Abstract
Increasing evidence suggests that the conditions of retinal microvessels are indicators to a variety of cerebrovascular, neurodegenerative, psychiatric, and developmental diseases. Thus noninvasive visualization of the human retinal microcirculation offers an exceptional opportunity for the investigation of not only the retinal but also cerebral microvasculature. In this review, we show how the conditions of the retinal microvessels could be used to assess the conditions of brain microvessels because the microvascular network of the retina and brain share, in many aspects, standard features in development, morphology, function, and pathophysiology. Recent techniques and imaging modalities, such as optical coherence tomography (OCT), allow more precise visualization of various layers of the retina and its microcirculation, providing a “microscope” to brain microvessels. We also review the potential role of retinal microvessels in the risk identification of cerebrovascular and neurodegenerative diseases. The association between vision problems and cerebrovascular and neurodegenerative diseases, as well as the possible role of retinal microvascular imaging biomarkers in cerebrovascular and neurodegenerative screening, their potentials, and limitations, are also discussed.
Keywords: retinal imaging biomarkers, optical imaging, cerebrovascular and neurodegenerative disorders, optical coherence tomography, brain
the link between retinal pathology and a variety of cerebrovascular and neurodegenerative diseases has been established by multiple studies (71). Patients with cerebrovascular and neurodegenerative diseases often have symptoms affecting visual function, including loss of best-corrected visual acuity, reduced contrast sensitivity, ocular motility abnormalities, and color vision defects (71). Most clinic- and population-based studies have revealed that cerebrovascular and neurodegenerative lesions are correlated with structural changes in the retinal microvasculature and its neural layers, supporting the hypothesis that the retina may be a window to the brain. Notably, this link reinforces the use of retinal microvessels as biomarkers to assess the conditions of the cerebral microvessels supplying brain tissues. Examining pathological alterations of retinal microvessels may be valuable for understanding the etiology of various cerebrovascular and neurodegenerative disorders; consequently, retinal imaging can be possibly translated into primary clinical care. Also, it may provide an affordable and faster possibility for potentially replacing and/or complementing magnetic resonance imaging (MRI) of the central nervous system.
Advances in optical imaging technologies have facilitated that the retinal microvasculature can be noninvasively visualized, quantified, and monitored. In this perspective, we intend to give a broad, but by no means a complete, review of current information on retinal vascular changes in correlation with cerebrovascular and neurodegenerative diseases. The possible role of retinal vascular imaging biomarkers in cerebrovascular and neurodegenerative screening is also discussed, as well as their role in the risk identification for cerebrovascular and neurodegenerative diseases. Only studies with the highest level of evidence for each disorder were reviewed and discussed. Based on the multifaceted nature of the pathophysiology of cerebrovascular and neurodegenerative diseases, it is expected that the ideal prediction models for the future development of cerebrovascular and neurodegenerative disorders, and risk of progression from early to late stages, will originate from approaches that combine multiple diagnostic methods. Therefore, integrating measures of the retinal vascular network and visual performance into the cerebrovascular and neurodegenerative assessment approaches that are currently used to investigate the mechanisms and alterations of brain function could lead to a better quantitative and qualitative assessment in the primary care setting.
Homology Between Cerebral and Retinal Microvasculature
The retina and optic nerve extend from the diencephalon during their embryonic development. Thus both are considered to be part of the central nervous system (CNS). The retina contains various neural elements, which are interconnected with each other and play a crucial role in visual processing, beginning already at the retinal level (98). The final processing stage occurs at the level of the retinal ganglion cells (RGCs), and the axons of many RGCs are collected in a bundle to form the optic nerve. The optic nerve, similarly to all fiber tracts in the CNS, is covered with oligodendrocyte-produced myelin and is being sheathed by all three meningeal layers.
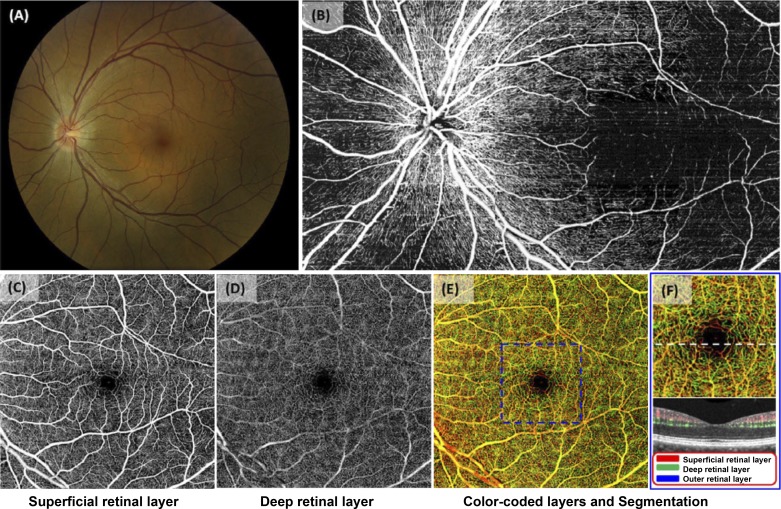
However, it is not only the neural structure that shares similarities with the CNS (85, 143). Similar to the CNS, the eye and the retina have a unique relationship with the immune system that involves specialized barriers, such as the inner blood-retinal barrier, the retinal analog of the blood-brain barrier (36). This analogy is in great part due to the same radial pattern of vascularization during development that is driven in part by the expression of vascular endothelial growth factors(s) VEGFs and other growth factors (36, 46, 52, 107). Accordingly, the retinal vasculature shares certain features with the vasculature of the brain because both networks have to provide sufficient levels of oxygen and substrates to the large demand of the tissues supplied. It is known that cerebral capillary density mirrors the metabolic demand and activity of the given brain regions (39, 63). Accordingly, retinal capillary density is greatest in the central, macular area, whereas the peripheral retina is practically avascular (Fig. 1). It is important to note that the retinal capillary networks consist of two distinct beds: the superficial capillary layer in the ganglion cell layer (GCL), and the deeper capillary layer at the level of the outer plexiform layer (OPL) (Fig. 1) (125). Interestingly, these capillaries are smaller than cerebral capillaries, with a diameter of ~5 μm (17, 121).
Fig. 1.
Details of the retinal microvasculature. A: fundus photograph of a healthy eye allowing visualization of the entire posterior pole of the ocular fundus, including the optic nerve, the macula, and the major retinal vessels. B: montage of images of the nerve fiber layer obtained by wide-field optical coherence tomography-based microangiography (OMAG) for the same eye. Note the radial pericapillary network within the RNFL. C: the superficial retinal layer slab contains the vascular network within the ganglion cell layer (GCL) and the outer plexiform layer (OPL). Both the arcade vessels and the fine capillaries are shown. D: the deep retinal layer demonstrates deeper capillary network. E: the whole retinal layer slab composed of the superficial retinal layer, the deep retinal layer, and the outer retinal layer allows visualization of the shallow, intermediate, and deep retinal capillary plexuses. Different colors identify various levels of the retina. F: the magnified image of the central macula (identified as in the blue box from E). The cross-sectional flow image of the area is marked with a white dashed line on the magnified OMAG image. Blood flow detected in the superficial retinal layer, the deep retinal layer, and the outer retinal layer (ORL) are in red, green, blue; respectively. Note that flow can be detected in the ORL. [Images adapted from Zhang et al. (143a) with permission.]
It is noteworthy that retinal blood vessels are so-called end arteries and arterioles having no anastomotic connections, and thus occlusion of these vessels leads to infarction and atrophy of the inner retina (139). In contrast, cerebral microvasculature, similarly to most human organs (except the heart), has a sufficient number of collateral vessels (17). Also, unlike cerebral arterioles, retinal arterioles often show sharp, 90-degree branching patterns allowing substantial plasma skimming, i.e., separation of plasma and cellular elements of blood, providing flow-perfusion through the smaller size of capillaries in the retina (17).
As mentioned above, the blood-retina barrier (BRB) is very similar to its cerebral counterpart, protecting the neural tissue of the retina from various large molecules and hematogenous cells. The BRB consists of both mechanical (tight junctional complexes) and metabolic components (metabolic transport acting as a metabolic barrier), similarly to the blood-brain barrier (BBB) (87, 124). Early ultrastructural studies in both brain and retinal vessels showed the presence of caveolae (also called plasmalemma vesicles) and certain membrane enzymes at the abluminal plasma membrane of endothelial cells. These vesicles are static structures, facilitating the transport of small ions and solutes across the BRB and BBB. The number of vesicles and their polarity are similar in retinal and cerebral microvessels, and likely contribute to their barriers, further supporting the idea that the two systems resemble each other (113).
The microvessels of the retina contain nonfenestrated endothelial cells and pericytes (125), both playing an important role in the maintenance of the BRB. Also, the retinal microvasculature includes many perivascular end-feet (also called astrocytic processes) that are actively involved in the regulation of barrier properties and the release of various pericyte-derived factors, which also influence angiogenesis, stabilize microvessels, and contribute to vasomotor control and neurovascular coupling (2, 55, 125, 144).
Finally, because both organs are enclosed in hard capsules, both cerebral and the retinal microcirculation have mechanisms to control vascular resistance in the face of changing systemic blood pressure (and flow) to maintain a relatively constant blood flow, called autoregulation (66).
Similar to the brain, in the eye this is supported by the retinal pericytes and the vascular smooth muscles, in part by the myogenic and perhaps flow-sensitive mechanisms (129, 142). Nevertheless, locally there is still sufficient room for metabolic factors to couple regulation of blood flow to regional neuronal activity: neurovascular coupling (50, 67). Indeed, the temporal retina that contains the macula has a greater blood flow compared with the nasal retina, reflecting the differences in metabolic activity (103). It is worth noting that both the retina and the brain are nonhomogeneous organs frequently exhibiting similar microvascular pathology suggesting that both bodies undergo similar disease developments.
Although there is no clear evidence regarding a neurogenic control of blood flow in the retina, there are data on the neurogenic control of cerebral blood flow. Under normal physiological conditions, gas, chemical. and metabolic vasomotor controls are dominant in the brain, whereas in severely challenging circumstances, such as delayed cerebral ischemia following subarachnoidal hemorrhage, these local mechanisms are suppressed, increasing the relative importance of neurogenic, sympathetic control of CBF (122).
Retinal Microvascular Changes in Cerebrovascular and Neurodegenerative Diseases
Brain diseases have a concomitant economic impact that costs societies trillions of dollars every year (45). Particularly, there is a need to develop a better understanding of the contribution of microvascular factors in cerebrovascular and neurodegenerative disorders and the identification of biomarkers of significant microvascular alterations. Although various minimally invasive and noninvasive in vivo imaging techniques have been introduced to examine brain functions either in healthy or diseased conditions, multimodal imaging of the eye is progressively investigated as a potential tool for identifying ocular biomarkers of primarily nonophthalmic disorders, ranging from neurovascular and neurodegenerative diseases to cardiovascular disease.
Although the concept that many cerebral diseases could be reflected in the retinal alterations were not widely appreciated when initially proposed (98), evidence has progressively accumulated to support this view after recent developments of multimodal imaging technologies in ophthalmology (Fig. 2). These technological advances, including the rapid and widespread use of optical coherence tomography applications, are discussed in detail elsewhere (26). Here we propose to examine the role of the retina as an anatomic and functional surrogate of the central brain (75) in further detail and to exemplify how the retina is an excellent entry point to directly visualize the CNS, both from microvascular and neural tissue aspects. For this reason, it is important to realize that a plausible hypothesis could be in place for retinal measures to provide surrogate metrics of CNS status; however, controversy still exists as to whether or not the degenerative alterations of the intracerebral microvessels are parallel to those of the retinal microvessels (98).
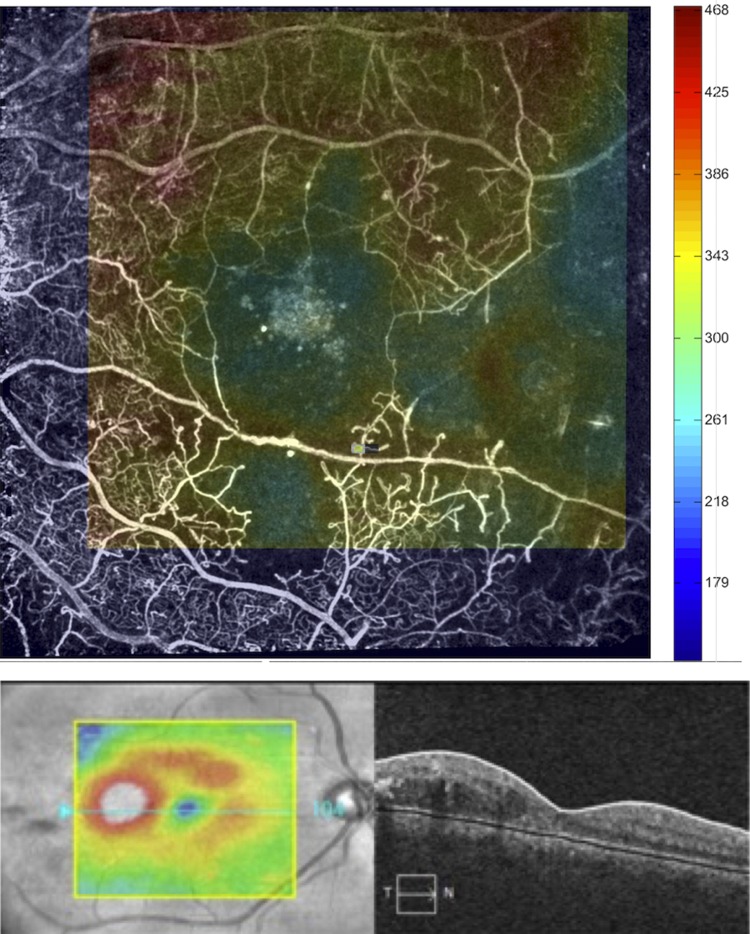
Fig. 2.
The advantage of combining multimodal optical imaging approaches: retinal images demonstrating functional and structural signatures of a diabetic patient with proliferative diabetic retinopathy (PDR). Similar signs of capillary dropout and retinal abnormalities are observed in retinal images from patients with cerebrovascular and neurodegenerative diseases with ocular manifestations. Top: noninvasive capillary perfusion map (nCPM) in an eye with extensive retinal ischemia due to the PDR obtained with the Retinal Function Imager (RFI, Optical Imaging Ltd., Rehovot, Israel). The OCT thickness map is overlayed on top of the nCPM. Note how the areas of thinning (represented in blue color) on the OCT map overlap with the capillary dropout. Bottom: focal diabetic macular edema in the temporal part of the macula as seen on the macular thickness map (left) and the horizontal B-scan (right) corresponding to the blue section line on the map. (Images courtesy of William E. Smiddy, MD, Thalmon R. Campagnoli, MD, Jing Tian, Ph.D., and Gabor M. Somfai, MD, Ph.D., University of Miami, Miami, FL.)
Stroke.
Notwithstanding current advancements in understanding cortical ischemic stroke (112), the pathogenesis and treatments of cerebral small vessel diseases are still unclear (6). The absence of development is mainly due to the difficulty in assessing and visualizing cerebral microvasculature. Interestingly, the retinal vasculature sits embedded in the retinal nerve fiber layer (RNFL), and the thinning of the RNFL and GCL has been proposed as an ocular biomarker of neurodegeneration in the CNS (9, 23, 54, 60, 72, 95, 108). There are different studies regarding retinal indicators of cerebrovascular diseases reporting changes of retinal vessels and the retinal nerve fiber layer (RNFL). Particularly, localized retinal defects have been found in patients with previous or acute stroke (130). Also, RNFL thinning was reported in patients after middle cerebral artery infarction and internal carotid artery occlusion (44). Moreover, abnormal microcirculatory function and alterations could play a significant role in the development of cerebral small vessel disease, such as lacunar infarcts (21). Specifically, population-based studies using retinal photography found that the alterations of the retinal microvascular structure are related to ischemic strokes (131, 137, 139), and they are more frequent among patients with lacunar stroke compared with those with cortical ischemic stroke (70).
A well-known prospective epidemiologic study [(Atherosclerosis Risk In Communities (ARIC)] conducted in four U.S. communities revealed that individuals were more probable to develop incident stroke when changes in the retinal microvascular network architecture, such as signs of retinopathy and decreased artery-vein ratio (AVR) at the control examination, were observed (138). Interestingly, the ARIC study also reported that individuals who had both retinopathy and cerebral white matter lesions were at significantly greater risk of incident stroke than those without either anomaly (139). There are also indications of potential stereotyping of stroke, based on microvascular changes in the retina. Mainly, distinctive retinal abnormalities have been linked with certain stroke subtypes. For instance, cerebral hemorrhages were associated with retinal hemorrhages, as well as the widening of retinal venules, and narrowing arterioles were related to lacunar stroke (3, 4, 70, 127, 141) whereas more tortuous retinal vessels and loss of branching complexity were associated with stroke (25, 59, 91). Also, a systematic review and individual-participant meta-analysis offered evidence that venules might play a significant role in the pathogenesis of stroke after confirming that wider retinal venular diameter, rather than narrower arteriolar caliber, is related to the risk of stroke (24, 80).
In a recent study, retinal microvascular diameters have been found to be associated with enlarged perivascular spaces in the brain (86). Although not yet confirmed by direct in vivo visualization of small vessels, perivascular enlargement is a putative imaging marker for microvascular brain damage. Therefore, these results confirm the alleged link between microvascular damage and enlarged perivascular spaces. The latest growing demand of telemedicine applications is also influencing the uses of retinal imaging to assess the possible atrophy of the optic nerve and retinal vessel anomalies in patients after a recent minor stroke (136). In particular, this study showed that optic nerve atrophy is common in patients who have suffered an ischemic stroke, and it appears to suggest microvascular damage, demonstrating the need for telemedicine-assisted assessment of the optic nerve.
Overall, these results suggest that microvascular network patterns of the retina can be associated with specific cerebral microvascular pathology and could advance the understanding of the fundamental microvascular mechanisms related to pathological features, both in the eye and brain. Future studies should utilize quantification of network morphology and geometry allowing the assessment of optimality and comparison of networks of healthy individuals and patients suffering from the development of various diseases (65, 79).
Dementia.
The dementias, which are characterized by extensive and complex heterogeneity in the etiology, have received significant attention in recent years, with clinicians employing ophthalmic imaging techniques. For example, cognitive impairment has been consistently reported to be associated with retinal abnormalities in both cross-sectional and longitudinal studies (5, 22, 90, 92, 138). Changes in retinal vascular network geometry, such as venular widening and arteriolar narrowing, rarefaction of the vasculature, and suboptimal bifurcation angles and suboptimal junctional branching coefficients, have been correlated with worse cognitive functioning (16, 38, 61, 69, 92, 99, 102). Such changes in network structure may result in deviation of optimal branching ratio as originally suggested by C. Murray in 1926 and later investigated by many modeling studies providing optimal retinovascular and neurovascular coupling (65, 85).
Several population-based studies have reported retinopathy signs associated with vascular dementia (AGES-Reykjavik Study), Alzheimer’s disease (AD), and dementia (Rotterdam Study and Cardiovascular Health Study) (5, 102, 117). Particularly, alterations in the retinal microvascular network were also reported as a probable manifestation of similar pathophysiological developments in the brain’s cerebral microvasculature of individuals with AD after assessing neocortical brain amyloid plaque burden with PiB-positron emission tomography imaging (15, 33). Numerous studies also reported RNFL thinning and reduction in the number of retinal ganglion cells in AD patients (8, 19, 47, 49, 54, 95, 97, 126). Particularly, controversy exists about the type of axon loss; some studies demonstrated a loss of unaffected myelinated axons, whereas others reported the loss of larger diameter axons (15, 49). A recent meta-analysis concluded that RNFL thickness measurements might be potentially useful in the diagnosis and discrimination of various types of dementias (123). Other OCT studies in AD demonstrated that RNFL thinning is associated with the disease (34, 78, 101, 119).
Recently, a new spectral imaging method has been used to extract spectral signatures in transgenic Alzheimer's mice produced by light-scatter changes with age (84). This new technique has the potential to aid as a marker for the buildup of amyloid aggregates in the retina. Moreover, abnormalities in retinal oxygen metabolism in AD have been observed in patients with moderate AD indicating that retinal oximetry may also offer new understandings into AD’s pathophysiology (27).
According to the above studies, the retinal changes may be used as objective markers making it possible to assess early microvascular and neurodegenerative changes in dementias and also providing some clues regarding the underlying microvascular mechanisms of the diseases.
Multiple sclerosis.
Retinal changes in multiple sclerosis (MS), a chronic inflammatory disease of the CNS of unknown etiology, are common and frequently reported as the first manifestation of this disease. Ocular findings in MS include optic neuritis, ocular motility dysfunction, peripheral vasculitis, retinitis, and pars planitis. Particularly, optic neuritis is the most significant condition due to its high frequency of association with MS; it occurs many times as the initial ocular manifestation. All of these changes may be attributable to alterations in the venular network of the retina.
Zamboni et al. have presented certain persuasive evidence that MS is mainly a microvascular disease, and that the neurologic impairment seen in MS patients has its origin in blood flow complications in the venous network (31, 143). Bolton and Smith (10) also reported considerable evidence demonstrating cerebral vasculitis in the early manifestation of MS pathology. These vascular alterations could play a role in neurodegeneration; however, measurements or even just the characterization of cerebral blood flow is a challenging task. On the other hand, retinal periphlebitis and stiffness of central retinal arteries have been reported in MS (64, 94). Our group recently reported significant differences in the nerve fiber layer and the ganglion cell/plexiform layer compound in MS patients compared with healthy subjects along with changes in the retinas affected by optic neuritis compared with those unaffected (128). The perceived dissimilarities in optical properties in our study can be related both to inflammatory or microvascular alterations of the inner retina. However, there is still little proof regarding first changes in the microcirculation of the retina in MS. A recent study by Wang et al. (133) using OCT angiography showed significantly reduced flow index around the optic nerve head (ONH) in eyes after optic neuritis compared with healthy controls, but no differences were shown in the parafoveal circulation. Although this may imply that their observations were not influenced by alterations in macular microcirculation, latest results also using hemodynamic evidence have shown impaired microcirculation in the macular region of the retina (56, 57, 146).
The inner retina also provides clinically important information, in which unmyelinated CNS axons can be measured and quantified (29, 37, 48, 96, 110, 111). However, methodological and meticulous pathological descriptions of retinal microcirculation in MS are still lacking. For example, it is particularly unclear whether or not the RNFL loss along with its embedded capillary network seen on OCT reflects loss of axons and neuronal elements, or atrophy of these structures (23, 40, 44). Of note, as inner retinal vessels lie in the RNFL, perivascular retinal inflammation and gliosis could lead to augmented thickness of retinal layers, which is not due to expansion or swelling of neuronal/axonal elements. Furthermore, despite advancements in optical imaging, it is unknown if there is any association between retinal atrophy and retinal inflammation, or if both occurrences are a result of processes that are initiated in the optic nerve as a consequence of inflammation, demyelination, and neurodegeneration.
Green et al. (42) reported that localized inflammatory cellular infiltrates surrounding retinal veins in the connective tissue of the RNFL and GCL were encountered in 29% of the relapsing remitting and secondary progressive multiple sclerosis eyes sampled but in only 5% of primary progressive multiple sclerosis eyes. They also observed perivenular gliosis and thickening of small veins and venules in the optic disk. Recently, Costello et al. (18) investigated the GCL and obtained novel understandings of the temporal association of damage following optic neuritis. Moreover, the Optic Neuritis Study Group (ONS) reported that the presence of MRI abnormalities at the time of an acute optic neuritis attack is a strong predictor of the 15-yr risk of multiple sclerosis (93). Interestingly, correlation of RNFL thickness with several MRI whole brain measures of brain atrophy has been demonstrated (40, 41, 118). Recently, a four-year study using OCT reported that as time goes on, atrophy of specific retinal layers and brain substructures are associated (114). Most studies cited above suggest that quantitative characterization of changes in the retinal microvascular network in MS would help to assess the role of vascular dysfunction in the pathogenesis of MS.
Retinal microvasculature alterations in other selected CNS disorders.
Ocular manifestations of CNS pathologies are not limited to cerebrovascular and neurodegenerative disorders. There are other studies involving psychiatric and developmental disorders that are looking at retinal changes as being another reflection of alterations in the brain tissues. For example, retinal imaging has been used as a tool for understanding the pathogenesis of schizophrenia. Particularly, wider retinal venules were observed in individuals who developed schizophrenia, suggesting microvascular abnormality reflective of deficient brain oxygen supply (73, 81). Also, retinal microvascular features have been reported as potential surrogates of brain damage in retinopathy-positive pediatric cerebral malaria (74). Subtle morphological changes of the optic nerve and retinal microvasculature were found in children with attention deficit hyperactivity disorder (AD/HD) (43). In contrast, another study showed that adult epileptic patients with elevated homocysteine levels do not show substantial changes in the diameter of retinal vessels assessed by the measurement of arteriolar-to-venular ratio (7). Yet, the authors noted that future studies should assess the changes of retinal microvessels in predicting the risk of cerebrovascular disease in patients with mild hyperhomocysteinemia, which is observed in 10–40% of epileptic patients (7). Indeed, central venous occlusion in the retina is frequently observed in hyperhomocysteinemia, which has been shown to impair the endothelial function of arterioles and venules, as well (3, 125).
Although retinal imaging studies on Parkinson disease have provided rather conflicting results, these studies have revealed thinning of the outer retinal layers and choroid that prompt the need of investigating the deep capillary plexus and choroidal circulation (1, 28, 76, 115). Recently, Normando et al. reported the first in vivo indication of RGCs loss and early retinal thickness changes in a PD model of neurodegenerative disease (88). This study revealed that the retina can be used as a surrogate marker for alterations in the brain.
Amyotrophic lateral sclerosis is another CNS disease that has been reported to present features of vascular compromise (82, 135). Recent studies using OCT have also demonstrated retinal involvement in ALS and suggested RNFL thinning as a potential marker of neurodegeneration, specifically in ganglion cell axons, in ALS (51, 120).
The above recent studies reveal the emerging interest in the analysis of retinal vessels to provide a low-cost, fast, and noninvasive approach for detecting and staging systemic microvascular damage. All in all, these exciting possibilities to couple neural changes along with microvascular and structural retinal alterations to brain diseases should generate future studies, in which quantitative biomarkers can be revealed and associated to particular diseases.
Optical Retinal Imaging Offers New Directions
There is a growing body of research due to the emerging technological developments in the field of optical imaging that can be beneficial to discover novel mechanisms regulating the vasomotor tone of not only retinal microvessels, but also that of the brain. Particularly, the innovations in optical technologies have made it easier to acquire high-quality retinal images even without the need for pupillary dilation (i.e., in a nonmydriatic fashion). This nonmydriatic capability in retinal imaging has lately gained relevance as a helpful tool for diagnosing acute medical, neuro-ophthalmologic, and neurologic conditions. Additionally, numerous population-based studies have used retinal imaging to relate ophthalmic anomalies to the risk of cardiovascular mortality, subclinical and clinical stroke, renal dysfunction, cognitive impairment, and hypertension. However, those epidemiologic reports on retinal vasculature were generally based on the assessment of retinal vessels with diameters greater than 70 µm. Recent advancements in optical imaging are facilitating the evaluation of smaller retinal vessels and have also increased the field of view of ocular fundus cameras, typically to capture an ultrawide field (UWF) image (200°). For example, current devices taking advantage of the UWF imaging capability are the Optomap 200Tx and Daytona Imaging Systems (Optos, Marlborough, MA) retinal imaging devices. Also, a noncontact UWF module that attaches easily to any Spectralis or HRA2 camera head was recently introduced by Heidelberg Engineering (Carlsbad, CA). Furthermore, Optical coherence angiography (OCA), one of the latest ophthalmic imaging developments, can be used to assess small vessel abnormalities, such as capillary loss or failure without the need for dye injection (77, 132, 134, 140). Recently, imaging capabilities of wide-field OCT-based microangiography OMAG in evaluating the vitreous cavity, retinal layers, retinal pigment epithelium, and choriocapillaris have been reported (144). Adaptive optics OCT and adaptive optics retinal cameras can also facilitate the assessment of noninvasive imaging of the retinal capillary network and also visualize both cone and rod photoreceptors (62, 106, 109).
The role of advanced retinal imaging in the assessment of cerebrovascular, neurodegenerative, psychiatric, and developmental diseases of the brain is an ongoing area of investigation. Quantitative investigation of the capillary network’s geometry in relation to structural, optical, and functional measures of the retinal neural tissue may provide a much better visualization of the retinal microcirculation (i.e., capillaries, arterioles, and venules) and allow a better insight on the increased risk of progression of brain disorders in the presence of vascular comorbidities (11, 83). It is also worth mentioning that imaging retinal microcirculation, in a noninvasive manner, could indicate cerebrovascular dysfunction due to their connected blood supply and close anatomical vicinity. Moreover, changes in the conjunctival microvasculature could also reflect systemic vascular dysfunction in concert with retinal microvascular alterations (12–14, 58, 89, 116, 131). Therefore, studying the microcirculation of both retina and conjunctiva, especially in a quantitative manner, at the level of the capillary network, might improve our ability to understand the pathogenesis and etiology of brain diseases, resulting in the development of sensitive ocular biomarkers of brain disorders.
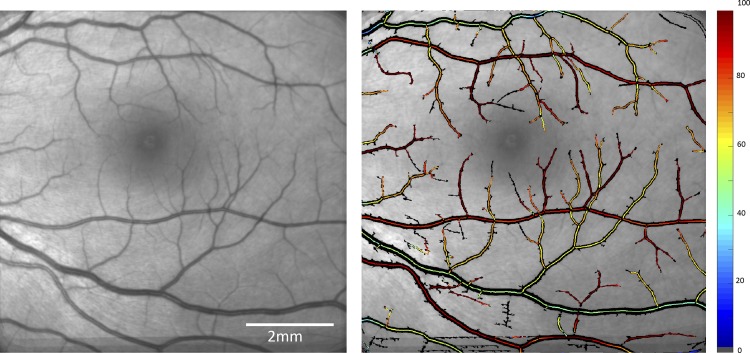
Other major advancements are the current dynamic retinal and cerebral imaging techniques that have the potential to allow more comprehensive analysis of pathophysiological and physiological function of the microvascular beds of the retina and the brain. These novel dynamic retinal imaging techniques facilitate the assessment of dynamic measurements of retinal blood flow, oxygen saturation, and vessel diameter (Fig. 3) (27, 35). This could pave the road for these technologies to introduce ocular dynamics measurements as a possible diagnostic tool in brain disorders (74).
Fig. 3.
Quantitative oximetry. Left: fundus image obtained from a healthy eye. Right: oxygen saturation map of the same eye. (Images courtesy of Optical Imaging Ltd., Rehovot, Israel.)
Moreover, the opportunity of telemedical consultation offered by current mobile retinal imaging devices has now improved the access not only to accurate, but timely subspecialty care, especially for underserved regions (68). This may open a new avenue for increasing retinal imaging applications in neurologic and psychiatric teleconsultations as it is currently in place for retinal diseases (32, 68).
Retino-Cerebrovascular Unit: Controversies and Challenges
A great challenge exists to understanding how changes in the retinal neurovascular unit reflect neurovascular pathology in the brain, which seems to play a critical role to understand cerebral neurovascular pathogenesis. Another challenge is the variation in retinal structural and microvascular features between individuals along with the existing confounding variables that may also result in retinal changes. For example, it is still vague whether the observed retinal changes represent mainly the brain microvessel abnormalities or rather reflect retinal impairment concomitant with the vascular risk features (e.g., features related to hypertension, diabetes, and aging).
Most of the controversies are related to contradictory results related to dilation or contraction of vessels, as well as thinning or thickening of neural layers in the retina. In part, these controversies are in place because of the lack of studies with parallel fundus imaging and direct in vivo data from the cerebral vasculature (i.e., CT, MRI images directly from the brain where the microvasculature could be analyzed). This is one of the reasons why there is no answer yet as to whether or not the degenerative alterations of the intracerebral microvessels are parallel to those of the retinal microvessels. Particularly, fundus imaging analyses making precise vessel measurements are difficult (although widely available), whereas, on the other hand, SLO imaging is not widely available for clinical use at the present time (30, 104, 105, 145). In addition, there are problems related to the imaging methodologies used in the ocular health assessment and therapeutic strategies due to the large variability in the overall location, size, shape, and intensity patterns of the retinal pathologies.
A major methodological challenge, commanded by the rapid growth and increasing variety of commercial retinal imaging devices with different image resolutions (24, 25, 53, 54, 59, 70, 74, 98), is the lack of a gold standard for defining which particular retinal feature could represent a unique abnormal feature among people with various CNS disorders. In general, the majority of published studies have reported heterogeneous distribution of retinal vessel and structural abnormalities related to brain disorders, which may be associated with different pathogenic mechanisms underlying CNS disorders (3, 5, 16, 20, 98). In this regard, it is also important to take into account that a particular brain disorder may manifest different phenotypes in different locations throughout the CNS. Another related challenge is the effect size of the observed changes in retinal vessel and structural abnormalities in relation to the particular image resolution, especially in longitudinal studies (98).
Last, but not least, a major challenge to software developers of image analyses is the constant improvement and diversity of retinal imaging technology. Particularly, a major undertaking is the demanding development of robust segmentation algorithms for both retinal vessels and intraretinal layer structures to extract valuable information to aid clinical diagnosis. For example, in contrast to other studies cited in the section Dementia above, a recent OCT cross-sectional study reported retinal markers that did not differ between PD, AD dementia, amnestic MCI, non-AD dementia, and age- and sex-matched controls in a well-characterized patient cohort (34, 100). Another recent study reported negative findings with respect to the RNFL and GCL thickness in the macula contradicting previous published studies reporting thinning of these cellular layers in different AD stages (8, 19, 47, 49, 54, 95, 97, 126).
The poor agreement among different studies using dissimilar OCT scanners may be a result of the use of different anatomical landmarks in manufacturer and custom-built segmentation algorithms that calculate structural and microvascular measures of the retina. However, in addition to providing a basis for objective comparison of different studies, challenges and controversies have always been stimulating for the development and achievements of a particular field.
Conclusions
The growing number of scientific reports providing evidence of abnormal retinal microvascular features in various disorders considered as being primarily nonocular pathologies indicates the importance of studying the retinal microvascular network in a quantitative manner. This may shed light on the current role of the microcirculatory networks of the neural tissues and their role in the development of brain diseases. In addition, currently available and widely accepted procedures, such as neuroimaging methods [e.g., magnetic resonance imaging (MRI) and positron emission tomography (PET)] are very high-priced, extremely invasive, and they lack specificity and are not readily accessible to most clinicians and affected individuals (patients). Thus the use of retinal imaging to diagnose brain neurovascular pathology might introduce a significant cost reduction compared with the current standard of care in neurology. However, further technological innovations along with future research are needed to increase our understanding of the association between the microvascular alterations of retina and brain, which, when prompted by identification of cerebrovascular, neurodegenerative, psychiatric, and developmental diseases, could enable the development of retinal imaging biomarkers, relevant to the identification of novel neuroprotective and neuroregenerative strategies in the treatment of both retinal and brain disorders.
GRANTS
This manuscript was supported by the Finker Frenkel Legacy Foundation, Inc. (D. Cabrera DeBuc); National Institutes of Health NIH Center Grant No. P30-EY014801 (D. Cabrera DeBuc); and by an unrestricted grant to the University of Miami from Research to Prevent Blindness, Inc. (D. Cabrera DeBuc); the Helen Keller Foundation (G. M. Somfai); and National Research, Development and Innovation Office (OTKA K108444), Hungary, Marie Curie Actions SMARTER 7th Framework Program of the European Union 606998 (A. Koller).
DISCLOSURES
D. Cabrera DeBuc is a member of the scientific advisory board of Optical Imaging, Ltd.
AUTHOR CONTRIBUTIONS
D.C.D. prepared figures; D.C.D., G.M.S., and A.K. drafted manuscript; D.C.D., G.M.S., and A.K. edited and revised manuscript; D.C.D., G.M.S., and A.K. approved final version of manuscript.
REFERENCES
- 1.Aaker GD, Myung JS, Ehrlich JR, Mohammed M, Henchcliffe C, Kiss S. Detection of retinal changes in Parkinson’s disease with spectral-domain optical coherence tomography. Clin Ophthalmol 4: 1427–1432, 2010. doi: 10.2147/OPTH.S15136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abbott NJ, Revest PA, Romero IA. Astrocyte-endothelial interaction: physiology and pathology. Neuropathol Appl Neurobiol 18: 424–433, 1992. doi: 10.1111/j.1365-2990.1992.tb00808.x. [DOI] [PubMed] [Google Scholar]
- 3.Baker ML, Hand PJ, Liew G, Wong TY, Rochtchina E, Mitchell P, Lindley RI, Hankey GJ, Wang JJ; Multi-Centre Retinal Stroke Study Group . Retinal microvascular signs may provide clues to the underlying vasculopathy in patients with deep intracerebral hemorrhage. Stroke 41: 618–623, 2010. doi: 10.1161/STROKEAHA.109.569764. [DOI] [PubMed] [Google Scholar]
- 4.Baker ML, Hand PJ, Wong TY, Liew G, Rochtchina E, Mitchell P, Lindley RI, Hankey GJ, Wang JJ; Multi-Centre Retinal Stroke Study Group . Retinopathy and lobar intracerebral hemorrhage: insights into pathogenesis. Arch Neurol 67: 1224–1230, 2010. doi: 10.1001/archneurol.2010.249. [DOI] [PubMed] [Google Scholar]
- 5.Baker ML, Marino Larsen EK, Kuller LH, Klein R, Klein BE, Siscovick DS, Bernick C, Manolio TA, Wong TY. Retinal microvascular signs, cognitive function, and dementia in older persons: the Cardiovascular Health Study. Stroke 38: 2041–2047, 2007. doi: 10.1161/STROKEAHA.107.483586. [DOI] [PubMed] [Google Scholar]
- 6.Behrouz R, Malek AR, Torbey MT. Small vessel cerebrovascular disease: the past, present, and future. Stroke Res Treat 2012: 839151, 2012. doi: 10.1155/2012/839151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belcastro V, Striano P, Ciampa C, Pierguidi L, Napoli M, Freno MC, Tenore R, Striano S, Pisani F, Trombetta CJ. Is retinal assessment useful in epileptic patients with hyperhomocysteinemia? Eye (Lond) 23: 1532–1534, 2009. doi: 10.1038/eye.2008.326. [DOI] [PubMed] [Google Scholar]
- 8.Blanks JC, Torigoe Y, Hinton DR, Blanks RH. Retinal pathology in Alzheimer’s disease. I. Ganglion cell loss in foveal/parafoveal retina. Neurobiol Aging 17: 377–384, 1996. doi: 10.1016/0197-4580(96)00010-3. [DOI] [PubMed] [Google Scholar]
- 9.Bodis-Wollner I. Retinopathy in Parkinson Disease. J Neural Transm (Vienna) 116: 1493–1501, 2009. doi: 10.1007/s00702-009-0292-z. [DOI] [PubMed] [Google Scholar]
- 10.Bolton C, Smith P. Defining and regulating acute inflammatory lesion formation during the pathogenesis of multiple sclerosis and experimental autoimmune encephalomyelitis. CNS Neurol Disord Drug Targets 14: 915–935, 2015. doi: 10.2174/1871527314666150716103629. [DOI] [PubMed] [Google Scholar]
- 11.Braaf B, Vermeer KA, Vienola KV, de Boer JF. Angiography of the retina and the choroid with phase-resolved OCT using interval-optimized backstitched B-scans. Opt Express 20: 20516–20534, 2012. doi: 10.1364/OE.20.020516. [DOI] [PubMed] [Google Scholar]
- 12.Cheung AT, Chen PC, Larkin EC, Duong PL, Ramanujam S, Tablin F, Wun T. Microvascular abnormalities in sickle cell disease: a computer-assisted intravital microscopy study. Blood 99: 3999–4005, 2002. doi: 10.1182/blood.V99.11.3999. [DOI] [PubMed] [Google Scholar]
- 13.Cheung AT, Harmatz P, Wun T, Chen PC, Larkin EC, Adams RJ, Vichinsky EP. Correlation of abnormal intracranial vessel velocity, measured by transcranial Doppler ultrasonography, with abnormal conjunctival vessel velocity, measured by computer-assisted intravital microscopy, in sickle cell disease. Blood 97: 3401–3404, 2001. doi: 10.1182/blood.V97.11.3401. [DOI] [PubMed] [Google Scholar]
- 14.Cheung AT, Ramanujam S, Greer DA, Kumagai LF, Aoki TT. Microvascular abnormalities in the bulbar conjunctiva of patients with type 2 diabetes mellitus. Endocr Pract 7: 358–363, 2001. doi: 10.4158/EP.7.5.358. [DOI] [PubMed] [Google Scholar]
- 15.Cheung CY, Ong S, Ikram MK, Ong YT, Chen CP, Venketasubramanian N, Wong TY. Retinal vascular fractal dimension is associated with cognitive dysfunction. J Stroke Cerebrovasc Dis 23: 43–50, 2014. doi: 10.1016/j.jstrokecerebrovasdis.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Cheung CY, Tay WT, Ikram MK, Ong YT, De Silva DA, Chow KY, Wong TY. Retinal microvascular changes and risk of stroke: the Singapore Malay Eye Study. Stroke 44: 2402–2408, 2013. doi: 10.1161/STROKEAHA.113.001738. [DOI] [PubMed] [Google Scholar]
- 17.Cogan DG, Kuwabara T. Comparison of retinal and cerebral vasculature in trypsin digest preparations. Br J Ophthalmol 68: 10–12, 1984. doi: 10.1136/bjo.68.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Costello F, Pan YI, Yeh EA, Hodge W, Burton JM, Kardon R. The temporal evolution of structural and functional measures after acute optic neuritis. J Neurol Neurosurg Psychiatry 86: 1369–1373, 2015. doi: 10.1136/jnnp-2014-309704. [DOI] [PubMed] [Google Scholar]
- 19.Davies DC, McCoubrie P, McDonald B, Jobst KA. Myelinated axon number in the optic nerve is unaffected by Alzheimer’s disease. Br J Ophthalmol 79: 596–600, 1995. doi: 10.1136/bjo.79.6.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Jong FJ, Schrijvers EM, Ikram MK, Koudstaal PJ, de Jong PT, Hofman A, Vingerling JR, Breteler MM. Retinal vascular caliber and risk of dementia: the Rotterdam study. Neurology 76: 816–821, 2011. doi: 10.1212/WNL.0b013e31820e7baa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Silva TM, Miller AA. Cerebral small vessel disease: targeting oxidative stress as a novel therapeutic strategy? Front Pharmacol 7: 61, 2016. doi: 10.3389/fphar.2016.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ding J, Strachan MW, Reynolds RM, Frier BM, Deary IJ, Fowkes FG, Lee AJ, McKnight J, Halpin P, Swa K, Price JF; Edinburgh Type 2 Diabetes Study Investigators . Diabetic retinopathy and cognitive decline in older people with type 2 diabetes: the Edinburgh Type 2 Diabetes Study. Diabetes 59: 2883–2889, 2010. doi: 10.2337/db10-0752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dörr J, Wernecke KD, Bock M, Gaede G, Wuerfel JT, Pfueller CF, Bellmann-Strobl J, Freing A, Brandt AU, Friedemann P. Association of retinal and macular damage with brain atrophy in multiple sclerosis. PLoS One 6: e18132, 2011. doi: 10.1371/journal.pone.0018132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doubal FN, Hokke PE, Wardlaw JM. Retinal microvascular abnormalities and stroke: a systematic review. J Neurol Neurosurg Psychiatry 80: 158–165, 2009. doi: 10.1136/jnnp.2008.153460. [DOI] [PubMed] [Google Scholar]
- 25.Doubal FN, MacGillivray TJ, Patton N, Dhillon B, Dennis MS, Wardlaw JM. Fractal analysis of retinal vessels suggests that a distinct vasculopathy causes lacunar stroke. Neurology 74: 1102–1107, 2010. doi: 10.1212/WNL.0b013e3181d7d8b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drexler W, Liu M, Kumar A, Kamali T, Unterhuber A, Leitgeb RA. Optical coherence tomography today: speed, contrast, and multimodality. J Biomed Opt 19: 071412, 2014. doi: 10.1117/1.JBO.19.7.071412. [DOI] [PubMed] [Google Scholar]
- 27.Einarsdottir AB, Hardarson SH, Kristjansdottir JV, Bragason DT, Snaedal J, Stefánsson E. Retinal oximetry imaging in Alzheimer’s disease. J Alzheimers Dis 49: 79–83, 2016. doi: 10.3233/JAD-150457. [DOI] [PubMed] [Google Scholar]
- 28.Eraslan M, Cerman E, Yildiz Balci S, Celiker H, Sahin O, Temel A, Suer D, Tuncer Elmaci N. The choroid and lamina cribrosa is affected in patients with Parkinson’s disease: enhanced depth imaging optical coherence tomography study. Acta Ophthalmol 94: e68–e75, 2016. doi: 10.1111/aos.12809. [DOI] [PubMed] [Google Scholar]
- 29.Fisher JB, Jacobs DA, Markowitz CE, Galetta SL, Volpe NJ, Nano-Schiavi ML, Baier ML, Frohman EM, Winslow H, Frohman TC, Calabresi PA, Maguire MG, Cutter GR, Balcer LJ. Relation of visual function to retinal nerve fiber layer thickness in multiple sclerosis. Ophthalmology 113: 324–332, 2006. doi: 10.1016/j.ophtha.2005.10.040. [DOI] [PubMed] [Google Scholar]
- 30.Fleming AD, Philip S, Goatman KA, Williams GJ, Olson JA, Sharp PF. Automated detection of exudates for diabetic retinopathy screening. Phys Med Biol 52: 7385–7396, 2007. doi: 10.1088/0031-9155/52/24/012. [DOI] [PubMed] [Google Scholar]
- 31.Franceschi C. The unsolved puzzle of multiple sclerosis and venous function. J Neurol Neurosurg Psychiatry 80: 358, 2009. doi: 10.1136/jnnp.2008.168179. [DOI] [PubMed] [Google Scholar]
- 32.Freeman WD, Barrett KM, Vatz KA, Demaerschalk BM. Future neurohospitalist: teleneurohospitalist. Neurohospitalist 2: 132–143, 2012. doi: 10.1177/1941874412450714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frost S, Kanagasingam Y, Sohrabi H, Vignarajan J, Bourgeat P, Salvado O, Villemagne V, Rowe CC, Macaulay SL, Szoeke C, Ellis KA, Ames D, Masters CL, Rainey-Smith S, Martins RN; AIBL Research Group . Retinal vascular biomarkers for early detection and monitoring of Alzheimer’s disease. Transl Psychiatry 3: e233, 2013. doi: 10.1038/tp.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garcia-Martin ES, Rojas B, Ramirez AI, de Hoz R, Salazar JJ, Yubero R, Gil P, Triviño A, Ramirez JM. Macular thickness as a potential biomarker of mild Alzheimer’s disease. Ophthalmology 121: 1149–1151.e3, 2014. doi: 10.1016/j.ophtha.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 35.Garhofer G, Bek T, Boehm AG, Gherghel D, Grunwald J, Jeppesen P, Kergoat H, Kotliar K, Lanzl I, Lovasik JV, Nagel E, Vilser W, Orgul S, Schmetterer L; Ocular Blood Flow Research Association . Use of the retinal vessel analyzer in ocular blood flow research. Acta Ophthalmol 88: 717–722, 2010. doi: 10.1111/j.1755-3768.2009.01587.x. [DOI] [PubMed] [Google Scholar]
- 36.Gariano RF, Gardner TW. Retinal angiogenesis in development and disease. Nature 438: 960–966, 2005. doi: 10.1038/nature04482. [DOI] [PubMed] [Google Scholar]
- 37.Gartner S. Optic neuropathy in multiple sclerosis; optic neuritis. AMA Arch Ophthalmol 50: 718–726, 1953. doi: 10.1001/archopht.1953.00920030729007. [DOI] [PubMed] [Google Scholar]
- 38.Gatto NM, Varma R, Torres M, Wong TY, Johnson PL, Segal-Gidan F, Mack WJ. Retinal microvascular abnormalities and cognitive function in Latino adults in Los Angeles. Ophthalmic Epidemiol 19: 127–136, 2012. doi: 10.3109/09286586.2011.615452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gjedde A, Diemer NH. Double-tracer study of the fine regional blood-brain glucose transfer in the rat by computer-assisted autoradiography. J Cereb Blood Flow Metab 5: 282–289, 1985. doi: 10.1038/jcbfm.1985.36. [DOI] [PubMed] [Google Scholar]
- 40.Gordon-Lipkin E, Chodkowski B, Reich DS, Smith SA, Pulicken M, Balcer LJ, Frohman EM, Cutter G, Calabresi PA. Retinal nerve fiber layer is associated with brain atrophy in multiple sclerosis. Neurology 69: 1603–1609, 2007. doi: 10.1212/01.wnl.0000295995.46586.ae. [DOI] [PubMed] [Google Scholar]
- 41.Grazioli E, Zivadinov R, Weinstock-Guttman B, Lincoff N, Baier M, Wong JR, Hussein S, Cox JL, Hojnacki D, Ramanathan M. Retinal nerve fiber layer thickness is associated with brain MRI outcomes in multiple sclerosis. J Neurol Sci 268: 12–17, 2008. doi: 10.1016/j.jns.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 42.Green AJ, McQuaid S, Hauser SL, Allen IV, Lyness R. Ocular pathology in multiple sclerosis: retinal atrophy and inflammation irrespective of disease duration. Brain 133: 1591–1601, 2010. doi: 10.1093/brain/awq080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grönlund MA, Aring E, Landgren M, Hellström A. Visual function and ocular features in children and adolescents with attention deficit hyperactivity disorder, with and without treatment with stimulants. Eye (Lond) 21: 494–502, 2007. doi: 10.1038/sj.eye.6702240. [DOI] [PubMed] [Google Scholar]
- 44.Gunes A, Demirci S, Umul A. Vision loss and RNFL thinning after internal carotid arter occlusion and middle cerebral artery infarction. Acta Inform Med 22: 413–414, 2014. doi: 10.5455/aim.2014.22.413-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gustavsson A, Svensson M, Jacobi F, Allgulander C, Alonso J, Beghi E, Dodel R, Ekman M, Faravelli C, Fratiglioni L, Gannon B, Jones DH, Jennum P, Jordanova A, Jönsson L, Karampampa K, Knapp M, Kobelt G, Kurth T, Lieb R, Linde M, Ljungcrantz C, Maercker A, Melin B, Moscarelli M, Musayev A, Norwood F, Preisig M, Pugliatti M, Rehm J, Salvador-Carulla L, Schlehofer B, Simon R, Steinhausen HC, Stovner LJ, Vallat JM, Van den Bergh P, van Os J, Vos P, Xu W, Wittchen HU, Jönsson B, Olesen J; CDBE2010Study Group . Cost of disorders of the brain in Europe 2010. Eur Neuropsychopharmacol 21: 718–779, 2011. doi: 10.1016/j.euroneuro.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 46.Haigh JJ, Morelli PI, Gerhardt H, Haigh K, Tsien J, Damert A, Miquerol L, Muhlner U, Klein R, Ferrara N, Wagner EF, Betsholtz C, Nagy A. Cortical and retinal defects caused by dosage-dependent reductions in VEGF-A paracrine signaling. Dev Biol 262: 225–241, 2003. doi: 10.1016/S0012-1606(03)00356-7. [DOI] [PubMed] [Google Scholar]
- 47.Hedges TR III, Perez Galves R, Speigelman D, Barbas NR, Peli E, Yardley CJ. Retinal nerve fiber layer abnormalities in Alzheimer’s disease. Acta Ophthalmol Scand 74: 271–275, 1996. doi: 10.1111/j.1600-0420.1996.tb00090.x. [DOI] [PubMed] [Google Scholar]
- 48.Henderson AP, Trip SA, Schlottmann PG, Altmann DR, Garway-Heath DF, Plant GT, Miller DH. An investigation of the retinal nerve fibre layer in progressive multiple sclerosis using optical coherence tomography. Brain 131: 277–287, 2008. doi: 10.1093/brain/awm285. [DOI] [PubMed] [Google Scholar]
- 49.Hinton DR, Sadun AA, Blanks JC, Miller CA. Optic-nerve degeneration in Alzheimer’s disease. N Engl J Med 315: 485–487, 1986. doi: 10.1056/NEJM198608213150804. [DOI] [PubMed] [Google Scholar]
- 50.Hoge RD, Atkinson J, Gill B, Crelier GR, Marrett S, Pike GB. Linear coupling between cerebral blood flow and oxygen consumption in activated human cortex. Proc Natl Acad Sci U S A 96: 9403–9408, 1999. doi: 10.1073/pnas.96.16.9403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hübers A, Müller HP, Dreyhaupt J, Böhm K, Lauda F, Tumani H, Kassubek J, Ludolph AC, Pinkhardt EH. Retinal involvement in amyotrophic lateral sclerosis: a study with optical coherence tomography and diffusion tensor imaging. J Neural Transm (Vienna) 123: 281–287, 2016. doi: 10.1007/s00702-015-1483-4. [DOI] [PubMed] [Google Scholar]
- 52.Hughes S, Yang H, Chan-Ling T. Vascularization of the human fetal retina: roles of vasculogenesis and angiogenesis. Invest Ophthalmol Vis Sci 41: 1217–1228, 2000. [PubMed] [Google Scholar]
- 53.Ikram MK, de Jong FJ, Bos MJ, Vingerling JR, Hofman A, Koudstaal PJ, de Jong PT, Breteler MM. Retinal vessel diameters and risk of stroke: the Rotterdam Study. Neurology 66: 1339–1343, 2006. doi: 10.1212/01.wnl.0000210533.24338.ea. [DOI] [PubMed] [Google Scholar]
- 54.Iseri PK, Altinaș O, Tokay T, Yüksel N. Relationship between cognitive impairment and retinal morphological and visual functional abnormalities in Alzheimer disease. J Neuroophthalmol 26: 18–24, 2006. doi: 10.1097/01.wno.0000204645.56873.26. [DOI] [PubMed] [Google Scholar]
- 55.Janzer RC. The blood-brain barrier: cellular basis. J Inherit Metab Dis 16: 639–647, 1993. doi: 10.1007/BF00711897. [DOI] [PubMed] [Google Scholar]
- 56.Jiang H, Delgado S, Liu C, Rammohan KW, DeBuc DC, Lam BL, Wang J. In vivo characterization of retinal microvascular network in multiple sclerosis. Ophthalmology 123: 437–438, 2016. doi: 10.1016/j.ophtha.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jiang H, Delgado S, Tan J, Liu C, Rammohan KW, DeBuc DC, Lam BL, Feuer WJ, Wang J. Impaired retinal microcirculation in multiple sclerosis. Mult Scler 22: 1812–1820, 2016. doi: 10.1177/1352458516631035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jiang H, Ye Y, DeBuc DC, Lam BL, Rundek T, Tao A, Shao Y, Wang J. Human conjunctival microvasculature assessed with a retinal function imager (RFI). Microvasc Res 85: 134–137, 2013. doi: 10.1016/j.mvr.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kawasaki R, Che Azemin MZ, Kumar DK, Tan AG, Liew G, Wong TY, Mitchell P, Wang JJ. Fractal dimension of the retinal vasculature and risk of stroke: a nested case-control study. Neurology 76: 1766–1767, 2011. doi: 10.1212/WNL.0b013e31821a7d7d. [DOI] [PubMed] [Google Scholar]
- 60.Kesler A, Vakhapova V, Korczyn AD, Naftaliev E, Neudorfer M. Retinal thickness in patients with mild cognitive impairment and Alzheimer’s disease. Clin Neurol Neurosurg 113: 523–526, 2011. doi: 10.1016/j.clineuro.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 61.Kim DH, Newman AB, Hajjar I, Strotmeyer ES, Klein R, Newton E, Sarnak MJ, Burke GL, Lipsitz LA. Retinal microvascular signs and functional loss in older persons: the cardiovascular health study. Stroke 42: 1589–1595, 2011. doi: 10.1161/STROKEAHA.110.605261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim JE, Chung M. Adaptive optics for retinal imaging: current status. Retina 33: 1483–1486, 2013. doi: 10.1097/IAE.0b013e31828cd053. [DOI] [PubMed] [Google Scholar]
- 63.Klein B, Kuschinsky W, Schröck H, Vetterlein F. Interdependency of local capillary density, blood flow, and metabolism in rat brains. Am J Physiol Heart Circ Physiol 251: H1333–H1340, 1986. [DOI] [PubMed] [Google Scholar]
- 64.Kochkorov A, Gugleta K, Kavroulaki D, Katamay R, Weier K, Mehling M, Kappos L, Flammer J, Orgül S. Rigidity of retinal vessels in patients with multiple sclerosis. Klin Monbl Augenheilkd 226: 276–279, 2009. doi: 10.1055/s-0028-1109291. [DOI] [PubMed] [Google Scholar]
- 65.Koller A, Dawant B, Liu A, Popel AS, Johnson PC. Quantitative analysis of arteriolar network architecture in cat sartorius muscle. Am J Physiol Heart Circ Physiol 253: H154–H164, 1987. [DOI] [PubMed] [Google Scholar]
- 66.Koller A, Toth P. Contribution of flow-dependent vasomotor mechanisms to the autoregulation of cerebral blood flow. J Vasc Res 49: 375–389, 2012. doi: 10.1159/000338747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Leenders KL, Perani D, Lammertsma AA, Heather JD, Buckingham P, Healy MJ, Gibbs JM, Wise RJ, Hatazawa J, Herold S, Beaney RP, Brooks DJ, Spinks T, Rhodes C, Frackowiak RS. Cerebral blood flow, blood volume and oxygen utilization. Normal values and effect of age. Brain 113: 27–47, 1990. doi: 10.1093/brain/113.1.27. [DOI] [PubMed] [Google Scholar]
- 68.Li HK, Horton M, Bursell SE, Cavallerano J, Zimmer-Galler I, Tennant M, Abramoff M, Chaum E, Debuc DC, Leonard-Martin T, Winchester M, Lawrence MG, Bauman W, Gardner WK, Hildebran L, Federman J; American Telemedicine Association Diabetic Retinopathy Telehealth Practice Recommendations Working Group . Telehealth practice recommendations for diabetic retinopathy, second edition. Telemed J E Health 17: 814–837, 2011. doi: 10.1089/tmj.2011.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liew G, Mitchell P, Wong TY, Lindley RI, Cheung N, Kaushik S, Wang JJ. Retinal microvascular signs and cognitive impairment. J Am Geriatr Soc 57: 1892–1896, 2009. doi: 10.1111/j.1532-5415.2009.02459.x. [DOI] [PubMed] [Google Scholar]
- 70.Lindley RI, Wang JJ, Wong MC, Mitchell P, Liew G, Hand P, Wardlaw J, De Silva DA, Baker M, Rochtchina E, Chen C, Hankey GJ, Chang HM, Fung VS, Gomes L, Wong TY; Multi-Centre Retina and Stroke Study (MCRS) Collaborative Group . Retinal microvasculature in acute lacunar stroke: a cross-sectional study. Lancet Neurol 8: 628–634, 2009. doi: 10.1016/S1474-4422(09)70131-0. [DOI] [PubMed] [Google Scholar]
- 71.London A, Benhar I, Schwartz M. The retina as a window to the brain-from eye research to CNS disorders. Nat Rev Neurol 9: 44–53, 2013. doi: 10.1038/nrneurol.2012.227. [DOI] [PubMed] [Google Scholar]
- 72.Lu Y, Li Z, Zhang X, Ming B, Jia J, Wang R, Ma D. Retinal nerve fiber layer structure abnormalities in early Alzheimer’s disease: evidence in optical coherence tomography. Neurosci Lett 480: 69–72, 2010. doi: 10.1016/j.neulet.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 73.Lucero M, Conger A, Conger D, Sobhanian M, Stokes V, Frohman T, White O, Balcer L, Calabresi P, Rennaker R, Frohman E, Beh S. Retinal oximetry-derived biomarkers of metabolic dysfunction in MS (Abstract). Neurology 84, Suppl: P5.307, 2015. [Google Scholar]
- 74.MacCormick IJ, Beare NA, Taylor TE, Barrera V, White VA, Hiscott P, Molyneux ME, Dhillon B, Harding SP. Cerebral malaria in children: using the retina to study the brain. Brain 137: 2119–2142, 2014. doi: 10.1093/brain/awu001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.MacGillivray TJ, Trucco E, Cameron JR, Dhillon B, Houston JG, van Beek EJ. Retinal imaging as a source of biomarkers for diagnosis, characterization and prognosis of chronic illness or long-term conditions. Br J Radiol 87: 20130832, 2014. doi: 10.1259/bjr.20130832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mailankody P, Battu R, Khanna A, Lenka A, Yadav R, Pal PK. Optical coherence tomography as a tool to evaluate retinal changes in Parkinson’s disease. Parkinsonism Relat Disord 21: 1164–1169, 2015. doi: 10.1016/j.parkreldis.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 77.Makita S, Hong Y, Yamanari M, Yatagai T, Yasuno Y. Optical coherence angiography. Opt Express 14: 7821–7840, 2006. doi: 10.1364/OE.14.007821. [DOI] [PubMed] [Google Scholar]
- 78.Marziani E, Pomati S, Ramolfo P, Cigada M, Giani A, Mariani C, Staurenghi G. Evaluation of retinal nerve fiber layer and ganglion cell layer thickness in Alzheimer’s disease using spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci 54: 5953–5958, 2013. doi: 10.1167/iovs.13-12046. [DOI] [PubMed] [Google Scholar]
- 79.Masters BR. Fractal analysis of the vascular tree in the human retina. Annu Rev Biomed Eng 6: 427–452, 2004. doi: 10.1146/annurev.bioeng.6.040803.140100. [DOI] [PubMed] [Google Scholar]
- 80.McGeechan K, Liew G, Macaskill P, Irwig L, Klein R, Klein BE, Wang JJ, Mitchell P, Vingerling JR, de Jong PT, Witteman JC, Breteler MM, Shaw J, Zimmet P, Wong TY. Prediction of incident stroke events based on retinal vessel caliber: a systematic review and individual-participant meta-analysis. Am J Epidemiol 170: 1323–1332, 2009. doi: 10.1093/aje/kwp306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Meier MH, Shalev I, Moffitt TE, Kapur S, Keefe RS, Wong TY, Belsky DW, Harrington H, Hogan S, Houts R, Caspi A, Poulton R. Microvascular abnormality in schizophrenia as shown by retinal imaging. Am J Psychiatry 170: 1451–1459, 2013. doi: 10.1176/appi.ajp.2013.13020234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Miloudi K, Dejda A, Binet F, Lapalme E, Cerani A, Sapieha P. Assessment of vascular regeneration in the CNS using the mouse retina. J Vis Exp 88: e51351, 2014. doi: 10.3791/51351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Minagar A, Jy W, Jimenez JJ, Alexander JS. Multiple sclerosis as a vascular disease. Neurol Res 28: 230–235, 2006. doi: 10.1179/016164106X98080. [DOI] [PubMed] [Google Scholar]
- 84.More SS, Beach JM, Vince R. Early Detection of Amyloidopathy in Alzheimer’s Mice by Hyperspectral Endoscopy. Invest Ophthalmol Vis Sci 57: 3231–3238, 2016. doi: 10.1167/iovs.15-17406. [DOI] [PubMed] [Google Scholar]
- 85.Murray CD. The physiological principle of minimum work. I. The vascular system and the cost of blood volume. Proc Natl Acad Sci USA 12: 207–214, 1926. doi: 10.1073/pnas.12.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mutlu U, Adams HH, Hofman A, Lugt A, Klaver CC, Vernooij MW, Ikram MK, Ikram MA. Retinal microvascular calibers are associated with enlarged perivascular spaces in the brain. Stroke 47: 1374–1376, 2016. doi: 10.1161/STROKEAHA.115.012438. [DOI] [PubMed] [Google Scholar]
- 87.Neuwelt EA. (Editor). Implications of the Blood-Brain Barrier and Its Manipulation. New York: Plenum, 1989. doi: 10.1007/978-1-4613-0701-3. [DOI] [Google Scholar]
- 88.Normando EM, Davis BM, De Groef L, Nizari S, Turner LA, Ravindran N, Pahlitzsch M, Brenton J, Malaguarnera G, Guo L, Somavarapu S, Cordeiro MF. The retina as an early biomarker of neurodegeneration in a rotenone-induced model of Parkinson’s disease: evidence for a neuroprotective effect of rosiglitazone in the eye and brain. Acta Neuropathol Commun 4: 86, 2016. doi: 10.1186/s40478-016-0346-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ohtani N. [Laser Doppler flowmetry of the bulbar conjunctiva as a monitor of the cerebral blood flow]. Nihon Kyobu Geka Gakkai Zasshi 44: 1721–1728, 1996. [PubMed] [Google Scholar]
- 90.Ong SY, Cheung CY, Li X, Lamoureux EL, Ikram MK, Ding J, Cheng CY, Haaland BA, Saw SM, Venketasubramanian N, Chen CP, Wong TY. Visual impairment, age-related eye diseases, and cognitive function: the Singapore Malay Eye study. Arch Ophthalmol 130: 895–900, 2012. doi: 10.1001/archophthalmol.2012.152. [DOI] [PubMed] [Google Scholar]
- 91.Ong YT, De Silva DA, Cheung CY, Chang HM, Chen CP, Wong MC, Wong TY, Ikram MK. Microvascular structure and network in the retina of patients with ischemic stroke. Stroke 44: 2121–2127, 2013. doi: 10.1161/STROKEAHA.113.001741. [DOI] [PubMed] [Google Scholar]
- 92.Ong YT, Hilal S, Cheung CY, Xu X, Chen C, Venketasubramanian N, Wong TY, Ikram MK. Retinal vascular fractals and cognitive impairment. Dement Geriatr Cogn Dis Extra 4: 305–313, 2014. doi: 10.1159/000363286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Optic Neuritis Study Group . Multiple sclerosis risk after optic neuritis: final optic neuritis treatment trial follow-up. Arch Neurol 65: 727–732, 2008. doi: 10.1001/archneur.65.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ortiz-Pérez S, Martínez-Lapiscina EH, Gabilondo I, Fraga-Pumar E, Martínez-Heras E, Saiz A, Sanchez-Dalmau B, Villoslada P. Retinal periphlebitis is associated with multiple sclerosis severity. Neurology 81: 877–881, 2013. doi: 10.1212/WNL.0b013e3182a3525e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Paquet C, Boissonnot M, Roger F, Dighiero P, Gil R, Hugon J. Abnormal retinal thickness in patients with mild cognitive impairment and Alzheimer’s disease. Neurosci Lett 420: 97–99, 2007. doi: 10.1016/j.neulet.2007.02.090. [DOI] [PubMed] [Google Scholar]
- 96.Parisi V, Manni G, Spadaro M, Colacino G, Restuccia R, Marchi S, Bucci MG, Pierelli F. Correlation between morphological and functional retinal impairment in multiple sclerosis patients. Invest Ophthalmol Vis Sci 40: 2520–2527, 1999. [PubMed] [Google Scholar]
- 97.Parisi V, Restuccia R, Fattapposta F, Mina C, Bucci MG, Pierelli F. Morphological and functional retinal impairment in Alzheimer’s disease patients. Clin Neurophysiol 112: 1860–1867, 2001. doi: 10.1016/S1388-2457(01)00620-4. [DOI] [PubMed] [Google Scholar]
- 98.Patton N, Aslam T, Macgillivray T, Pattie A, Deary IJ, Dhillon B. Retinal vascular image analysis as a potential screening tool for cerebrovascular disease: a rationale based on homology between cerebral and retinal microvasculatures. J Anat 206: 319–348, 2005. doi: 10.1111/j.1469-7580.2005.00395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Patton N, Pattie A, MacGillivray T, Aslam T, Dhillon B, Gow A, Starr JM, Whalley LJ, Deary IJ. The association between retinal vascular network geometry and cognitive ability in an elderly population. Invest Ophthalmol Vis Sci 48: 1995–2000, 2007. doi: 10.1167/iovs.06-1123. [DOI] [PubMed] [Google Scholar]
- 100.Pillai JA, Bermel R, Bonner-Jackson A, Rae-Grant A, Fernandez H, Bena J, Jones SE, Ehlers JP, Leverenz JB. Retinal nerve fiber layer thinning in Alzheimer’s disease: a case-control study in comparison to normal aging, Parkinson’s disease, and Non-Alzheimer’s dementia. Am J Alzheimers Dis Other Demen 31: 430–436, 2016. doi: 10.1177/1533317515628053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Polo V, Garcia-Martin E, Bambo MP, Pinilla J, Larrosa JM, Satue M, Otin S, Pablo LE. Reliability and validity of Cirrus and Spectralis optical coherence tomography for detecting retinal atrophy in Alzheimer’s disease. Eye (Lond) 28: 680–690, 2014. doi: 10.1038/eye.2014.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Qiu C, Cotch MF, Sigurdsson S, Jonsson PV, Jonsdottir MK, Sveinbjrnsdottir S, Eiriksdottir G, Klein R, Harris TB, van Buchem MA, Gudnason V, Launer LJ. Cerebral microbleeds, retinopathy, and dementia: the AGES-Reykjavik Study. Neurology 75: 2221–2228, 2010. doi: 10.1212/WNL.0b013e3182020349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rassam SM, Patel V, Chen HC, Kohner EM. Regional retinal blood flow and vascular autoregulation. Eye (Lond) 10: 331–337, 1996. doi: 10.1038/eye.1996.69. [DOI] [PubMed] [Google Scholar]
- 104.Rasta SH, Manivannan A, Sharp PF. Spectral imaging technique for retinal perfusion detection using confocal scanning laser ophthalmoscopy. J Biomed Opt 17: 116005, 2012. doi: 10.1117/1.JBO.17.11.116005. [DOI] [PubMed] [Google Scholar]
- 105.Rasta SH, Partovi ME, Seyedarabi H, Javadzadeh A. A comparative study on preprocessing techniques in diabetic retinopathy retinal images: illumination correction and contrast enhancement. J Med Signals Sens 5: 40–48, 2015. [PMC free article] [PubMed] [Google Scholar]
- 106.Rha J, Jonnal RS, Thorn KE, Qu J, Zhang Y, Miller DT. Adaptive optics flood-illumination camera for high speed retinal imaging. Opt Express 14: 4552–4569, 2006. doi: 10.1364/OE.14.004552. [DOI] [PubMed] [Google Scholar]
- 107.Risau W. Mechanisms of angiogenesis. Nature 386: 671–674, 1997. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 108.Rohani M, Langroodi AS, Ghourchian S, Falavarjani KG, SoUdi R, Shahidi G. Retinal nerve changes in patients with tremor dominant and akinetic rigid Parkinson’s disease. Neurol Sci 34: 689–693, 2013. doi: 10.1007/s10072-012-1125-7. [DOI] [PubMed] [Google Scholar]
- 109.Roorda A, Romero-Borja F, Donnelly Iii W, Queener H, Hebert T, Campbell M. Adaptive optics scanning laser ophthalmoscopy. Opt Express 10: 405–412, 2002. doi: 10.1364/OE.10.000405. [DOI] [PubMed] [Google Scholar]
- 110.Rucker CW. Sheathing of the retinal veins in multiple sclerosis. Res Publ Assoc Res Nerv Ment Dis 28: 396–402, 1950. [PubMed] [Google Scholar]
- 111.Rucker CW. Sheathing of the retinal veins in multiple sclerosis. Review of pertinent literature. Mayo Clin Proc 47: 335–340, 1972. [PubMed] [Google Scholar]
- 112.Sacco RL, Rundek T. Cerebrovascular disease. Curr Opin Neurol 25: 1–4, 2012. doi: 10.1097/WCO.0b013e32834f89b1. [DOI] [PubMed] [Google Scholar]
- 113.Sagaties MJ, Raviola G, Schaeffer S, Miller C. The structural basis of the inner blood-retina barrier in the eye of Macaca mulatta. Invest Ophthalmol Vis Sci 28: 2000–2014, 1987. [PubMed] [Google Scholar]
- 114.Saidha S, Al-Louzi O, Ratchford JN, Bhargava P, Oh J, Newsome SD, Prince JL, Pham D, Roy S, van Zijl P, Balcer LJ, Frohman EM, Reich DS, Crainiceanu C, Calabresi PA. Optical coherence tomography reflects brain atrophy in multiple sclerosis: A four-year study. Ann Neurol 78: 801–813, 2015. doi: 10.1002/ana.24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Satue M, Garcia-Martin E, Fuertes I, Otin S, Alarcia R, Herrero R, Bambo MP, Pablo LE, Fernandez FJ. Use of Fourier-domain OCT to detect retinal nerve fiber layer degeneration in Parkinson’s disease patients. Eye (Lond) 27: 507–514, 2013. doi: 10.1038/eye.2013.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schaser KD, Settmacher U, Puhl G, Zhang L, Mittlmeier T, Stover JF, Vollmar B, Menger MD, Neuhaus P, Haas NP. Noninvasive analysis of conjunctival microcirculation during carotid artery surgery reveals microvascular evidence of collateral compensation and stenosis-dependent adaptation. J Vasc Surg 37: 789–797, 2003. doi: 10.1067/mva.2003.139. [DOI] [PubMed] [Google Scholar]
- 117.Schrijvers EM, Buitendijk GH, Ikram MK, Koudstaal PJ, Hofman A, Vingerling JR, Breteler MM. Retinopathy and risk of dementia: the Rotterdam Study. Neurology 79: 365–370, 2012. doi: 10.1212/WNL.0b013e318260cd7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sepulcre J, Murie-Fernandez M, Salinas-Alaman A, García-Layana A, Bejarano B, Villoslada P. Diagnostic accuracy of retinal abnormalities in predicting disease activity in MS. Neurology 68: 1488–1494, 2007. doi: 10.1212/01.wnl.0000260612.51849.ed. [DOI] [PubMed] [Google Scholar]
- 119.Shen Y, Liu L, Cheng Y, Feng W, Shi Z, Zhu Y, Wu W, Li C. Retinal nerve fiber layer thickness is associated with episodic memory deficit in mild cognitive impairment patients. Curr Alzheimer Res 11: 259–266, 2014. doi: 10.2174/1567205011666140131114418. [DOI] [PubMed] [Google Scholar]
- 120.Simonett JM, Huang R, Siddique N, Farsiu S, Siddique T, Volpe NJ, Fawzi AA. Macular sub-layer thinning and association with pulmonary function tests in Amyotrophic Lateral Sclerosis. Sci Rep 6: 29187, 2016. doi: 10.1038/srep29187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.T L.. Zirkulations- and Ernahrungsverhaltnisse des Auges. In: Handbuch der Gesamten Augenheilkunde, edited by Graefe AST. Leipzig: Sopringer-Verlag, 1903. [Google Scholar]
- 122.ter Laan M, van Dijk JM, Elting JW, Staal MJ, Absalom AR. Sympathetic regulation of cerebral blood flow in humans: a review. Br J Anaesth 111: 361–367, 2013. doi: 10.1093/bja/aet122. [DOI] [PubMed] [Google Scholar]
- 123.Thomson KL, Yeo JM, Waddell B, Cameron JR, Pal S. A systematic review and meta-analysis of retinal nerve fiber layer change in dementia, using optical coherence tomography. Alzheimers Dement (Amst) 1: 136–143, 2015. doi: 10.1016/j.dadm.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Törnquist P, Alm A. Carrier-mediated transport of amino acids through the blood-retinal and the blood-brain barriers. Graefes Arch Clin Exp Ophthalmol 224: 21–25, 1986. doi: 10.1007/BF02144127. [DOI] [PubMed] [Google Scholar]
- 125.Toussaint D, Kuwabara T, Cogan DG. Retinal vascular patterns. II. Human retinal vessels studied in three dimensions. Arch Ophthalmol 65: 575–581, 1961. doi: 10.1001/archopht.1961.01840020577022. [DOI] [PubMed] [Google Scholar]
- 126.Tsai CS, Ritch R, Schwartz B, Lee SS, Miller NR, Chi T, Hsieh FY. Optic nerve head and nerve fiber layer in Alzheimer’s disease. Arch Ophthalmol 109: 199–204, 1991. doi: 10.1001/archopht.1991.01080020045040. [DOI] [PubMed] [Google Scholar]
- 127.van Hecke MV, Dekker JM, Nijpels G, Moll AC, Heine RJ, Bouter LM, Polak BC, Stehouwer CD. Inflammation and endothelial dysfunction are associated with retinopathy: the Hoorn Study. Diabetologia 48: 1300–1306, 2005. doi: 10.1007/s00125-005-1799-y. [DOI] [PubMed] [Google Scholar]
- 128.Varga BE, Gao W, Laurik KL, Tátrai E, Simó M, Somfai GM, Cabrera DeBuc D. Investigating Tissue Optical Properties and Texture Descriptors of the Retina in Patients with Multiple Sclerosis. PLoS One 10: e0143711, 2015. doi: 10.1371/journal.pone.0143711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wallis SJ, Firth J, Dunn WR. Pressure-induced myogenic responses in human isolated cerebral resistance arteries. Stroke 27: 2287–2290, 1996. doi: 10.1161/01.STR.27.12.2287. [DOI] [PubMed] [Google Scholar]
- 130.Wang D, Li Y, Wang C, Xu L, You QS, Wang YX, Zhao L, Wei WB, Zhao X, Jonas JB. Localized retinal nerve fiber layer defects and stroke. Stroke 45: 1651–1656, 2014. doi: 10.1161/STROKEAHA.113.004629. [DOI] [PubMed] [Google Scholar]
- 131.Wang JJ, Baker ML, Hand PJ, Hankey GJ, Lindley RI, Rochtchina E, Wong TY, Liew G, Mitchell P. Transient ischemic attack and acute ischemic stroke: associations with retinal microvascular signs. Stroke 42: 404–408, 2011. doi: 10.1161/STROKEAHA.110.598599. [DOI] [PubMed] [Google Scholar]
- 132.Wang RK, Jacques SL, Ma Z, Hurst S, Hanson SR, Gruber A. Three dimensional optical angiography. Opt Express 15: 4083–4097, 2007. doi: 10.1364/OE.15.004083. [DOI] [PubMed] [Google Scholar]
- 133.Wang X, Jia Y, Spain R, Potsaid B, Liu JJ, Baumann B, Hornegger J, Fujimoto JG, Wu Q, Huang D. Optical coherence tomography angiography of optic nerve head and parafovea in multiple sclerosis. Br J Ophthalmol 98: 1368–1373, 2014. doi: 10.1136/bjophthalmol-2013-304547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang Y, Fawzi A, Tan O, Gil-Flamer J, Huang D. Retinal blood flow detection in diabetic patients by Doppler Fourier domain optical coherence tomography. Opt Express 17: 4061–4073, 2009. doi: 10.1364/OE.17.004061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Winkler EA, Sengillo JD, Sullivan JS, Henkel JS, Appel SH, Zlokovic BV. Blood-spinal cord barrier breakdown and pericyte reductions in amyotrophic lateral sclerosis. Acta Neuropathol 125: 111–120, 2013. doi: 10.1007/s00401-012-1039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wolz J, Audebert H, Laumeier I, Ahmadi M, Steinicke M, Ferse C, Michelson G. Telemedical assessment of optic nerve head and retina in patients after recent minor stroke or TIA. Int Ophthalmol; Epub ahead of print, 2016. doi: 10.1007/s10792-016-0222-7. [DOI] [PubMed] [Google Scholar]
- 137.Wong TY. Is retinal photography useful in the measurement of stroke risk? Lancet Neurol 3: 179–183, 2004. doi: 10.1016/S1474-4422(04)00682-9. [DOI] [PubMed] [Google Scholar]
- 138.Wong TY, Klein R, Couper DJ, Cooper LS, Shahar E, Hubbard LD, Wofford MR, Sharrett AR. Retinal microvascular abnormalities and incident stroke: the Atherosclerosis Risk in Communities Study. Lancet 358: 1134–1140, 2001. doi: 10.1016/S0140-6736(01)06253-5. [DOI] [PubMed] [Google Scholar]
- 139.Wong TY, Klein R, Sharrett AR, Couper DJ, Klein BE, Liao DP, Hubbard LD, Mosley TH; ARIC Investigators. Atheroslerosis Risk in Communities Study . Cerebral white matter lesions, retinopathy, and incident clinical stroke. JAMA 288: 67–74, 2002. doi: 10.1001/jama.288.1.67. [DOI] [PubMed] [Google Scholar]
- 140.Yasuno Y, Hong Y, Makita S, Yamanari M, Akiba M, Miura M, Yatagai T. In vivo high-contrast imaging of deep posterior eye by 1-microm swept source optical coherence tomography and scattering optical coherence angiography. Opt Express 15: 6121–6139, 2007. doi: 10.1364/OE.15.006121. [DOI] [PubMed] [Google Scholar]
- 141.Yatsuya H, Folsom AR, Wong TY, Klein R, Klein BE, Sharrett AR; ARIC Study Investigators . Retinal microvascular abnormalities and risk of lacunar stroke: Atherosclerosis Risk in Communities Study. Stroke 41: 1349–1355, 2010. doi: 10.1161/STROKEAHA.110.580837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yu DY, Su EN, Cringle SJ, Yu PK. Isolated preparations of ocular vasculature and their applications in ophthalmic research. Prog Retin Eye Res 22: 135–169, 2003. doi: 10.1016/S1350-9462(02)00044-7. [DOI] [PubMed] [Google Scholar]
- 143.Zamboni P, Galeotti R, Menegatti E, Malagoni AM, Tacconi G, Dall’Ara S, Bartolomei I, Salvi F. Chronic cerebrospinal venous insufficiency in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry 80: 392–399, 2009. doi: 10.1136/jnnp.2008.157164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143a.Zhang Q, Lee CS, Chao J, Chen CL, Zhang T, Sharma U, Zhang A, Liu J, Rezaei K, Pepple KL, Munsen R, Kinyoun J, Johnstone M, Van Gelder RN, Wang RK. Wide-field optical coherence tomography based microangiography for retinal imaging. Sci Rep 6: 22017, 2016. doi: 10.1038/srep22017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhang Y, Stone J. Role of astrocytes in the control of developing retinal vessels. Invest Ophthalmol Vis Sci 38: 1653–1666, 1997. [PubMed] [Google Scholar]
- 145.Zheng Y, Gandhi JS, Stangos AN, Campa C, Broadbent DM, Harding SP. Automated segmentation of foveal avascular zone in fundus fluorescein angiography. Invest Ophthalmol Vis Sci 51: 3653–3659, 2010. doi: 10.1167/iovs.09-4935. [DOI] [PubMed] [Google Scholar]
- 146.Zipp F, Aktas O. The brain as a target of inflammation: common pathways link inflammatory and neurodegenerative diseases. Trends Neurosci 29: 518–527, 2006. doi: 10.1016/j.tins.2006.07.006. [DOI] [PubMed] [Google Scholar]