Abstract
Hippo/YAP signaling is implicated in tumorigenesis and progression of various cancers. By inhibiting a plethora signaling cascades, resveratrol has strong anti-tumorigenic and anti-metastatic activity. In the present study, we demonstrate that resveratrol decreases the expression of YAP target genes. In addition, our data showed that resveratrol attenuates breast cancer cell invasion through the activation of Lats1 and consequent inactivation of YAP. Strikingly, we also demonstrate that resveratrol inactivates RhoA, leading to the activation of Lats1 and induction of YAP phosphorylation. Further, resveratrol in combination with other agents that inactivate RhoA or YAP showed more marked suppression of breast cancer cell invasion compared with single treatment. Collectively, these findings indicate the beneficial effects of resveratrol on breast cancer patients by suppressing the RhoA/Lats1/YAP signaling axis and subsequently inhibiting breast cancer cell invasion.
Introduction
The Hippo signaling pathway was initially shown to regulate organ size, and its dysregulation contributes to tumorigenesis.1, 2, 3 Components of the Hippo signaling pathway include Mst1/2 and Lats1/2. Mst1/2 forms a complex with the adapter protein Sav1 to phosphorylate and activate Lats1/2,4, 5 leading to inactivation of yes-associated protein (YAP) and TAZ, the key downstream effectors of the Hippo signaling pathway.6, 7, 8, 9, 10 Although phosphorylated YAP and TAZ are retained in the cytosol, unphosphorylated YAP and TAZ translocate into the nucleus and function as transcriptional activators by binding to TEA domain (TEADs). Notably, recent studies have shown that YAP11 and TAZ,12 located downstream of Lats, are associated with tumor progression in breast cancer cells.
The Rho GTPase family is a member of the Ras superfamily and is critical for the invasion and metastasis of various cancers, including breast cancer. Recently, RhoA has been implicated in the Hippo signaling pathway. Indeed, RhoA was suggested to mediate the lysophosphatidic acid (LPA) signal to YAP through Lats1/2 kinase activity.13 In addition, the gernaylgeranyl pyrophosphate produced by the mevalonate cascade is required for the activation of RhoA, which results in activation of YAP/TAZ in breast cancer cells.14 Furthermore, RhoA is critical for LPA- and epidermal growth factor (EGF)-induced breast cancer invasion.15, 16
The natural polyphenolic compound resveratrol (REV, trans-3,5,4-trihydroxystilbene) is found in peanuts, grapes and a variety of berries, as well as in food products, such as wine.17, 18 REV has been shown to inhibit the initiation and progression of several cancers, including breast cancer.19, 20, 21 We recently showed that REV inhibits EGF receptor phosphorylation to attenuate LPA-induced ovarian cancer invasion.22
Although Hippo/YAP signaling is closely associated with breast cancer cell progression, little is known the effect of REV on Hippo signaling and its association with breast cancer progression. Considering the anti-proliferative and anti-metastatic effects of REV on multiple tumors, exploring the function of REV on Hippo/YAP signaling is invaluable for uncovering the therapeutic potential of REV on cancer patients. In the present study, we demonstrated that REV attenuates LPA- and EGF-induced breast cancer invasion by inactivating YAP, leading to the downregulation of transcriptional products of YAP, such as amphiregulin (AREG), cysteine-rich angiogenic inducer 61 (CYR61) and connective tissue growth factor (CTGF). In addition, we observed that REV efficiently reduces LPA- and EGF-induced dephosphorylation of Lats1. Finally, and more importantly, our present data showed that REV directly inactivates RhoA. Collectively, this study indicates that REV reduces LPA- and EGF-induced breast cancer cell invasion through coordinated inactivation of RhoA with YAP phosphorylation, providing novel targets for breast cancer progression and indicating the potential therapeutic importance of REV for breast cancer patients.
Materials and methods
Reagents
REV, EGF and simvastatin were purchased from Sigma-Aldrich (St Louis, MO, USA). LPA was purchased from Avanti Polar Lipids (Albaster, AL, USA). All reagents were of the purest grade available.
Cell culture
The human breast cancer MDA-MB-231 and MDA-MB-468 cells were purchased from American Type Culture Collection (Manassas, VA, USA) and used between the 10th passage and 30th passage. The cells were cultured in RPMI 1640 with 10% fetal bovine serum (HyClone, Logan, UT, USA) and 1% penicillin/streptomycin (HyClone) in a humidified incubator under 5% CO2 at 37 °C.
Cell viability assay
The cells (5 × 104 cells per well) were seeded in 96-well plates for 24 h and then pretreated with the indicated amounts of REV for 24 h in serum-free medium. The cells were mixed with 0.5 mg ml−1 MTT ((3,4,5-dimethylthiazol-2yl)-5-diphenyl-tetrazolium bromide). After incubating for 3 h, 100 μl of dimethylsulphoxide was added to each well to extract the dye. The absorbance was subsequently measured at 540 nm using an enzyme-linked immunosorbent assay plate reader (Bio-Rad, Berkeley, CA, USA).
Reverse transcription-PCR quantification
Total RNA (1 μg) was primed with oligo (dT) and reverse-transcribed into complementary DNA using a Moloney murine leukemia virus reverse transcriptase (Promega, Madison, WI, USA). The complementary DNA was then subjected to PCR amplification with primer sets for AREG, CTGF, CYR61 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH): AREG forward (5′-TGGGGAAAAGTCCATGAAAA-3′), AREG reverse (5′-TGTGAGGATCACAGCAGACA-3′); CTGF forward (5′-CCAATGACAACGCCTCCTG-3′), CTGF reverse (5′-TGGTGCAGCCAGAAAGCTC-3′); CYR61 forward (5′-AGCCTCGCATCCTATACAACC-3′), CYR61 reverse (5′-TTCTTTCACAAGGCGGCACTC-3′); and GAPDH forward (5′-ACAGTCAGCCGCATCTTCTT-3′), GAPDH reverse (5′-ACGACCAAATCCGTTGACTC-3′).
Immunoblotting
After cells were washed with phosphate-buffered saline, they were incubated 10 min at room temperature in radioimmunoprecipitation assay lysis buffer with added protease inhibitor cocktail tablets. The cell lysates were scraped from the plates. Then, they were centrifuged at 13000 r.p.m. for 15 min at 4 °C, and protein concentrations were determined using BCA protein assay reagent (Thermo Fisher Scientific Inc., Rockford, IL, USA). Total cellular proteins were resolved by 8% SDS-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes (Millipore, Temecula, CA, USA). The membranes were blocked with 5% nonfat-dried milk in TBS-T, incubated for 2 h and then probed with the indicated primary antibodies. Then, the membranes were washed twice with washing buffer (0.1% Tween 20 in TBS) and incubated with secondary antibodies for 2 h. The membrane was washed twice again with washing buffer, and the immunoreactive bands were visualized by ECL (Thermo Fisher Scientific Inc.). The primary antibodies were used as follows: p-Lats1 (#9157, Cell Signaling Technology, Danvers, MA, USA), Lats1 (#3477, Cell Signaling Technology), p-YAP (#4911, Cell Signaling Technology), YAP (sc-15407, Santa Cruz Biotechnology, Texas, CA, USA), AREG (sc-25436, Santa Cruz Biotechnology), CTGF (sc-25440, Santa Cruz Biotechnology), CYR61 (sc-13100, Santa Cruz Biotechnology), RhoA (sc-179, Santa Cruz Biotechnology) and GAPDH (sc-25778, Santa Cruz Biotechnology).
Immunofluorescence
Cells were fixed with cold methanol for 15 min and washed twice with phosphate-buffered saline. Immunofluorescence was carried out with an anti-YAP antibody (1:100; sc-179, Santa Cruz Biotechnology) overnight as described previously.23 The nuclei were counterstained with 4′,6-diamidino-2-phenylindole. The cells were washed with ice-cold phosphate-buffered saline three times for 10 min and then incubated with goat anti-rabbit secondary antibody (green, 1:500; Jackson Immuno Research, West Grove, PA, USA) at room temperature. The cells were examined by confocal microscopy (× 200, LSM710; Carl Zeiss, Jena, Germany).
In vitro invasion and migration assay
In vitro invasion assays were performed in triplicate using an invasion assay kit with Matrigel-coated inserts (BD Bioscience, San Jose, CA, USA) as described previously.24 A volume of 1 × 106 cells per well was added to the upper compartment of the invasion chamber with or without pharmacological inhibitors in transfected cells. To the lower compartment, we added serum-free conditioned medium with or without attractants. In vitro migration assays were performed in triplicate using a 48-well microchemotaxis chamber as described previously.25 Type I collagen-coated polyvinyl pyrrolidine-free polycarbonate filters with 8 μm pore membranes (Neuro Probe, Gaithersburg, MD, USA) were used in these modified Boyden chambers. After the samples were incubated for 6 (for migration) and 17 h (for invasion) at 37 °C, the migrated or invaded cells were sequentially fixed, stained with Diff-Quik reagents (Dade Behring Inc., Newark, DE, USA) and quantitated by counting the number of cells in five random high-power fields for each replicate (× 200) with a light microscope.
Transfection of plasmid DNAs and siRNAs
The cells were transfected with the indicated plasmids and short interfering RNAs (siRNAs) with Lipofectamine RNAiMAX and Lipofectamine 2000 (Invitrogen Carlsbad, Temecula, CA, USA), according to the manufacturer's instructions, respectively. A RhoAV14 complementary DNA was subcloned into a pEGFPC1 vector, and the empty pEGFPC1 vector was used as a negative control. The siRNAs targeting YAP and TAZ were purchased from Sigma-Aldrich.
Analysis of RhoA GTPase activity
For the analysis of RhoA GTPase activity, the amount of RhoA-GTP bound to the Rhotekin RBD was determined using a RhoA Activation Assay kit (Millipore), according to the manufacturer's instructions as described previously.26
RhoA GLISA activation assay
The GLISA RhoA Activation Assay kit (Cytoskeleton, Denver, CO, USA) was used to analyze the GTP-RhoA in the cells, according to the manufacturer's instructions.
Statistics
Data are shown as the mean±s.d. Differences between two groups were assessed using Student's t-test. Differences among three or more groups were evaluated by analysis of variance, followed by Bonferroni multiple comparison tests.
Results
REV reduces the expression of YAP target genes
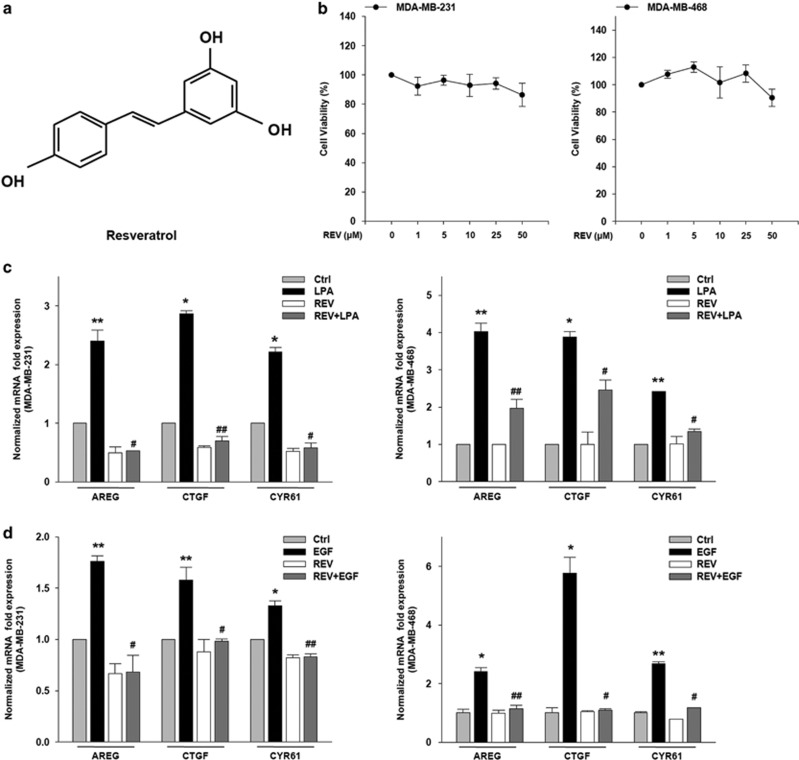
REV is a small molecule with the chemical structure of 3,5,4′-trihydroxy-trans-stilbene (Figure 1a). To identify an optimal concentration of REV that does not induce apoptosis, we first determined the toxicity of REV against breast cancer cells. Treatment of the cells with REV at 25 μM showed >90% (±s.d., n=3) of the cells survived (Figure 1b). Given that LPA27 and EGF have been shown to induce cancer cell invasion through the expression of YAP target genes, including AREG, CTGF and CYR61, we next determined the effect of REV on the LPA- and EGF-induced expression of YAP target genes. Stimulation of the breast cancer cells with LPA or EGF significantly increased the messenger RNA expression of transcriptional target genes of YAP (Figure 1c and d). However, pretreatment of the cells with REV efficiently inhibited the LPA- or EGF-induced expression of YAP target genes. Therefore, these data strongly suggest that REV, at a concentration with no significant toxicity to the tested cancer cells, attenuates LPA- and EGF-induced messenger RNA expression of YAP target genes.
Figure 1.
Resveratrol (REV) reduces the expression of yes-associated protein target genes. (a) The chemical structure of REV. (b) The viability of breast cancer cell was analyzed by (3,4,5-dimethylthiazol-2yl)-5-diphenyl-tetrazolium bromide (MTT) assays. The serum-starved cells were treated with REV for 24 h. (c, d) The serum-starved MDA-MB-231 and MDA-MB-468 cells were pretreated with REV (25 μM) for 1 h and then stimulated with lysophosphatidic acid (LPA; 5 μM) and epidermal growth factor (EGF; 100 ng ml−1) for 2 and 0.5 h, respectively. Quantitative reverse transcription-PCR (*P<0.05 and **P<0.01 versus control, #P<0.05 and ##P<0.01 versus LPA or epidermal growth factor (EGF) treatment only). All experiments were repeated three times.
REV inhibits LPA- and EGF-induced YAP activation
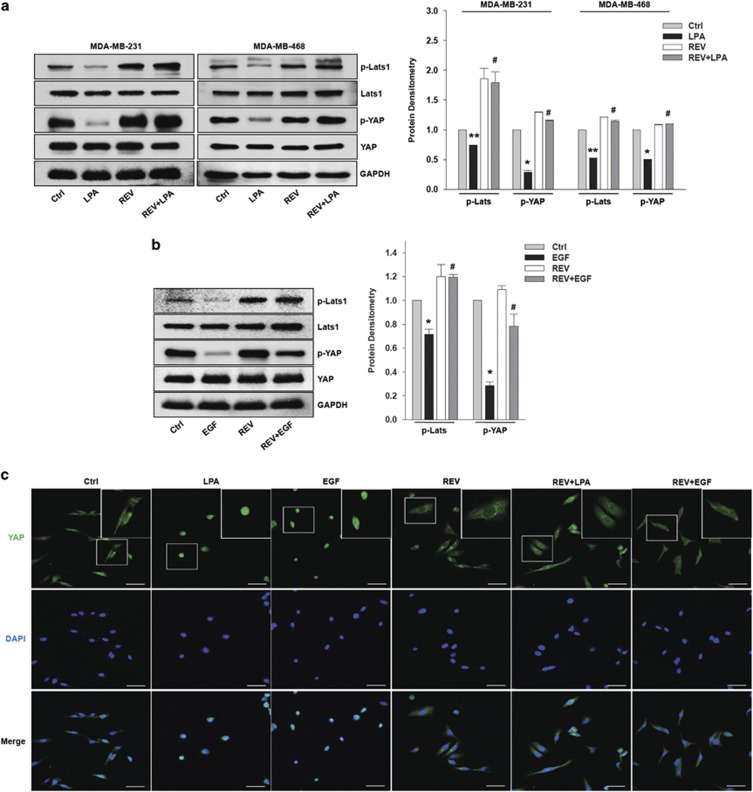
Given that LPA13 and EGF28, 29 induce AREG, CTGF and CYR61 expression through YAP activation, we next determined the effect of REV on LPA- and EGF-induced YAP activation. Pretreatment of the cells with REV strongly inhibited LPA- and EGF-induced YAP dephosphorylation (Figure 2a and b), suggesting that REV inactivates YAP, thereby inhibiting the messenger RNA expression of YAP target genes. In addition, we observed that REV recovers LPA- and EGF-induced Lats1 dephosphorylation. Considering that Lats1 phosphorylation governs YAP inactivation, our present results suggest that REV attenuates LPA- and EGF-induced YAP activation by restoring Lats1 phosphorylation. Consistent with this observation, immunofluorescence analyses showed that LPA and EGF translocate YAP from the cytosol to the nucleus, and that REV efficiently blocks LPA- and EGF-induced translocation of YAP into the nucleus (Figure 2c). Therefore, these results indicate that REV suppresses LPA- and EGF-induced YAP activation, leading to a reduction in the expression of YAP target genes.
Figure 2.
Resveratrol (REV) inhibits lysophosphatidic acid (LPA)- and epidermal growth factor (EGF)-induced YAP activation. (a–c) The serum-starved MDA-MB-231 and MDA-MB-468 cells were pretreated with REV (25 μM) for 1 h and then stimulated with LPA (5 μM) and EGF (100 ng ml−1) for 2 h and 0.5 h, respectively. (a, b) Immunoblotting. Immunoblot bands were quantified by ImageJ densitometric analysis and normalized to Lats1 and YAP, respectively (*P<0.05, **P<0.01 versus control, #P<0.05 versus LPA and EGF treatment only). (c) Immunofluorescence. Original magnification × 200; scale bar=50 μm. All experiments were repeated three times.
REV suppresses LPA- and EGF-induced breast cancer cell invasion
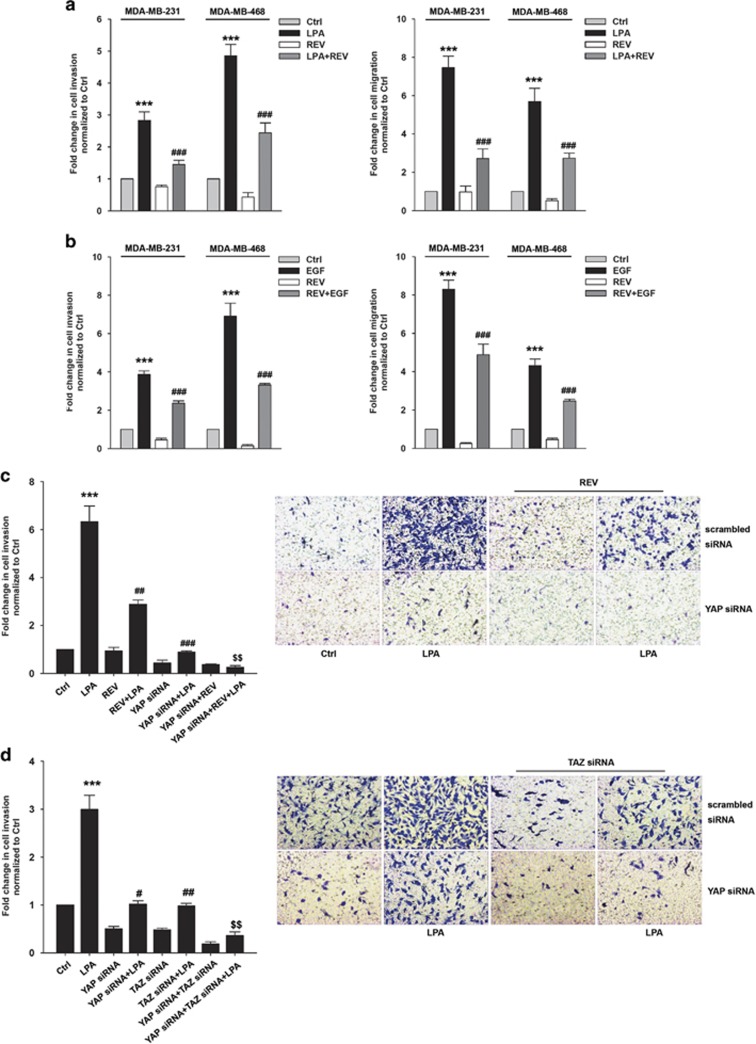
Previously, LPA was shown to induce YAP activation, leading to the secretion of AREG and ovarian cancer cell migration.27 Therefore, we next determined whether REV attenuates LPA- and EGF-induced breast cancer cell invasion and migration. Pretreatment of the cells with REV significantly reduced LPA- and EGF-induced breast cancer cell invasion and migration (Figure 3a and b). More importantly, combined treatment of the cells with REV and YAP siRNA showed stronger inhibition of LPA-induced breast cancer cell invasion compared with the single treatment (Figure 3c). To determine the roles of YAP and TAZ in LPA-induced breast cancer cell invasion, we transfected the cells with YAP- and TAZ-specific siRNAs. Silencing the YAP or TAZ expression significantly reduced LPA-induced breast cancer cell invasion (Figure 3d). In addition, silencing both YAP and TAZ expression showed a more marked inhibition of LPA-induced breast cancer cell invasion than silencing single protein expression. Therefore, these data suggest that both YAP and TAZ are important for LPA-induced breast cancer cell invasion, and that REV efficiently suppresses LPA-induced breast cancer cell invasion.
Figure 3.
Resveratrol (REV) suppresses lysophosphatidic acid (LPA)- and epidermal growth factor (EGF)-induced breast cancer cell invasion. (a, b) The serum-starved MDA-MB-231 and MDA-MB-468 cells were pretreated with REV (25 μM) for 1 h and in vitro invasion and migration were analyzed against LPA (5 μM) (a) and EGF (100 ng ml−1) (b) (***P<0.001 versus control, ###P<0.001 versus LPA and EGF treatment only). (c) The MDA-MB-231 cells were transfected with the indicated siRNA. Then, the cells were pretreated with REV (25 μM) for 1 h, followed by stimulation with or without LPA (5 μM; ***P<0.001 versus control, ##P<0.01 versus LPA treatment only, ###P<0.001 versus LPA treatment only, $$P<0.01 versus LPA treatment and YAP siRNA). Original magnification × 200. (d) The MDA-MB-231 cells were transfected with the indicated siRNA and then stimulated with or without LPA (5 μM; ***P<0.001 versus control, #P<0.05 versus LPA treatment only, ##P<0.01 versus LPA treatment only, $$P<0.01 versus TAZ siRNA and LPA treatment). Original magnification of all images, × 200. All experiments were repeated three times.
REV inactivates RhoA
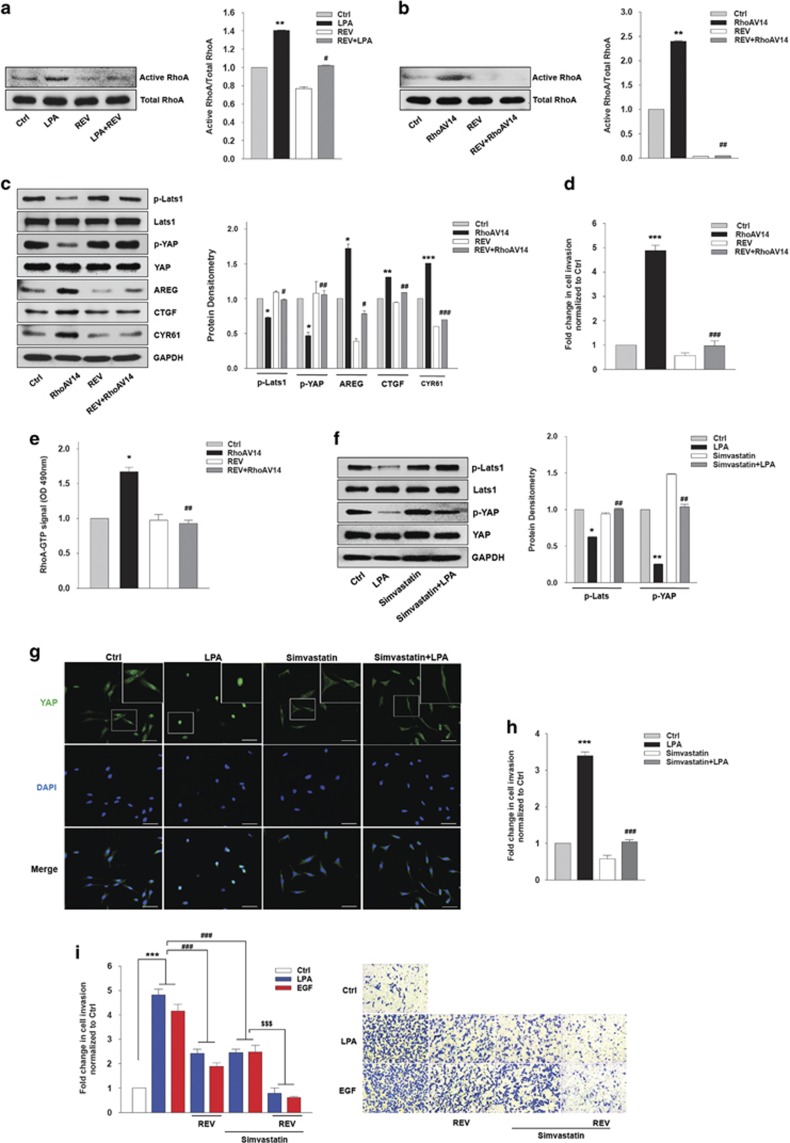
RhoA has been associated with LPA30- and EGF31,32,33-induced inhibition of Lats1/2 and consequent YAP activation. Therefore, we investigated whether REV activates LPA-induced Lats1 by inhibiting RhoA activation. Strikingly, REV strongly attenuated LPA-induced RhoA activation (Figure 4a). To determine whether REV directly inactivates RhoA, we utilized a constitutively active mutant of RhoA (RhoAV14). REV reduced the level of active RhoA induced by RhoAV14 (Figure 4b). In addition, transfection of the cells with RhoAV14 substantially reduced YAP phosphorylation and subsequently increased the expression of YAP target genes (Figure 4c). However, pretreatment of the cells with REV significantly recovered the YAP phosphorylation suppressed by RhoAV14. Furthermore, we observed that REV suppressed RhoAV14-induced breast cancer cell invasion (Figure 4d). In addition, REV reduced the RhoA activity induced by RhoAV14 (Figure 4e), suggesting that REV directly inactivates RhoA. Consistent with these observations, pretreatment of the cells with simvastatin, an inhibitor of geranlygeranylation of RhoA,34, 35 significantly inhibited LPA-induced dephosphorylation of Lats1 and consequent YAP activation (Figure 4f). In addition, simvastatin strongly inhibited LPA-induced YAP translocation into the nucleus (Figure 4g) as well as breast cancer cell invasion (Figure 4h). Furthermore, pretreatment of the cells with both REV and simvastatin showed more significant suppression of LPA- and EGF-induced breast cancer cell invasion compared with that of the single treatment (Figure 4i). Therefore, these results strongly suggest that REV inhibits LPA-induced YAP activation by inactivating RhoA, and that REV in combination with simvastatin can be used to improve survival of patients with breast cancer.
Figure 4.
Resveratrol (REV) inactivates RhoA. (a) The serum-starved MDA-MB-231 cells were pretreated with REV (25 μM) and then stimulated with lysophosphatidic acid (LPA; 5 μM) for 3 min. Active RhoA bands were quantified by ImageJ densitometric analysis and normalized to total RhoA (**P<0.01 versus control, #P<0.05 versus LPA treatment). (b–e) The MDA-MB-231 cells were transfected with the indicated vectors for 48 h and then treated with REV (25 μM) for 1 h. (b) RhoA-GTP pull-down assays. Active RhoA bands were quantified by ImageJ densitometric analysis and normalized to total RhoA (**P<0.01 versus control, ##P<0.05 versus transfection of RhoAV14). (c) Immunoblotting. Immunoblot bands were quantified by ImageJ densitometric analysis and normalized to the control (*P<0.05 versus control vector, **P<0.01 versus control vector, ***P<0.001 versus control vector, #P<0.05 versus transfection of RhoAV14, ##P<0.01 versus transfection of RhoAV14, ###P<0.001 versus transfection of RhoAV14). (d) In vitro invasion assay (***P<0.001 versus control vector, ###P<0.001 versus transfection of RhoAV14). (e) RhoA GLISA activation assay (*P<0.05 versus control, ##P<0.01 versus V14 RhoA treatment only). (f–h) The serum-starved MDA-MB-231 cells were pretreated with simvastatin (5 μM) for 1 h and then stimulated with LPA (5 μM) for 2 h. (f) Immunoblotting. Immunoblot bands were quantified by ImageJ densitometric analysis and normalized to Lats1 and YAP (*P<0.05, **P<0.01 versus control, ##P<0.01 versus LPA treatment only). (g) The expression of YAP was visualized by immunofluorescence. Original magnification × 200; scale bar, 50 μm. (h) In vitro invasion assay (***P<0.001 versus control, ###P<0.001 versus LPA treatment only). (i) The serum-starved MDA-MB-231 cells were pretreated with REV (25 μM) or simvastatin (5 μM) for 1 h and in vitro invasion was analyzed against LPA (5 μM) or epidermal growth factor (EGF; 100 ng ml−1; ***P<0.001 versus control, ###P<0.001 versus LPA or EGF treatment only, $$$P<0.001 versus LPA or EGF and simvastatin treatment). All experiments were repeated three times.
Discussion
The Hippo/YAP signaling pathway has been implicated in the tumorigenesis and progression of various types of cancer.36, 37, 38, 39 In the present study, we elucidated additional anti-invasive effects of REV on breast cancer cells. REV suppressed the LPA- and EGF-induced expression of YAP target genes. In addition, we observed that REV attenuates LPA- and EGF-induced breast cancer cell invasion via activation of Lats1 and consequent inactivation of YAP. More interestingly, we demonstrated for the first time that REV inactivates RhoA, leading to the activation of Lats1 and induction of YAP phosphorylation. Therefore, the present data provide another underlying mechanism by which REV attenuates breast cancer invasion, indicating the beneficial effects of REV on this deadly disease.
A natural compound, REV enhances apoptosis and suppresses tumor growth through multiple signaling pathways in various cancer cells.40, 41, 42, 43 Notably, REV decreases breast cancer cell invasion by inhibiting FAK,44 Rac and Cdc4245 activities. In addition, REV has been shown to downregulate MMP-9 expression46 and suppresses PI3K/Akt/Wnt/beta-catenin signaling.47 Furthermore, REV enhances the anticancer effects of doxorubicin in breast cancer cells.48 In the present study, we showed that REV inhibits the Hippo/YAP signaling pathway, leading to the downregulation of YAP target gene expression and consequent attenuation of breast cancer cell invasion. We observed that REV reduces the LPA- and EGF-induced expression of AREG, CTGF and CYR61. In addition, our data showed that REV recovers LPA- and EGF-induced Lats1 inactivation and subsequent YAP dephosphorylation. It is noteworthy that REV increased the phosphorylation of Lats1 and YAP, suggesting that REV also inhibits a basal Hippo/YAP signaling axis.
Previously, cerivastatin, which is used in the treatment of hypercholesterolemia by inhibiting HMG-CoA reductase, was reported to inhibit translocation of RhoA to the cell membrane and consequent RhoA activation and breast cancer cell invasion.34 Interestingly, geranylgeranyl pyrophosphate produced by the mevalonate cascade was required for activation of RhoA, leading to the activation of YAP/TAZ.14 Recent studies have shown discordant effects of REV on RhoA activation. Although indirect effects of REV on RhoA have been found, we reported that REV suppresses LPA-induced EGF receptor activation and subsequent inhibition a Ras/RhoA/ROCK signaling in ovarian cancer cells.22 In contrast, REV has been shown to induce RhoA activation in glioblastoma cells.49 In the present study, we demonstrated that REV directly reduces RhoA activity. REV treatment reduced the basal RhoA activity. In addition, REV inhibited LPA-induced RhoA activity and subsequent YAP activation in breast cancer cells. Furthermore, REV reduced RhoAV14-induced YAP dephosphorylation. Therefore, the effect of REV on RhoA activity appears to be context-dependent. Whether REV inactivates RhoA by suppressing HMG-CoA reductase is under current investigation.
In conclusion, our results demonstrate that REV directly inactivates RhoA, and that inactivated RhoA in turn phosphorylates YAP through the activation of Lats1, leading to the suppression of YAP target gene expression and consequent inhibition of breast cancer cell invasion. Therefore, REV may be used to reduce invasion and metastasis of breast cancer cells to improve outcomes for this devastating disease.
Acknowledgments
This study was supported by a grant from the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2014R1A2A1A11051091).
Footnotes
The authors declare no conflict of interest.
References
- Pan D. The hippo signaling pathway in development and cancer. Dev Cell 2010; 19: 491–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nat Rev Cancer 2013; 13: 246–257. [DOI] [PubMed] [Google Scholar]
- Zhao B, Tumaneng K, Guan KL. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat Cell Biol 2011; 13: 877–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilili GK, Kyriakis JM. Mammalian Ste20-like kinase (Mst2) indirectly supports Raf-1/ERK pathway activity via maintenance of protein phosphatase-2A catalytic subunit levels and consequent suppression of inhibitory Raf-1 phosphorylation. J Biol Chem 2010; 285: 15076–15087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praskova M, Xia F, Avruch J. MOBKL1A/MOBKL1B phosphorylation by MST1 and MST2 inhibits cell proliferation. Curr Biol 2008; 18: 311–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y, Chun A, Cheung K, Rashidi B, Yang X. Tumor suppressor LATS1 is a negative regulator of oncogene YAP. J Biol Chem 2008; 283: 5496–5509. [DOI] [PubMed] [Google Scholar]
- Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 2007; 130: 1120–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev 2007; 21: 2747–2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei QY, Zhang H, Zhao B, Zha ZY, Bai F, Pei XH et al. TAZ promotes cell proliferation and epithelial-mesenchymal transition and is inhibited by the hippo pathway. Mol Cell Biol 2008; 28: 2426–2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka T, Mazack V, Sudol M. Mst2 and Lats kinases regulate apoptotic function of Yes kinase-associated protein (YAP). J Biol Chem 2008; 283: 27534–27546. [DOI] [PubMed] [Google Scholar]
- Lamar JM, Stern P, Liu H, Schindler JW, Jiang ZG, Hynes RO. The Hippo pathway target, YAP, promotes metastasis through its TEAD-interaction domain. Proc Natl Acad Sci USA 2012; 109: E2441–E2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordenonsi M, Zanconato F, Azzolin L, Forcato M, Rosato A, Frasson C et al. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell 2011; 147: 759–772. [DOI] [PubMed] [Google Scholar]
- Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell 2012; 150: 780–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrentino G, Ruggeri N, Specchia V, Cordenonsi M, Mano M, Dupont S et al. Metabolic control of YAP and TAZ by the mevalonate pathway. Nat Cell Biol 2014; 16: 357–366. [DOI] [PubMed] [Google Scholar]
- Kim JH, Adelstein RS. LPA(1)-induced migration requires nonmuscle myosin II light chain phosphorylation in breast cancer cells. J Cell Physiol 2011; 226: 2881–2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison SM, Knifley T, Chen M, O'Connor KL. LPA, HGF, and EGF utilize distinct combinations of signaling pathways to promote migration and invasion of MDA-MB-231 breast carcinoma cells. BMC Cancer 2013; 13: 501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Catana F, Yang Y, Roderick R, van Breemen RB. An LC-MS method for analyzing total resveratrol in grape juice, cranberry juice, and in wine. J Agric Food Chem 2002; 50: 431–435. [DOI] [PubMed] [Google Scholar]
- Burns J, Yokota T, Ashihara H, Lean ME, Crozier A. Plant foods and herbal sources of resveratrol. J Agric Food Chem 2002; 50: 3337–3340. [DOI] [PubMed] [Google Scholar]
- Lin HY, Tang HY, Keating T, Wu YH, Shih A, Hammond D et al. Resveratrol is pro-apoptotic and thyroid hormone is anti-apoptotic in glioma cells: both actions are integrin and ERK mediated. Carcinogenesis 2008; 29: 62–69. [DOI] [PubMed] [Google Scholar]
- Chin YT, Hsieh MT, Yang SH, Tsai PW, Wang SH, Wang CC et al. Anti-proliferative and gene expression actions of resveratrol in breast cancer cells in vitro. Oncotarget 2014; 5: 12891–12907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HY, Delmas D, Vang O, Hsieh TC, Lin S, Cheng GY et al. Mechanisms of ceramide-induced COX-2-dependent apoptosis in human ovarian cancer OVCAR-3 cells partially overlapped with resveratrol. J Cell Biochem 2013; 114: 1940–1954. [DOI] [PubMed] [Google Scholar]
- Jeong KJ, Cho KH, Panupinthu N, Kim H, Kang J, Park CG et al. EGFR mediates LPA-induced proteolytic enzyme expression and ovarian cancer invasion: inhibition by resveratrol. Mol Oncol 2013; 7: 121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi MJ, Cho KH, Lee S, Bae YJ, Jeong KJ, Rha SY et al. hTERT mediates norepinephrine-induced Slug expression and ovarian cancer aggressiveness. Oncogene 2015; 34: 3402–3412. [DOI] [PubMed] [Google Scholar]
- Jeong KJ, Park SY, Cho KH, Sohn JS, Lee J, Kim YK et al. The Rho/ROCK pathway for lysophosphatidic acid-induced proteolytic enzyme expression and ovarian cancer cell invasion. Oncogene 2012; 31: 4279–4289. [DOI] [PubMed] [Google Scholar]
- Seo JH, Jeong KJ, Oh WJ, Sul HJ, Sohn JS, Kim YK et al. Lysophosphatidic acid induces STAT3 phosphorylation and ovarian cancer cell motility: their inhibition by curcumin. Cancer Lett 2010; 288: 50–56. [DOI] [PubMed] [Google Scholar]
- Jeong KJ, Park SY, Cho KH, Sohn JS, Lee J, Kim YK et al. The Rho/ROCK pathway for lysophosphatidic acid-induced proteolytic enzyme expression and ovarian cancer cell invasion. Oncogene 2012; 31: 4279–4289. [DOI] [PubMed] [Google Scholar]
- Cai H, Xu Y. The role of LPA and YAP signaling in long-term migration of human ovarian cancer cells. Cell Commun Signal 2013; 11: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Ji JY, Yu M, Overholtzer M, Smolen GA, Wang R et al. YAP-dependent induction of amphiregulin identifies a non-cell-autonomous component of the Hippo pathway. Nat Cell Biol 2009; 11: 1444–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan R, Kim NG, Gumbiner BM. Regulation of Hippo pathway by mitogenic growth factors via phosphoinositide 3-kinase and phosphoinositide-dependent kinase-1. Proc Natl Acad Sci USA 2013; 110: 2569–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward Y, Lake R, Yin JJ, Heger CD, Raffeld M, Goldsmith PK et al. LPA receptor heterodimerizes with CD97 to amplify LPA-initiated RHO-dependent signaling and invasion in prostate cancer cells. Cancer Res 2011; 71: 7301–7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li KJ, Siao SC, Wu CH, Shen CY, Wu TH, Tsai CY et al. EGF receptor-dependent mechanism may be involved in the Tamm-Horsfall glycoprotein-enhanced PMN phagocytosis via activating Rho family and MAPK signaling pathway. Molecules 2013; 19: 1328–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlienger S, Campbell S, Claing A. ARF1 regulates the Rho/MLC pathway to control EGF-dependent breast cancer cell invasion. Mol Biol Cell 2014; 25: 17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilcrease MZ, Zhou X, Lu X, Woodward WA, Hall BE, Morrissey PJ. Alpha6beta4 integrin crosslinking induces EGFR clustering and promotes EGF-mediated Rho activation in breast cancer. J Exp Clin Cancer Res 2009; 28: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denoyelle C, Vasse M, Korner M, Mishal Z, Ganne F, Vannier JP et al. Cerivastatin, an inhibitor of HMG-CoA reductase, inhibits the signaling pathways involved in the invasiveness and metastatic properties of highly invasive breast cancer cell lines: an in vitro study. Carcinogenesis 2001; 22: 1139–1148. [DOI] [PubMed] [Google Scholar]
- Wang Z, Wu Y, Wang H, Zhang Y, Mei L, Fang X et al. Interplay of mevalonate and Hippo pathways regulates RHAMM transcription via YAP to modulate breast cancer cell motility. Proc Natl Acad Sci USA 2014; 111: E89–E98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall CA, Wang R, Miao J, Oliva E, Shen X, Wheeler T et al. Hippo pathway effector Yap is an ovarian cancer oncogene. Cancer Res 2010; 70: 8517–8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Dong Q, Zhang Q, Li Z, Wang E, Qiu X. Overexpression of yes-associated protein contributes to progression and poor prognosis of non-small-cell lung cancer. Cancer Sci 2010; 101: 1279–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T, Murakami H, Fujii M, Ishiguro F, Tanaka I, Kondo Y et al. YAP induces malignant mesothelioma cell proliferation by upregulating transcription of cell cycle-promoting genes. Oncogene 2012; 31: 5117–5122. [DOI] [PubMed] [Google Scholar]
- Ge L, Smail M, Meng W, Shyr Y, Ye F, Fan KH et al. Yes-associated protein expression in head and neck squamous cell carcinoma nodal metastasis. PLoS ONE 2011; 6: e27529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement MV, Hirpara JL, Chawdhury SH, Pervaiz S. Chemopreventive agent resveratrol, a natural product derived from grapes, triggers CD95 signaling-dependent apoptosis in human tumor cells. Blood 1998; 92: 996–1002. [PubMed] [Google Scholar]
- Carbo N, Costelli P, Baccino FM, Lopez-Soriano FJ, Argiles JM. Resveratrol, a natural product present in wine, decreases tumour growth in a rat tumour model. Biochem Biophy Res Commun 1999; 254: 739–743. [DOI] [PubMed] [Google Scholar]
- Huang C, Ma WY, Goranson A, Dong Z. Resveratrol suppresses cell transformation and induces apoptosis through a p53-dependent pathway. Carcinogenesis 1999; 20: 237–242. [DOI] [PubMed] [Google Scholar]
- Hsieh TC, Wu JM. Differential effects on growth, cell cycle arrest, and induction of apoptosis by resveratrol in human prostate cancer cell lines. Experi Cell Res 1999; 249: 109–115. [DOI] [PubMed] [Google Scholar]
- Azios NG, Dharmawardhane SF. Resveratrol and estradiol exert disparate effects on cell migration, cell surface actin structures, and focal adhesion assembly in MDA-MB-231 human breast cancer cells. Neoplasia 2005; 7: 128–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azios NG, Krishnamoorthy L, Harris M, Cubano LA, Cammer M, Dharmawardhane SF. Estrogen and resveratrol regulate Rac and Cdc42 signaling to the actin cytoskeleton of metastatic breast cancer cells. Neoplasia 2007; 9: 147–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang FY, Chiang EP, Sun YC. Resveratrol inhibits heregulin-beta1-mediated matrix metalloproteinase-9 expression and cell invasion in human breast cancer cells. J Nutr Biochem 2008; 19: 287–294. [DOI] [PubMed] [Google Scholar]
- Tsai JH, Hsu LS, Lin CL, Hong HM, Pan MH, Way TD et al. 3,5,4'-Trimethoxystilbene, a natural methoxylated analog of resveratrol, inhibits breast cancer cell invasiveness by downregulation of PI3K/Akt and Wnt/beta-catenin signaling cascades and reversal of epithelial-mesenchymal transition. Toxicol Appl Pharmacol 2013; 272: 746–756. [DOI] [PubMed] [Google Scholar]
- Rai G, Mishra S, Suman S, Shukla Y. Resveratrol improves the anticancer effects of doxorubicin in vitro and in vivo models: A mechanistic insight. Phytomedicine 2016; 23: 233–242. [DOI] [PubMed] [Google Scholar]
- Xiong W, Yin A, Mao X, Zhang W, Huang H, Zhang X. Resveratrol suppresses human glioblastoma cell migration and invasion via activation of RhoA/ROCK signaling pathway. Oncol Lett 2016; 11: 484–490. [DOI] [PMC free article] [PubMed] [Google Scholar]