Abstract
Thyroid hormones (THs) regulate metabolism, but are typically suppressed during times of stressful physiological conditions, including fasting. Interestingly, prolonged fasting in northern elephant seal pups is associated with reliance on a lipid-based metabolism and increased levels of circulating THs that are partially attributed to active secretion as opposed to reduced clearance. This apparent paradox is coupled with complementary increases in cellular TH-mediated activity, suggesting that in mammals naturally adapted to prolonged fasting, THs are necessary to support metabolism. However, the functional relevance of this physiological paradox has remained largely unexplored, especially as it relates to the regulation of lipids. To address the hypothesis that TSH-mediated increase in THs contributes to lipid metabolism, we infused early and late-fasted pups with TSH and measured several key genes in adipose and muscle, and plasma hormones associated with regulation of lipid metabolism. TSH infusion increased the mRNA expressions of peroxisome proliferator-activated receptor gamma coactivator-1α (PGC-1α) more than 6.5-fold at 60 min in muscle, and expression of uncoupling protein 2 (UCP2) more than 27-fold during the early fast at 60 min, in adipose. Additionally, during the late fast period, the protein content of adipose CD36 increased 1.1-fold, and plasma nonesterified fatty acid (NEFA) concentrations increased 25% at 120 min, with NEFA levels returning to baseline after 24 h. We show that the TSH-induced increases in THs in fasting pups are functional and likely contribute to the maintenance of a lipid-based metabolism.
Keywords: fasting, lipids, metabolism, thyroid, uncoupling protein
thyroid hormones (THs) exert many physiological and metabolic effects (3, 40, 60). Physiologically, they regulate skeletal, cardiovascular, and nervous system homeostasis; metabolically, they stimulate cellular metabolism in most tissues (except the brain, spleen, and testicles) through acceleration of protein, carbohydrate, and lipid metabolism (both anabolic and catabolic pathways) (15, 37, 45–47, 60). Peroxisome proliferator-activated receptor gamma coactivator-1α (PGC-1α) is a master coregulator of both glucose and lipid metabolism and an important cofactor for the peroxisome proliferator-activated receptor α (PPARα). As it pertains to lipid metabolism, PGC-1α regulates the transcriptional activation of genes associated with fatty acid oxidation, directly increasing palmitate oxidation rates (51). Interestingly, given that PGC-1α expression is upregulated through genomic-mediated TH action, not only is PGC-1α a direct target of THs, but PGC-1α itself also coactivates liganded thyroid hormone receptor (62).
As a promoter of oxidative metabolism, upregulation of PGC-1α maintains the potential to upregulate the expression of genes that regulate the tricarboxylic acid cycle, β-oxidation of free fatty acids, and oxidative phosphorylation, thereby promoting oxygen consumption. These cellular responses are induced in times of physiological stress, coupled with energetic burdens such as fasting and exposure to cold (21, 61). To meet such demands, PGC-1α facilitates fatty acid transport through increases in the fatty acid transporter (CD36) (5). In terms of glucose metabolism, studies using PGC-1α knockout mice have suggested that PGC-1α is associated with glucose intolerance and insulin resistance (26). Although the specific role of PGC-1α remains unclear, it has been established that PGC-1α drives anabolic processes such as glucose refueling and whole body lactate homeostasis (48).
Mitochondrial uncoupling is another biochemical process that can mediate glucose homeostasis and lipid metabolism. Although mitochondrial uncoupling protein 2 (UCP2) has been suggested to regulate lipid metabolism, it may alter glucose homeostasis as well as insulin secretion through metabolite transport, which also implicates UCP2 in insulin resistance and glucose utilization (54).
Previous studies using northern elephant seal pups have identified some unique physiological responses to prolonged fasting that would accommodate their natural adaptations; however, the functional or evolutionary purpose of some of these perplexing responses remain elusive. For example, northern elephant seals exhibit tissue-specific insulin resistance, hypertriglyceridemia, paradoxical increases in circulating THs and upregulation of TH-associated cellular signaling (33, 34, 55–58). To address the hypothesis that TSH-mediated increases in TH regulates other endocrine systems and substrate metabolism, we compared the changes in several key genes in adipose and muscle associated with lipid metabolism, plasma hormones, and metabolites following an acute TSH infusion in pups that were fasted early and late. The aim of this study was to assess the contributions of TSH-induced increases in THs on cellular function and subsequent changes in substrate metabolism in a large mammal in which TH activity is increased despite prolonged fasting.
MATERIALS AND METHODS
All procedures were reviewed and approved by the Institutional Animal Care and Use Committee of both the University of California, Merced, and Sonoma State University. All research was conducted under National Marine Fisheries Service marine mammal permit 87–1743.
Animals.
Northern elephant seal (Mirounga angustirostris) pups were studied at the Año Nuevo State Reserve (30 km north of Santa Cruz, CA) during their natural postweaning fast while they are still on land. Ten pups were sampled during the early fasting period (1–2 wk after weaning) and 10 were sampled during the late fasting period (6–8 wk after weaning). The elephant seal pups were isolated on the beach during the procedures to protect them from the much larger adults. The pups were initially sedated with 1 mg/kg tiletamine/zolazepam HCl (Telazol; Fort Dodge Laboratories, Fort Dodge, IA) administered intramuscularly, and once immobilized, an 18-gauge, 3.5-inch spinal needle was inserted into the extradural spinal vein to facilitate infusion of saline (control) or TSH. Body mass was measured using a hanging-load cell suspended from a tripod following the procedures.
Intravenous TSH infusion.
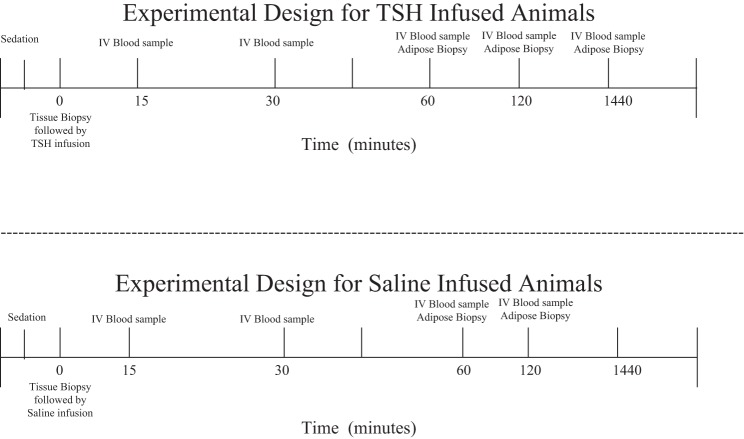
Before infusion (T0, baseline) blood samples were collected in chilled, EDTA-treated vacutainer sample tubes and kept on ice until they could be centrifuged. Preinfusion adipose and muscle biopsies were collected as previously described (33, 50). After the initial sample collection, animals were infused with sterile saline (n = 4) or bovine TSH (8 IU, n = 6; Sigma, St. Louis, MO) concurrently, for a total of 10 during the early fasting period, and similarly a total of 10 during the late fasting period. After the infusions of both saline and TSH for control and study groups, respectively, blood samples were collected at 15, 30, 60, 120, and 1,440 min after infusion (Fig. 1). The study assessed acute responses (up to 120 min) and prolonged responses (1,440 min, or 24 h after infusion). The study was terminated after 24 h, when the last sample was collected following infusion. Subsequent adipose and muscle biopsies were collected at 60, 120, and 1,440 min. Blood and tissue samples were prepared in the field as previously described (33, 50). Total protein content in nuclear, cytosolic, and membrane-bound fractions was measured by Bradford assay (Bio-Rad, Hercules, CA) to normalize loading of samples into gel wells (Bio-Rad).
Fig. 1.
Protocol diagram representing the sampling period for both TSH- and saline-treated animals. The pups were sampled during either the early fasting period (1–2 wk after weaning) or the late fasting period (6–8 wk after weaning).
Quantification of mRNA expressions.
Tissue samples were processed for quantification of mRNA expressions, and PCR reactions were performed as previously described (33, 34). Specific primers for PGC-1α and UCP2 were designed based on homologous mammalian nucleotide sequences, and partial sequences were confirmed. Expression of β-actin was used as an internal standard to normalize the expression of each target gene. Gene expression was measured by quantitative PCR using PGC-1αFw1 + PGC-1αRv1, UCP2Fw1 + UCP2Rv1, and β-actinFw1 + β-actinRv1 primers, respectively. Positive (with cDNA) and negative (no cDNA) controls were included in each assay. Statistical analyses were performed to confirm that β-actin expression did not change with fasting duration or in response to exogenous infusions, confirming its utility for normalizing as a reference gene.
Quantification of protein expression by Western blotting.
Protein expression was quantified by standard Western blot as previously described (33, 50). The primary antibodies for CD36 and β-actin (Santa Cruz Biotechnology, Santa Cruz, CA) were diluted 1:500 to 1:5,000. Blots were visualized using an Odyssey Clx Li-Cor Imager (Li-Cor Biosciences, Lincoln, NE). In addition to consistently loading the same amount of total protein (15 μg) per well, densitometry values were further normalized by the densitometry values of β-actin. Again, statistical analyses confirmed the appropriateness of using β-actin expression to normalize the CD36 values.
Plasma analyses.
Glucose and lactate were measured in duplicate on a YSI 2300 autoanalyzer. Insulin (SRI-13K; Linco Research, St. Charles, MO) and IGF-1 (22-IGFHU-E01; ALPCO Diagnostics, Salem, NH) were measured in duplicate as previously described (7, 42, 43, 55). Triglycerides were measured in duplicate on an Analox GM7 analyzer (Analox Instruments, London, UK). Nonesterified fatty acids (NEFAs) were also measured using a commercial kit (Wako Chemicals, Richmond, VA). All samples were analyzed in duplicate and run in a single assay with intra-assay percent coefficients of variability of <10% for all assays.
Statistical analysis.
Means (± SE) were compared using repeated-measures ANOVA to determine changes following infusions. Changes were considered significantly different at P < 0.05. Statistical analyses were performed using R software.
RESULTS
TSH-stimulated increase in THs is increased with fasting.
We have previously published these data demonstrating the TSH-induced increase in THs in these animals (32). In summary, in response to the same TSH stimuli during both early and late fasting duration, mean concentrations of total (t) and free (f) tT4, fT4, and tT3 increased (P < 0.05), with levels peaking after 120 min. Although concentrations returned to baseline after 24 h in the early fast period, in the late fast period mean circulating levels of tT4 and fT4 remained elevated after 24 h, demonstrating sustained elevation of tT4 with acute TSH infusion (32).
Fasting differentially altered TSH-induced changes in mRNA expression of PGC-1α and UCP2.
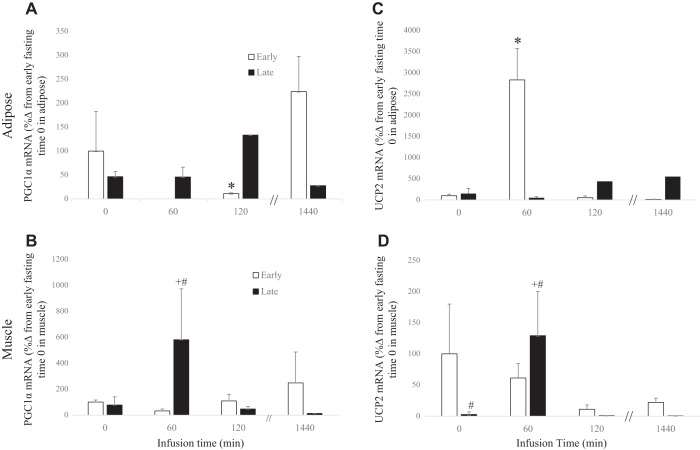
The mean mRNA expression of adipose PGC-1α decreased 90% (P < 0.05) during the early fast period at 120 min, with levels returning to baseline after 24 h, whereas during the late fast period there was no detectable change in expression after TSH infusion (Fig. 2A). The mean mRNA expression of muscle PGC-1α decreased 32% (P < 0.10) initially at 60 min, then increased 1.5-fold at 1,440 min during the early fast period. During the late fast period, mean expression levels increased approximately 6.5-fold (P < 0.05) at 60 min and returned to baseline at 120 min (Fig. 2B).
Fig. 2.
Messenger RNA expressions (means ± SE) of adipose peroxisome proliferator activated receptor gamma coactivator α (PGC-1α) (A), adipose uncoupling protein 2 (UCP2) (B), muscle peroxisome proliferator activated receptor gamma coactivator α (PGC-1α) (C), and muscle uncoupling protein 2 (UCP2) (D) from northern elephant seal pups before (0) and 60 and 120 min and 24 h after TSH infusion in early fasted (n = 6; 2–3 wk after weaning) and late fasted (n = 6; 6–8 wk after weaning) elephant seal pups. #Significant (P < 0.05) difference from 2 to 3 wk after weaning. *Significant (P < 0.05) difference from T0 within a group.
The mean mRNA expression of adipose UCP2 increased more than 27-fold (P < 0.05) in response to TSH during the early fast period at 60 min but returning to baseline after 120 min, whereas there was no detectable change during the late fast period (Fig. 2C). The mean mRNA expression of muscle UCP2 decreased to 11% (P < 0.10) at 120 min in the early fast period in response to TSH, but levels increased more than 50-fold (P < 0.05) at 60 min in the late fast period, returning to baseline after 120 min (Fig. 2D).
TSH-infusion increased adipose CD36 and plasma NEFA during late fasting.
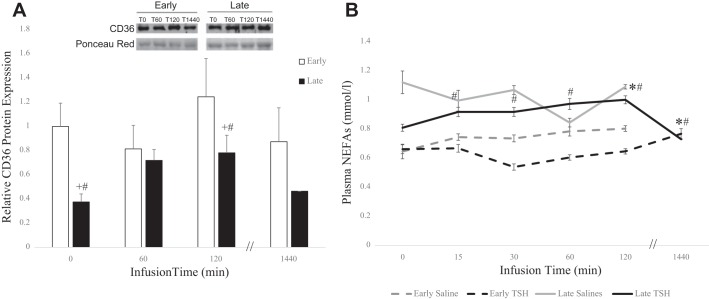
Neither the mean protein content of adipose CD36 nor plasma NEFA concentrations changed significantly in response to TSH in the early fast period (Fig. 3, A and B). However, during the late fast the mean protein content of adipose CD36 increased 1.1-fold (P < 0.05) and mean plasma NEFA concentrations increased 25% (P < 0.05) at 120 min, with NEFA levels returning to baseline after 24 h (Fig. 3, A and B).
Fig. 3.
Relative protein expressions (means ± SE) of fatty acid translocase/CD36 (CD36) (A) and plasma circulating levels of nonesterified fatty acids (NEFAs) (B) from northern elephant seal pups before (0) and 60 and 120 min and 24 h after TSH infusion in early fasted (n = 6; 2–3-wk after weaning) and late fasted (n = 6; 6–8 wk after weaning) elephant seal pups. #Significant (P < 0.05) difference from 2 to 3 wk after weaning. *Significant (P < 0.05) difference from T0 within a group.
TSH increased circulating glucose and insulin during late fasting.
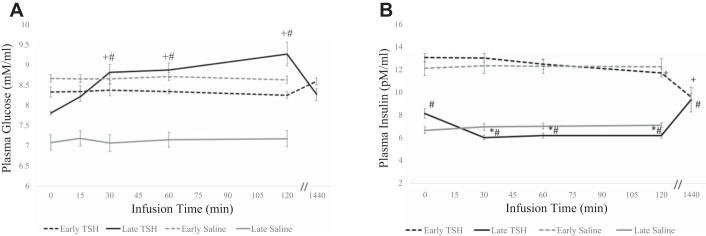
Although mean plasma glucose concentrations were not significantly altered in the early fast period in response to TSH, mean plasma glucose levels gradually increased 5% (P < 0.10), 12% (P < 0.05), and 12% (P < 0.05) at 15, 30, and 60 min, respectively, peaking at 18% at 120 min, and returning to baseline after 24 h in the late-fast period. In response to TSH, mean plasma insulin decreased 10% (P < 0.05) at 120 min, and 27% (P < 0.05) at 24 h in the early fast period. Conversely, in late fasting, mean plasma insulin decreased 26% (P < 0.05) at 30 min, and remained suppressed at 60 and 120 min, and increased 16% (P < 0.05) at 24 h (Fig. 4).
Fig. 4.
Plasma circulating levels (means ± SE) of glucose (A) and insulin (B) from northern elephant seal pups before (0) and 60 and 120 min and 24 h after TSH infusion in early fasted (n = 6; 2–3 wk after weaning) and late fasted (n = 6; 6–8 wk after weaning) elephant seal pups. #Significant (P < 0.05) difference from 2 to 3 wk after weaning. *Significant (P < 0.05) difference from T0 within a group.
Fasting differentially altered the TSH-associated responses in circulating metabolites.
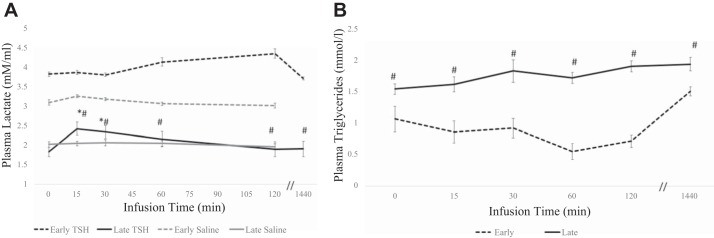
Mean plasma lactate concentrations increased 13% (P < 0.05) at 120 min during the early fast period in response to TSH, with levels returning to baseline at 24 h. However, during the late-fast period, mean circulating lactate levels increased 33% (P < 0.05) at 15 min in response to TSH, and gradually returned to baseline levels at 120 min (Fig. 5A). Mean plasma triglyceride levels did not change significantly in response to TSH in either early or late fasting (Fig. 5B).
Fig. 5.
Plasma circulating levels (means ± SE) of lactate (A) and triglycerides (B) from northern elephant seal pups before (0) and 60 and 120 min and 24 h after TSH infusion in early fasted (n = 6; 2–3 wk after weaning) and late fasted (n = 6; 6–8 wk after weaning) elephant seal pups. #Significant (P < 0.05) difference from 2 to 3 wk after weaning. *Significant (P < 0.05) difference from T0 within a group.
TSH decreases IGF-1.
To assess the capacity for cellular TH-mediated events to contribute to and regulate other endocrine systems such as the growth hormone/IGF-1 axis, we measured circulating levels of IGF-1 in response to TSH. In response to TSH, circulating levels of IGF-1 decreased 10% after 24 h in the early fast period (Fig. 6).
Fig. 6.
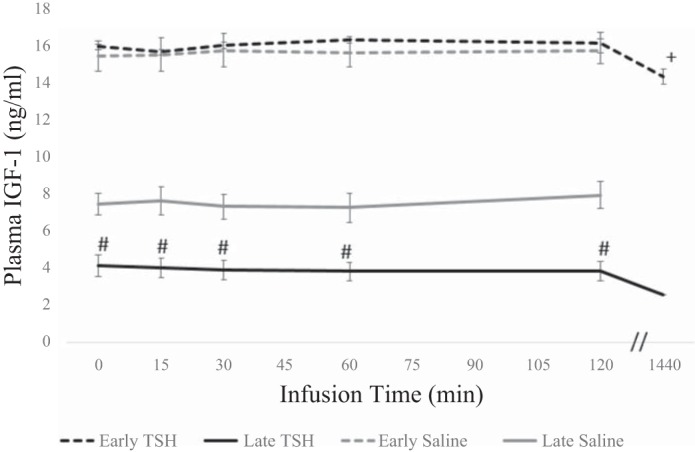
Plasma circulating levels (means ± SE) of IGF-1 from northern elephant seal pups before (0) and 60 and 120 min and 24 h after TSH infusion in early fasted (n = 6; 2–3 wk after weaning) and late fasted (n = 6; 6–8 wk after weaning) elephant seal pups. #Significant (P < 0.05) difference from 2 to 3 wk after weaning. *Significant (P < 0.05) difference from T0 within a group.
DISCUSSION
Recent studies have demonstrated that northern elephant seal pups undergo a physiologically perplexing fast, characterized by increases in THs as a direct result of markedly increased thyroidal production (32), which is coupled with fasting-induced adipose-specific insulin resistance (55–58) and other shifts in cellular metabolism (6, 23). Given the contributions of TH signaling to substrate metabolism, including lipid and glucose mobilization (4, 14, 16, 39, 45), this study investigated the potential of increased cellular TH-mediated events during prolonged fasting to drive tissue-specific metabolism. The central hypothesis is that these changes in cellular events with fasting duration elicit, or at the very least, enable life history transitions facilitating the survival of northern elephant seal pups during a period of adaptive fasting.
TSH-mediated effects on adipose.
Though several studies have related UCP2 function (which is regulated by TH levels) with lipid metabolism, more recently, its involvement in glucose and insulin regulation has been revealed (8, 11, 24, 31, 59). It seems no coincidence that the gene location of UCP2 lies in The obesity and Type 2 diabetes trait loci (18). Specifically, increases in UCP2 have been associated with impaired glucose-stimulated insulin secretion, leading to insulin resistance (2). This is further corroborated by other studies showing that inhibiting UCP2 expression reversed diet-induced diabetes and improved insulin signaling in adipose in both ob/ob and diet-induced obesity mouse models (12). Given that northern elephant seal pups develop adipose-specific insulin resistance (55–58), coupled with our most recent data, which demonstrated that plasma T3 levels were increased and metabolized as a function of increased thyroidal production and peripheral deiodination (32), respectively, UCP2 is likely contributing to adipose-specific insulin resistance in fasted pups.
The mechanism by which UCP2 regulates adipose-specific insulin resistance during prolonged fasting may be associated with adiponectin expression because adiponectin is markedly reduced in UCP2 knockout studies (9). Adiponectin is an abundant adipokine expressed in adipose and sensitizes tissues to insulin, with low levels corresponding with insulin resistance (25). In northern elephant seal pups, adiponectin is decreased with fasting duration, suggesting that it likely contributes to adipose-specific insulin resistance observed in late-fasted pups (57). Additionally, recent next-generation whole transcriptome analyses in elephant seals show that the adiponectin receptor is also decreased with fasting duration in adipose (Martinez B, Rutherford K, Crocker DE, Gemmell N, and Ortiz RM; unpublished data from our laboratory in collaboration with the Otago Medical University in New Zealand). Interestingly, UCP2 has been shown to mediate glucose signaling through the direct regulation of adiponectin gene expression and vice versa (9, 31, 63). In UCP2-null mice, both adipose and plasma adiponectin are reduced (9). In this study, we showed that the TSH-induced increase in T3 (and accompanied increase in TH-mediated signaling) was associated with a more than 27-fold increase in adipose UCP2 mRNA expression during early fasting when animals are not insulin resistant. However, during the late fast period, there are no detectable effects of TSH on UCP2, and it is during this time when northern elephant seal pups have been shown to exhibit insulin resistance. This would suggest that the fasting-associated desensitization of UCP2 expression to TSH is a deliberate mechanism to help maintain tissue-specific insulin resistance, which is also likely facilitated by suppression of plasma adiponectin, and adipose adiponectin and adiponectin receptor levels. This tightly controlled mechanism also seems to be mediated by suppression of PPARγ activation, which also decreased with fasting duration in northern elephant seal pups (57) rendering any increase in PGC-1α incapable of mediating UCP2 gene expression, at least through this mechanism.
Additionally, PGC-1α may contribute to lipid metabolism through fatty acid oxidation (FAO), oxidative phosphorylation, and lipid secretion (17), as well as regulating glucose metabolism (41). PGC-1α couples β-cell lipid metabolism to facilitate efficient insulin secretion. This is demonstrated in β-cell-specific PGC-1α knockout mice exhibiting decreased insulin secretion attributed to a lack in fatty acid potentiation (41). Additional studies corroborate that decreases in peripheral PGC-1α expression lead to insulin resistance and glucose intolerance (38). Adipose-specific PGC-1α knockout mice are more insulin resistant than wild-type mice, providing strong evidence that adipose PGC-1α regulates whole body glucose homeostasis (26). In this study, although we did not observe statistically different increases in mean PGC1-α in adipose, the increasing trend during the late fast period is suggestive of the potential for PGC-1α to serve as a cofactor that contributes to a shift from glucose to fatty acid oxidation which would spare glucose for the duration of the prolonged fast. Moreover, PGC1-α increases the mean protein expression of CD36 (10). Furthermore, T3 alone has the capacity to increase expression of CD36 and drive lipid metabolism (36), and in humans, TSH stimulates mRNA expression of CD36 (20). In this study, TSH increased CD36 protein expression at 120 min after infusion, possibly corroborating the trending increase in PGC1-α. Nonetheless, these data suggest that lipid mobilization is more sensitive to thyroidal stimulation in the late fast period. Given that CD36 shuttles NEFAs into the cell, increased CD36 should decrease plasma NEFA levels, a response that was observed during the late fast period at 24 h after infusion after a modest increase at 120 min. Although the contribution of PGC1-α to the TH-induced increase in CD36-mediated regulation of NEFA remains elusive, our data corroborate that of previous studies suggesting that the fasting-associated increase in TH-mediated cellular activity contributes to lipid metabolism via CD36.
TSH-mediated effects on muscle.
The effects of TSH infusion on muscle PGC1-α and UCP2 are intriguing because these data support the concept that UCP2 contributes to mediating the development of insulin resistance in adipose; however, its role in insulin-sensitive muscle is likely differential. This is likely the case for PGC1-α as well, because decreased PGC-1α is associated with insulin resistance and glucose intolerance (38). In this study, TSH increased PGC-1α expression in muscle (noninsulin resistant) at 60 min, but not adipose (insulin resistant). Interestingly, T3 directly increases the expression of PGC-1α, which in turn coactivated liganded thyroid hormone receptor, suggesting that an autoregulatory loop may exist in muscle that implicates PGC-1α (62). The potential for PGC-1α to serve as a coactivator in muscle is increased because both the peripheral receptor, THrβ1, and coreceptor, PGC-1α, are simultaneously upregulated in response to TSH in muscle, resulting in upregulation of the key TH target, UCP2 (32). These data highlight the dynamic, tissue-specific contributions of PGC-1α in regulation of UCP2, which likely translates to differential metabolism of lipid by tissues in fasted elephant seal pups.
Studies have shown that increases in NEFAs not only interfere with insulin-mediated glucose uptakes, especially uptake in muscle, but they also have the capacity to drive mitochondrial uncoupling events (53). Specifically, studies show that infusion of lipid increases NEFA concentrations, which inhibits insulin-mediated glucose uptake in muscle (53), suggesting that increased lipolysis contributes to the induction of insulin resistance. The increase in NEFA in response to TSH stimulation suggests that THs are capable of driving lipid metabolism, which is further corroborated by increases in protein levels of CD36, possibly mediated by PGC1-α. Moreover, glucose disposal in muscle seems plausible given the increases in UCP2 expression at 60 min, which potentially. may mediate glucose utilization through the insulin-sensitizing actions of adiponectin. However, the most intriguing aspect of these findings is that despite the TSH-mediated increase in NEFA and increased CD36 to facilitate lipid mobilization in the muscle, the muscle remains insulin sensitive during prolonged fasting, suggesting that the insulin-desensitizing ability of NEFA is mitigated with fasting duration in seal muscle.
Study limitations.
This study was performed to complement another in which we thoroughly examined the effects of fasting duration on thyroid gland secretion and TH-mediated cellular signaling using a TSH-infusion protocol described here. Thus the sharing of biopsies for both studies limited the availability of sample for each time point, which ultimately restricted our ability to statistically compare some time points to detect a change. For example, at 120 min after TSH infusion, although there appears to be a trend that agrees with our conclusions, sample size hindered our ability to detect significance. However, the ability to detect significant changes for a majority of our analyses with the given sample sizes underscores the value of these changes.
Perspectives and Significance
Northern elephant seals have evolved unique endocrine responses to facilitate the adaptation to life history-related events such as prolonged fasting. Whereas the development of fasting-induced insulin resistance is not entirely unusual, and often provides some survival advantage (13, 19, 34, 35, 44, 49, 52, 56–58), the functional relevance of increased TH production and subsequent cellular TH-mediated effects are far less common for a fasting mammal (1, 22, 27–30, 49). Given the confounding relationship between thyroidal derangements and insulin resistance, the 2- to 3-mo fasting period of the elephant seal offers an invaluable opportunity to assess the integration of multiple endocrine systems under naturally adapted conditions. How this relationship enables the maintenance of a primarily lipid-based metabolism can potentially have far reaching implications in the elucidation of the mechanisms that contribute to an array of metabolic disorders. The present study highlights the effects of prolonged fasting in a naturally adapted mammal on the dynamic alterations in TH-mediated cellular events that contribute to the maintenance of a lipid-based metabolism. The main relevance of this study is that it highlights a TH-mediated reliance on lipid metabolism that is not otherwise observed in human patients with diabetes. The phenotype of northern elephant seal pups make them an intriguing model for elucidating such mechanisms by which endocrine systems have evolved to exert cellular effects that protect tissues, and ergo, function, against the consequences of dyslipidemia and insulin resistance.
GRANTS
B. Martinez was supported by the Dennis R. Washington Graduate Achievement Scholarship. J. G. Soñanez-Organis was supported by a postdoctoral fellowship from the University of California Institute for Mexico and the United States, and Mexico’s National Council for Science and Technology (UC MEXUS-CONACYT). L. Horin was supported by the Integrative Organismal Systems Physiology (IOSP) Fellowship from the American Physiological Society. R. M. Ortiz was partially supported by National Heart, Lung, and Blood Institute Grant K02 HL-103787. Support for this study was also provided by National Heart, Lung, and Blood Institute Grant R01 HL-09176 and by Office of Naval Research Grant N000141110434.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.M., D.E.C., and R.M.O. conceived and designed research; B.M., J.G.S.-O., J.A.G.-L., L.H., and D.E.C. performed experiments; B.M., analyzed data; B.M. interpreted results of experiments; B.M. prepared figures; B.M. drafted manuscript; B.M., J.G.S.-O., J.A.G.-L., L.H., D.E.C., and R.M.O. edited and revised manuscript; B.M., J.G.S.-O., J.A.G.-L., L.H., D.E.C., and R.M.O. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank D. Somo, D. Ensminger, H. Peck, and D. Lee for their help in the field.
REFERENCES
- 1.Azizi F. Effect of dietary composition on fasting-induced changes in serum thyroid hormones and thyrotropin. Metabolism 27: 935–942, 1978. doi: 10.1016/0026-0495(78)90137-3. [DOI] [PubMed] [Google Scholar]
- 2.Barbe P, Larrouy D, Boulanger C, Chevillotte E, Viguerie N, Thalamas C, Oliva Trastoy M, Roques M, Vidal H, Langin D. Triiodothyronine-mediated up-regulation of UCP2 and UCP3 mRNA expression in human skeletal muscle without coordinated induction of mitochondrial respiratory chain genes. FASEB J 15: 13–15, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Bauer K. Adenohypophyseal degradation of thyrotropin releasing hormone regulated by thyroid hormones. Nature 330: 375–377, 1987. doi: 10.1038/330375a0. [DOI] [PubMed] [Google Scholar]
- 4.Boerner AR, Voth E, Theissen P, Wienhard K, Wagner R, Schicha H. Glucose metabolism of the thyroid in Graves’ disease measured by F-18-fluoro-deoxyglucose positron emission tomography. Thyroid 8: 765–772, 1998. doi: 10.1089/thy.1998.8.765. [DOI] [PubMed] [Google Scholar]
- 5.Bonen A. PGC-1α-induced improvements in skeletal muscle metabolism and insulin sensitivity. Appl Physiol Nutr Metab 34: 307–314, 2009. doi: 10.1139/H09-008. [DOI] [PubMed] [Google Scholar]
- 6.Champagne CD, Boaz SM, Fowler MA, Houser DS, Costa DP, Crocker DE. A profile of carbohydrate metabolites in the fasting northern elephant seal. Comp Biochem Physiol Part D Genomics Proteomics 8: 141–151, 2013. doi: 10.1016/j.cbd.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Champagne CD, Houser DS, Crocker DE. Glucose production and substrate cycle activity in a fasting adapted animal, the northern elephant seal. J Exp Biol 208: 859–868, 2005. doi: 10.1242/jeb.01476. [DOI] [PubMed] [Google Scholar]
- 8.Chan CB, Harper ME. Uncoupling proteins: role in insulin resistance and insulin insufficiency. Curr Diabetes Rev 2: 271–283, 2006. doi: 10.2174/157339906777950660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chevillotte E, Giralt M, Miroux B, Ricquier D, Villarroya F. Uncoupling protein-2 controls adiponectin gene expression in adipose tissue through the modulation of reactive oxygen species production. Diabetes 56: 1042–1050, 2007. doi: 10.2337/db06-1300. [DOI] [PubMed] [Google Scholar]
- 10.Choi CS, Befroy DE, Codella R, Kim S, Reznick RM, Hwang Y-J, Liu Z-X, Lee H-Y, Distefano A, Samuel VT, Zhang D, Cline GW, Handschin C, Lin J, Petersen KF, Spiegelman BM, Shulman GI. Paradoxical effects of increased expression of PGC-1α on muscle mitochondrial function and insulin-stimulated muscle glucose metabolism. Proc Natl Acad Sci USA 105: 19926–19931, 2008. doi: 10.1073/pnas.0810339105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D’Adamo M, Perego L, Cardellini M, Marini MA, Frontoni S, Andreozzi F, Sciacqua A, Lauro D, Sbraccia P, Federici M, Paganelli M, Pontiroli AE, Lauro R, Perticone F, Folli F, Sesti G. The -866A/A genotype in the promoter of the human uncoupling protein 2 gene is associated with insulin resistance and increased risk of type 2 diabetes. Diabetes 53: 1905–1910, 2004. doi: 10.2337/diabetes.53.7.1905. [DOI] [PubMed] [Google Scholar]
- 12.De Souza CT, Araújo EP, Stoppiglia LF, Pauli JR, Ropelle E, Rocco SA, Marin RM, Franchini KG, Carvalheira JB, Saad MJ, Boschero AC, Carneiro EM, Velloso LA. Inhibition of UCP2 expression reverses diet-induced diabetes mellitus by effects on both insulin secretion and action. FASEB J 21: 1153–1163, 2007. doi: 10.1096/fj.06-7148com. [DOI] [PubMed] [Google Scholar]
- 13.Delgiudice GD, Seal US, Mech LD. Effects of feeding and fasting on wolf blood and urine characteristics. J Wildl Manage 51: 1–10, 1987. doi: 10.2307/3801619. [DOI] [Google Scholar]
- 14.Dimitriadis GD, Raptis SA. Thyroid hormone excess and glucose intolerance. Exp Clin Endocrinol Diabetes 109, Suppl 2: S225–S239, 2001. doi: 10.1055/s-2001-18584. [DOI] [PubMed] [Google Scholar]
- 15.Duncan Bassett JH, Williams GR. The molecular actions of thyroid hormone in bone. Trends Endocrinol Metab 14: 356–364, 2003. doi: 10.1016/S1043-2760(03)00144-9. [DOI] [PubMed] [Google Scholar]
- 16.Duntas LH, Brenta G. The effect of thyroid disorders on lipid levels and metabolism. Med Clin North Am 96: 269–281, 2012. doi: 10.1016/j.mcna.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 17.Estall JL, Kahn M, Cooper MP, Fisher FM, Wu MK, Laznik D, Qu L, Cohen DE, Shulman GI, Spiegelman BM. Sensitivity of lipid metabolism and insulin signaling to genetic alterations in hepatic peroxisome proliferator-activated receptor-γ coactivator-1α expression. Diabetes 58: 1499–1508, 2009. doi: 10.2337/db08-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fleury C, Neverova M, Collins S, Raimbault S, Champigny O, Levi-Meyrueis C, Bouillaud F, Seldin MF, Surwit RS, Ricquier D, Warden CH. Uncoupling protein-2: a novel gene linked to obesity and hyperinsulinemia. Nat Genet 15: 269–272, 1997. doi: 10.1038/ng0397-269. [DOI] [PubMed] [Google Scholar]
- 19.Freitas MB, Queiroz JF, Dias Gomes CI, Collares-Buzato CB, Barbosa HC, Boschero AC, Gonçalves CA, Pinheiro EC. Reduced insulin secretion and glucose intolerance are involved in the fasting susceptibility of common vampire bats. Gen Comp Endocrinol 183: 1–6, 2013. doi: 10.1016/j.ygcen.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 20.Gagnon A, Lochnan HA, Tran CS, Sorisky A. Thyroid-stimulating hormone acutely increases monocyte gene expression in vivo. Neuro Endocrinol Lett 37: 121–123, 2016. [PubMed] [Google Scholar]
- 21.Handschin C, Spiegelman BM. Peroxisome proliferator-activated receptor γ coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev 27: 728–735, 2006. doi: 10.1210/er.2006-0037. [DOI] [PubMed] [Google Scholar]
- 22.Herlihy JT, Stacy C, Bertrand HA. Long-term food restriction depresses serum thyroid hormone concentrations in the rat. Mech Ageing Dev 53: 9–16, 1990. doi: 10.1016/0047-6374(90)90030-J. [DOI] [PubMed] [Google Scholar]
- 23.Houser DS, Crocker DE, Tift MS, Champagne CD. Glucose oxidation and nonoxidative glucose disposal during prolonged fasts of the northern elephant seal pup (Mirounga angustirostris). Am J Physiol Regul Integr Comp Physiol 303: R562–R570, 2012. doi: 10.1152/ajpregu.00101.2012. [DOI] [PubMed] [Google Scholar]
- 24.Huriyati E, Luglio HF, Ratrikaningtyas PD, Tsani AF, Sadewa AH, Juffrie M. Dyslipidemia, insulin resistance and dietary fat intake in obese and normal weight adolescents: the role of uncoupling protein 2 -866G/A gene polymorphism. Int J Mol Epidemiol Genet 7: 67–73, 2016. [PMC free article] [PubMed] [Google Scholar]
- 25.Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest 116: 1784–1792, 2006. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kleiner S, Mepani RJ, Laznik D, Ye L, Jurczak MJ, Jornayvaz FR, Estall JL, Chatterjee Bhowmick D, Shulman GI, Spiegelman BM. Development of insulin resistance in mice lacking PGC-1α in adipose tissues. Proc Natl Acad Sci USA 109: 9635–9640, 2012. doi: 10.1073/pnas.1207287109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kmiec Z, Kotlarz G, Smiechowska B, Mysliwski A. Thyroid hormones homeostasis in rats refed after short-term and prolonged fasting. J Endocrinol Invest 19: 304–311, 1996. doi: 10.1007/BF03347867. [DOI] [PubMed] [Google Scholar]
- 28.LoPresti JS, Gray D, Nicoloff JT. Influence of fasting and refeeding on 3,3′,5′-triiodothyronine metabolism in man. J Clin Endocrinol Metab 72: 130–136, 1991. doi: 10.1210/jcem-72-1-130. [DOI] [PubMed] [Google Scholar]
- 29.Magnus TH, Henderson NE. Thyroid hormone resistance in hibernating ground squirrels, Spermophilus richardsoni. I. Increased binding of triiodo-L-thyronine and L-thyroxine by serum proteins. Gen Comp Endocrinol 69: 352–360, 1988. doi: 10.1016/0016-6480(88)90025-1. [DOI] [PubMed] [Google Scholar]
- 30.Magnus TH, Henderson NE. Thyroid hormone resistance in hibernating ground squirrels, Spermophilus richardsoni. II. Reduction of hepatic nuclear receptors. Gen Comp Endocrinol 69: 361–371, 1988. doi: 10.1016/0016-6480(88)90026-3. [DOI] [PubMed] [Google Scholar]
- 31.Mahadik SR, Lele RD, Saranath D, Seth A, Parikh V. Uncoupling protein-2 (UCP2) gene expression in subcutaneous and omental adipose tissue of Asian Indians: relationship to adiponectin and parameters of metabolic syndrome. Adipocyte 1: 101–107, 2012. doi: 10.4161/adip.19671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martinez B, Soñanez-Organis J, MacKenzie D, Crocker D, Ortiz R. Thyroid gland remains responsive to thyroid stimulating hormone with fasting duration. FASEB J 29 Suppl: 686.6, 2015. [Google Scholar]
- 33.Martinez B, Soñanez-Organis JG, Vázquez-Medina JP, Viscarra JA, MacKenzie DS, Crocker DE, Ortiz RM. Prolonged food deprivation increases mRNA expression of deiodinase 1 and 2, and thyroid hormone receptor β-1 in a fasting-adapted mammal. J Exp Biol 216: 4647–4654, 2013. doi: 10.1242/jeb.085290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martinez B, Soñanez-Organis JG, Viscarra JA, Jaques JT, MacKenzie DS, Crocker DE, Ortiz RM. Glucose delays the insulin-induced increase in thyroid hormone-mediated signaling in adipose of prolong-fasted elephant seal pups. Am J Physiol Regul Integr Comp Physiol 310: R502–R512, 2016. doi: 10.1152/ajpregu.00054.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCain S, Ramsay E, Kirk C. The effects of hibernation and captivity on glucose metabolism and thyroid hormones in American black bear (Ursus americanus). J Zoo Wildl Med 44: 324–332, 2013. doi: 10.1638/2012-0146R1.1. [DOI] [PubMed] [Google Scholar]
- 36.Miklosz A, Chabowski A, Zendzian-Piotrowska M, Gorski J. Effects of hyperthyroidism on lipid content and composition in oxidative and glycolytic muscles in rats. J Physiol Pharmacol 63: 403–410, 2012. [PubMed] [Google Scholar]
- 37.Mittag J, Davis B, Vujovic M, Arner A, Vennström B. Adaptations of the autonomous nervous system controlling heart rate are impaired by a mutant thyroid hormone receptor-α1. Endocrinology 151: 2388–2395, 2010. doi: 10.1210/en.2009-1201. [DOI] [PubMed] [Google Scholar]
- 38.Mootha VK, Lindgren CM, Eriksson K-F, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstråle M, Laurila E, Houstis N, Daly MJ, Patterson N, Mesirov JP, Golub TR, Tamayo P, Spiegelman B, Lander ES, Hirschhorn JN, Altshuler D, Groop LC. PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 34: 267–273, 2003. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 39.Obregon M-J. Adipose tissues and thyroid hormones. Front Physiol 5: 479, 2014. doi: 10.3389/fphys.2014.00479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oppenheimer JH, Schwartz HL, Lane JT, Thompson MP. Functional relationship of thyroid hormone-induced lipogenesis, lipolysis, and thermogenesis in the rat. J Clin Invest 87: 125–132, 1991. doi: 10.1172/JCI114961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oropeza D, Jouvet N, Bouyakdan K, Perron G, Ringuette L-J, Philipson LH, Kiss RS, Poitout V, Alquier T, Estall JL. PGC-1 coactivators in β-cells regulate lipid metabolism and are essential for insulin secretion coupled to fatty acids. Mol Metab 4: 811–822, 2015. doi: 10.1016/j.molmet.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ortiz RM, Noren DP, Ortiz CL, Talamantes F. GH and ghrelin increase with fasting in a naturally adapted species, the northern elephant seal (Mirounga angustirostris). J Endocrinol 178: 533–539, 2003. doi: 10.1677/joe.0.1780533. [DOI] [PubMed] [Google Scholar]
- 43.Ortiz RM, Wade CE, Ortiz CL. Effects of prolonged fasting on plasma cortisol and TH in postweaned northern elephant seal pups. Am J Physiol Regul Integr Comp Physiol 280: R790–R795, 2001. [DOI] [PubMed] [Google Scholar]
- 44.Palumbo PJ, Wellik DL, Bagley NA, Nelson RA. Insulin and glucagon responses in the hibernating bear. Int Conf Bear Res Manage 5: 291–296, 1983. [Google Scholar]
- 45.Pucci E, Chiovato L, Pinchera A. Thyroid and lipid metabolism. Int J Obes Relat Metab Disord 24, Suppl 2: S109–S112, 2000. doi: 10.1038/sj.ijo.0801292. [DOI] [PubMed] [Google Scholar]
- 46.Romero R, Casanova B, Pulido N, Suarez AI, Rodriguez E, Rovira A. Stimulation of glucose transport by thyroid hormone in 3T3-L1 adipocytes: increased abundance of GLUT1 and GLUT4 glucose transporter proteins. J Endocrinol 164: 187–195, 2000. doi: 10.1677/joe.0.1640187. [DOI] [PubMed] [Google Scholar]
- 47.Samuels HH, Tsai JS. Thyroid hormone action in cell culture: demonstration of nuclear receptors in intact cells and isolated nuclei. Proc Natl Acad Sci USA 70: 3488–3492, 1973. doi: 10.1073/pnas.70.12.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Summermatter S, Santos G, Pérez-Schindler J, Handschin C. Skeletal muscle PGC-1α controls whole-body lactate homeostasis through estrogen-related receptor α-dependent activation of LDH B and repression of LDH A. Proc Natl Acad Sci USA 110: 8738–8743, 2013. doi: 10.1073/pnas.1212976110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tomasi TE, Hellgren EC, Tucker TJ. Thyroid hormone concentrations in black bears (Ursus americanus): hibernation and pregnancy effects. Gen Comp Endocrinol 109: 192–199, 1998. doi: 10.1006/gcen.1997.7018. [DOI] [PubMed] [Google Scholar]
- 50.Vázquez-Medina JP, Crocker DE, Forman HJ, Ortiz RM. Prolonged fasting does not increase oxidative damage or inflammation in postweaned northern elephant seal pups. J Exp Biol 213: 2524–2530, 2010. doi: 10.1242/jeb.041335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vega RB, Huss JM, Kelly DP. The coactivator PGC-1 cooperates with peroxisome proliferator-activated receptor α in transcriptional control of nuclear genes encoding mitochondrial fatty acid oxidation enzymes. Mol Cell Biol 20: 1868–1876, 2000. doi: 10.1128/MCB.20.5.1868-1876.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Verrier D, Atkinson S, Guinet C, Groscolas R, Arnould JP. Hormonal responses to extreme fasting in subantarctic fur seal (Arctocephalus tropicalis) pups. Am J Physiol Regul Integr Comp Physiol 302: R929–R940, 2012. doi: 10.1152/ajpregu.00370.2011. [DOI] [PubMed] [Google Scholar]
- 53.Vettor R, Fabris R, Serra R, Lombardi AM, Tonello C, Granzotto M, Marzolo MO, Carruba MO, Ricquier D, Federspil G, Nisoli E. Changes in FAT/CD36, UCP2, UCP3 and GLUT4 gene expression during lipid infusion in rat skeletal and heart muscle. Int J Obes Relat Metab Disord 26: 838–847, 2002. [DOI] [PubMed] [Google Scholar]
- 54.Vimaleswaran KS, Radha V, Ghosh S, Majumder PP, Sathyanarayana Rao MR, Mohan V. Uncoupling protein 2 and 3 gene polymorphisms and their association with type 2 diabetes in Asian Indians. Diabetes Technol Ther 13: 19–25, 2011. doi: 10.1089/dia.2010.0091. [DOI] [PubMed] [Google Scholar]
- 55.Viscarra JA, Champagne CD, Crocker DE, Ortiz RM. 5'AMP-activated protein kinase activity is increased in adipose tissue of northern elephant seal pups during prolonged fasting-induced insulin resistance. J Endocrinol 209: 317–325, 2011. doi: 10.1530/JOE-11-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Viscarra JA, Rodriguez R, Vazquez-Medina JP, Lee A, Tift MS, Tavoni SK, Crocker DE, Ortiz RM. Insulin and GLP-1 infusions demonstrate the onset of adipose-specific insulin resistance in a large fasting mammal: potential glucogenic role for GLP-1. Physiol Rep 1: e00023, 2013. doi: 10.1002/phy2.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Viscarra JA, Vázquez-Medina JP, Crocker DE, Ortiz RM. Glut4 is upregulated despite decreased insulin signaling during prolonged fasting in northern elephant seal pups. Am J Physiol Regul Integr Comp Physiol 300: R150–R154, 2011. doi: 10.1152/ajpregu.00478.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Viscarra JA, Vázquez-Medina JP, Rodriguez R, Champagne CD, Adams SH, Crocker DE, Ortiz RM. Decreased expression of adipose CD36 and FATP1 are associated with increased plasma non-esterified fatty acids during prolonged fasting in northern elephant seal pups (Mirounga angustirostris). J Exp Biol 215: 2455–2464, 2012. doi: 10.1242/jeb.069070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang H, Chu WS, Lu T, Hasstedt SJ, Kern PA, Elbein SC. Uncoupling protein-2 polymorphisms in type 2 diabetes, obesity, and insulin secretion. Am J Physiol Endocrinol Metab 286: E1–E7, 2004. doi: 10.1152/ajpendo.00231.2003. [DOI] [PubMed] [Google Scholar]
- 60.Wrutniak-Cabello C, Casas F, Cabello G. Thyroid hormone action in mitochondria. J Mol Endocrinol 26: 67–77, 2001. doi: 10.1677/jme.0.0260067. [DOI] [PubMed] [Google Scholar]
- 61.Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98: 115–124, 1999. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 62.Wulf A, Harneit A, Kröger M, Kebenko M, Wetzel MG, Weitzel JM. T3-mediated expression of PGC-1α via a far upstream located thyroid hormone response element. Mol Cell Endocrinol 287: 90–95, 2008. doi: 10.1016/j.mce.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 63.Zhou M, Xu A, Tam PK, Lam KS, Huang B, Liang Y, Lee IK, Wu D, Wang Y. Upregulation of UCP2 by adiponectin: the involvement of mitochondrial superoxide and hnRNP K. PLoS One 7: e32349, 2012. doi: 10.1371/journal.pone.0032349. [DOI] [PMC free article] [PubMed] [Google Scholar]