Abstract
Experimental studies in rodents have shown that females are more susceptible to exhibiting fat expansion and metabolic disease compared with males in several models of fetal programming. This study tested the hypothesis that female rat pups exposed to maternal separation (MatSep), a model of early-life stress, display an exacerbated response to diet-induced obesity compared with male rats. Also, we tested whether the postnatal treatment with metyrapone (MTP), a corticosterone synthase inhibitor, would attenuate this phenotype. MatSep was performed in WKY offspring by separation from the dam (3 h/day, postnatal days 2–14). Upon weaning, male and female rats were placed on a normal (ND; 18% kcal fat) or high-fat diet (HFD; 60% kcal fat). Nondisturbed littermates served as controls. In male rats, no diet-induced differences in body weight (BW), glucose tolerance, and fat tissue weight and morphology were found between MatSep and control male rats. However, female MatSep rats displayed increased BW gain, fat pad weights, and glucose intolerance compared with control rats (P < 0.05). Also, HFD increased plasma corticosterone (196 ± 51 vs. 79 ± 18 pg/ml, P < 0.05) and leptin levels (1.8 ± 0.4 vs. 1.3 ± 0.1 ng/ml, P < 0.05) in female MatSep compared with control rats, whereas insulin and adiponectin levels were similar between groups. Female control and MatSep offspring were treated with MTP (50 µg/g ip) 30 min before the daily separation. MTP treatment significantly attenuated diet-induced obesity risk factors, including elevated adiposity, hyperleptinemia, and glucose intolerance. These findings show that exposure to stress hormones during early life could be a key event to enhance diet-induced obesity and metabolic disease in female rats. Thus, pharmacological and/or behavioral inflection of the stress levels is a potential therapeutic approach for prevention of early life stress-enhanced obesity and metabolic disease.
Keywords: early-life stress, high fat diet, maternal separation, metyrapone, sex differences in developmental programming of metabolic disease
trends in obesity among adults in the US are showing that the age-adjusted prevalence in 2013–2014 was higher in women (40.4%) than in men (35%) (15, 51). The underlying mechanisms for this health disparity are not completely understood. However, hypothesized contributors include sex differences in lean muscle mass, glucose metabolism, body fat distribution, adipocyte size and function, hormonal regulation of body weight, adiposity influence of menopause transition, and altered susceptibility to peripheral insulin resistance (42, 47, 49).
Besides the well-established influence of genetic and lifestyle traditional risk factors, early-life stress (ELS) has been recognized as an independent risk factor for cardiovascular and metabolic disease in adulthood (1, 10, 38, 50, 61). Numerous clinical studies have documented that ELS can induce long-lasting effects by misprograming the immune, neuroendocrine, and vascular function. Recent data from the national longitudinal survey of the Americans’ Changing Lives show that women who experienced emotional stress during childhood gained weight more rapidly than men (30). Also, the Ovarian Aging Study (OVA) reports that socioeconomic disadvantage experienced during puberty may have a stronger impact on adulthood cardiometabolic risk, which is greater in women (6). Therefore, these recent findings suggest that ELS could contribute to the projected epidemic rates of obesity in a sex-specific manner.
In some physiological conditions, behavioral stress can reduce body weight (17). Besides experimental studies that combine a hypercaloric diet with behavioral stress showing a synergistic effect in body weight gain and fat expansion in adult rodents (25, 65, 66), little is known about the mechanism by which ELS, may exert permanent modifications on the metabolic homeostasis.
It is widely accepted that maternal separation (MatSep), a chronic behavioral stress model during postnatal life in rodents, exerts long-term effects on the hypothalamic-pituitary-adrenal (HPA) axis function, negatively influencing behavioral responses (34, 39). A growing number of studies have shown that this paradigm also exaggerates cardiovascular, neuroendocrine, and immune responses to “secondary insults” during adult life (7, 24, 36, 56). Importantly, the hyporesponsive period is a stage of resilience to mild stressors during early postnatal life (days 0–10) (39, 63). This phenomenon allows a natural protection of the offspring from excessive glucocorticoid levels to ensure normal tissue/organ development during this critical window of plasticity. Several studies have reported a short-term rise in plasma corticosterone levels in rat pups undergoing the MatSep procedure (5, 53, 58). Hence, rats subjected to chronic stress as offspring display exaggerated acute stress-induced plasma levels of corticosterone and ACTH as adults (21, 29, 40, 53, 55), providing evidence for persistent activation of the HPA axis in these responses.
Notably, chronic treatment with a corticosterone synthase inhibitor, metyrapone (MTP), has been used to attenuate the behavioral, cardiovascular, and metabolic responses to various models of developmental programming (3, 26, 54). Similarly, targeting corticosterone has been used in adult life as an approach for the treatment of metabolic disease in adult db/db mice, a genetic model of obesity and diabetes. Because db/db mice display increased circulating corticosterone levels compared with wild-type mice, chronic treatment with MTP reduced body weight and improved glycemic control in these mice (12). Furthermore, it has been shown that MTP treatment attenuates the behavioral and neurochemical alterations secondary to stressful experiences (8). However, there are no reports showing that the long-lasting effects of emotional stress-induced elevations in corticosterone during postnatal life can be prevented by MTP administration.
Taken together, the current study tested the hypothesis that exposure to MatSep would exaggerate diet-induced obesity phenotype in female rats compared with male rats. Also, we investigated whether the administration of MTP during postnatal life reverts the stress-induced programming of the metabolic function. Therefore, we determined the effect of diet-induced obesity on adipocyte number and morphology, plasma metabolic markers, blood pressure, glucose sensitivity, and tissue gene expression in adult female rats that were untreated or treated with MTP during postnatal life.
METHODS
Animal model and design.
All animal protocols received prior approval by the Institutional Animal Care and Use Committee at the University of Kentucky. Maternal separation (MatSep) protocol was performed as described previously (36). Briefly, approximately half of the male and female pups were separated from their mothers and littermates by having the pups transferred to a clean cage in an incubator (30 ± 1°C) for 3 h from days 2 to 14 of life. Normally reared and unhandled littermates remained with their mother and served as the control group. At weaning (4 wk of age), female rats were placed on special diets for 12 wk. Body weight and fat depot weights were determined gravimetrically and expressed in grams. Blood was obtained from a direct puncture into the abdominal aorta under anesthesia.
MTP treatment.
At postnatal day 2 (1st day of separation), a daily administration of MTP (50 µg/g body wt ip injection, solubility in water: 100 mM; Tocris, Bristol, UK) was conducted at 8:30 AM, 30 min before the exposure to MatSep (9 AM-12 PM). From postnatal days 2 to 14, 3 µl of MTP stock solution (17 mg/ml) were injected, increasing 5-µl volume with every 2 g of weight gain (3, 62).
Diet composition.
The high-fat diet (HFD) consisted of calories from 14% protein, 26% carbohydrates, and 60% fat, 5.49 kcal/g gross energy, and 0.4% NaCl (F3282; Bioserv, Frenchtown, NJ). Regular chow (ND) consisted of calories from 24% protein, 58% carbohydrates, and 18% fat, 3.1 kcal/g gross energy, and 0.4% NaCl (Teklad 8604; Teklad, Madison, WI). All rats were given tap water ad libitum.
Plasma and urine measurements.
Plasma was collected using EDTA 7.5% solution. Corticosterone (Cayman, Ann Arbor, MI), leptin (Cayman), insulin (Cayman), and adiponectin (EMD Millipore, CA) EIA kits were performed, following the manufacturer’s specifications. Glucose was determined in total blood using a glucometer (Accu-check; Roche). Plasma cholesterol and triglyceride were determined using an enzymatic kit (Wako Diagnostics, Richmond, VA).
Rats were placed in metabolic cages for determination of water and food intake as well as urine output. After 2 days of acclimation, 24-h urine collections were obtained. Proteinuria was determined by Coomassie Blue colorimetric assay.
Oral glucose tolerance test.
An oral glucose tolerance test (OGTT) was performed in rats after 9 wk of ND or HFD. Rats were fasted overnight for 16 h before glucose tolerance test. Rats were administered d-glucose (2 g/kg body wt, oral gavage), and blood glucose measurements were taken via tail prick at 0,15, 30, 60, and 120 min using a glucometer (Accu-check; Roche).
Histological analysis of adipose tissue morphology.
Adipose tissue depots, including subcutaneous, epididymal, and perirenal, were collected in 10% buffered formalin, fixed for 24 h, and paraffin-embedded. Following this, 5-µm sections were cut and stained with hematoxylin and eosin. Images of slides were obtained at ×10 magnification. Using the “detect edges,” image threshold, and object count features of NIS Elements software (Nikon Instruments, Tokyo, Japan), the area of each adipocyte and cell number within a 1,500 × 1,500 µm2 measurement frame was quantified. Adipocyte size and number were quantified on two measurement frames within each section of adipose tissue (n = 2 sections/rat) from rats in each group (n = 5 rats/group).
Blood pressure, heart rate, and activity measurements.
Rats fed a HFD (4/group) were implanted with radiotelemetry transmitters at 10 wk of age (Data Sciences, St. Paul, MN) (32, 35). Briefly, rats were anesthetized with isoflurane (1–4% in O2) in a sterile surgical field. The abdominal aorta was then exposed by a midline incision and briefly occluded. The transmitter catheter was inserted into a hole made by a 21-gauge needle just proximal to the iliac bifurcation and secured in place with tissue glue (Vetbond). The transmitter body was attached to the abdominal wall along the incision line with a 4-O proline suture as the incision was closed. The skin was closed with staples that were removed 7 days after the incision had healed. After recovery (14 days), mean arterial pressure (MAP), heart rate (HR), and activity were recorded by radiotelemetry (Data Sciences International, New Brighton, MN) in 10-s bursts every 10 min throughout the experiment.
Quantitative analysis of mRNA expression.
Total RNA was extracted from rat adipose tissue and liver using Purzol Reagent (Bio-Rad) and the RNA isolation kit Aurum Total RNA. cDNA was prepared from 1µg of total RNA. The mRNA levels in tissue were measured using I-Taq Universal SYBR Green (Bio-Rad) with a CFX96 Real Time System (Bio-Rad). The oligonucleotide primers for the different genes were designed using the NCBI Pick Primer program and made by Integrated DNA Technologies. An optimal primer concentration was determined, and a single melting curve was obtained. The co-amplification of rat GAPDH mRNA was used as the housekeeping gene. The assays were performed in triplicate, and the results were normalized to the GAPDH mRNA levels using the method.
Study in male rats.
Male control and MatSep rats were placed on a ND or a HFD upon weaning as well. Body weight was recorded weekly (n = 12/group). Glucose tolerance test (n = 6/group), white adipose tissue morphometrical analysis (n = 5/group), and plasma metabolic profile were measured as described above. Because of the lack of MatSep effects observed after 12 wk, the study in male rats was extended for an additional 5 wk.
Statistical analysis.
Data were expressed in means ± SE. Statistical analysis was performed using Graph Pad Prism 6 for Mac, Version 6.0 (GraphPad Software, La Jolla, CA). For blood pressure and body weight gain analysis, two-way repeated-measures ANOVA was performed for differences in blood pressure over time. A value of P < 0.05 was considered statistically significant. Two-way ANOVA was used to assess the interaction between MatSep and MTP treatment. Differences between control and MatSep groups were compared using unpaired Student’s t-test. The criterion for significance was P < 0.05.
RESULTS
MatSep does not affect metabolic parameters in female rats fed a ND.
Female MatSep rats showed similar body weight, plasma parameters, and metabolic profile (Table 1) compared with control rats fed a ND diet. MatSep did not increase tissue weight or alter adipose tissue morphology as well. Furthermore, gene expression in liver and gonadal white adipose tissue (gWAT) was not different between groups at baseline conditions (Table 2).
Table 1.
Metabolic parameters in 12-wk-old female rats fed a ND are not different in control or MatSep rats
| Control | MatSep | |
|---|---|---|
| Metabolic profile | ||
| Body weight, g | 205 ± 7 | 206 ± 5 |
| Food intake, g/day | 17.1 ± 1.5 | 16.3 ± 2.5 |
| Water intake, ml/day | 24.1 ± 2.3 | 23.1 ± 4.1 |
| Urine flow, ml/day | 10.2 ± 0.9 | 13.5 ± 1.0 |
| Proteinuria, mg/day | 4.3 ± 0.5 | 4.9 ± 0.6 |
| Plasma profile | ||
| Fasting glucose, mg/dl | 78 ± 3 | 80 ± 2 |
| Insulin, ng/ml | 1.6 ± 0.2 | 1.6 ± 0.4 |
| Leptin, ng/ml | 4.1 ± 0.3 | 5.3 ± 0.6 |
| Adiponectin, ng/ml | 29.2 ± 2.2 | 27.5 ± 1.3 |
| Corticosterone, ng/ml | 60 ± 16 | 74 ± 22 |
| Cholesterol, ml/dl | 189 ± 15 | 183 ± 10 |
| Triglycerides, ml/dl | 111 ± 17 | 115 ± 15 |
| Tissue weight | ||
| Liver weight, g | 4.4 ± 0.5 | 4.1 ± 0.7 |
| Adrenal weight, mg | 67 ± 12 | 71 ± 14 |
| Kidney weight, g | 1.3 ± 0.2 | 1.4 ± 0.3 |
| Spleen weight, g | 0.4 ± 0.6 | 0.4 ± 0.2 |
Values are means ± SE; n = 6–8. ND, normal diet; MatSep, maternal separation.
Table 2.
Gene expression is not different in 12-wk-old female control or MatSep rats fed a ND
| Control | MatSep | |
|---|---|---|
| Liver | ||
| 11β-HSD1 | 1.10 ± 0.20 | 1.57 ± 0.20 |
| GR | 1.01 ± 0.05 | 1.02 ± 0.06 |
| MR | 1.01 ± 0.05 | 0.85 ± 0.18 |
| PEPCK | 1.07 ± 0.18 | 1.37 ± 0.10 |
| PC | 1.08 ± 0.19 | 0.96 ± 0.05 |
| G6Pase | 1.11 ± 0.18 | 1.24 ± 0.17 |
| gWAT | ||
| 11β-HSD1 | 1.11 ± 0.23 | 0.96 ± 0.17 |
| GR | 1.09 ± 0.17 | 1.72 ± 0.28 |
| MR | 1.15 ± 0.25 | 1.81 ± 0.38 |
| GLUT4 | 1.01 ± 0.05 | 1.67 ± 0.18 |
| PCG-1α | 1.14 ± 0.29 | 1.09 ± 0.17 |
| IR | 1.24 ± 0.44 | 1.01 ± 0.18 |
Values are means ± SE; n = 6–8. 11β-HSD1, 11β-hydroxysteroid dehydrogenase type 1; GR, glucocorticoid receptor; MR, mineralocorticoid receptor; PEPCK, phosphoenolpyruvate carboxykinase; PC, pyruvate carboxylase; G6Pase, glucose-6-phosphatase; GLUT4, glucose transporter 4; PGC-1α, peroxisome proliferator-activated receptor-γ coactivator-1α; IR, insulin receptor.
MTP abolishes diet-induced increases in body weight and adiposity in MatSep rats.
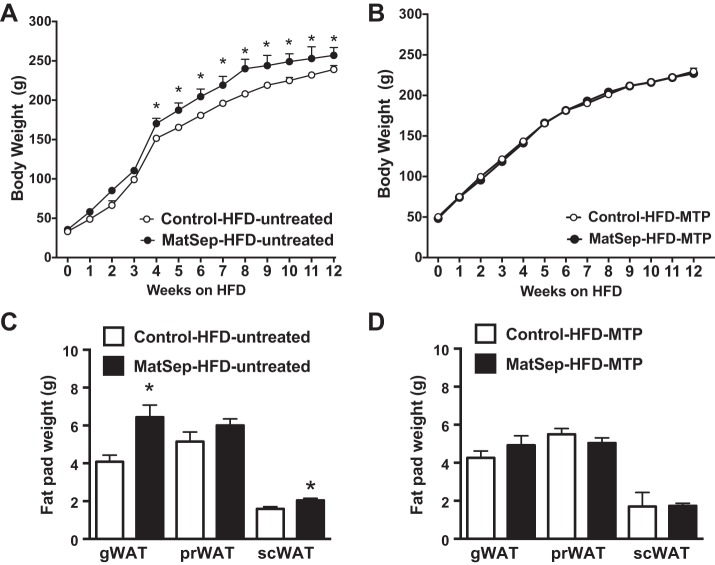
After 4 wk on a HFD, female MatSep rats started gaining significantly more weight than control rats (Fig. 1A). This difference was maintained throughout the 12 wk of the study, showing an overall effect of MatSep [F(1, 31) = 7.1, P < 0.05], time [F(10, 310) = 841, P < 0.05], and interaction effect [F(10, 310) = 3.84, P < 0.05] on body weight. In addition, we found that MatSep increases gonadal white adipose tissue (gWAT; P < 0.05, t = 3.4, df = 11) and subcutaneous WAT (scWAT) weight in response to HFD (gWAT; P < 0.05, t = 2.7, df = 11), with no changes in perirenal WAT (prWAT; Fig. 1C). Despite this increase in adiposity, 24-h systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP), and heart rate (HR) were similar between groups (Table 3). However, MTP treatment blunted the MatSep effect to exacerbate diet-induced-obesity (Fig. 1B) and fat pad weight (Fig. 1D). Importantly, postnatal treatment with MTP did not affect the body weight gain in control rats fed a HFD.
Fig. 1.
Body weight and fat pad weights in rats fed for 12 wk on a high-fat (HFD). Untreated rats (A and C) and metyrapone (MTP)-treated rats (B and D); n = 12 (A and C) and 6 (B and D). *P < 0.05 vs. control. MatSep, maternal separation; gWAT, gonadal white adipose tissue; prWAT, perirenal white adipose tissue; scWAT, subcutaneous white adipose tissue.
Table 3.
Blood pressure, HR, and locomotor activity obtained by radiotelemetry in 12-wk-old female rats fed a HFD
| Control | MatSep | |
|---|---|---|
| MAP, mmHg | 107 ± 5 | 110 ± 4 |
| SBP, mmHg | 118 ± 6 | 122 ± 4 |
| DBP, mmHg | 96 ± 6 | 99 ± 6 |
| HR, beats/min | 340 ± 5 | 349 ± 8 |
| Locomtor activity (AU) | 1.8 ± 0.1 | 2.0 ± 0.1 |
Values are means ± SE; n = 4/group. HR, heart rate; MAP, mean arterial pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; AU, arbitrary units.
Histological analysis (Fig. 2, A and B) determined that MatSep rats fed a HFD diet display lower adipocyte number per frame of measurement in gWAT compared with control rats (373 ± 62 vs. 491 ± 49), and this effect was abolished in MTP-treated rats [553 ± 14 vs. 562 ± 33; MatSep: F(1, 62) = 11.7, P < 0.05; MTP: F(1, 62) = 7.2, P < 0.05; interaction: F(1, 62) = 0.6, P = not significant (NS)]. Similar results were obtained in scWAT (data not shown). Whereas HFD-fed rats displayed increased cell area in gWAT (Fig. 2C), MTP-treated MatSep rats showed a significant attenuation in these parameters [MatSep: F(1, 62) = 12.9, P < 0.05; MTP: F(1, 62) = 7.4, P < 0.05; interaction: F(1, 62) = 1.9, P = NS]. The same observations were made in cell diameter [MatSep: F(1, 62) = 11.7, P < 0.05; MTP: F(1, 62) = 7.2, P < 0.05; interaction: F(1, 62) = 0.6, P = NS]. In scWAT, increased cell area in response to HFD was attenuated in MTP-treated rats [MatSep: F(1, 49) = 11.7, P < 0.05; MTP: F(1, 49) = 1.7 P = NS; interaction: F(1, 49) = 20.6, P < 0.05; Fig. 2C], whereas MTP treatment showed a significant attenuation in these parameters [MatSep: F(1, 49) = 8.2, P < 0.05; MTP: F(1, 49) = 1.4, P = NS; interaction: F(1, 49) = 6.2, P < 0.05]. These data indicate that adipocytes are bigger in MatSep rats fed a HFD, and MTP treatment abolishes fat expansion in gWAT and scWAT.
Fig. 2.
White adipose tissue morphology in HFD-fed rats untreated and treated with MTP. A and B: representative pictures. C and D: cell area. E and F: cell diameter. *P < 0.05 (separation), #P < 0.05 (treatment), and †P < 0.05 (interaction); n = 5/group. gWAT, gonadal white adipose tissue; scWAT, subcutaneous adipose tissue.
MTP reduces plasma corticosterone and leptin in MatSep rats.
MatSep enhanced corticosterone and leptin levels in untreated rats fed a HFD, whereas MTP treatment had a robust effect on lowering their levels in both groups [MatSep: F(1, 26) = 5.1, P < 0.05; MTP: F(1, 26) = 12.2, P < 0.05; interaction: F(1, 26) = 5.4, P < 0.05; Fig. 3, A and C]. MTP treatment reduced insulin levels in both groups [MatSep: F(1, 19) = 0.01, P = NS; MTP: F(1, 19) = 12.5, P < 0.05; interaction: F(1, 19) = 1.2, P = NS; Fig. 3B]. Also, no changes in adiponectin were observed between groups in untreated rats fed a HFD. Furthermore, MTP treatment increased plasma adiponectin similarly in both groups [MatSep: F(1, 20) = 3.5, P = NS; MTP: F(1,20) = 9.4, P < 0.05; interaction: F(1, 20) = 0.2, P = NS; Fig. 3D]. Taken together, these data indicate that MTP treatment exerted an overall improvement in several metabolic hormones implicated in the advancement of diet-induced metabolic derangements, particularly in MatSep rats.
Fig. 3.
Plasma profile in untreated and MTP-treated rats. A: corticosterone (CORT). B: insulin. C: leptin. D: adiponectin. *P < 0.05 (separation); #P < 0.05 (treatment); †P <0.05 (interaction). Untreated, n = 6–12; MTP, n = 6.
MTP attenuates MatSep-induced glucose intolerance.
After 12 wk of HFD feeding, OGTT was increased in MatSep rats compared with control rats (Fig. 4A). Likewise, the postnatal treatment with MTP significantly improved the OGTT in MatSep rats [MatSep: F(1, 21) = 5.6, P < 0.05; MTP: F(1, 21) = 0.1, P = NS; interaction: F(1,21) = 0.7, P = NS; Fig. 4B]. These data support the notion that MTP treatment is associated with an improvement of the glucose sensitivity in MatSep rats.
Fig. 4.
Oral glucose tolerance test in 16-h-fasted untreated and MTP-treated rats fed a HFD for 9 wk. A: plasma glucose time course. B: area under the curve. *P < 0.05 (separation) vs. control. Untreated, n = 6; MTP, n = 6. AUC, area under the curve; AU, arbitrary units.
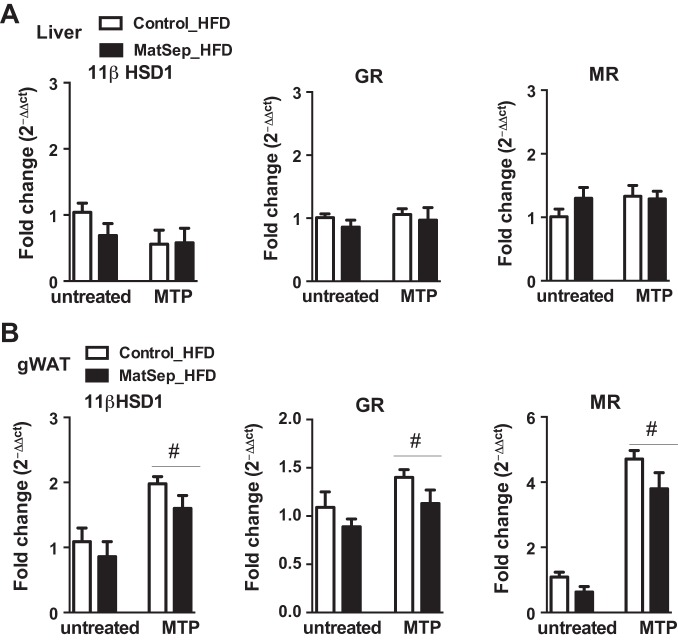
MTP treatment exerts an effect on the glucocorticoid metabolism in adipose tissue.
MatSep did not influence hepatic mRNA levels of 11βHSD1, GR, or MR from rats fed a HFD, and MTP treatment did not have a significant effect on these genes expression (Fig. 5A). However, rats exposed to MTP treatment had significantly higher levels of adipose gWAT, 11βHSD1, GR, and MR mRNA expression, and this effect was similar between control and MatSep groups [MatSep: F(1, 22) = 1.9, P = NS; MTP: F(1, 22) = 13.9, P < 0.05; interaction: F(1, 22) = 1.8, P = NS; Fig. 5B]. Thus, these data indicate that the adipose tissue may respond to the lower plasma circulating levels of corticosterone by increasing the expression of the local glucocorticoid metabolism components. Oligonucleotide primer sequences for the different genes are presented in Table 4.
Fig. 5.
Tissue gene expression of 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1), glucocorticoid receptor (GR), and mineralocorticoid receptor (MR) mRNA levels (quantitative RT-PCR) in liver (A) and gonadal white adipose tissue (gWAT; B) from rats fed a HFD. #P < 0.05 (treatment). Untreated, n = 6–8; MTP, n = 6.
Table 4.
Primers designed for qRT-PCR
| 5′–3′ (Forward) | 5′–3′ (Reverse) | |
|---|---|---|
| Liver | ||
| 11β-HSD1 | GAAGAAGCATGGAGGTCAAC | GCAATCAGAGGTTGGGTCAT |
| GR | AGGGGAGGGGGAGCGTAATGG | CCTCTGCTGCTTGGAATCTGC |
| MR | ACCACATACATCGCTCCGAC | ATGGTGTGAGTGCATGCGTA |
| PEPCK | CTTCCCTCTGGGAACACACC | GTGCCACCTTTCTTCCTCCT |
| PC | CCAACATCCCATTCCAGATGC | CCACCTCACAGAACTTGAAGAC |
| G6Pase | AAGTCGCTCCCATTCCGTTT | GGGCTTCAGCGAGTCAAAGA |
| gWAT | ||
| GLUT4 | TGCAGTGCCTGAGTCTTCTT | GGTTCCCCATCTTCAGAGCC |
| PCG-1α | CATTCAGGAGCTGGATGGCT | CAAAGAGGCTGGTCCTCACC |
| IR | TGCTGAGGACACTAGGCCAT | GGGTGTAGTGGCTGTCACAT |
| Housekeeper | ||
| GAPDH | TCTCTGCTCCTCCCTGTTCT | TACGGCCAAATCCGTTCACA |
11β-HSD1, 11β-hydroxysteroid dehydrogenase type 1; GR, glucocorticoid receptor; MR, mineralocorticoid receptor; PEPCK, phosphoenolpyruvate carboxykinase; PC, pyruvate carboxylase; G6Pase, glucose-6-phosphatase; gWAT, gonadal white adipose tissue; GLUT4, glucose transporter 4; PGC-1α, peroxisome proliferator-activated receptor-γ coactivator-1α; IR, insulin receptor.
MTP normalizes gene expression associated with gluconeogenesis.
In HFD-fed rats, whereas no changes were found in PEPCK mRNA abundance, MTP treatment reduced the PC mRNA levels [MatSep: F(1, 20) = 3.4, P = NS; MTP: F(1, 20) = 4.4, P = NS; interaction: F(1, 20) = 13.4, P < 0.05; Fig. 6A]. Also, MatSep increased G6Pase mRNA expression in liver, which was attenuated by the postnatal MTP treatment [MatSep: F(1, 20) = 6.7, P < 0.05; MTP: F(1, 20) = 1.8, P = NS; interaction: F(1, 20) = 4.4, P = 0.0502; Fig. 6A].
Fig. 6.
Tissue gene expression of glucose metabolism in liver (A) and gonadal white adipose tissue (gWAT; B) from rats fed a HFD. *P < 0.05 (separation); #P <0.05 (treatment); †P < 0.05 (interaction). Untreated, n = 6–8; MTP, n = 6.
In gWAT, whereas MTP treatment significantly reduced GLUT4 expression [MatSep: F(1, 22) = 0.9, P = NS; MTP: F(1, 22) = 6.4, P < 0.05; interaction: F(1, 22) = 0.5, P = NS; Fig. 6B], it did not affect IR or PGC-1α (Fig. 6B). These data indicate that MatSep could induce glucose intolerance in response to HFD by enhancing the hepatic gluconeogenic activity. Lower IR expression in fat tissue most likely contributes to impairments in glucose uptake; however MTP treatment did not change its mRNA expression [MatSep: F(1, 22) = 18.1, P < 0.05; MTP: F(1, 22) = 0.2, P = NS; interaction: F(1, 22) = 0.3, P = NS].
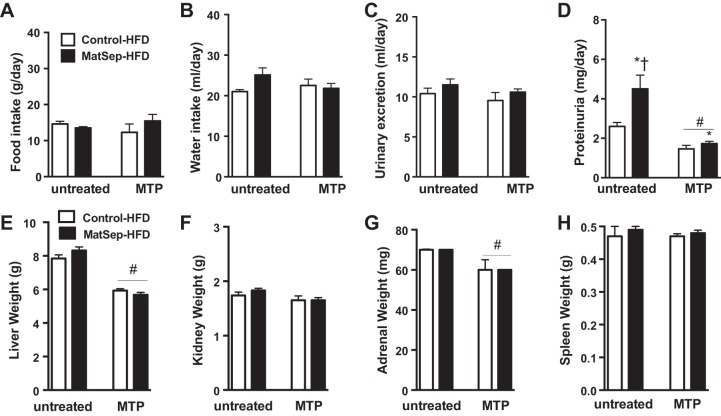
MTP normalizes signs of proteinuria and reduces liver and adrenal weight.
MatSep and control rats fed a HFD showed similar 24-h food intake and water intake (Fig. 7, A and B). Although urinary excretion was not increased significantly in MatSep rats (Fig. 7C), it was accompanied by signs of renal damage, as evidenced by increased proteinuria in MatSep rats (Fig. 7D); however, these outcomes were attenuated by the MTP treatment [MatSep: F(1, 20) = 8.3, P < 0.05; MTP: F(1, 20) = 26.5, P < 0.05; interaction: F(1, 20) = 4.7, P < 0.05]. In addition, there were no differences between groups in liver, kidney, adrenal, and spleen weight from rats fed a HFD (Fig. 7, E–H), and yet MTP treatment significantly lowered liver (~25%) and adrenal weight (~20%) in control and MatSep rats [MTP: F(1, 20) = 56.5, P < 0.05]. These data indicate that the MTP treatment affects the organs that are especially sensitive to glucocorticoid levels during postnatal life.
Fig. 7.
Metabolic profile. A–D: parameters obtained in 24-h metabolism cage housing for untreated and MTP-treated rats. E–H: organ weight after 12 wk on a HFD in untreated and MTP-treated rats. *P < 0.05 (separation); #P < 0.05 (treatment); †P <0.05 (interaction). Untreated, n = 6–12; MTP, n = 6.
MatSep does not influence body weight or glucose tolerance in male rats.
In a period of 12 wk on a HFD, male MatSep did not show significant increases in body weight compared with control rats. In week 17, body weight gain remained similar between MatSep and control rats (Fig. 8A), and fat pad weights were not different between groups (Fig. 8B). Exposure to MatSep during postnatal life did not induce overall changes on WAT morphology in response to HFD. MatSep did not influence cell area or diameter in gWAT and scWAT compared with control rats (Fig. 8, C and D). Accordingly, cell number was not different between MatSep and control groups in gWAT (555 ± 44 vs. 562 ± 30 cells/measurement frame, respectively, P = NS, t = 1.5, df = 18) or scWAT (344 ± 25 vs. 291 ± 40 cells/measurement frame, respectively, P = NS, t = 1.2, df = 16).
Fig. 8.
Diet-induced obesity in MatSep and control male rats. A: body weight gain over the course of 17 wk (n = 12). B: fat pad weight (n = 6).C: gonadal white adipose tissue (gWAT) morphology (n = 5). D: subcutaneous white adipose tissue (scWAT) morphology (n = 5). No significant differences were observed.
After 15 wk of HFD feeding, OGTT was not statistically different between MatSep and control rats fed a ND [area under the curve (AUC): 24.3 ± 0.7 vs. 23.2 ± 3.3 mg·dl−1·120 min × 103]. In response to HFD, the elevation in the total AUC was not statistically significant between groups (AUC: 31.2 ± 0.8 vs. 33.6 ± 1.6 mg·dl−1·120 min × 103). In addition, plasma metabolic profile in MatSep and control male rats showed similar fasting glucose (100 ± 9 vs. 105 ± 7 mg/dl, P = NS, t = 2.1, df = 18), total plasma cholesterol E (133 ± 5 vs. 128 ± 6 mg/dl, P = NS, t = 0.4, df = 14), and plasma triglycerides (121 ± 38 vs. 120 ± 21 mg/dl, P = NS, t = 0.1, df = 14).
DISCUSSION
The major finding of this study is that MatSep, well known to induce stress during postnatal life, exacerbates diet-induced obesity in female rats. Most importantly, this phenomenon can be abrogated by a postnatal treatment with MTP, a corticosterone synthase inhibitor. Specifically, female MatSep rats fed a HFD upon weaning displayed exacerbated (1) body weight gain (2), adipocyte hypertrophy (3), plasma corticosterone and leptin levels (4), glucose intolerance, and gluconeogenic gene expression (5). On the contrary, male rats exposed to MatSep did not show an increased susceptibility to develop diet-induced obesity compared with control counterparts. Our data suggest that targeting the stress-induced neuroendocrine response may serve as a mechanism to attenuate the effects of MatSep in the developmental programming of metabolic disease, specifically in female rats.
Traditional risk factors such as smoking, high cholesterol, hypertension, and diabetes have been shown to contribute to women’s cardiometabolic risk more than men; however, these traditional risk factors account for only ~40% of the variance of cardiovascular disease (2). This percentage strongly suggests that there are other veiled factors that may provide a critical contribution. Numerous studies have recognized psychosocial stress as an independent risk factor for chronic adult disease, including depression, anxiety, obesity, and high blood pressure (13, 14, 43, 46, 52, 64), all important factors associated with the development of cardiovascular disease. A recent study using 6,714 members of the 1958 British Birth Cohort Study revealed that cardiometabolic risk was higher among people with psychological distress in childhood only and persistent across the life course (64). The strengths of this study to avoid the risk of bias include the large unselected sample, a followup spanning 45 yr, prospective measures of psychological distress, and multiple data collection sources/methods. However, a number of limitations are present: 1) the measures of psychological distress were not comprehensive, 2) it is difficult to ensure no exposure to stress in the control group, 3) and the observational nature of the data raises the possibility of unmeasured confounding variables. An important next step will be to evaluate whether interventions to reduce psychological distress in childhood indeed improve subsequent risk for development of obesity and cardiovascular disease.
The influence of ELS on long-term health conditions has several origins. The multifactorial interaction among the HPA axis, the central nervous system, and nutritional status all impact the overall glucocorticoid levels. The role of glucocorticoids as stress-induced hormones is well established, in addition to their role in the regulation of energy balance (11) and the development of obesity with central distribution of fat (12). The actions of glucocorticoids mediating diet-induced obesity have been attributed in part to its capacity to activate lipogenesis and induce fat deposition (19, 27, 41). Experimental studies have shown that local (45) and systemic (16, 18) glucocorticoid inhibition attenuate obesity and derangements of the metabolic function. Based on the studies showing that MatSep deeply affects the neuroendocrine responses in rodents (21, 28, 33), we inhibited the systemic corticosterone synthesis in female rat offspring. As a result, we observed a systemic improvement in diet-induced obesity outcomes in MatSep and control rats, but importantly, we were able to attenuate the MatSep-enhanced obesity and metabolic dysfunction. Therefore, our studies suggest that ELS can sensitize the glucocorticoid metabolism in response to HFD. In addition, our data reveal specific changes at the WAT and liver level, two metabolically active tissues potentially responsible, along with the skeletal muscle, for the impaired metabolic function.
Diet composition is also a main factor to take into consideration. In an elegant study by Bernadi et al., it has been shown that MatSep male rats consuming an ω-3-deficient diet have elevated food intake and body weight gain, plasma insulin, leptin, impaired glucose tolerance, and elevated hepatic levels of PEPCK (4). Thus, these data indicate that a nutritional approach involving diet supplementation could have a therapeutic effect on the metabolic derangements induced by exposure to MatSep.
We also acknowledge some discrepancies in the literature. Paternain et al. (48) have shown that female MatSep rats fed a high-fat and sucrose diet for 10 wk exhibit significant improvements in visceral fat, insulin, and leptin levels as well as impairments in HOMA-IR compared with control rats. A potential explanation may relate to the experimental design utilized, which involved exposure to MatSep between postnatal day 2 and weaning day, showing a longer time frame of separation protocol compared with the classical protocol widely used in the literature. Also, the hypercaloric diet was initiated 4 wk following weaning, suggesting that early changes in dietary composition may play a critical role exacerbating metabolic derangements in our model. Taken together, this particular extended separation protocol may confer metabolic resilience instead of endurance, as reported previously by others (23, 59).
There is a growing body of literature addressing sex-specific comorbidities. Recent outcomes from the national longitudinal survey Americans’ Changing Lives showed for the first time that women who experienced adverse childhood experiences gained weight more rapidly throughout the 15-yr followup period compared with men (30). Since sex hormones affect the HPA axis function, it is notable that women are two to three times more likely to experience depression during the perimenopausal period, with the onset of schizophrenia and other psychotic disorders presenting after the age of 40 (44). Coincidently, this period of life is characterized by increases in BMI. In that sense, glucocorticoid metabolism has been consistently reported as one of the major factors contributing to the sex differences in glucose tolerance and adiposity in humans (39, 63).
Finally, despite the changes in fat mass, MAP and HR were not different in MatSep and control rats. Previously, it has been shown that female MatSep rats have MAP and HR that are comparable with control rats fed a regular chow as well (37). Thus, these data suggest that chronic blood pressure control is still achieved during diet-induced obesity, indicating that the metabolic derangements may precede cardiovascular dysfunction in female rats. Our studies in male rats show that MatSep does not influence diet-induced obesity or glucose intolerance, even though HFD feeding was extended for a 5-wk period. In these conditions, we have shown that blood pressure is not different between male MatSep and control rats either (31). A role for sex hormones in this model remains to be elucidated. Reduced physiological levels of estrogens during menopause are well known to increase BMI and metabolic risk in clinical (9, 57) and experimental models (11, 20, 22, 60). Therefore, the study of the mechanisms by which MatSep may impact the reproductive function could contribute to decipher the long-lasting effects of ELS on the metabolic function.
The limitations of the current study include the specificity of the treatment, the induced protein breakdown, and the role of potential precursors in the steroidogenic pathway secondary to the corticosterone synthase enzyme inhibition. Something to consider is that both control and MatSep rats subjected to postnatal treatment with MTP showed lower plasma corticosterone levels and reduced adrenal gland weight as adults. These data indicate that MTP-treated rats display a permanent adjustment on the HPA axis regulation. Therefore, a comprehensive assessment of these factors would provide the mechanistic insights by which MatSep worsens diet-induced obesity in female rats.
Overall, this study further supports the concept that ELS induces permanent changes in adult health status, reinforcing the experimental evidence that links glucocorticoids with exaggerated fat expansion and metabolic dysfunction exposed to stress during developmental stages of life in a sex-specific manner.
PERSPECTIVES
Postnatal exposure to psychosocial stress is believed to exacerbate the levels of stress hormones, inducing metabolic derangements. Growing evidence of a positive correlation among ELS and risk for obesity and diabetes, as well as depression and anxiety, may contribute to reduced quality of life and life expectancy. A greater understanding of how ELS affects metabolism might assist in the development of behavioral enrichment strategies that overcome the negative effects of ELS on the endocrine system in a sex-specific manner.
GRANTS
This study was supported by grants from the National Heart, Lung, and Blood Institute (to A. S. Loria; R00-HL-111354) and the University of Kentucky Center of Research in Obesity and Cardiovascular Disease (COBRE P20 GM103527-06; Pathology Core).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.O.M., C.T.W., S.G.U., D.M.C., and A.S.L. performed experiments; M.O.M., J.B.H., C.T.W., D.M.C., and A.S.L. analyzed data; M.O.M., J.B.H., and A.S.L. edited and revised manuscript; A.S.L. interpreted results of experiments; A.S.L. prepared figures; A.S.L. drafted manuscript; A.S.L. approved final version of manuscript.
ACKNOWLEDGMENTS
We gratefully acknowledge the outstanding technical support in the histological sectioning and staining from Wendy Katz. Thanks to Dr. Phillip Landfield and Dr. Phillip Kern for the scientific feedback.
REFERENCES
- 1.Alciati A, Gesuele F, Casazza G, Foschi D. The relationship between childhood parental loss and metabolic syndrome in obese subjects. Stress Health 29: 5–13, 2013. doi: 10.1002/smi.1435. [DOI] [PubMed] [Google Scholar]
- 2.Appelman Y, van Rijn BB, Ten Haaf ME, Boersma E, Peters SAE. Sex differences in cardiovascular risk factors and disease prevention. Atherosclerosis 241: 211–218, 2015. doi: 10.1016/j.atherosclerosis.2015.01.027. [DOI] [PubMed] [Google Scholar]
- 3.Baitharu I, Deep SN, Jain V, Barhwal K, Malhotra AS, Hota SK, Prasad D, Ilavazhagan G. Corticosterone synthesis inhibitor metyrapone ameliorates chronic hypobaric hypoxia induced memory impairment in rat. Behav Brain Res 228: 53–65, 2012. doi: 10.1016/j.bbr.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 4.Bernardi JR, Ferreira CF, Senter G, Krolow R, de Aguiar BW, Portella AK, Kauer-Sant’anna M, Kapczinski F, Dalmaz C, Goldani MZ, Silveira PP. Early life stress interacts with the diet deficiency of omega-3 fatty acids during the life course increasing the metabolic vulnerability in adult rats. PLoS One 8: e62031, 2013. doi: 10.1371/journal.pone.0062031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biagini G, Pich EM, Carani C, Marrama P, Agnati LF. Postnatal maternal separation during the stress hyporesponsive period enhances the adrenocortical response to novelty in adult rats by affecting feedback regulation in the CA1 hippocampal field. Int J Dev Neurosci 16: 187–197, 1998. doi: 10.1016/S0736-5748(98)00019-7. [DOI] [PubMed] [Google Scholar]
- 6.Bleil ME, Appelhans BM, Latham MD, Irving MA, Gregorich SE, Adler NE, Cedars MI. Neighborhood socioeconomic status during childhood versus puberty in relation to endogenous sex hormone levels in adult women. Nurs Res 64: 211–220, 2015. doi: 10.1097/NNR.0000000000000096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bobrovskaya L, Maniam J, Ong LK, Dunkley PR, Morris MJ. Early life stress and post-weaning high fat diet alter tyrosine hydroxylase regulation and AT1 receptor expression in the adrenal gland in a sex dependent manner. Neurochem Res 38: 826–833, 2013. doi: 10.1007/s11064-013-0985-4. [DOI] [PubMed] [Google Scholar]
- 8.Calvo N, Martijena ID, Molina VA, Volosin M. Metyrapone pretreatment prevents the behavioral and neurochemical sequelae induced by stress. Brain Res 800: 227–235, 1998. doi: 10.1016/S0006-8993(98)00515-0. [DOI] [PubMed] [Google Scholar]
- 9.Carr MC. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab 88: 2404–2411, 2003. doi: 10.1210/jc.2003-030242. [DOI] [PubMed] [Google Scholar]
- 10.Danese A, Moffitt TE, Harrington H, Milne BJ, Polanczyk G, Pariante CM, Poulton R, Caspi A. Adverse childhood experiences and adult risk factors for age-related disease: depression, inflammation, and clustering of metabolic risk markers. Arch Pediatr Adolesc Med 163: 1135–1143, 2009. doi: 10.1001/archpediatrics.2009.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Danilovich N, Babu PS, Xing W, Gerdes M, Krishnamurthy H, Sairam MR. Estrogen deficiency, obesity, and skeletal abnormalities in follicle-stimulating hormone receptor knockout (FORKO) female mice. Endocrinology 141: 4295–4308, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Dey A, Hao S, Erion JR, Wosiski-Kuhn M, Stranahan AM. Glucocorticoid sensitization of microglia in a genetic mouse model of obesity and diabetes. J Neuroimmunol 269: 20–27, 2014. doi: 10.1016/j.jneuroim.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong M, Giles WH, Felitti VJ, Dube SR, Williams JE, Chapman DP, Anda RF. Insights into causal pathways for ischemic heart disease: adverse childhood experiences study. Circulation 110: 1761–1766, 2004. doi: 10.1161/01.CIR.0000143074.54995.7F. [DOI] [PubMed] [Google Scholar]
- 14.Felitti VJ. Adverse childhood experiences and adult health. Acad Pediatr 9: 131–132, 2009. doi: 10.1016/j.acap.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA 307: 491–497, 2012. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 16.Gross C, Blasey CM, Roe RL, Belanoff JK. Mifepristone reduces weight gain and improves metabolic abnormalities associated with risperidone treatment in normal men. Obesity (Silver Spring) 18: 2295–2300, 2010. doi: 10.1038/oby.2010.51. [DOI] [PubMed] [Google Scholar]
- 17.Hall ME, do Carmo JM, da Silva AA, Juncos LA, Wang Z, Hall JE. Obesity, hypertension, and chronic kidney disease. Int J Nephrol Renovasc Dis 7: 75–88, 2014. doi: 10.2147/IJNRD.S39739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hashimoto T, Igarashi J, Hasan AU, Ohmori K, Kohno M, Nagai Y, Yamashita T, Kosaka H. Mifepristone promotes adiponectin production and improves insulin sensitivity in a mouse model of diet-induced-obesity. PLoS One 8: e79724, 2013. doi: 10.1371/journal.pone.0079724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hauner H, Schmid P, Pfeiffer EF. Glucocorticoids and insulin promote the differentiation of human adipocyte precursor cells into fat cells. J Clin Endocrinol Metab 64: 832–835, 1987. doi: 10.1210/jcem-64-4-832. [DOI] [PubMed] [Google Scholar]
- 20.Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proc Natl Acad Sci U S A 97: 12729–12734, 2000. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huot RL, Gonzalez ME, Ladd CO, Thrivikraman KV, Plotsky PM. Foster litters prevent hypothalamic-pituitary-adrenal axis sensitization mediated by neonatal maternal separation. Psychoneuroendocrinology 29: 279–289, 2004. doi: 10.1016/S0306-4530(03)00028-3. [DOI] [PubMed] [Google Scholar]
- 22.Intapad S, Dasinger JH, Brown AD, Fahling JM, Esters J, Alexander BT. Glucose intolerance develops prior to increased adiposity and accelerated cessation of estrous cyclicity in female growth-restricted rats. Pediatr Res 79: 962–970, 2016. doi: 10.1038/pr.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korosi A, Shanabrough M, McClelland S, Liu Z-W, Borok E, Gao X-B, Horvath TL, Baram TZ. Early-life experience reduces excitation to stress-responsive hypothalamic neurons and reprograms the expression of corticotropin-releasing hormone. J Neurosci 30: 703–713, 2010. doi: 10.1523/JNEUROSCI.4214-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kruschinski C, Skripuletz T, Bedoui S, Raber K, Straub RH, Hoffmann T, Grote K, Jacobs R, Stephan M, Pabst R, von Hörsten S. Postnatal life events affect the severity of asthmatic airway inflammation in the adult rat. J Immunol 180: 3919–3925, 2008. doi: 10.4049/jimmunol.180.6.3919. [DOI] [PubMed] [Google Scholar]
- 25.Kuo LE, Kitlinska JB, Tilan JU, Li L, Baker SB, Johnson MD, Lee EW, Burnett MS, Fricke ST, Kvetnansky R, Herzog H, Zukowska Z. Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress-induced obesity and metabolic syndrome. Nat Med 13: 803–811, 2007. doi: 10.1038/nm1611. [DOI] [PubMed] [Google Scholar]
- 26.Langley-Evans SC, Welham SJ, Jackson AA. Fetal exposure to a maternal low protein diet impairs nephrogenesis and promotes hypertension in the rat. Life Sci 64: 965–974, 1999. doi: 10.1016/S0024-3205(99)00022-3. [DOI] [PubMed] [Google Scholar]
- 27.Lee MJ, Pramyothin P, Karastergiou K, Fried SK. Deconstructing the roles of glucocorticoids in adipose tissue biology and the development of central obesity. Biochim Biophys Acta 1842: 473–481, 2014. doi: 10.1016/j.bbadis.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lehmann J, Stöhr T, Feldon J. Long-term effects of prenatal stress experiences and postnatal maternal separation on emotionality and attentional processes. Behav Brain Res 107: 133–144, 2000. doi: 10.1016/S0166-4328(99)00122-9. [DOI] [PubMed] [Google Scholar]
- 29.Lippmann M, Bress A, Nemeroff CB, Plotsky PM, Monteggia LM. Long-term behavioural and molecular alterations associated with maternal separation in rats. Eur J Neurosci 25: 3091–3098, 2007. doi: 10.1111/j.1460-9568.2007.05522.x. [DOI] [PubMed] [Google Scholar]
- 30.Liu H, Umberson D. Gender, stress in childhood and adulthood, and trajectories of change in body mass. Soc Sci Med 139: 61–69, 2015. doi: 10.1016/j.socscimed.2015.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loria AS, Fox B, Pollock D, Pollock J. High fat feeding induces exaggerated endothelial dysfunction and insulin resistance in rats exposed to maternal separation. FASEB J 28: 1085.7, 2014. http://www.fasebj.org/content/28/1_Supplement/1085.7 [Google Scholar]
- 32.Loria AS, D’Angelo G, Pollock DM, Pollock JS. Early life stress downregulates endothelin receptor expression and enhances acute stress-mediated blood pressure responses in adult rats. Am J Physiol Regul Integr Comp Physiol 299: R185–R191, 2010. doi: 10.1152/ajpregu.00333.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loria AS, D’Angelo G, Pollock DM, Pollock JS. Early life stress downregulates endothelin receptor expression and enhances acute stress-mediated blood pressure responses in adult rats. Am J Physiol Regul Integr Comp Physiol 299: R185–R191, 2010. doi: 10.1152/ajpregu.00333.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loria AS, Ho DH, Pollock JS. A mechanistic look at the effects of adversity early in life on cardiovascular disease risk during adulthood. Acta Physiol (Oxf) 210: 277–287, 2014. doi: 10.1111/apha.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loria AS, Kang KT, Pollock DM, Pollock JS. Early life stress enhances angiotensin II-mediated vasoconstriction by reduced endothelial nitric oxide buffering capacity. Hypertension 58: 619–626, 2011. doi: 10.1161/HYPERTENSIONAHA.110.168674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loria AS, Pollock DM, Pollock JS. Early life stress sensitizes rats to angiotensin II-induced hypertension and vascular inflammation in adult life. Hypertension 55: 494–499, 2010. doi: 10.1161/HYPERTENSIONAHA.109.145391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loria AS, Yamamoto T, Pollock DM, Pollock JS. Early life stress induces renal dysfunction in adult male rats but not female rats. Am J Physiol Regul Integr Comp Physiol 304: R121–R129, 2013. doi: 10.1152/ajpregu.00364.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luecken LJ, Kraft A, Appelhans BM, Enders C. Emotional and cardiovascular sensitization to daily stress following childhood parental loss. Dev Psychol 45: 296–302, 2009. doi: 10.1037/a0013888. [DOI] [PubMed] [Google Scholar]
- 39.Maniam J, Antoniadis C, Morris MJ. Early-Life Stress, HPA Axis Adaptation, and Mechanisms Contributing to Later Health Outcomes. Front Endocrinol (Lausanne) 5: 73, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marais L, van Rensburg SJ, van Zyl JM, Stein DJ, Daniels WMU. Maternal separation of rat pups increases the risk of developing depressive-like behavior after subsequent chronic stress by altering corticosterone and neurotrophin levels in the hippocampus. Neurosci Res 61: 106–112, 2008. doi: 10.1016/j.neures.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 41.Masuzaki H, Yamamoto H, Kenyon CJ, Elmquist JK, Morton NM, Paterson JM, Shinyama H, Sharp MGF, Fleming S, Mullins JJ, Seckl JR, Flier JS. Transgenic amplification of glucocorticoid action in adipose tissue causes high blood pressure in mice. J Clin Invest 112: 83–90, 2003. doi: 10.1172/JCI17845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mauvais-Jarvis F. Sex differences in metabolic homeostasis, diabetes, and obesity. Biol Sex Differ 6: 14, 2015. doi: 10.1186/s13293-015-0033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Möller-Leimkühler AM. Higher comorbidity of depression and cardiovascular disease in women: a biopsychosocial perspective. World J Biol Psychiatry 11: 922–933, 2010. doi: 10.3109/15622975.2010.523481. [DOI] [PubMed] [Google Scholar]
- 44.Möller-Leimkühler AM. Higher comorbidity of depression and cardiovascular disease in women: a biopsychosocial perspective. World J Biol Psychiatry 11: 922–933, 2010. doi: 10.3109/15622975.2010.523481. [DOI] [PubMed] [Google Scholar]
- 45.Morgan SA, McCabe EL, Gathercole LL, Hassan-Smith ZK, Larner DP, Bujalska IJ, Stewart PM, Tomlinson JW, Lavery GG. 11β-HSD1 is the major regulator of the tissue-specific effects of circulating glucocorticoid excess. Proc Natl Acad Sci U S A 111: E2482–E2491, 2014. doi: 10.1073/pnas.1323681111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Orth-Gomér K, Schneiderman N, Wang HX, Walldin C, Blom M, Jernberg T. Stress reduction prolongs life in women with coronary disease: the Stockholm Women’s Intervention Trial for Coronary Heart Disease (SWITCHD). Circ Cardiovasc Qual Outcomes 2: 25–32, 2009. doi: 10.1161/CIRCOUTCOMES.108.812859. [DOI] [PubMed] [Google Scholar]
- 47.Patel MJ, Batch BC, Svetkey LP, Bain JR, Turer CB, Haynes C, Muehlbauer MJ, Stevens RD, Newgard CB, Shah SH. Race and sex differences in small-molecule metabolites and metabolic hormones in overweight and obese adults. OMICS 17: 627–635, 2013. doi: 10.1089/omi.2013.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paternain L, Martisova E, Milagro FI, Ramírez MJ, Martínez JA, Campión J. Postnatal maternal separation modifies the response to an obesogenic diet in adulthood in rats. Dis Model Mech 5: 691–697, 2012. doi: 10.1242/dmm.009043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pradhan AD. Sex differences in the metabolic syndrome: implications for cardiovascular health in women. Clin Chem 60: 44–52, 2014. doi: 10.1373/clinchem.2013.202549. [DOI] [PubMed] [Google Scholar]
- 50.Pretty C, O’Leary DD, Cairney J, Wade TJ. Adverse childhood experiences and the cardiovascular health of children: a cross-sectional study. BMC Pediatr 13: 208, 2013. doi: 10.1186/1471-2431-13-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Räikkönen K, Matthews KA, Kuller LH. The relationship between psychological risk attributes and the metabolic syndrome in healthy women: antecedent or consequence? Metabolism 51: 1573–1577, 2002. doi: 10.1053/meta.2002.36301. [DOI] [PubMed] [Google Scholar]
- 52.Räikkönen K, Matthews KA, Kuller LH. Depressive symptoms and stressful life events predict metabolic syndrome among middle-aged women: a comparison of World Health Organization, Adult Treatment Panel III, and International Diabetes Foundation definitions. Diabetes Care 30: 872–877, 2007. doi: 10.2337/dc06-1857. [DOI] [PubMed] [Google Scholar]
- 53.Rees SL, Steiner M, Fleming AS. Early deprivation, but not maternal separation, attenuates rise in corticosterone levels after exposure to a novel environment in both juvenile and adult female rats. Behav Brain Res 175: 383–391, 2006. doi: 10.1016/j.bbr.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 54.Rehan VK, Li Y, Corral J, Saraswat A, Husain S, Dhar A, Sakurai R, Khorram O, Torday JS. Metyrapone blocks maternal food restriction-induced changes in female rat offspring lung development. Reprod Sci 21: 517–525, 2014. doi: 10.1177/1933719113503404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roque S, Mesquita AR, Palha JA, Sousa N, Correia-Neves M. The behavioral and immunological impact of maternal separation: a matter of timing. Front Behav Neurosci 8: 192, 2014. doi: 10.3389/fnbeh.2014.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sanders BJ, Anticevic A. Maternal separation enhances neuronal activation and cardiovascular responses to acute stress in borderline hypertensive rats. Behav Brain Res 183: 25–30, 2007. doi: 10.1016/j.bbr.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Santosa S, Jensen MD. Adipocyte fatty acid storage factors enhance subcutaneous fat storage in postmenopausal women. Diabetes 62: 775–782, 2013. doi: 10.2337/db12-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schmidt MV, Levine S, Oitzl MS, van der Mark M, Müller MB, Holsboer F, de Kloet ER. Glucocorticoid receptor blockade disinhibits pituitary-adrenal activity during the stress hyporesponsive period of the mouse. Endocrinology 146: 1458–1464, 2005. doi: 10.1210/en.2004-1042. [DOI] [PubMed] [Google Scholar]
- 59.Steptoe A, Dockray S, Wardle J. Positive affect and psychobiological processes relevant to health. J Pers 77: 1747–1776, 2009. doi: 10.1111/j.1467-6494.2009.00599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stubbins RE, Holcomb VB, Hong J, Núñez NP. Estrogen modulates abdominal adiposity and protects female mice from obesity and impaired glucose tolerance. Eur J Nutr 51: 861–870, 2012. doi: 10.1007/s00394-011-0266-4. [DOI] [PubMed] [Google Scholar]
- 61.Su S, Wang X, Kapuku GK, Treiber FA, Pollock DM, Harshfield GA, McCall WV, Pollock JS. Adverse childhood experiences are associated with detrimental hemodynamics and elevated circulating endothelin-1 in adolescents and young adults. Hypertension 64: 201–207, 2014. doi: 10.1161/HYPERTENSIONAHA.113.02755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Upton KJ, Sullivan RM. Defining age limits of the sensitive period for attachment learning in rat pups. Dev Psychobiol 52: 453–464, 2010. doi: 10.1002/dev.20448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vierhapper H, Heinze G, Nowotny P. Sex-specific difference in the interconversion of cortisol and cortisone in men and women. Obesity (Silver Spring) 15: 820–824, 2007. doi: 10.1038/oby.2007.592. [DOI] [PubMed] [Google Scholar]
- 64.Winning A, Glymour MM, McCormick MC, Gilsanz P, Kubzansky LD. Psychological Distress Across the Life Course and Cardiometabolic Risk: Findings From the 1958 British Birth Cohort Study. J Am Coll Cardiol 66: 1577–1586, 2015. doi: 10.1016/j.jacc.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 65.Zhang L, Lee ICJ, Enriquez RF, Lau J, Vähätalo LH, Baldock PA, Savontaus E, Herzog H. Stress- and diet-induced fat gain is controlled by NPY in catecholaminergic neurons. Mol Metab 3: 581–591, 2014. doi: 10.1016/j.molmet.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zukowska-Grojec Z. Neuropeptide Y. A novel sympathetic stress hormone and more. Ann N Y Acad Sci 771: 219–233, 1995. doi: 10.1111/j.1749-6632.1995.tb44683.x. [DOI] [PubMed] [Google Scholar]