Abstract
Increased sugar consumption, particularly fructose, in the form of sweetened beverages and sweeteners in our diet adversely affects metabolic health. Because these effects are associated with features of the metabolic syndrome in humans, the direct effect of fructose on pancreatic islet function is unknown. Therefore, we examined the islet phenotype of mice fed excess fructose. Fructose-fed mice exhibited fasting hyperglycemia and glucose intolerance but not hyperinsulinemia, dyslipidemia, or hyperuricemia. Islet function was impaired, with decreased glucose-stimulated insulin secretion and increased glucagon secretion and high fructose consumption leading to α-cell proliferation and upregulation of the fructose transporter GLUT5, which was localized only in α-cells. Our studies demonstrate that excess fructose consumption contributes to hyperglycemia by affecting both β- and α-cells of islets in mice.
fructose consumption has risen dramatically in the past three decades, contributing to the increasing prevalence of the metabolic syndrome (47). The adverse peripheral effects of excess fructose consumption include hyperuricemia, increased adiposity, hepatic steatosis, dyslipidemia, insulin resistance, and increased risk of developing type 2 diabetes (47). However, the mechanisms by which these effects occur are unclear. Although fructose-mediated susceptibility to developing type 2 diabetes is attributed mainly to hyperuricemia, which is strongly associated with insulin resistance (21), insulin resistance does not cause type 2 diabetes unless pancreatic islet function is perturbed (12). Oral ingestion of a bolus of fructose can enhance glucose-stimulated insulin secretion (24). Because fructose and glucose both effectively induce secretion of the gut hormone glucagon-like peptide-1 (GLP-1), and GLP-1 is known to potentiate insulin secretion, the fructose-mediated increase in insulin secretion is thought to occur via GLP-1 (24). However, intravenous infusion of fructose, which bypasses the gut, can also increase insulin secretion by an unknown mechanism (26). Moreover, pancreatic islets in vitro exposed to high fructose show augmented glucose-stimulated insulin secretion (10, 25, 42, 46), suggesting that fructose directly affects insulin-producing islet β-cells. Finally, consumption of dietary fructose, but not isocaloric amounts of glucose, for more than 24 h leads to decreased insulin secretion (48).
Because fructose consumption leads to various metabolic derangements, assessing the direct effect of fructose consumption on pancreatic islet function is difficult. Controlled studies in mice have shown that exposure to a high-fructose diet impairs glucose tolerance but does not affect serum insulin levels (8, 33, 51). Caton et al. (6) reported impaired glucose-stimulated insulin secretion in vitro after long-term (16 wk) fructose feeding; however, in contrast to other studies, these authors also observed serum hyperinsulinemia. We recently showed that a 6-wk fructose feeding paradigm was sufficient to impair glucose tolerance without affecting insulin sensitivity (3, 39). Glucose homeostasis is normally maintained in a narrow physiological range by the opposing actions of the pancreatic hormones insulin and glucagon during postprandial and postabsorptive states, respectively. It is well known that the absence of insulin precipitates hyperglycemia; however, hyperglycemia can also occur in the presence of insulin when endogenous hepatic glucose production is stimulated by an increase in plasma glucagon (49). In support of this, pancreatic islets from organ donors with type 2 diabetes have an increased number of glucagon-producing α-cells relative to insulin-producing β-cells (53). Although the effect of high fructose consumption on α-cells has not been well documented, one study showed that acute infusion of fructose in lean nondiabetic subjects augmented glucagon release during hypoglycemia (18), suggesting that fructose consumption could promote glucagon secretion.
Here, we aimed to test the direct effects of fructose on pancreatic islet function by exposing mice to a high-fructose (HFr) diet for 6 wk, at which point the mice had increased adiposity but were not obese or hyperuricemic. We demonstrated that fructose feeding led to decreased insulin secretion in response to glucose challenge in vivo and that isolated islets from fructose-fed mice were dysfunctional. The fructose-exposed islets showed reduced glucose-stimulated insulin secretion and mitochondrial metabolism. ATP production by mitochondrial oxidative phosphorylation is critical for glucose-stimulated insulin secretion (30), and our data suggest that high fructose consumption impairs mitochondrial function and integrity. In addition to disrupted insulin secretion, islets from fructose-fed mice exhibited increased glucagon secretion and content as well as increased α-cell proliferation. To our knowledge, this is the first study showing that dysregulated glucagon secretion contributes to fructose-mediated hyperglycemia.
MATERIALS AND METHODS
Maintenance and diet composition of mice.
Male C57BL6 mice (Jackson Laboratories) were fed standard chow (control) or a high-fructose diet (60% fructose; Harlan-Teklad, Madison, WI) for 6 wk beginning at 8–10 wk of age. The high-fructose group consumed water supplemented with 30% fructose (HFr), whereas the control group consumed nonsupplemented water. Mice were maintained on a 12-h light-dark cycle. All animal procedures were approved by the Washington University School of Medicine Animal Studies Committee.
Body weight and composition.
Whole body weights were measured weekly during the 6 wk the mice were on the control or HFr diets. Percent body fat and lean mass were measured in awake animals by magnetic resonance imaging (EchoMRI Instrument; Echo Medical Systems, Houston, TX) at the Washington University Diabetes Phenotyping Core.
Glucose tolerance tests with insulin release and insulin tolerance test.
For the glucose tolerance test, mice were fasted for 6 h and then injected intraperitoneally with 2 mg glucose/g body wt, as described previously (2). Blood glucose was measured at 0, 15, 30, 60, 90, and 120 min postinjection. To measure insulin release during the glucose challenge, blood was collected in heparinized tubes at the times indicated and analyzed by using a rat/mouse insulin ELISA kit (Crystal Chem). For insulin tolerance tests, mice were fasted for 4 h and then injected intraperitoneally with 1 U insulin/g body wt. Blood glucose levels were measure over the next 90 min. The One-Touch Bayer Contour T5 glucometer (Mishiwaka, IN) was used for all glucose measurements.
Serum measurements.
After mice were fasted for 4 h, serum levels of triglyceride, free fatty acid, and uric acid were measured by using the infinity triglyceride reagent kit (Fisher Diagnostics), the NEFA-HR (2) assay kit (Wako Diagnostics), and the Amplex Red Uric Acid Assay Kit (Invitrogen, Carlsbad, CA), respectively, per the manufacturers’ instructions. Plasma for insulin and glucagon was collected under fed, fasted, and refed conditions, as described previously (22), and measured using the rat/mouse insulin ELISA kit (Crystal Chem) and glucagon ELISA kit (RayBiotech).
Islet isolation and assessment of insulin and glucagon secretion.
For islet isolation, the bile duct of euthanized mice was perfused with RPMI media (ThermoFisher Scientific) containing 0.8 mg/ml collagenase type V (Sigma, St. Louis, MO) and 2% BSA. The distended pancreata were dissected out and incubated at 37°C for 15 min with gentle shaking. The islets were dispersed and handpicked under a stereo microscope and cultured in RPMI media (ThermoFisher Scientific). Islets were cultured overnight before all experiments, except for insulin secretion; in this case, islets were cultured for 2 h. For glucose or leucine/glutamine-induced insulin secretion, islets were preincubated in Krebs Ringer buffer solution containing 2% BSA and 2.8 mM glucose for 1 h before stimulation. Glucagon secretion was performed as described previously (1). Levels of hormones released into the medium were measured by using rat/mouse insulin (Crystal Chem) or glucagon (RayBiotech) ELISA kit. Total insulin and glucagon were extracted from islets in 0.2 N/85% acid-ethanol. Protein content was determined by the Pierce BCA protein assay kit (ThermoFisher Scientific).
Mitochondrial bioenergetics.
Seahorse flux analysis was performed as described (32). Briefly, 50 islets/well were plated in XF24 islet capture microplates and equilibrated at 37°C for 1 h before oxygen consumption rate (OCR) measurements under basal (2 mM glucose) and glucose-stimulated (20 mM glucose) conditions. The reagent concentrations were 10 µM oligomycin, 7.5 µM FCCP, 2 µM antimycin, and 1 µM rotenone. The OCR in each well was normalized to total protein quantified by the Pierce BCA protein assay kit (ThermoFisher Scientific).
Histology and confocal imaging.
After animal euthanasia, whole pancreata were fixed overnight in 10% neutral buffered formalin (Fisher Scientific), serially dehydrated in ethanol, processed into paraffin blocks, and cut into 5-µm sections. After heat-induced antigen retrieval in 10 mM sodium citrate, pH 6, pancreatic sections were permeabilized in 0.1% Triton X, blocked in 2% BSA and 10% normal serum, and incubated overnight in combinations of rabbit anti-insulin (1:400; Cell Signaling Technology), guinea pig anti-insulin (1:400; Abcam), guinea pig anti-glucagon (1:400; Abcam), goat anti-GLUT5 (1:100; Santa Cruz Biotechnology), and rabbit anti-Ki-67 (1:200; Abcam), as appropriate. The slides were washed with PBS and incubated with Alexa Fluor 488-conjugated goat anti-rabbit, Alexa Fluor 546-conjugated anti-guinea pig, or Alexa Fluor 647-conjugated anti-guinea pig (1:500; Life Technologies) for 1 h and mounted in prolong gold antifade mounting medium containing DAPI (Thermo Fisher Scientific). Fluorescence was observed by confocal microscopy (Leica Microsystems DMI4000 B; North Central Instruments). To measure islet areas, three sections (50 µM apart) per pancreas (stained for insulin and glucagon) were analyzed.
Quantitative PCR analysis.
Isolated islets were cultured overnight, and then RNA was extracted by using the RNeasy Plus Mini kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. The Quantitect Qiagen reverse transcriptase kit (Qiagen, Valencia, CA) was used to synthesize cDNA. Quantitative PCR (7500/7500 fast real-time PCR system, software version 2.0.3; Applied Biosystems) was performed with SYBR Green master mix (Applied Biosystems, Carlsbad, CA). The PCR conditions were follows: 95°C for 20 s, followed by 40 cycles at 95°C for 3 s, and 60°C for 30 s. Gene expression levels were determined by using the ΔΔCT approach with normalization to β-actin. Primers used were as follows: GLUT5, 5′-TCTTTGTGGTAGAGCTTTGGG-3′ and 5′-GACAATGACACAGACAATGCTG3′; and β-actin, 5′-ACC TTC TAC AAT GAG CTG CG-3′ and 5′-CTG GAT GGC TAC GTA CAT GG-3′.
Statistical analysis.
Data are expressed as means ± SE. Statistical difference between groups was determined by Student’s t-test with GraphPad PRISM version 6.0 (La Jolla, CA). For all data, P < 0.05 was considered statistically significant.
RESULTS
Metabolic profile of male mice exposed to high fructose.
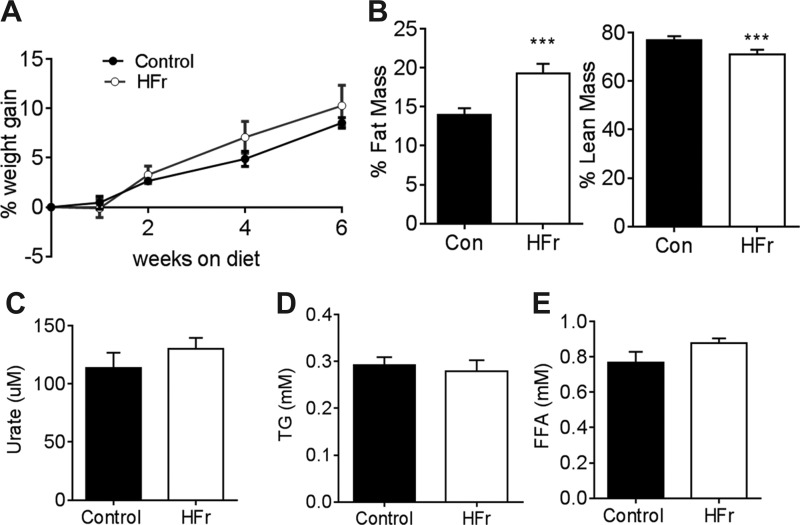
We demonstrated previously that both male and female mice exposed to a HFr (60%) diet for 6 wk did not gain excess weight or develop triglyceridemia, hyperuricemia, or hyperuricemia (3, 11). Here, we exposed 8- to 10-wk-old C57BL6 males to a 60% fructose diet supplemented with 30% fructose in the water (HFr) to magnify the metabolic insults induced by fructose. After 6 wk of exposure to HFr or control (Con) chow diet, HFr-fed mice had body weight similar to control mice (Fig. 1A). Mice on HFr demonstrate a reduction of ~60% in food consumption with respect to chow-fed control mice (1.82 ± 0.07 g HFr vs. 4.69 ± 0.50 g Con). Water consumption was not significantly different between the two groups (5.83 ± 0.42 ml HFr vs 4.58 ± 0.42 ml Con). Importantly, HFr group showed significantly higher fat mass and lower lean mass compared with the control group (Fig. 1B). Serum uric acid (Fig. 1C), triglyceride (Fig. 1D), or free fatty acid (Fig. 1E) levels were not different between the two groups.
Fig. 1.
High fructose consumption increases adiposity. Beginning at 6–8 wk of age, male mice were fed standard chow diet and water [control (Con); ● and black bars] or 60% fructose diet + 30% fructose water (HFr; ○ and open bars) for 6 wk. A: body weights of HFr and control mice; n = 8–10. B: %body fat and lean mass; n = 7 mice in each group. C–E: fasting serum uric acid (C), triglyceride (D), and free fatty acid (E) levels; n = 7 in each group. ***P < 0.001 by Student's t-test.
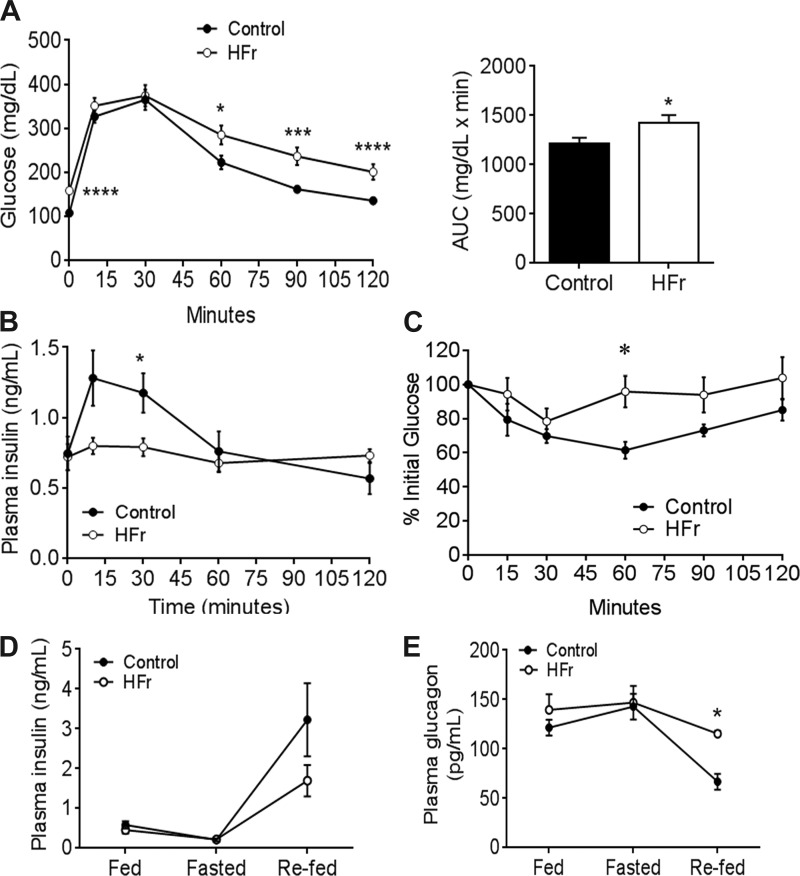
In our previous study, mice exposed to high-fructose diet but nonsupplemented water developed impaired glucose tolerance but not insulin resistance. Here, we found that HFr-exposed mice exhibited impaired glucose tolerance (Fig. 2A), impaired insulin response to glucose challenge (Fig. 2B), and modest insulin resistance as determined by an insulin tolerance test (Fig. 2C). Moreover, fed and fasting insulin and glucagon levels were not different between the control and HFr groups; however, under refed conditions, HFr mice exhibited decreased, albeit not statistically significant, levels in plasma insulin (Fig. 2D) and a significant increase in glucagon levels (Fig. 2E).
Fig. 2.
High-fructose consumption impairs glucose homeostasis. A, left: blood glucose levels over time following an intraperitoneal injection of 2 mg/g glucose; n = 16 in each group. A, right: area under the curve (AUC). B: insulin response during the glucose tolerance test; n = 11–12 mice. C: blood glucose levels following an intraperitoneal injection of 0.5 U insulin; n = 6 in each group. D and E: fed, fasting, and refed plasma insulin (D) and glucagon (E) levels; n = 4–6. Data are presented as means ± SE. *P < 0.05, ***P < 0.01, and ****P < 0.0001 by Student’s t-test.
Fructose exposure impairs glucose-stimulated insulin secretion by pancreatic islets in vitro.
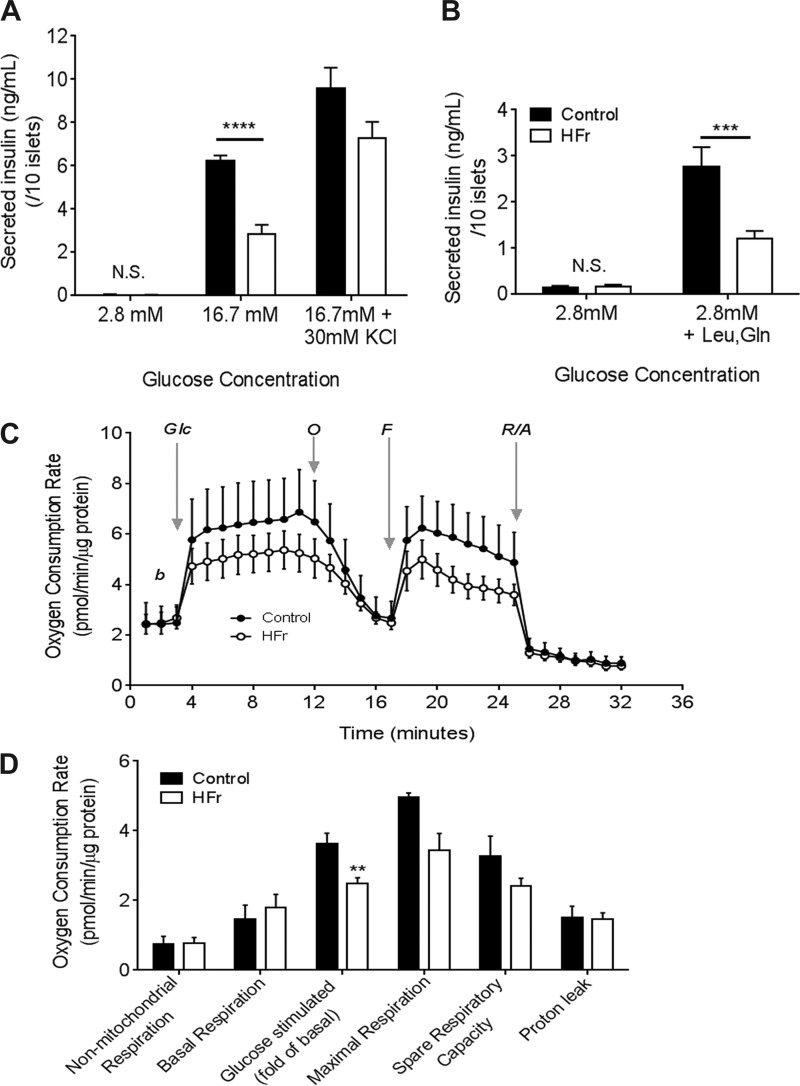
To determine whether the decreased plasma insulin in response to glucose challenge in vivo was due to decreased insulin secretion, we examined glucose-stimulated insulin secretion in freshly isolated islets from HFr- and control-exposed mice. Islets from HFr-exposed mice showed lower glucose-stimulated insulin secretion than islets from control chow-fed mice (Fig. 3A). Importantly, KCl-mediated insulin secretion was not significantly different between islets isolated from mice exposed to HFr or control diets (Fig. 3A). Total insulin content was similar in islets from both groups (37,652 ± 2,171 ng/mg Con vs. 31,765 ± 1,979 ng/mg HFr). Together, these data suggest that the decrease in insulin secretion in HFr islets is likely due to an impaired ability of β-cells to respond to glucose challenge. More importantly, islets isolated from HFr mice show a blunted insulin secretory response to leucine and glutamine, which effectively bypasses glycolysis (Fig. 3B), suggesting a compromised mitochondrial function. Therefore, we performed Seahorse flux analyses of mitochondrial bioenergetics to test whether glucose metabolism was altered in islets from HFr-exposed mice (Fig. 3C). We found that the basal oxygen consumption rate (OCR), nonmitochondrial OCR, and proton leak measurements were similar in islets from HFr- and control chow-exposed mice. However, after induction with 20 mM glucose, the OCR was significantly lower in islets from HFr-exposed mice than in control islets (Fig. 3D). Islets from HFr-exposed mice exhibited lower maximal respiration rate and spare respiratory capacity than control islets, but these differences were not statistically significant. Together, these data suggest that HFr exposure can impair mitochondrial function in islets, and the defect in insulin secretion lies downstream of glycolysis.
Fig. 3.
High fructose consumption leads to decreased glucose-stimulated insulin secretion and impaired mitochondrial response. A: glucose-stimulated insulin secretion in freshly isolated islets; n = 6–7 replicates of islet batches from ≥4 mice in each group. B: insulin secretion simulated with 10 mM leucine and 10 mM glutamine under nonstimulatory (2.8 mM) glucose conditions; n = 7 replicates of islet batches from 3 mice in each group. C: oxygen consumption rates in the basal (b) state, after glucose stimulation (+20 mM glc), after addition of the mitochondrial uncoupler FCCP (F), and after addition of the electron transport chain inhibitors oligomycin (O) and rotenone/antimycin (R/A). D: mitochondrial bioenergetics parameters were calculated based on measurements from those in C. Results are expressed as means ± SE; n = 4–7/group. **P < 0.01, ***P < 0.001, and ****P < 0.0001 by Student’s t-test.
Exposure to high fructose leads to increased glucagon secretion and more α-cells.
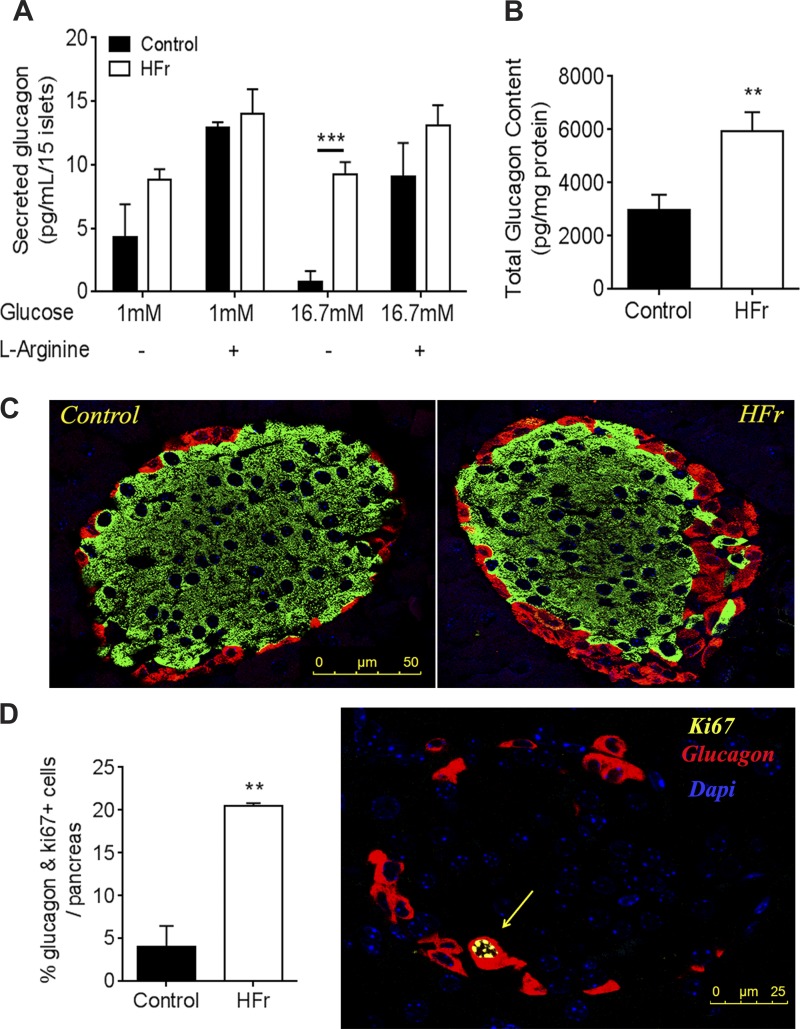
Given our observation that HFr-exposed mice had elevated plasma glucagon levels, we investigated whether HFr exposure affected glucagon secretion in vitro. We found that, under both low and high glucose conditions, as well as when stimulated with the amino acid arginine, islets from HFr-exposed mice secreted more glucagon than those from control mice (Fig. 4A). Additionally, the islets from HFr-exposed mice contained significantly more glucagon than those from controls (Fig. 4B). We questioned whether this finding reflected altered architecture of islets, which consists of glucagon-secreting α-cells surrounding insulin-secreting β-cells. We found that although islets from control and HFr-exposed mice were similar in size (9.02 ± 0.73 vs. 11.06 ± 2.48 mm2) and contained similar levels of protein (0.11 ± 0.031 vs. 0.10 ± 0.030 mg/ml), islets from HFr-exposed mice contained a larger proportion of glucagon-positive α-cells than control islets (Fig. 4C). Additionally, staining for the proliferation marker Ki-67 revealed increased proliferation of α-cells in islets from HFr-exposed mice (Fig. 4D).
Fig. 4.
High fructose consumption leads to increases in glucagon secretion and α-cell expansion. A: glucagon secretion from cultured islets under low (1 mM) and high glucose (16.7 mM) with or without 20 mM l-arginine; n = 5 replicates of islet batches from 4 mice in each group. B: total islet glucagon content; n = 12 batches of islets. C: representative immunofluorescence image of control and HFr islets. Green, insulin; red, glucagon. D: quantification of Ki-67-positive red stained α-cells (left) and representative image (right). Red, glucagon; yellow, Ki-67; Dapi, blue (n = 3 mice pancreata examined by pancreatic sections separated by 80 µm from each of 3 mice). **P < 0.01 and ***P < 0.001 by Student’s t-test.
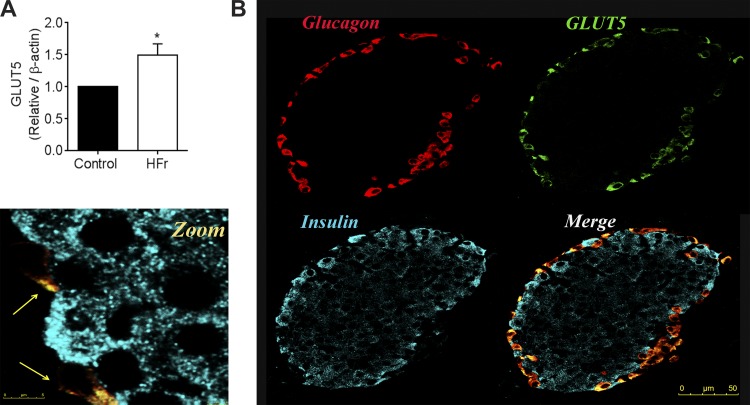
Four fructose transporters are known: glucose transporters (GLUT) 2, 5, 8, and 9. GLUT2 (20) and GLUT8 (14) are not expressed in α-cells, and expression of GLUT9 in islets seems to be β-cell specific (16). GLUT5 has been reported to be expressed only in α-cells of rat islets (41), but expression in mouse islets has not been documented. Our immunofluorescence analysis revealed that GLUT5 is expressed solely within the α-cells of the islet (Fig. 5A). Finally, we found that GLUT5 mRNA expression was higher in islets from HFr-exposed mice than in those from control mice (Fig. 5B).
Fig. 5.
GLUT5 is expressed in α-cells and is upregulated in response to high fructose consumption. A: mRNA expression of GLUT5 (normalized to β-actin) in islets from control and HFr-exposed mice. B: representative immunofluorescence image of control islets at low and high magnification. Cyan, insulin; red, glucagon; green, GLUT5 (n = 4 replicates from 4 mice). *P< 0.05 by Student’s t-test.
DISCUSSION
We report here that mice that consumed excess fructose (HFr) for 6 wk did not develop hyperuricemia but did develop pancreatic islet dysfunction and impaired glucose homeostasis. Islets from HFr-exposed mice exhibited decreased glucose and amino acid-stimulated insulin secretion and decreased mitochondrial oxygen consumption rates, suggesting that mitochondrial function was compromised. Islets from HFr-exposed mice also showed increased glucagon secretion under both low and high glucose concentrations in vitro; in vivo, this could worsen hyperglycemia. We also found that α-cell proliferation was increased in islets from HFr-exposed mice. Finally, we report that expression of the fructose transporter GLUT5 was upregulated in islets from HFr-exposed mice. Thus, we speculate that fructose acts directly on α-cells by gaining entry via GLUT5, leading to α-cell proliferation.
Our findings from this work, in which we exposed mice to 60% fructose diet plus 30% fructose water (HFr), are similar to those in which mice consumed only a 60% fructose diet (HFrD) (39) but differ somewhat from those in rats. Whereas rats on a high-fructose diet developed several features of metabolic syndrome, such as hyperuricemia, insulin resistance, hyperlipidemia, and weight gain, neither the HFr nor the HFrD mice developed these features. In the HFr mouse model described here, we observed a slight decrease in insulin sensitivity, increased adiposity, and fasting hyperglycemia, features that were not observed in HFrD-fed female mice (39). However pregnant HFrD-exposed mice did develop fasting hyperglycemia (3), suggesting that either the excess calories obtained from the 30% fructose water or metabolic stress brought about by pregnancy is sufficient to precipitate fasting hyperglycemia. Nonetheless, serum insulin remained within normal levels in both HFrD and HFr mice. In the HFr model, we did not observe a compensatory increase in islet size or insulin content. In contrast, rats fed either 10% fructose water or a 60% fructose diet had increased size and number of islets and became hyperinsulinemic and exhibited increased glucose-stimulated insulin secretion (27, 36).
HFr exposure led to decreased glucose-stimulated insulin secretion both in vivo and in isolated islets. Given that the islets from HFr-exposed mice showed reduction in both glucose-dependent mitochondrial oxygen consumption rates and leucine/glutamine-stimulated insulin secretion, the defect in insulin secretion was likely due to impaired mitochondrial function. Although we did not examine the mechanisms underlying impaired mitochondrial function, one study showed that high-fructose feeding in rats led to decreased pancreatic expression of sirtuin 1, a transcriptional regulator of mitochondrial genes that control metabolic coupling (9). Expression of the mitochondrial uncoupling protein UCP2 is associated with a failure of cells to increase ATP levels after glucose stimulation, and Sirtuin 1 suppresses Ucp2 transcription (5). Increase in UCP2 levels as a result of fructose feeding has been observed in enterocytes (11), but we found no increase in UCP2 transcript levels in the islets (data not shown), suggesting that this mechanism does not explain the decreased insulin secretion.
Another possibility that we cannot rule out is that 6-wk exposure to HFr caused a defect in glucose-stimulated insulin secretion as a result of a shift in substrate preference. This seems unlikely given that fructose cannot induce insulin secretion in the absence of stimulatory concentrations of glucose in animals maintained on a normal diet. Nonetheless, future work could address this possibility by examining whether fructose-exposed mice respond to a fructose challenge in the same way they responded to a glucose challenge.
In addition to impaired insulin secretion, HFr-exposed mice showed increased glucagon secretion, which could occur in one of several ways. First, in the absence of adequate insulin, elevated glucose might stimulate glucagon production and secretion, which aggravates hyperglycemia by favoring hepatic endogenous glucose production, mimicking the fasting state (52). Second, insulin signaling may regulate glucagon secretion. In support of this idea, mice lacking the insulin receptor specifically in α-cells develop hyperglucagonemia, and their islets exhibit abnormal glucagon secretion under both normo- and hypoglycemic conditions (17, 22). Therefore, the fructose-induced increase in glucagon secretion could be explained by a general decrease in glucose-stimulated insulin secretion. Third, the increased glucagon secretion could be due to the increased number of α-cells in HFr-exposed mice. Finally, glucagon could positively stimulate glucagon secretion, as glucagon receptors are expressed on α-cells (29).
It is well established that fasting hyperglycemia is due to increased gluconeogenesis, which is inhibited by insulin and activated by glucagon (40). In humans, suppression of glucagon secretion can eliminate postprandial hyperglycemia (37). In contrast, acute disruption of glucagon secretion or action does not improve glucose tolerance in an insulin-deficient mouse model of diabetes (44). Relevant to our study, humans that ingested fructose and were infused with glucagon showed increased gluconeogenesis (35). In our mouse model, the sequence of events leading to hyperglycemia and hyperglucagonemia remain unclear. Fructose could directly affect the islets, leading to glucagon hypersecretion and hyperglycemia. Alternatively, fructose-induced hepatic circulating factors could contribute to hyperglycemia by inducing α-cell proliferation.
Glucose is a well-established energy source for cell growth and proliferation. Although the role of fructose as an energy source is unclear, some studies have shown that it can promote cell proliferation to a similar extent as glucose (23). In addition to increased α-cell proliferation in islets from HFr-exposed mice, we found that expression of the fructose transporter GLUT5 was increased in islets from these mice. Similarly, increased expression of GLUT5 was reported in intestine and kidney upon fructose exposure in rodents (13). Perhaps similarly, levels of GLUT5 mRNA and protein are increased in skeletal muscle (45) and intestine (15) of humans with type 2 diabetes. There is also evidence that fructose and GLUT5 enhance the proliferative and invasive properties of tumor cells (19, 34) and that suppressing GLUT5 expression can inhibit cell proliferation (7). Importantly, fructose preferentially promotes proliferation of pancreatic tumor cells (14).
The mechanisms leading to α-cell proliferation and regulation of function are not well understood. Possibilities include upregulation of forkhead transcription factors (FOXO1, -2, and -4) (43), triglyceride metabolism in adipose tissue (4), or glucagon receptor inhibition in the liver (28). In general, chronic exposure to fructose is linked to inflammation and oxidative damage (38). However, α-cells are less susceptible than β-cells to toxic and metabolic stress (31, 50), which could explain why fructose induces α-cell expansion but decreases β-cell function. Future in vitro work could begin to address this question by incubating α-cells with fructose.
GRANTS
This work was supported by American Diabetes Association Research Grant 76274 to K. H. Moley and by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-098584 to M. S. Remedi.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Z.A.A., A.C., and Z.Y. performed experiments; Z.A.A., A.C., Z.Y., and M.S.R. analyzed data; Z.A.A., M.S.R., and K.H.M. interpreted results of experiments; Z.A.A. and A.C. prepared figures; Z.A.A. drafted manuscript; Z.A.A., M.S.R., and K.H.M. edited and revised manuscript; K.H.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Deborah Frank, Obstetrics and Gynecology, Washington University in St. Louis, for critical reading and editing of the manuscript.
REFERENCES
- 1.Allister EM, Robson-Doucette CA, Prentice KJ, Hardy AB, Sultan S, Gaisano HY, Kong D, Gilon P, Herrera PL, Lowell BB, Wheeler MB. UCP2 regulates the glucagon response to fasting and starvation. Diabetes 62: 1623–1633, 2013. doi: 10.2337/db12-0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrikopoulos S, Blair AR, Deluca N, Fam BC, Proietto J. Evaluating the glucose tolerance test in mice. Am J Physiol Endocrinol Metab 295: E1323–E1332, 2008. doi: 10.1152/ajpendo.90617.2008. [DOI] [PubMed] [Google Scholar]
- 3.Asghar ZA, Thompson A, Chi M, Cusumano A, Scheaffer S, Al-Hammadi N, Saben JL, Moley KH. Maternal fructose drives placental uric acid production leading to adverse fetal outcomes. Sci Rep 6: 25091, 2016. doi: 10.1038/srep25091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ben-Zvi D, Barrandon O, Hadley S, Blum B, Peterson QP, Melton DA. Angptl4 links α-cell proliferation following glucagon receptor inhibition with adipose tissue triglyceride metabolism. Proc Natl Acad Sci U S A 112: 15498–15503, 2015. doi: 10.1073/pnas.1513872112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bordone L, Motta MC, Picard F, Robinson A, Jhala US, Apfeld J, McDonagh T, Lemieux M, McBurney M, Szilvasi A, Easlon EJ, Lin SJ, Guarente L. Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic β cells. PLoS Biol 4: e31, 2006. doi: 10.1371/journal.pbio.0040031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caton PW, Kieswich J, Yaqoob MM, Holness MJ, Sugden MC. Nicotinamide mononucleotide protects against pro-inflammatory cytokine-mediated impairment of mouse islet function. Diabetologia 54: 3083–3092, 2011. doi: 10.1007/s00125-011-2288-0. [DOI] [PubMed] [Google Scholar]
- 7.Chan KK, Chan JY, Chung KK, Fung KP. Inhibition of cell proliferation in human breast tumor cells by antisense oligonucleotides against facilitative glucose transporter 5. J Cell Biochem 93: 1134–1142, 2004. doi: 10.1002/jcb.20270. [DOI] [PubMed] [Google Scholar]
- 8.Chan SM, Sun RQ, Zeng XY, Choong ZH, Wang H, Watt MJ, Ye JM. Activation of PPARα ameliorates hepatic insulin resistance and steatosis in high fructose-fed mice despite increased endoplasmic reticulum stress. Diabetes 62: 2095–2105, 2013. doi: 10.2337/db12-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen YR, Lai YL, Lin SD, Li XT, Fu YC, Xu WC. SIRT1 interacts with metabolic transcriptional factors in the pancreas of insulin-resistant and calorie-restricted rats. Mol Biol Rep 40: 3373–3380, 2013. doi: 10.1007/s11033-012-2412-3. [DOI] [PubMed] [Google Scholar]
- 10.Curry DL, Curry KP, Gomez M. Fructose potentiation of insulin secretion. Endocrinology 91: 1493–1498, 1972. doi: 10.1210/endo-91-6-1493. [DOI] [PubMed] [Google Scholar]
- 11.DeBosch BJ, Chen Z, Finck BN, Chi M, Moley KH. Glucose transporter-8 (GLUT8) mediates glucose intolerance and dyslipidemia in high-fructose diet-fed male mice. Mol Endocrinol 27: 1887–1896, 2013. doi: 10.1210/me.2013-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Del Prato S, Marchetti P. Beta- and alpha-cell dysfunction in type 2 diabetes. Horm Metab Res 36: 775–781, 2004. doi: 10.1055/s-2004-826163. [DOI] [PubMed] [Google Scholar]
- 13.Douard V, Ferraris RP. Regulation of the fructose transporter GLUT5 in health and disease. Am J Physiol Endocrinol Metab 295: E227–E237, 2008. doi: 10.1152/ajpendo.90245.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Douard V, Ferraris RP. The role of fructose transporters in diseases linked to excessive fructose intake. J Physiol 591: 401–414, 2013. doi: 10.1113/jphysiol.2011.215731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dyer J, Wood IS, Palejwala A, Ellis A, Shirazi-Beechey SP. Expression of monosaccharide transporters in intestine of diabetic humans. Am J Physiol Gastrointest Liver Physiol 282: G241–G248, 2002. doi: 10.1152/ajpgi.00310.2001. [DOI] [PubMed] [Google Scholar]
- 16.Evans SA, Doblado M, Chi MM, Corbett JA, Moley KH. Facilitative glucose transporter 9 expression affects glucose sensing in pancreatic beta-cells. Endocrinology 150: 5302–5310, 2009. doi: 10.1210/en.2009-0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farhy LS, McCall AL. Optimizing reduction in basal hyperglucagonaemia to repair defective glucagon counterregulation in insulin deficiency. Diabetes Obes Metab 13, Suppl 1: 133–143, 2011. doi: 10.1111/j.1463-1326.2011.01455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gabriely I, Hawkins M, Vilcu C, Rossetti L, Shamoon H. Fructose amplifies counterregulatory responses to hypoglycemia in humans. Diabetes 51: 893–900, 2002. doi: 10.2337/diabetes.51.4.893. [DOI] [PubMed] [Google Scholar]
- 19.Godoy A, Ulloa V, Rodríguez F, Reinicke K, Yañez AJ, García ML, Medina RA, Carrasco M, Barberis S, Castro T, Martínez F, Koch X, Vera JC, Poblete MT, Figueroa CD, Peruzzo B, Pérez F, Nualart F. Differential subcellular distribution of glucose transporters GLUT1-6 and GLUT9 in human cancer: ultrastructural localization of GLUT1 and GLUT5 in breast tumor tissues. J Cell Physiol 207: 614–627, 2006. doi: 10.1002/jcp.20606. [DOI] [PubMed] [Google Scholar]
- 20.Heimberg H, De Vos A, Pipeleers D, Thorens B, Schuit F. Differences in glucose transporter gene expression between rat pancreatic alpha- and beta-cells are correlated to differences in glucose transport but not in glucose utilization. J Biol Chem 270: 8971–8975, 1995. doi: 10.1074/jbc.270.15.8971. [DOI] [PubMed] [Google Scholar]
- 21.Johnson RJ, Perez-Pozo SE, Sautin YY, Manitius J, Sanchez-Lozada LG, Feig DI, Shafiu M, Segal M, Glassock RJ, Shimada M, Roncal C, Nakagawa T. Hypothesis: could excessive fructose intake and uric acid cause type 2 diabetes? Endocr Rev 30: 96–116, 2009. doi: 10.1210/er.2008-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawamori D, Kurpad AJ, Hu J, Liew CW, Shih JL, Ford EL, Herrera PL, Polonsky KS, McGuinness OP, Kulkarni RN. Insulin signaling in alpha cells modulates glucagon secretion in vivo. Cell Metab 9: 350–361, 2009. doi: 10.1016/j.cmet.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim J, Song G, Wu G, Bazer FW. Functional roles of fructose. Proc Natl Acad Sci U S A 109: E1619–E1628, 2012. doi: 10.1073/pnas.1204298109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuhre RE, Gribble FM, Hartmann B, Reimann F, Windeløv JA, Rehfeld JF, Holst JJ. Fructose stimulates GLP-1 but not GIP secretion in mice, rats, and humans. Am J Physiol Gastrointest Liver Physiol 306: G622–G630, 2014. doi: 10.1152/ajpgi.00372.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kyriazis GA, Soundarapandian MM, Tyrberg B. Sweet taste receptor signaling in beta cells mediates fructose-induced potentiation of glucose-stimulated insulin secretion. Proc Natl Acad Sci U S A 109: E524–E532, 2012. doi: 10.1073/pnas.1115183109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lawrence JR, Gray CE, Grant IS, Ford JA, McIntosh WB, Dunnigan MG. The insulin response to intravenous fructose in maturity-onset diabetes mellitus and in normal subjects. Diabetes 29: 736–741, 1980. doi: 10.2337/diab.29.9.736. [DOI] [PubMed] [Google Scholar]
- 27.Li JM, Wang W, Fan CY, Wang MX, Zhang X, Hu QH, Kong LD. Quercetin Preserves β -Cell Mass and Function in Fructose-Induced Hyperinsulinemia through Modulating Pancreatic Akt/FoxO1 Activation. Evid Based Complement Alternat Med 2013: 303902, 2013. doi: 10.1155/2013/303902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li M, Dean ED, Zhao L, Nicholson WE, Powers AC, Chen W. Glucagon receptor inactivation leads to α-cell hyperplasia in zebrafish. J Endocrinol 227: 93–103, 2015. doi: 10.1530/JOE-15-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Z, Kim W, Chen Z, Shin YK, Carlson OD, Fiori JL, Xin L, Napora JK, Short R, Odetunde JO, Lao Q, Egan JM. Insulin and glucagon regulate pancreatic α-cell proliferation. PLoS One 6: e16096, 2011. doi: 10.1371/journal.pone.0016096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu H, Koshkin V, Allister EM, Gyulkhandanyan AV, Wheeler MB. Molecular and metabolic evidence for mitochondrial defects associated with beta-cell dysfunction in a mouse model of type 2 diabetes. Diabetes 59: 448–459, 2010. doi: 10.2337/db09-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marroqui L, Masini M, Merino B, Grieco FA, Millard I, Dubois C, Quesada I, Marchetti P, Cnop M, Eizirik DL. Pancreatic α Cells are Resistant to Metabolic Stress-induced Apoptosis in Type 2 Diabetes. EBioMedicine 2: 378–385, 2015. doi: 10.1016/j.ebiom.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitchell T, Johnson MS, Ouyang X, Chacko BK, Mitra K, Lei X, Gai Y, Moore DR, Barnes S, Zhang J, Koizumi A, Ramanadham S, Darley-Usmar VM. Dysfunctional mitochondrial bioenergetics and oxidative stress in Akita(+/Ins2)-derived β-cells. Am J Physiol Endocrinol Metab 305: E585–E599, 2013. doi: 10.1152/ajpendo.00093.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montgomery MK, Fiveash CE, Braude JP, Osborne B, Brown SH, Mitchell TW, Turner N. Disparate metabolic response to fructose feeding between different mouse strains. Sci Rep 5: 18474, 2015. doi: 10.1038/srep18474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Monzavi-Karbassi B, Hine RJ, Stanley JS, Ramani VP, Carcel-Trullols J, Whitehead TL, Kelly T, Siegel ER, Artaud C, Shaaf S, Saha R, Jousheghany F, Henry-Tillman R, Kieber-Emmons T. Fructose as a carbon source induces an aggressive phenotype in MDA-MB-468 breast tumor cells. Int J Oncol 37: 615–622, 2010. doi: 10.3892/ijo_00000710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paquot N, Schneiter P, Jéquier E, Gaillard R, Lefèbvre PJ, Scheen A, Tappy L. Effects of ingested fructose and infused glucagon on endogenous glucose production in obese NIDDM patients, obese non-diabetic subjects, and healthy subjects. Diabetologia 39: 580–586, 1996. doi: 10.1007/BF00403305. [DOI] [PubMed] [Google Scholar]
- 36.Pokrywczynska M, Flisinski M, Jundzill A, Krzyzanowska S, Brymora A, Deptula A, Bodnar M, Kloskowski T, Stefanska A, Marszalek A, Manitius J, Drewa T. Impact of fructose diet and renal failure on the function of pancreatic islets. Pancreas 43: 801–808, 2014. doi: 10.1097/MPA.0000000000000111. [DOI] [PubMed] [Google Scholar]
- 37.Raskin P, Unger RH. Hyperglucagonemia and its suppression. Importance in the metabolic control of diabetes. N Engl J Med 299: 433–436, 1978. doi: 10.1056/NEJM197808312990901. [DOI] [PubMed] [Google Scholar]
- 38.Roncal-Jimenez CA, Lanaspa MA, Rivard CJ, Nakagawa T, Sanchez-Lozada LG, Jalal D, Andres-Hernando A, Tanabe K, Madero M, Li N, Cicerchi C, Mc Fann K, Sautin YY, Johnson RJ. Sucrose induces fatty liver and pancreatic inflammation in male breeder rats independent of excess energy intake. Metabolism 60: 1259–1270, 2011. doi: 10.1016/j.metabol.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saben JL, Asghar Z, Rhee JS, Drury A, Scheaffer S, Moley KH. Excess Maternal Fructose Consumption Increases Fetal Loss and Impairs Endometrial Decidualization in Mice. Endocrinology 157: 956–968, 2016. doi: 10.1210/en.2015-1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Samuel VT, Beddow SA, Iwasaki T, Zhang XM, Chu X, Still CD, Gerhard GS, Shulman GI. Fasting hyperglycemia is not associated with increased expression of PEPCK or G6Pc in patients with Type 2 Diabetes. Proc Natl Acad Sci U S A 106: 12121–12126, 2009. doi: 10.1073/pnas.0812547106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sato Y, Ito T, Udaka N, Kanisawa M, Noguchi Y, Cushman SW, Satoh S. Immunohistochemical localization of facilitated-diffusion glucose transporters in rat pancreatic islets. Tissue Cell 28: 637–643, 1996. doi: 10.1016/S0040-8166(96)80067-X. [DOI] [PubMed] [Google Scholar]
- 42.Seino Y, Ogata H, Maekawa R, Izumoto T, Iida A, Harada N, Miki T, Seino S, Inagaki N, Tsunekawa S, Oiso Y, Hamada Y. Fructose induces glucose-dependent insulinotropic polypeptide, glucagon-like peptide-1 and insulin secretion: Role of adenosine triphosphate-sensitive K(+) channels. J Diabetes Investig 6: 522–526, 2015. doi: 10.1111/jdi.12356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spaeth JM, Hunter CS, Bonatakis L, Guo M, French CA, Slack I, Hara M, Fisher SE, Ferrer J, Morrisey EE, Stanger BZ, Stein R. The FOXP1, FOXP2 and FOXP4 transcription factors are required for islet alpha cell proliferation and function in mice. Diabetologia 58: 1836–1844, 2015. doi: 10.1007/s00125-015-3635-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steenberg VR, Jensen SM, Pedersen J, Madsen AN, Windeløv JA, Holst B, Quistorff B, Poulsen SS, Holst JJ. Acute disruption of glucagon secretion or action does not improve glucose tolerance in an insulin-deficient mouse model of diabetes. Diabetologia 59: 363–370, 2016. doi: 10.1007/s00125-015-3794-2. [DOI] [PubMed] [Google Scholar]
- 45.Stuart CA, Howell ME, Yin D. Overexpression of GLUT5 in diabetic muscle is reversed by pioglitazone. Diabetes Care 30: 925–931, 2007. doi: 10.2337/dc06-1788. [DOI] [PubMed] [Google Scholar]
- 46.Sussman KE, Vaughan GD, Timmer RF. An in vitro method for studying insulin secretion in the perfused isolated rat pancreas. Metabolism 15: 466–476, 1966. doi: 10.1016/0026-0495(66)90089-8. [DOI] [PubMed] [Google Scholar]
- 47.Tappy L, Lê KA. Metabolic effects of fructose and the worldwide increase in obesity. Physiol Rev 90: 23–46, 2010. doi: 10.1152/physrev.00019.2009. [DOI] [PubMed] [Google Scholar]
- 48.Teff KL, Elliott SS, Tschöp M, Kieffer TJ, Rader D, Heiman M, Townsend RR, Keim NL, D’Alessio D, Havel PJ. Dietary fructose reduces circulating insulin and leptin, attenuates postprandial suppression of ghrelin, and increases triglycerides in women. J Clin Endocrinol Metab 89: 2963–2972, 2004. doi: 10.1210/jc.2003-031855. [DOI] [PubMed] [Google Scholar]
- 49.Unger RH. Role of glucagon in the pathogenesis of diabetes: the status of the controversy. Metabolism 27: 1691–1709, 1978. doi: 10.1016/0026-0495(78)90291-3. [DOI] [PubMed] [Google Scholar]
- 50.Vasu S, Moffett RC, McClenaghan NH, Flatt PR. Differential molecular and cellular responses of GLP-1 secreting L-cells and pancreatic alpha cells to glucotoxicity and lipotoxicity. Exp Cell Res 336: 100–108, 2015. doi: 10.1016/j.yexcr.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 51.Wang H, Sun RQ, Zeng XY, Zhou X, Li S, Jo E, Molero JC, Ye JM. Restoration of autophagy alleviates hepatic ER stress and impaired insulin signalling transduction in high fructose-fed male mice. Endocrinology 156: 169–181, 2015. doi: 10.1210/en.2014-1454. [DOI] [PubMed] [Google Scholar]
- 52.Wang MY, Yan H, Shi Z, Evans MR, Yu X, Lee Y, Chen S, Williams A, Philippe J, Roth MG, Unger RH. Glucagon receptor antibody completely suppresses type 1 diabetes phenotype without insulin by disrupting a novel diabetogenic pathway. Proc Natl Acad Sci U S A 112: 2503–2508, 2015. doi: 10.1073/pnas.1424934112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yoon KH, Ko SH, Cho JH, Lee JM, Ahn YB, Song KH, Yoo SJ, Kang MI, Cha BY, Lee KW, Son HY, Kang SK, Kim HS, Lee IK, Bonner-Weir S. Selective beta-cell loss and alpha-cell expansion in patients with type 2 diabetes mellitus in Korea. J Clin Endocrinol Metab 88: 2300–2308, 2003. doi: 10.1210/jc.2002-020735. [DOI] [PubMed] [Google Scholar]