Abstract
Respiration varies from breath to breath. On the millisecond timescale of spiking, neuronal circuits exhibit variability due to the stochastic properties of ion channels and synapses. Does this fast, microscopic source of variability contribute to the slower, macroscopic variability of the respiratory period? To address this question, we modeled a stochastic oscillator with forcing; then, we tested its predictions experimentally for the respiratory rhythm generated by the in situ perfused preparation during vagal nerve stimulation (VNS). Our simulations identified a relationship among the gain of the input, entrainment strength, and rhythm variability. Specifically, at high gain, the periodic input entrained the oscillator and reduced variability, whereas at low gain, the noise interacted with the input, causing events known as “phase slips”, which increased variability on a slow timescale. Experimentally, the in situ preparation behaved like the low-gain model: VNS entrained respiration but exhibited phase slips that increased rhythm variability. Next, we used bilateral muscimol microinjections in discrete respiratory compartments to identify areas involved in VNS gain control. Suppression of activity in the nucleus tractus solitarii occluded both entrainment and amplification of rhythm variability by VNS, confirming that these effects were due to the activation of the Hering-Breuer reflex. Suppressing activity of the Kölliker-Fuse nuclei (KFn) enhanced entrainment and reduced rhythm variability during VNS, consistent with the predictions of the high-gain model. Together, the model and experiments suggest that the KFn regulates respiratory rhythm variability via a gain control mechanism.
Keywords: stochastic nonlinear oscillator, Hering-Breuer reflex, vagal nerve stimulation, in situ preparation, respiratory rhythmogenesis
variability is a fundamental property of biologic rhythms (54, 82). Since 1963, when Hon and Lee (52) published their article describing a loss of heart rate variability (HRV) associated with poor prognosis, interest has developed in HRV (58, 62, 107a) and, more recently, respiratory rhythm variability as biomarkers for defining pathophysiological states in patients (6, 11, 15, 16, 21, 79, 108, 109, 114). Traditionally, we, as physiologists, have investigated causes of variability at the macroscopic level, in the context of reflexes and behaviors. In these contexts, we work to understand the circuit mechanisms affecting the variability of the rhythm (as well as the patterning of motor activity). In the case of reflexes, we are confident that these changes will return the organism to homeostasis, whereas in behaviors, variability is a component of the behavior. However, our physiological approach has not yet integrated the biophysics of microscopic mechanisms of variability, such as the stochastic properties of ion channel gating and synaptic neurotransmission (40, 44, 86, 95, 96, 117), which is often considered inconsequential, given its low-amplitude and high-frequency compared with the respiratory rhythm. However, these mechanisms may play a key role in breathing variability by providing a background of physiological noise. Here, we ask whether microscopic mechanisms of variability are evident in the variability of the respiratory rhythm at the macroscopic level by examining a model of a noisy oscillator, subject to periodic forcing, and testing its predictions experimentally in the in situ, perfused preparation during forcing by rhythmic vagal nerve stimulation (VNS).
Mechanistically, respiratory rhythm variability at the macroscopic level is partially determined by pulmonary stretch receptor inputs to the rhythm-generating network. Elimination of these inputs via vagotomy reduces respiratory rhythm variability. Specifically, information theory-based measures quantifying nonlinear variability showed decreased nonlinear variability in the respiratory rhythm after removal of pulmonary stretch receptor inputs by vagotomy (25, 93).
During both spontaneous breathing and mechanical ventilation, activation of pulmonary stretch receptors by lung inflation evokes the Hering-Breuer reflex (HBR), which consists of inhibition of inspiratory motor activity, transition from inspiration to expiration, and prolongation of expiration (23, 60, 65). Additionally, phasic stimulation of the HBR circuit increases the frequency of respiration (47, 93). The fundamental neural substrate of this reflex is well known. Briefly, pulmonary stretch receptors are neural afferent endings in the tracheobronchial smooth muscle layer (94). Their axons travel in the vagal nerves and terminate in the nucleus tractus solitarius (nTS) on pump (P) cells (14). P cells project to neurons in the pontine Kölliker-Fuse nucleus (KFn) and in the ventrolateral medullary Bötzinger and Pre-Bötzinger complexes of the respiratory network. Additionally, each of these distinct regions are connected reciprocally (37). In summary, the HBR is normally a peripheral feedback loop that depends on the activity of pulmonary stretch receptors (PSRs), the nTS, KFn, and ventrolateral medulla (48, 68, 119).
Rhythmic activation of the HBR circuit can entrain the respiratory rhythm, which occurs commonly during mechanical ventilation; e.g., periodic lung inflations entrain respiration in several species, including humans (45, 76, 81, 98, 99). Not surprisingly, activation of the HBR circuit via rhythmic VNS also entrains the respiratory rhythm in the in situ arterially perfused brain stem preparation (26, 33). Entrainment of an oscillator in the absence of noise depends on the magnitude of the input and the difference in the frequencies of the oscillator forcing the input. These parameters determine whether phase locking of an oscillator to a periodic input occurs at a 1:1 ratio or at higher harmonic ratios. Previous experimental studies have demonstrated the existence of various phase-locking ratios during mechanical ventilation (4, 5, 12, 81, 99). Further, in theoretical studies of entrainment, Glass and Mackey and their colleagues (42, 43, 67, 80) demonstrated that both a simple van der Pol oscillator and a variant of the classic inhibitory-ring model of the respiratory central pattern generator can entrain to rhythmic PSR inputs over several phase-locking ratios, depending on the stimulus parameters.
In the present study, we focus on the dynamics within the 1:1 entrainment regime. We anticipated that during 1:1 entrainment between breathing and mechanical ventilation that variability of the breathing pattern would decrease because the variability of the vagal input to which respiration is entrained is negligible. However, breathing variability increased, rather than decreased when we entrained respiration 1:1 with periodic VNS. Previous theoretical investigations of the periodic forcing of a noisy van der Pol oscillator has shown that noise interacts with the forcing input to transiently desynchronize the oscillation from the periodic input to result in phase slips that occur at a defined rate (91). Therefore, we have used a noisy Stuart-Landau oscillator (SLO) (52) with periodic forcing to illustrate the relationship between an oscillator’s input gain and its rhythm variability. Physiologically, the oscillator terms of the model represent the collective mechanisms underlying respiratory rhythmicity; the noise term represents the influence of ion-channel and/or synaptic noise on the rhythm; and the periodic forcing term represents the Hering-Breuer inputs that occur during mechanical ventilation or periodic VNS. The model predicts that at low gain, the oscillator entrains to the input, but rhythm variability is enhanced by phase slips, whereas at high gain, the oscillator is strongly entrained to the input, and rhythm variability is reduced. We next test these predictions experimentally by recording respiratory motor output in situ during rhythmic VNS. We find that the intact respiratory network is consistent with the low-gain model. Next, we use pharmacologic suppression of neuronal activity in distinct respiratory compartments to test how activity in these compartments relates to the parameters of the model. Importantly, we find that suppression of neuronal activity in the KFn transforms the HBR circuit from the low- to high-gain state, wherein rhythmic VNS entrains the respiratory rhythm without phase slips and, thereby, reduces overall respiratory rhythm variability. We conclude that the KFn regulates the variability of the respiratory rhythm during HBR forcing via a gain control mechanism.
MATERIALS AND METHODS
Forced stochastic Stuart-Landau oscillator simulations.
To illustrate the dynamical mechanism underlying the amplification of respiratory oscillation variability by periodic HBR forcing, we conducted numerical simulations of a generic nonlinear oscillator, the Stuart-Landau oscillator (SLO) (61). To mimic our experimental approach, the SLO model was modified to include periodic forcing. Endogenous variability due to channel noise and stochastic synaptic release was modeled as Gaussian white noise, which includes random fluctuations at faster timescales (milliseconds) than that of respiration (seconds). On the complex plane, the SLO oscillator is described by the following dynamical equation:
| (1) |
where a, b, c, and d are real parameters of the oscillator, F(t) is the periodic forcing, η(t) is a Gaussian white noise process with zero mean and unitary variance, and α and σ are scaling parameters for these two time-dependent inputs, respectively.
Expressing the model in polar coordinates reveals the parameter controlling the gain of the oscillator to the noise and forcing terms. In polar coordinates, z is replaced with reiφ in (Eq. 1), and after separating the real and imaginary parts, one obtains
| (2) |
Note that the steady states of the deterministic part of the equation in r satisfy ar − cr3 = 0, whose solutions are r = 0 and . The former is unstable, and the latter is stable, which implies that if the noise level σ is not very large, the amplitude of the oscillation will be roughly constant with amplitude r(t) ≈ R. This allows us to rewrite Eq. 2 as
| (3) |
with ω0 = b − R2d, G = 1/R, and Z(φ) = −sin φ. Note that determines the influence of the forcing (and the noise) on the phase and can be thought of as the gain of the oscillator’s input. Our experiments below show that G models the level of activity in the Kölliker-Fuse nuclei. Equation 3 is formally equivalent to the Kuramoto model of a generic nonlinear, phase oscillator, in which Z(φ) is the phase-dependent sensitivity (61), or phase-response curve. To test whether the gain of the oscillator’s input could account for the changes in variability that we observed experimentally (Fig. 3), we conducted numerical simulations of the SLO model with different G, keeping all other parameters constant. To mimic our experimental conditions, we modeled the forcing F(t) as a series of periodic pulses with a frequency close to the natural frequency of the oscillation, ωin ≈ ω0. The parameters used in all simulations are presented in Table 1 in arbitrary units.
Fig. 3.
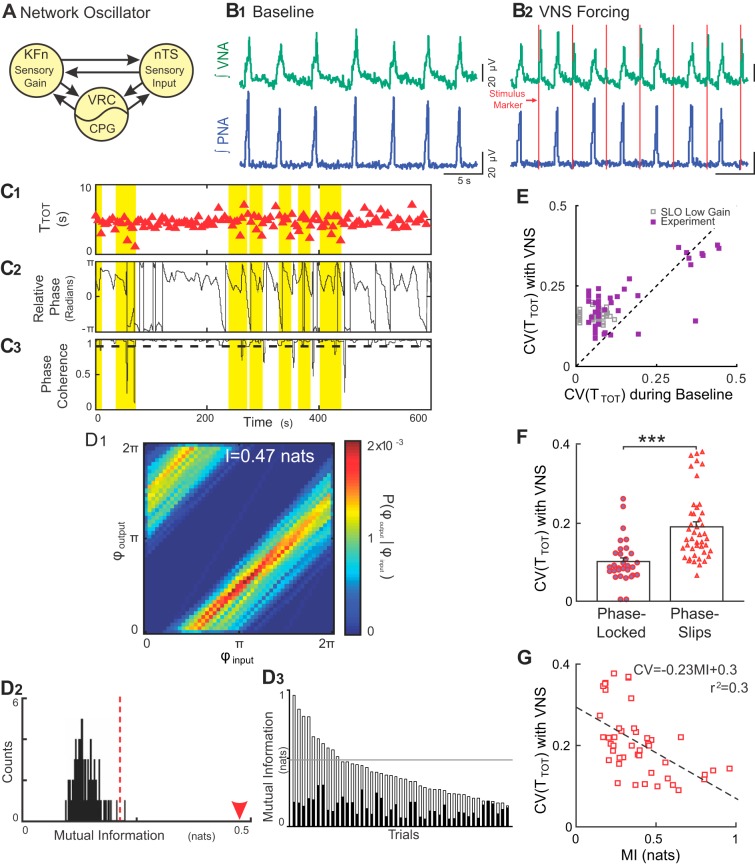
Rhythmic VNS evokes stable input-output entrainment and increases respiratory rhythm variability in situ. A: schematic model of the Hering-Breuer reflex circuit is shown. The nucleus tractus solitarii (nTS) relays vagal sensory input to the lateral respiratory column, specifically, the ventral respiratory column (VRC) including the central pattern generator (CPG) and Kölliker-Fuse nuclei (KFn). These three regions are connected reciprocally and can be thought of collectively as a single oscillator when analyzing phrenic motor output. B: representative traces of PNA and VNA are shown during baseline (B1) and VNS (B2). B1: at baseline, the in situ arterially perfused preparation generated an in vivo-like pattern of activities, wherein VNA peaked after PNA. B2: during VNS (red lines), each train of VNS evoked bursts of efferent VNA. C: representative plots of the respiratory period (C1, TTOT), instantaneous relative phase (C2, ϕoutput-ϕinput) and phase coherence during VNS (C3) are shown. Flat regions in the instantaneous relative phase (C2) indicate periods of perfect phase locking. The phase coherence (C3) measures the mean direction of the relative phase time series in a 20-s sliding window. A phase slip event was defined as a window with a phase coherence less than 0.9 (dashed line in C3) and are highlighted across all three plots. Importantly, the variability in the respiratory period increased selectively during phase slip events. D: joint probability histogram (D1) of the instantaneous phase of the output (PNA; ϕoutput) given the instantaneous phase of the input (VNS stimulus; ϕinput) had strong banding, indicative of stable input-output entrainment. The mutual information for this epoch was 0.47 nats (arrowhead in D2). To determine the significance of the entrainment interaction, the data were bootstrapped by shuffling the interbreath intervals (n = 100) and recomputing the mutual information of the instantaneous phases (D2). The observed value (arrowhead) was greater than the 99% confidence interval of the bootstrapped distribution (dashed line), indicating that the observed input-output entrainment was significant. The mutual information of the instantaneous phases (open bars) and their corresponding 99% confidence intervals (solid bars) was significant for 44 of 45 trials from eight experiments (D3). The distribution of the mutual information of the instantaneous phases derived from simulations of the low-gain SLO model (gray lines) fell slightly above the median of the experimental data set. E: VNS increased the coefficient of variation (CV) of the instantaneous period (purple squares) (Wilcoxon signed-rank test; P = 2.14 × 10–5). For qualitative comparison, we have also plotted the distribution of CVs from the simulations of the low-gain SLO model (gray squares), which overlap with the experimental distribution. F: for the group of experiential preparations, the CV during phase-locked windows was less than the CV during phase-slip windows, suggesting that the source of the increase in variability during VNS was the phase slip events (***P < 0.001). G: accordingly, the strength of entrainment during rhythmic VNS (as quantified by mutual information) was inversely correlated with the magnitude of respiratory rhythm variability. Together, these results are consistent with the predictions of the low-gain, forced, stochastic SLO model, suggesting that the intact respiratory rhythm-generating network similarly maintains a low-input gain for vagal afferent input.
Table 1.
Parameters used in stochastic SLO simulations
| Low-Gain State | High-Gain State | |
|---|---|---|
| a | 22.86 | 11.43 |
| b | 1.43 | 1.43 |
| c | 11.43 | 11.43 |
| d | 0 | 0 |
| σ | 2.86 | 2.86 |
| α (with forcing) | 11.43 | 11.43 |
| G | 0.71 | 1 |
The parameters used in low-gain and high-gain state SLO simulations (Eq. 3) are presented in arbitrary units.
Experimental protocol.
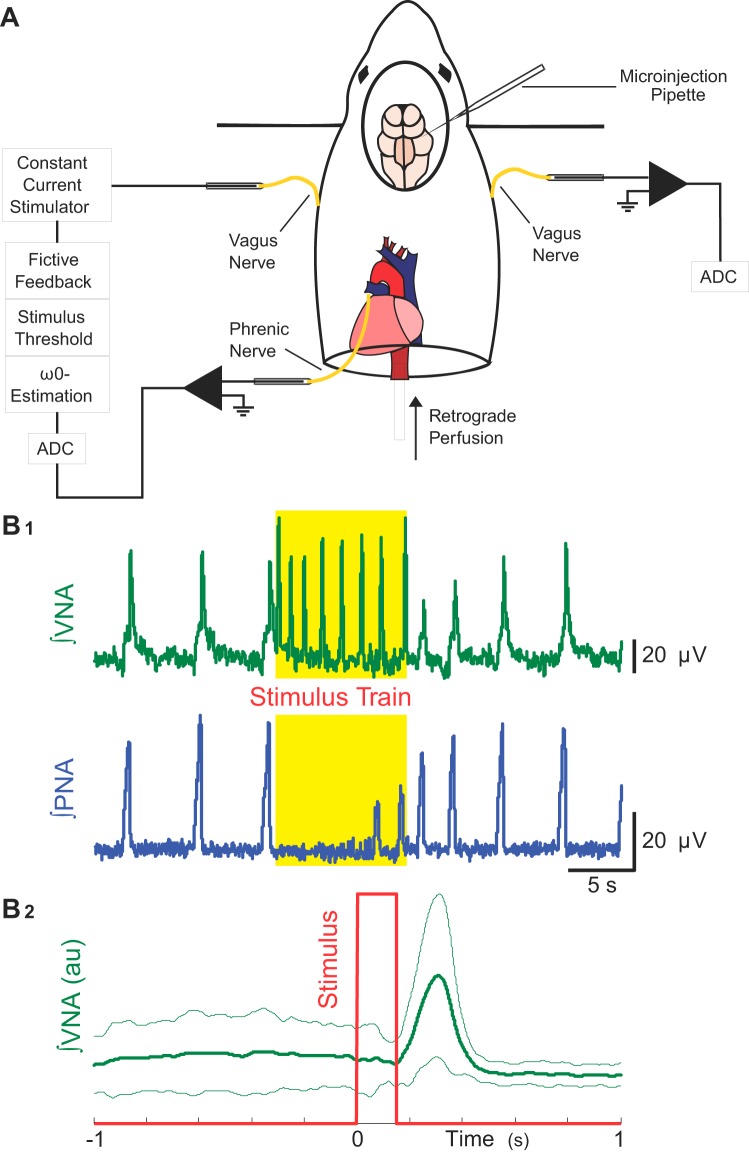
Experimental protocols were approved by the Case Western Reserve University Institutional Animal Care and Use Committee. Experiments were performed in juvenile (17–24 days postnatal age, n = 15), male Sprague-Dawley rats using the in situ arterially perfused brain stem preparation. Briefly, rats were deeply anesthetized with isoflurane (1.5–2%, Piramal Healthcare, Andra Pradesh, India). After the rats reached a surgical plane of anesthesia, the rats were transected below the diaphragm and transferred to an ice-cold bath of artificial cerebrospinal fluid (aCSF) for precollicular decerebration and cerebellectomy. After removal of the lungs, the descending aorta, the phrenic and both vagal nerves were dissected for cannulation, recording, or electrical stimulation. The preparation was then transferred to a recording chamber. After cannulation of the descending aorta with a double lumen catheter (no. 4 French, Braintree Scientific, Braintree, MA), the preparation was perfused with aCSF (in mM: 125 NaCl, 3 KCl, 1.25 KH2PO4, 2.5 CaCl2, 1.25 MgSO4, 25 NaHCO3, 10 d-glucose, 1.25% Ficoll) warmed to 31°C using a peristaltic pump (Watson & Marlow 505S, Cornwall, UK), circulating water bath and heat exchanger (Thermo Fisher Scientific SC150, Waltham, MA). The perfusate was continually bubbled with 95% O2-5% CO2 to maintain constant chemosensory drive. After the resumption of respiratory movements, the preparation was paralyzed with vecuronium bromide (1 mg/250 ml perfusate). If the respiratory rhythm was apneustic after achieving adequate perfusion, the respiratory pattern of the preparation was tuned to eupnea-like activity with a single bolus of NaCN (0.1 ml, 0.03% wt/vol).
Phrenic (PNA) and contralateral vagal nerve (VNA) activities were recorded via suction electrodes to assess respiratory network output. The resultant potential was amplified (20,000 times, P511; Grass Instruments, West Warwick, RI), filtered (3 Hz to 3 kHz), digitized [fS = 10 kHz, Power1401, Cambridge Electronic Design (CED), Cambridge, UK], and stored on a computer using Spike2 software (CED, Cambridge, UK).
Vagal nerve stimulation.
To test the predictions of the model, we delivered rhythmic bursts of electrical stimuli to the ipsilateral vagal nerve (n = 8 preparations). Because stable 1:1 entrainment of an oscillator with a rhythmic forcing input depends on the difference between the frequency of the forcing stimulus, ωin, and the frequency of the intrinsic oscillation, ω0, and (2) the strength of the forcing stimulus, we measured these parameters to construct a stimulus that would evoke maximal 1:1 entrainment. Before each rhythmic forcing trial, a 2-min baseline epoch was recorded for measurement of ω0. The current amplitude used for VNS was set to the threshold current necessary to evoke both activity on the contralateral vagus nerve and an increase in the respiratory period (10-s train, 75 Hz, 0.5-ms pulse duration). This response is consistent with previous reports in which the HBR was evoked by VNS (48). The threshold current was measured first after tuning of the respiratory rhythm and also periodically during the acquisition of baseline and forcing trial data to ensure that the chosen current was still sufficient to evoke HBR-like responses. The range of threshold current was 1–15 µA. After determination of these two parameters, a 10-min forcing trial that consisted of 75-Hz bursts [0.5-ms pulse duration (26)], occurring with a burst frequency near ω0 was generated using custom MATLAB scripts. Subsequently, custom Spike2 scripts were used to deliver these rhythmic bursts of current pulses to the ipsilateral vagus nerve by triggering a stimulator (Grass Instruments, S11).
Muscimol microinjections.
To test whether VNS-induced amplification of respiratory rhythm variability and entrainment of the respiratory rhythm depended on the ability of nTS pump cells to relay sensory afferent information to the network, we microinjected the GABAA receptor (GABAAR) agonist muscimol (10 mM) in the nTS (n = 4 preparations) (Fig. 1). A triple-barreled micropipette containing glutamate (10 mM), muscimol (10 mM), and 2% (wt/vol) pontamine sky blue/aCSF was used to allow mapping of respiratory responses to local glutamate application (20–50 nl). Effective sites displayed HBR-like responses that were defined by a prolongation of the respiratory period, suppression of inspiratory activity, and a transient enhancement of vagal efferent nerve activity. Such sites were typically found at the following stereotaxic coordinates relative to calamus scriptorius (CS): AP, CS + 0.5–0.7 mm, ML, 0.7–1 mm lateral to midline; and depth, 0.5 mm below surface of brain stem. After identification of the nTS, muscimol (100 nl) was injected to suppress local neuronal activity. Pontamine sky blue (100 nl) was also injected to confirm the anatomic location of the injection site by post hoc histologic analysis (Fig. 4E).
Fig. 1.
Methods to assess whether vagal nerve stimulation (VNS) is sufficient for respiratory rhythm variability and Hering-Breuer input-output entrainment in situ. A: we measured left phrenic (PNA) and right vagal nerve (VNA) activities, while rhythmically forcing the network at its intrinsic oscillation frequency (ω0) via left VNS. B1: threshold amplitude for the electrical stimulus to evoke the Hering-Breuer reflex (HBR) was defined not only by the suppression of inspiratory activity on PNA (bottom), but also by the presence of evoked bursts of efferent VNA from the contralateral vagus nerve (top). B2: stimulus-triggered average of integrated efferent right VNA during a rhythmic VNS trial is shown. The rhythmic VNS (red trace) consistently evoked bursts of efferent VNA (green traces: thick line is the averaged activity; and thin lines, plus and minus standard deviation) at a delay of ~250 ms.
Fig. 4.
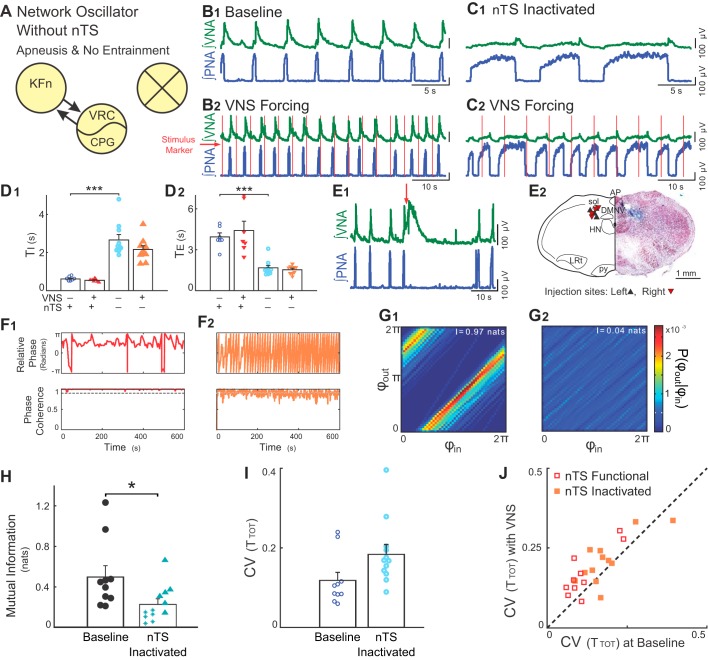
Suppressing nTS neuronal activity occludes VNS-evoked entrainment and variability amplification. A: we suppressed neuronal activity in discrete components of the Hering-Breuer reflex circuitry via local microinjections of the GABAA receptor agonist, muscimol. Subsequent panels demonstrate that suppression of nTS neuronal activity (marked with an X) occludes both entrainment and variability amplification evoked by rhythmic VNS in situ. B and C: representative traces of PNA and VNA during baseline (B1 and C1) and VNS (B2 and C2) before (B) and after (C) microinjection of muscimol in the nTS. Silencing nTS activity induced apneusis, a prolongation of inspiration. However, a postinspiratory peak in VNA remained, suggesting the presence of an intact three-phase rhythm (Fig. 4C). Furthermore, during VNS, PNA had no consistent phase relation between vagal stimuli and respiration. D: for this experimental group, microinjections of muscimol in the nTS increased inspiratory time (TI) (D1, ***P < 0.001) and decreased expiratory time (TE) (D2, ***P < 0.001). E: respiratory related nTS subnuclei were identified during the experiment by the respiratory responses to local glutamate microinjection (E1) and after the experiment by histologic identification of the injection site (E2). Injections of glutamate (20–50 nl, 10 mM, at red arrow) at the target site evoked VNA and prolonged TE. E2, right: a hemisection counterstained with neutral red is shown indicating the location of a representative muscimol microinjection site marked by pontamine sky blue. E2, left: the locations of the recovered microinjection sites for experiments included in the subsequent analyses are shown. Left and right injection sites are indicated by upward- and downward-pointing triangles, respectively. AP, area postrema; DMNV, dorsal motor nucleus of the vagus; HN, hypoglossal nucleus; LRt, lateral reticular formation; py, pyramidal tract; sol, solitary tract. F: instantaneous relative phase (top) and phase coherence (bottom) are shown before (F1) and after (F2) microinjection of muscimol in the nTS. After suppression of nTS, the respiration did not entrain to VNS. Accordingly, phase coherence was below 0.9, indicating that the underlying instantaneous relative phase window had little directionality. G: joint probability histograms of the instantaneous phase before (G1) and after (G2) microinjection of muscimol in the nTS are shown. Suppression of nTS activity abolished the banding associated with entrainment. H: for the group, bilateral microinjections of muscimol in the nTS decreased the strength of entrainment as measured by the mutual information of the instantaneous phases (*P < 0.05). Note that starred data points indicate VNS stimulation trials that failed the bootstrap test. I: suppression of nTS activity with muscimol tended to increase the variability in TTOT. J: VNS stimulation evoked a significant amplification of CV before (P = 0.004), but not after (P = 0.15) nTS muscimol microinjection. Together, these results confirm that entrainment between VNS and respiration depends on the ability of nTS neurons to relay VNS input to the network. In the context of the model, activity in the nTS is related to the parameter α.
To test whether rhythmic VNS-evoked modulation of respiratory rhythm variability and entrainment of the respiratory rhythm required KFn activity, we suppressed KFn activity by local microinjection of muscimol (n = 4 preparations). As above, the local area was first mapped via respiratory responses to glutamate microinjection (20–50 nl). Effective sites within the KFn were also characterized by a transient prolongation of the respiratory period, suppression of inspiratory activity, and enhancement of vagal nerve activity. These sites were found at the following coordinates relative to the inferior colliculus (IC): AP, 0.5–1.0 mm rostral to caudal extent of IC; ML, 0.5 mm from lateral extent of brain stem; and depth, 2.5–3 mm below the surface of IC. After identification of the KFn, we microinjected muscimol (100 nl) to suppress local neuronal activity and pontamine sky blue (100 nl) for post hoc histologic verification of the injection sites (Fig. 5E). After microinjection of muscimol in either the nTS or the KFn, several baseline and rhythmic VNS trials were recorded to test whether these sites modulated the VNS-induced amplification of respiratory rhythm variability and VNS-induced entrainment of the respiratory rhythm.
Fig. 5.
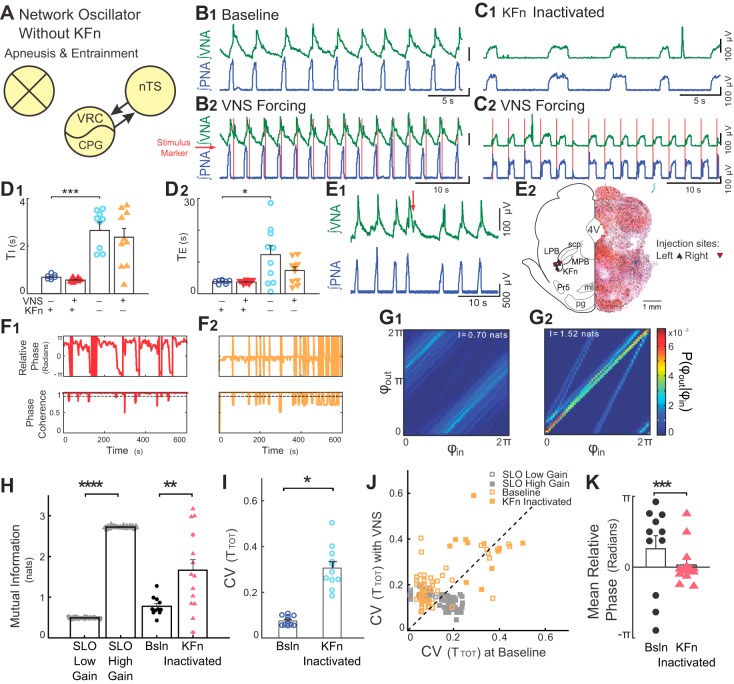
Suppression of KFn activity evokes high-gain responses to rhythmic VNS. A: suppression of KFn activity spared nTS-CPG connectivity selectively and was sufficient to switch the network state from a low- to high-gain state. B and C: representative traces of PNA and VNA are shown during baseline (B1 and C1) and VNS (B2 and C2) before (B) and after (C) microinjection of muscimol in the KFn. C: suppression of KFn activity increased TI and TE and transformed the respiratory rhythm to a two-phase rhythm without postinspiratory output on vagal efferent fibers. During rhythmic VNS, entrainment strength increased, and the phase angle between VNS and respiration cycle shifted from postinspiratory to preinspiratory coupling. D: for the group, silencing the KFn increased TI (D1, ***P < 0.001) and TE (D2, *P < 0.05). E: respiratory related dorsolateral pontine subnuclei were identified during the experiment by the respiratory responses to local glutamate microinjection (E1) and after the experiment by histological identification of the injection site (E2). E1: injections of glutamate (20–50 nl, 10 mM, at red arrow) at the target site evoked VNA and prolonged TE. E2, right: a representative hemisection counterstained with neutral red shows a KFn-targeted muscimol microinjection site marked by pontamine sky blue. E2, left: locations of the recovered microinjection sites for experiments included in the subsequent analyses are shown. Left and right injection sites are indicated by upward- and downward-pointing triangles, respectively. 4V, fourth ventricle; KFn, Kölliker-Fuse nucleus; LPB, lateral parabrachial nucleus; MPB, medial parabrachial nucleus; ml, medial lemniscus; Pr5, principal sensory of CN V; pg, pontine gray; scp, superior cerebellar peduncle. F: instantaneous relative phase (top) and phase coherence (bottom) are shown before (F1) and after (F2) microinjection of muscimol in the KFn. After suppression of KFn, respiration entrained to VNS with a relative phase near 0 (i.e., the start of inspiration). The reduction in respiratory drive evoked by suppressing KFn activity caused a qualitative change in the desynchronization events, such that they occurred abruptly when respiratory drive was not great enough to evoke an inspiratory burst. G: nonetheless, joint probability histograms of the instantaneous phases before (G1) and after (G2) microinjection of muscimol in the KFn suggest the presence of stronger entrainment after muscimol microinjection. H: for the group, KFn interventions increased the strength entrainment as measured by the mutual information of the instantaneous phases (**P < 0.01), which is consistent with a transition of the state of the network oscillator from low to high gain. For qualitative comparison, we have replotted the effect of increasing gain from the SLO model (****P < 0.001; see Fig. 2). Note that starred data points indicate VNS stimulation trials that failed the bootstrap test. I: at baseline, in the absence of VNS, suppression of KFn activity with muscimol significantly increased the variability in TTOT (*P < 0.05). J: VNS stimulation amplified respiratory rhythm variability before (open orange squares; P < 10–3), but not after (solid orange squares; P = 0.15) KFn muscimol microinjections. For qualitative comparison, we have also plotted the distribution of CVTOT) from simulations of the low- (open gray squares) and high- (solid gray squares) gain SLO models (see Fig. 2). In both model and experimental data, the perturbation (change of gain, or muscimol microinjection) shifted the distribution to lie along the line of identity, suggesting that the amplification of rhythm variability no longer occurred. However, the model failed to capture the increase in rhythm variability evoked by KFn muscimol microinjection. K: suppression of KFn activity shifted the preferred phase during entrainment from a postinspiratory to a preinspiratory relative phase angle (***P = ≪ 0.0001). Together, these observations suggest that activity in the KFn regulates the gain of vagal inputs and is responsible for the amplification of respiratory rhythm variability during VNS forcing.
At the conclusion of the experiment, the brain stem was dissected from the preparation and postfixed in 4% (wt/vol) paraformaldehyde/PBS. After 24 h of fixation, the brain stem was transferred to 20% (wt/vol) sucrose/PBS. After cryoprotection, the brain stem was sectioned (50-µm sections) using a freezing microtome (SM2010R; Leica Biosystems, Buffalo Grove, IL). Freely floating sections were then mounted on gelatin-coated slides and counterstained with 1% (wt/vol) Neutral Red. Slides were imaged using an upright microscope (Eclipse 80i, Nikon Instruments, Melville, NY; Retiga 2000R, QImaging, Surrey, BC) to confirm accurate delivery of muscimol to the target sites.
Data analysis.
Two-minute epochs of baseline and the following 10-min epochs of respiratory activities during forcing VNS trials were analyzed as before and during treatment pairs. Raw PNA and VNA signals were rectified and integrated with a zero-phase low-pass filter (0.01 Hz cut-off, 100- and 50-ms time constants, respectively). PNA and stimulus onset times were determined using a threshold-crossing algorithm and were visually inspected to ensure that there were no artifacts. These onset times were used to compute the instantaneous respiratory period (TTOT). The coefficient of variation (CV) of TTOT was used to measure respiratory rhythm variability during baseline and during VNS stimulation.
Entrainment measures.
From the stimulus and PNA onset times, the instantaneous phase of the input (forcing stimulus (VNS) and output (integrated PNA) was computed assuming a linear change of phase within each cycle using the following equation:
where tk is the time of the kth event, and tk+1 is the time of the next event.
From the instantaneous phase time series, the instantaneous relative phase time series, φout − φin, was computed. Regions where the slope of this time series approaches zero are associated with stable input-output entrainment.
To define regions associated with stable phase locking more precisely, we computed the phase coherence time series, a windowed statistic that measures the squared magnitude of the mean phase:
where T is the number of points in a window of size w (20 s). Windows with a phase coherence greater than 0.9 were considered to show stable phase locking.
Finally, to quantify the strength of input-output entrainment and determine the presence of a significant interaction in a given forcing trial, we computed the mutual information of the instantaneous phases (118). Mutual information, a measure of the statistical dependence between two variables, was computed from the joint-probability histogram, according to the following equation:
where P(φout, φin) is the joint probability distribution of the instantaneous phases, and Po(φout) and Pi(φin) are the marginal probability distributions of either variable. For all measurements of mutual information of the instantaneous phases, we used a fixed bin width of 0.126 rad to discretize the probability distributions. Because we use the natural logarithm in computing mutual information, the reported mutual information values are presented in the corresponding unit of nats. Values of mutual information near zero indicate that the variables are independent and have no coupling, whereas high values of mutual information are associated with high dependence between the instantaneous phase variables and, thereby, are associated with high entrainment strength between the oscillator and forcing stimuli.
To assess whether a given forcing trial had a statistically significant interaction between the input and the output, we bootstrapped the distribution to represent the null hypothesis that the two instantaneous phases were independent by randomly shuffling the interevent intervals and recomputing the instantaneous phases and their mutual information. The bootstrapping procedure was iterated 100 times to estimate the distribution that represented the null hypothesis. If a forcing trial had a mutual information greater than the 99% confidence interval of the bootstrap distribution, it was considered significant.
Unless stated otherwise, all data are presented as means ± SE. For statistical comparisons of group data, a one-way or two-way ANOVA was used as appropriate. If significant, a Bonferroni post hoc test was used to determine specific differences. A Wilcoxon-signed rank test was used to test whether VNS forcing significantly altered a given statistic on a trial-by-trial basis. A Williams-Watson test was used to assess the significance of changes in circular variables.
RESULTS
Stuart-Landau oscillator simulations with forcing and noise.
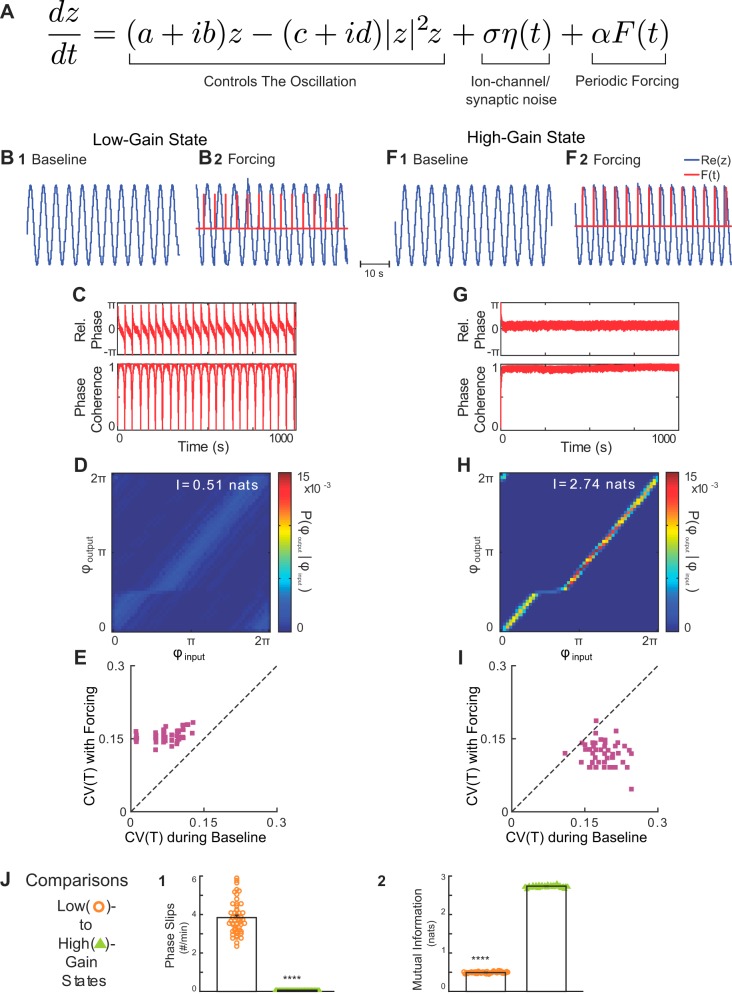
To illustrate how rhythmic forcing can interact with intrinsic noise to increase respiratory variability via phase slips, we conducted numerical simulations using a Stuart-Landau oscillator as a minimal model for respiratory rhythm generation. Model equations were adapted to mimic our in situ experimental protocols by incorporating an intrinsic source of noise, and a rhythmic forcing input (Fig. 2A). As shown in materials and methods, when expressed in terms of instantaneous phase and amplitude, a single parameter G determines the gain of the SLO phase to both the forcing and noise terms. Consequently, we explored the effect of changing G on the variability and input-output coupling in the forced, stochastic SLO model.
Fig. 2.
A stochastic, forced Stuart-Landau Oscillator (SLO) model predicts that variability during forcing depends on input gain. A: The Stuart-Landau oscillator was modified to include a Gaussian noise term that represents the influence of ion channel gating- and/or synaptic noise on the respiratory oscillation mechanism and a periodic forcing term that represents the influence of periodic vagal input that occurs during mechanical ventilation. B and F: representative outputs of the SLO model (blue traces) in the low- (B) and high- (F) gain states at baseline (B1 and F1) and during rhythmic forcing input (red traces, B2 and F2). C and G: instantaneous relative phase (top) and phase coherence (bottom) of the low- (B) and high- (F) gain states. In the low-gain state, forcing resulted in a coupling pattern that was characterized by epochs of stable coupling interrupted by phase slips qualitatively similar to the experimentally observed coupling pattern (see Fig. 3C). In contrast, in the high-gain state, forcing evoked stronger input-output entrainment without phase slips. D and H: in the low-gain state during forcing (D), the mutual information of the instantaneous phases revealed a significant VNS-evoked entrainment interaction despite the presence of phase slips; whereas, in the high-gain state (H), the mutual information of the instantaneous phases identified a stronger VNS-evoked entrainment interaction. E and I: effect of forcing on rhythm variability also depended on the gain of the model. In the low-gain state (E), forcing increased the variability of the oscillation (P = 9.1 × 10–10). In contrast, in the high-gain state (I), forcing decreased the variability of the oscillation (P = 9.6 × 10–1). J: for the forced, stochastic SLO model, increasing gain reduced the rate of phase slips (****P < 0.0001, J1), and increased the strength of input-output entrainment (****P < < 0.0001, J2). Thus the model predicts that the presence of ion channel- and synaptic-noise sources in the biologic VNS-forced respiratory rhythm generator should generate either stable entrainment characterized by the presence of phase slips and an increase in respiratory rhythm variability, or perfectly phase-locked entrainment without phase slips and a decrease in respiratory rhythm variability depending on the input gain of vagal nerve impulses.
Representative simulations in the low-gain (G = 0.71, arbitrary units) state are presented in Fig. 2, B–E. In the absence of forcing (α = 0), the variability in the SLO model was determined by the gain of the forcing input and the intrinsic noise process, ση(t). Consequently, the intrinsic rhythm had little variability (Fig. 2E). With the addition of periodic forcing input (α = 11.4), variability of the oscillation period increased [CV(T) during baseline, 0.05 ± 0.005 vs. CV(T) with forcing, 0.16 ± 0.002; Wilcoxon signed-rank test, P = 9.1 × 10−10; Fig. 2E]. Furthermore, the entrainment to the forcing input was characterized by episodes of stable phase-locking that were interrupted by regularly occurring phase slips (Fig. 2C), as suggested by Ryter et al. (91). This entrainment interaction was significant despite the presence of phase slips (Fig. 2D).
Representative simulations in the high-gain state (G = 1, arbitrary units) are presented in Fig. 2, F–I. At baseline, the oscillation produced by the high-gain state model had increased variability compared with that of the low-gain state model (compare Fig. 2I with Fig. 2E). With periodic forcing, the high-gain state model strongly entrained to the forcing input without phase-slip events (compare Fig. 2G with Fig. 2C). In contrast to the low-gain state, the variability of the oscillation period decreased during forcing [CV(T) during baseline, 0.19 ± 0.003 vs. CV(T) with forcing, 0.11 ± 0.003; Wilcoxon signed-rank test, P = 9.6 × 10−10; Fig. 2I]. Thus, the effect of the forcing on rhythm variability depended on the gain of the oscillator to this input.
When comparing the entrainment properties of the low- vs. high-gain states in the model, we observed a significantly lower rate of phase-slip events in the high-gain state (3.83 ± 0.06 phase slips/min in the low-gain state vs. 0.06 ± 0.00 phase slips/min in the high-gain state; P < 10−10; Fig. 2J1). Finally, the reduction in phase slips was associated with an increase in the entrainment strength in the high-gain state model (0.49 ± 0.003 nats in the low-gain state vs. 2.73 ± 0.004 nats in the high-gain state, P < 10−10, Fig. 2J2). Together, these simulations suggest that phase slip events, which only transiently desynchronize entrainment, also account for the increased oscillation variability in the forced, stochastic SLO model.
Rhythmic VNS is sufficient to entrain the respiratory rhythm and amplify respiratory rhythm variability in situ.
To test whether the responses of the intact respiratory rhythm-generating network to rhythmic VNS are consistent with the predictions of the low- or high-gain SLO model, we measured the strength of entrainment between the rhythmic VNS and the respiratory rhythm and compared the variability in the respiratory period (TTOT) between a 2-min baseline epoch and a 10-min epoch during VNS. Representative traces of PNA and VNA are shown during baseline (Fig. 3B1) and in the presence of VNS (Fig. 3B2). Importantly, providing the preparation with rhythmic VNS evoked short bursts of efferent VNA (as detailed in Fig. 1B2), confirming that our stimulus was above the threshold to activate vagal fibers innervating pulmonary stretch receptors. Additionally, VNS reduced inspiratory duration (TI; 0.62 ± 0.02 s during baseline vs. 0.44 ± 0.01 s during stimulation, P < 0.05). During stimulation, HBR-like effects on TI were particularly apparent when comparing the data on a trial-by-trial basis (Wilcoxon signed-rank test, P = 2.6 × 10−8).
To assess the effect of VNS on the entrainment pattern and respiratory rhythm variability, we plotted TTOT, the relative phase time series, φout − φin, and the phase coherence sequentially (Fig. 3, C1–C3). Regions with constant relative phase indicated stable input-output phase locking. To define windows associated with stable phase locking vs. phase-slip events, we measured the phase coherence of the relative phase time series (Fig. 3C3). A phase coherence greater than 0.9 identified underlying windows (20 s) that had stable entrainment between the respiratory rhythm and the rhythmic VNS input, whereas those less than 0.9 were defined as phase-slip events (Fig. 3C, highlighted in yellow). When we examined TTOT during the rhythmic VNS trial, enhanced variability associated with VNS was limited to windows containing phase-slip events, consistent with the predictions of the low-gain SLO model. Additionally, inspection of the respiratory period in conjunction with the phase coherence suggested that the recovery time from a phase-slip event was on the order of 2–3 respiratory cycles. To accurately quantify the strength and significance of the VNS-evoked entrainment of respiration, we measured the mutual information of the instantaneous phases of the input and output (Fig. 3D). A representative joint probability histogram (Fig. 3D1) showed strong banding, suggesting the presence of a stable entrainment interaction (Fig. 3D1). Mutual information, which quantifies the dependence of the phase of the output on the phase of the input, i.e., the intensity of the banding, in this representative experiment was 0.47 nats. To determine whether this value reflected a significant entrainment dynamic, we bootstrapped the data by shuffling the interevent intervals before computing the instantaneous phases and their mutual information. As expected, the mutual information of the surrogate data sets represents the null hypothesis of no input-output coupling interaction. The mutual information of this example (arrowhead in Fig. 3D2, 0.47 nats) was greater than the 99% confidence interval of the bootstrap distribution (Fig. 3D2, dashed line), suggesting the presence of a significant VNS-evoked input-output entrainment interaction. In 44/45 rhythmic VNS trials, the mutual information of the instantaneous phases for the original data (open bars) was greater than the 99% confidence interval of their corresponding surrogate data sets (solid bars), indicating the presence of reproducible, significant entrainment dynamics (Fig. 3D3). When compared with the experimental data set, the means ± SE of the mutual information of the instantaneous phases from the low-gain SLO model (gray lines) fell slightly above the median of the experimental distribution.
To assess whether rhythmic VNS was associated with changes in respiratory rhythm variability, we compared the coefficient of variation (CV) of the respiratory period (TTOT). For the group, VNS increased the CV(TTOT) from 0.14 ± 0.02 during baseline to 0.21 ± 0.01 during forcing (Fig. 3E). This amplification of respiratory rhythm variability was highly significant (Wilcoxon signed rank test, P = 2.1 × 10−5, Fig. 3E). Qualitatively, the distribution of CV(T) in the low-gain SLO model overlapped with that of the experimental data set (Fig. 3E).
Next, to confirm that phase slips caused respiratory rhythm variability amplification in situ, we defined the relationship between the variability of TTOT and phase slips by quantifying the CV(TTOT) in windows associated with stable phase locking vs. windows containing phase slips (Figs. 3F). In analyzing the group data, we identified a significant, yet selective, increase in respiratory rhythm variability during phase slip-associated windows [CV(TTOT) during phase-locked windows, 0.10 ± 0.01, vs. during phase-slip windows, 0.19 ± 0.03, P < 0.001, Fig. 3F]. This observation confirmed that, like the model, the increase in rhythm variability during forcing was due to the presence of phase-slip dynamics.
Finally, we directly measured the relationship between variability of TTOT and the strength of the entrainment interaction via least-squares fitting. Using this approach, we observed a significant inverse correlation between the strength of the entrainment interaction (mutual information of the instantaneous phases) and the variability in the respiratory oscillation (R2 = 0.30, P < 0.0001, Fig. 3G).
Taken together, rhythmic VNS entrained the respiratory rhythm but amplified respiratory rhythm variability. These findings are consistent with the predictions of the low-gain stochastic SLO model, supporting our hypothesis that respiratory rhythm variability during mechanical ventilation or periodic VNS occurs as a consequence of phase slips between the brain stem respiratory rhythm generator and rhythmic vagal inputs.
Neuronal activity in the nTS is required to entrain respiration and amplify its variability.
Gain control is a potential mechanism that could account for VNS-evoked amplification of variability. We took advantage of the compartmentalized neuroanatomy of the respiratory network to experimentally test whether respiratory compartments that mediate the HBR (10, 30) also regulate the gain of vagal sensory inputs. To do this, we microinjected muscimol to suppress neuronal activity bilaterally in either the nTS (Fig. 4) or KFn (Fig. 5) and measured input-output entrainment and respiratory rhythm variability before and after local suppression of neuronal activity. Perturbations of the neuronal activity in the nTS additionally served as a useful control to validate that the low-gain state-like responses observed in the intact network are, in fact, due to forcing of the respiratory rhythm via the HBR pathway.
The respiration-related subregion of the nTS was identified by the response to microinjections of glutamate (10 mM, 20–50 nl). These responses were characterized by large bursts of VNA associated with a prolongation of the interburst interval of PNA (Fig. 4E1). After the experiment, the injection site was confirmed by visualizing the pontamine sky blue injection sites, which were clustered in the caudal nTS and in similar locations bilaterally (Fig. 4E2).
Representative traces before and after local administration of muscimol in the nTS showed the expected effect of occluding the HBR, (Fig. 4, B2 and C2, respectively). With nTS activity blocked, baseline inspiratory time (TI) (0.61 ± 0.04 s) increased to 2.66 ± 0.28 s (P < 0.001, Fig. 4, C1 and D1); baseline expiratory time (TE) (3.93 ± 0.29 s) decreased to 1.66 ± 0.18 s, (P < 0.001, Fig. 4, C1 and D2). Consistent with the change in respiratory phase durations, blocking nTS neuronal activity reduced, but did not eliminate, the peak of efferent VNA that occurs during the postinspiratory phase. Together, these observations suggest that suppressing nTS activity causes an apneustic breathing pattern characterized by increased inspiratory duration.
Representative traces during VNS before and after local administration of muscimol in the nTS (Fig. 4, B2 and C2, respectively) showed the expected effect of blocking the integration of pulmonary stretch receptor input. The 1:1 entrainment between VNS and respiratory rhythm at baseline (Fig. 4, B2 and F1) was not apparent after blocking nTS activity despite the optimization of the VNS to maximize entrainment (Fig. 4, C2 and F2). Instantaneous relative phase and phase coherence plots were consistent with the presence of entrainment before, but not after nTS muscimol administration (Fig. 4, F1 and F2). With the network intact (Fig. 4F1), the relative phase between the respiratory oscillator and periodic vagal input revealed long periods of stable entrainment. In contrast, after silencing the respiratory subregion of the nTS, the relative phases of the input and output varied continuously and did not converge on an entrained state, with a phase coherence consistently below 0.9 (Fig. 4F2). Representative joint probability histograms also confirmed evidence of input-output entrainment before (Fig. 4G1), but not after suppression of nTS activity (Fig. 4G2). For the group, silencing nTS activity significantly reduced the mutual information of the instantaneous phases (0.50 ± 0.11 nats before muscimol microinjection vs. 0.23 ± 0.05 nats after muscimol microinjection, P < 0.05, Fig. 4H), confirming that neurons within this compartment are necessary for coupling the respiratory rhythm with VNS.
Finally, to assess whether activity within the nTS was also necessary for the VNS-evoked amplification of respiratory rhythm variability observed in intact preparations, we measured the CV(TTOT) before and after nTS muscimol microinjections in the presence or absence of VNS. At baseline, without VNS, there was a tendency for nTS suppression to increase the intrinsic variability of the respiratory rhythm [CV(TTOT), P = 0.25, Fig. 4I]. With VNS, we observed the existence of significant amplification of respiratory rhythm variability with the network intact (Wilcoxon signed-rank test, P = 0.004, Fig. 4J), but not after suppression of nTS neuronal activity (Wilcoxon signed-rank test, P = 0.15, Fig. 4J). Consistent with the role of the nTS in the HBR, these observations suggest that rhythmic VNS-induced entrainment of respiration and amplification of respiratory rhythm variability depends on intact synaptic transmission within this compartment. In the context of the forced, stochastic SLO model, neuronal activity in the nTS is related to the parameter, α, which controls the influence of the rhythmic forcing input to the oscillator.
Suppression of KFn neuronal activity shifts the network to a high-gain state.
We next tested whether synaptic transmission in the KFn could regulate the gain of sensory VNS inputs because this respiratory compartment has been proposed to gate vagal sensory feedback to the respiratory CPG (Pre-Bötzinger and Bötzinger complexes) (30). Again, we addressed this hypothesis by measuring respiratory network-VNS entrainment and respiratory rhythm variability before and after suppressing KFn activity bilaterally with muscimol. The target site in the KFn was identified by the responses to glutamate microinjection, which, like the nTS, evoked a prolongation of TE, and was associated with a transient increase in VNA (Fig. 5E1). A hemi-section showing a representative pontamine sky blue microinjection confirmed that the injection site was in the KFn (Fig. 5E2). The injection sites for all data obtained after KFn suppression are shown on the contralateral side of Fig. 5E2.
Representative traces before and after microinjection of muscimol in the KFn show that this perturbation transformed the respiratory pattern from a three- to a two-phase rhythm that lacked postinspiratory activity in the VNA (Fig. 5, B1 and C1), as previously shown by other groups (31, 89, 101). With activity of the KFn suppressed, TI increased (0.65 ± 0.03 s during baseline vs. 2.63 ± 0.37 s after KFn suppression, P < 0.001, Fig. 5D1). Suppression of KFn activity also increased TE (3.42 ± 0.22 s during baseline vs. 11.99 ± 2.90 s after KFn suppression, P < 0.05, Fig. 5D2). As expected, these changes suggest that silencing KFn activity abolished IE phase-transition mechanisms in the absence of vagal pulmonary stretch receptor inputs.
Representative traces of a VNS trial before (Fig. 5B2) and after (Fig. 5C2) silencing KFn activity showed that the entrainment pattern was transformed radically from a postinspiratory to a preinspiratory pattern, in which the VNS preceded and perhaps evoked PNA. Instantaneous relative phase and phase coherence time series confirmed this effect (Fig. 5F). With the KFn intact, the respiratory network converged to a stable phase-locked state in which PNA entrained to VNS during the postinspiratory phase. Consistent with earlier intact experiments and the low-gain SLO model, the instantaneous relative phase remained constant for many cycles (Fig. 5F1). After KFn activity was suppressed, the preferred phase during VNS entrainment was preinspiratory: the onset of the stimulus preceded the onset of PNA, yielding an instantaneous relative phase near 0. For the group, suppression of KFn activity decreased the mean instantaneous relative phase (0.81 ± 0.58 rad before muscimol to 0.11 ± 0.23 rad after muscimol, P = 9.9 × 10−5; Fig. 5K). Furthermore, because of the reduction in respiratory drive associated with the suppression of KFn activity, the desynchronization events observed after this perturbation were qualitatively different than the phase slips observed in the intact preparation. These events seemed to occur because there was not enough drive for VNS to evoke a respiratory cycle, rather than a true phase slip, in which the respiratory rhythm became progressively desynchronized and resynchronized with the VNS.
Accordingly, representative joint-probability histograms of the instantaneous phases showed stronger banding after silencing KFn activity, suggesting an increase in entrainment strength (compare Fig. 5G2 to Fig. 5G1). For the group, measurement of mutual information of the instantaneous phases confirmed that entrainment strength was greater after suppression of KFn activity (0.77 ± 0.07 nats before muscimol vs. 1.66 ± 0.26 nats after muscimol, P < 0.01, Fig. 5H). Qualitatively, this observation agrees with the predictions of the forced, stochastic SLO model, where an increase in the gain evoked a significant increase in the mutual information of the instantaneous phases (P < 0.001; Figs. 5H and 2J2).
Finally, we assessed the role of the KFn on VNS-evoked amplification of respiratory rhythm variability by examining the CV(TTOT) (Fig. 5K) before and after muscimol microinjection. After suppressing KFn activity, the respiratory rhythm was more variable during baseline conditions (0.08 ± 0.01 before muscimol vs. 0.31 ± 0.03 after muscimol, P < 0.05, Fig. 5I). Consistent with earlier experiments, VNS amplified respiratory rhythm variability with the network intact (Fig. 5I). For the group, the CV(TTOT) was amplified before, but not after, suppression of KFn activity (Wilcoxon signed rank test: before muscimol, P = 9.8 × 10−4 vs. after muscimol, P = 0.24, Fig. 5J). This result agreed with the predictions of the forced, stochastic SLO model, in which increasing the gain of the oscillator was associated with a reduction of variability during forcing (Figs. 5J and 2, E and I). However, the model failed to fully account for the increase in respiratory rhythm variability observed during baseline after suppression of KFn activity (Fig. 5J).
Taken together, these data suggest that the KFn limits the strength and regulates the phase of VNS-evoked input-output entrainment and is necessary for the amplification of respiratory rhythm variability. These experimental observations after suppression of KFn activity are consistent with the predictions of the high-gain SLO model. Overall, these experimental findings and forced, stochastic SLO simulations suggest that the KFn regulates the variability of the forced respiratory network by regulating the gain of vagal sensory afferent inputs.
DISCUSSION
Using the in situ arterially perfused preparation, we have tested the predictions of a minimalist mathematical model of the respiratory network on how variability in the respiratory rhythm is modulated by vagal sensory input. We have shown experimentally that rhythmic VNS is sufficient not only to entrain the respiratory rhythm, but also to increase the variability of respiratory motor output. Guided by the model, the experiments have also allowed us to identify the KFn as the respiratory network compartment that controls the gain of vagal sensory inputs onto the rhythm-generating network, and is, hence, the key player in enhancing or attenuating respiratory rhythm variability during vagal forcing, as occurs during mechanical ventilation. To our knowledge, this is the first study providing a mechanistic explanation of physiological variability at the systems level. Our results have several implications for the interpretation and modeling of respiratory network oscillations in the arterially perfused preparation, as well as for our understanding of dorsolateral pontine control of respiration.
One limitation of our findings stems from our use of the GABAAR agonist muscimol to suppress local neuronal activity. It is possible that the extent of activity suppression via this perturbation is less than that achieved via administration of Na channel blockers like tetrodotoxin or lidocaine. However, GABAARs are expressed on neuronal cell bodies in both the KFn and nTS, suggesting that muscimol administration likely suppressed local neuronal activity in our experiments (46, 64, 92). Moreover, our observations regarding the change in respiratory patterning after muscimol microinjections agreed with several other studies that have used the GABAAR agonists isoguavacine or muscimol to suppress activity in cardiorespiratory brain areas, including the KFn and nTS (22, 31, 85). Most importantly, our protocols evoked respiratory responses consistent with lesion of either the KFn or nTS (13, 14, 56, 57, 75). Thus, it is likely that our muscimol microinjections suppressed local neuronal activity.
Another limitation of our study is that the gain-dependent phase-slip mechanism could be one of many mechanisms influencing the variability of the respiratory rhythm during forcing via the HBR. For instance, the frequency of respiration could itself be a time-varying process, or the coupling function of respiration to VNS inputs could also vary in time (102, 103, 107). Our analytic methods fail to distinguish noise-induced phase slips from these other mechanisms. Despite this limitation, our conclusions were supported by the demonstration that the variability associated with putative phase slips was, indeed, greater than that observed during stable coupling. Moreover, this enhanced variability during phase slip windows entirely accounted for the amplification of respiratory rhythm variability observed during VNS forcing. Nonetheless, in the future, we could disambiguate these possibilities by employing dynamic inference, a Bayesian approach that enables one to distinguish time-varying processes and phase slips in a continuous manner (103, 111).
Phase-slip dynamics generate variability during periodically forced respiration.
How is entrainment associated with variability? This question was addressed theoretically and experimentally in physics throughout the 20th century and promoted the development and application of stochastic theory (106). Physicists realized that the van der Pol oscillator, a simple nonlinear circuit that sustains self-oscillations and which was originally proposed as an electronic model of the heart (110a), displays variations of its natural period due to thermal noise in its resistive elements (106). One may naively think that these variations would be minimized by forcing the oscillator at its natural frequency. However, this may lead to the very opposite effect of increasing variability. The reason for this was identified by the numerical analysis and simulations of a noisy van der Pol oscillator driven by a periodic forcing (91). For a small relative phase difference between the oscillator and the periodic input, the oscillator does entrain to the input. However, the additional stochastic perturbation from Gaussian white noise causes the coupled system to exhibit phase slips, which may be larger than the period fluctuations without forcing. In the respiratory network, we observe similar dynamics of stable entrainment interrupted by phase slips due to the presence of noise in this rhythmogenic network. In Fig. 3C, the periodic drops of the phase coherence of the instantaneous relative phase mark the phase slips that interrupt the stable input-output coupling. The existence of phase slips within the VNS trials implies that the respiratory periods following these events must be lengthened and/or shortened for the system to return to a stable coupled state, which we assessed by measuring CV(TTOT) during stable entrained regimes vs. during phase-slip events (Fig. 3H). Thus, this process of phase slipping and returning to stable input-output interaction introduces significant slow-timescale variability to the respiratory rhythm.
Modeling respiratory rhythmogenesis and control.
Current models of respiratory rhythmogenesis consist of a group of three inhibitory populations and one excitatory population arranged in a mutually inhibitory ring, with equally distributed excitatory drive from chemosensory and pontine populations (72, 88–90). These models nicely account for the generation of the three-phase respiratory rhythm and exhibit phasic population activity patterns consistent with experimental recordings of medullary respiratory neuron activity. Recently, we have shown that adding ion-channel noise to this model of the respiratory central pattern generation can influence not only the variability of respiratory rhythm, but also its pattern, suggesting that noise alone may mediate pathological respiratory patterns, such as apneusis (117). Other investigators have extended this same model of rhythmogenesis to incorporate lung mechanics and chemosensory regulation (8, 9, 72). Because we focused on understanding the interaction between noise and a periodic input, it was sufficient to use a more general model in our study that captures the dynamics of entrainment and rhythm variability that we observed experimentally. Our reduced approach had the benefit of limiting the parameter space of the model, which helped to identify sensory input gain as the key parameter that changes the dynamics of the HBR-forced respiratory network. Because of the robustness of the model’s predictions, we could later identify the existence and neuroanatomy of this mechanism experimentally in the arterially perfused preparation. Petrillo and Glass (80) showed that an inhibitory-ring CPG can be derived from the forced van der Pol equations, which suggests that the findings that we present using a similar nonlinear oscillator are likely consistent with the dynamics of conductance-based, inhibitory-ring CPG models, and are relevant for closed-loop models of respiratory control. In the future, these modeling approaches could be combined to investigate how phase-slip dynamics affect other respiratory parameters in a more detailed neurophysiological model when stochastic fluctuations are introduced.
Dorsolateral pontine control of vagal HBR inputs.
Dorsolateral (dl) pontine mechanisms are essential for shaping the respiratory rhythm (30). In particular, while the generation of the respiratory rhythm depends on the medullary CPG, its activity is converted into the final motor pattern through interactions with a larger pontomedullary network. In this context, the dl pons has been shown to mediate various aspects of pattern formation, including regulation of the stability of the rhythm (78), the coupling with sympathetic rhythms (7, 27), and the neural plasticity modulating respiratory reflexes (53, 84, 100). Importantly, these mechanisms emerge over the course of postnatal development (29, 33).
Pulmonary vagal reflexes affect the transition from inspiration to expiration (inspiratory off-switch, IOS). One hundred and fifty years ago, Breuer, working in Hering’s laboratory, identified that lung inflation acting via vagal nerves inhibited inspiration and facilitated expiration, defining the Hering-Breuer reflex (17, 50, 60). Blocking the HBR by vagotomy, vagal nerve cooling, or prevention of lung inflation during inspiration increases inspiratory duration [TI (23, 25, 60)]. Through the activation of pump cells in the nTS, the HBR acts directly on the medullary rhythm, generating nuclei that excite postinspiratory neurons in the Bötzinger complex, inhibiting inspiratory neurons in the Pre-Bötzinger Complex, and acting indirectly through the dorsolateral (dl) pons (including the KFn). The indirect pathway was demonstrated as bilateral lesioning of the dl pons in vagotomized animals that caused apneustic breathing, a severe prolongation of inspiration (69, 104). These data shaped the view that pons and vagal sensory inputs interact to facilitate the IOS (30, 109a). Recent electrophysiological characterization of respiration-related neurons in the dl pons (37) identified bilateral connectivity between the nTS and dl pons, and unidirectional connectivity from the dl pons to the ventrolateral medulla, hypoglossal nucleus, and spinal cord, confirming previous tracing studies in rats (35, 41, 49). Thus, the neuroanatomy of the KFn is consistent with a potential role in mediating responses to HBR activation.
The findings of the present study provide additional support for this view. Our simulations show that an increase in oscillation variability, evoked by a forcing input, depends on the input gain. Consistent with this model, our experimental perturbations to the respiratory network identify that the sensory input gain of vagal inputs is determined by neuronal activity in the KFn. Therefore, our data suggest that projections from the KFn to the nTS regulate the gain of vagal HBR inputs on the resetting of the respiratory rhythm, and in so doing, increase the variability of the forced respiratory rhythm.
Our findings are also consistent with earlier investigation of the plasticity evoked by repetitive (33) VNS. Repetitive short trains of VNS evoke a long-term plasticity that emerges during postnatal development (33). In this study, neonatal preparations readily entrained to periodic forcing within the first trial, whereas juvenile preparations required several vagal stimulation trials before achieving 1:1 entrainment. Further, the postnatal reduction in entrainment strength was associated with an “anticipatory IOS”—a change in the preferred relative phase—such that, at neonatal ages, the inspiratory burst was terminated by the rhythmic vagal input, whereas at juvenile stages, the inspiratory burst was terminated before the arrival of vagal stimuli. By using the methods that we developed in the present study, future experimental work could use phase-slip dynamics as a measure to quantify the developmental plasticity of KFn gain control during periodic VNS.
Respiratory rhythm variability as a biomarker and therapeutic target.
Respiratory rhythm variability is emerging as a novel biomarker of pathophysiological state in a diverse array of human disorders. Before identification of Mecp2 mutations as genetic markers for Rett syndrome, increased respiratory rhythm variability was one of the primary diagnostic criteria (112, 113). Patients with restrictive lung disease display reduced respiratory rhythm variability (16). Accordingly, treatment with bronchodilators increases respiratory rhythm variability in patients diagnosed with chronic obstructive pulmonary disease (108). Respiratory rhythm variability has also been noted to decline during opioid-induced respiratory depression (15). Respiratory rhythm variability has also been proposed as a marker for aging in humans (97). Similar changes in respiratory rhythm variability have also been observed in animal models of stroke, cystic fibrosis, and sepsis (28, 55, 59).
The most investigated clinical use of respiratory rhythm variability has been its potential as a biomarker for weaning patients from mechanical ventilation (21), which is also highly relevant to the experimental and analytic approach presented herein. A number of studies have identified a positive correlation between the strength of respiratory rhythm variability during spontaneous breathing trials and successful extubation from mechanical ventilation in patients with various underlying pathology (6, 11, 79, 109, 114). A likely culprit for the difficulty in weaning critically ill patients from mechanical ventilation is the nonassociative plasticity within the HBR circuit, which depends on the presence of a functional KFn (66). The findings of the present study support this hypothesis by identifying that the KFn regulates entrainment to periodic vagal pulmonary stretch receptor inputs and, thereby, enhances respiratory rhythm variability through a gain control mechanism in the healthy, physiological state. Moreover, our findings suggest that serotonergic drugs, which modulate activity of the KFn, may be effective to increase compliance during mechanical ventilation (1, 24, 51, 105).
Given these suggestive clinical observations, numerous studies have sought to define the physiological and pathophysiological mechanisms underlying changes in respiratory rhythm variability to enhance its clinical utility. Chemosensory-, arousal-, baroreceptor-, and lung stretch receptor-inputs to the pontomedullary respiratory network have been identified as potent modulators of respiratory rhythm variability in humans and rodents in the healthy state (18–20, 39, 63, 70, 73, 110). Additionally, respiratory rhythm variability may also be modified by genetic factors since strain differences and at least one gene have been associated with differing levels of respiratory rhythm variability (1–3, 74, 105, 115, 116).
Therapeutically, the KFn nuclei has been identified as a key target to modify respiratory rhythm variability in disease by this study and others. This brain area plays an important role in a variety of neurogenic breathing disorders, including Rett syndrome (1, 3, 105) and tauopathy-linked neurodegeneration (32, 71). Serotonergic drugs have proven exceptionally effective at beneficially modulating the activity of the KFn to enhance physiological respiratory rhythm variability and already represent an ideal pharmacologic therapy (1, 24, 51, 105). One limitation of this approach is due to the desensitization of serotonergic receptors. Alternatively, ampakines, which have also been shown to counteract opioid-induced respiratory depression and respiratory irregularity in Rett syndrome (77, 87), may potentially mediate their effects through modulation of KFn activity and, therefore, might also prove useful in treating respiratory diseases that are associated with changes in respiratory rhythm variability. Finally, through the results of the present study, we have identified a novel approach to enhance respiratory rhythm variability via a KFn-dependent mechanism using VNS, which has recently emerged as a key target for the development of “electroceutical” therapies (38).
Perspectives and Significance
The respiratory rhythm is generated by the ventrolateral respiratory column and patterned by a distributed pontomedullary network that actively processes visceral sensory inputs to maintain homeokinesis. A side effect of this input processing is the generation of respiratory rhythm variability. The findings of the present study suggest that the KFn is likely critical for both viscerosensory input processing and the generation of respiratory rhythm variability.
GRANTS
This work was supported by National Institutes of Health Grants HL-087377 and 5P01HL-101871-06 (to T. E. Dick), T32HL-007913 (to R. R. Dhingra), the Future Fellowship from Australian Research Council (ARC) and the ARC Project Grant DP170104861, CIA (to M. Dutschmann), and The Hartwell Foundation (to R. F. Galán).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.R.D., R.F.G., and T.E.D. conceived and designed research; R.R.D. performed experiments; R.R.D. analyzed data; R.R.D., M.D., R.F.G., and T.E.D. interpreted results of experiments; R.R.D. prepared figures; R.R.D. drafted manuscript; R.R.D., M.D., R.F.G., and T.E.D. edited and revised manuscript; R.R.D., M.D., R.F.G., and T.E.D. approved final version of manuscript.
REFERENCES
- 1.Abdala APL, Dutschmann M, Bissonnette JM, Paton JFR. Correction of respiratory disorders in a mouse model of Rett syndrome. Proc Natl Acad Sci USA 107: 18,208–18,213, 2010. doi: 10.1073/pnas.1012104107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdala APP, Bissonnette JM, Newman-Tancredi A. Pinpointing brainstem mechanisms responsible for autonomic dysfunction in Rett syndrome: therapeutic perspectives for 5-HT1A agonists. Front Physiol 5: 205, 2014. doi: 10.3389/fphys.2014.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdala AP, Toward MA, Dutschmann M, Bissonnette JM, Paton JFR. Deficiency of GABAergic synaptic inhibition in the Kölliker-Fuse area underlies respiratory dysrhythmia in a mouse model of Rett syndrome. J Physiol 594: 223–237, 2016. doi: 10.1113/JP270966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Achour L, Letellier C, Cuvelier A, Vérin E, Muir J-F. Asynchrony and cyclic variability in pressure support noninvasive ventilation. Comput Biol Med 37: 1308–1320, 2007. doi: 10.1016/j.compbiomed.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Akoumianaki E, Lyazidi A, Rey N, Matamis D, Perez-Martinez N, Giraud R, Mancebo J, Brochard L, Marie Richard JC. Mechanical ventilation-induced reverse-triggered breaths: a frequently unrecognized form of neuromechanical coupling. Chest 143: 927–938, 2013. doi: 10.1378/chest.12-1817. [DOI] [PubMed] [Google Scholar]
- 6.Arcentales A, Giraldo BF, Caminal P, Benito S, Voss A. Recurrence quantification analysis of heart rate variability and respiratory flow series in patients on weaning trials. Conf Proc IEEE Eng Med Biol Soc 2011: 2724–2727, 2011. [DOI] [PubMed] [Google Scholar]
- 7.Baekey DM, Dick TE, Paton JFR. Pontomedullary transection attenuates central respiratory modulation of sympathetic discharge, heart rate and the baroreceptor reflex in the in situ rat preparation. Exp Physiol 93: 803–816, 2008. doi: 10.1113/expphysiol.2007.041400. [DOI] [PubMed] [Google Scholar]
- 8.Ben-Tal A. Simplified models for gas exchange in the human lungs. J Theor Biol 238: 474–495, 2006. doi: 10.1016/j.jtbi.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Ben-Tal A, Smith JC. Control of breathing: two types of delays studied in an integrated model of the respiratory system. Respir Physiol Neurobiol 170: 103–112, 2010. doi: 10.1016/j.resp.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berger AJ, Dick TE. Connectivity of slowly adapting pulmonary stretch receptors with dorsal medullary respiratory neurons. J Neurophysiol 58: 1259–1274, 1987. [DOI] [PubMed] [Google Scholar]
- 11.Bien M-Y, Hseu S-S, Yien H-W, Kuo BI-T, Lin Y-T, Wang J-H, Kou YR. Breathing pattern variability: a weaning predictor in postoperative patients recovering from systemic inflammatory response syndrome. Intensive Care Med 30: 241–247, 2004. doi: 10.1007/s00134-003-2073-8. [DOI] [PubMed] [Google Scholar]
- 12.Bignall S, Dixon P, Quinn C, Kitney R. Monitoring interactions between spontaneous respiration and mechanical inflations in preterm neonates. Crit Care Med 25: 545–553, 1997. doi: 10.1097/00003246-199703000-00027. [DOI] [PubMed] [Google Scholar]
- 13.Bonham AC, Coles SK, McCrimmon DR. Pulmonary stretch receptor afferents activate excitatory amino acid receptors in the nucleus tractus solitarii in rats. J Physiol 464: 725–745, 1993. doi: 10.1113/jphysiol.1993.sp019660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonham AC, McCrimmon DR. Neurones in a discrete region of the nucleus tractus solitarius are required for the Breuer-Hering reflex in rat. J Physiol 427: 261–280, 1990. doi: 10.1113/jphysiol.1990.sp018171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bouillon T, Bruhn J, Roepcke H, Hoeft A. Opioid-induced respiratory depression is associated with increased tidal volume variability. Eur J Anaesthesiol 20: 127–133, 2003. doi: 10.1097/00003643-200302000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Brack T, Jubran A, Tobin MJ. Dyspnea and decreased variability of breathing in patients with restrictive lung disease. Am J Respir Crit Care Med 165: 1260–1264, 2002. doi: 10.1164/rccm.2201018. [DOI] [PubMed] [Google Scholar]
- 17.Breuer J. Die Selbststeurung der Athmung durch den Nervus vagus. Sitzungsberichte Kais Akad Wiss 57: 909–937, 1868. [Google Scholar]
- 18.Bruce EN. Chemoreflex and vagal afferent mechanisms enhance breath to breath variability of breathing. Respir Physiol 110: 237–244, 1997. doi: 10.1016/S0034-5687(97)00088-1. [DOI] [PubMed] [Google Scholar]
- 19.Burioka N, Cornélissen G, Halberg F, Kaplan DT, Suyama H, Sako T, Shimizu E. Approximate entropy of human respiratory movement during eye-closed waking and different sleep stages. Chest 123: 80–86, 2003. doi: 10.1378/chest.123.1.80. [DOI] [PubMed] [Google Scholar]
- 20.BuSha BF, Stella MH. State and chemical drive modulate respiratory variability. J Appl Physiol (1985) 93: 685–696, 2002. doi: 10.1152/japplphysiol.00951.2001. [DOI] [PubMed] [Google Scholar]
- 21.Casaseca-de-la-Higuera P, Martín-Fernández M, Alberola-López C. Weaning from mechanical ventilation: a retrospective analysis leading to a multimodal perspective. IEEE Trans Biomed Eng 53: 1330–1345, 2006. doi: 10.1109/TBME.2006.873695. [DOI] [PubMed] [Google Scholar]
- 22.Chitravanshi VC, Kachroo A, Sapru HN. A midline area in the nucleus commissuralis of NTS mediates the phrenic nerve responses to carotid chemoreceptor stimulation. Brain Res 662: 127–133, 1994. doi: 10.1016/0006-8993(94)90804-4. [DOI] [PubMed] [Google Scholar]
- 23.Clark FJ, von Euler C. On the regulation of depth and rate of breathing. J Physiol 222: 267–295, 1972. doi: 10.1113/jphysiol.1972.sp009797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dhingra RR, Dutschmann M, Dick TE. Blockade of dorsolateral pontine 5HT1A receptors destabilizes the respiratory rhythm in C57BL6/J wild-type mice. Respir Physiol Neurobiol 226: 110–114, 2016. doi: 10.1016/j.resp.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dhingra RR, Jacono FJ, Fishman M, Loparo KA, Rybak IA, Dick TE. Vagal-dependent nonlinear variability in the respiratory pattern of anesthetized, spontaneously breathing rats. J Appl Physiol (1985) 111: 272–284, 2011. doi: 10.1152/japplphysiol.91196.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dhingra RR, Zhu Y, Jacono FJ, Katz DM, Galán RF, Dick TE. Decreased Hering-Breuer input-output entrainment in a mouse model of Rett syndrome. Front Neural Circuits 7: 42, 2013. doi: 10.3389/fncir.2013.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dick TE, Baekey DM, Paton JFR, Lindsey BG, Morris KF. Cardio-respiratory coupling depends on the pons. Respir Physiol Neurobiol 168: 76–85, 2009. doi: 10.1016/j.resp.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 28.Dick TE, Molkov YI, Nieman G, Hsieh YH, Jacono FJ, Doyle J, Scheff JD, Calvano SE, Androulakis IP, An G, Vodovotz Y. Linking inflammation, cardiorespiratory variability, and neural control in acute inflammation via computational modeling. Front Physiol 3: 222, 2012. doi: 10.3389/fphys.2012.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dutschmann M, Bautista TG, Mörschel M, Dick TE. Learning to breathe: habituation of Hering-Breuer inflation reflex emerges with postnatal brainstem maturation. Respir Physiol Neurobiol 195: 44–49, 2014. doi: 10.1016/j.resp.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dutschmann M, Dick TE. Pontine mechanisms of respiratory control. Compr Physiol 2: 2443–2469, 2012. doi: 10.1002/cphy.c100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dutschmann M, Herbert H. The Kölliker-Fuse nucleus gates the postinspiratory phase of the respiratory cycle to control inspiratory off-switch and upper airway resistance in rat. Eur J Neurosci 24: 1071–1084, 2006. doi: 10.1111/j.1460-9568.2006.04981.x. [DOI] [PubMed] [Google Scholar]
- 32.Dutschmann M, Menuet C, Stettner GM, Gestreau C, Borghgraef P, Devijver H, Gielis L, Hilaire G, Van Leuven F. Upper airway dysfunction of Tau-P301L mice correlates with tauopathy in midbrain and ponto-medullary brainstem nuclei. J Neurosci 30: 1810–1821, 2010. doi: 10.1523/JNEUROSCI.5261-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dutschmann M, Mörschel M, Rybak IA, Dick TE. Learning to breathe: control of the inspiratory-expiratory phase transition shifts from sensory- to central-dominated during postnatal development in rats. J Physiol 587: 4931–4948, 2009. doi: 10.1113/jphysiol.2009.174599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ellenberger HH, Vera PL, Haselton JR, Haselton CL, Schneiderman N. Brainstem projections to the phrenic nucleus: an anterograde and retrograde HRP study in the rabbit. Brain Res Bull 24: 163–174, 1990. doi: 10.1016/0361-9230(90)90201-A. [DOI] [PubMed] [Google Scholar]
- 36.von Euler C. On the central pattern generator for the basic breathing rhythmicity. J Appl Physiol Respir Environ Exerc Physiol 55: 1647–1659, 1983. [DOI] [PubMed] [Google Scholar]
- 37.Ezure K, Tanaka I. Distribution and medullary projection of respiratory neurons in the dorsolateral pons of the rat. Neuroscience 141: 1011–1023, 2006. doi: 10.1016/j.neuroscience.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 38.Famm K, Litt B, Tracey KJ, Boyden ES, Slaoui M. Drug discovery: a jump-start for electroceuticals. Nature 496: 159–161, 2013. doi: 10.1038/496159a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fiamma M-N, Straus C, Thibault S, Wysocki M, Baconnier P, Similowski T. Effects of hypercapnia and hypocapnia on ventilatory variability and the chaotic dynamics of ventilatory flow in humans. Am J Physiol Regul Integr Comp Physiol 292: R1985–R1993, 2007. doi: 10.1152/ajpregu.00792.2006. [DOI] [PubMed] [Google Scholar]
- 40.Galán RF. Cellular mechanisms underlying spike-time reliability and stochastic synchronization: insights and predictions from the phase-response curve [Online]. In: Phase Response Curves in Neuroscience, edited by Schultheiss NW, Prinz AA, Butera RJ. New York: Springer, 2011, p. 237–255. doi: 10.1007/978-1-4614-0739-3_10. [DOI] [Google Scholar]
- 41.Gaytán SP, Calero F, Núñez-Abades PA, Morillo AM, Pásaro R. Pontomedullary efferent projections of the ventral respiratory neuronal subsets of the rat. Brain Res Bull 42: 323–334, 1997. doi: 10.1016/S0361-9230(96)00292-4. [DOI] [PubMed] [Google Scholar]
- 42.Glass L, Graves C, Petrillo GA, Mackey MC. Unstable dynamics of a periodically driven oscillator in the presence of noise. J Theor Biol 86: 455–475, 1980. doi: 10.1016/0022-5193(80)90345-8. [DOI] [PubMed] [Google Scholar]
- 43.Glass L, Mackey MC. A simple model for phase locking of biological oscillators. J Math Biol 7: 339–352, 1979. doi: 10.1007/BF00275153. [DOI] [PubMed] [Google Scholar]
- 44.Goldwyn JH, Imennov NS, Famulare M, Shea-Brown E. Stochastic differential equation models for ion channel noise in Hodgkin-Huxley neurons. Phys Rev E Stat Nonlin Soft Matter Phys 83: 041908, 2011. doi: 10.1103/PhysRevE.83.041908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Graves C, Glass L, Laporta D, Meloche R, Grassino A. Respiratory phase locking during mechanical ventilation in anesthetized human subjects. Am J Physiol Regul Integr Comp Physiol 250: R902–R909, 1986. [DOI] [PubMed] [Google Scholar]
- 46.Guthmann A, Fritschy J-M, Ottersen OP, Torp R, Herbert H. GABA, GABA transporters, GABAA receptor subunits, and GAD mRNAs in the rat parabrachial and Kölliker-Fuse nuclei. J Comp Neurol 400: 229–243, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 47.Guz A, Noble MI, Trenchard D, Cochrane HL, Makey AR. Studies on the vagus nerves in man: their role in respiratory and circulatory control. Clin Sci 27: 293–304, 1964. [PubMed] [Google Scholar]
- 48.Hayashi F, Coles SK, McCrimmon DR. Respiratory neurons mediating the Breuer-Hering reflex prolongation of expiration in rat. J Neurosci 16: 6526–6536, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Herbert H, Moga MM, Saper CB. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J Comp Neurol 293: 540–580, 1990. doi: 10.1002/cne.902930404. [DOI] [PubMed] [Google Scholar]
- 50.Hering KEK. Die Selbststeuerung der Athmung durch den Nervus vagus. Sitzungsberichte Kais Akad Wiss 57: 672–677, 1868. [Google Scholar]
- 51.Hilaire G, Voituron N, Menuet C, Ichiyama RM, Subramanian HH, Dutschmann M. The role of serotonin in respiratory function and dysfunction. Respir Physiol Neurobiol 174: 76–88, 2010. doi: 10.1016/j.resp.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hon EH, Lee ST. Electronic evaluation of the fetal heart rate. VIII. Patterns preceding fetal death, further observations. Am J Obstet Gynecol 87: 814–826, 1963. [PubMed] [Google Scholar]
- 53.Hsieh YH, Siegel RE, Dick TE. Pontine GABAergic pathways: role and plasticity in the hypoxic ventilatory response. Respir Physiol Neurobiol 143: 141–153, 2004. doi: 10.1016/j.resp.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 54.Jacono FJ, Dick TE. Variability, measuring the spice of life. J Appl Physiol (1985) 111: 351–352, 2011. doi: 10.1152/japplphysiol.00786.2011. [DOI] [PubMed] [Google Scholar]
- 55.Jacono FJ, Mayer CA, Hsieh YH, Wilson CG, Dick TE. Lung and brainstem cytokine levels are associated with breathing pattern changes in a rodent model of acute lung injury. Respir Physiol Neurobiol 178: 429–438, 2011. doi: 10.1016/j.resp.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jodkowski JS, Coles SK, Dick TE. Prolongation in expiration evoked from ventrolateral pons of adult rats. J Appl Physiol (1985) 82: 377–381, 1997. [DOI] [PubMed] [Google Scholar]
- 57.St. John WM, Glasser RL, King RA. Apneustic breathing after vagotomy in cats with chronic pneumotaxic center lesions. Respir Physiol 12: 239–250, 1971. doi: 10.1016/0034-5687(71)90056-9. [DOI] [PubMed] [Google Scholar]
- 58.Kleiger RE, Miller JP, Bigger JT Jr, Moss AJ. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol 59: 256–262, 1987. doi: 10.1016/0002-9149(87)90795-8. [DOI] [PubMed] [Google Scholar]
- 59.Koo BB, Strohl KP, Gillombardo CB, Jacono FJ. Ventilatory patterning in a mouse model of stroke. Respir Physiol Neurobiol 172: 129–135, 2010. doi: 10.1016/j.resp.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kubin L, Alheid GF, Zuperku EJ, McCrimmon DR. Central pathways of pulmonary and lower airway vagal afferents. J Appl Physiol (1985) 101: 618–627, 2006. doi: 10.1152/japplphysiol.00252.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kuramoto Y. Chemical Oscillations, Waves, and Turbulence. Berlin: Springer Verlag, 1984. doi: 10.1007/978-3-642-69689-3. [DOI] [Google Scholar]
- 62.Lake DE, Richman JS, Griffin MP, Moorman JR. Sample entropy analysis of neonatal heart rate variability. Am J Physiol Regul Integr Comp Physiol 283: R789–R797, 2002. doi: 10.1152/ajpregu.00069.2002. [DOI] [PubMed] [Google Scholar]
- 63.Li A, Nattie E. Catecholamine neurones in rats modulate sleep, breathing, central chemoreception and breathing variability. J Physiol 570: 385–396, 2006. doi: 10.1113/jphysiol.2005.099325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu Q, Wong-Riley MTT. Postnatal developmental expressions of neurotransmitters and receptors in various brain stem nuclei of rats. J Appl Physiol (1985) 98: 1442–1457, 2005. doi: 10.1152/japplphysiol.01301.2004. [DOI] [PubMed] [Google Scholar]
- 65.Luck JC. Afferent vagal fibres with an expiratory discharge in the rabbit. J Physiol 211: 63–71, 1970. doi: 10.1113/jphysiol.1970.sp009266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.MacDonald SM, Song G, Poon C-S. Nonassociative learning promotes respiratory entrainment to mechanical ventilation. PLoS One 2: e865, 2007. doi: 10.1371/journal.pone.0000865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mackey MC, Glass L. Oscillation and chaos in physiological control systems. Science 197: 287–289, 1977. doi: 10.1126/science.267326. [DOI] [PubMed] [Google Scholar]
- 68.Manabe M, Ezure K. Decrementing expiratory neurons of the Bötzinger complex. I. Response to lung inflation and axonal projection. Exp Brain Res 72: 150–158, 1988. doi: 10.1007/BF00248510. [DOI] [PubMed] [Google Scholar]
- 69.Marckwald M. Die Athembewegungen und deren Innervation beim Kaninchen. Z Biol 23: 149–283, 1887. [Google Scholar]
- 70.McMullan S, Dick TE, Farnham MMJ, Pilowsky PM. Effects of baroreceptor activation on respiratory variability in rat. Respir Physiol Neurobiol 166: 80–86, 2009. doi: 10.1016/j.resp.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Menuet C, Cazals Y, Gestreau C, Borghgraef P, Gielis L, Dutschmann M, Van Leuven F, Hilaire G. Age-related impairment of ultrasonic vocalization in Tau.P301L mice: possible implication for progressive language disorders. PLoS One 6: e25770, 2011. doi: 10.1371/journal.pone.0025770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Molkov YI, Shevtsova NA, Park C, Ben-Tal A, Smith JC, Rubin JE, Rybak IA. A closed-loop model of the respiratory system: focus on hypercapnia and active expiration. PLoS One 9: e109894, 2014. doi: 10.1371/journal.pone.0109894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moore J, Haouzi P, Van de Louw A, Bell HJ. Hypocapnia-dependent facilitation of augmented breaths: observations in awake vs. anesthetized rats. Respir Physiol Neurobiol 180: 105–111, 2012. doi: 10.1016/j.resp.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 74.Moore MW, Chai S, Gillombardo CB, Carlo A, Donovan LM, Netzer N, Strohl KP. Two weeks of buspirone protects against posthypoxic ventilatory pauses in the C57BL/6J mouse strain. Respir Physiol Neurobiol 183: 35–40, 2012. doi: 10.1016/j.resp.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 75.Morrison SF, Cravo SL, Wilfehrt HM. Pontine lesions produce apneusis in the rat. Brain Res 652: 83–86, 1994. doi: 10.1016/0006-8993(94)90320-4. [DOI] [PubMed] [Google Scholar]
- 76.Muzzin S, Baconnier P, Benchetrit G. Entrainment of respiratory rhythm by periodic lung inflation: effect of airflow rate and duration. Am J Physiol Regul Integr Comp Physiol 263: R292–R300, 1992. [DOI] [PubMed] [Google Scholar]
- 77.Ogier M, Wang H, Hong E, Wang Q, Greenberg ME, Katz DM. Brain-derived neurotrophic factor expression and respiratory function improve after ampakine treatment in a mouse model of Rett syndrome. J Neurosci 27: 10,912–10,917, 2007. doi: 10.1523/JNEUROSCI.1869-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Oku Y, Dick TE. Phase resetting of the respiratory cycle before and after unilateral pontine lesion in cat. J Appl Physiol (1985) 72: 721–730, 1992. [DOI] [PubMed] [Google Scholar]
- 79.Papaioannou VE, Chouvarda IG, Maglaveras NK, Pneumatikos IA. Study of multiparameter respiratory pattern complexity in surgical critically ill patients during weaning trials. BMC Physiol 11: 2, 2011. doi: 10.1186/1472-6793-11-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Petrillo GA, Glass L. A theory for phase locking of respiration in cats to a mechanical ventilator. Am J Physiol Regul Integr Comp Physiol 246: R311–R320, 1984. [DOI] [PubMed] [Google Scholar]
- 81.Petrillo GA, Glass L, Trippenbach T. Phase locking of the respiratory rhythm in cats to a mechanical ventilator. Can J Physiol Pharmacol 61: 599–607, 1983. doi: 10.1139/y83-092. [DOI] [PubMed] [Google Scholar]
- 82.Pincus SM, Goldberger AL. Physiological time-series analysis: what does regularity quantify? Am J Physiol Heart Circ Physiol 266: H1643–H1656, 1994. [DOI] [PubMed] [Google Scholar]
- 83.van der Pol B, van der Mark J. LXXII. The heartbeat considered as a relaxation oscillation, and an electrical model of the heart. Lond Edinb Dublin Philos Mag J Sci 6: 763–775, 1928. doi: 10.1080/14786441108564652. [DOI] [Google Scholar]
- 84.Poon C-S, Song G. Bidirectional plasticity of pontine pneumotaxic postinspiratory drive: implication for a pontomedullary respiratory central pattern generator. Prog Brain Res 209: 235–254, 2014. doi: 10.1016/B978-0-444-63274-6.00012-6. [DOI] [PubMed] [Google Scholar]
- 85.Potts JT, Rybak IA, Paton JFR. Respiratory rhythm entrainment by somatic afferent stimulation. J Neurosci 25: 1965–1978, 2005. doi: 10.1523/JNEUROSCI.3881-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Puzerey PA, Galán RF. On how correlations between excitatory and inhibitory synaptic inputs maximize the information rate of neuronal firing. Front Comput Neurosci 8: 59, 2014. doi: 10.3389/fncom.2014.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ren J, Poon BY, Tang Y, Funk GD, Greer JJ. Ampakines alleviate respiratory depression in rats. Am J Respir Crit Care Med 174: 1384–1391, 2006. doi: 10.1164/rccm.200606-778OC. [DOI] [PubMed] [Google Scholar]
- 88.Rubin JE, Shevtsova NA, Ermentrout GB, Smith JC, Rybak IA. Multiple rhythmic states in a model of the respiratory central pattern generator. J Neurophysiol 101: 2146–2165, 2009. doi: 10.1152/jn.90958.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rybak IA, Abdala APL, Markin SN, Paton JFR, Smith JC. Spatial organization and state-dependent mechanisms for respiratory rhythm and pattern generation. Prog Brain Res 165: 201–220, 2007. doi: 10.1016/S0079-6123(06)65013-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rybak IA, Shevtsova NA, Paton JFR, Dick TE. St. John WM, Mörschel M, Dutschmann M. Modeling the ponto-medullary respiratory network. Respir Physiol Neurobiol 143: 307–319, 2004. doi: 10.1016/j.resp.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 91.Ryter D, Talkner P, Hänggi P. Rate of phase slips of a driven Van der Pol oscillator at low noise. Phys Lett A 93: 447–450, 1983. doi: 10.1016/0375-9601(83)90627-8. [DOI] [Google Scholar]
- 92.Saha S, Sieghart W, Fritschy J-M, McWilliam PN, Batten TFC. γ-Aminobutyric acid receptor (GABAA) subunits in rat nucleus tractus solitarii (NTS) revealed by polymerase chain reaction (PCR) and immunohistochemistry. Mol Cell Neurosci 17: 241–257, 2001. doi: 10.1006/mcne.2000.0919. [DOI] [PubMed] [Google Scholar]
- 93.Sammon MP, Bruce EN. Vagal afferent activity increases dynamical dimension of respiration in rats. J Appl Physiol (1985) 70: 1748–1762, 1991. [DOI] [PubMed] [Google Scholar]
- 94.Schelegle ES, Green JF. An overview of the anatomy and physiology of slowly adapting pulmonary stretch receptors. Respir Physiol 125: 17–31, 2001. doi: 10.1016/S0034-5687(00)00202-4. [DOI] [PubMed] [Google Scholar]
- 95.Schmandt NT, Galán RF. Stochastic-shielding approximation of Markov chains and its application to efficiently simulate random ion-channel gating. Phys Rev Lett 109: 118101, 2012. doi: 10.1103/PhysRevLett.109.118101. [DOI] [PubMed] [Google Scholar]
- 96.Schneidman E, Freedman B, Segev I. Ion channel stochasticity may be critical in determining the reliability and precision of spike timing. Neural Comput 10: 1679–1703, 1998. doi: 10.1162/089976698300017089. [DOI] [PubMed] [Google Scholar]
- 97.Shiogai Y, Stefanovska A, McClintock PVE. Nonlinear dynamics of cardiovascular ageing. Phys Rep 488: 51–110, 2010. doi: 10.1016/j.physrep.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Simon PM, Habel AM, Daubenspeck JA, Leiter JC. Vagal feedback in the entrainment of respiration to mechanical ventilation in sleeping humans. J Appl Physiol 89: 760–769, 2000. [DOI] [PubMed] [Google Scholar]
- 99.Simon PM, Zurob AS, Wies WM, Leiter JC, Hubmayr RD, Jensen ML, Stroetz RW. Entrainment of respiration in humans by periodic lung inflations. Effect of state and CO2. Am J Respir Crit Care Med 160: 950–960, 1999. doi: 10.1164/ajrccm.160.3.9712057. [DOI] [PubMed] [Google Scholar]
- 100.Siniaia MS, Young DL, Poon C-S. Habituation and desensitization of the Hering-Breuer reflex in rat. J Physiol 523: 479–491, 2000. doi: 10.1111/j.1469-7793.2000.t01-1-00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Smith JC, Abdala APL, Koizumi H, Rybak IA, Paton JFR. Spatial and functional architecture of the mammalian brain stem respiratory network: a hierarchy of three oscillatory mechanisms. J Neurophysiol 98: 3370–3387, 2007. doi: 10.1152/jn.00985.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stankovski T, Cooke WH, Rudas L, Stefanovska A, Eckberg DL. Time-frequency methods and voluntary ramped-frequency breathing: a powerful combination for exploration of human neurophysiological mechanisms. J Appl Physiol (1985) 115: 1806–1821, 2013. doi: 10.1152/japplphysiol.00802.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Stankovski T, Duggento A, McClintock PVE, Stefanovska A. Inference of time-evolving coupled dynamical systems in the presence of noise. Phys Rev Lett 109: 024101, 2012. doi: 10.1103/PhysRevLett.109.024101. [DOI] [PubMed] [Google Scholar]
- 104.Stella G. On the mechanism of production, and the physiological significance of “apneusis”. J Physiol 93: 10–23, 1938. doi: 10.1113/jphysiol.1938.sp003621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Stettner GM, Zanella S, Hilaire G, Dutschmann M. 8-OH-DPAT suppresses spontaneous central apneas in the C57BL/6J mouse strain. Respir Physiol Neurobiol 161: 10–15, 2008. doi: 10.1016/j.resp.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 106.Stratonovich R. Topics in the Theory of Random Noise. New York: Gordon and Breach, 1963. [Google Scholar]
- 107.Suprunenko YF, Clemson PT, Stefanovska A. Chronotaxic systems: a new class of self-sustained nonautonomous oscillators. Phys Rev Lett 111: 024101, 2013. doi: 10.1103/PhysRevLett.111.024101. [DOI] [PubMed] [Google Scholar]
- 107a.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology . Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation 93: 1043–1065, 1996. doi: 10.1161/01.CIR.93.5.1043. [DOI] [PubMed] [Google Scholar]
- 108.Teulier M, Fiamma M-N, Straus C, Similowski T. Acute bronchodilation increases ventilatory complexity during resting breathing in stable COPD: toward mathematical biomarkers of ventilatory function? Respir Physiol Neurobiol 185: 477–480, 2013. doi: 10.1016/j.resp.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 109.Vallverdú M, Tibaduisa O, Clariá F, Hoyer D, Giraldo B, Benito S, Caminal P. Information flow to assess cardiorespiratory interactions in patients on weaning trials. Conf Proc IEEE Eng Med Biol Soc 1: 1462–1465, 2006. [DOI] [PubMed] [Google Scholar]
- 110.Van den Aardweg JG, Karemaker JM. Influence of chemoreflexes on respiratory variability in healthy subjects. Am J Respir Crit Care Med 165: 1041–1047, 2002. doi: 10.1164/ajrccm.165.8.2104100. [DOI] [PubMed] [Google Scholar]
- 111.Voss HU, Timmer J, Kurths J. Nonlinear dynamical system identification from uncertain and indirect measurements. Int J Bifurcat Chaos 14: 1905–1933, 2004. doi: 10.1142/S0218127404010345. [DOI] [Google Scholar]
- 112.Weese-Mayer DE, Lieske SP, Boothby CM, Kenny AS, Bennett HL, Ramirez J-M. Autonomic dysregulation in young girls with Rett Syndrome during nighttime in-home recordings. Pediatr Pulmonol 43: 1045–1060, 2008. doi: 10.1002/ppul.20866. [DOI] [PubMed] [Google Scholar]
- 113.Weese-Mayer DE, Lieske SP, Boothby CM, Kenny AS, Bennett HL, Silvestri JM, Ramirez J-M. Autonomic nervous system dysregulation: breathing and heart rate perturbation during wakefulness in young girls with Rett syndrome. Pediatr Res 60: 443–449, 2006. doi: 10.1203/01.pdr.0000238302.84552.d0. [DOI] [PubMed] [Google Scholar]
- 114.White CE, Batchinsky AI, Necsoiu C, Nguyen R, Walker KP III, Chung KK, Wolf SE, Cancio LC. Lower interbreath interval complexity is associated with extubation failure in mechanically ventilated patients during spontaneous breathing trials. J Trauma 68: 1310–1316, 2010. doi: 10.1097/TA.0b013e3181da90db. [DOI] [PubMed] [Google Scholar]
- 115.Yamauchi M, Dostal J, Strohl KP. Post-hypoxic unstable breathing in the C57BL/6J mouse: effects of acetazolamide. Adv Exp Med Biol 605: 75–79, 2008. doi: 10.1007/978-0-387-73693-8_13. [DOI] [PubMed] [Google Scholar]
- 116.Yamauchi M, Ocak H, Dostal J, Jacono FJ, Loparo KA, Strohl KP. Post-sigh breathing behavior and spontaneous pauses in the C57BL/6J (B6) mouse. Respir Physiol Neurobiol 162: 117–125, 2008. doi: 10.1016/j.resp.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yu H, Dhingra RR, Dick TE, Galan RF. Effects of ion-channel noise on neural circuits: an application to the respiratory pattern generator to investigate breathing variability. J Neurophysiol 2016, 2016. doi: 10.1152/jn.00416.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhu Y, Hsieh Y-H, Dhingra RR, Dick TE, Jacono FJ, Galán RF. Quantifying interactions between real oscillators with information theory and phase models: application to cardiorespiratory coupling. Phys Rev E Stat Nonlin Soft Matter Phys 87: 022709, 2013. doi: 10.1103/PhysRevE.87.022709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zuperku EJ, Hopp FA. Control of discharge patterns of medullary respiratory neurons by pulmonary vagal afferent inputs. Am J Physiol Regul Integr Comp Physiol 253: R809–R820, 1987. [DOI] [PubMed] [Google Scholar]