Abstract
Sustained hypertension is an important consequence of obstructive sleep apnea. An animal model of the hypoxemia associated with sleep apnea, chronic intermittent hypoxia (CIH), produces increased sympathetic nerve activity (SNA) and sustained increases in blood pressure. Many mechanisms have been implicated in the hypertension associated with CIH, including the role of ΔFosB within the median preoptic nucleus (MnPO). Also, the renin-angiotensin system (RAS) has been associated with CIH hypertension. We conducted experiments to determine the possible association of FosB/ΔFosB with a RAS component, angiotensin-converting enzyme 1 (ACE1), within the MnPO following 7 days of CIH. Retrograde tract tracing from the paraventricular nucleus (PVN), a downstream region of the MnPO, was used to establish a potential pathway for FosB/ΔFosB activation of MnPO ACE1 neurons. After CIH, ACE1 cells with FosB/ΔFosB expression increased colocalization with a retrograde tracer that was injected unilaterally within the PVN. Also, Western blot examination showed ACE1 protein expression increasing within the MnPO following CIH. Chromatin immunoprecipitation (ChIP) assays demonstrated an increase in FosB/ΔFosB association with the ACE1 gene within the MnPO following CIH. FosB/ΔFosB may transcriptionally target ACE1 within the MnPO following CIH to affect the downstream PVN region, which may influence SNA and blood pressure.
Keywords: sleep apnea, hypertension, median preoptic nucleus, angiotensin
sleep apnea is characterized by frequent interruptions in breathing that are specific to sleep and lead to hypoxemia, hypercapnia, increased thoracic pressure, arousal, and sleep fragmentation (12). According to a recent poll from The National Sleep Foundation, 26% of U.S. individuals are at high risk for developing obstructive sleep apnea (OSA) (24). Certain demographic groups have seen increases in OSA by as much as 55% over the last 20 yr (45). Furthermore, OSA is associated with multiple cardiovascular diseases, including hypertension (12, 21, 37–39, 41, 42, 46, 47, 58, 62, 65). Patients with OSA have a sustained hypertension that fails to subside even during waking hours (38). Increased sympathetic nerve activity (SNA) that may lead to this sustained hypertension has been observed in OSA patients (6, 56, 57, 68). The increase in SNA associated with OSA suggests that central nervous system (CNS) mechanisms may contribute to the cardiovascular sequelae associated with this disorder.
An experimental model of sleep apnea, chronic intermittent hypoxia (CIH), was first developed by Fletcher et al. (18, 19) and mimics the arterial hypoxemia seen in sleep apnea patients and results in an analogous increase in SNA and sustained hypertension (15, 16, 63). Several CNS mechanisms have been proposed that contribute to CIH hypertension, such as chemoreceptor sensitization (12, 18). The renin-angiotensin system (RAS) has been shown to contribute to CIH hypertension (11, 17, 20, 30). In addition, several CNS regions that are not directly related to the chemoreceptor reflex have been investigated for their role in CIH hypertension (22, 28). The paraventricular nucleus (PVN) of the hypothalamus has been shown to contribute to CIH hypertension (9, 11, 28, 54). Several studies indicate that the lamina terminalis, which has been linked with several models of neurogenic hypertension and the RAS (5), may contribute to CIH hypertension (10, 52). The lamina terminalis contains two circumventricular organs, the subfornical organ (SFO) and the organum vasculosum of the lamina terminalis (OVLT), located dorsally and ventrally along the anterior wall of the third ventricle. The median preoptic nucleus (MnPO) is situated between the two circumventricular organs and has a functional blood-brain-barrier. The MnPO projects to the PVN (2, 49) and may influence SNA and blood pressure (14, 49, 59–61). In CIH, the transcription factor FosB/ΔFosB is significantly increased in the MnPO, and inhibition of ΔFosB in this region blocks the sustained component of CIH hypertension (10, 29). These observations suggest that FosB/ΔFosB-mediated changes in gene expression play an essential role specifically in the sustained hypertension associated with CIH (10). Our working hypothesis is that changes in the expression of one or more of these FosB/ΔFosB target genes in the MnPO contribute to the sustained component of CIH hypertension (10). One of the candidate genes identified in this study is angiotensin-converting enzyme 1 (ACE1).
Increased ACE1 expression within the MnPO may be a prohypertensive switch that gets chronically activated during CIH by FosB/ΔFosB promoting the inappropriate sustained blood pressure increase. ACE1 is a well-known component of RAS that converts ANG I to ANG II. The CNS has been shown to have a RAS that is separate from the systemic RAS (3, 66). Furthermore, ANG II has been shown to affect SNA (1, 4, 13, 31, 33, 67). We conducted experiments to test the hypothesis that PVN-projecting MnPO neurons are activated by CIH, leading to FosB/ΔFosB-dependent increases in ACE1 expression. To test this hypothesis, we used retrograde tract tracing in combination with immunohistochemistry to determine the colocalization of ACE1 and FosB/ΔFosB in MnPO neurons that project to the PVN. We used ChIP to test the association of FosB/ΔFosB with the ACE1 gene in MnPO following CIH treatment.
METHODS
Animals.
Adult male Sprague-Dawley rats (250–300 g body wt; Charles River Laboratories, Wilmington, MA) were maintained on a 12:12-h light-dark cycle (lights on at 0600) and individually housed with ad libitum food and water. All animal procedures were conducted in accordance with current National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use Committee at the University of North Texas Health Science Center.
Retrograde tract tracing.
Rats were anesthetized with isoflurane (2–3%) and were placed in a stereotaxic frame after their scalps were shaved and cleaned with betadine and alcohol. Each rat received a unilateral PVN injection with a retrograde tracer, Fluorogold (Fluorochrome, Denver, CO). Each injection was made using a 30-gauge injector at a volume of 200 nl over a 5-min period at atlas-defined coordinates of −1.80 mm (anterior/posterior), −0.4 mm (medial/lateral), and −7.6 mm (dorsal/ventral) (44). After the injection, the holes made in their skulls were filled with sterile gel foam, and their scalps were closed with sterile absorbable suture. Rats were given at least 1 wk to recover before telemetry implantation.
Radio telemetry transmitter implantation.
Rats were implanted with an abdominal aortic catheter attached to a CA11PA-C40 radio-telemetry transmitter using isoflurane anesthesia (2–3%), as described previously (29). Each rat was allowed to recover for at least 1 wk after the telemetry surgery. A Dataquest radio-telemetry system (Data Sciences, St. Paul, MN) was used to record mean arterial blood pressure (MAP), respiratory rate (RR), and heart rate (HR). All physiological measurements were monitored by radio telemetry and sampled for 10 s every 10 min. Data are expressed as changes from baseline.
Chronic intermittent hypoxia treatment.
Baseline radio-telemetry recording occurred for 5 days before the start of the CIH protocol. CIH exposure was applied for 8 h beginning at 0800 of the light phase using nitrogen to generate a 3-min hypoxia (10% O2)/3-min normoxia (21% O2) cycle, as described previously (29). During the remaining 16 h (1600–0800), the chambers were open to normoxic room air (21% O2). Rats were exposed to CIH for 7 days. Controls were placed in identical chambers within the same room but were only exposed to room air (21% O2). All animals were euthanized on the 8th day following the 7-day CIH protocol.
Immunohistochemistry.
Rats used for the immunohistochemical studies were anesthetized with Inactin (100 mg/kg ip) and perfused with 0.1 M PBS (100–200 ml) followed by 4% paraformaldehyde (400–500 ml), as previously described (29). Brains were then postfixed overnight and dehydrated in 30% sucrose. Each brain was cut into three sets of serial 40-μm coronal sections using a crytostat (Leica Biosystems, Buffalo Grove, IL). The sections were stored in cryoprotectant at −20°C until the immunohistochemistry protocol. Sections were stained, as previously described (29, 30), for FosB (1:1,000 goat polyclonal sc-48869; Santa Cruz Biotechnology, Dallas, TX), GFAP (1:1,500 mouse monoclonal G3893, Sigma-Aldrich, St. Louis, MO), or ACE1 (1:500 rabbit polyclonal sc-20791; Santa Cruz Biotechnology). The FosB antibody binds to both FosB and the more stable splice variant ΔFosB, and thus, staining will be denoted as FosB/ΔFosB. Staining for FosB/ΔFosB was processed with a biotinylated secondary antibody and avidin-biotin conjugated with horseradish peroxidase and diaminobenzidine (DAB) staining. Tissue processed for FosB/ΔFosB DAB staining were incubated with a biotinylated horse anti-goat IgG (1:200; Vector Laboratories, Burlingame, CA), then treated with an avidin-peroxidase conjugate (Vectastain ABC kit; Vector Laboratories) following PBS containing 0.04% 3,3′-diaminobenzidine hydrochloride and 0.04% nickel ammonium sulfate for 11 min. Tissue sections were then mounted to gel-coated slides, allowed to dry for one day, and then dehydrated with serial ethanol solutions and xylene. Slides were then coverslipped with Permount mounting medium (ThermoScientific, Waltham, MA). Staining of ACE1 was visualized using a Cy3 anti-rabbit (1:200; Jackson ImmunoResearch, West Grove, PA), and GFAP staining was visualized using a Cy2 anti-mouse (1:200; Jackson ImmunoResearch). Tissue was then imaged using an Olympus (Olympus BX41) fluorescent microscope or an inverted microscope (Olympus BX50) equipped with a spinning disk confocal unit (Olympus IX 2-DSU) and epifluorescence. Images were collected using a Retiga-SRV camera (Q-imaging, Surrey, British Columbia, Canada). ImageJ was used to analyze and count labeled cells for each section.
Western blot analysis.
The day after our 7-day CIH protocol, inactin (100 mg/kg ip)-anesthetized rats were decapitated, and each brain was placed dorsal surface down in a commercially available brain matrix (Stoelting) in order to cut the brain into 1-mm coronal slabs with razor blades. Punch samples were collected from the slabs using 1-ml syringes equipped with blunt 23-gauge needles. The punches were ejected into microcentrifuge tubes and frozen at −80°C until protein isolations and Western blot analysis were performed, as previously described (7, 51). Two to three MnPO punches from each rat were dissolved in Laemmli buffer and run on a 12% acrylamide gradient SDS gel (Nupage Bris-Tris, Invitrogen, Carlsbad, CA) and transferred to a nitrocellulose membrane. Blots were washed, blocked, and then incubated with primary antibodies for ACE1 (1:100 rabbit polyclonal; SantaCruz Biotechnology) and GAPDH (1:2,000 mouse monoclonal; EMD Millipore, Millipore Sigma, St. Louis, MO) as a control. Blots were washed again then incubated with secondary antibodies, according to the primary host species. Proteins were detected by chemiluminescent reagents (Thermo Fisher Scientific), imaged using G-Box (Syngene, Fredrick, MD), and then analyzed for densitometry using ImageJ.
Chromatin immunoprecipitation analysis.
Punch samples of the MnPO were collected as described above for Western blot analysis. Two MnPO punches (dorsal and ventral to the anterior commissure) from the same rat were taken from the slabs using 1-ml syringes with blunt 23-gauge needles. Punches containing the MnPO from two rats from the same treatment group were pooled and placed into the same microcentrifuge tube. All samples were kept frozen at −80°C until chromatin immunoprecipitation analysis (ChIP) assays were performed on the following day. Pooled punches from two rats, four punches total, were needed to obtain sufficient material to execute the ChIP assay. The LowCell# ChIP kit (kch-maglow-G16; Diagenode, Denville, NJ) was utilized according to the manufacturer’s tissue protocol in order to analyze the association between FosB/ΔFosB and ACE1. Samples were sheared on ice using a model 150 Sonic Dismembrator (Fisher Scientific, Pittsburg, PA) and were subjected to four rounds of 10 s on 30 s off at 100% AMPS to achieve a base pair size between 100 and 1,000 base pairs. A ChIP grade FosB primary antibody [2 µg, (102: sc-48) GX goat polyclonal IgG, Santa Cruz Biotechnology] was used to detect FosB/ΔFosB bound to DNA, while a normal goat IgG polyclonal antibody (2 µg; Abcam, Cambridge, MA) was used as a negative control. FosB and goat IgG isolated DNA samples were then subjected to quantitative PCR (qPCR) using iQ SYBR green Supermix (Bio-Rad Laboratories, Hercules, CA) and ACE1 primers (forward 5′-CCCGGAAATACGAAGAATTGC-3′ and reverse 5′-GGCTCTCCCCACCTTGTCTC-3′). Data were normalized to input background.
Statistical analysis.
Data from immunohistochemistry studies were analyzed using one-way ANOVA or Kruskal-Wallis nonparametric tests. Data from the radio-telemetry experiments were analyzed using two-way repeated-measures ANOVA with Student-Newman-Keuls t-tests for post hoc analysis. Data from Western blot analyses and ChIP qPCR were analyzed using independent t-tests. All tests were performed using SigmaPlot (v. 12.0, Systat Software, San Diego, CA). Differences were considered statistically significant at P < 0.05. Data are reported as means ± SE.
RESULTS
MAP, HR, and RR measurements following CIH.
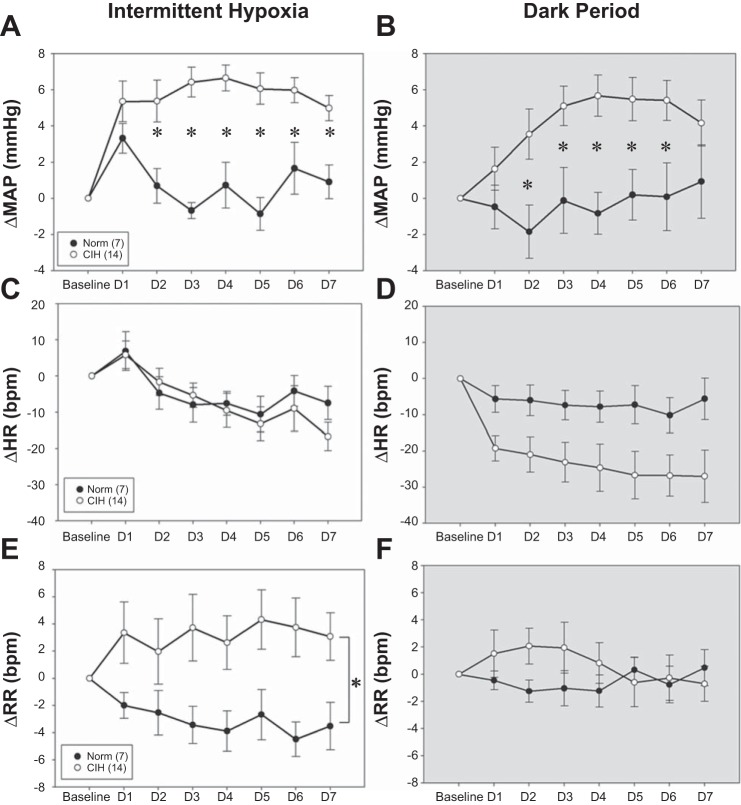
Baseline MAP and RR measurements were not significantly different between the groups before CIH exposure, but baseline HR was significantly lower in the CIH group (Table 1). On the first day of treatment, MAP was not significantly different between the normoxic- (CON = 7) and hypoxia (CIH = 14)-treated rats for either the dark phase or the light phase during intermittent hypoxia administration. However, changes in MAP of the CIH treatment group significantly increased from day 2 to day 7, as compared with the CON controls in both the light phase [Fig. 1A, Treatment × Day F(6,114) = 4.21; P < 0.05] and the normoxic dark phase [Fig. 1B, Treatment × Day F(6,114) = 2.63; P < 0.05]. These results are comparable to our previous studies (29). There were no significant differences in the changes in HR, CON, and CIH treatment groups in the light phase [Fig. 1C, Treatment × Day F(1,19) = 0.83, Treatment × Day F(6,114) = 0.95; P > 0.05] or the dark phase [Fig. 1D, Treatment × Day F(1,19) = 3.93, P = 0.062; Treatment × Day F(6,114) = 0.716, P > 0.05], although there was a trend for HR to be lower in the CIH-treated rats during the normoxic dark phase. Significant increases in RR were observed in the CIH treatment group during the light phase as compared with CON controls [Fig. 1E, Treatment × Day F(1,19) = 14.38, P < 0.05], which was expected since CIH is known to activate the chemoreflex (48). No significant difference was found among the RR in the dark period, while CIH treatment was not active [Fig. 1F, Treatment × Day F(1,19) = 0.084, P > 0.05].
Table 1.
Mean arterial pressure, heart rates, and respiratory rates recorded from rats during four baseline periods before 7 days of normoxia or chronic intermittent hypoxia
| Baseline Period | MAP, mmHg | HR, bpm | RR, bpm | |
|---|---|---|---|---|
| CON | 0800–1600 | 93.9 ± 3.8 | 346 ± 6 | 100 ± 2 |
| n = 7 | 1800–0600 | 98.2 ± 4.5 | 411 ± 3 | 99 ± 1 |
| CIH | 0800–1600 | 91.1 ± 1.4 | 318 ± 4* | 99 ± 2 |
| n = 14 | 1800–0600 | 94.7 ± 1.5 | 372 ± 4* | 101 ± 1 |
Data are expressed as means ± SE. Averages were calculated for 0800–1600 when the intermittent hypoxia exposures occurred during the chronic intermittent hypoxia (CIH) protocol or for 1800–0600, which was the dark phase. MAP, mean arterial pressure; HR, heart rate; RR, respiratory rates; CON, control.
P < 0.05.
Fig. 1.
Effects of chronic intermittent hypoxia (CIH) on changes in mean arterial pressure (MAP; A and B), heart rate (HR; C and D), and respiratory rate (RR; E and F) in normoxic controls (Norm; ●, n = 7) and rats exposed to CIH for 7 days (CIH, ○, n = 14). Brackets and symbols indicate significant differences (P < 0.05, two-way repeated-measures ANOVA and Student-Newman-Keuls test).
ACE1 immunohistochemistry.
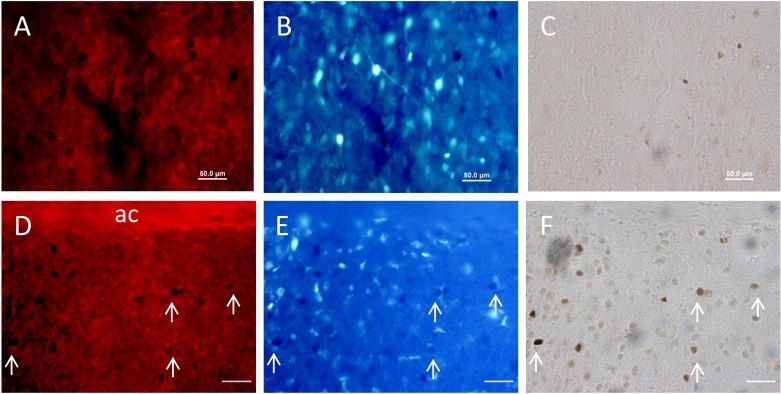
ACE1 staining was visually expressed in both dorsal and ventral portions of the MnPO. The dorsal portion on the MnPO lies between the anterior commissure and the lower border of the SFO, while the ventral portion exists between the anterior commissure and the dorsal cap of the OVLT, as previously described by McKinley et al. (36). ACE1 staining was not colocalized with GFAP in the MnPO of rats from either the normoxic control or the CIH treatment group (Fig. 2). These results suggest that in the MnPO, ACE1 is not expressed by GFAP-positive astrocytes (Fig. 2).
Fig. 2.
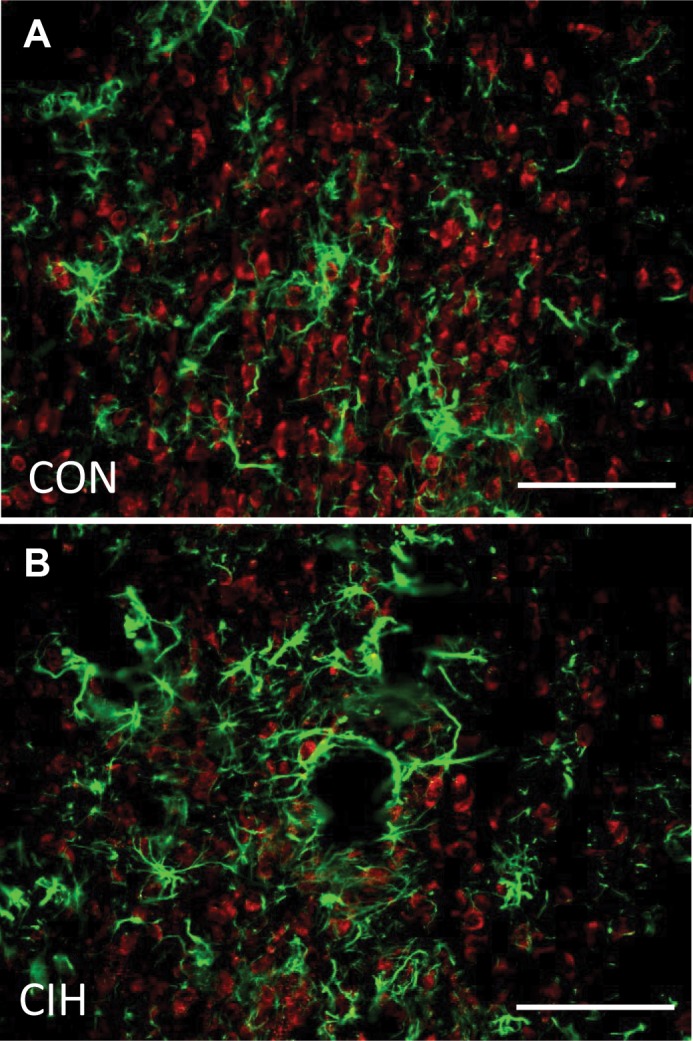
Digital images of the same sections showing immunohistochemical staining for merged images of ACE1 (red) and GFAP (green) in the ventral median preoptic nucleus (MnPO) from a normoxic control (CON; A) and a rat exposed to CIH (B).
Effect of CIH on ACE1/FosB projecting to the PVN.
Figure 3 shows a representative Fluorogold injection site that included the PVN. Fluorogold was injected primarily into the dorsal and the medial parvocellular region of the PVN with some inclusion of the lateral magnocellular region. Fluorogold spread was noted going dorsally and laterally beyond the PVN injection site in the reuniens thalamic nucleus region, as well as the zona incerta. Brains with similar injection sites were used in the analysis of ACE1 and FosB/ΔFosB colocalization with Fluorogold. Rats with injections sites that did not include the PVN were excluded from the analysis. The average numbers of retrogradely labeled MnPO neurons were similar between the two groups [CON (n = 5) 41.4 ± 10.6 cells per section; CIH (n = 6) 56.7 ± 11.9; t (9) = 0.25, P > 0.05]. The Fluorogold-positive cells were distributed in both the dorsal and ventral portions of the MnPO. A significant CIH-dependent increase in the numbers of FosB/ΔFosB-positive cells within the MnPO was observed as compared with normoxic controls [H(1) = 7.50, P < 0.05; Figs. 3 and 4]. FosB/ΔFosB-positive cells seemed to be in both the dorsal and ventral regions of the MnPO, with the majority being in the ventral portion of the MnPO. MnPO cells that were positive for both FosB/ΔFosB and ACE1 significantly increased following CIH as compared with normoxic controls [H(1) = 7.569, P < 0.05; Figs. 3 and 4]. Also CIH increased FosB/ΔFosB staining in PVN-projecting cells, as indicated by a significant increase in the numbers of Fluorogold-positive cells that were stained for FosB/ΔFosB [H(1) = 7.534, P < 0.05; Figs. 3 and 4]. The number of cells expressing all three (FosB/ΔFosB, ACE1, and Fluorogold) labels within the MnPO significantly increased following CIH and were mainly found within the ventral portion of the MnPO [F(1, 9) = 26.4, P < 0.05; Figs. 3 and 4].
Fig. 3.
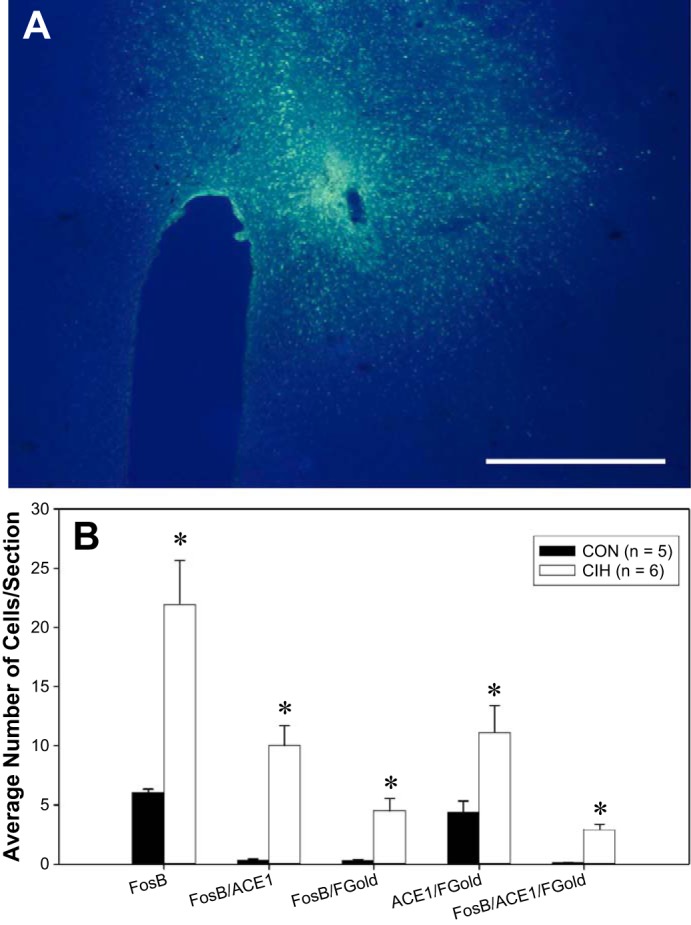
A: digital image of a representative unilateral injection site containing the paraventricular nucleus of the hypothalamus (PVN). Labeling is evident in both parvocellular and magnocellular portions of the PVN, while some Fluorogold is also dorsal and lateral to nucleus. B: summary data showing the average numbers of FosB, ACE1, or Fluorogold (Fgold)-labeled cells in the MnPO of rats exposed to normoxia (CON, n = 5) or chronic intermittent hypoxia (CIH, n = 6). *Significant difference, P < 0.05.
Fig. 4.
Digital images of ACE1 immunostaining (A and D), Fluorogold retrograde labeling from the PVN (B and E) and FosB immunostaining (C and F) in the ventral MnPO. The top row of images are all from the same section from a normoxic control rat (A–C) and the bottom row of images are from a rat exposed to 7 days of CIH (D–F). Arrows illustrate triple-labeled cells in the images from the CIH-treated rat (D–F). Scale bars in D–F are 75 µm. ac, anterior commissure.
Association of FosB with ACE1.
Western blot analysis demonstrated that ACE1 protein abundance in MnPO significantly increased following CIH (n = 9) as compared with normoxic controls (n = 6) when normalized to GAPDH [t (13) = −2.551, P < 0.05, Fig. 5A]. Studies utilizing ChIP analyses were used so that we could examine the possible interaction of FosB/ΔFosB with ACE1 since the gene for ACE1 contains an AP-1 regulatory site. MnPO samples were pooled from two rats of identical treatment, either normoxic or CIH, to get sufficient material to perform ChIP. Samples were also sonicated and sheared to obtain an optimal size of base pairs between 100 and 1,000 base pairs. qPCR was used to examine the expression of ACE1 of FosB/ΔFosB precipitated DNA following CIH or normoxia along with input genomic controls and relevant IgG-negative controls. Data were normalized to input background. ACE1 association with FosB/ΔFosB was significantly increased in the MnPO following CIH as compared with the normoxic controls [t (6) = −2.516, P < 0.05; Fig. 5B].
Fig. 5.
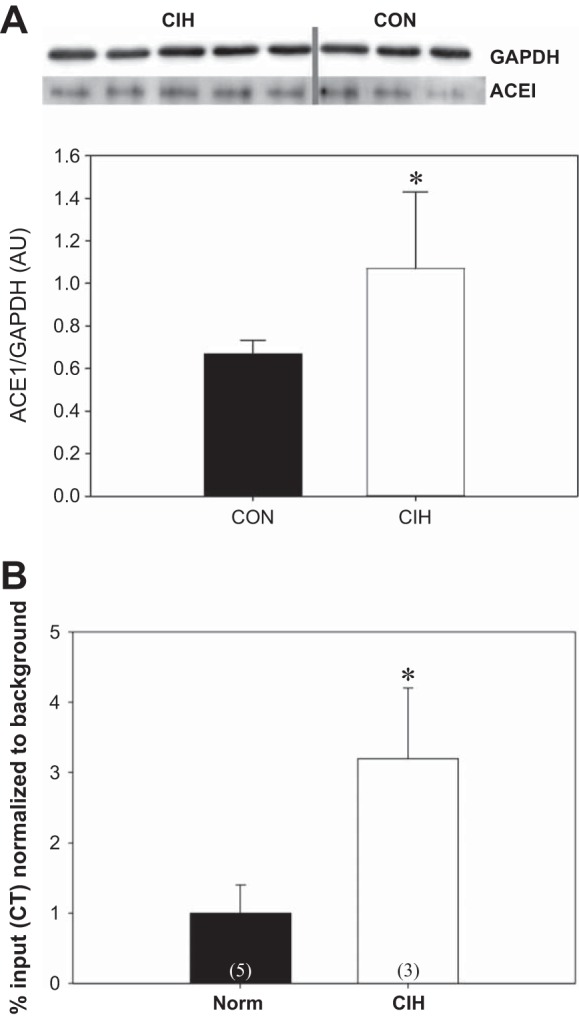
A: Western blot analysis of ACE1 abundance in the MnPO of punch samples collected from normoxic controls (CON; n = 6) and rats exposed to 7 days of chronic intermittent hypoxia (CIH, n = 7) treatment. Densitometric analysis of ACE1 immunoreactivity was normalized to GAPDH. Data are expressed as means ± SE; *P < 0.05. B: results of the chromatin immunoprecipitation (ChIP) analysis of the association of FosB with ACE1 in punch samples containing the MnPO. Each sample used for the analysis represented MnPO sample pooled from two individuals from the same treatment group (CIH, n = 3 samples from 6 rats; CON, n = 5 samples from 10 rats). CIH significantly increased the association of FosB with ACE1 in the MnPO compared with normoxic controls. *P < 0.05.
DISCUSSION
Many neuronal adaptations have been implicated in the hypertension observed in animal models of CIH (12, 55). The current study tested whether ACE1 could be a FosB/ΔFosB-regulated gene in the MnPO that contributes to CIH hypertension. ΔFosB has been shown to participate in long-term neural adaptations seen in many behaviors, including drug addiction (34). As part of the AP-1 transcription factor family, FosB/ΔFosB has been linked to sustained changes in gene expression due to its long half-life and accumulation with intermittent or repeated stimulation (8, 25, 34, 40). In CIH, FosB/ΔFosB significantly increases in many autonomic regions, including the MnPO (10, 29). Our previous work suggests that changes in gene expression that are mediated by FosB/ΔFosB in the MnPO play an essential role in the sustained component of CIH hypertension that occurs during normoxia (10). The present study focused on one of these potential FosB/ΔFosB gene targets in MnPO to better characterize its possible role in CIH hypertension. Additionally, retrograde tract tracing was also used to explore how FosB/ΔFosB activated neurons within the MnPO may influence the PVN. The main finding of this study is that CIH is associated with increased FosB/ΔFosB staining in ACE1-positive MnPO cells that project to the PVN. Furthermore, CIH significantly increases FosB/ΔFosB association with ACE1 in the MnPO, suggesting that increases in ACE1 gene expression associated with CIH are due to the direct interaction of FosB/ΔFosB with ACE1. These findings are consistent with the hypothesis that FosB/ΔFosB regulation of ACE1 within the MnPO contributes to CIH hypertension possibly via the PVN, which may be a pathway for increasing SNA and, thus, sustained elevations of MAP. Additional experiments will be required to test the functional significance of MnPO ACE1 in CIH hypertension.
Immunostaining for ACE1 was evident within the MnPO and did not overlap with GFAP. That ACE1 expression failed to colabel with GFAP suggests ACE1 is not localized in astrocytes and may be expressed by neurons in the MnPO. Also, ACE1 protein abundance significantly increased within the MnPO following 7 days of CIH treatment as compared with normoxic controls. These results are consistent with our earlier report that ACE1 gene expression was upregulated in the MnPO following CIH and that this effect was blocked by virally mediated dominant negative inhibition of ΔFosB in the MnPO (10). We have previously shown that CIH-induced FosB/ΔFosB staining in the MnPO is dependent on angiotensin 1a receptors in the SFO (52). Together, these results indicate that activation of the peripheral RAS by CIH and the subsequent stimulation of the SFO leads to activation of the MnPO and FosB/ΔFosB-mediated upregulation of specific components of the brain RAS. This leads to the speculation that the peripheral RAS and brain RAS work together in concert to maintain CIH hypertension during normoxia. Further studies will be needed to fully understand the role of ACE1 within the MnPO and its involvement in the hypertension seen in CIH.
Furthermore, the MnPO has been implicated in various neural networks that regulate cardiovascular control and fluid and electrolyte balance (26, 35, 49, 64). Activation of the MnPO has been shown to result in sympathoexcitation and increased blood pressure (32, 60). The MnPO projects directly to the PVN (2, 49), more specifically the parvocellular division of the PVN that, in turn, projects to the nucleus of the solitary tract, the rostral ventrolateral medulla, and sympathetic preganglionic neurons in the intermediolateral column of the spinal cord (23, 27, 43, 50, 53). The MnPO to PVN projection may very well regulate increases in SNA and MAP during CIH stimulation. Cells positive for FosB/ΔFosB significantly increased within the MnPO following CIH, as compared with the normoxic controls, which agree with our previous experiments (29). The increase in FosB/ΔFosB is consistent with the hypothesis that the MnPO is activated during CIH. ACE1 and FosB/ΔFosB immunohistochemistry colocalization also significantly increased within the MnPO following CIH. This suggests that increases in FosB/ΔFosB could increase ACE1, resulting in changes in angiotensin metabolism, which could change the strength of angiotensinergic synapses. ANG II has been shown to activate MnPO neurons that project to the PVN (60) and, therefore, increased FosB/ΔFosB activation of MnPO neurons containing ACE1 may project to the PVN following CIH. The results of our retrograde tract tracing study from the PVN support this hypothesis.
As mentioned before, dominant-negative inhibition of ΔFosB in the MnPO blocked the increase in mRNA expression of ACE1 in CIH-treated rats (10). Also, the increase in colocalization of FosB/ΔFosB and ACE1 within the MnPO after CIH suggest a possible relationship between the two. To test for a possible interaction between FosB/ΔFosB and the ACE1 gene, ChIP experiments were conducted to test for increases in the association between ACE1 and FosB/ΔFosB in the MnPO following CIH. Our results indicate that there was a significant increase in association between FosB/ΔFosB to the ACE1 gene region in the MnPO after 7 days of CIH. The increase in association suggests that an increase in FosB/ΔFosB activation of the MnPO targets the RAS, specifically ACE1, and that interaction may upregulate ACE1 message. The upregulation of ACE1 within the MnPO then may go on to influence downstream regions, such as the PVN, and ultimately MAP. ACE1 was not the only gene affected by dominant-negative inhibition of ΔFosB within the MnPO, and other genes may contribute to CIH hypertension in the MnPO along with ACE1. The other genes identified in this study included map3K3, ace2, nos1, and nos3 (10). Overall, downstream regulation of ACE1 by FosB/ΔFosB within the MnPO may play an important role following CIH stimulation. However, functional studies will be required to determine the importance of MnPO ACE1 to CIH hypertension.
Perspectives and Significance
OSA is associated with a sustained hypertension even in the absence of a hypoxia, leading to increased risk for multiple cardiovascular diseases (38). The increase in prevalence and cardiovascular health risks accompanying OSA makes it important to understand the underlying mechanisms of its associated hypertension. Previous studies have suggested the importance of the CNS, as well as the brain RAS, which promotes various forms of hypertension, and the sustained hypertension seen in CIH may be no different (55, 66). This study suggests that FosB may utilize an AP-1 target gene, ACE1, in order to influence the PVN downstream of the MnPO in CIH. Investigating the role of ACE1 within the MnPO may provide a better understanding of the pathogenesis of CIH hypertension, which could improve our ability to treat OSA hypertension.
GRANTS
This study was supported by National Institutes of Health Grant P01 HL-88052.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.F., T.P.N., and J.T.C. conceived and designed research; K.F., B.S., and T.P.N. performed experiments; K.F., B.S., T.P.N., and J.T.C. analyzed data; K.F., B.S., T.P.N., and J.T.C. interpreted results of experiments; K.F., T.P.N., and J.T.C. prepared figures; K.F. drafted manuscript; K.F., B.S., T.P.N., and J.T.C. edited and revised manuscript; J.T.C. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors acknowledge the technical assistance of J. Little, A. Niu, M. Dutta, and Dr. G. E. Farmer for comments about the manuscript.
REFERENCES
- 1.Aars H, Akre S. Effect of angiotensin on sympathetic nerve activity. Acta Physiol Scand 74: 134–141, 1968. doi: 10.1111/j.1748-1716.1968.tb04222.x. [DOI] [PubMed] [Google Scholar]
- 2.Abbott SB, Machado NL, Geerling JC, Saper CB. Reciprocal control of drinking behavior by median preoptic neurons in mice. J Neurosci 36: 8228–8237, 2016. doi: 10.1523/JNEUROSCI.1244-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baltatu OC, Campos LA, Bader M. Local renin-angiotensin system and the brain—a continuous quest for knowledge. Peptides 32: 1083–1086, 2011. doi: 10.1016/j.peptides.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 4.Barrett CJ, Ramchandra R, Guild SJ, Lala A, Budgett DM, Malpas SC. What sets the long-term level of renal sympathetic nerve activity: a role for angiotensin II and baroreflexes? Circ Res 92: 1330–1336, 2003. doi: 10.1161/01.RES.0000078346.60663.A0. [DOI] [PubMed] [Google Scholar]
- 5.Buggy J, Fink GD, Haywood JR, Johnson AK, Brody MJ. Interruption of the maintenance phase of established hypertension by ablation of the anteroventral third ventricle (AV3V) in rats. Clin Exp Hypertens 1: 337–353, 1978. doi: 10.3109/10641967809068612. [DOI] [PubMed] [Google Scholar]
- 6.Carlson JT, Hedner J, Elam M, Ejnell H, Sellgren J, Wallin BG. Augmented resting sympathetic activity in awake patients with obstructive sleep apnea. Chest 103: 1763–1768, 1993. doi: 10.1378/chest.103.6.1763. [DOI] [PubMed] [Google Scholar]
- 7.Carreño FR, Ji LL, Cunningham JT. Altered central TRPV4 expression and lipid raft association related to inappropriate vasopressin secretion in cirrhotic rats. Am J Physiol Regul Integr Comp Physiol 296: R454–R466, 2008. doi: 10.1152/ajpregu.90460.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen J, Kelz MB, Hope BT, Nakabeppu Y, Nestler EJ. Chronic Fos-related antigens: stable variants of deltaFosB induced in brain by chronic treatments. J Neurosci 17: 4933–4941, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coleman CG, Wang G, Park L, Anrather J, Delagrammatikas GJ, Chan J, Zhou J, Iadecola C, Pickel VM. Chronic intermittent hypoxia induces NMDA receptor-dependent plasticity and suppresses nitric oxide signaling in the mouse hypothalamic paraventricular nucleus. J Neurosci 30: 12103–12112, 2010. doi: 10.1523/JNEUROSCI.3367-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cunningham JT, Knight WD, Mifflin SW, Nestler EJ. An essential role for ΔFosB in the median preoptic nucleus in the sustained hypertensive effects of chronic intermittent hypoxia. Hypertension 60: 179–187, 2012. doi: 10.1161/HYPERTENSIONAHA.112.193789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.da Silva AQG, Fontes MAP, Kanagy NL. Chronic infusion of angiotensin receptor antagonists in the hypothalamic paraventricular nucleus prevents hypertension in a rat model of sleep apnea. Brain Res 1368: 231–238, 2011. doi: 10.1016/j.brainres.2010.10.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP. Pathophysiology of sleep apnea. Physiol Rev 90: 47–112, 2010. doi: 10.1152/physrev.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DiBona GF. Central angiotensin modulation of baroreflex control of renal sympathetic nerve activity in the rat: influence of dietary sodium. Acta Physiol Scand 177: 285–289, 2003. doi: 10.1046/j.1365-201X.2003.01074.x. [DOI] [PubMed] [Google Scholar]
- 14.Ferguson AV, Latchford KJ, Samson WK. The paraventricular nucleus of the hypothalamus - a potential target for integrative treatment of autonomic dysfunction. Expert Opin Ther Targets 12: 717–727, 2008. doi: 10.1517/14728222.12.6.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fletcher EC. Invited review: Physiological consequences of intermittent hypoxia: systemic blood pressure. J Appl Physiol (1985) 90: 1600–1605, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Fletcher EC. Sympathetic over activity in the etiology of hypertension of obstructive sleep apnea. Sleep 26: 15–19, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Fletcher EC, Bao G, Li R. Renin activity and blood pressure in response to chronic episodic hypoxia. Hypertension 34: 309–314, 1999. doi: 10.1161/01.HYP.34.2.309. [DOI] [PubMed] [Google Scholar]
- 18.Fletcher EC, Lesske J, Behm R, Miller CC III, Stauss H, Unger T. Carotid chemoreceptors, systemic blood pressure, and chronic episodic hypoxia mimicking sleep apnea. J Appl Physiol (1985) 72: 1978–1984, 1992. [DOI] [PubMed] [Google Scholar]
- 19.Fletcher EC, Lesske J, Qian W, Miller CC III, Unger T. Repetitive, episodic hypoxia causes diurnal elevation of blood pressure in rats. Hypertension 19: 555–561, 1992. doi: 10.1161/01.HYP.19.6.555. [DOI] [PubMed] [Google Scholar]
- 20.Fletcher EC, Orolinova N, Bader M. Blood pressure response to chronic episodic hypoxia: the renin-angiotensin system. J Appl Physiol (1985) 92: 627–633, 2002. doi: 10.1152/japplphysiol.00152.2001. [DOI] [PubMed] [Google Scholar]
- 21.Garpestad E, Katayama H, Parker JA, Ringler J, Lilly J, Yasuda T, Moore RH, Strauss HW, Weiss JW. Stroke volume and cardiac output decrease at termination of obstructive apneas. J Appl Physiol (1985) 73: 1743–1748, 1992. [DOI] [PubMed] [Google Scholar]
- 22.Greenberg HE, Sica AL, Scharf SM, Ruggiero DA. Expression of c-fos in the rat brainstem after chronic intermittent hypoxia. Brain Res 816: 638–645, 1999. doi: 10.1016/S0006-8993(98)01222-0. [DOI] [PubMed] [Google Scholar]
- 23.Haselton JR, Goering J, Patel KP. Parvocellular neurons of the paraventricular nucleus are involved in the reduction in renal nerve discharge during isotonic volume expansion. J Auton Nerv Syst 50: 1–11, 1994. doi: 10.1016/0165-1838(94)90117-1. [DOI] [PubMed] [Google Scholar]
- 24.Hiestand DM, Britz P, Goldman M, Phillips B. Prevalence of symptoms and risk of sleep apnea in the US population: Results from the national sleep foundation sleep in America 2005 poll. Chest 130: 780–786, 2006. doi: 10.1378/chest.130.3.780. [DOI] [PubMed] [Google Scholar]
- 25.Hope BT, Nye HE, Kelz MB, Self DW, Iadarola MJ, Nakabeppu Y, Duman RS, Nestler EJ. Induction of a long-lasting AP-1 complex composed of altered Fos-like proteins in brain by chronic cocaine and other chronic treatments. Neuron 13: 1235–1244, 1994. doi: 10.1016/0896-6273(94)90061-2. [DOI] [PubMed] [Google Scholar]
- 26.Johnson AK, Cunningham JT, Thunhorst RL. Integrative role of the lamina terminalis in the regulation of cardiovascular and body fluid homeostasis. Clin Exp Pharmacol Physiol 23: 183–191, 1996. doi: 10.1111/j.1440-1681.1996.tb02594.x. [DOI] [PubMed] [Google Scholar]
- 27.Kannan H, Yamashita H. Connections of neurons in the region of the nucleus tractus solitarius with the hypothalamic paraventricular nucleus: their possible involvement in neural control of the cardiovascular system in rats. Brain Res 329: 205–212, 1985. doi: 10.1016/0006-8993(85)90526-8. [DOI] [PubMed] [Google Scholar]
- 28.Kc P, Balan KV, Tjoe SS, Martin RJ, Lamanna JC, Haxhiu MA, Dick TE. Increased vasopressin transmission from the paraventricular nucleus to the rostral medulla augments cardiorespiratory outflow in chronic intermittent hypoxia-conditioned rats. J Physiol 588: 725–740, 2010. doi: 10.1113/jphysiol.2009.184580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knight WD, Little JT, Carreno FR, Toney GM, Mifflin SW, Cunningham JT. Chronic intermittent hypoxia increases blood pressure and expression of FosB/ΔFosB in central autonomic regions. Am J Physiol Regul Integr Comp Physiol 301: R131–R139, 2011. doi: 10.1152/ajpregu.00830.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knight WD, Saxena A, Shell B, Nedungadi TP, Mifflin SW, Cunningham JT. Central losartan attenuates increases in arterial pressure and expression of FosB/ΔFosB along the autonomic axis associated with chronic intermittent hypoxia. Am J Physiol Regul Integr Comp Physiol 305: R1051–R1058, 2013. doi: 10.1152/ajpregu.00541.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lim K, Burke SL, Moretti JL, Head GA. Differential activation of renal sympathetic burst amplitude and frequency during hypoxia, stress and baroreflexes with chronic angiotensin treatment. Exp Physiol 100: 1132–1144, 2015. doi: 10.1113/EP085312. [DOI] [PubMed] [Google Scholar]
- 32.Llewellyn T, Zheng H, Liu X, Xu B, Patel KP. Median preoptic nucleus and subfornical organ drive renal sympathetic nerve activity via a glutamatergic mechanism within the paraventricular nucleus. Am J Physiol Regul Integr Comp Physiol 302: R424–R432, 2012. doi: 10.1152/ajpregu.00403.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsukawa T, Gotoh E, Minamisawa K, Kihara M, Ueda S, Shionoiri H, Ishii M. Effects of intravenous infusions of angiotensin II on muscle sympathetic nerve activity in humans. Am J Physiol 261: R690–R696, 1991. [DOI] [PubMed] [Google Scholar]
- 34.McClung CA, Ulery PG, Perrotti LI, Zachariou V, Berton O, Nestler EJ. DeltaFosB: a molecular switch for long-term adaptation in the brain. Brain Res Mol Brain Res 132: 146–154, 2004. doi: 10.1016/j.molbrainres.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 35.McKinley MJ, Gerstberger R, Mathai ML, Oldfield BJ, Schmid H. The lamina terminalis and its role in fluid and electrolyte homeostasis. J Clin Neurosci 6: 289–301, 1999. doi: 10.1016/S0967-5868(99)90050-4. [DOI] [PubMed] [Google Scholar]
- 36.McKinley MJ, Yao ST, Uschakov A, McAllen RM, Rundgren M, Martelli D. The median preoptic nucleus: front and centre for the regulation of body fluid, sodium, temperature, sleep and cardiovascular homeostasis. Acta Physiol (Oxf) 214: 8–32, 2015. doi: 10.1111/apha.12487. [DOI] [PubMed] [Google Scholar]
- 37.McNicholas WT. Cardiovascular outcomes of CPAP therapy in obstructive sleep apnea syndrome. Am J Physiol Regul Integr Comp Physiol 293: R1666–R1670, 2007. doi: 10.1152/ajpregu.00401.2007. [DOI] [PubMed] [Google Scholar]
- 38.Morrell MJ, Finn L, Kim H, Peppard PE, Badr MS, Young T. Sleep fragmentation, awake blood pressure, and sleep-disordered breathing in a population-based study. Am J Respir Crit Care Med 162: 2091–2096, 2000. doi: 10.1164/ajrccm.162.6.9904008. [DOI] [PubMed] [Google Scholar]
- 39.Narkiewicz K, Wolf J, Lopez-Jimenez F, Somers VK. Obstructive sleep apnea and hypertension. Curr Cardiol Rep 7: 435–440, 2005. doi: 10.1007/s11886-005-0061-z. [DOI] [PubMed] [Google Scholar]
- 40.Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci 2: 119–128, 2001. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- 41.Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, D’Agostino RB, Newman AB, Lebowitz MD, Pickering TG. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA 283: 1829–1836, 2000. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 42.Parati G, Lombardi C, Narkiewicz K. Sleep apnea: epidemiology, pathophysiology, and relation to cardiovascular risk. Am J Physiol Regul Integr Comp Physiol 293: R1671–R1683, 2007. doi: 10.1152/ajpregu.00400.2007. [DOI] [PubMed] [Google Scholar]
- 43.Patel KP, Schmid PG. Role of paraventricular nucleus (PVH) in baroreflex-mediated changes in lumbar sympathetic nerve activity and heart rate. J Auton Nerv Syst 22: 211–219, 1988. doi: 10.1016/0165-1838(88)90109-9. [DOI] [PubMed] [Google Scholar]
- 44.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic, 1986. [DOI] [PubMed] [Google Scholar]
- 45.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 177: 1006–1014, 2013. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 342: 1378–1384, 2000. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 47.Phillips BG, Somers VK. Hypertension and obstructive sleep apnea. Curr Hypertens Rep 5: 380–385, 2003. doi: 10.1007/s11906-003-0083-0. [DOI] [PubMed] [Google Scholar]
- 48.Prabhakar NR, Kumar GK. Mechanisms of sympathetic activation and blood pressure elevation by intermittent hypoxia. Respir Physiol Neurobiol 174: 156–161, 2010. doi: 10.1016/j.resp.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saper CB, Levisohn D. Afferent connections of the median preoptic nucleus in the rat: anatomical evidence for a cardiovascular integrative mechanism in the anteroventral third ventricular (AV3V) region. Brain Res 288: 21–31, 1983. doi: 10.1016/0006-8993(83)90078-1. [DOI] [PubMed] [Google Scholar]
- 50.Sawchenko PE, Swanson LW. The organization of forebrain afferents to the paraventricular and supraoptic nuclei of the rat. J Comp Neurol 218: 121–144, 1983. doi: 10.1002/cne.902180202. [DOI] [PubMed] [Google Scholar]
- 51.Saxena A, Bachelor M, Park YH, Carreno FR, Nedungadi TP, Cunningham JT. Angiotensin II induces membrane trafficking of natively expressed transient receptor potential vanilloid type 4 channels in hypothalamic 4B cells. Am J Physiol Regul Integr Comp Physiol 307: R945–R955, 2014. doi: 10.1152/ajpregu.00224.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saxena A, Little JT, Nedungadi TP, Cunningham JT. Angiotensin II type 1a receptors in subfornical organ contribute towards chronic intermittent hypoxia-associated sustained increase in mean arterial pressure. Am J Physiol Heart Circ Physiol 308: H435–H446, 2015. doi: 10.1152/ajpheart.00747.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shafton AD, Ryan A, Badoer E. Neurons in the hypothalamic paraventricular nucleus send collaterals to the spinal cord and to the rostral ventrolateral medulla in the rat. Brain Res 801: 239–243, 1998. doi: 10.1016/S0006-8993(98)00587-3. [DOI] [PubMed] [Google Scholar]
- 54.Sharpe AL, Calderon AS, Andrade MA, Cunningham JT, Mifflin SW, Toney GM. Chronic intermittent hypoxia increases sympathetic control of blood pressure: role of neuronal activity in the hypothalamic paraventricular nucleus. Am J Physiol Heart Circ Physiol 305: H1772–H1780, 2013. doi: 10.1152/ajpheart.00592.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shell B, Faulk K, Cunningham JT. Neural control of blood pressure in chronic intermittent hypoxia. Curr Hypertens Rep 18: 19, 2016. doi: 10.1007/s11906-016-0627-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith ML, Niedermaier ON, Hardy SM, Decker MJ, Strohl KP. Role of hypoxemia in sleep apnea-induced sympathoexcitation. J Auton Nerv Syst 56: 184–190, 1996. doi: 10.1016/0165-1838(95)00062-3. [DOI] [PubMed] [Google Scholar]
- 57.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 96: 1897–1904, 1995. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, Daniels S, Floras JS, Hunt CE, Olson LJ, Pickering TG, Russell R, Woo M, Young T, American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology; American Heart Association Stroke Council; American Heart Association Council on Cardiovascular Nursing; American College of Cardiology Foundation . Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation 118: 1080–1111, 2008. doi: 10.1161/CIRCULATIONAHA.107.189420. [DOI] [PubMed] [Google Scholar]
- 59.Stocker SD, Hunwick KJ, Toney GM. Hypothalamic paraventricular nucleus differentially supports lumbar and renal sympathetic outflow in water-deprived rats. J Physiol 563: 249–263, 2005. doi: 10.1113/jphysiol.2004.076661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stocker SD, Toney GM. Median preoptic neurones projecting to the hypothalamic paraventricular nucleus respond to osmotic, circulating Ang II and baroreceptor input in the rat. J Physiol 568: 599–615, 2005. doi: 10.1113/jphysiol.2005.094425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stocker SD, Toney GM. Vagal afferent input alters the discharge of osmotic and ANG II-responsive median preoptic neurons projecting to the hypothalamic paraventricular nucleus. Brain Res 1131: 118–128, 2007. doi: 10.1016/j.brainres.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stoohs R, Guilleminault C. Cardiovascular changes associated with obstructive sleep apnea syndrome. J Appl Physiol (1985) 72: 583–589, 1992. [DOI] [PubMed] [Google Scholar]
- 63.Tamisier R, Gilmartin GS, Launois SH, Pépin JL, Nespoulet H, Thomas R, Lévy P, Weiss JW. A new model of chronic intermittent hypoxia in humans: effect on ventilation, sleep, and blood pressure. J Appl Physiol (1985) 107: 17–24, 2009. doi: 10.1152/japplphysiol.91165.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Westerhaus MJ, Loewy AD. Sympathetic-related neurons in the preoptic region of the rat identified by viral transneuronal labeling. J Comp Neurol 414: 361–378, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- 65.Wolk R, Kara T, Somers VK. Sleep-disordered breathing and cardiovascular disease. Circulation 108: 9–12, 2003. doi: 10.1161/01.CIR.0000072346.56728.E4. [DOI] [PubMed] [Google Scholar]
- 66.Wright JW, Harding JW. The brain renin-angiotensin system: a diversity of functions and implications for CNS diseases. Pflügers Arch 465: 133–151, 2013. doi: 10.1007/s00424-012-1102-2. [DOI] [PubMed] [Google Scholar]
- 67.Yoshimoto M, Miki K, Fink GD, King A, Osborn JW. Chronic angiotensin II infusion causes differential responses in regional sympathetic nerve activity in rats. Hypertension 55: 644–651, 2010. doi: 10.1161/HYPERTENSIONAHA.109.145110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Young T, Peppard P, Palta M, Hla KM, Finn L, Morgan B, Skatrud J. Population-based study of sleep-disordered breathing as a risk factor for hypertension. Arch Intern Med 157: 1746–1752, 1997. doi: 10.1001/archinte.1997.00440360178019. [DOI] [PubMed] [Google Scholar]