Abstract
Insulin action on hippocampus improves cognitive function, and obesity and type 2 diabetes are associated with decreased cognitive function. Cerebral microvasculature plays a critical role in maintaining cerebral vitality and function by supplying nutrients, oxygen, and hormones such as insulin to cerebral parenchyma, including hippocampus. In skeletal muscle, insulin actively regulates microvascular opening and closure, and this action is impaired in the insulin-resistant states. To examine insulin’s action on hippocampal microvasculature and parenchyma and the impact of diet-induced obesity, we determined cognitive function and microvascular insulin responses, parenchyma insulin responses, and capillary density in the hippocampus in 2- and 8-mo-old rats on chow diet and 8-mo-old rats on a long-term high-fat diet (6 mo). Insulin infusion increased hippocampal microvascular perfusion in rats on chow diet by ~80–90%. High-fat diet feeding completely abolished insulin-mediated microvascular responses and protein kinase B phosphorylation but did not alter the capillary density in the hippocampus. This was associated with a significantly decreased cognitive function assessed using both the two-trial spontaneous alternation behavior test and the novel object recognition test. As the microvasculature provides the needed endothelial surface area for delivery of nutrients, oxygen, and insulin to hippocampal parenchyma, we conclude that hippocampal microvascular insulin resistance may play a critical role in the development of cognitive impairment seen in obesity and diabetes. Our results suggest that improvement in hippocampal microvascular insulin sensitivity might help improve or reverse cognitive function in the insulin-resistant states.
Keywords: blood flow, cognition, hippocampus, insulin resistance, microvasculature
patients with type 2 diabetes mellitus (T2DM) tend to develop cognitive dysfunction of various forms, including mild cognitive deficit, mild cognitive impairment, and dementia. Clinical studies have clearly linked T2DM and insulin resistance to cognitive dysfunction and dementia (1, 52). Animals with T2DM and insulin resistance also exhibit deficits in a wide range of hippocampus-dependent tasks such as water maze, object recognition, contextual cue conditioning, and discrimination reversal learning (8). Although the underlying mechanisms remain largely unknown, brain insulin resistance, particularly in the hippocampus, has been implicated in the development of cognitive dysfunction in patients with T2DM (8, 45).
Intact insulin signaling supports normal cognitive function (7, 38, 41, 50), and insulin acts on neuronal insulin receptors to regulate ion channels, neurotransmitters, and synaptic transmission and control glucose utilization by increasing glucose uptake (8, 24, 49). It has been demonstrated that impaired cerebral glucose utilization and energy metabolism precede or accompany the initial stages of cognitive impairment (28, 43), and hyperinsulinemia is associated with memory improvement in Alzheimer’s disease patients (13). Indeed, hippocampal insulin resistance has been shown to impair spatial learning and synaptic plasticity and is likely a key mediator of cognitive deficits independent of glycemic control (21). Studies aimed at addressing the cellular and molecular mechanisms underpinning insulin resistance in the hippocampus suggest that mitochondrial dysfunction, increased production of reactive oxygen species, and neuroinflammation may have contributed to this process (8).
Microvasculature controls tissue delivery of vital nutrients, oxygen, and hormones such as insulin and provides the endothelial surface area for their extraction into the tissue interstitium (4, 6). In peripheral tissues such as skeletal muscle, insulin potently recruits muscle microvasculature to increase tissue perfusion and thus the delivery of nutrients, oxygen, and insulin to myocytes, and this action is clearly impaired in the insulin-resistant states (36). We have reported recently that microvascular insulin resistance is an early event in diet-induced obesity and may contribute to the development of metabolic insulin resistance by causing tissue hypoxia and decreased insulin delivery (55). Insulin resistance also is associated with a decreased capillary density in the muscle, which further reduces the endothelial surface area. Insulin also increases cerebral blood flow (11), and a recent clinical study has shown that insulin resistance is associated with a reduced cortical perfusion in cognitively asymptomatic middle-aged adults (27). Whether insulin regulates microvascular perfusion in the hippocampus and whether insulin resistance decreases hippocampal insulin-mediated microvascular perfusion and capillary density are not known. This is an important question, as abnormalities in microvasculature may result in decreased endothelial surface area and less delivery of nutrients, oxygen, and insulin, thus contributing to the development of hippocampal insulin resistance and cognitive dysfunction.
In the current study, we used contrast-enhanced ultrasound (CEU) to assess insulin-mediated hippocampal microvascular perfusion and aimed to examine the impact of long-term high-fat diet (HFD) on insulin action in hippocampus in rats together with hippocampus-dependent cognitive function and hippocampal microvascular density. Diet-induced obesity rodent models are commonly used in the studies of T2DM and insulin resistance as they reflect more closely human physiology than genetic rodent models (31). Our results indicate that insulin could potently increase hippocampal microvascular perfusion in rats fed a chow diet but failed to do so in HFD-fed animals. HFD feeding also impaired hippocampus-dependent cognitive function and caused a selective insulin resistance in the protein kinase B (Akt) signaling pathway in the hippocampus.
MATERIALS AND METHODS
Animal Preparations and Experimental Protocols
Adult male Sprague-Dawley rats (2 mo old) were purchased from Charles River Laboratories (Wilmington, MA), housed in groups of two in 480 × 280 × 240 mm3 cages (Allentown, Inc., Allentown, NJ) at 22 ± 2°C on a 12:12-h light-dark cycle (lights on at 7 AM and off at 7 PM) and humidity of ~50% and had access to water ad libitum. Environmental enrichment included aspen wood chip bedding (Harlan Laboratories, Madison, WI), and one 70 × 70 × 150 mm3 polycarbonate rat retreat (Bioserv, Flemington, NJ). Animals were monitored twice daily for health status. No adverse events were observed. After a 7-day acclimation period with free access to a standard chow diet (protein 28 kcal%, carbohydrate 60 kcal%, and fat 12 kcal%) and water ad libitum, rats (n = 28) were randomly assigned to two groups of 14 each, with one half fed the standard chow diet (chow) and the other half HFD (protein 20 kcal%, carbohydrate 20 kcal%, and fat 60 kcal%; Research Diets) for 6 mo. Additionally, 14 2-mo-old rats were used as age control. Animals in each group were randomly assigned a number from 1 to 14. Among each group, one-half of the rats randomly received insulin infusion and the other half saline as control. The sample size was calculated to give 80% power to detect a 30% difference in glucose infusion rate. At the end of dietary intervention, seven to eight rats in each group were randomly selected and subjected to hippocampus-dependent learning and memory performance tests.
All rats (sequentially by randomly assigned number) were then fasted overnight, weighed, and anesthetized with thiobutabarbital (Inactin, 120 mg/kg ip; Sigma-Aldrich, St. Louis, MO), which was well documented not to affect blood glucose, blood pressure, or blood flow (26). They were placed in a prone position on a heating pad to ensure euthermia and intubated to maintain a patent airway. A craniotomy (8 × 3 mm) was performed with an RA No. 2 Round Carbide Bur in the left parietal bone between bregma and lamda to provide an acoustic window for ultrasound imaging of the brain as described previously (42). The right carotid artery and jugular vein were cannulated with polyethylene tubing (PE-50; Fisher Scientific, Newark, DE) for arterial blood pressure monitoring, arterial blood sampling, and various infusions.
After a 30-min baseline period to ensure hemodynamic stability and a stable level of anesthesia, baseline hippocampal microvascular parameters, including microvascular blood volume (MBV) and microvascular flow velocity (MFV), were determined via the acoustic window using contrast-enhanced ultrasound (CEU) and microbubble as the contrast agent (Definity; Lantheus Medical Imaging), as described previously (9, 18). In brief, microbubbles were diluted with normal saline (1:10 vol:vol) and infused intravenously at a rate of 0.8 ml/hr. Once the systemic microbubble concentrations reached steady state (~6 min), high-power pulse-inversion ultrasound (HDI-5000; Philips Ultrasound) was used to image the hippocampus area (Fig. 1). Intermittent imaging was performed at pulsing intervals ranging from 0.5 to 10 s. At least three images were acquired at each pulsing interval, and the images obtained at 0.5 s were used as background. Pulsing interval vs. background-subtracted video intensity was fitted to the function y = A (1 − e−βt), with y being video intensity at pulsing interval t. A as the plateau video intensity measures MBV, and β measures the rate of microvascular filling (MFV). Microvascular blood flow (MBF) was calculated by multiplying MBV and MFV.
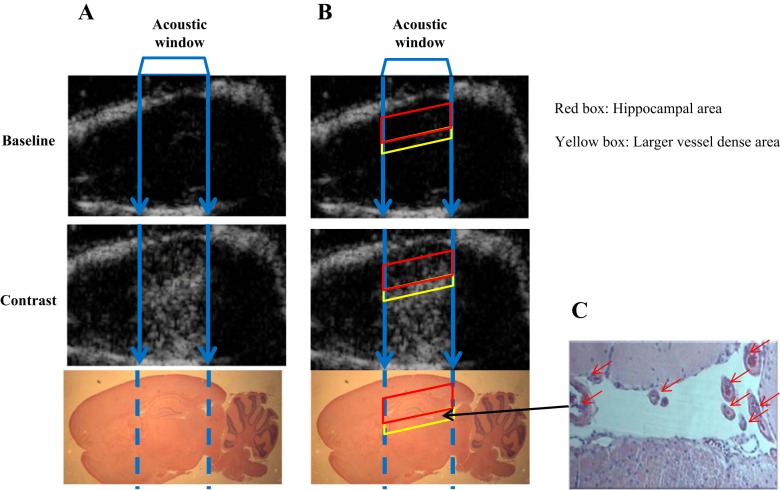
Fig. 1.
Anatomic mapping of ultrasound imaging. A: ultrasound images (top: baseline; middle: microbubble contrast), with corresponding sagittal section of rat brain stained with hematoxylin (bottom). B: same images as in A, with red box delineating the hippocampal area and yellow box the larger vessel dense area. C: hemotoxylin stain of the larger vessel dense area (red arrows, ×40 magnification) below the hippocampus. The middle line between the roof of the skull and larger vessel dense area (area with higher video intensity during microbubble infusion) was used to define the top boundary of hippocampus and the larger vessel dense area the lower boundary of hippocampus on the ultrasound image (B, red box).
After the baseline hippocampal microvascular parameters were obtained, rats then received a continuous intravenous infusion of either saline or a hyperinsulinemic euglycemic clamp (10 mU·kg−1·min−1) for 120 min. Hippocampal microvascular parameters (MBV, MFV, and MBF) were again determined every 30 min using CEU. During the insulin clamp, arterial blood glucose was determined every 10 min using an Accu-Chek Advantage glucometer (Roche Diagnostics, Indianapolis, IN), and 30% dextrose (30% wt/vol) was infused at a variable rate to maintain blood glucose within 10% of basal.
Throughout the study, mean arterial blood pressure (MAP) was monitored via a sensor connected to the arterial catheter (Harvard Apparatus, Holliston, MA; and ADInstruments, Colorado Springs, CO). Heart rate and body temperature were monitored using electrocardiography (ADInstruments, Sydney, Australia) and rectal thermometer, respectively (World Precision Instruments, Sarasota, FL). Thiobutabarbital was infused at a variable rate to maintain steady levels of anesthesia and blood pressure throughout the study. No adverse event was observed during the study. At the end of each study the rat was euthanized by an overdose of thiobutabarbital. The left hippocampus was isolated and freeze-clamped in liquid nitrogen for later measurement of Akt and ERK1/2 phosphorylation using Western blot analysis. Right hemisphere of the brain from rats that received saline infusion was preserved in 4% paraformaldehyde to determine capillary density in the hippocampus.
This study conformed to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (publication No. 85-23, revised 1996). The study protocols were approved by the Animal Care and Use Committee of the University of Virginia.
Determination of Hippocampus-Dependent Learning and Memory Performance
To determine the effect of long-term HFD feeding on cognitive function, we assessed hippocampus-dependent learning and memory performance (between 8 and 12 AM) using both two-trial spontaneous alternation behavior test and novel object recognition test, as described previously (2, 40).
Two-trial spontaneous alternation behavior test.
Spontaneous alternation behavior was determined using a 5-min session Y-maze test, which is a nonspatial learning paradigm that learning performance of each subject can be tested based on attention, working memory, and motor activity performance. The Y-maze has three arms, each 45 cm long, 10 cm wide, and 35 cm high. The arms were positioned at equal angles and converged in an equilateral triangular central area. Each rat was placed at the end of an arm and allowed to move freely through the Y-maze. The series of arm entries was recorded by the Any-Maze video tracking system (Stoelting, Wood Dale, IL). Alternation behavior (actual alternations) was defined as successive entries into the three arms with overlapping triplet sets. The percent alternation was calculated as the ratio of actual alternation to possible alternations (total number of arm entries – 2) × 100.
Novel object recognition test.
This comprised a habituation phase, an acquisition phase, and a test phase separated by a delay. During the habituation phase, each rat was exposed to an empty arena (82 × 32 × 38 cm with no objects) for 5 min. After the 24-h delay period, in the familiarization phase each rat was allowed to explore the same arena but with two identical objects (5 cm from the walls) for 5 min. After a short (30 min) or a long (24 h) delay period, in the test phase, rats were allowed to explore the arena with one of the original objects replaced with a novel object for 5 min. Before and after all behavioral procedures, all objects and arena were cleaned with 75% ethanol. Frequency (entry score to the novel and familiar object area) and duration (time spent in the novel and familiar object area) were recorded by Any-Maze tracking system. The percentage of recognition index was calculated from the ratio of duration of visits to the novel object divided by the total duration of visits to novel plus familiar objects, and the ratio was multiplied by 100.
Determination of Protein Phosphorylation
Hipocampal Akt and ERK1/2 phosphorylation were determined by Western blot analysis, as described previously (18, 32). Primary antibodies against phospho-Akt (Ser473), total Akt, phospho-ERK (Thr202/Tyr204), total ERK1/2, and total eNOS were obtained from Cell Signaling Technology (Beverly, MA). All blots were developed using enhanced chemiluminescence (GE Healthcare Bio-Sciences, Piscataway, NJ). Autoradiographic films were scanned densitometrically and quantified using ImageJ software (National Institutes of Health; https://imagej.nih.gov/ij/). Both the total and phosphospecific densities were quantified and the ratios of phosphospecific to total protein density calculated.
Measurement of Hippocampal Capillary Density
Rat brain was fixed in 4% paraformaldehyde, embedded in paraffin, and then sectioned sagittally at 5-µm thickness. Five to six sections from each brain sample were deparaffinized and rehydrated. After antigen retrieval with sodium citrate buffer (10 mM sodium citrate, 0.05% Tween-20, pH 6.0), sections were incubated with fluorescein Griffonia Simplcifolia Lectin I (Vector Laboratory, Burlingame, CA) overnight at 4°C in a humidity chamber and counterstained with 4,6-diamidino-2-phenylindole. The images of the anterior and posterior of the hippocampus on the section were captured (×40 magnification) to determine the capillary area using ImageJ software (National Institutes of Health; https://imagej.nih.gov/ij/). Capillary densities in both anterior (CA3 region) and posterior (DG region) hippocampus from all sections were calculated by dividing the capillary area with the total area of the image and then averaged.
Statistical Analysis
All data are presented as means ± SE. Statistical analyses were performed with SigmaStat 11.0 software (Systat Software). Data were analyzed using either Student’s t-test or ANOVA with post hoc analysis as appropriate. A P value of <0.05 was considered statistically significant.
RESULTS
Long-Term HFD Feeding Impairs Hippocampal Function in Rats
Prior to the hippocampal CEU and insulin clamp procedures, rats were subjected to hippocampus-dependent cognitive function test using both the two-trial spontaneous alternation behavior test (Y-maze) and the novel object recognition test (NOR). As shown in Fig. 2A, 2- and 8-mo-old rats on chow diet had the same percentage of spontaneous alternation behavior (~40%), but rats on long-term HFD had an ~50% increase (~60%) in spontaneous alternation behavior (P < 0.05) due to random entries to the blocked and unblocked arms in the Y-maze test. Similarly in NOR test (Fig. 2B), both 2- and 8-mo-old rats on chow diet spent ~80% of the time with the novel object in the test phase at either short (30 min) or long (24 h) delay periods. On the contrary, HFD-fed rats showed a significantly reduced time spent with the novel object (~50%, P < 0.05). Thus, both tests demonstrated that long-term HFD impairs hippocampal cognitive function in rats.
Fig. 2.
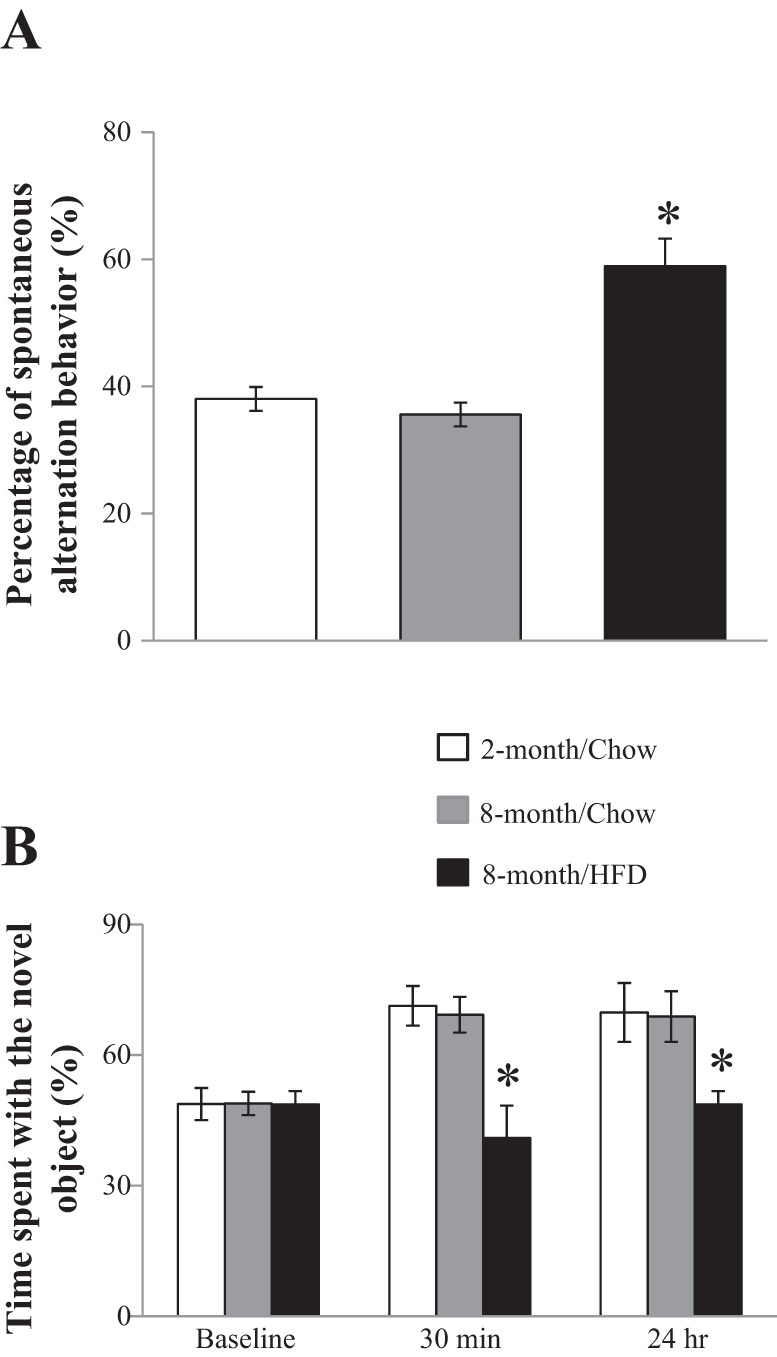
Long-term high-fat diet (HFD) feeding impairs cognitive function. Eight-month-old rats fed either a HFD or standard chow diet for 6 mo were subject to both a 2-trial spontaneous alternation behavior test (Y-maze; A) and a novel object recognition test (B). Two-month-old rats on chow diet were used as age controls. *P < 0.05; n = 7–8 each.
Long-term HFD Feeding Causes Metabolic Insulin Resistance
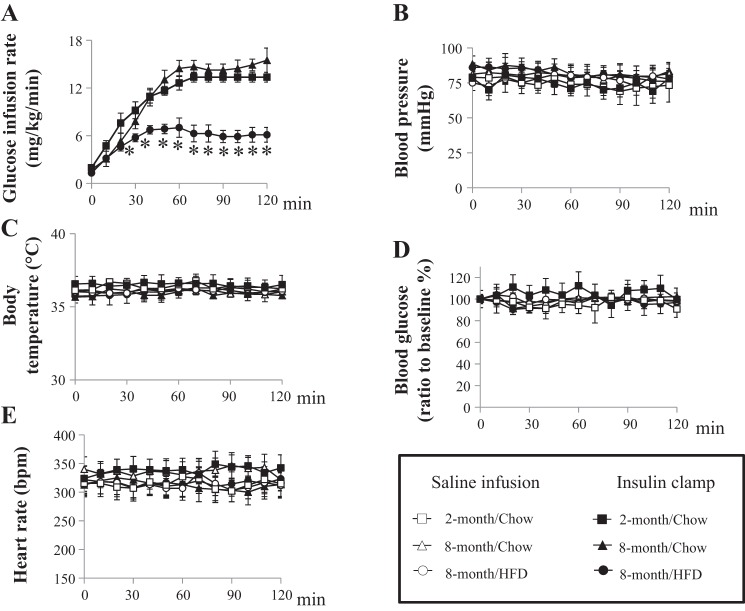
As expected, HFD feeding for 6 mo caused significant peripheral insulin resistance, as demonstrated by an ~50% decrease in insulin-mediated whole body glucose disposal during insulin clamp (P < 0.01; Fig. 3A) when compared with 2- or 8-mo-old rats on chow diet. During the insulin clamp, blood glucose, blood pressure, heart rate, and body temperature remained steady across all three groups (Fig. 3, B–D). Both 2- and 8-mo-old rats on chow diet had similar fasting plasma insulin, triglyceride, and total cholesterol levels, whereas HFD-fed rats exhibited significantly higher fasting plasma insulin, triglyceride, and total cholesterol levels (P < 0.05; Table 1). Insulin infusion lowered plasma triglyceride levels by 50% (P < 0.05) in 2- and 8-mo-old rats on chow diet but by a much smaller percentage (~30%) in the HFD group.
Fig. 3.
Long-term HFD feeding decreases insulin-mediated whole body glucose disposal. Rats from each treatment group received a 120-min euglycemic hyperinsulinemic clamp (10 mU·kg−1·min−1) or saline infusion. A: glucose infusion rate was determined every 10 min during insulin clamp. B–E: blood pressure (B), body temperature (C), blood glucose concentrations (D), and heart rate (E) remained steady during insulin clamp or saline infusion. *P < 0.01; n = 7 each.
Table 1.
Plasma insulin, triglyceride, and total cholesterol levels
| Infusion | Total cholesterol, mM | Triglyceride, mg/dl | Insulin, pM | Weight, g |
|---|---|---|---|---|
| 2-Mo chow | ||||
| Saline 0 min | 2.33 ± 0.21a | 46.82 ± 9.73a | 134.97 ± 8.02a | 342.43 ± 6.94a |
| Saline 2 h | 2.35 ± 0.24a | 42.04 ± 5.21a | 139.79 ± 8.2a | |
| Insulin 0 min | 2.66 ± 0.24a | 46.46 ± 9.09a | 134.89 ± 11.31a | 348.86 ± 7.11a |
| Insulin 2 h | 2.5 ± 0.08a | 17.02 ± 2.29c | 1,431.6 ± 131.85c | |
| 8-Mo chow | ||||
| Saline 0 min | 2.94 ± 0.4a | 45.75 ± 5.54a | 152.97 ± 3.03a | 610.57 ± 12.43b |
| Saline 2 h | 3.07 ± 0.37a | 50.71 ± 9.7a | 128.47 ± 7.97a | |
| Insulin 0 min | 2.68 ± 0.38a | 49.56 ± 10.86a | 134.95 ± 9.23a | 616.43 ± 19.12b |
| Insulin 2 h | 2.76 ± 0.39a | 24.62 ± 2.09c | 1,324.63 ± 130.94c | |
| 8-Mo HFD | ||||
| Saline 0 min | 4.14 ± 0.46b | 71.66 ± 7.93b | 640.59 ± 111.54b | 890.57 ± 21.24c |
| Saline 2 h | 4.64 ± 0.63b | 70.51 ± 8.33b | 579.29 ± 78.66b | |
| Insulin 0 min | 4.79 ± 0.52b | 78.65 ± 9.96b | 639.39 ± 93.96b | 925.29 ± 24.58c |
| Insulin 2 h | 4.48 ± 0.43b | 50.97 ± 7.7c | 2,675.76 ± 115.6c | |
Data are presented as means ± SE; n = 7. Chow, standard chow diet; HFD, high-fat diet. Rats from each group received either a saline infusion or euglycemic hyperinsulinemic clamp (10 mU·kg−1·min−1) for 2 h. Plasma insulin, triglyceride, and total cholesterol concentrations and body weight were measured. For each parameter, values without a common letter denote P < 0.05; n = 7 each.
Long-Term HFD Feeding Impairs Insulin-Mediated Microvascular Perfusion in Hippocampus
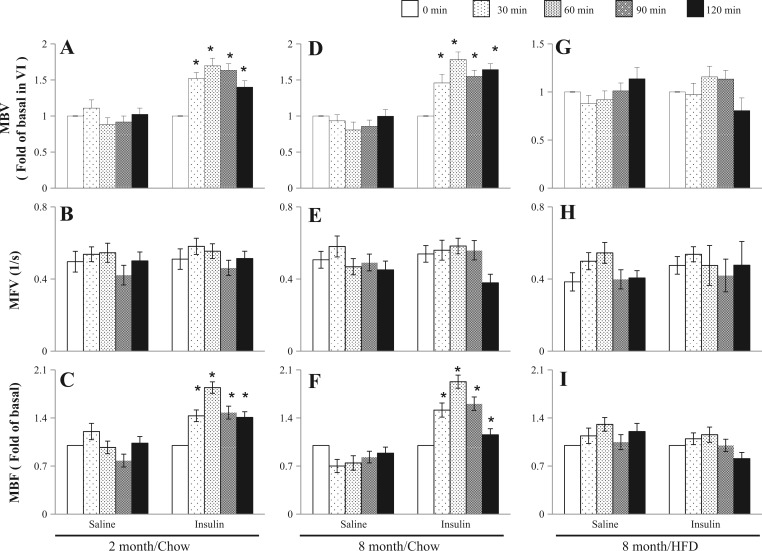
Insulin exerts vasodilatory actions on both conduit artery and microvasculature in the periphery (34, 35, 37), and a study has shown that it also dilates carotid artery to increase cerebral blood flow (11). To assess insulin action on the hippocampal microvasculature and the effect of long-term HFD, we quantified both basal and insulin-mediated microvascular perfusion in the hippocampus using CEU. Figure 4 shows both basal and insulin-mediated changes in hippocampal MBV, MFV, and MBF in 2- and 8-mo-rats on chow diet and 8-mo-old rats on long-term HFD. Insulin promptly increased both hippocampal MBV and MBF without affecting MFV at 30 min in both 2- and 8-mo-old rats on chow diet, and this effect reached maximum at 60 min: ~80% increase in MBV (P < 0.05) and ~90% increase in MBF (P < 0.05). HFD did not alter basal hippocampal microvascular parameters but completely abolished insulin-mediated increases in MBV and MBF in hippocampus.
Fig. 4.
Insulin increases hippocampal microvascular perfusion, which is impaired by long-term HFD feeding. Hippocampal microvascular blood volume (MBV; A, D, and G), microvascular flow velocity (MFV; B, E, and H), and microvascular blood flow (MBF; C, F, and I) were determined at 0, 30, 60, 90, and 120 min during either saline infusion or euglycemic hyperinsulinemic clamp (10 mU·kg−1·min−1) in each treatment group. *P < 0.05 vs. time 0; n = 7 each.
Long-Term HFD Feeding Does Not Affect Capillary Density in the Hippocampus
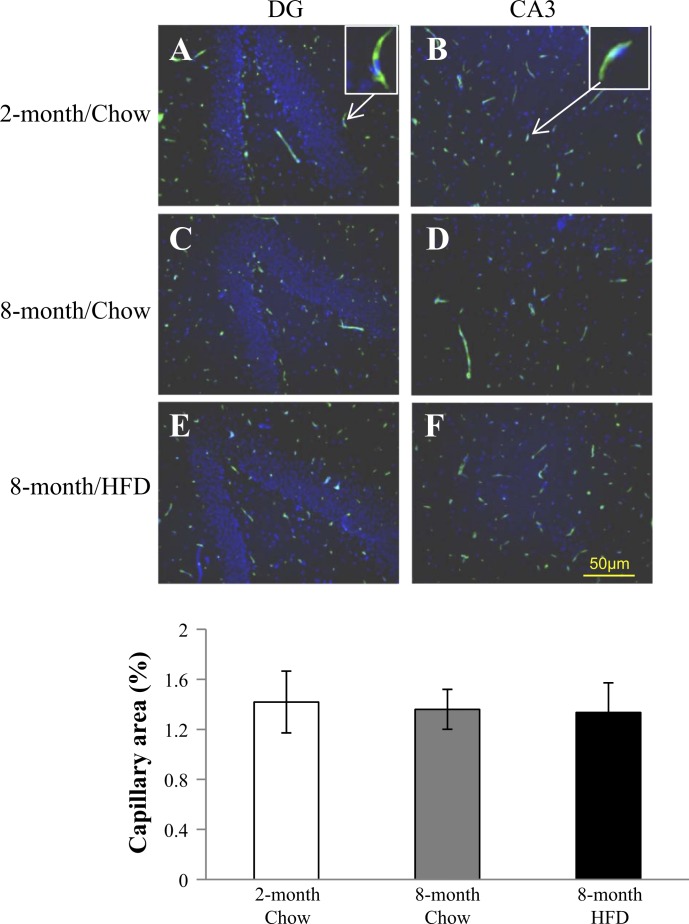
Capillary density is another major determinant of endothelial exchange surface area in addition to microvascular perfusion, and prior studies have shown that HFD decreases capillary density in peripheral tissues such as skeletal muscle (19, 33). As such, we also determined the capillary density in the hippocampus using fluorescein Griffonia Simplicifolia Lectin I staining (Fig. 5). The areas that capillaries cover (green) were comparable among all three groups (P = 0.962, 1-way ANOVA), suggesting that long-term HFD feeding did not alter capillary density in the hippocampus.
Fig. 5.
Long-term HFD does not affect hippocampal capillary density. Sagittal section of rat brain from each treatment group was stained with fluorescein Griffonia Simplicifolia Lectin I (green) and counterstained with 4,6-diamidino-2-phenylindole (blue). Image was sampled and analyzed in DG and CA3 regions of hippocampus from each section. Five to six sections from each rat were used to determine the capillary density. Top: examples of fluorescein Griffonia Simplicifolia Lectin I staining. Bottom: quantification of capillary density; n = 7 each.
Effects of Long-Term HFD on Insulin-Mediated Hippocampal Akt and ERK1/2 Phosphorylation and eNOS Expression
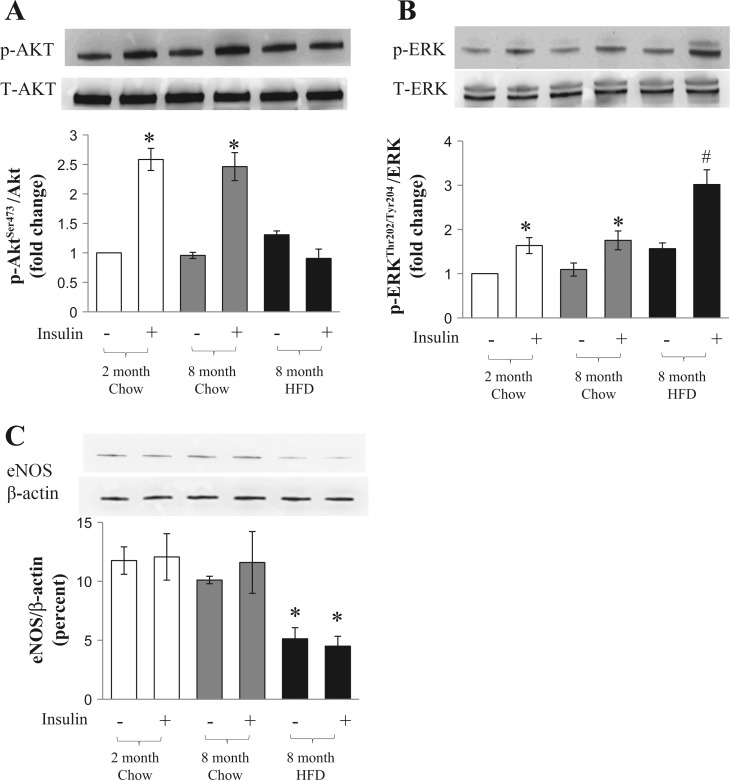
As evidence suggests that hippocampal insulin resistance results from a combination of decreased insulin transport across the blood-brain barrier and an impaired insulin receptor signaling in the hippocampus (3, 8, 20), and the above results demonstrated that HFD feeding abolishes insulin-mediated increases in hippocampal MBV and thus the endothelial exchange surface area, we examined insulin-mediated phosphorylation of Akt and ERK1/2, two key signal intermediates in the insulin-signaling pathways as well as the eNOS expression level, which is critical in governing vessel function (48) in the isolated hippocampus from all study animals (Fig. 6). Insulin infusion potently increased hippocampal Akt (~1.5-fold increase, P < 0.05) and ERK1/2 (~1-fold increase, P < 0.05) phosphorylation in both 2- and 8-mo-old rats on the chow diet. However, insulin-induced Akt phosphorylation was completely abolished in rats that received a 6-mo HFD feeding. Insulin-stimulated ERK1/2 phosphorylation remained elevated in these HFD-fed rats (~2-fold increase, P < 0.01). Six-month HFD feeding reduced hippocampal eNOS expression by ~50% (P < 0.05) when compared with rats fed a chow diet.
Fig. 6.
Effects of long-term HFD feeding on insulin-mediated Akt and ERK1/2 phosphorylation and endothelial nitric oxide synthase (eNOS) expression in hippocampus. Rat hippocampus from each treatment group was collected after either a saline infusion or euglycemic hyperinsulinemic clamp (10 mU·kg−1·min−1). Ratios of phosphorylated/total Akt (A) or ERK1/2 (B) and eNOS/β-actin (C) were determined using Western blot. *P < 0.05 and #P < 0.01 vs. respective saline control; n = 7 each.
DISCUSSION
Given the importance of microvasculature in maintaining tissue vitality and function, the clear link between vascular insulin resistance and metabolic insulin resistance in muscle, and the evidence suggesting hippocampal insulin resistance in the development of cognitive dysfunction, in the current study we examined the effect of insulin on hippocampal microvascular perfusion and the effect of long-term HFD feeding on cognitive function and insulin’s microvascular function and capillary density in the hippocampus. Our results indicate that insulin is able to potently increase microvascular perfusion in the hippocampus, but this action is impaired in long-term HFD-fed rodents, along with decreased cognitive function and hippocampal insulin resistance but preserved hippocampal capillary density.
In healthy humans, insulin at physiological concentrations induces vasodilation of internal carotid artery (11), thus increasing cerebral blood flow. Using the CEU technique, we in the current study found that insulin potently increases the microvascular blood volume (MBV) in the hippocampus without affecting MFV, resulting in increased blood flow. Similar to peripheral vasculature, in brain the microvascular tone is also regulated by the vascular endothelium, which releases both vasodilator nitric oxide (NO) and vasoconstrictor endothelin-1 (12, 16). Insulin in the insulin-sensitive state increases NO production by activating eNOS through the phosphatidylinositol (PI) 3-kinase-Akt signal pathway (15, 39, 53), leading to increased blood flow to tissue. This finding is of physiological significance, as increased microvascular endothelial exchange surface area increases the delivery of nutrients, oxygen, and hormones such as insulin to the hippocampal parenchyma.
In the current study, we chose to use a long-term HFD rodent model, as we intended to examine not only the functional changes (insulin responses) but also structural changes (capillary density) in hippocampal microvasculature as well as the development of cognitive dysfunction, which is a slow process. We used two behavioral tests (Y-Maze Spontaneous Alternation and Novel Object Recognition) to assess the willingness of rodents to explore new environments and recognition memory, both of which are hippocampus dependent (2, 40). Our results showed that these animals exhibited not only peripheral insulin resistance as reflected by a significant decrease in insulin-mediated whole body glucose disposal but also decreased cognitive function and hippocampal insulin resistance in both the microvasculature and the parenchyma, as evidenced by decreased insulin-mediated microvascular perfusion and parenchymal Akt phosphorylation.
Microvascular endothelium forms the first barrier for the central nervous system and is the “first responder” to environmental insults such as excessive free fatty acids and inflammatory cytokines that have been proven to cause insulin resistance (5). Insulin delivery to the capillaries nurturing myocytes and transendothelial insulin transport is the rate-limiting step in insulin action in muscle (25, 51), and microvascular insulin resistance precedes metabolic insulin resistance in muscle (55). Although there is evidence suggesting that neurons are able to produce insulin (22), studies confirm that peripheral insulin is the main source of insulin to brain via a saturable process (3, 20). Our finding that long-term HFD feeding completely abolished insulin-mediated hippocampal microvascular perfusion confirms the presence of insulin resistance in hippocampal microcirculation. Since in the vasculature the insulin resistance is selective only in the PI 3-kinase/Akt/eNOS pathway, insulin-mediated NO production is decreased but insulin-mediated endothelin-1 production is unchanged or even increased, tilting the balance to vasoconstriction and resulting in decreased tissue perfusion. Indeed, insulin-mediated vasodilation of internal carotid artery in humans with T2DM is absent (10), and in mice fed a HFD for 8.5 mo cerebral blood flow assessed using MRI is decreased (56). Whether this impairment by HFD led to decreased insulin delivery to the hippocampus remains to be studied. That insulin-mediated hippocampal Akt phosphorylation is decreased by HFD feeding supports such a possibility.
Tissue capillary density is another major determinant of the endothelial surface area. In skeletal muscle the number of capillaries in muscle directly correlates with insulin-stimulated glucose uptake (23, 33), and insulin resistance is associated with a reduction of muscle capillary content (capillary rarefaction) (19, 33) that is proportional to the severity of insulin resistance (33, 44). It is of great interest to note that in the hippocampus long-term HFD feeding caused significant microvascular insulin resistance, but the capillary density was preserved. This suggests that the decrease in hippocampal endothelial surface area results mainly from functional abnormality and points to the possibility that correction of hippocampal microvascular insulin resistance might be a therapeutic possibility in improving or even reversing cognitive function in patients with T2DM. One caveat is that although in young (7 mo of age) mice HFD feeding does not decrease capillary density, aging does exacerbate obesity-induced microvascular rarefaction in the hippocampus (47), arguing for a special emphasis in obesity prevention in the aged.
The mechanisms underpinning the association between insulin resistance and cognitive dysfunction are largely unknown, and our results suggest that hippocampal microvascular insulin resistance might contribute to decreased hippocampus-dependent cognitive functions seen in patients with T2DM and insulin resistance. Insulin has been shown repeatedly to improve cognitive function but only in healthy humans and rodents, not in the insulin-resistant states (8). This is not surprising, as microvascular insulin resistance, as seen in the current study, results in decreased hippocampal microvascular perfusion, thus resulting in less delivery of nutrients from blood to brain in addition to oxygen and insulin. It is not clear whether the microvascular insulin resistance seen in the current study was secondary to HFD and/or HFD-induced obesity. Future studies using obesity-prone and -resistant rat models may help clarify this.
Similar to peripheral tissues such as muscle and vasculature (14, 29, 30), HFD-induced insulin resistance is also selective in the hippocampus in that it blunted only insulin-mediated Akt phosphorylation but not the ERK1/2 phosphorylation. The physiological significance of this selective insulin resistance in the hippocampus is not clear, as ERK cascade regulates multiple functions, including gene expression, cell proliferation, and other functions such as long-term depression, which decreases protein phosphorylation (46). The marked decrease in hippocampal eNOS expression in HFD-fed rats provides further explanation of the lack of microvascular response to insulin in those animals, as eNOS is the major molecule regulating vascular tension (17, 54).
In conclusion, insulin potently increases hippocampal microvascular perfusion in rats, and this action is impaired by long-term HFD feeding, which also engenders hippocampus-dependent cognitive deficits without decreasing hippocampal capillary density. Because the microvasculature provides endothelial surface area for delivery of nutrients, oxygen, and insulin to hippocampal parenchyma, hippocampal microvascular insulin resistance may play a critical role in the development of cognitive impairment seen in obesity and T2DM.
GRANTS
This work was supported by the American Diabetes Association Grants 1-15-CE-32 (to Z. Liu) and National Institutes of Health Grants R01-HL-094722 and R01-DK-102359 (to Z. Liu). Z. Fu is supported by a fellowship award from the American Diabetes Association.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Z.F., J.W., T.N., K.W.A., and Z.L. performed experiments; Z.F., M.D.L., and Z.L. analyzed data; Z.F., M.D.L., and Z.L. interpreted results of experiments; Z.F. and Z.L. prepared figures; Z.F. and Z.L. drafted manuscript; Z.F. and Z.L. edited and revised manuscript; Z.F., J.W., T.N., M.D.L., K.W.A., and Z.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Rick I. Meijer for technical assistance in hippocampal contrast-enhanced ultrasonography.
REFERENCES
- 1.Allen KV, Frier BM, Strachan MW. The relationship between type 2 diabetes and cognitive dysfunction: longitudinal studies and their methodological limitations. Eur J Pharmacol 490: 169–175, 2004. doi: 10.1016/j.ejphar.2004.02.054. [DOI] [PubMed] [Google Scholar]
- 2.Antunes M, Biala G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process 13: 93–110, 2012. doi: 10.1007/s10339-011-0430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banks WA, Owen JB, Erickson MA. Insulin in the brain: there and back again. Pharmacol Ther 136: 82–93, 2012. doi: 10.1016/j.pharmthera.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrett EJ, Eggleston EM, Inyard AC, Wang H, Li G, Chai W, Liu Z. The vascular actions of insulin control its delivery to muscle and regulate the rate-limiting step in skeletal muscle insulin action. Diabetologia 52: 752–764, 2009. doi: 10.1007/s00125-009-1313-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barrett EJ, Liu Z. The endothelial cell: an “early responder” in the development of insulin resistance. Rev Endocr Metab Disord 14: 21–27, 2013. doi: 10.1007/s11154-012-9232-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barrett EJ, Wang H, Upchurch CT, Liu Z. Insulin regulates its own delivery to skeletal muscle by feed-forward actions on the vasculature. Am J Physiol Endocrinol Metab 301: E252–E263, 2011. doi: 10.1152/ajpendo.00186.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benedict C, Hallschmid M, Schultes B, Born J, Kern W. Intranasal insulin to improve memory function in humans. Neuroendocrinology 86: 136–142, 2007. doi: 10.1159/000106378. [DOI] [PubMed] [Google Scholar]
- 8.Biessels GJ, Reagan LP. Hippocampal insulin resistance and cognitive dysfunction. Nat Rev Neurosci 16: 660–671, 2015. doi: 10.1038/nrn4019. [DOI] [PubMed] [Google Scholar]
- 9.Chai W, Wang W, Liu J, Barrett EJ, Carey RM, Cao W, Liu Z. Angiotensin II type 1 and type 2 receptors regulate basal skeletal muscle microvascular volume and glucose use. Hypertension 55: 523–530, 2010. doi: 10.1161/HYPERTENSIONAHA.109.145409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaudhuri A, Kanjwal Y, Mohanty P, Rao S. Absence of insulin-induced vasodilation of internal carotid artery in type 2 diabetes. Metab Syndr Relat Disord 1: 69–73, 2003. doi: 10.1089/154041903321648261. [DOI] [PubMed] [Google Scholar]
- 11.Chaudhuri A, Kanjwal Y, Mohanty P, Rao S, Sung BH, Wilson MF, Dandona P. Insulin-induced vasodilatation of internal carotid artery. Metabolism 48: 1470–1473, 1999. doi: 10.1016/S0026-0495(99)90161-0. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, McCarron RM, Golech S, Bembry J, Ford B, Lenz FA, Azzam N, Spatz M. ET-1- and NO-mediated signal transduction pathway in human brain capillary endothelial cells. Am J Physiol Cell Physiol 284: C243–C249, 2003. doi: 10.1152/ajpcell.00305.2002. [DOI] [PubMed] [Google Scholar]
- 13.Craft S, Newcomer J, Kanne S, Dagogo-Jack S, Cryer P, Sheline Y, Luby J, Dagogo-Jack A, Alderson A. Memory improvement following induced hyperinsulinemia in Alzheimer’s disease. Neurobiol Aging 17: 123–130, 1996. doi: 10.1016/0197-4580(95)02002-0. [DOI] [PubMed] [Google Scholar]
- 14.Cusi K, Maezono K, Osman A, Pendergrass M, Patti ME, Pratipanawatr T, DeFronzo RA, Kahn CR, Mandarino LJ. Insulin resistance differentially affects the PI 3-kinase- and MAP kinase-mediated signaling in human muscle. J Clin Invest 105: 311–320, 2000. doi: 10.1172/JCI7535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399: 601–605, 1999. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 16.Durieu-Trautmann O, Fédérici C, Créminon C, Foignant-Chaverot N, Roux F, Claire M, Strosberg AD, Couraud PO. Nitric oxide and endothelin secretion by brain microvessel endothelial cells: regulation by cyclic nucleotides. J Cell Physiol 155: 104–111, 1993. doi: 10.1002/jcp.1041550114. [DOI] [PubMed] [Google Scholar]
- 17.Förstermann U, Münzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation 113: 1708–1714, 2006. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- 18.Fu Z, Zhao L, Chai W, Dong Z, Cao W, Liu Z. Ranolazine recruits muscle microvasculature and enhances insulin action in rats. J Physiol 591: 5235–5249, 2013. doi: 10.1113/jphysiol.2013.257246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gavin TP, Stallings HW 3rd, Zwetsloot KA, Westerkamp LM, Ryan NA, Moore RA, Pofahl WE, Hickner RC. Lower capillary density but no difference in VEGF expression in obese vs. lean young skeletal muscle in humans. J Appl Physiol 98: 315–321, 2005. doi: 10.1152/japplphysiol.00353.2004. [DOI] [PubMed] [Google Scholar]
- 20.Gray SM, Meijer RI, Barrett EJ. Insulin regulates brain function, but how does it get there? Diabetes 63: 3992–3997, 2014. doi: 10.2337/db14-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grillo CA, Piroli GG, Lawrence RC, Wrighten SA, Green AJ, Wilson SP, Sakai RR, Kelly SJ, Wilson MA, Mott DD, Reagan LP. Hippocampal Insulin Resistance Impairs Spatial Learning and Synaptic Plasticity. Diabetes 64: 3927–3936, 2015. doi: 10.2337/db15-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Havrankova J, Schmechel D, Roth J, Brownstein M. Identification of insulin in rat brain. Proc Natl Acad Sci USA 75: 5737–5741, 1978. doi: 10.1073/pnas.75.11.5737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hedman A, Berglund L, Essén-Gustavsson B, Reneland R, Lithell H. Relationships between muscle morphology and insulin sensitivity are improved after adjustment for intra-individual variability in 70-year-old men. Acta Physiol Scand 169: 125–132, 2000. doi: 10.1046/j.1365-201x.2000.00722.x. [DOI] [PubMed] [Google Scholar]
- 24.Heni M, Kullmann S, Preissl H, Fritsche A, Häring H-U. Impaired insulin action in the human brain: causes and metabolic consequences. Nat Rev Endocrinol 11: 701–711, 2015. doi: 10.1038/nrendo.2015.173. [DOI] [PubMed] [Google Scholar]
- 25.Herkner H, Klein N, Joukhadar C, Lackner E, Langenberger H, Frossard M, Bieglmayer C, Wagner O, Roden M, Müller M. Transcapillary insulin transfer in human skeletal muscle. Eur J Clin Invest 33: 141–146, 2003. doi: 10.1046/j.1365-2362.2003.01106.x. [DOI] [PubMed] [Google Scholar]
- 26.Hindlycke M, Jansson L. Glucose tolerance and pancreatic islet blood flow in rats after intraperitoneal administration of different anesthetic drugs. Ups J Med Sci 97: 27–35, 1992. doi: 10.3109/03009739209179279. [DOI] [PubMed] [Google Scholar]
- 27.Hoscheidt SM, Kellawan JM, Berman SE, Rivera-Rivera LA, Krause RA, Oh JM, Beeri MS, Rowley HA, Wieben O, Carlsson CM, Asthana S, Johnson SC, Schrage WG, Bendlin BB. Insulin resistance is associated with lower arterial blood flow and reduced cortical perfusion in cognitively asymptomatic middle-aged adults. J Cereb Blood Flow Metab 0271678X16663214, 2016. doi: 10.1177/0271678X16663214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iwangoff P, Armbruster R, Enz A, Meier-Ruge W. Glycolytic enzymes from human autoptic brain cortex: normal aged and demented cases. Mech Ageing Dev 14: 203–209, 1980. doi: 10.1016/0047-6374(80)90120-7. [DOI] [PubMed] [Google Scholar]
- 29.Jiang ZY, Lin YW, Clemont A, Feener EP, Hein KD, Igarashi M, Yamauchi T, White MF, King GL. Characterization of selective resistance to insulin signaling in the vasculature of obese Zucker (fa/fa) rats. J Clin Invest 104: 447–457, 1999. doi: 10.1172/JCI5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim JA, Montagnani M, Koh KK, Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation 113: 1888–1904, 2006. doi: 10.1161/CIRCULATIONAHA.105.563213. [DOI] [PubMed] [Google Scholar]
- 31.King AJ. The use of animal models in diabetes research. Br J Pharmacol 166: 877–894, 2012. doi: 10.1111/j.1476-5381.2012.01911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li G, Barrett EJ, Wang H, Chai W, Liu Z. Insulin at physiological concentrations selectively activates insulin but not insulin-like growth factor I (IGF-I) or insulin/IGF-I hybrid receptors in endothelial cells. Endocrinology 146: 4690–4696, 2005. doi: 10.1210/en.2005-0505. [DOI] [PubMed] [Google Scholar]
- 33.Lillioja S, Young AA, Culter CL, Ivy JL, Abbott WG, Zawadzki JK, Yki-Järvinen H, Christin L, Secomb TW, Bogardus C. Skeletal muscle capillary density and fiber type are possible determinants of in vivo insulin resistance in man. J Clin Invest 80: 415–424, 1987. doi: 10.1172/JCI113088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu J, Jahn LA, Fowler DE, Barrett EJ, Cao W, Liu Z. Free fatty acids induce insulin resistance in both cardiac and skeletal muscle microvasculature in humans. J Clin Endocrinol Metab 96: 438–446, 2011. doi: 10.1210/jc.2010-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Z. Insulin at physiological concentrations increases microvascular perfusion in human myocardium. Am J Physiol Endocrinol Metab 293: E1250–E1255, 2007. doi: 10.1152/ajpendo.00451.2007. [DOI] [PubMed] [Google Scholar]
- 36.Liu Z, Ko SH, Chai W, Cao W. Regulation of muscle microcirculation in health and diabetes. Diabetes Metab J 36: 83–89, 2012. doi: 10.4093/dmj.2012.36.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Z, Liu J, Jahn LA, Fowler DE, Barrett EJ. Infusing lipid raises plasma free fatty acids and induces insulin resistance in muscle microvasculature. J Clin Endocrinol Metab 94: 3543–3549, 2009. doi: 10.1210/jc.2009-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moosavi M, Naghdi N, Choopani S. Intra CA1 insulin microinjection improves memory consolidation and retrieval. Peptides 28: 1029–1034, 2007. doi: 10.1016/j.peptides.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 39.Muniyappa R, Montagnani M, Koh KK, Quon MJ. Cardiovascular actions of insulin. Endocr Rev 28: 463–491, 2007. doi: 10.1210/er.2007-0006. [DOI] [PubMed] [Google Scholar]
- 40.Nesil T, Cao J, Yang Z, Chang SL, Li MD. Nicotine attenuates the effect of HIV-1 proteins on the neural circuits of working and contextual memories. Mol Brain 8: 43, 2015. doi: 10.1186/s13041-015-0134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park CR, Seeley RJ, Craft S, Woods SC. Intracerebroventricular insulin enhances memory in a passive-avoidance task. Physiol Behav 68: 509–514, 2000. doi: 10.1016/S0031-9384(99)00220-6. [DOI] [PubMed] [Google Scholar]
- 42.Rim S-J, Leong-Poi H, Lindner JR, Couture D, Ellegala D, Mason H, Durieux M, Kassel NF, Kaul S. Quantification of cerebral perfusion with “Real-Time” contrast-enhanced ultrasound. Circulation 104: 2582–2587, 2001. doi: 10.1161/hc4601.099400. [DOI] [PubMed] [Google Scholar]
- 43.Sims NR, Bowen DM, Smith CC, Flack RH, Davison AN, Snowden JS, Neary D. Glucose metabolism and acetylcholine synthesis in relation to neuronal activity in Alzheimer’s disease. Lancet 315: 333–336, 1980. doi: 10.1016/S0140-6736(80)90884-3. [DOI] [PubMed] [Google Scholar]
- 44.Solomon TP, Haus JM, Li Y, Kirwan JP. Progressive hyperglycemia across the glucose tolerance continuum in older obese adults is related to skeletal muscle capillarization and nitric oxide bioavailability. J Clin Endocrinol Metab 96: 1377–1384, 2011. doi: 10.1210/jc.2010-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, Xu XJ, Wands JR, de la Monte SM. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease—is this type 3 diabetes? J Alzheimers Dis 7: 63–80, 2005. [DOI] [PubMed] [Google Scholar]
- 46.Thiels E, Kanterewicz BI, Norman ED, Trzaskos JM, Klann E. Long-term depression in the adult hippocampus in vivo involves activation of extracellular signal-regulated kinase and phosphorylation of Elk-1. J Neurosci 22: 2054–2062, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tucsek Z, Toth P, Tarantini S, Sosnowska D, Gautam T, Warrington JP, Giles CB, Wren JD, Koller A, Ballabh P, Sonntag WE, Ungvari Z, Csiszar A. Aging exacerbates obesity-induced cerebromicrovascular rarefaction, neurovascular uncoupling, and cognitive decline in mice. J Gerontol A Biol Sci Med Sci 69: 1339–1352, 2014. doi: 10.1093/gerona/glu080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vecoli C, Novelli M, Pippa A, Giacopelli D, Beffy P, Masiello P, L’Abbate A, Neglia D. Partial deletion of eNOS gene causes hyperinsulinemic state, unbalance of cardiac insulin signaling pathways and coronary dysfunction independently of high fat diet. PLoS One 9: e104156, 2014. doi: 10.1371/journal.pone.0104156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wan Q, Xiong ZG, Man HY, Ackerley CA, Braunton J, Lu WY, Becker LE, MacDonald JF, Wang YT. Recruitment of functional GABA(A) receptors to postsynaptic domains by insulin. Nature 388: 686–690, 1997. doi: 10.1038/41792. [DOI] [PubMed] [Google Scholar]
- 50.Wickelgren I. Tracking insulin to the mind. Science 280: 517–519, 1998. doi: 10.1126/science.280.5363.517. [DOI] [PubMed] [Google Scholar]
- 51.Yang YJ, Hope ID, Ader M, Bergman RN. Insulin transport across capillaries is rate limiting for insulin action in dogs. J Clin Invest 84: 1620–1628, 1989. doi: 10.1172/JCI114339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Young SE, Mainous AG III, Carnemolla M. Hyperinsulinemia and cognitive decline in a middle-aged cohort. Diabetes Care 29: 2688–2693, 2006. doi: 10.2337/dc06-0915. [DOI] [PubMed] [Google Scholar]
- 53.Zeng G, Quon MJ. Insulin-stimulated production of nitric oxide is inhibited by wortmannin. Direct measurement in vascular endothelial cells. J Clin Invest 98: 894–898, 1996. doi: 10.1172/JCI118871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang Y, Janssens SP, Wingler K, Schmidt HH, Moens AL. Modulating endothelial nitric oxide synthase: a new cardiovascular therapeutic strategy. Am J Physiol Heart Circ Physiol 301: H634–H646, 2011. doi: 10.1152/ajpheart.01315.2010. [DOI] [PubMed] [Google Scholar]
- 55.Zhao L, Fu Z, Wu J, Aylor KW, Barrett EJ, Cao W, Liu Z. Inflammation-induced microvascular insulin resistance is an early event in diet-induced obesity. Clin Sci (Lond) 129: 1025–1036, 2015. doi: 10.1042/CS20150143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zuloaga KL, Johnson LA, Roese NE, Marzulla T, Zhang W, Nie X, Alkayed FN, Hong C, Grafe MR, Pike MM, Raber J, Alkayed NJ. High fat diet-induced diabetes in mice exacerbates cognitive deficit due to chronic hypoperfusion. J Cereb Blood Flow Metab 36: 1257–1270, 2016. doi: 10.1177/0271678X15616400. [DOI] [PMC free article] [PubMed] [Google Scholar]