human VCAM-1 extracellular domain, and VCAM-1-D6-Fc-HRP was then incubated in the plates in the absence and presence of increasing concentrations of anti-VCAM-1-D6 IgG (Figure 6f). We also confirmed that the direct interaction of VCAM-1-D6-Fc-HRP with the extracellular domain of recombinant human VCAM-1 was specifically inhibited by anti-VCAM-1-D6 IgG in a concentration-dependent manner. In summary, these data suggest that the anti-VCAM-1-D6 antibody may suppress endothelial cell–cell contact by directly inhibiting VCAM-1-D6-mediated interactions between VCAM-1 molecules on adjacent endothelial cells in TNFα-induced angiogenesis.
Discussion
Angiogenesis is the process by which new blood vessels form from pre-existing vessels and is pivotal in many biological processes, including development, reproduction and wound repair. Under pathological conditions, angiogenesis is regulated by the complex coordinated actions of multiple pro- and anti-angiogenic regulators.5 Despite increasing attention to TNFα-induced angiogenesis, novel therapeutic targets and their molecular mechanisms in TNFα-induced angiogenesis have not been intensively studied yet. This is the first study to suggest that VCAM-1-D6 may be a novel potential angiogenic target in TNFα-induced angiogenesis and that antibody-based modulation of VCAM-1-D6 may be an effective strategy for suppressing TNFα-induced angiogenesis.
VCAM-1 is a type I transmembrane protein that is inducible and exclusively expressed on activated endothelial cells in response to extracellular stimuli, including numerous pro-inflammatory cytokines.20, 21 VCAM-1 plays a key role in leukocyte binding to activated endothelial cells and transendothelial migration during inflammatory responses. Prior studies have suggested that VCAM-1 is closely associated with a variety of diseases, including cancers, atherosclerosis, arthritis and immune rejection.24, 25, 26, 27, 28 However, to date, the relevance and role of VCAM-1 in TNFα-induced angiogenesis have not been clearly identified. Here we propose that VCAM-1-D6 may play a key role and be a potential novel therapeutic target in TNFα-induced angiogenesis. Several lines of evidence support our hypothesis. First, the results of VCAM-1 knockdown in TNFα-treated HUVECs and VCAM-1 overexpression in HEK293F cells, which demonstrated VCAM-1-mediated cell–cell contact, imply the possible role of VCAM-1 in TNFα-induced angiogenesis. Second, using competitive blocking experiments with a VCAM-1-D6 protein, we identified VCAM-1-D6 as a key domain regulating TNFα-induced vascular tube formation. Third, we found that hTNFα-induced HUVEC migration and tube formation were specifically inhibited by anti-VCAM-1-D6 IgG but not by control IgG. Fourth, VCAM-1-mediated cell–cell contact in HEK293 cells and VCAM-1-overexpressing HUVEC tube formation (Supplementary Figure S4) were specifically inhibited by anti-VCAM-1-D6 IgG but not by control IgG. Fifth, angiogenic sprouting from TNFα-induced rat aortic rings was significantly inhibited by anti-VCAM-1-D6 IgG but not by control IgG. Sixth, the finding that VCAM-1-D6 protein or anti-VCAM-1-D6 IgG significantly inhibited tube formation induced by TNFα (Supplementary Figure S3), but not by VEGF (Supplementary Figure S2) also suggests that VCAM-1 may be a major player in hTNFα-induced, but not in VEGF-induced angiogenesis. Seventh, similar to findings from hTNFα-induced HUVEC tube formation assays, we also confirmed the significant inhibitory effect of anti-VCAM-1-D6 IgG on hTNFα-induced tube formation in human retinal endothelial cells (Supplementary Figure S5).
VCAM-1 forms complexes with several binding partners, including α4β1 integrin, moesin, ezrin, and secreted protein acidic and rich in cysteine.34, 35, 36 However, the direct binding partner of VCAM-1-D6 in TNFα-induced angiogenesis had not yet been identified. Here we suggest that VCAM-1-D6 may directly interact with VCAM-1 and that this interaction is, at least in part, important for VCAM-1-mediated endothelial cell–cell contact in TNFα-induced angiogenesis. Through the use of the cell aggregation assay, we demonstrated that VCAM-1-mediated cell–cell contact was specifically and significantly inhibited by anti-VCAM-1-D6 IgG but not by control IgG. Furthermore, we also demonstrated that HUVEC tube formation was increased by the overexpression of VCAM-1 and that its increase was significantly inhibited by anti-VCAM-1-D6 IgG, but not by control IgG (Supplementary Figure S4). Our cell ELISA experiment also showed that VCAM-1-D6 bound specifically to the surface of TNFα-activated HUVECs and that this binding was specifically inhibited by anti-VCAM-1-D6 IgG in a concentration-dependent manner. Furthermore, we confirmed the direct interaction between purified VCAM-1-D6 and VCAM-1, and showed specific inhibition of this interaction by anti-VCAM-1-D6 IgG. However, although we focused on the VCAM-1-D6-mediated interaction between VCAM-1 molecules in TNFα-induced angiogenesis, we cannot exclude the possibility that VCAM-1 may bind to other binding partners implicated in this angiogenesis.
Monoclonal antibody-based therapy is now one of the most important strategies for treating patients with various diseases, including solid tumors, hematological malignancies, immunological disorders and eye diseases. As of November 2014, a total of 48 monoclonal antibodies had been approved in Europe and/or the United States for various indications, and many more are currently being evaluated in clinical trials.37 Although, most successful anti-angiogenic antibody drugs target VEGF-dependent angiogenesis.6, 7 TNFα-induced angiogenesis is closely associated with the progression of several diseases, including cancer, wet AMD, and rheumatoid arthritis.8, 9, 10, 11, 12, 13 On the basis of our present results, we suggest that antibody-based modulation of VCAM-1-D6 may be a novel and effective strategy to suppress TNFα-induced abnormal angiogenesis in vivo. We previously reported that the anti-VCAM-1-D6 IgG we developed specifically binds, with subnanomolar affinity and broad cross-species reactivity, to VCAM-1-D6.30 In this study, we found that anti-VCAM-1-D6 IgG specifically and significantly inhibits angiogenic properties in vitro, including HUVEC migration, tube formation and VCAM-1-mediated cell–cell contact. Furthermore, we demonstrated that anti-VCAM-1-D6 IgG also specifically inhibits TNFα-induced vessel sprouting from rat aorta ex vivo, but not EGM-dependent vessel sprouting. We also found that the antibody seems to suppress VCAM-1-mediated endothelial cell–cell contact in TNFα-induced angiogenesis by directly blocking VCAM-1-D6-mediated interactions between VCAM-1 molecules on adjacent endothelial cells. Finally, we confirmed that anti-VCAM-1-D6 IgG had little effect on HUVEC viability, morphology or activation, suggesting that the antibody would be associated with a low level of endothelial cell toxicity in vivo.
In conclusion, we have shown that VCAM-1-D6 is a novel potential angiogenic target in TNFα-induced angiogenesis and that antibody targeting of VCAM-1-D6 may be effective in suppressing TNFα-induced angiogenesis. On the basis of currently available evidence, we suggest a mode of action whereby, in pathological conditions that are predominantly affected by TNFα-induced angiogenesis, anti-VCAM-1-D6 antibody binds to VCAM-1 expressed on the surface of TNFα-activated endothelial cells and directly blocks VCAM-1-D6-mediated interactions between VCAM-1 molecules on adjacent cells, resulting in efficient suppression of TNFα-induced angiogenesis. In future studies, we plan to investigate the mechanism of action of the anti-VCAM-1-D6 antibody and evaluate its in vivo efficacy in greater depth.
Acknowledgments
This work was supported by a research grant (10TS03) from the Scripps Korea Antibody Institute and partly by the Bio & Medical Technology Development Program of the National Research Foundation funded by the Korean government (Ministry of Science, ICT and Future Planning) (2015M3A9D9074279).
Footnotes
Supplementary Information accompanies the paper on Experimental & Molecular Medicine website (http://www.nature.com/emm)
The authors declare no conflict of interest.
Supplementary Material
References
- Nowak JZ. Age-related macular degeneration (AMD): pathogenesis and therapy. Pharmacol Rep 2006; 58: 353–363. [PubMed] [Google Scholar]
- Elshabrawy HA, Chen Z, Volin MV, Ravella S, Virupannavar S, Shahrara S. The pathogenic role of angiogenesis in rheumatoid arthritis. Angiogenesis 2015; 18: 433–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J. Diagnostic and therapeutic applications of angiogenesis research. C R Acad Sci III 1993; 316: 909–918. [PubMed] [Google Scholar]
- Aref AA. Current management of glaucoma and vascular occlusive disease. Curr Opin Ophthalmol 2015; 27: 140–145. [DOI] [PubMed] [Google Scholar]
- Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell 2011; 146: 873–887. [DOI] [PubMed] [Google Scholar]
- Hsu JY, Wakelee HA. Monoclonal antibodies targeting vascular endothelial growth factor: current status and future challenges in cancer therapy. BioDrugs 2009; 23: 289–304. [DOI] [PubMed] [Google Scholar]
- Syed BA, Evans JB, Bielory L. Wet AMD market. Nat Rev Drug Discov 2012; 11: 827. [DOI] [PubMed] [Google Scholar]
- Hammam O, Mahmoud O, Zahran M, Sayed A, Salama R, Hosny K et al. A possible role for TNF-alpha in coordinating inflammation and angiogenesis in chronic liver disease and hepatocellular carcinoma. Gastrointest Cancer Res 2013; 6: 107–114. [PMC free article] [PubMed] [Google Scholar]
- DeBusk LM, Chen Y, Nishishita T, Chen J, Thomas JW, Lin PC. Tie2 receptor tyrosine kinase, a major mediator of tumor necrosis factor alpha-induced angiogenesis in rheumatoid arthritis. Arthritis Rheum 2003; 48: 2461–2471. [DOI] [PubMed] [Google Scholar]
- Xiao Z, Liu Q, Mao F, Wu J, Lei T. TNF-alpha-induced VEGF and MMP-9 expression promotes hemorrhagic transformation in pituitary adenomas. Int J Mol Sci 2011; 12: 4165–4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodossiadis PG, Liarakos VS, Sfikakis PP, Vergados IA, Theodossiadis GP. Intravitreal administration of the anti-tumor necrosis factor agent infliximab for neovascular age-related macular degeneration. Am J Ophthalmol 2009; 147: 825–830. [DOI] [PubMed] [Google Scholar]
- Lichtlen P, Lam TT, Nork TM, Streit T, Urech DM. Relative contribution of VEGF and TNF-alpha in the cynomolgus laser-induced CNV model: comparing the efficacy of bevacizumab, adalimumab, and ESBA105. Invest Ophthalmol Vis Sci 2010; 51: 4738–4745. [DOI] [PubMed] [Google Scholar]
- Lai KC, Liu CJ, Lin TJ, Mar AC, Wang HH, Chen CW et al. Blocking TNF-alpha inhibits angiogenesis and growth of IFIT2-depleted metastatic oral squamous cell carcinoma cells. Cancer Lett 2016; 370: 207–215. [DOI] [PubMed] [Google Scholar]
- Welti J, Loges S, Dimmeler S, Carmeliet P. Recent molecular discoveries in angiogenesis and antiangiogenic therapies in cancer. J Clin Invest 2013; 123: 3190–3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong V. Ranibizumab for the treatment of wet AMD: a summary of real-world studies. Eye (Lond) 2015; 30: 270–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon SD, Lindsley KB, Krzystolik MG, Vedula SS, Hawkins BS. Intravitreal bevacizumab versus ranibizumab for treatment of neovascular age-related macular degeneration: findings from a Cochrane systematic review. Ophthalmology 2015; 123: 70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdi MH, Faramarzi MA, Nikfar S, Falavarjani KG, Abdollahi M. Ranibizumab and aflibercept for the treatment of wet age-related macular degeneration. Expert Opin Biol Ther 2015; 15: 1349–1358. [DOI] [PubMed] [Google Scholar]
- Kopetz S, Hoff PM, Morris JS, Wolff RA, Eng C, Glover KY et al. Phase II trial of infusional fluorouracil, irinotecan, and bevacizumab for metastatic colorectal cancer: efficacy and circulating angiogenic biomarkers associated with therapeutic resistance. J Clin Oncol 2010; 28: 453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucio-Eterovic AK, Piao Y, de Groot JF. Mediators of glioblastoma resistance and invasion during antivascular endothelial growth factor therapy. Clin Cancer Res 2009; 15: 4589–4599. [DOI] [PubMed] [Google Scholar]
- Carlos TM, Schwartz BR, Kovach NL, Yee E, Rosa M, Osborn L et al. Vascular cell adhesion molecule-1 mediates lymphocyte adherence to cytokine-activated cultured human endothelial cells. Blood 1990; 76: 965–970. [PubMed] [Google Scholar]
- Lee S, Chung J, Ha IS, Yi K, Lee JE, Kang HG et al. Hydrogen peroxide increases human leukocyte adhesion to porcine aortic endothelial cells via NFkappaB-dependent up-regulation of VCAM-1. Int Immunol 2007; 19: 1349–1359. [DOI] [PubMed] [Google Scholar]
- Polte T, Newman W, Raghunathan G, Gopal TV. Structural and functional studies of full-length vascular cell adhesion molecule-1: internal duplication and homology to several adhesion proteins. DNA Cell Biol 1991; 10: 349–357. [DOI] [PubMed] [Google Scholar]
- Ulbrich H, Eriksson EE, Lindbom L. Leukocyte and endothelial cell adhesion molecules as targets for therapeutic interventions in inflammatory disease. Trends Pharmacol Sci 2003; 24: 640–647. [DOI] [PubMed] [Google Scholar]
- Gorcyznski RM, Chung S, Fu XM, Levy G, Sullivan B, Chen Z. Manipulation of skin graft rejection in alloimmune mice by anti-VCAM-1:VLA-4 but not anti-ICAM-1:LFA-1 monoclonal antibodies. Transpl Immunol 1995; 3: 55–61. [DOI] [PubMed] [Google Scholar]
- Kanwar JR, Kanwar RK, Wang D, Krissansen GW. Prevention of a chronic progressive form of experimental autoimmune encephalomyelitis by an antibody against mucosal addressin cell adhesion molecule-1, given early in the course of disease progression. Immunol Cell Biol 2000; 78: 641–645. [DOI] [PubMed] [Google Scholar]
- Stegall MD, Dean PG, Ninova D, Cohen AJ, Shepard GM, Gup C et al. alpha4 integrin in islet allograft rejection. Transplantation 2001; 71: 1549–1555. [DOI] [PubMed] [Google Scholar]
- Carter RA, Campbell IK, O'Donnel KL, Wicks IP. Vascular cell adhesion molecule-1 (VCAM-1) blockade in collagen-induced arthritis reduces joint involvement and alters B cell trafficking. Clin Exp Immunol 2002; 128: 44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cybulsky MI, Iiyama K, Li H, Zhu S, Chen M, Iiyama M et al. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J Clin Invest 2001; 107: 1255–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Kim JH, Lee CS, Kim Y, Heo K, Ihara Y et al. Collapsin response mediator protein-2 inhibits neuronal phospholipase D(2) activity by direct interaction. J Biol Chem 2002; 277: 6542–6549. [DOI] [PubMed] [Google Scholar]
- Lee S, Yoon IH, Yoon A, Cook-Mills JM, Park CG, Chung J. An antibody to the sixth Ig-like domain of VCAM-1 inhibits leukocyte transendothelial migration without affecting adhesion. J Immunol 2012; 189: 4592–4601. [DOI] [PubMed] [Google Scholar]
- Ki MK, Jeoung MH, Choi JR, Rho SS, Kwon YG, Shim H et al. Human antibodies targeting the C-type lectin-like domain of the tumor endothelial cell marker clec14a regulate angiogenic properties in vitro. Oncogene 2013; 32: 5449–5457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TK, Na HJ, Lee WR, Jeoung MH, Lee S. Heat shock protein 70-1A is a novel angiogenic regulator. Biochem Biophys Res Commun 2015; 469: 222–228. [DOI] [PubMed] [Google Scholar]
- Kim TK, Park CS, Jeoung MH, Lee WR, Go NK, Choi JR et al. Generation of a human antibody that inhibits TSPAN8-mediated invasion of metastatic colorectal cancer cells. Biochem Biophys Res Commun 2015; 468: 774–780. [DOI] [PubMed] [Google Scholar]
- Klemke M, Weschenfelder T, Konstandin MH, Samstag Y. High affinity interaction of integrin alpha4beta1 (VLA-4) and vascular cell adhesion molecule 1 (VCAM-1) enhances migration of human melanoma cells across activated endothelial cell layers. J Cell Physiol 2007; 212: 368–374. [DOI] [PubMed] [Google Scholar]
- Kelly KA, Allport JR, Yu AM, Sinh S, Sage EH, Gerszten RE et al. SPARC is a VCAM-1 counter-ligand that mediates leukocyte transmigration. J Leukoc Biol 2007; 81: 748–756. [DOI] [PubMed] [Google Scholar]
- Barreiro O, Yanez-Mo M, Serrador JM, Montoya MC, Vicente-Manzanares M, Tejedor R et al. Dynamic interaction of VCAM-1 and ICAM-1 with moesin and ezrin in a novel endothelial docking structure for adherent leukocytes. J Cell Biol 2002; 157: 1233–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker DM, Jones SD, Levine HL. The therapeutic monoclonal antibody market. MAbs 2015; 7: 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.