Abstract
Free fatty acids (FFAs) are important substrates for mitochondrial oxidative metabolism and ATP synthesis but also cause serious stress to various tissues, contributing to the development of metabolic diseases. CD36 is a major mediator of cellular FFA uptake. Inside the cell, saturated FFAs are able to induce the production of cytosolic and mitochondrial reactive oxygen species (ROS), which can be prevented by co-exposure to unsaturated FFAs. There are close connections between oxidative stress and organellar Ca2+ homeostasis. Highly oxidative conditions induced by palmitate trigger aberrant endoplasmic reticulum (ER) Ca2+ release and thereby deplete ER Ca2+ stores. The resulting ER Ca2+ deficiency impairs chaperones of the protein folding machinery, leading to the accumulation of misfolded proteins. This ER stress may further aggravate oxidative stress by augmenting ER ROS production. Secondary to ER Ca2+ release, cytosolic and mitochondrial matrix Ca2+ concentrations can also be altered. In addition, plasmalemmal ion channels operated by ER Ca2+ depletion mediate persistent Ca2+ influx, further impairing cytosolic and mitochondrial Ca2+ homeostasis. Mitochondrial Ca2+ overload causes superoxide production and functional impairment, culminating in apoptosis. This vicious cycle of lipotoxicity occurs in multiple tissues, resulting in β-cell failure and insulin resistance in target tissues, and further aggravates diabetic complications.
Introduction
Free fatty acids (FFAs) are important sources of fuel required for efficient cellular energy production. FFAs enter mitochondria via carnitine palmitoyltransferase 1 (CPT1) and undergo β-oxidation to generate acetyl-CoA, which serves as a substrate for the Krebs cycle. Fatty acid metabolism generates reducing equivalents used by the electron transport chain (ETC) for ATP synthesis.1 Increased β-oxidation attenuates further mitochondrial FFA uptake through the formation of malonyl CoA, an inhibitor of CPT1. Excess FFA critically induces reactive oxygen species (ROS) generation, resulting in lipotoxicity associated with ER stress, calcium dysregulation, mitochondrial dysfunction and cell death.
Palmitate, stearate and oleate are the most abundant FFAs, accounting for 70–80% of total plasma FFAs.2 FFA concentrations in patients with type 2 diabetes are significantly higher than in healthy subjects.3, 4 Compared with normal subjects, rates of palmitate appearance in plasma are 1.5- and 3-fold higher in type 2 diabetic individuals during nocturnal and postprandial states, respectively.4 In the Paris Prospective Study, increased plasma FFA concentration and decreased 2-h plasma insulin levels are considered to be independent predictors of type 2 diabetes in subjects with a history of impaired glucose tolerance. Among impaired glucose tolerance subjects who develop type 2 diabetes, 78% are in the highest tertile of fasting FFA concentrations. It has been suggested that lipotoxicity is associated with uncompensated insulin secretion in patients with insulin resistance, leading to overt type 2 diabetes.5
In this review, we summarize the molecular mechanisms leading to palmitate-induced toxicity in type 2 diabetes, including sources of ROS generation and Ca2+-mediated pathogenic changes. These mechanisms show harmful cross-interactions. Endoplasmic reticulum (ER) Ca2+ release due to palmitate-induced oxidative stress results in cytosolic and mitochondrial Ca2+ overload, which may further accelerate ROS generation from mitochondria and facilitate permeability transition (PT) pore opening. The activation of store-operated Ca2+ (SOC) entry triggered by ER Ca2+ depletion augments the persistent Ca2+ load. The interruption of such vicious cycles of ROS formation and Ca2+ dysregulation may be a good therapeutic target for the prevention and treatment of metabolic diseases related to lipotoxicity.
CD36: Fatty acid transporter or receptor?
CD36 is an 88-kDa, ditopic, heavily N-linked glycosylated transmembrane protein that is also known as fatty acid translocase (FAT).6 CD36 is abundantly expressed in tissues with a high capacity for fatty acid metabolism (for example, adipose tissue, cardiac and skeletal muscles).6, 7, 8 Other cells and tissues including liver,9 endothelial cells,10 monocytes, macrophages,11, 12 pancreatic β-cells13 and podocytes14 also express CD36.
Muscle-specific over-expression of CD36 enhances FFA uptake and thus decreases plasma triglyceride and fatty acids levels.15 Conversely, FFA uptake is impaired in CD36 null mice with high plasma concentrations of cholesterol and triglyceride.16 CD36 expression is low in normal hepatocytes and does not have a significant role in FFA uptake.8, 9, 17, 18, 19 The Pro90Ser CD36 mutation in humans perturbs the FFA uptake of muscle and adipose tissue, but hepatic uptake is not affected under suppressed or slightly increased concentrations of palmitate.18 Consistently, hepatic FFA uptake is not disturbed in CD36 knockout mice.8 Under a high-fat diet or in hepatic steatosis, CD36 is highly inducible by activation of nuclear receptors, including liver X receptor, pregnane X receptor, peroxisome proliferator-activated receptor γ and the aryl hydrocarbon receptor.9, 17, 19 However, controversies arise concerning the impact of CD36 on fatty liver disease. Hepatocyte-specific CD36 disruption significantly reduces hepatic triacylglycerol, diacylglycerol (DAG) and cholesterol ester content and improves insulin sensitivity when a high-fat diet is consumed.19 However, liver-specific CD36 overexpression attenuated hepatic steatosis and insulin resistance in another study with transgenic mice.17, 19
In addition to its role in FFA transport, CD36 has an important role in signal transduction through the activation of non-receptor tyrosine kinases of the Src family, including Fyn and Lyn.20, 21 The binding of long chain (LC)-FFAs to CD36 stimulates the tyrosine phosphorylation of downstream proteins, inducing pro-inflammatory and atherogenic responses associated with diabetes, atherosclerosis, thrombosis, and Alzheimer disease.20 Ligand binding to CD36 also stimulates phospholipase C (PLC) and, as a consequence, IP3-mediated ER Ca2+ release. This signaling pathway contributes, for example, to the sensing of LC-FFA in taste buds.22 In addition, CD36 stimulates SOC influx. The associated increase in Ca2+ activates Ca2+-dependent phospholipase A2 and prostaglandin synthesis involved in inflammatory responses.21
Interestingly, CD36 is upregulated in response to high glucose in insulin-secreting cells and in patients with diabetic nephropathy. Such regulation of CD36 expression may lead to the exacerbation of glucolipotoxicity via increased FFA uptake.23, 24 In insulinoma cells, CD36 induction increases the uptake of FFA, leading to the blunting of the functional interplay between glucose and lipids in insulin secretion as a consequence of impaired oxidative metabolism.25 The disruption of the CD36 gene, however, protects from obesity-associated steatosis and insulin resistance.26 In diabetic animals, a lack of CD36 attenuates NADPH oxidase (NOX)-dependent ROS generation. Moreover, the targeted disruption of CD36 in macrophages shows protective action against atherosclerosis.27 Therefore, CD36 could be a therapeutic target for the treatment of metabolic dysfunction worsened by dyslipidemia.
Sulfo-N-succinimidyl derivatives have been developed as selective inhibitors for CD36.28, 29 Preincubation with a CD36 inhibitor prevents saturated FFA-induced ROS production and cytotoxicity.24, 30 Sulfo-N-succinimidyl derivatives also inhibit oxidized low-density lipoprotein (oxLDL) uptake in macrophages.21 Recently, Souza et al.31 demonstrated that the 5A peptide antagonizes oxLDL binding to CD36, inhibiting inflammation and oxidative stress in vascular tissues. The 5A peptide, through its inhibition of CD36, also reduces glomerular injury and tubule-interstitial fibrosis in animal models of chronic kidney disease.31
Oxidative stress induced by fatty acids
Reactive oxygen species are essential signaling molecules that regulate physiological cell functions.32 However, the overproduction of ROS in pathologic conditions has detrimental consequences, causing organellar stress, injury and cell death.33, 34 Palmitate is a potent inducer of ROS in a number of cell types, including pancreatic β cells,35, 36, 37 cardiomyocytes,34, 38 vascular smooth muscle cells,39 endothelial cells,40 skeletal muscle cells,41 glomerular podocytes,30 hepatocytes42 and adipocytes.43 CD36 appears to be required for fatty acid-induced ROS production due to the fact that the knockdown of CD36 prevents palmitate-dependent oxidative stress.23
Increased mitochondrial fatty acid oxidation has been proposed as the main process leading to ROS generation in lipotoxicity (Figure 1). The oxidation of palmitate delivers excess electrons to the ETC, which thus causes superoxide overproduction.44, 45, 46 There are, however, conflicting data in the literature showing that the acceleration of β-oxidation actually relieves oxidative stress, and the inhibition of mitochondrial fatty acid uptake aggravates ROS production.47, 48 The molecular mechanisms for cellular ROS generation by palmitate, therefore, remain to be fully elucidated.
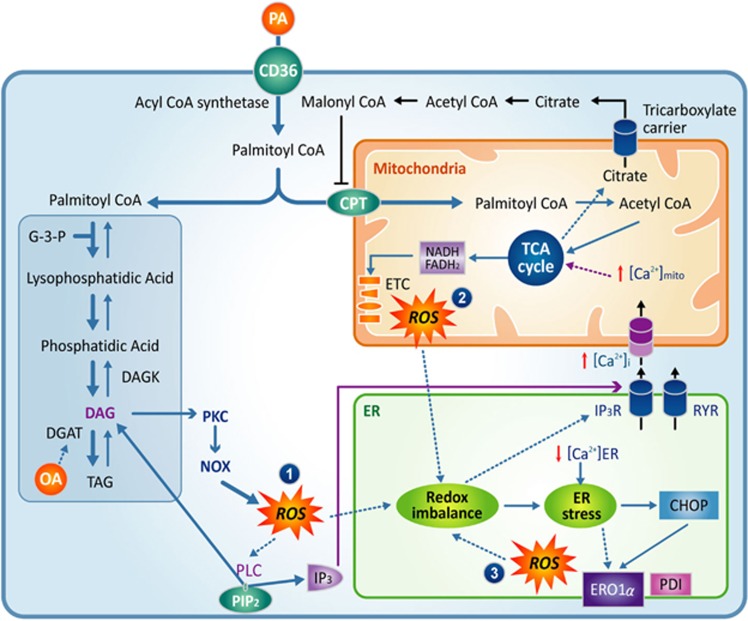
Figure 1.
Palmitate induces ROS overproduction. (1) Increased β-oxidation, (2) DAG-PKC-NOX, (3) CHOP-ERO1α and PDI under ER stress. ROS produced by palmitate triggers PLC activation, ER Ca2+ release, ER stress and mitochondrial dysfunction, which, in turn, aggravate ROS generation. CHOP, CCAAT-enhancer-binding protein homologous protein; DAG, diacylglycerol; ERO1α, ER oxidoreductin 1 alpha; NOX, NADPH oxidase; PDI, protein disulfide isomerase; PKC, protein kinase C; PLC, phospholipase C; ROS, reactive oxygen species.
Palmitate-induced superoxide cannot be fully eliminated by the addition of the complex III inhibitor antimycin A, revealing that ROS are also generated through sources other than the ETC.44 In chondrocytes, a mixture of oleate and palmitate enhances ROS production and induces cell apoptosis, mainly by upregulating the protein levels of NOX4.49 Notably, NOX4 is expressed in mitochondria and contributes to mitochondrial ROS production.50, 51 A recent study suggests that the activation of protein kinase Cα (PKCα) by palmitate increases ROS production through NOX2 upregulation in cardiomyocytes.38 An upstream stimulus of PKC is DAG, which is produced either in a membrane-delimited manner with Gq/11-coupled PLC or by enzymatic synthesis from phosphatidic acid. Palmitate increases DAG in a number of cell types.52, 53, 54 The formation of this signaling molecule may be responsible for palmitate-induced PKC activation and ROS generation (Figure 1). Moreover, there is crosstalk between mitochondria and the NADPH oxidase system via feed-forward amplification of ROS production.55 The involvement of ER oxidoreductin 1 alpha (ERO1α) and disulfide isomerase during ER stress, as well as ER-mitochondrial Ca2+ dysregulation in ROS overproduction, will be discussed later in this review.
Unlike palmitate, oleate is an unsaturated fatty acid with a cis double bond at position 9. Oleate may stimulate ROS generation but may also protect from oxidative stress. Oleate has been reported to increase intracellular H2O2 production in rat smooth muscle cells,56 pancreatic β-cells,57 and human hepatoma HepG2 cells.58 Other studies, however, reported no effect of oleate on ROS generation in smooth muscle cells from the human coronary artery59 or Chang liver cells.60 Oleate is even able to attenuate or abolish palmitate-induced ROS synthesis when the two fatty acids are used in combination.30, 61 Despite apparently conflicting data, there is some convincing evidence that oleate does not increase mitochondrial ROS level when employing a technique specifically detecting mitochondrial ROS.38, 61 Reduced ROS generation in the presence of oleate is correlated with a protective effect of unsaturated FFAs on ER stress and cytotoxicity.
Palmitate induces ER stress
Approximately one-third of all newly synthesized proteins are imported into the ER.62 Proteins trafficking through the ER undergo post-translational processing modifications, including glycosylation and chaperone-assisted protein folding. The oxidative folding process, especially the generation of disulfide bonds, generates a large amount of ROS.63 Therefore, redox homeostasis is vital to maintain ER folding capacity. Palmitate-induced ROS formation impairs ER redox status and leads to the accumulation of misfolded or unfolded proteins.64, 65 The associated excess workload beyond the protein folding capacity of the ER activates the unfolded protein response in an attempt to reestablish normal ER function.66 Unfolded protein response -dependent signaling is initiated by three ER transmembrane proteins: inositol-requiring protein 1α (IRE1α), protein kinase RNA-like endoplasmic reticulum kinase (PERK) and activating transcription factor 6 (ATF6). These stress sensors normally bind to a luminal ER chaperone called the binding immunoglobulin protein (BiP or GRP78). BiP has a high affinity for unfolded proteins. As unfolded proteins accumulate in the ER lumen, BiPs detach from the stress sensors activating downstream signaling, leading to three main outcomes: (1) the overall attenuation of translation, with the simultaneous (2) promotion of the translation of ER chaperones and (3) the restoration of the ER-associated degradation (ERAD) system.67, 68 If the stress is too severe to be resolved by the unfolded protein response, the cell triggers a death program to be eliminated.
The condensation of palmitoyl-CoA, the activated form of palmitate and serine, is the first step in the biosynthesis of ceramide, which is catalyzed by serine palmitoyltransferase. Ceramide activates protein phosphatase 2A and PKC, both of which can inhibit Akt activation, leading to insulin resistance in skeletal muscle and adipose tissue.69, 70 This pathogenic process activates pro-apoptotic signaling and cytochrome c release from mitochondrial inter-membrane space.71 Ceramide also inhibits mitochondrial beta-oxidation, which aggravates palmitate-induced lipotoxicity.72 Intriguingly, ceramide induces the loss of the ER calcium pool and ER stress. The inhibition of the sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) by ceramide has been suggested as the main mechanism of ER calcium depletion.73
Unlike palmitate, oleate does not cause a significant ER stress response.30, 38, 61 Moreover, oleate prevents palmitate-induced ER stress, c-Jun N-terminal kinase (JNK) activation and cell death,74, 75 all of which are consistent with reduced ROS generation. A key difference between the two fatty acids is that oleate, but not palmitate, activates diacylglycerol acyl transferase (DGAT). The stimulation of DGAT lessens DAG accumulation by converting it to triacylglycerol.76, 77, 78 Using 3H-labeled palmitate, it was shown that oleate attenuates palmitate-induced DAG formation and instead leads to the preferential accumulation of triacylglycerol.79 Oleate promotes the mitochondrial oxidation of palmitate by increasing CPT1 expression. This mechanism contributes to diminished total palmitate and palmitate-derived toxic metabolites.78
The ER stress response could be a therapeutic target to prevent palmitate-induced lipotoxicity. There have been attempts to tackle diseases of protein misfolding, such as cystic fibrosis, α1 antitrypsin deficiency, Alzheimer disease and type 2 diabetes, using the chemical chaperone 4-phenylbutyric acid.80, 81, 82, 83 Taurine-conjugated ursodeoxycholic acid (TUDCA) has also been tested as a chaperone to protect hepatocytes from palmitate-induced ER stress and apoptosis.84 Salubrinal, a selective chemical inhibitor of eIF2α phosphatase, was introduced to prevent ER stress.85 Further studies revealed, however, that salubrinal treatment shows deleterious effects in pancreatic β-cells and other cell types.86, 87
Several studies have demonstrated that knockdown of ER stress proteins (for example, CCAAT-enhancer-binding protein homologous protein (CHOP)) has protective effects on palmitate-induced apoptosis in insulin-secreting cells,68, 88 podocytes75 and other cell types.84, 89 However, CHOP knockout mice suffer from steatohepatitis and fibrosis due to the pro-inflammatory actions of CHOP-deleted macrophages in the liver. Therefore, more research is required to find better interventions to prevent palmitate-induced ER stress without serious adverse events.
ER calcium depletion by palmitate
Luminal ER Ca2+ concentration is particularly important for protein folding. High levels of Ca2+ in the ER lumen (>400 μM) are required for interactions among ER chaperones and between chaperones and unfolded proteins.66 SERCA maintains high ER Ca2+ concentrations. A chronic reduction of ER Ca2+ stores elicits the accumulation of unfolded or misfolded proteins and initiates an ER stress response (Figure 2).66, 90 Exposure to thapsigargin, an inhibitor of SERCA, is applied to induce ER stress experimentally by depleting the ER Ca2+ stores. Palmitate-induced ER stress is also associated with a sustained reduction of the ER Ca2+ pool, which has been demonstrated directly through cytosolic and ER Ca2+ measurements.30, 68, 88, 91 ER Ca2+ loss caused by FFA triggers the unfolded protein response to rescue cells from misfolded protein overload or programmed cell death.92
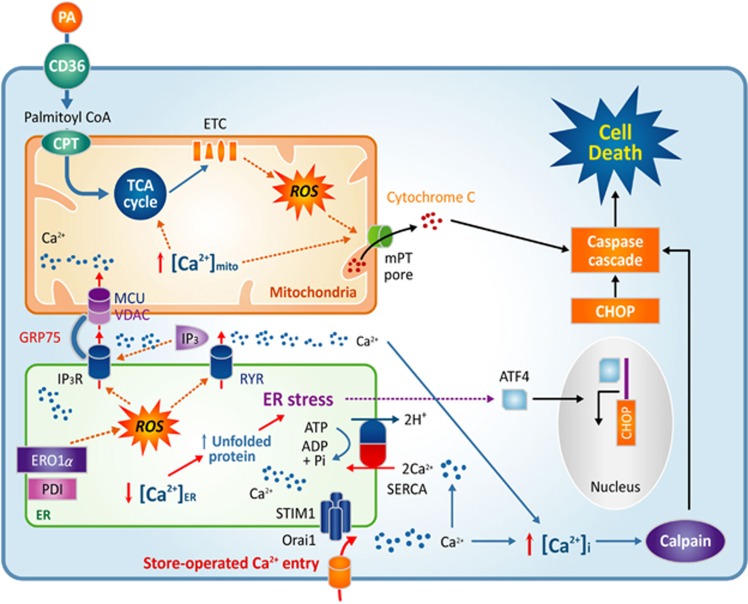
Figure 2.
Palmitate disturbs intracellular Ca2+ homeostasis. ROS activate IP3R and RYR, which release Ca2+ from the ER. The deprivation of ER Ca2+ leads to ER stress and CHOP upregulation. Ca2+ is transported into mitochondria through a specialized structure composed of IP3R, VDAC, MCU and GRP75. Excessive Ca2+ in mitochondria leads to cytochrome c release. SOC entry triggered by ER Ca2+ depletion elicits the persistent influx of Ca2+ into cytosol and mitochondria. High intracellular calcium activates calpain signaling. Cytochrome c, CHOP and calpain all provoke caspase activation and cell death. CHOP, CCAAT-enhancer-binding protein homologous protein; GRP75, 75 kDa glucose-regulated protein; MCU, mitochondrial Ca2+ uniporter; ROS, reactive oxygen species; SOC, store-operated Ca2+; VDAC, voltage-dependent anion channel.
ER proteome analysis in the liver of ob/ob mice shows a fundamental shift in ER function in obesity from protein synthesis to lipid synthesis and metabolism.93 One important factor inducing ER calcium depletion in obesity is the increased phosphatidylcholine/phosphatidylethanolamine ratio, which disrupts ER calcium refilling capacity by inhibiting SERCA activity. This regulation did not occur at the level of expression, as the SERCA protein was slightly more abundant in ob/ob mice compared to lean mice. The suppression of phosphatidylcholine synthesis from phosphatidylethanolamine normalized the phosphatidylcholine / phosphatidylethanolamine ratio, protected against ER stress and improved systemic glucose homeostasis.
The accumulation of misfolded proteins causes ROS generation from the oxidative folding machineries in the ER and mitochondria.94 Defective disulfide bond formation depletes glutathione in the ER and produces oxygen radicals via ERO1α and protein disulfide isomerase.63 Intriguingly, ROS produced by ERO1α activates type 1 IP3 receptors (IP3R) and stimulates ER Ca2+ release.95 Consequent ER Ca2+ loss further deteriorates the protein-folding process and augments ROS generation. Furthermore, prolonged ER stress increases CHOP expression, which upregulates ERO1α, causing additional oxidative stress. This positive feedback mechanism amplifies oxidation-triggered IP3R activation and ER Ca2+ release (Figure 1). Blocking this vicious cycle between ER ROS formation and ER Ca2+ release could be a pertinent therapeutic strategy. In support of this approach, we observed that palmitate-induced ER Ca2+ loss was prevented by both ROS scavengers or the inhibition of IP3 generation.30
It is noteworthy that H2O2-mediated oxidative stress can activate PLCγ and generate IP3 and DAG in astrocytes and lung endothelial cells.96, 97 Consistent with these findings, PLC activation was observed in podocytes treated with either palmitate or H2O2. Pretreatment with a PLC inhibitor attenuated palmitate-induced ER Ca2+ loss, suggesting that IP3 generation from phosphatidylinositol 4,5-bisphosphate (PIP2) contributes to ER Ca2+ release via the IP3R.30 In addition, DAG, the other signaling molecule produced by PLC activity, may also participate in palmitate-dependent ER Ca2+ loss. This hypothesis was supported by experimental evidence showing that palmitate-induced ER Ca2+ depletion and ER stress were surprisingly augmented by treatment with a DAG kinase blocker, leading to DAG accumulation.30 DAG accumulation activates PKCδ.74 PKC activity upregulates NOX (Figure 1), and more ROS are thus generated, as discussed earlier, leading to further ER Ca2+ loss. The inhibition of PKC blunted the effect of palmitate on ER Ca2+, suggesting a critical pathogenic role for DAG-mediated PKC activation.30 We propose a synergistic stimulation of Ca2+ release from the ER by IP3 and DAG, although further detailed studies are required to substantiate our working model.
Palmitate disturbs intracellular calcium homeostasis
Plasma membrane Ca2+ ATPase (PMCA) and SERCA establish 1000- to 10 000-fold change gradients of Ca2+ concentrations across the plasma membrane and the ER membrane.90 Therefore, inappropriate and uncontrolled cytosolic Ca2+ increases that result from Ca2+ influx from the extracellular space or release from the ER are a burden for the cell as it tries to maintain intracellular Ca2+ homeostasis. Ca2+ stress may initiate pathogenic processes such as calpain-mediated cell death. In β-cells, for instance, it was observed that palmitate-induced ER Ca2+ release activates the calcium-dependent pro-apoptotic protease calpain-2.92
Ca2+ release from the ER participates in cell death mechanisms. The luminal ER Ca2+ level is an important factor determining susceptibility to apoptosis triggered by different kinds of proapoptotic stimuli, including ceramides and arachidonic acid.98, 99 Recent discoveries support these observations by revealing a role for ER-mitochondrial contacts, known as the mitochondria-associated ER membrane (MAM) in apoptosis (Figure 2). MAMs are specialized subcompartments of the ER where the distance between the ER and the mitochondrial membranes is <25 nm.100 MAMs have been reported as either larger or tighter in diabetic mice on a high-fat diet.101, 102 The physical contact points between the ER and mitochondria are enriched for specific membrane proteins such as the IP3 receptor, the voltage-dependent anion channel and the mitochondrial Ca2+ uniporter (MCU). Additional adaptor proteins, including GRP75, are also required to establish high capacity Ca2+ transfer from the ER to the mitochondria.103 The increased density of MAMs in the cells of animals fed a high-fat diet may aggravate ER Ca2+ depletion and mitochondrial Ca2+ overload, although this hypothesis requires further experimentation.104
The activation of Ca2+ signals via PLC-mediated IP3 generation depletes ER Ca2+ and may thus have a negative impact on ER function and cell survival.90 To prevent this pathogenic consequence, there is an innate response to refill the ER Ca2+ reservoir. In the ER membrane, the stromal interaction molecule (STIM), a transmembrane protein with luminal EF hands, senses ER Ca2+ levels. A decrease in ER Ca2+ leads to STIM translocation to the plasma membrane-ER junctions. In these sub-plasma membrane areas, STIM proteins oligomerize to form clusters to recruit Orai1, a plasmalemmal Ca2+ channel. Orai1 mediates SOC entry until ER Ca2+ stores are refilled, at which point STIM oligomers again become dispersed. Notably, palmitate-treated cells maintain STIM1 oligomerization, signifying that ER Ca2+ release and depletion of stores persist.30 Upon extracellular Ca2+ addition, palmitate-treated cells show strong and sustained increases in cytosolic Ca2+, whereas there is a negligible influence on Ca2+ influx in control cells (Figure 2).30 This evidence suggests that ER Ca2+ depletion by palmitate induces sustained SOC entry, which may raise cytosolic and mitochondrial Ca2+ to an intolerable level. Low extracellular Ca2+ conditions protect against palmitate-induced cytotoxicity, suggesting that SOC contributes to the harmful effects of palmitate.105 It should be noted that Ca2+ influx via SOC entry is essential for physiological process such as immune cell activation. Moreover, there have been no reports of a truly selective SOC inhibitor until now. Nevertheless, we suggest that the prevention of sustained SOC activation could be a candidate for therapeutic targets to prevent lipotoxicity.
Mitochondrial dysfunction by palmitate
Mitochondria have an essential role in energy metabolism, biosynthetic processes, Ca2+ homeostasis and the integration of apoptotic signals.106, 107 Ca2+ in the mitochondrial matrix and extramitochondrial locations modulates mitochondrial functions, including intermediary metabolism and ATP synthesis. Mitochondrial Ca2+ activates pyruvate dehydrogenase, Krebs cycle activity, mitochondrial transporters, and ATP synthase.108, 109, 110 MCU is the main molecule responsible for mitochondrial Ca2+ uptake and the activation of mitochondrial metabolism.111, 112 Unexpectedly, no obvious phenotype was initially observed in mice lacking MCU.113 In animals with a cardiac muscle-specific deletion, however, MCU deficiency induces defects in acute metabolic stimulation and protects against ischemia-reperfusion injury.114 Local increases in cytosolic Ca2+ arrest the movement of mitochondria, allowing the organelle to efficiently take up and sequester Ca2+ into its matrix to stimulate mitochondrial energetics.115 Excess mitochondrial Ca2+ uptake, in contrast, induces mitochondrial permeability transition pore opening, followed by cytochrome c release and apoptotic cell death (Figure 2).116
Accumulating evidence suggests that human subjects with obesity or insulin resistance exhibit reduced oxygen consumption rates, decreased expression of mitochondrial proteins, and impaired ATP synthesis.117, 118 Mitochondrial dysfunction decreases β-oxidation and may elevate plasma FFA concentration, thereby aggravating lipotoxicity. The supplementation of tricarboxylic acid cycle substrates to facilitate mitochondrial FFA metabolism rescues lipotoxicity in insulin-secreting cells.47, 119 However, excessive FFA in mitochondria stimulates superoxide generation from the ETC, leading to cytotoxicity.44
Mitochondria are dynamic organelles that undergo continuous fusion and fission.120 During this process, dysfunctional mitochondria are separated and degraded by mitophagy, which acts as a quality control mechanism.121 Palmitate induces mitochondrial depolarization, morphodynamic fragmentation and impaired ATP synthesis.30 Furthermore, palmitate suppresses autophagic activity, which may increase the proportion of dysfunctional mitochondria. Defective fission allows mitochondria to become more elongated but also more susceptible to glucolipotoxicity.121, 122 The deterioration of mitochondrial function induces PT pore opening followed by caspase activation and apoptosis. The major known stimuli for PT pore opening are oxidative stress and matrix Ca2+ overload, both of which are observed during palmitate overload (Figure 2). Mitochondrial antioxidants effectively protect from palmitate-induced ER Ca2+ depletion, IP3 generation, ER stress and cell death.30 These findings demonstrate the important role of mitochondrial ROS in the palmitate-induced vicious cycle of calcium dysregulation and apoptosis.
Functional consequences of lipotoxicity and implications
Pancreatic beta cell failure and diabetes
During the glucose stimulation of pancreatic β-cells, insulin synthesis represents more than half of total protein synthesis in this highly specialized cell type. This high synthesis rate of insulin is further exaggerated in the context of insulin resistance, when proinsulin production is approximately 1 000 000 molecules per minute.123 Therefore, ER function in β-cells is prone to be overloaded in individuals on a high-calorie diet with limited physical activity. Saturated FFAs exert extra stress on β-cells due to the induction of ROS production. In an attempt to overcome this stress, β-cells upregulate the expression of chaperone proteins and reduce the ER workload as part of the ER stress response. Once a threshold of ER stress has been reached, palmitate may shift the β-cell response from physiologic adaptation to a pro-apoptotic program.124
ER stress in β-cells is a critical step in the pathogenesis of type 2 diabetes (Figure 3). Both high glucose and lipid stimulation produce mitochondrial ROS synergistically. Compared to other cell types, β-cells are highly susceptible to oxidative stress. High glucose and/or palmitate have been reported to decrease SERCA2b expression and ER Ca2+ level in β-cells.92 Inflammatory cytokines, acting as pathogenic molecules in type 1 diabetes, also attenuate SERCA2b expression in β-cells.125 Therefore, ER stress caused by insufficient ER Ca2+ content may be an important factor in the development of diabetes. In addition, the deletion or inactivation of WFS1, which is mutated in Wolfram syndrome, results in reduced ER Ca2+ content and increases ER stress in β-cells.92, 126 Genome studies revealed a link between WFS1 polymorphism and a high risk of type 2 diabetes,127 which may be due to the reported ER stress in β-cells. Palmitate, but not oleate, has been shown to trigger NF-κB activation and ER stress, which may be one mechanism to induce interleukin 1β (IL-1β) and downstream chemokines and cytokines, culminating in mild inflammation in human islets, although this does not directly cause β-cell dysfunction and apoptosis.128
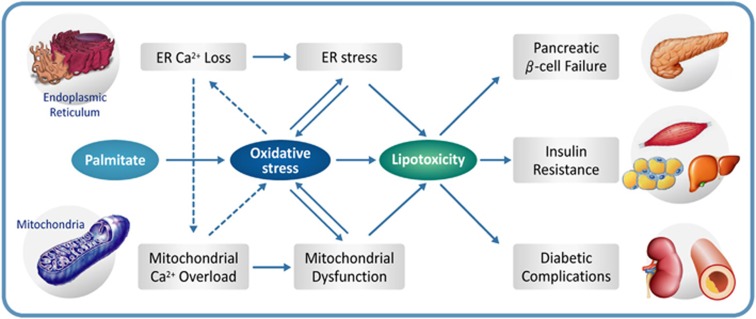
Figure 3.
Proposed mechanism of lipotoxicity in type 2 diabetes. Oxidative stress and calcium dysregulation form a vicious cycle that disturbs critical organelle function. Lipotoxicity resulting from ER stress and mitochondrial dysfunction contributes to pancreatic β-cell failure, insulin resistance in target tissues and diabetic complications.
Mitochondrial function in β-cells is particularly important because glucose/lipid/amino acid metabolism and insulin secretion depend on mitochondrial function.129 It has been demonstrated that mitochondrial morphodynamics protect β-cells from lipotoxicity.130 The inhibition of mitochondrial fission and/or defective mitophagy augments sensitivity to glucolipotoxicity.122 Mitochondrial Ca2+ is a crucial regulator of mitochondrial energy metabolism,108 as mentioned earlier. Therefore, Ca2+ transport from the ER to the mitochondria can affect mitochondrial metabolism as well as β-cell death. The pathogenic role of the ER-mitochondrial Ca2+ connection in mitochondrial dysfunction and β-cell failure by palmitate deserves further investigation.
Insulin resistance in target tissues
It is well-known that palmitate induces insulin resistance by disrupting intracellular insulin signaling in diverse cell types such as hepatocytes, cardiac and skeletal muscle cells, adipocytes, podocytes, hypothalamic neurons, and pancreatic α-cells.42, 131, 132, 133, 134, 135, 136 Palmitate exposure activates JNK, which phosphorylates IRS-1 on serine307 and decreases Akt phosphorylation, leading to the impairment of downstream signaling. Intriguingly, neuronal cells are more prone to the cytotoxic effects of palmitate. Compared to other cell types, neuronal cells are sensitive to lower doses and shorter exposure time.135 Oleate, again, prevents palmitate-induced insulin resistance in many cases.137, 138, 139
Palmitate-induced oxidative stress is the main mechanism disrupting insulin signaling (Figure 3). As discussed above, ROS are derived from multiple sources: mitochondrial ETC, DAG-PKC-NOX and CHOP-ERO1α. ROS can activate not only JNK but also other serine kinases, such as p38 MAPK, GSK-3β and IKKβ in skeletal muscle.140 In HepG2 cells treated with palmitate, p38 MAPK and JNK activities are significantly attenuated by siRNA-mediated NOX3 silencing.42 However, in another hepatic cell line, ROS-induced JNK activation was not completely reversed, even when efficiently suppressing ROS levels using antioxidants.45 The findings suggest that other mechanisms are also involved in palmitate-induced insulin resistance. One possible explanation is the intracellular accumulation of ceramide, which may activate JNK via mixed lineage kinase-3.141, 142
Ca2+ is another modulator of insulin signaling, the molecular mechanisms of which are still poorly understood.143 Ca2+/calmodulin was suggested to have an important role in the insulin-mediated translocation and exocytosis of glucose transporter type 4 (GLUT4) vesicles in 3T3-L1 adipocytes. A more recent study found that the Ca2+ chelator BAPTA144 operates through the depolarization of microtubules rather than Ca2+ chelation.145 In L6 myotubes, ER Ca2+ release through both ryanodine receptor 1 (RYR1) and IP3R promotes insulin-dependent GLUT4 trafficking to the plasma membrane.146 Palmitate impairs mitochondrial calcium retention capacity and impairs insulin-stimulated GLUT4 translocation in L6 myotubes, which was fully restored by adding an inhibitor of PT pore opening.147 Finally, several studies have suggested that either enlarged or insufficient MAMs fail to maintain normal ER-mitochondrial Ca2+ homeostasis. Altered MAM structures may, therefore, indirectly affect the translocation and fusion of GLUT4 vesicles with the plasma membrane.148 Does palmitate-induced ER-mitochondrial Ca2+ dysregulation affect GLUT4 trafficking in a ROS-independent manner? Does palmitate affect insulin-dependent and/or contraction-dependent GLUT4 translocation in muscle? More studies are needed to address such potential Ca2+-mediated mechanisms of lipotoxicity.
Diabetic complications
Chronic diabetic complications have traditionally been attributed to long-term exposure to high glucose. The four classical pathways of hyperglycemia-induced complications include (1) increased polyol pathway flux, (2) increased intracellular formation of advanced glycation end products (AGE), (3) the activation of PKC and (4) the stimulation of the hexosamine pathway. Those pathways are connected by the fact that intracellular high glucose induces elevated mitochondrial ROS production, which leads to a decrease in GAPDH activity. As a result, upstream glycolytic metabolites are diverted into the pathogenic pathways described above.149 In addition, high glucose augments the expression and activity of members of the NOX family.150, 151, 152 A detailed description of this crosstalk between NOX and mitochondrial ROS generation has been described elsewhere.55, 153
Intriguingly, accumulating evidence supports a synergistic effect between palmitate and high glucose leading to diabetic complications. Such findings have led to the concept of glucolipotoxicity. Oxidative stress may be a common mechanism explaining the harmful synergistic effects of the two nutrients. In bovine and human retinal endothelial cells, NOX2-derived ROS overproduction was significantly higher when the cells were exposed to palmitate and high glucose rather than high glucose alone. Consequently, mitochondrial DNA damage is observed as early as 48 h when bovine retinal cells are exposed to palmitate and high glucose. Similar mtDNA damage was only observed after 96 h when high glucose was added alone.154 The separate exposure of HUVEC cells to either palmitate or high glucose increases ROS production, but the highest ROS levels were observed upon treatment with both.155 These experiments clearly demonstrate that glucolipotoxicity, which was originally proposed to affect β-cells, may be similarly harmful to other tissues.
In diabetic nephropathy, functional and structural alterations in podocytes accompany disease progression. In this cell type, palmitate reduces tyrosine phosphorylation following insulin stimulation.156 This defect downstream of insulin receptor signaling also impairs GLUT4 translocation in podocytes. Palmitate-induced intracellular calcium dysregulation also participates in diabetic nephropathy. In podocytes, elevated cytosolic Ca2+ concentrations induce actin remodeling, which increases albumin permeability. These structural alterations in podocytes also have a critical role in the pathogenesis of proteinuric glomerular disease.30
Furthermore, elevated palmitate may also exert harmful effects in periodontitis linked to type 2 diabetes. In mice fed a high-fat diet, which serve as a model of type 2 diabetes, CD36 is overexpressed in gingival fibroblasts. In human gingival fibroblasts, palmitate also provokes mRNA expression of pro-inflammatory cytokines and chemokines, as well as IL-6, IL-8 and CXCL1.157 This evidence supports the hypothesis that palmitate exposure may worsen diabetic complications.
Conclusion
The accumulation of palmitate and derived metabolites, e.g., DAG, induces oxidative stress and ER Ca2+ depletion, leading to ER stress and mitochondrial dysfunction. Excessive ER Ca2+ release and mitochondrial Ca2+ overload further amplify oxidative stress. This close interaction between oxidative stress and Ca2+ dysregulation results in a vicious cycle of increasingly impaired cell function and death. The activation of stores-operated Ca2+ entry may chronically disturb cytosolic and organellar Ca2+ homeostasis; this hypothesis will require further investigation. The disruption of Ca2+ regulation by oxidative stress also contributes to insulin resistance. These hypotheses provide an integrated mechanistic view of lipotoxicity, which has pivotal roles during the progress of diabetes and its complications. In this review, we have suggested future therapeutic approaches to type 2 diabetes via interference with the basic molecular mechanisms overstimulated during lipotoxicity.
Acknowledgments
This research was supported by a grant (HI16C1501) from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (awarded to IKL).
Footnotes
The authors declare no conflict of interest.
References
- Choi CS, Savage DB, Kulkarni A, Yu XX, Liu ZX, Morino K et al. Suppression of diacylglycerol acyltransferase-2 (DGAT2), but not DGAT1, with antisense oligonucleotides reverses diet-induced hepatic steatosis and insulin resistance. J Biol Chem 2007; 282: 22678–22688. [DOI] [PubMed] [Google Scholar]
- Hagenfeldt L, Wahren J, Pernow B, Raf L. Uptake of individual free fatty acids by skeletal muscle and liver in man. J Clin Invest 1972; 51: 2324–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraze E, Donner CC, Swislocki AL, Chiou YA, Chen YD, Reaven GM. Ambient plasma free fatty acid concentrations in noninsulin-dependent diabetes mellitus: evidence for insulin resistance. J Clin Endocrinol Metab 1985; 61: 807–811. [DOI] [PubMed] [Google Scholar]
- Miles JM, Wooldridge D, Grellner WJ, Windsor S, Isley WL, Klein S et al. Nocturnal and postprandial free fatty acid kinetics in normal and type 2 diabetic subjects: effects of insulin sensitization therapy. Diabetes 2003; 52: 675–681. [DOI] [PubMed] [Google Scholar]
- Charles MA, Eschwege E, Thibult N, Claude JR, Warnet JM, Rosselin GE et al. The role of non-esterified fatty acids in the deterioration of glucose tolerance in Caucasian subjects: results of the Paris Prospective Study. Diabetologia 1997; 40: 1101–1106. [DOI] [PubMed] [Google Scholar]
- Abumrad NA, el-Maghrabi MR, Amri EZ, Lopez E, Grimaldi PA. Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. Homology with human CD36. J Biol Chem 1993; 268: 17665–17668. [PubMed] [Google Scholar]
- Van Nieuwenhoven FA, Verstijnen CP, Abumrad NA, Willemsen PH, Van Eys GJ, Van der Vusse GJ et al. Putative membrane fatty acid translocase and cytoplasmic fatty acid-binding protein are co-expressed in rat heart and skeletal muscles. Biochem Biophys Res Commun 1995; 207: 747–752. [DOI] [PubMed] [Google Scholar]
- Coburn CT, Knapp FFJr, Febbraio M, Beets AL, Silverstein RL, Abumrad NA. Defective uptake and utilization of long chain fatty acids in muscle and adipose tissues of CD36 knockout mice. J Biol Chem 2000; 275: 32523–32529. [DOI] [PubMed] [Google Scholar]
- He J, Lee JH, Febbraio M, Xie W. The emerging roles of fatty acid translocase/CD36 and the aryl hydrocarbon receptor in fatty liver disease. Exp Biol Med (Maywood) 2011; 236: 1116–1121. [DOI] [PubMed] [Google Scholar]
- Swerlick RA, Lee KH, Wick TM, Lawley TJ. Human dermal microvascular endothelial but not human umbilical vein endothelial cells express CD36 in vivo and in vitro. J Immunol 1992; 148: 78–83. [PubMed] [Google Scholar]
- Endemann G, Stanton LW, Madden KS, Bryant CM, White RT, Protter AA. CD36 is a receptor for oxidized low density lipoprotein. J Biol Chem. 1993; 268: 11811–11816. [PubMed] [Google Scholar]
- Huh HY, Pearce SF, Yesner LM, Schindler JL, Silverstein RL. Regulated expression of CD36 during monocyte-to-macrophage differentiation: potential role of CD36 in foam cell formation. Blood 1996; 87: 2020–2028. [PubMed] [Google Scholar]
- Noushmehr H, D'Amico E, Farilla L, Hui H, Wawrowsky KA, Mlynarski W et al. Fatty acid translocase (FAT/CD36) is localized on insulin-containing granules in human pancreatic beta-cells and mediates fatty acid effects on insulin secretion. Diabetes 2005; 54: 472–481. [DOI] [PubMed] [Google Scholar]
- Mayrhofer C, Krieger S, Huttary N, Chang MW, Grillari J, Allmaier G et al. Alterations in fatty acid utilization and an impaired antioxidant defense mechanism are early events in podocyte injury: a proteomic analysis. Am J Pathol 2009; 174: 1191–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahimi A, Bonen A, Blinn WD, Hajri T, Li X, Zhong K et al. Muscle-specific overexpression of FAT/CD36 enhances fatty acid oxidation by contracting muscle, reduces plasma triglycerides and fatty acids, and increases plasma glucose and insulin. J Biol Chem 1999; 274: 26761–26766. [DOI] [PubMed] [Google Scholar]
- Febbraio M, Abumrad NA, Hajjar DP, Sharma K, Cheng W, Pearce SF et al. A null mutation in murine CD36 reveals an important role in fatty acid and lipoprotein metabolism. J Biol Chem 1999; 274: 19055–19062. [DOI] [PubMed] [Google Scholar]
- Garbacz WG, Lu P, Miller TM, Poloyac SM, Eyre NS, Mayrhofer G et al. Hepatic overexpression of CD36 improves glycogen homeostasis and attenuates high-fat diet induced hepatic steatosis and insulin resistance. Mol Cell Biol 2016; 36: 2715–2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hames KC, Vella A, Kemp BJ, Jensen MD. Free fatty acid uptake in humans with CD36 deficiency. Diabetes 2014; 63: 3606–3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CG, Tran JL, Erion DM, Vera NB, Febbraio M, Weiss EJ. Hepatocyte-Specific Disruption of CD36 Attenuates Fatty Liver and Improves Insulin Sensitivity in HFD-Fed Mice. Endocrinology 2016; 157: 570–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein RL, Li W, Park YM, Rahaman SO. Mechanisms of cell signaling by the scavenger receptor CD36: implications in atherosclerosis and thrombosis. Trans Am Clin Climatol Assoc 2010; 121: 206–220. [PMC free article] [PubMed] [Google Scholar]
- Kuda O, Jenkins CM, Skinner JR, Moon SH, Su X, Gross RW et al. CD36 protein is involved in store-operated calcium flux, phospholipase A2 activation, and production of prostaglandin E2. J Biol Chem 2011; 286: 17785–17795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdoul-Azize S, Selvakumar S, Sadou H, Besnard P, Khan NA. Ca2+ signaling in taste bud cells and spontaneous preference for fat: unresolved roles of CD36 and GPR120. Biochimie 2014; 96: 8–13. [DOI] [PubMed] [Google Scholar]
- Kim YW, Moon JS, Seo YJ, Park SY, Kim JY, Yoon JS et al. Inhibition of fatty acid translocase cluster determinant 36 (CD36), stimulated by hyperglycemia, prevents glucotoxicity in INS-1 cells. Biochem Biophys Res Commun 2012; 420: 462–466. [DOI] [PubMed] [Google Scholar]
- Hua W, Huang HZ, Tan LT, Wan JM, Gui HB, Zhao L et al. CD36 mediated fatty acid-induced podocyte apoptosis via oxidative stress. PLoS ONE 2015; 10: e0127507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin T, Ma Z, Ogata H, Jorgensen IH, Iezzi M, Wang H et al. Facilitation of fatty acid uptake by CD36 in insulin-producing cells reduces fatty-acid-induced insulin secretion and glucose regulation of fatty acid oxidation. Biochim Biophys Acta 2010; 1801: 191–197. [DOI] [PubMed] [Google Scholar]
- Gharib M, Tao H, Fungwe TV, Hajri T. Cluster differentiating 36 (CD36) deficiency attenuates obesity-associated oxidative stress in the heart. PLoS ONE 2016; 11: e0155611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febbraio M, Podrez EA, Smith JD, Hajjar DP, Hazen SL, Hoff HF et al. Targeted disruption of the class B scavenger receptor CD36 protects against atherosclerotic lesion development in mice. J Clin Invest 2000; 105: 1049–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon CM, Luce P, Beth AH, Abumrad NA. Labeling of adipocyte membranes by sulfo-N-succinimidyl derivatives of long-chain fatty acids: inhibition of fatty acid transport. J Membr Biol 1991; 121: 261–268. [DOI] [PubMed] [Google Scholar]
- Coort SL, Willems J, Coumans WA, van der Vusse GJ, Bonen A, Glatz JF et al. Sulfo-N-succinimidyl esters of long chain fatty acids specifically inhibit fatty acid translocase (FAT/CD36)-mediated cellular fatty acid uptake. Mol Cell Biochem 2002; 239: 213–219. [PubMed] [Google Scholar]
- Xu S, Nam SM, Kim JH, Das R, Choi SK, Nguyen TT et al. Palmitate induces ER calcium depletion and apoptosis in mouse podocytes subsequent to mitochondrial oxidative stress. Cell Death Dis 2015; 6: e1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza AC, Bocharov AV, Baranova IN, Vishnyakova TG, Huang YG, Wilkins KJ et al. Antagonism of scavenger receptor CD36 by 5A peptide prevents chronic kidney disease progression in mice independent of blood pressure regulation. Kidney Int 2016; 89: 809–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachootham D, Lu W, Ogasawara MA, Nilsa RD, Huang P. Redox regulation of cell survival. Antioxid Redox Signal 2008; 10: 1343–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehinger S, Ortiz R, Diaz MI, Aguirre A, Valenzuela M, Llanos P et al. Phosphorylation of caveolin-1 on tyrosine-14 induced by ROS enhances palmitate-induced death of beta-pancreatic cells. Biochim Biophys Acta 2015; 1852: 693–708. [DOI] [PubMed] [Google Scholar]
- Liu J, Chang F, Li F, Fu H, Wang J, Zhang S et al. Palmitate promotes autophagy and apoptosis through ROS-dependent JNK and p38 MAPK. Biochem Biophys Res Commun 2015; 463: 262–267. [DOI] [PubMed] [Google Scholar]
- Carlsson C, Borg LA, Welsh N. Sodium palmitate induces partial mitochondrial uncoupling and reactive oxygen species in rat pancreatic islets in vitro. Endocrinology 1999; 140: 3422–3428. [DOI] [PubMed] [Google Scholar]
- Sato Y, Fujimoto S, Mukai E, Sato H, Tahara Y, Ogura K et al. Palmitate induces reactive oxygen species production and beta-cell dysfunction by activating nicotinamide adenine dinucleotide phosphate oxidase through Src signaling. J Diabetes Investig 2014; 5: 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow J, Affourtit C. Novel insights into pancreatic beta-cell glucolipotoxicity from real-time functional analysis of mitochondrial energy metabolism in INS-1E insulinoma cells. Biochem J 2013; 456: 417–426. [DOI] [PubMed] [Google Scholar]
- Joseph LC, Barca E, Subramanyam P, Komrowski M, Pajvani U, Colecraft HM et al. Inhibition of NAPDH oxidase 2 (NOX2) Prevents oxidative stress and mitochondrial abnormalities caused by saturated fat in cardiomyocytes. PLoS ONE 2016; 11: e0145750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodeur MR, Bouvet C, Barrette M, Moreau P. Palmitic acid increases medial calcification by inducing oxidative stress. J Vasc Res 2013; 50: 430–441. [DOI] [PubMed] [Google Scholar]
- Yamagishi S, Okamoto T, Amano S, Inagaki Y, Koga K, Koga M et al. Palmitate-induced apoptosis of microvascular endothelial cells and pericytes. Mol Med 2002; 8: 179–184. [PMC free article] [PubMed] [Google Scholar]
- Taheripak G, Bakhtiyari S, Rajabibazl M, Pasalar P, Meshkani R. Protein tyrosine phosphatase 1B inhibition ameliorates palmitate-induced mitochondrial dysfunction and apoptosis in skeletal muscle cells. Free Radic Biol Med 2013; 65: 1435–1446. [DOI] [PubMed] [Google Scholar]
- Gao D, Nong S, Huang X, Lu Y, Zhao H, Lin Y et al. The effects of palmitate on hepatic insulin resistance are mediated by NADPH Oxidase 3-derived reactive oxygen species through JNK and p38MAPK pathways. J Biol Chem 2010; 285: 29965–29973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JE, Gabler NK, Walker-Daniels J, Spurlock ME. The c-Jun N-terminal kinase mediates the induction of oxidative stress and insulin resistance by palmitate and toll-like receptor 2 and 4 ligands in 3T3-L1 adipocytes. Horm Metab Res 2009; 41: 523–530. [DOI] [PubMed] [Google Scholar]
- Lambertucci RH, Hirabara SM, Silveira Ldos R, Levada-Pires AC, Curi R, Pithon-Curi TC. Palmitate increases superoxide production through mitochondrial electron transport chain and NADPH oxidase activity in skeletal muscle cells. J Cell Physiol 2008; 216: 796–804. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Takamura T, Matsuzawa-Nagata N, Takayama H, Misu H, Noda H et al. Palmitate induces insulin resistance in H4IIEC3 hepatocytes through reactive oxygen species produced by mitochondria. J Biol Chem 2009; 284: 14809–14818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudina S, Sena S, Theobald H, Sheng X, Wright JJ, Hu XX et al. Mitochondrial energetics in the heart in obesity-related diabetes: direct evidence for increased uncoupled respiration and activation of uncoupling proteins. Diabetes 2007; 56: 2457–2466. [DOI] [PubMed] [Google Scholar]
- Choi SE, Jung IR, Lee YJ, Lee SJ, Lee JH, Kim Y et al. Stimulation of lipogenesis as well as fatty acid oxidation protects against palmitate-induced INS-1 beta-cell death. Endocrinology 2011; 152: 816–827. [DOI] [PubMed] [Google Scholar]
- Namgaladze D, Lips S, Leiker TJ, Murphy RC, Ekroos K, Ferreiros N et al. Inhibition of macrophage fatty acid beta-oxidation exacerbates palmitate-induced inflammatory and endoplasmic reticulum stress responses. Diabetologia 2014; 57: 1067–1077. [DOI] [PubMed] [Google Scholar]
- Fu D, Lu J, Yang S. Oleic/palmitate induces apoptosis in human articular chondrocytes via upregulation of NOX4 expression and ROS production. Ann Clin Lab Sci 2016; 46: 353–359. [PubMed] [Google Scholar]
- Das R, Xu S, Quan X, Nguyen TT, Kong ID, Chung CH et al. Upregulation of mitochondrial Nox4 mediates TGF-beta-induced apoptosis in cultured mouse podocytes. Am J Physiol Renal Physiol 2014; 306: F155–F167. [DOI] [PubMed] [Google Scholar]
- Ago T, Kuroda J, Pain J, Fu C, Li H, Sadoshima J. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ Res 2010; 106: 1253–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson ML, Macrae K, Marley AE, Hundal HS. Chronic effects of palmitate overload on nutrient-induced insulin secretion and autocrine signalling in pancreatic MIN6 beta cells. PLoS ONE 2011; 6: e25975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrae K, Stretton C, Lipina C, Blachnio-Zabielska A, Baranowski M, Gorski J et al. Defining the role of DAG, mitochondrial function, and lipid deposition in palmitate-induced proinflammatory signaling and its counter-modulation by palmitoleate. J Lipid Res 2013; 54: 2366–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leamy AK, Egnatchik RA, Shiota M, Ivanova PT, Myers DS, Brown HA et al. Enhanced synthesis of saturated phospholipids is associated with ER stress and lipotoxicity in palmitate treated hepatic cells. J Lipid Res 2014; 55: 1478–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikalov S. Cross talk between mitochondria and NADPH oxidases. Free Radic Biol Med 2011; 51: 1289–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu G, Greene EL, Nagai T, Egan BM. Reactive oxygen species are critical in the oleic acid-mediated mitogenic signaling pathway in vascular smooth muscle cells. Hypertension 1998; 32: 1003–1010. [DOI] [PubMed] [Google Scholar]
- Koulajian K, Desai T, Liu GC, Ivovic A, Patterson JN, Tang C et al. NADPH oxidase inhibition prevents beta cell dysfunction induced by prolonged elevation of oleate in rodents. Diabetologia 2013; 56: 1078–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang J, Cui J, Gong H, Xi C, Zhang TM. Effect of NAD on PARP-mediated insulin sensitivity in oleic acid treated hepatocytes. J Cell Physiol 2015; 230: 1607–1613. [DOI] [PubMed] [Google Scholar]
- Lamers D, Schlich R, Horrighs A, Cramer A, Sell H, Eckel J. Differential impact of oleate, palmitate, and adipokines on expression of NF-kappaB target genes in human vascular smooth muscle cells. Mol Cell Endocrinol 2012; 362: 194–201. [DOI] [PubMed] [Google Scholar]
- Park EJ, Lee AY, Chang SH, Yu KN, Kim JH, Cho MH. Role of p53 in the cellular response following oleic acid accumulation in Chang liver cells. Toxicol Lett 2014; 224: 114–120. [DOI] [PubMed] [Google Scholar]
- Yuzefovych L, Wilson G, Rachek L. Different effects of oleate vs. palmitate on mitochondrial function, apoptosis, and insulin signaling in L6 skeletal muscle cells: role of oxidative stress. Am J Physiol Endocrinol Metab 2010; 299: E1096–E1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellgaard L, Molinari M, Helenius A. Setting the standards: quality control in the secretory pathway. Science 1999; 286: 1882–1888. [DOI] [PubMed] [Google Scholar]
- Tu BP, Weissman JS. Oxidative protein folding in eukaryotes: mechanisms and consequences. J Cell Biol 2004; 164: 341–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal 2007; 9: 2277–2293. [DOI] [PubMed] [Google Scholar]
- Cao SS, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid Redox Signal 2014; 21: 396–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem 2005; 74: 739–789. [DOI] [PubMed] [Google Scholar]
- Back SH, Kaufman RJ. Endoplasmic reticulum stress and type 2 diabetes. Annu Rev Biochem 2012; 81: 767–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cnop M, Ladriere L, Igoillo-Esteve M, Moura RF, Cunha DA. Causes and cures for endoplasmic reticulum stress in lipotoxic beta-cell dysfunction. Diabetes Obes Metab 2010; 12(Suppl 2): 76–82. [DOI] [PubMed] [Google Scholar]
- Hage Hassan R, Bourron O, Hajduch E. Defect of insulin signal in peripheral tissues: Important role of ceramide. World J Diabetes 2014; 5: 244–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaurasia B, Summers SA. Ceramides—lipotoxic inducers of metabolic disorders. Trends Endocrinol Metab 2015; 26: 538–550. [DOI] [PubMed] [Google Scholar]
- Ueda N. Ceramide-induced apoptosis in renal tubular cells: a role of mitochondria and sphingosine-1-phoshate. Int J Mol Sci 2015; 16: 5076–5124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichur S, Wang ST, Chan PW, Li Y, Ching J, Chaurasia B et al. CerS2 haploinsufficiency inhibits beta-oxidation and confers susceptibility to diet-induced steatohepatitis and insulin resistance. Cell Metab 2014; 20: 687–695. [DOI] [PubMed] [Google Scholar]
- Liu Z, Xia Y, Li B, Xu H, Wang C, Liu Y et al. Induction of ER stress-mediated apoptosis by ceramide via disruption of ER Ca(2+) homeostasis in human adenoid cystic carcinoma cells. Cell Biosci 2014; 4: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soumura M, Kume S, Isshiki K, Takeda N, Araki S, Tanaka Y et al. Oleate and eicosapentaenoic acid attenuate palmitate-induced inflammation and apoptosis in renal proximal tubular cell. Biochem Biophys Res Commun 2010; 402: 265–271. [DOI] [PubMed] [Google Scholar]
- Sieber J, Lindenmeyer MT, Kampe K, Campbell KN, Cohen CD, Hopfer H et al. Regulation of podocyte survival and endoplasmic reticulum stress by fatty acids. Am J Physiol Renal Physiol 2010; 299: F821–F829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Listenberger LL, Han X, Lewis SE, Cases S, Farese RVJr, Ory DS et al. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci USA 2003; 100: 3077–3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn K, Bergsten P. Fatty acid-induced oxidation and triglyceride formation is higher in insulin-producing MIN6 cells exposed to oleate compared to palmitate. J Cell Biochem 2010; 111: 497–507. [DOI] [PubMed] [Google Scholar]
- Henique C, Mansouri A, Fumey G, Lenoir V, Girard J, Bouillaud F et al. Increased mitochondrial fatty acid oxidation is sufficient to protect skeletal muscle cells from palmitate-induced apoptosis. J Biol Chem 2010; 285: 36818–36827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieber J, Weins A, Kampe K, Gruber S, Lindenmeyer MT, Cohen CD et al. Susceptibility of podocytes to palmitic acid is regulated by stearoyl-CoA desaturases 1 and 2. Am J Pathol 2013; 183: 735–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heard A, Thompson J, Carver J, Bakey M, Wang XR. Targeting molecular chaperones for the treatment of cystic fibrosis: is it a viable approach? Curr Drug Targets 2015; 16: 958–964. [DOI] [PubMed] [Google Scholar]
- Silverman GA, Pak SC, Perlmutter DH. Disorders of protein misfolding: alpha-1-antitrypsin deficiency as prototype. J Pediatr 2013; 163: 320–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee A, Morales-Scheihing D, Butler PC, Soto C. Type 2 diabetes as a protein misfolding disease. Trends Mol Med 2015; 21: 439–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JA, Radford H, Peretti D, Steinert JR, Verity N, Martin MG et al. Sustained translational repression by eIF2alpha-P mediates prion neurodegeneration. Nature 2012; 485: 507–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffenbach KT, Gentile CL, Nivala AM, Wang D, Wei Y, Pagliassotti MJ. Linking endoplasmic reticulum stress to cell death in hepatocytes: roles of C/EBP homologous protein and chemical chaperones in palmitate-mediated cell death. Am J Physiol Endocrinol Metab 2010; 298: E1027–E1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce M, Bryant KF, Jousse C, Long K, Harding HP, Scheuner D et al. A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science 2005; 307: 935–939. [DOI] [PubMed] [Google Scholar]
- Cnop M, Ladriere L, Hekerman P, Ortis F, Cardozo AK, Dogusan Z et al. Selective inhibition of eukaryotic translation initiation factor 2 alpha dephosphorylation potentiates fatty acid-induced endoplasmic reticulum stress and causes pancreatic beta-cell dysfunction and apoptosis. J Biol Chem 2007; 282: 3989–3997. [DOI] [PubMed] [Google Scholar]
- Ladriere L, Igoillo-Esteve M, Cunha DA, Brion JP, Bugliani M, Marchetti P et al. Enhanced signaling downstream of ribonucleic Acid-activated protein kinase-like endoplasmic reticulum kinase potentiates lipotoxic endoplasmic reticulum stress in human islets. J Clin Endocrinol Metab 2010; 95: 1442–1449. [DOI] [PubMed] [Google Scholar]
- Cunha DA, Hekerman P, Ladriere L, Bazarra-Castro A, Ortis F, Wakeham MC et al. Initiation and execution of lipotoxic ER stress in pancreatic beta-cells. J Cell Sci. 2008; 121((Pt 14)): 2308–2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhi H, Kropp EM, Clavo VF, Kobrossi CR, Han J, Mauer AS et al. C/EBP homologous protein-induced macrophage apoptosis protects mice from steatohepatitis. J Biol Chem 2013; 288: 18624–18642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekahli D, Bultynck G, Parys JB, De Smedt H, Missiaen L. Endoplasmic-reticulum calcium depletion and disease. Cold Spring Harb Perspect Biol 2011; 3: a004317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwiazda KS, Yang TL, Lin Y, Johnson JD. Effects of palmitate on ER and cytosolic Ca2+ homeostasis in beta-cells. Am J Physiol Endocrinol Metab 2009; 296: E690–E701. [DOI] [PubMed] [Google Scholar]
- Hara T, Mahadevan J, Kanekura K, Hara M, Lu S, Urano F. Calcium efflux from the endoplasmic reticulum leads to beta-cell death. Endocrinology 2014; 155: 758–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu S, Yang L, Li P, Hofmann O, Dicker L, Hide W et al. Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature 2011; 473: 528–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes CM, Titus EA, Cooper AA. Degradation of misfolded proteins prevents ER-derived oxidative stress and cell death. Mol Cell 2004; 15: 767–776. [DOI] [PubMed] [Google Scholar]
- Li G, Mongillo M, Chin KT, Harding H, Ron D, Marks AR et al. Role of ERO1-alpha-mediated stimulation of inositol 1,4,5-triphosphate receptor activity in endoplasmic reticulum stress-induced apoptosis. J Cell Biol 2009; 186: 783–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissmann N, Sydykov A, Kalwa H, Storch U, Fuchs B, Mederos y Schnitzler M et al. Activation of TRPC6 channels is essential for lung ischaemia-reperfusion induced oedema in mice. Nat Commun 2012; 3: 649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong JH, Moon SJ, Byun HM, Kim MS, Jo H, Bae YS et al. Critical role of phospholipase Cgamma1 in the generation of H2O2-evoked [Ca2+]i oscillations in cultured rat cortical astrocytes. J Biol Chem 2006; 281: 13057–13067. [DOI] [PubMed] [Google Scholar]
- Scorrano L, Oakes SA, Opferman JT, Cheng EH, Sorcinelli MD, Pozzan T et al. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science 2003; 300: 135–139. [DOI] [PubMed] [Google Scholar]
- Demaurex N, Distelhorst C. Cell biology. Apoptosis--the calcium connection. Science 2003; 300: 65–67. [DOI] [PubMed] [Google Scholar]
- Csordas G, Renken C, Varnai P, Walter L, Weaver D, Buttle KF et al. Structural and functional features and significance of the physical linkage between ER and mitochondria. J Cell Biol 2006; 174: 915–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arruda AP, Pers BM, Parlakgul G, Guney E, Inouye K, Hotamisligil GS. Chronic enrichment of hepatic endoplasmic reticulum-mitochondria contact leads to mitochondrial dysfunction in obesity. Nat Med 2014; 20: 1427–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubbs E, Theurey P, Vial G, Bendridi N, Bravard A, Chauvin MA et al. Mitochondria-associated endoplasmic reticulum membrane (MAM) integrity is required for insulin signaling and is implicated in hepatic insulin resistance. Diabetes 2014; 63: 3279–3294. [DOI] [PubMed] [Google Scholar]
- Rizzuto R, De Stefani D, Raffaello A, Mammucari C. Mitochondria as sensors and regulators of calcium signalling. Nat Rev Mol Cell Biol 2012; 13: 566–578. [DOI] [PubMed] [Google Scholar]
- Arruda AP, Hotamisligil GS. Calcium homeostasis and organelle function in the pathogenesis of obesity and diabetes. Cell Metab 2015; 22: 381–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SE, Kim HE, Shin HC, Jang HJ, Lee KW, Kim Y et al. Involvement of Ca2+-mediated apoptotic signals in palmitate-induced MIN6N8a beta cell death. Mol Cell Endocrinol 2007; 272: 50–62. [DOI] [PubMed] [Google Scholar]
- Park KS, Wiederkehr A, Kirkpatrick C, Mattenberger Y, Martinou JC, Marchetti P et al. Selective actions of mitochondrial fission/fusion genes on metabolism-secretion coupling in insulin-releasing cells. J Biol Chem 2008; 283: 33347–33356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiederkehr A, Wollheim CB. Minireview: implication of mitochondria in insulin secretion and action. Endocrinology 2006; 147: 2643–2649. [DOI] [PubMed] [Google Scholar]
- Wiederkehr A, Wollheim CB. Impact of mitochondrial calcium on the coupling of metabolism to insulin secretion in the pancreatic beta-cell. Cell Calcium 2008; 44: 64–76. [DOI] [PubMed] [Google Scholar]
- Del Arco A, Contreras L, Pardo B, Satrustegui J. Calcium regulation of mitochondrial carriers. Biochim Biophys Acta 2016; 1863: 2413–2421. [DOI] [PubMed] [Google Scholar]
- De Marchi U, Thevenet J, Hermant A, Dioum E, Wiederkehr A. Calcium co-regulates oxidative metabolism and ATP synthase-dependent respiration in pancreatic beta cells. J Biol Chem 2014; 289: 9182–9194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan X, Nguyen TT, Choi SK, Xu S, Das R, Cha SK et al. Essential role of mitochondrial Ca2+ uniporter in the generation of mitochondrial pH gradient and metabolism-secretion coupling in insulin-releasing cells. J Biol Chem 2015; 290: 4086–4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendin D, Greotti E, Pozzan T. The elusive importance of being a mitochondrial Ca(2+) uniporter. Cell Calcium 2014; 55: 139–145. [DOI] [PubMed] [Google Scholar]
- Pan X, Liu J, Nguyen T, Liu C, Sun J, Teng Y et al. The physiological role of mitochondrial calcium revealed by mice lacking the mitochondrial calcium uniporter. Nat Cell Biol 2013; 15: 1464–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong JQ, Lu X, Correll RN, Schwanekamp JA, Vagnozzi RJ, Sargent MA et al. The mitochondrial calcium uniporter selectively matches metabolic output to acute contractile stress in the heart. Cell Rep 2015; 12: 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi M, Weaver D, Hajnoczky G. Control of mitochondrial motility and distribution by the calcium signal: a homeostatic circuit. J Cell Biol 2004; 167: 661–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med 2000; 6: 513–519. [DOI] [PubMed] [Google Scholar]
- Turner N, Heilbronn LK. Is mitochondrial dysfunction a cause of insulin resistance? Trends Endocrinol Metab 2008; 19: 324–330. [DOI] [PubMed] [Google Scholar]
- Szendroedi J, Phielix E, Roden M. The role of mitochondria in insulin resistance and type 2 diabetes mellitus. Nat Rev Endocrinol 2012; 8: 92–103. [DOI] [PubMed] [Google Scholar]
- Choi SE, Lee YJ, Hwang GS, Chung JH, Lee SJ, Lee JH et al. Supplement of TCA cycle intermediates protects against high glucose/palmitate-induced INS-1 beta cell death. Arch Biochem Biophys 2011; 505: 231–241. [DOI] [PubMed] [Google Scholar]
- Archer SL. Mitochondrial dynamics–mitochondrial fission and fusion in human diseases. N Engl J Med 2013; 369: 2236–2251. [DOI] [PubMed] [Google Scholar]
- Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J 2008; 27: 433–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina AJ, Wikstrom JD, Stiles L, Las G, Mohamed H, Elorza A et al. Mitochondrial networking protects beta-cells from nutrient-induced apoptosis. Diabetes 2009; 58: 2303–2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuit FC, In't Veld PA, Pipeleers DG. Glucose stimulates proinsulin biosynthesis by a dose-dependent recruitment of pancreatic beta cells. Proc Natl Acad Sci USA 1988; 85: 3865–3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 2007; 8: 519–529. [DOI] [PubMed] [Google Scholar]
- Cardozo AK, Ortis F, Storling J, Feng YM, Rasschaert J, Tonnesen M et al. Cytokines downregulate the sarcoendoplasmic reticulum pump Ca2+ ATPase 2b and deplete endoplasmic reticulum Ca2+, leading to induction of endoplasmic reticulum stress in pancreatic beta-cells. Diabetes 2005; 54: 452–461. [DOI] [PubMed] [Google Scholar]
- Fonseca SG, Fukuma M, Lipson KL, Nguyen LX, Allen JR, Oka Y et al. WFS1 is a novel component of the unfolded protein response and maintains homeostasis of the endoplasmic reticulum in pancreatic beta-cells. J Biol Chem 2005; 280: 39609–39615. [DOI] [PubMed] [Google Scholar]
- Sandhu MS, Weedon MN, Fawcett KA, Wasson J, Debenham SL, Daly A et al. Common variants in WFS1 confer risk of type 2 diabetes. Nat Genet 2007; 39: 951–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igoillo-Esteve M, Marselli L, Cunha DA, Ladriere L, Ortis F, Grieco FA et al. Palmitate induces a pro-inflammatory response in human pancreatic islets that mimics CCL2 expression by beta cells in type 2 diabetes. Diabetologia 2010; 53: 1395–1405. [DOI] [PubMed] [Google Scholar]
- Wiederkehr A, Wollheim CB. Mitochondrial signals drive insulin secretion in the pancreatic beta-cell. Mol Cell Endocrinol 2012; 353: 128–137. [DOI] [PubMed] [Google Scholar]
- Wiederkehr A, Wollheim CB. Linking fatty acid stress to beta-cell mitochondrial dynamics. Diabetes 2009; 58: 2185–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobuhara M, Saotome M, Watanabe T, Urushida T, Katoh H, Satoh H et al. Mitochondrial dysfunction caused by saturated fatty acid loading induces myocardial insulin-resistance in differentiated H9c2 myocytes: a novel ex vivo myocardial insulin-resistance model. Exp Cell Res 2013; 319: 955–966. [DOI] [PubMed] [Google Scholar]
- Souto Padron de Figueiredo A, Salmon AB, Bruno F, Jimenez F, Martinez HG, Halade GV et al. Nox2 mediates skeletal muscle insulin resistance induced by a high fat diet. J Biol Chem 2015; 290: 13427–13439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Qian Z, Ji H, Yang R, Wang Y, Xi L et al. Inhibitory effect on protein kinase Ctheta by Crocetin attenuates palmitate-induced insulin insensitivity in 3T3-L1 adipocytes. Eur J Pharmacol 2010; 642: 47–55. [DOI] [PubMed] [Google Scholar]
- Martinez-Garcia C, Izquierdo-Lahuerta A, Vivas Y, Velasco I, Yeo TK, Chen S et al. Renal lipotoxicity-associated inflammation and insulin resistance affects actin cytoskeleton organization in podocytes. PLoS ONE 2015; 10: e0142291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer CM, Belsham DD. Palmitate attenuates insulin signaling and induces endoplasmic reticulum stress and apoptosis in hypothalamic neurons: rescue of resistance and apoptosis through adenosine 5′ monophosphate-activated protein kinase activation. Endocrinology 2010; 151: 576–585. [DOI] [PubMed] [Google Scholar]
- Piro S, Maniscalchi ET, Monello A, Pandini G, Mascali LG, Rabuazzo AM et al. Palmitate affects insulin receptor phosphorylation and intracellular insulin signal in a pancreatic alpha-cell line. Endocrinology 2010; 151: 4197–4206. [DOI] [PubMed] [Google Scholar]
- Salvado L, Coll T, Gomez-Foix AM, Salmeron E, Barroso E, Palomer X et al. Oleate prevents saturated-fatty-acid-induced ER stress, inflammation and insulin resistance in skeletal muscle cells through an AMPK-dependent mechanism. Diabetologia 2013; 56: 1372–1382. [DOI] [PubMed] [Google Scholar]
- Kwon B, Lee HK, Querfurth HW. Oleate prevents palmitate-induced mitochondrial dysfunction, insulin resistance and inflammatory signaling in neuronal cells. Biochim Biophys Acta 2014; 1843: 1402–1413. [DOI] [PubMed] [Google Scholar]
- Perdomo L, Beneit N, Otero YF, Escribano O, Diaz-Castroverde S, Gomez-Hernandez A et al. Protective role of oleic acid against cardiovascular insulin resistance and in the early and late cellular atherosclerotic process. Cardiovasc Diabetol 2015; 14: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen EJ, Diamond-Stanic MK, Marchionne EM. Oxidative stress and the etiology of insulin resistance and type 2 diabetes. Free Radic Biol Med 2011; 51: 993–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galadari S, Rahman A, Pallichankandy S, Galadari A, Thayyullathil F. Role of ceramide in diabetes mellitus: evidence and mechanisms. Lipids Health Dis 2013; 12: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyanarayana P, Barthwal MK, Kundu CN, Lane ME, Bergmann A, Tzivion G et al. Activation of the Drosophila MLK by ceramide reveals TNF-alpha and ceramide as agonists of mammalian MLK3. Mol Cell 2002; 10: 1527–1533. [DOI] [PubMed] [Google Scholar]
- Lanner JT, Katz A, Tavi P, Sandstrom ME, Zhang SJ, Wretman C et al. The role of Ca2+ influx for insulin-mediated glucose uptake in skeletal muscle. Diabetes 2006; 55: 2077–2083. [DOI] [PubMed] [Google Scholar]
- Whitehead JP, Molero JC, Clark S, Martin S, Meneilly G, James DE. The role of Ca2+ in insulin-stimulated glucose transport in 3T3-L1 cells. J Biol Chem 2001; 276: 27816–27824. [DOI] [PubMed] [Google Scholar]
- Furuta A, Tanaka M, Omata W, Nagasawa M, Kojima I, Shibata H. Microtubule disruption with BAPTA and dimethyl BAPTA by a calcium chelation-independent mechanism in 3T3-L1 adipocytes. Endocr J 2009; 56: 235–243. [DOI] [PubMed] [Google Scholar]
- Contreras-Ferrat A, Llanos P, Vasquez C, Espinosa A, Osorio-Fuentealba C, Arias-Calderon M et al. Insulin elicits a ROS-activated and an IP(3)-dependent Ca(2)(+) release, which both impinge on GLUT4 translocation. J Cell Sci 2014; 127((Pt 9)): 1911–1923. [DOI] [PubMed] [Google Scholar]
- Taddeo EP, Laker RC, Breen DS, Akhtar YN, Kenwood BM, Liao JA et al. Opening of the mitochondrial permeability transition pore links mitochondrial dysfunction to insulin resistance in skeletal muscle. Mol Metab 2014; 3: 124–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CH, Tsai TF, Wei YH. Role of mitochondrial dysfunction and dysregulation of Ca(2+) homeostasis in insulin insensitivity of mammalian cells. Ann NY Acad Sci 2015; 1350: 66–76. [DOI] [PubMed] [Google Scholar]
- Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001; 414: 813–820. [DOI] [PubMed] [Google Scholar]
- Shah A, Xia L, Goldberg H, Lee KW, Quaggin SE, Fantus IG. Thioredoxin-interacting protein mediates high glucose-induced reactive oxygen species generation by mitochondria and the NADPH oxidase, Nox4, in mesangial cells. J Biol Chem 2013; 288: 6835–6848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manea A, Manea SA, Todirita A, Albulescu IC, Raicu M, Sasson S et al. High-glucose-increased expression and activation of NADPH oxidase in human vascular smooth muscle cells is mediated by 4-hydroxynonenal-activated PPARalpha and PPARbeta/delta. Cell Tissue Res 2015; 361: 593–604. [DOI] [PubMed] [Google Scholar]
- Cheng DW, Jiang Y, Shalev A, Kowluru R, Crook ED, Singh LP. An analysis of high glucose and glucosamine-induced gene expression and oxidative stress in renal mesangial cells. Arch Physiol Biochem 2006; 112: 189–218. [DOI] [PubMed] [Google Scholar]
- Daiber A. Redox signaling (cross-talk) from and to mitochondria involves mitochondrial pores and reactive oxygen species. Biochim Biophys Acta 2010; 1797: 897–906. [DOI] [PubMed] [Google Scholar]
- Kumar B, Kowluru A, Kowluru RA. Lipotoxicity augments glucotoxicity-induced mitochondrial damage in the development of diabetic retinopathy. Invest Ophthalmol Vis Sci 2015; 56: 2985–2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong OK, Yoo SJ, Son JW, Kim MK, Baek KH, Song KH et al. High glucose and palmitate increases bone morphogenic protein 4 expression in human endothelial cells. Korean J Physiol Pharmacol 2016; 20: 169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennon R, Pons D, Sabin MA, Wei C, Shield JP, Coward RJ et al. Saturated fatty acids induce insulin resistance in human podocytes: implications for diabetic nephropathy. Nephrol Dial Transplant 2009; 24: 3288–3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikama Y, Kudo Y, Ishimaru N, Funaki M. Possible Involvement of Palmitate in Pathogenesis of Periodontitis. J Cell Physiol 2015; 230: 2981–2989. [DOI] [PubMed] [Google Scholar]