Using a multidimensional approach, we present evidence supporting that aged microvascular networks display vessel density and patterning similar to adult networks despite also being characterized by a decreased capacity to undergo angiogenesis. Thus, vessel loss is not necessarily a characteristic of aging.
Keywords: Microcirculation, microvascular dysfunction, endothelial cells
Abstract
A big problem associated with aging is thought to be impaired microvascular growth or angiogenesis. However, to link the evidence for impaired angiogenesis to microvascular dysfunction in aged tissues, we must compare adult vs. aged microvascular networks in unstimulated scenarios. The objective of this study was to test the hypothesis that aged microvascular networks are characterized by both fewer vessels and the impaired ability to undergo angiogenesis. Mesentery tissues from adult (9-mo) and aged (24-mo) male Fischer 344 rats were harvested and immunolabeled for platelet/endothelial cell adhesion molecule (an endothelial cell marker) according to two scenarios: unstimulated and stimulated. For unstimulated groups, tissues harvested from adult and aged rats were compared. For stimulated groups, tissues were harvested 3 or 10 days after compound 48/80-induced mast cell degranulation stimulation. Unstimulated aged microvascular networks displayed larger mean vascular area per tissue area compared with the unstimulated adult networks. The lack of a decrease in vessel density was supported at the gene expression level with RNA-Seq analysis and with comparison of vessel densities in soleus muscle. Following stimulation, capillary sprouting and vessel density were impaired in aged networks at 3 and 10 days, respectively. Our results suggest that aging associated with impaired angiogenesis mechanisms might not influence normal microvascular function, since unstimulated aged microvascular networks can display a “normal adult-like” vessel density and architecture.
NEW & NOTEWORTHY Using a multidimensional approach, we present evidence supporting that aged microvascular networks display vessel density and patterning similar to adult networks despite also being characterized by a decreased capacity to undergo angiogenesis. Thus, vessel loss is not necessarily a characteristic of aging.
aging is associated with microvascular dysfunction characterized by impaired vasoreactivity (14, 30, 39), altered neurovascular coupling (45), vessel biomechanical properties (12), increased oxidative stress (22), and reduced blood flow (22, 25). A big problem, however, is that the mechanisms leading to these dysfunctional characteristics and the functional link between changed phenotypes and flow through microvascular networks remain unclear. One potential link could be a reduced number of microvessels and an altered microvascular network architecture. This hypothesis would be consistent with the common conception that angiogenesis, defined as the growth of new blood vessels, is impaired in aged tissues (22, 25). Angiogenesis in aged populations is indeed commonly thought to be impaired (20, 22, 24, 34, 39), yet the link between impaired angiogenesis to microvascular dysfunction remains understudied.
An essential fundamental step in making this link is to determine whether an altered microvascular network architecture in aged tissues correlates with its impaired ability to undergo angiogenesis. The potential significance of such a correlation is highlighted, for example, by what we have learned about the role of the microvasculature in the age-related progression of hypertension. In hypertension, microvascular rarefaction defined as the loss of microvessels has been associated in various tissues with multiple microvascular dysfunction characteristics, including increased wall-to-lumen ratio, increased microvessel-specific oxidative stress, elevated matrix metalloproteinase (MMP) levels, elevated microvascular tone, deficient leukocyte–endothelial interaction, and extensive nonuniform endothelial cell apoptosis (1, 19, 43). Rarefaction has even been shown to precede the onset of elevated blood pressure (28). Therefore, similarly for aging research, evidence for vessel rarefaction in aged vs. adult tissues would motivate a new area of research focused on altered network structure as a cause for age-associated microvascular dysfunction.
The objective of this study was to test the hypothesis that aged microvascular networks are characterized by both fewer vessels and the impaired ability to undergo angiogenesis. Using a multidimensional approach, including network quantification, RNA-Seq, and computational methods, we show that “unstimulated” aged networks in tissues not exogenously induced to undergo angiogenesis are characterized neither by a decreased number of vessels nor by patterning alterations. Importantly, impaired angiogenesis in aging is supported by metric and temporally specific responses in aged vs. adult tissues after an inflammatory stimulation of network growth. The inclusion of gene expression and computational analyses strengthen our finding that microvascular density is not different between aged and adult networks. This is important given the discrepancies in the literature across studies using seemingly similar metrics and analysis. Microvascular densities in unstimulated aged tissues have been reported to be lower than (11), higher than (10), and equal to (21) unstimulated adult tissues. Moreover, the computational data provide support that microvascular resistance is, also, comparable between adult and aged scenarios, suggesting that the network patterns in aged tissues might not be a contributor to any dysfunctional characteristics. Our results further suggest that aged microvascular networks can display “normal” “adult-like” patterns in spite of their decreased capacity to undergo angiogenesis and that vessel loss may not be a contributor to age-related microvascular dysfunction. Thus, our findings motivate new questions for aging research. Why, if angiogenesis is impaired during aging, is microvascular network structure normal? And how is microvascular network structure linked to microvascular dysfunction?
MATERIALS AND METHODS
Animals.
All animal experiments were approved by Tulane University Institutional Animal Care and Use Committee. Adult (9-mo-old) and aged (24-mo-old) male Fischer 344 rats were acquired from the National Institute on Aging for use in all experiments. The Fischer 344 rat is a common strain used for aging studies and the 9-mo and 24-mo ages were selected for this study based on previously reported studies of aging and microvascular function (7, 18, 40).
Tissue harvesting.
Animals were anesthetized by intramuscular injection of ketamine (80 mg/kg body wt; Fort Dodge Animal Health, Fort Dodge, IA) and xylazine (8 mg/kg body wt; Lloyd, Shenandoah, IA). Following anesthesia, animals were euthanized by intracardiac injection of Beuthanasia (Merck, Kenilworth, NJ). The mesentery was surgically exposed, and 12 mesenteric tissue windows were harvested starting from the ileum as previously described (2). Mesenteric tissue windows were defined as the thin translucent connective tissues attached to the small intestine between the larger feeding vessels. Mesentery tissues were whole mounted on glass slides, fixed in 100% methanol for 30 min at −20°C, and washed for 30 min with phosphate-buffered saline (PBS; Sigma-Aldrich, St. Louis, MO) containing 0.1% saponin (Sigma-Aldrich) while the wash solution was changed every 10 min. Soleus muscles were harvested from the animals by reflecting the gastrocnemius, plantaris, and soleus from the right sural region and then removing the soleus from the two superficial muscles. After harvesting, each soleus muscle was cut transversely at its widest point, and 2 mm of the superior side was embedded in optimum cutting temperature (OCT) compound (Sakura Finetek, Torrance, CA) with its cut facing down in the cassette. Each muscle specimen was placed for 1 min in 2-methylbutane (Sigma-Aldrich) cooled in a liquid nitrogen bath. Muscles were cryosectioned using a Leica CM3050S cryostat (Leica Biosystems, Buffalo Grove, IL) at −20°C; 10-μm sections were taken from two locations, one at the cut face and one at a location 200 μm in the specimen. All sections were placed on slides and washed with drops of PBS for 10 min at room temperature to remove the OCT compound. Muscle sections were then fixed in 100% methanol for 30 min at −20°C and washed for 30 min with PBS + 0.1% saponin while the wash solution was changed every 10 min.
Angiogenesis stimulation.
Angiogenesis was stimulated in rat mesentery tissues using a previously utilized model of mast cell degranulation (42, 44). Compound 48/80 (Sigma-Aldrich) was administered via five 2.5-ml intraperitoneal injections of increasing concentration over 3 days (160, 320, 480, 640, and 800 µg/ml in sterile saline). Mesentery tissues were harvested, as described above, 3 or 10 days following the final injection (n = 4 rats/age group for each time point). The main reason 48/80 was selected for our study was because it causes a well-described robust multifactorial microvascular network growth in rat mesentery tissues (42, 44) that can be used to compare adult vs. aged angiogenic responses.
Immunohistochemistry.
Mesentery and muscle tissues were prepared for fluorescent imaging by labeling with biotinylated mouse monoclonal anti-rat platelet/endothelial cell adhesion molecule (PECAM) antibody (TLD-3A12; BD PharMingen, San Diego, CA) to identify blood vessels. Tissues were incubated for 1 h at room temperature with PECAM antibody diluted 1:200 in an antibody buffer solution (ABS) containing PBS + 0.1% saponin + 2% bovine serum albumin (Jackson ImmunoResearch, West Grove, PA) + 5% normal goat serum (Jackson ImmunoResearch). Tissues were then washed with PBS + 0.1% saponin that was changed every 10 min for 30 min. PECAM antibody was detected by incubating tissues for 1 h at room temperature with Cy3-conjugated streptavidin diluted 1:500 in ABS. Tissues were then washed with PBS and mounted with 1:1 glycerol and PBS and glass cover slips.
Intraluminal blood vessel labeling.
Mesenteric microvascular network perfusion was assessed by intraluminal fluorescent labeling. A 2-ml bolus of 40 kDa lysine-fixable FITC-dextran (10 mg/ml; Molecular Probes, Eugene, OR) was injected via the femoral vein of anesthetized animals. Animals were then euthanized, and the mesentery tissue was fixed by soaking the intraperitoneal space with 4% paraformaldehyde for 1 h. Mesenteric tissue windows were then harvested, mounted, and labeled for PECAM as described above.
Quantification of microvascular network characteristics and angiogenesis.
Mesentery tissues containing microvascular networks were randomly selected for analysis from adult and aged rats according to the following groups: unstimulated [adult: n = 17 total tissues from 8 rats (2–3 tissues/rat); aged: n = 17 total tissues from 6 rats (2–5 tissues/rat)], 3-day stimulation [adult: n = 16 total tissues from 4 rats (4 tissues/rat); aged: n = 16 total tissues from 4 rats (4 tissues/rat)], and 10-day stimulation [adult: n = 8 total tissues from 4 rats (2 tissues/rat); aged: n = 8 total tissues from 4 rats (2 tissues/rat)]. Blood vessel networks were identified based on PECAM expression and characterized based on the following quantitative metrics: vascular area, vascular length density, and capillary sprouting (44, 49). Arterioles were identified against paired venules by smaller diameters and the associated endothelial cell elongated morphology (41). Vascular area was defined as the total sum of the areas circumscribed by the perimeters of each vascular network within a tissue and normalized to the total tissue area. Vascular length density was defined as the total length of vessels normalized to the vascular area. Capillary sprouting was defined as the number of blind-ended segments extending from existing blood vessels in a network and normalized to the vascular area.
Soleus muscle sections from adult and aged rats were quantified for blood vessel density. The number of PECAM+ blood vessels were counted from four ×10 fields of view per 10-μm section and normalized to the muscle area or the number of muscle fibers. The density from two sections of the same muscle, 200 µm apart, was averaged per rat (n = 6/age group).
Computational modeling of vascular network resistance.
For each network, the segment length, segment diameter, vessel type, and nodal connections were measured per vessel segment. Nodes were defined as any point of vessel bifurcation or convergence or as the terminal end of a feeding arteriole or draining venule. Arteriolar vs. venular vessel type was identified by relative position in the vascular network, relative vessel diameter, and endothelial cell morphology. Endothelial cells along arterioles appeared more elongated and aligned with the direction of flow when compared with venules (27, 42).
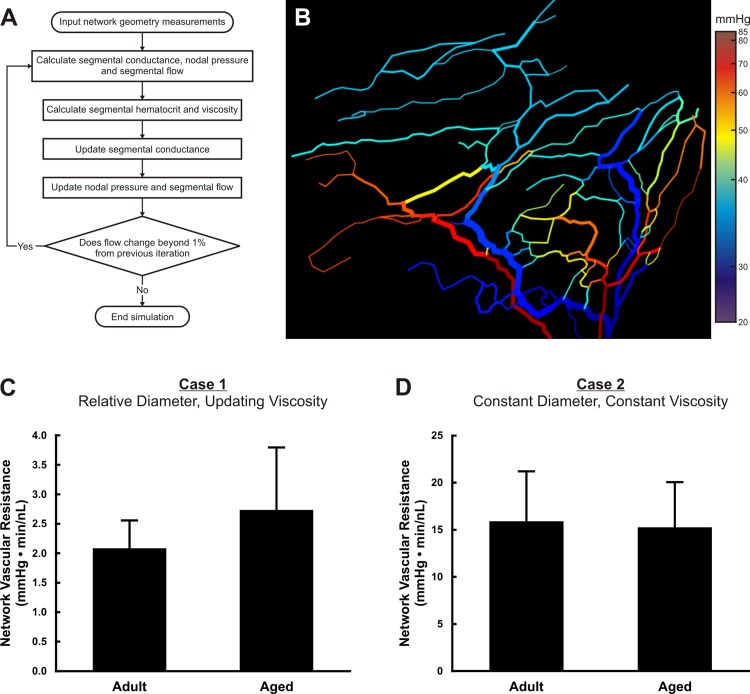
Total vascular resistance per network was calculated for adult and aged tissues (n = 6 networks/age group) using a computational model established in literature (5, 33) and previously used by our laboratory (50) (see Fig. 3A). Because network resistance is closely tied to the series-parallel organization of vessels in a network, it can also be used as an indirect quantification of network patterning. The model inputs included vessel segment lengths, segment diameters, nodal connections, and an assumed pressure drop of 65 mmHg. Network resistance was calculated as total pressure drop (65 mmHg) divided by total flow. The 65-mmHg pressure drop was created by assigning a pressure of 75 mmHg to input segments (feeding arterioles) and 10 mmHg to outlet segments (draining venules). This total pressure drop along with the input and outlet pressures was chosen based on previously reported values used for hemodynamic analysis of microvascular networks in the rat mesentery (15, 33). The apparent viscosity for each segment was either held constant or updated based on empirical data that accounted for changes in hematocrit in branching vessels (32, 38). Segmental flow and pressure drop are calculated in the model based on conservation of mass and a Poiseuille relationship. This study considered two cases. For case 1, vessel segment diameter inputs were those measured during network analysis, and viscosity was updated per segment. For case 2, all segment diameters were set to 10 µm, allowing for delineation of microvascular pattern alteration effects from potential diameter change effects, and viscosity per segment was set constant to 4 cP. It also allowed for analysis of pattern alteration effects independent of potential changes in diameter associated with fixation and labeling.
Fig. 3.
Computational modeling of adult and aged mesenteric microvascular network resistance. A: algorithm for determining the total network vascular resistance for mesenteric microvascular networks. B: example color map of the calculated segmental pressures output by the computational model. C: quantification of the total network vascular resistance for case 1 in which measured anatomical diameters were used and viscosity was iteratively updated per vessel segment within the algorithm. D: quantification of the total network vascular resistance for case 2 in which a constant vessel diameter of 10 µm was used and viscosity was held constant.
RNA-Seq and bioinformatics analysis.
Mesentery tissues harvested from additional adult (9-mo-old) and aged (24-mo-old) male Fischer 344 rats were used for differential gene expression analysis (n = 4 tissues from 4 rats/group). The RNA-Seq procedure was initiated with total RNA isolation from fragments of tissue homogenized in liquid nitrogen. Tripure Reagent (Roche, Basel, Switzerland), chloroform with Heavy Phase Lock Gel (5 Prime, Gaithersburg, MD), and RNEasy silica columns (Qiagen, Valencia, CA) were used during total RNA isolation. The quality and concentration of isolated total RNA was estimated with a BioAnalyzer RNA Pico Kit (Agilent Technologies, Santa Clara, CA), and only samples with RNA Integrity Number greater than seven were included in further analysis. Total RNA samples were depleted of up to 99.9% of the RNA from the 5S, 5.8S, 18S, and 28S ribosomal subunits using the RiboMinus Eukaryote System version (v) 2 (Thermofisher, Grand Island, NY) and analyzed on the BioAnalyzer using the RNA Pico Kit (Agilent Technologies) to verify the absence of ribosomal peaks. Barcoded cDNA libraries were prepared from the ribodepleted samples using the Ion Total RNA-Seq Kit v2 (Thermofisher/Life Technologies). First, the ribodepleted RNA was fragmented with RNase III at 37°C for 3 min. The fragmented RNA was purified on nucleic acid-binding beads and then analyzed on the BioAnalyzer using the RNA 6000 Pico Kit (Agilent Technologies). Next, the fragmented RNA was hybridized with adaptors and then ligated at 30°C for 1 h. The adaptor-ligated libraries were preincubated with a reverse transcription primer at 70°C for 10 min and then converted to cDNA by reverse transcription at 42°C for 30 min. The cDNA libraries were purified on nucleic acid-binding beads and then amplified by PCR using barcoded primers (Ion Xpress RNA-Seq Barcode 01–16 Kit; Thermofisher/Life Technologies). Barcoded and amplified libraries were purified on nucleic acid-binding beads, using a solvent ratio of 33% ethanol optimized to capture 300-bp fragments and analyzed on the BioAnalyzer using the High Sensitivity DNA Kit (Agilent Technologies). Barcoded amplified cDNA libraries were diluted to 100 pM according to the quantification from the BioAnalyzer and amplified on Ion Sphere Particles (ISPs) by emulsion PCR on the Ion One Touch 2 system using the Ion PI Template OT2 200 Kit v3 (Thermofisher/Life Technologies). ISPs were enriched for template-positive particles using the Ion OneTouch ES automated bead purification station, in which the biotinylated adaptor sequences are selected by binding to streptavidin beads. The template-positive ISPs were prepared for sequencing using the Ion PI Sequencing Kit v3 (Thermofisher/Life Technologies). Sequencing primer was annealed to template fragments attached to ISPs, which were loaded on an Ion PI v2 chip and incubated with polymerase. Finally, the Ion PI v2 chip was placed on the Ion Proton system for sequencing by the collection of pH readings from a high-density array of wells as successive single nucleotides are incorporated by the polymerase.
Single-end reads were mapped to the rat genome (Rattus norvegicus, Rnor_5.0, Ensembl release 79) using a two-step alignment protocol. First reads were mapped using STAR (v2.3.0) with default parameters. Unmapped reads from this alignment were then remapped using bowtie2 (v2.1.0), also with default parameters. Mapped reads from STAR and bowtie2 were then merged. Reads from the merged aligned files were then processed for read counts and normalized abundance measurements using SAMMate v2.6.1. Next, the read counts were fed into EBSeq (v1.4.0) differential expression testing at a false discovery rate of 5% or less.
Statistical analysis.
Data from unstimulated vascular network characterization and computational modeling were analyzed using mixed-models regression to control for multiple samples from the same rat. Results were confirmed with van der Waerden scores test assuming independence of scores per rat. Data from unstimulated muscle characterization were analyzed using van der Waerden scores (2-group analyses). Data from angiogenesis quantification were analyzed using mixed-models regression. Statistical significance was defined as P < 0.05. All values are presented as means ± SE.
RESULTS
Comparison of mesenteric microvascular networks in adult and aged rats.
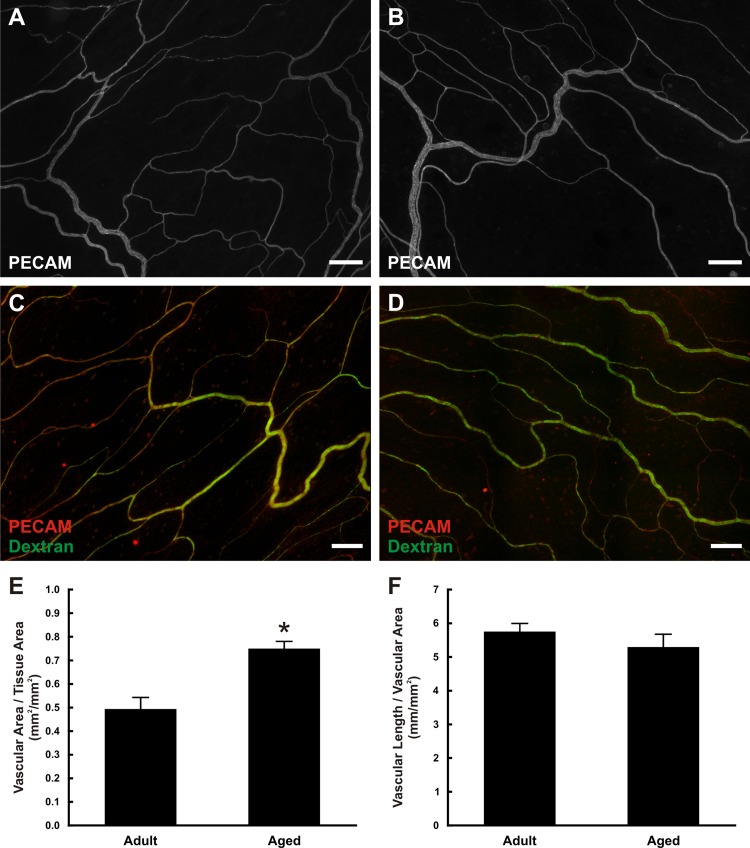
Rat mesenteric microvascular networks qualitatively displayed similar structure and morphology (Fig. 1, A and B). Injection of fluorescent dextran identified patent vessels across the hierarchy of both adult and aged networks down to the capillary level (Fig. 1, C and D). Quantification of the size and density of the microvascular networks showed that the vascular area per tissue area was significantly larger in aged tissues compared with adults (adult: 0.49 ± 0.05 mm2/mm2, aged: 0.75 ± 0.03 mm2/mm2, P = 0.0012; Fig. 1E). The average tissue size was comparable between age groups (data not shown). The densities of adult and aged networks were not significantly different based on quantification of vascular length per vascular area (adult: 5.76 ± 0.26 mm/mm2, aged: 5.30 ± 0.38 mm/mm2, P = 0.6649; Fig. 1F).
Fig. 1.
Structure of adult and aged mesenteric microvascular networks. A and B: representative examples of the structure of adult (A) and aged (B) mesenteric microvascular networks. C and D: infusion of fluorescent dextran was used to assess vessel perfusion and permeability in adult (C) and aged (D) microvascular networks. E and F: quantification of the total vascular area normalized to tissue area (E) and vascular length normalized to vascular area (F). *Significant difference between adult and aged groups (P < 0.05). Scale bars = 200 µm.
Transcriptome analysis of adult and aged rat mesentery tissue.
The lack of a decrease in microvascular density in aged vs. adult rat mesentery tissues is supported at the gene expression level via RNA-Seq analysis. Based on a 95% false discovery rate, 386 genes were differentially expressed. Of these genes, 170 exhibited a greater than twofold change in expression. To compare the expression levels of angioregulators, we evaluated 68 genes currently known to be associated with the promotion or inhibition of microvascular growth (Table 1). Only 3 (Hgf, Pdgfrb, and Tgfbr1) of the 68 genes were identified as being differentially expressed based on a 95% false discovery rate, and they exhibited a less than twofold change in expression.
Table 1.
Selected genes from RNAseq analysis of adult and aged rat mesentery tissues
| Gene ID | PPDE | Post-FC | Gene ID | PPDE | Post-FC |
|---|---|---|---|---|---|
| Angpt1 | 0.107 | 0.397 | Mdk | 0.023 | 1.934 |
| Angpt2 | 0.300 | 2.083 | Mmp14 | 0.022 | 2.053 |
| Ccl2 | 0.038 | 2.208 | Mmp19 | 0.029 | 1.513 |
| Col18a1 | 0.002 | 0.943 | Mmp2 | 0.003 | 1.736 |
| Col4a3 | 0.012 | 0.816 | Mmp9 | 0.015 | 1.218 |
| Ctgf | 0.010 | 1.366 | Nos3 | 0.021 | 1.233 |
| Cxcl1 | 0.017 | 0.814 | Nrp1 | 0.014 | 1.208 |
| Cxcl10 | 0.037 | 1.912 | Pdgfa | 0.120 | 0.865 |
| Cxcl12 | 0.183 | 1.923 | Pdgfb | 0.019 | 1.235 |
| Cxcl2 | 0.119 | 2.114 | Pdgfc | 0.016 | 0.830 |
| Cxcl9 | 0.048 | 1.675 | Pdgfra | 0.002 | 1.235 |
| Cxcr2 | 0.124 | 0.642 | Pdgfrb | 0.977* | 1.799 |
| Cxcr3 | 0.334 | 1.684 | Pgf | 0.016 | 1.081 |
| Cxcr4 | 0.029 | 1.441 | Ptgs1 | 0.002 | 1.033 |
| Dll4 | 0.009 | 1.019 | Ptgs2 | 0.060 | 1.531 |
| Edn1 | 0.009 | 1.027 | Ptn | 0.058 | 3.731 |
| Eng | 0.042 | 1.412 | S1pr1 | 0.547 | 1.739 |
| Erbb2 | 0.009 | 1.103 | Sphk1 | 0.017 | 1.031 |
| Fgf1 | 0.002 | 1.235 | Tek | 0.545 | 1.397 |
| Fgf2 | 0.007 | 0.943 | Tgfa | 0.016 | 1.099 |
| Fgfr1 | 0.003 | 1.022 | Tgfb1 | 0.015 | 1.186 |
| Fgfr2 | 0.346 | 1.506 | Tgfb2 | 0.008 | 0.945 |
| Fgfr3 | 0.024 | 1.149 | Tgfb3 | 0.002 | 1.101 |
| Figf | 0.051 | 1.449 | Tgfbr1 | 0.999* | 1.259 |
| Flt1 | 0.009 | 1.067 | Thbs1 | 0.050 | 2.247 |
| Flt4 | 0.006 | 1.117 | Thbs2 | 0.143 | 2.525 |
| Fn1 | 0.000 | 1.222 | Tie1 | 0.004 | 1.252 |
| Hgf | 0.964* | 1.647 | Timp1 | 0.003 | 0.970 |
| Hif1a | 0.031 | 1.131 | Timp2 | 0.001 | 0.954 |
| Igf1 | 0.004 | 1.183 | Timp3 | 0.003 | 0.977 |
| Jag1 | 0.003 | 0.855 | Tnf | 0.025 | 1.241 |
| Kdr | 0.007 | 1.038 | Vegfa | 0.005 | 1.149 |
| Lep | 0.003 | 0.407 | Vegfb | 0.012 | 0.727 |
| Mapk14 | 0.004 | 0.948 | Vegfc | 0.013 | 0.984 |
ID, identification; PPDE, posterior probability that a gene is differentially expressed; post-FC, posterior fold change (aged over adult) for a gene, defined as the ratio between posterior mean estimates of the gene for each condition.
Differentially expressed genes (>95% probability).
Comparison of microvascular networks in adult and aged skeletal muscle.
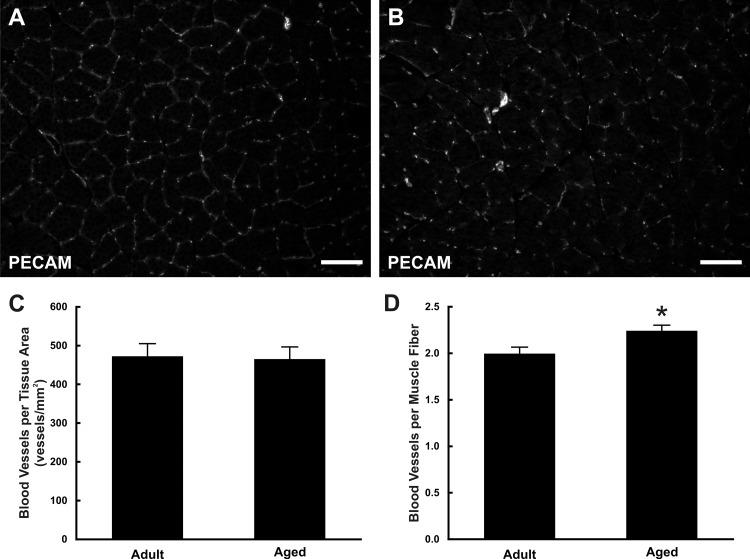
Blood vessel density was assessed in skeletal muscle of adult and aged rats (Fig. 2, A and B). The number of blood vessels in rat soleus muscles was quantified and normalized to the field of view. There was no significant difference between the number of blood vessels normalized to the tissue area in adult and aged muscles (adult: 473 ± 33 vessels/mm2, aged: 465 ± 31 vessels/mm2, P = 0.7166; Fig. 2C); aged muscles did display a significant increase in the number of blood vessels normalized to the number of muscle fibers vs. adult muscles (adult: 2.00 ± 0.07 vessels/muscle fiber, aged: 2.24 ± 0.06 vessels/muscle fiber, P = 0.0179; Fig. 2D). We speculate that the increased vessel per muscle fiber ratio might be explained by lower muscle counts. This is supported by a nonsignificant decrease in the number of muscle fibers per area in aged tissues (adult: 236.7 ± 14.6 vs. aged: 207.7 ± 13.3 fibers/mm2; P = 0.17).
Fig. 2.
Blood vessel density in adult and aged soleus muscle. A and B: blood vessels were identified in cross sections of adult (A) and aged (B) soleus muscle based on platelet/endothelial cell adhesion molecule (PECAM) expression. C and D: quantification of the number of blood vessels normalized to the tissue area (C) or normalized to the number of muscle fibers (D). *Significant difference between adult and aged groups (P < 0.05). Scale bars = 100 µm.
Computational modeling of vascular resistance in adult and aged microvascular networks.
To evaluate potential network structural alterations associated with aging, we evaluated network resistance using a previously established segmental flow computational model (Fig. 3, A and B) (6, 32). For the case of relative diameters where viscosity was iteratively updated, no significant difference in resistance was observed between adult and aged microvascular networks (adult: 2.08 ± 0.47 mmHg·min−1·nl−1, aged: 2.73 ± 1.06 mmHg·min−1·nl−1, P = 0.6022; Fig. 3C). These values are similar to what is reported by Pries et al. in which adult rat mesenteric networks were analyzed using a similar computational model (31). To isolate network patterning effects vs. possible diameter effects, we considered a case of constant diameters and constant viscosity. Again, no significant difference was observed between aged and adult resistances (adult: 15.90 ± 5.29 mmHg·min−1·nl−1, aged: 15.27 ± 4.78 mmHg·min−1·nl−1, P = 0.9024; Fig. 3D). Similar to the results of the network resistance comparison, capillary flow heterogeneity values, another indicator of patterning alterations, were not significantly different between aged and adult groups (data not shown).
Angiogenesis is impaired in aged rat microvascular networks.
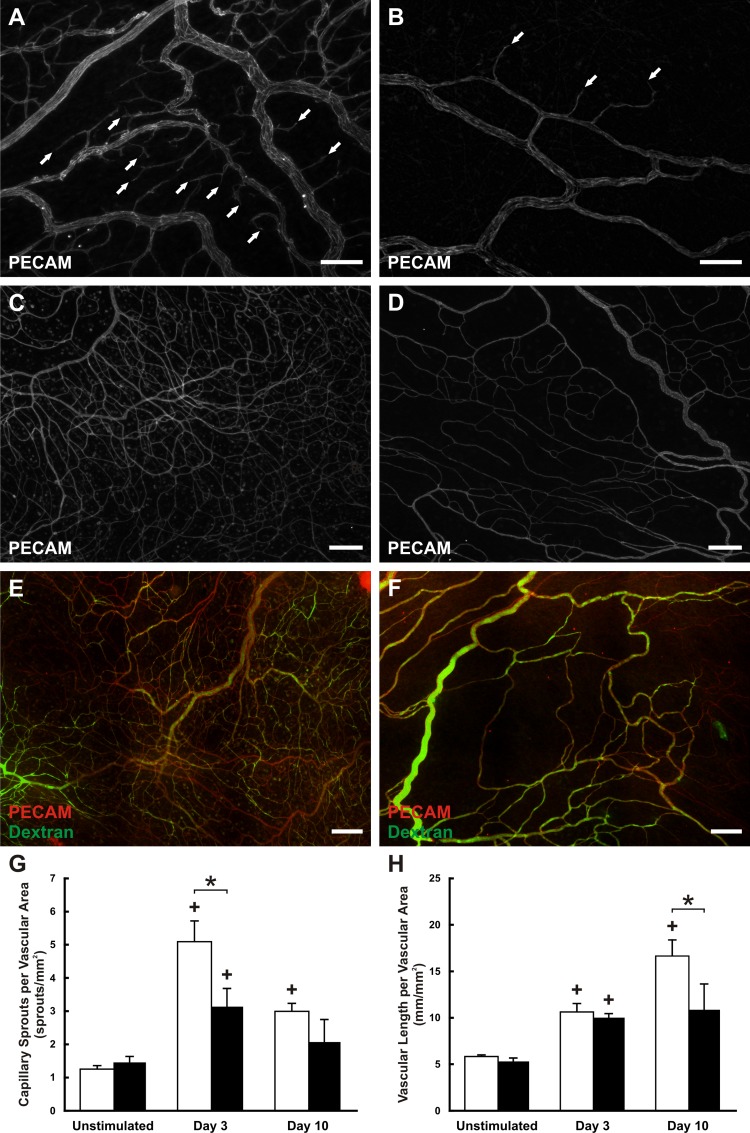
The effect of aging on angiogenesis was investigated by using a compound 48/80- stimulation model of inflammation previously described (42, 44). This particular model was chosen for this study because it produces a robust networkwide angiogenic response over a relatively short time duration (16, 29). In mesentery tissues stimulated with compound 48/80, evidence of capillary sprouting was observed in microvascular networks after 3 days (Fig. 4, A and B). Ten days following stimulation, tissues contained high-density vascular networks (Fig. 4, C and D). Adult and aged angiogenic networks showed no apparent difference in patent vessels as evidenced by the presence of injected fluorescent dextran within the vessel lumens throughout the hierarchy of the microvascular networks at day 10 poststimulation (Fig. 4, E and F). Quantification of capillary sprouting showed that both adult and aged networks had a significant increase in the number of sprouts per vascular area at day 3. However, sprouting in aged networks was significantly reduced compared with adult networks (adult: 5.07 ± 0.64 sprouts/mm2, aged: 3.13 ± 0.55 sprouts/mm2, P = 0.0124; Fig. 4G). Quantification of vascular length density indicated that both adult and aged networks increased in density over time, yet adult networks were significantly denser at day 10 while aged density had plateaued (adult: 16.6 ± 1.9 sprouts/mm2, aged: 10.9 ± 2.8 sprouts/mm2, P = 0.0089; Fig. 4H). These results suggest that angiogenesis is impaired in aged microvascular networks; however, observation of this impairment is time and metric dependent.
Fig. 4.
Angiogenesis in adult and aged mesenteric microvascular networks. A and B: representative examples of adult (A) and aged (B) microvascular networks 3 days following compound 48/80 stimulation. C and D: representative examples of adult (C) and aged (D) microvascular networks 10 days following compound 48/80 stimulation. E and F: infusion of fluorescent dextran was used to assess vessel perfusion and permeability in adult (E) and aged (F) microvascular networks 10 days following compound 48/80 stimulation. G and H: quantification of the capillary sprouting (G) and vascular length density (H) at different time points following compound 48/80 stimulation in adult (white bars) and aged (black bars) microvascular networks. *Significant difference between adult and aged groups (P < 0.05). +Significant difference between the previous time point. Scale bars = 100 µm in A and B and 200 µm in C-F.
DISCUSSION
Impaired angiogenesis in aged populations has been characterized by decreased endothelial cell proliferation, decreased endothelial cell migration, and decreased sprouting (8, 25, 34, 46, 48, 51). Potential mechanisms for age-related impaired angiogenesis include increased oxidative stresses (8, 22) and decreased growth factor signaling (22, 35). However, it remains unclear whether this altered response capability affects microvessel number and normal maturation of microvascular network structures in unchallenged scenarios. The contribution of this study is the multidimensional evaluation of microvascular network structure and remodeling in adult vs. aged scenarios. The importance of evaluating microvascular structure and the number of vessels in a microvascular network is supported by the established link between microvascular structure and microvascular dysfunction (4, 13, 27). Our study highlights the complexities of assessing microvascular networks in adult and aged tissues beyond the common assertion that angiogenesis is impaired with age. Microvascular density in aged networks can be comparable to adult networks and, importantly, can still be characterized by impaired angiogenesis.
Network resistance comparisons based on intact network geometries enabled us to elucidate whether aged networks experience patterning alterations. Aged and adult networks displayed comparable resistances, suggesting that, in addition to microvascular networks having similar vessel length density, they have similar patterns. Validation of this approach is supported by the comparison of theoretical predictions and experimental measurements in the same tissue (31–33). Our modeling approach also enabled the isolation of potential patterning effects from diameter effects. While more direct measurements of patterning changes in aged networks (e.g., vessel tortuosity, vessel branch order, etc.) may be warranted in the future, our resistance results suggest that microvascular networks in this tissue do not undergo changes significant enough to alter local network resistance. For the networks analyzed in this study, no significant difference in vascular area was observed between the adult and aged networks (data not shown). Based on our tissue level analysis suggesting that vascularized area per tissue area is increased during aging, we speculate that aged tissues might contain an increased number of networks. Future analysis will be required to determine whether these networks are interconnected and how an increased number of networks influences tissue level hemodynamics. A limitation of our approach is also that the analysis does not account for potential patterning changes in vessel tortuosity or branching angle. Another limitation is that we did not compare network resistances between aged and adult networks after 48/80 stimulation. Such an analysis could potentially provide new information about how impaired angiogenesis could influence tissue function. Interestingly, the aged and adult day 10 remodeled networks displayed comparable (i.e., nonsignificant) differences for arteriole, venule, and small vessel (<20 μm) density per total vascular length (data not shown), suggesting that the relative distribution of the different vessel types remains similar. Because the total vascular length per vascular area is increased in adult networks vs. aged networks, we speculate that the adult networks contain more vessels and thus an increased number of parallel pathways leading to a decreased resistance.
The mesentery tissue was selected for this study because it offers a unique wholemount view of intact microvascular networks. In spite of unknown physiological function and low energetic requirement compared with other tissues like the heart or brain, it has been used as a model to investigate microvascular network growth and remodeling dynamics that are applicable to other tissue types (3, 16, 29) and has been a valuable tissue bed for identifying characteristics of microvascular alterations associated with pathological conditions, such as hypertension (6, 17, 27, 49). In spontaneously hypertensive rats, the mesentery tissue displays microvascular rarefaction and a multitude of dysfunctional characteristics, including microvessel loss, specific oxidative stress, elevated MMP levels, elevated microvascular tone, and deficient leukocyte–endothelial interaction (1, 19). Future studies are needed to identify whether aged networks similarly display microvascular dysfunctional characteristics.
In the current study, the observation of comparable microvascular density in unstimulated mesenteric networks is supported by our finding that vessel densities are similar in aged and adult soleus muscles. While the soleus muscle only represents a subtype of muscle and is characterized by a high proportion of red vs. white muscle fibers (37), it does offer a physiologically relevant tissue type to compare with the mesentery. Additional studies will be needed to confirm whether microvascular networks develop normally during aging in other tissue beds. Additional studies are also needed to characterize potential sex-dependent differences and strain-dependent differences in network architecture and the potential effects on aging phenotypes.
The unstimulated network comparison data are supported by our RNA-Seq analysis as well, which suggests that most of the regulators currently known to be associated with angiogenesis are not necessarily differentially expressed in aged vs. adult mesentery tissues. This is important because much of the support for altered angiogenesis during aging is based on reports on altered pro- or anti-angiogenic regulators at the gene or protein levels in unstimulated tissues (22). Although a limitation of our analysis is that gene expression differences were not determined between aged and adult tissues poststimulation, we were focused on identifying potential differences in a normal unstimulated scenario. Still, we recognize that potential insights might still be gained for identifying potential causes of microvascular dysfunction outside of network structural changes and causes of impaired angiogenesis by further focusing on the genes that did display a difference at the unstimulated (i.e., day 0) state. As an example, performing Ingenuity Pathway Analysis with the list of differentially expressed genes identified altered activation of pathways that have been associated with regulating angiogenesis and linked to dysfunction [mitochondrial dysfunction (9), oxidative phosphorylation (26), and inhibition of angiogenesis by thrombospondin1 (23)]. These pathways provide evidence of potential gene expression differences in an aged tissue with normal adult-like vessel density and warrant further investigation into their specific function in the aging vasculature.
Our quantification of capillary sprouting and microvascular length density metrics at 3 and 10 days following the 48/80 inflammatory stimulus supports that angiogenesis is impaired in the aged rat mesentery and, importantly, also demonstrates the need for studies to consider multiple time points. As indicated in materials and methods, 48/80 was used for this study because it produces a robust angiogenic response over a defined duration (42, 44). Future studies will be needed to determine if angiogenesis is also impaired with other stimuli.
In spite of the common association of impaired angiogenesis in aged tissues, discrepant results from the literature exist. For example, Rivard et al. reported a decreased capillary density response in aged mice after 28 days of hindlimb ischemia (36). In contrast, Westvik et al. reported increased capillary densities in aged mice 7 days after hindlimb ischemia (47). Based on our results, possible explanations for this discrepancy could be different temporal end points. At 3 days, stimulated aged rat mesenteric networks displayed impaired capillary sprouting but comparable vascular length densities. Meanwhile, the aged networks at day 10 displayed comparable sprouting and a decreased vascular density response. Thus, conclusions regarding whether angiogenesis is impaired with aging need to consider multiple time points, and for that matter multiple angiogenesis metrics, since conclusions might differ based on only single time snapshots. For example, it might be important to consider whether or not the impaired capillary sprouting is specific to local network regions. Comparisons of capillary sprouts per total vascular length provided similar trends to our data for sprout per area analysis (day 3 adult: 0.57 ± 0.09 vs. 3 day aged: 0.34 ± 0.06 sprouts/mm; P = 0.06). Potential new information, however, is gained by comparing the capillary sprout per vessel length density per vessel type. The largest reduction of sprouting in aged networks occurs along vessels <20 μm (day 3 adult: 0.50 ± 0.12 vs. 3 day aged: 0.22 ± 0.13 sprouts/mm; P = 0.08). Thus, the evidence for altered angiogenesis during aging can even be influenced by analysis location.
In summary, the results from this study suggest that aged microvascular networks can display both normal vascular density and impaired angiogenesis. Undoubtedly, the microcirculation plays a big role in aging pathologies, yet the big challenge will be identifying the underlying triggers. Our results implicate fundamental different mechanisms associated with microvascular network remodeling during maturation over time vs. an angiogenic response to a stimulus. This motivates the need for future investigations focused on answering what are the mechanisms for age-related microvascular dysfunction outside of vessel loss or altered vessel interconnectivity that are often linked to altered hemodynamics.
GRANTS
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Numbers 5-P20-GM-103629–04, P20-GM-103518, and R01-AG-049821. Statistical analysis and next-generation sequencing were completed in the Genomics and Biostatistics Core at the Tulane Center for Aging, which is supported by National Institute of General Medical Sciences Grant P20-GM-103629 to S. M. Jazwinski. NGS data analysis was supported by the Cancer Crusaders Next Generation Sequence Analysis Core at Tulane Cancer Center.
DISCLOSURES
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.S.S., D.C.S., S.A.S., M.C.-R., M.B., J.R.E., A.D.S.-M., H.E.B., L.M., and W.L.M. analyzed data; R.S.S., D.C.S., S.A.S., M.C.-R., M.B., A.D.S.-M., H.E.B., and W.L.M. interpreted results of experiments; R.S.S., A.D.S.-M., M.S.A., and W.L.M. edited and revised manuscript; D.C.S., S.A.S., M.C.-R., J.R.E., L.O.C., and W.L.M. performed experiments; D.C.S., S.A.S., M.S.A., H.E.B., and W.L.M. prepared figures; A.D.S.-M., M.S.A., and W.L.M. approved final version of manuscript; L.O.C. and W.L.M. were responsible for the conception of the study; and W.L.M. was responsible for the overall design of the experiments.
REFERENCES
- 1.Antonios TFT, Singer DRJ, Markandu ND, Mortimer PS, MacGregor GA. Rarefaction of skin capillaries in borderline essential hypertension suggests an early structural abnormality. Hypertension 34: 655–658, 1999. doi: 10.1161/01.HYP.34.4.655. [DOI] [PubMed] [Google Scholar]
- 2.Azimi MS, Myers L, Lacey M, Stewart SA, Shi Q, Katakam PV, Mondal D, Murfee WL. An ex vivo model for anti-angiogenic drug testing on intact microvascular networks. PLoS One 10: e0119227, 2015. doi: 10.1371/journal.pone.0119227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benest AV, Harper SJ, Herttuala SY, Alitalo K, Bates DO. VEGF-C induced angiogenesis preferentially occurs at a distance from lymphangiogenesis. Cardiovasc Res 78: 315–323, 2008. doi: 10.1093/cvr/cvm094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhutto IA, Amemiya T. Vascular changes in retinas of spontaneously hypertensive rats demonstrated by corrosion casts. Ophthalmic Res 29: 12–23, 1997. doi: 10.1159/000267986. [DOI] [PubMed] [Google Scholar]
- 5.Binder KW, Murfee WL, Song J, Laughlin MH, Price RJ. Computational network model prediction of hemodynamic alterations due to arteriolar remodeling in interval sprint trained skeletal muscle. Microcirculation 14: 181–192, 2007. doi: 10.1080/10739680601139237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bohlen HG. Intestinal microvascular adaptation during maturation of spontaneously hypertensive rats. Hypertension 5: 739–745, 1983. doi: 10.1161/01.HYP.5.5.739. [DOI] [PubMed] [Google Scholar]
- 7.Chatterjee V, Gashev AA. Aging-associated shifts in functional status of mast cells located by adult and aged mesenteric lymphatic vessels. Am J Physiol Heart Circ Physiol 303: H693–H702, 2012. doi: 10.1152/ajpheart.00378.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Csiszar A, Gautam T, Sosnowska D, Tarantini S, Banki E, Tucsek Z, Toth P, Losonczy G, Koller A, Reglodi D, Giles CB, Wren JD, Sonntag WE, Ungvari Z. Caloric restriction confers persistent anti-oxidative, pro-angiogenic, and anti-inflammatory effects and promotes anti-aging miRNA expression profile in cerebromicrovascular endothelial cells of aged rats. Am J Physiol Heart Circ Physiol 307: H292–H306, 2014. doi: 10.1152/ajpheart.00307.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davidson SM, Duchen MR. Endothelial mitochondria: contributing to vascular function and disease. Circ Res 100: 1128–1141, 2007. doi: 10.1161/01.RES.0000261970.18328.1d. [DOI] [PubMed] [Google Scholar]
- 10.Davidson YS, Clague JE, Horan MA, Pendleton N. The effect of aging on skeletal muscle capillarization in a murine model. J Gerontol A Biol Sci Med Sci 54: B448–B451, 1999. doi: 10.1093/gerona/54.10.B448. [DOI] [PubMed] [Google Scholar]
- 11.Degens H, Turek Z, Hoofd L, van’t Hof MA, Binkhorst RA. Capillarisation and fibre types in hypertrophied m. plantaris in rats of various ages. Respir Physiol 94: 217–226, 1993. doi: 10.1016/0034-5687(93)90049-G. [DOI] [PubMed] [Google Scholar]
- 12.Diaz-Otero JM, Garver H, Fink GD, Jackson WF, Dorrance AM. Aging is associated with changes to the biomechanical properties of the posterior cerebral artery and parenchymal arterioles. Am J Physiol Heart Circ Physiol 310: H365–H375, 2016. doi: 10.1152/ajpheart.00562.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engelson ET, Schmid-Schönbein GW, Zweifach BW. The microvasculature in skeletal muscle. II. Arteriolar network anatomy in normotensive and spontaneously hypertensive rats. Microvasc Res 31: 356–374, 1986. doi: 10.1016/0026-2862(86)90024-5. [DOI] [PubMed] [Google Scholar]
- 14.Feher A, Broskova Z, Bagi Z. Age-related impairment of conducted dilation in human coronary arterioles. Am J Physiol Heart Circ Physiol 306: H1595–H1601, 2014. doi: 10.1152/ajpheart.00179.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fenger-Gron J, Mulvany MJ, Christensen KL. Mesenteric blood pressure profile of conscious, freely moving rats. J Physiol 488: 753–760, 1995. doi: 10.1113/jphysiol.1995.sp021006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franzén L, Ghassemifar R, Malcherek P. Experimental mast cell activation improves connective tissue repair in the perforated rat mesentery. Agents Actions 33: 371–377, 1991. doi: 10.1007/BF01986588. [DOI] [PubMed] [Google Scholar]
- 17.Henrich H, Hertel R, Assmann R. Structural differences in the mesentery microcirculation between normotensive and spontaneously hypertensive rats. Pflugers Arch 375: 153–159, 1978. doi: 10.1007/BF00584238. [DOI] [PubMed] [Google Scholar]
- 18.Hughes S, Gardiner T, Hu P, Baxter L, Rosinova E, Chan-Ling T. Altered pericyte-endothelial relations in the rat retina during aging: implications for vessel stability. Neurobiol Aging 27: 1838–1847, 2006. doi: 10.1016/j.neurobiolaging.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 19.Hutchins PD, Darnell AE. Observations of a decreased number of small arterioles in spontaneously hypertensive rats. Circ Res 34: 161–165, 1974. [Google Scholar]
- 20.Iemitsu M, Maeda S, Jesmin S, Otsuki T, Miyauchi T. Exercise training improves aging-induced downregulation of VEGF angiogenic signaling cascade in hearts. Am J Physiol Heart Circ Physiol 291: H1290–H1298, 2006. doi: 10.1152/ajpheart.00820.2005. [DOI] [PubMed] [Google Scholar]
- 21.Kano Y, Shimegi S, Furukawa H, Matsudo H, Mizuta T. Effects of aging on capillary number and luminal size in rat soleus and plantaris muscles. J Gerontol A Biol Sci Med Sci 57: B422–B427, 2002. doi: 10.1093/gerona/57.12.B422. [DOI] [PubMed] [Google Scholar]
- 22.Lähteenvuo J, Rosenzweig A. Effects of aging on angiogenesis. Circ Res 110: 1252–1264, 2012. doi: 10.1161/CIRCRESAHA.111.246116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lawler PR, Lawler J. Molecular basis for the regulation of angiogenesis by thrombospondin-1 and -2. Cold Spring Harb Perspect Med 2: a006627, 2012. doi: 10.1101/cshperspect.a006627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin CHS, Chen J, Ziman B, Marshall S, Maizel J, Goligorsky MS. Endostatin and kidney fibrosis in aging: a case for antagonistic pleiotropy? Am J Physiol Heart Circ Physiol 306: H1692–H1699, 2014. doi: 10.1152/ajpheart.00064.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Long DA, Mu W, Price KL, Johnson RJ. Blood vessels and the aging kidney. Nephron Exp Nephrol 101: e95–e99, 2005. doi: 10.1159/000087146. [DOI] [PubMed] [Google Scholar]
- 26.Madamanchi NR, Vendrov A, Runge MS. Oxidative stress and vascular disease. Arterioscler Thromb Vasc Biol 25: 29–38, 2005. doi: 10.1161/01.ATV.0000150649.39934.13. [DOI] [PubMed] [Google Scholar]
- 27.Murfee WL, Schmid-Schönbein GW. Chapter 12. Structure of microvascular networks in genetic hypertension. Methods Enzymol 444: 271–284, 2008. doi: 10.1016/S0076-6879(08)02812-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noon JP, Walker BR, Webb DJ, Shore AC, Holton DW, Edwards HV, Watt GCM. Impaired microvascular dilatation and capillary rarefaction in young adults with a predisposition to high blood pressure. J Clin Invest 99: 1873–1879, 1997. doi: 10.1172/JCI119354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Norrby K, Jakobsson A, Sörbo J. Mast-cell-mediated angiogenesis: a novel experimental model using the rat mesentery. Virchows Arch B Cell Pathol Incl Mol Pathol 52: 195–206, 1986. doi: 10.1007/BF02889963. [DOI] [PubMed] [Google Scholar]
- 30.Park SY, Ives SJ, Gifford JR, Andtbacka RHI, Hyngstrom JR, Reese V, Layec G, Bharath LP, Symons JD, Richardson RS. Impact of age on the vasodilatory function of human skeletal muscle feed arteries. Am J Physiol Heart Circ Physiol 310: H217–H225, 2016. doi: 10.1152/ajpheart.00716.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pries AR, Reglin B, Secomb TW. Remodeling of blood vessels: responses of diameter and wall thickness to hemodynamic and metabolic stimuli. Hypertension 46: 725–731, 2005. doi: 10.1161/01.HYP.0000184428.16429.be. [DOI] [PubMed] [Google Scholar]
- 32.Pries AR, Secomb TW, Gaehtgens P, Gross JF. Blood flow in microvascular networks. Experiments and simulation. Circ Res 67: 826–834, 1990. doi: 10.1161/01.RES.67.4.826. [DOI] [PubMed] [Google Scholar]
- 33.Pries AR, Secomb TW, Gessner T, Sperandio MB, Gross JF, Gaehtgens P. Resistance to blood flow in microvessels in vivo. Circ Res 75: 904–915, 1994. doi: 10.1161/01.RES.75.5.904. [DOI] [PubMed] [Google Scholar]
- 34.Reed MJ, Corsa AC, Kudravi SA, McCormick RS, Arthur WT. A deficit in collagenase activity contributes to impaired migration of aged microvascular endothelial cells. J Cell Biochem 77: 116–126, 2000. doi:. [DOI] [PubMed] [Google Scholar]
- 35.Reed MJ, Edelberg JM. Impaired angiogenesis in the aged. Sci Aging Knowledge Environ 2004: pe7, 2004. doi: 10.1126/sageke.2004.7.pe7. [DOI] [PubMed] [Google Scholar]
- 36.Rivard A, Fabre JE, Silver M, Chen D, Murohara T, Kearney M, Magner M, Asahara T, Isner JM. Age-dependent impairment of angiogenesis. Circulation 99: 111–120, 1999. doi: 10.1161/01.CIR.99.1.111. [DOI] [PubMed] [Google Scholar]
- 37.Schiaffino S, Reggiani C. Fiber types in mammalian skeletal muscles. Physiol Rev 91: 1447–1531, 2011. doi: 10.1152/physrev.00031.2010. [DOI] [PubMed] [Google Scholar]
- 38.Secomb TP, Gaehtgens P, Gross JF. Theoretical and Experimental Analysis of Hematocrit Distribution in Microcirculatory Networks. New York: Springer, 1989. [Google Scholar]
- 39.Sinkler SY, Segal SS. Aging alters reactivity of microvascular resistance networks in mouse gluteus maximus muscle. Am J Physiol Heart Circ Physiol 307: H830–H839, 2014. doi: 10.1152/ajpheart.00368.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stablein M, Meyer J. Age-related changes in the epithelial dimensions and capillaries of the oral mucosa of the rat. Arch Oral Biol 31: 609–616, 1986. doi: 10.1016/0003-9969(86)90085-3. [DOI] [PubMed] [Google Scholar]
- 41.Stapor PC, Azimi MS, Ahsan T, Murfee WL. An angiogenesis model for investigating multicellular interactions across intact microvascular networks. Am J Physiol Heart Circ Physiol 304: H235–H245, 2013. doi: 10.1152/ajpheart.00552.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stapor PC, Murfee WL. Spatiotemporal distribution of neurovascular alignment in remodeling adult rat mesentery microvascular networks. J Vasc Res 49: 299–308, 2012. doi: 10.1159/000336714. [DOI] [PubMed] [Google Scholar]
- 43.Suematsu M, Suzuki H, Delano FA, Schmid-Schönbein GW. The inflammatory aspect of the microcirculation in hypertension: oxidative stress, leukocytes/endothelial interaction, apoptosis. Microcirculation 9: 259–276, 2002. doi: 10.1038/sj.mn.7800141. [DOI] [PubMed] [Google Scholar]
- 44.Sweat RS, Stapor PC, Murfee WL. Relationships between lymphangiogenesis and angiogenesis during inflammation in rat mesentery microvascular networks. Lymphat Res Biol 10: 198–207, 2012. doi: 10.1089/lrb.2012.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Toth P, Tarantini S, Tucsek Z, Ashpole NM, Sosnowska D, Gautam T, Ballabh P, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Resveratrol treatment rescues neurovascular coupling in aged mice: role of improved cerebromicrovascular endothelial function and downregulation of NADPH oxidase. Am J Physiol Heart Circ Physiol 306: H299–H308, 2014. doi: 10.1152/ajpheart.00744.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wagatsuma A. Effect of aging on expression of angiogenesis-related factors in mouse skeletal muscle. Exp Gerontol 41: 49–54, 2006. doi: 10.1016/j.exger.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 47.Westvik TS, Fitzgerald TN, Muto A, Maloney SP, Pimiento JM, Fancher TT, Magri D, Westvik HH, Nishibe T, Velazquez OC, Dardik A. Limb ischemia after iliac ligation in aged mice stimulates angiogenesis without arteriogenesis. J Vasc Surg 49: 464–473, 2009. doi: 10.1016/j.jvs.2008.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williamson KA, Hamilton A, Reynolds JA, Sipos P, Crocker I, Stringer SE, Alexander YM. Age-related impairment of endothelial progenitor cell migration correlates with structural alterations of heparan sulfate proteoglycans. Aging Cell 12: 139–147, 2013. doi: 10.1111/acel.12031. [DOI] [PubMed] [Google Scholar]
- 49.Yang M, Aragon M, Murfee WL. Angiogenesis in mesenteric microvascular networks from spontaneously hypertensive versus normotensive rats. Microcirculation 18: 574–582, 2011. doi: 10.1111/j.1549-8719.2011.00117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang M, Murfee WL. The effect of microvascular pattern alterations on network resistance in spontaneously hypertensive rats. Med Biol Eng Comput 50: 585–593, 2012. doi: 10.1007/s11517-012-0912-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zanetti M, Zwacka R, Engelhardt J, Katusic Z, O’Brien T. Superoxide anions and endothelial cell proliferation in normoglycemia and hyperglycemia. Arterioscler Thromb Vasc Biol 21: 195–200, 2001. doi: 10.1161/01.ATV.21.2.195. [DOI] [PubMed] [Google Scholar]