Abstract
The signaling cross talk between the tracheal mesenchyme and epithelium has not been researched extensively, leaving a substantial gap of knowledge in the mechanisms dictating embryonic development of the proximal airways by the adjacent mesenchyme. Recently, we reported that embryos lacking mesenchymal expression of Sox9 did not develop tracheal cartilage rings and showed aberrant differentiation of the tracheal epithelium. Here, we propose that tracheal cartilage provides local inductive signals responsible for the proper differentiation, metabolism, and inflammatory status regulation of the tracheal epithelium. The tracheal epithelium of mesenchyme-specific Sox9Δ/Δ mutant embryos showed altered mRNA expression of various epithelial markers such as Pb1fa1, surfactant protein B (Sftpb), secretoglobulin, family 1A, member 1 (Scgb1a1), and trefoil factor 1 (Tff1). In vitro tracheal epithelial cell cultures confirmed that tracheal chondrocytes secrete factors that inhibit club cell differentiation. Whole gene expression profiling and ingenuity pathway analysis showed that the tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), and transforming growth factor-β (TGF-β) signaling pathways were significantly altered in the Sox9 mutant trachea. TNF-α and IFN-γ interfered with the differentiation of tracheal epithelial progenitor cells into mature epithelial cell types in vitro. Mesenchymal knockout of Tgf-β1 in vivo resulted in altered differentiation of the tracheal epithelium. Finally, mitochondrial enzymes involved in fat and glycogen metabolism, cytochrome c oxidase subunit VIIIb (Cox8b) and cytochrome c oxidase subunit VIIa polypeptide 1 (Cox7a1), were strongly upregulated in the Sox9 mutant trachea, resulting in increases in the number and size of glycogen storage vacuoles. Our results support a role for tracheal cartilage in modulation of the differentiation and metabolism and the expression of inflammatory-related genes in the tracheal epithelium by feeding into the TNF-α, IFN-γ, and TGF-β signaling pathways.
Keywords: basal cell, club cells, embryonic trachea, Tgf-β, Sox9
the developmental biology of the tracheal and bronchial airways is not well understood, despite its important protective role against many serious lung diseases, including tracheomalacia, asthma, cough, chronic obstructive pulmonary disease, bronchiectasis, and cancer. The proximal airway mesenchyme comprises smooth muscle for elasticity and cartilage for prevention of airway collapse during respiration (10, 20, 41). The categories of cells found in the tracheal epithelium are basal, ciliated, club (formerly Clara), and goblet cells. Basal [transformation-related protein 63-positive (Trp63+)] cells are attached to the epithelial basement membrane and have been shown to act as a stem cell population during postnatal growth and tracheal injury repair (20, 34). Ciliated cells expel inhaled particles and mucus from the trachea and lungs (2, 44); club and goblet secretory cells are the trachea’s innate immune defense cells: they secrete mucus and immunoprotective proteins that are used as defense mechanisms against infections, allergens, and toxic inhalants, such as air pollutants and cigarette smoke (14, 27, 32). The number of each of these cell types differs from the proximal to the distal airway: the distal lung contains a greater number of club cells, while the proximal airway contains more basal cells (13). A study of the mechanisms of development and differentiation of tracheal cells is a necessary step toward advancing regenerative therapies for tracheal diseases and injuries. Recent studies have aimed to pinpoint signaling pathways that are critical during development and repair of the trachea. Two well-characterized signaling pathways that induce epithelial differentiation during development are WNT and fibroblast growth factor 10 (FGF10), which are produced by the mesenchymal cells underlying the tracheal epithelium (4). In addition, the tracheal mesenchyme is the site at which the cartilage rings develop (10, 45). The maturation of chondrocytes in the lung mesenchyme is regulated by a combination of WNT and transforming growth factor (TGF)-β signaling pathways, which enhance and inhibit, respectively, bone morphogenetic protein signaling (29, 42).
Transgenic mice lacking Sox9 expression, specifically in the tracheal mesenchyme, were born without cartilage rings along the trachea and died of collapsed airways. Interestingly, the surrounding tracheal epithelium also showed significant alterations: fewer basal and goblet cells and more club cells (10, 45). These results suggest that interaction between the tracheal cartilage and epithelium plays a critical role in normal tracheal tissue formation.
In the present study we further characterized the phenotype of the compartment-specific knockout of Sox9 in the tracheal mesenchyme, which yields further insights into the molecular mechanisms of cellular cross talk between the tracheal cartilage and the overlying airway epithelium. In the Sox9-knockout trachea, morphology in the epithelium was strongly altered (also reported in Ref. 45), as marked by altered expression of lung epithelium-specific markers, including surfactant protein B (Sftpb), trefoil factor 1 (Tff1), and Pb1fa1 [palate, lung, and nasal epithelium clone (Plunc)]. We hypothesized that the presence of cartilage rings is necessary for proper tracheal epithelium differentiation, and we used a genome-wide approach to clarify which signaling pathways mediate the cross talk between the tracheal cartilage and the tracheal epithelium. Ingenuity pathway analysis (IPA) highlighted deregulation of the interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), and TGF-β signaling pathways in the cartilage-deficient trachea. Moreover, IFN-γ or TNF-α can strongly inhibit differentiation of tracheal club cells, while IFN-γ can also inhibit basal and ciliated cell differentiation. In vivo deletion of mesenchymal expression of Tgf-β1 gene impaired tracheal cartilage development and tracheal epithelial differentiation. Finally, we showed that the lack of cartilage rings impairs oxidative metabolism in the tracheal epithelium, with increased expression of mitochondrial respiratory genes such as cytochrome c oxidase subunit VIIIb (Cox8b) and cytochrome c oxidase subunit VIIa polypeptide 1 (Cox7a1), together with an increase in cytoplasmic glycogen storage.
MATERIALS AND METHODS
Ethics statement.
We adhered to the National Institutes of Health guidelines for animal care and safety. All animal experimental protocols were approved by the Animal Care and Use Committee at the Children’s Hospital of Los Angeles.
Mouse breeding and histology.
Sox9fl/fl/Tbx4-rtTA/Tet-On-Cre male mice were bred with Sox9fl/fl female mice; pregnant females were fed doxycycline chow from embryonic day 7.5 (E7.5) to E18.5. Embryos were collected at E18.5, and lungs were fixed in formalin and embedded in paraffin (45). Tgfb1fl/fl mice were purchased from Jackson Laboratory and bred with Tbx4-rtTA/Tet-On-Cre mice to generate male Tgfb1fl/fl/Tbx4-rtTA/Tet-On-Cre mice. Tgfb1fl/fl/Tbx4-rtTA/Tet-On-Cre male mice were bred with Tgfb1fl/fl female mice; pregnant females were fed doxycycline chow from E7.5 to E18.5, and embryos were collected at E18.5 for analysis. Paraffin-embedded sections (4 μm thick) were stained with Alcian blue, and nuclei were counterstained with nuclear fast red. The periodic acid-Schiff (PAS) staining kit (Sigma-Aldrich) was used according to the manufacturer's standard procedure for staining tissue sections to detect glycogen storage. Whole mount Alcian blue staining was performed as previously described (10, 45).
Quantitative real-time PCR.
RNA was extracted from E18.5 mutant and control tracheas. Embryonic tracheas were severed between the larynx and the bronchi. RNA was extracted with TRIzol reagent, and 500-1,000 ng of total RNA were reverse-transcribed using the iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). Real-time quantitative PCR was performed on a real-time PCR system (LightCycler 480 II, Roche Applied Science) using gene-specific primers (Operon, Huntsville, AL) and TaqMan probes (Roche Applied Science). The primer sequences are as follows: CCCTGGCTCAGGGTCTGCCT (sense) and TGGGCCCCTTTCTGTGAGCCA (antisense) for Pb1fa1, gatcgccatcacaatcactg (sense) and gaagaaatcctgggcagatg (antisense) for secretoglobulin, family 1A, member 1 (Scgb1a1), catcagattctgcaaacaatgg (sense) and tggtatcaaagttgactgactgc (antisense) for surfactant protein A (Sftpa), aagccgtgatccccaagt (sense) and ctagctggggcaccacac (antisense) for surfactant protein B (Sftpb), gggtgagaagggtgatcca (sense) and ttctccctttggtccaggtt (antisense) for surfactant protein D (Sftpd), gtcctcatgctggccttc (sense) and tctctccgtgcactgctg (antisense) for Tff1, gctctcaaggagggtgatga (sense) and cggtccagtaagggtcgtag (antisense) for complement C1q TNF-related protein 2 (C1qtnf2), tggagattatggatttcgtggt (sense) and ccattgttcccattgtttcc (antisense) for complement C1q TNF-related protein 3 (C1qtnf3), agctggctgactggaagc (sense) and tagtcctggagggccaca (antisense) for melanoma inhibitory activity 1 (Mia1), agccaaaactcccacttcc (sense) and gctctccaagtgggctaaga (antisense) for Cox8b, and ctgaggacgcaaaatgagg (sense) and gtcattgtcggcctggaa (antisense) for Cox7a1.
Isolation of mouse tracheal epithelial cells, immunofluorescence, and cell count.
Eight-week-old mice were euthanized, and the tracheas were immediately removed and placed in cold DMEM/F-12 containing penicillin-streptomycin. The tracheas were cleaned by removal of mesenchyme, fat, and smooth muscle and cut vertically. Isolated tracheas were incubated overnight at 4°C in 1.5 mg/ml pronase solution (Roche Applied Science). On the following day, epithelial cells were collected and seeded onto a polycarbonate 0.4-mm Transwell insert in proliferation medium [10 μg/ml insulin, 5 μg/ml apotransferrin, 0.1 μg/ml cholera toxin, 25 ng/ml epidermal growth factor (EGF), 30 μg/ml bovine pituitary extract (BPE), 5% FBS, and 0.01 μM retinoic acid] as previously described (8, 15, 46). Medium was changed every other day. Cells were cultured for 9–10 days until transepithelial resistance reached >1,300 Ω/cm2 (day 0), at which time medium in the inner top chamber was removed to create an air-liquid interface (ALI) and induce differentiation of the epithelial cells. Differentiation cell medium was added on the bottom chamber (1:1 DMEM/F-12, 2% NuSerum, 5 μg/ml insulin, 0–5 μg/ml apotransferrin, 5 μg/ml EGF, 0.025 μg/ml cholera toxin, 30 μg/ml BPE, and 0.01 μM retinoic acid). Cells were collected at day 14 and fixed in 4% formalin for 15 min. Cells were permeabilized in PBS-0.4% Triton X-100-3% BSA for 30 min at room temperature and then incubated with the primary antibody overnight. For cell counting, images were taken using a confocal microscope (×10 magnification) and analyzed with ImageJ: four 200-μm2 (400-μm2 for ciliated cells) regions were randomly selected, and cells within these regions were counted. Counts are expressed as average ± SD.
Chondrocyte purification.
E18.5 tracheas were isolated and digested with collagenase II (2 mg/ml) for 10 min until single cartilage rings were released from the tissue. Isolated cartilage rings were removed and further digested with fresh collagenase II solution (2 mg/ml) until a single-cell suspension was obtained (30 min) (24). The cells were centrifuged and resuspended in DMEM-10% FBS + antibiotics and seeded on Primaria plates (BD Biosciences) for 3 days. On day 3, supernatant was collected from the plated cells, separated into aliquots, and stored at −80°C. Mouse tracheal epithelial cells were seeded on Transwell culture inserts (Corning). The total volume of medium on the bottom compartment of the ALI culture was 520 μl; 100 μl of the volume consisted of the chondrocyte supernatant. For the control, 100 μl of DMEM-10% FBS + antibiotics were added to the bottom compartment of the ALI culture. The remainder (420 μl) of the medium contained 1:1 DMEM/F-12, 2% NuSerum, 5 μg/ml insulin, 0–5 μg/ml apotransferrin, 5 μg/ml EGF, 0.025 μg/ml cholera toxin, 30 μg/ml BPE, and 0.01 μM retinoic acid.
Antibodies and reagents.
The following primary antibodies were used: Trp63 (Genetex), Plunc (R & D Systems), tubulin-β4B, class IVb (Tubb4b; Biogenics), phosphorylated histone 3 (Cell Signaling), and 2′,5′-oligoadenylate synthetase 2 (Oas2; Proteintech). Secondary antibodies (donkey Alexa 488 anti-rabbit, donkey Alexa 555 anti-mouse, donkey Alexa 633 anti-sheep, and donkey Alexa 633 anti-goat) were purchased from Life Technologies. Recombinant IFN-γ and TNF-α were obtained from Peprotech and used at a final concentration of 10 ng/ml.
Microarray analysis.
Total RNA was extracted from mouse tracheas using TRIzol reagent (Life Technologies). RNA was extracted from a pool of three tracheas to obtain sufficient good-quality RNA for expression analysis. Three mutant and three control tracheal pools were used. The total of nine mutant and nine control embryonic tracheas were collected from seven different pregnant females and randomly pooled immediately before extraction of the total RNA. The embryonic heart was used to confirm the genotype of the embryos. Gene expression profiling was performed on Illumina mouse Ref 8 v2.0 expression chip at the University of California Los Angeles Neuroscience Genomics Core. Gene expression profiling was performed in triplicate. The R/Bioconductor software package Limma (33) was used to process Illumina bead chip data (36) and generate false discovery rate-corrected (1a) differential gene expression statistics (39).
Setting a cutoff (P < 0.05) for statistically significant changes, we identified 550 genes of interest. To identify signaling pathways affected by the lack of tracheal cartilage, the data set was analyzed with the online program IPA.
Statistics.
Real-time PCR was performed in triplicate. To obtain sufficient RNA for analysis, two to three tracheas were pooled. Tracheal epithelial culture was repeated at least four times. The statistical P value was determined by t-test.
RESULTS
Epithelium of the mesenchyme-specific mutant Sox9 trachea shows altered epithelial cell morphology and expression of epithelial markers.
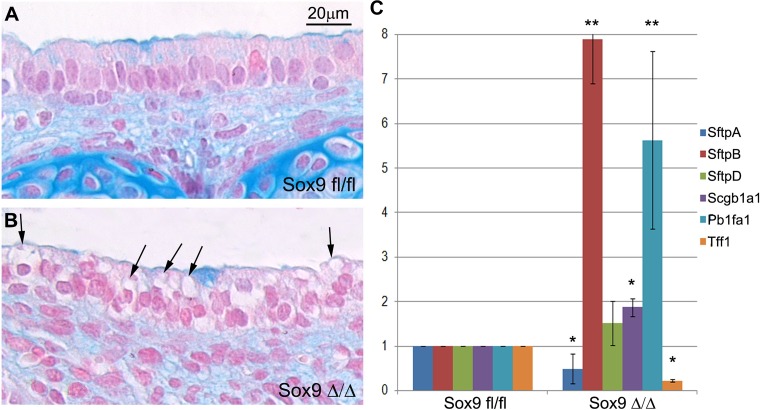
We previously reported that the Sox9Δ/Δ mutant tracheas lacked cartilage rings and showed an altered morphology in the tracheal epithelium and that the number of basal and club cells was increased and decreased, respectively, compared with normal tracheal epithelium (45). As previously reported by our group and others (10, 45), the mutant Sox9Δ/Δ tracheal epithelium appears unhealthy: it is characterized by the presence of large and numerous vacuoles within the cytoplasm of the epithelial cells (Fig. 1B). We quantified the expression of several lung epithelium-specific markers to determine whether the absence of cartilage rings affected differentiation of the tracheal epithelium. RT-PCR results showed drastically altered gene expression of tracheal epithelial cell markers in the mutant Sox9 compared with the wild-type trachea. While Sftpa (9) and Tff1 (31) expression levels were downregulated in the mutant trachea, expression levels of Sftpb (23), Scgb1a1 (38), and Pb1fa1 (6) were significantly increased (Fig. 1C), and there was no statistically significant difference in the expression level of Sftpd (19) mRNA. Sftpa, Sftpb, Scgb1a1, and Pb1fa1 are expressed by the club cells, while Tff1 is mostly expressed by the lung goblet cells.
Fig. 1.
Lack of tracheal cartilage rings alters expression of tracheal epithelial markers. A and B: tracheal sections from wild-type (Sox9fl/fl) and Sox9 knockout (Sox9Δ/Δ) mice at embryonic day 18.5 (E18.5) stained with Alcian blue to show cartilage. In transgenic Sox9 knockout mice, note the lack of cartilage and the presence of unidentified vacuoles (black arrows) within cells lining the surface of the epithelium. Each image is representative of 4 tracheas. C: surfactant protein B (Sftpb) and Pb1fa1 dramatically increased, by ~8- and 5-fold, respectively, and secretoglobulin, family 1A, member 1 (Scgb1a1) modestly, but significantly, increased in the absence of Sox9 in vivo. Expression levels of surfactant protein A (Sftpa) and trefoil factor 1 (Tff1) were significantly downregulated, but expression of surfactant protein D (Sftpd) did not change significantly, in mutant tracheas. *P < 0.05, **P < 0.01. Values are means ± SD of 4 mutant and 4 control tracheas.
Tracheal chondrocytes secrete factors that alter tracheal epithelial differentiation.
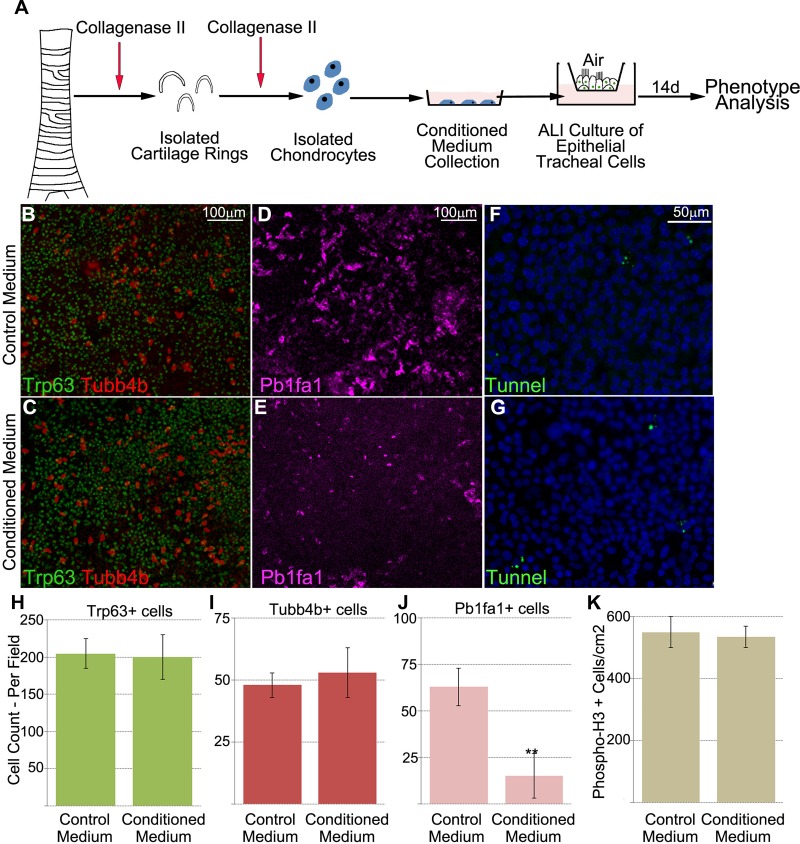
Next, we tested whether airway cartilage could affect the development and homeostasis of tracheal epithelial differentiation in vitro. We hypothesized that embryonic tracheal cartilage secretes specific signaling factors that have the ability to drive proper differentiation of the tracheal epithelium. To test our hypothesis, we used an in vitro culture system comprising mouse tracheal epithelial cells fed with medium containing supernatant from 72-h-cultured primary chondrocytes (Fig. 2A) and supplied to tracheal cells during the differentiation phase (from day 0 to day 14). DMEM-10% FBS was used as control medium (see materials and methods). Cells fed chondrocyte-conditioned medium showed a decrease in club cells and no change in basal and ciliated cells (Fig. 2, B–E and H–J).
Fig. 2.
Chondrocyte-conditioned medium inhibits club cell differentiation. A: concept diagram of the protocol for chondrocyte isolation and differentiation of tracheal epithelial cells. ALI, air-liquid interface. B–E: analysis of respective population differences between control and conditioned cells by fluorescence imaging of ALI cell cultures. Cells fed conditioned medium from chondrocytes showed no significant change in frequency between basal [transformation-related protein 63-positive (Trp63+)] and ciliated [tubulin-β4B, class IVb (Tubb4b+)] cells and control cells. However, the number of club (Pb1fa1+) cells was significantly decreased in chondrocyte-conditioned medium. F and G: no increase in apoptotic rate in tracheal cells cultured with chondrocyte-conditioned medium. Images are representative of 4 different samples. H–J: quantification of results from fluorescence imaging (B–E) illustrating reduced number of club (Pb1fa1+) cells, but no change in the number of basal or ciliated cells, fed chondrocyte-conditioned medium. K: no statistically significant difference in proliferation or apoptosis between cells fed chondrocyte-conditioned medium and cells fed control medium. phospho-H3, phosphorylated histone. **P < 0.01. Values are means ± SD of 4 separate experiments.
However, chondrocyte-conditioned medium altered neither the proliferation nor the apoptosis of tracheal epithelial cells (Fig. 2, F, G, and K), suggesting that decreased number of club cells was a result of impaired differentiation.
Gene expression profiling of the mutant trachea identified prominent alteration of the TNF-α, IFN-γ, and TGF-β signaling pathways.
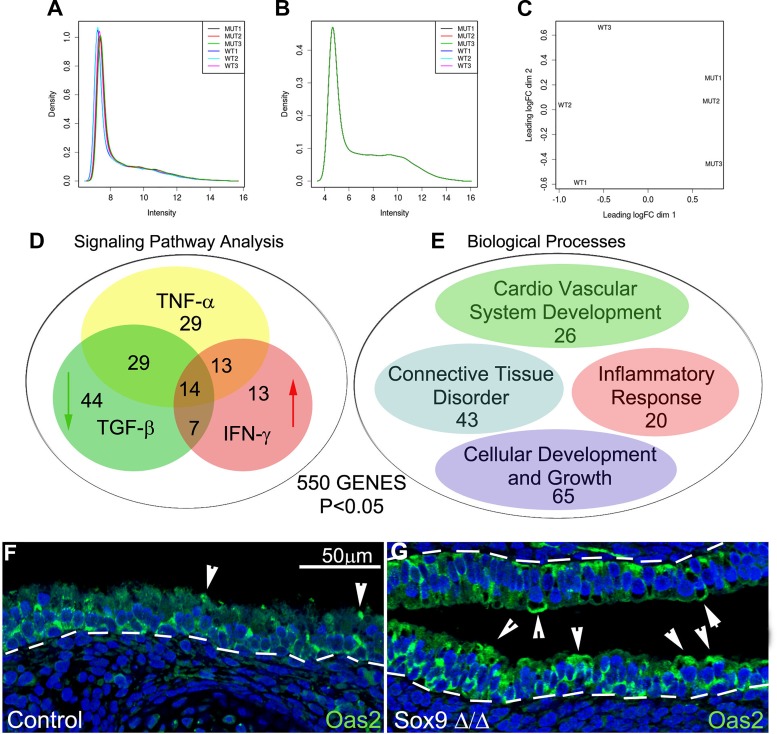
We used a genome-wide approach for insight into the molecular mechanisms linking the absence of cartilage to altered epithelial differentiation in the mutant trachea. Total RNA was extracted from mutant and control tracheas, and differentially expressed genes were identified using a beads-based Illumina platform. After normalization of gene expression, the expression profile of three mutant tracheas showed clusters that were well separated from the expression profile of the three control tracheas (see Fig. 4, A–C). We identified 550 differentially expressed genes (P < 0.05, no constraint on fold change; see Supplemental Data Set S1 in Supplemental Material for this article available online at the Journal website). Most (347 of 550) of the identified genes were downregulated in the mutant trachea, and they were related to cartilage development.
Fig. 4.
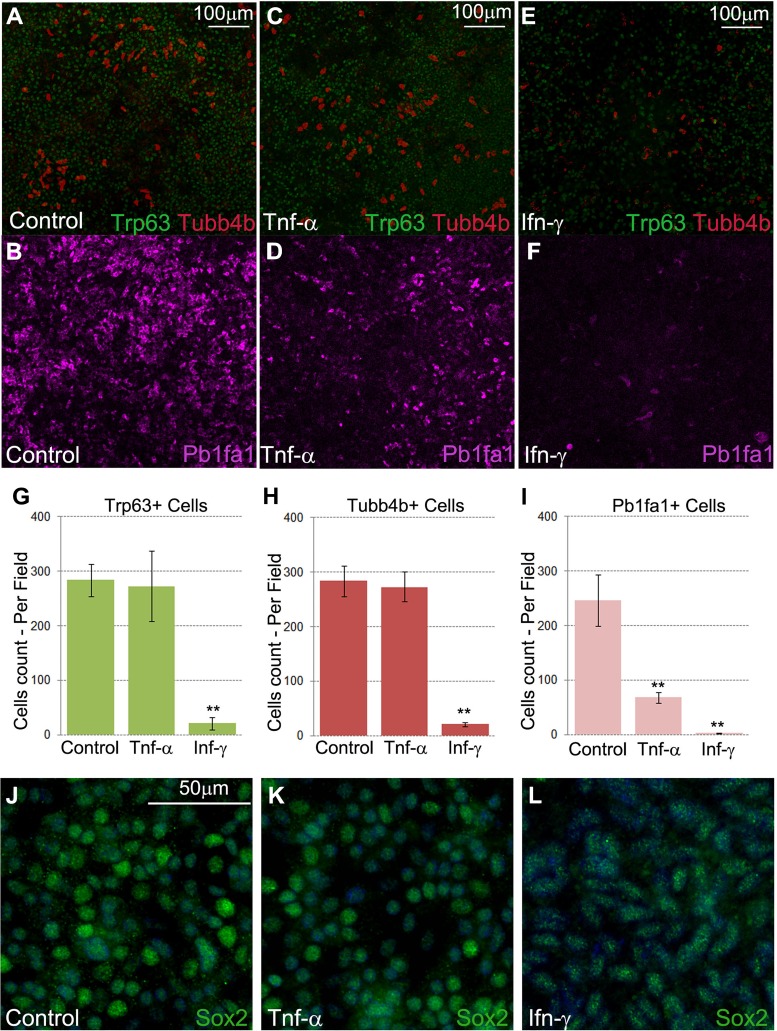
Recombinant TNF-α and IFN-γ alter differentiation of the tracheal epithelial cell in vitro. A and B: images showing the frequency of basal (Trp63+), ciliated (Tubb4b+), and club (Pb1fa1+) cells fed the control medium. C and D: TNF-α inhibited club cell differentiation in vitro. E and F: IFN-γ reduced the number of basal cells and strongly diminished the number of ciliated and club cells. G–I: quantification of images (A–F) showing the frequency of Trp63+, Tubb4b+, and Pb1fa1+ cells under each condition. *P < 0.05, **P < 0.01. Values are means ± SD from 3 independent experiments. J–L: control and TNF-α- and IFN-γ-treated tracheal epithelial cells expressed Sox2 at the end of the 14-day treatment period. However, expression of the Sox2 marker appeared slightly reduced after treatment with recombinant IFN-γ. Images are representative of 3 independent experiments.
Application of IPA software to the list of 550 differentially expressed genes showed that the TNF-α, IFN-γ, and TGF-β signaling pathways were altered in the mutant Sox9 trachea (Fig. 3D; see Supplemental Data Set S1). According to the IPA software, IFN-γ and TGF-β signaling pathways were activated and inhibited, respectively, in the mutant trachea, while a definite result was not obtained regarding activation of the TNF-α signaling pathway.
Fig. 3.
Gene expression profiles of the mutant trachea. A and B: expression signals before and after normalization. C: gene expression profiles of mutant tracheas cluster separately from those of control tracheas. D: ingenuity pathway analysis (IPA) software was applied to the 550 differentially expressed genes. IPA identified the IFN-γ signaling pathway as being significantly stimulated in the mutant trachea. According to IPA, a group of genes feeding into the TNF-α signaling pathway were also deregulated, and the TGF-β signaling pathway was inhibited in the mutant Sox9Δ/Δ trachea. E: GeneNetwork-based analysis showed that most of the genes with changes in expression belonged to the following categories: connective tissue disorder, inflammatory response, cardiovascular system development, and cellular development and growth. F and G: staining for 2′,5′-oligoadenylate synthetase 2 (Oas2), a downstream gene of the TNF-α and IFN-γ signaling pathways, was increased in the epithelium of the mutant trachea. Arrowheads indicate apical expression of Oas2. Images are representative of 3 separate stained sections.
Genes related to the IFN-γ signaling pathway included viral genes, such as Oas2, Oasl2, and Oas1g, the cytokine genes Ccl6 and Cxcl9, and the inflammatory transcriptional factor genes Irf7 and Stat1 (see Supplemental Data Set S1). Genes related to the TGF-β signaling pathway (see Supplemental Data Set S1) included Tgfb1i1, Twist1, Vim, Wisp, and Grem1, all of which were downregulated. We confirmed the microarray data for the Oas2 gene, which feeds the TNF-α and IFN-γ signaling pathways: in accordance with the microarray data, Oas2 protein expression was increased in the mutant compared with the control tracheal epithelium (Fig. 3, F and G).
A large portion of the genes differentially expressed in the mutant trachea were related to connective tissue development/connective tissue disorders, inflammatory responses, cellular development and growth, and cardiovascular system development (Fig. 3E; see Supplemental Data Set S1).
TNF-α and IFN-γ regulate differentiation of tracheal epithelial cells in vitro.
Using IPA software, we found that the lack of cartilage rings impairs expression of genes related to the inflammatory TNF-α and INF-γ pathways. Cartilage expresses members of the TNF-α superfamily, such as C1qtnf2 and C1qtnf3 (52); however, expression of IFN-γ by cartilage has not been reported. We speculate that lack of cartilage expression of C1qtnf2, C1qtnf3, and Mia1, all of which are anti-inflammatory cytokines (26, 28, 52), and inhibition of the TGF-β signaling pathway, which suppresses the IFN-γ signaling pathway (21, 22, 37), may contribute to regulation of expression of inflammatory-related genes in the embryonic tracheal mesenchyme and epithelium.
Next, we determined the effect of the TNF-α or IFN-γ signaling pathways on differentiation of tracheal epithelial cells. Tracheal epithelial cells were isolated from adult mice as previously described and cultured until confluent, at which time the ALI was established. Cells were fed medium that contained recombinant TNF-α or IFN-γ for 14 days. We then stained for club, basal, and ciliated cell markers. TNF-α reduced the amount of club cell differentiation, while IFN-γ inhibited differentiation of all three cell types (basal, club, and ciliated; Fig. 4). The Tubb4b− or Pb1fa1− cells treated with IFN-γ still expressed Sox2 protein, suggesting an undifferentiated status of airway epithelial cell lineage (Fig. 4, J–L).
TGF-β signaling originating in the tracheal mesenchyme regulates tracheal cartilage development and differentiation of the club and basal cells of the tracheal epithelium.
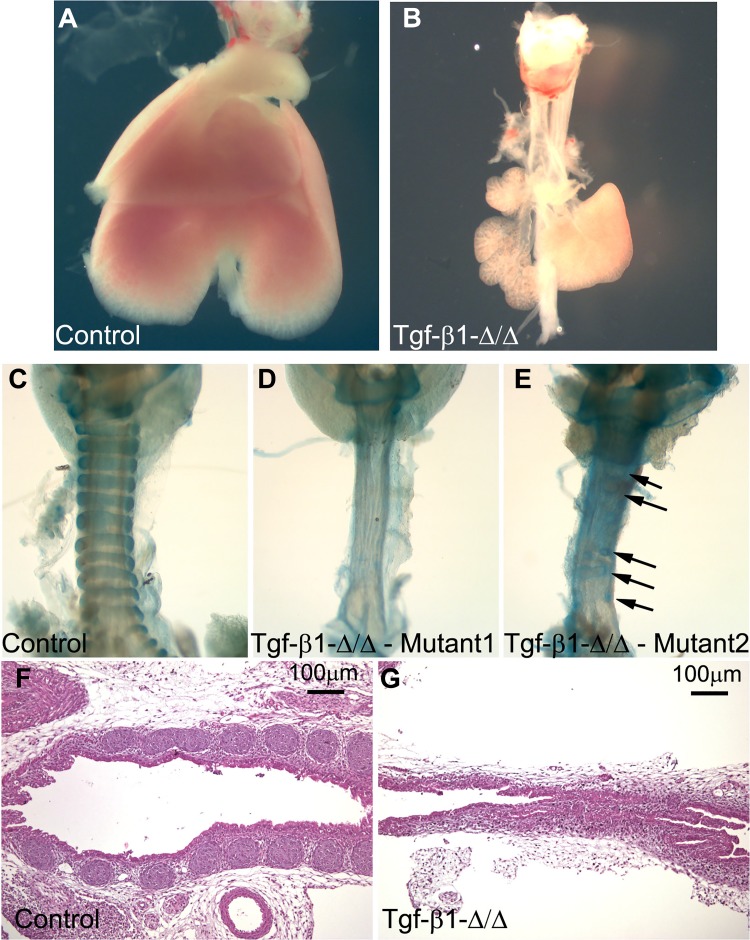
To provide insight into the specific role of the TGF-β signaling pathway during embryonic tracheal development, we used the inducible Tbx4-rtTA/Tet-On-Cre mouse line to knock out Tgf-β1 in the embryonic tracheal mesenchyme (55). E18.5 mutant lungs were hypoplastic, and tracheas were collected for further analysis: mutant Tgf-β1Δ/Δ trachea showed complete or nearly complete absence of cartilaginous rings (Fig. 5, A–E), the tracheal lumen was narrowed, and the numbers of club and basal cells were increased and decreased, respectively (Fig. 6, A–E). We did not observe a change in the number of ciliated cells between the mutant Tgf-β1Δ/Δ and the control tracheal epithelium (Fig. 6E). Deletion of the Tgf-β1 gene in the lung mesenchyme also reduced expression of Sox9 protein by the tracheal mesenchyme (Fig. 6, F and G).
Fig. 5.
Mesenchymal knockout of Tgf-β1 impairs normal tracheal cartilage development and induces lung hypoplasia. A and B: at E18.5, Tgf-β1 mutant lungs are severely hypoplastic. C and D: Alcian blue staining of control and mutant Tgf-β1 embryonic tracheas. Lack of Tgf-β1 expression prevented cartilage ring development. E: small cartilaginous rudiments (arrows) in the trachea of a few mutant embryos. F and G: hematoxylin-eosin staining showing narrowing of the lumen and lack of cartilage in mutant embryonic trachea (E). Images are representative of 4 separate embryonic lungs.
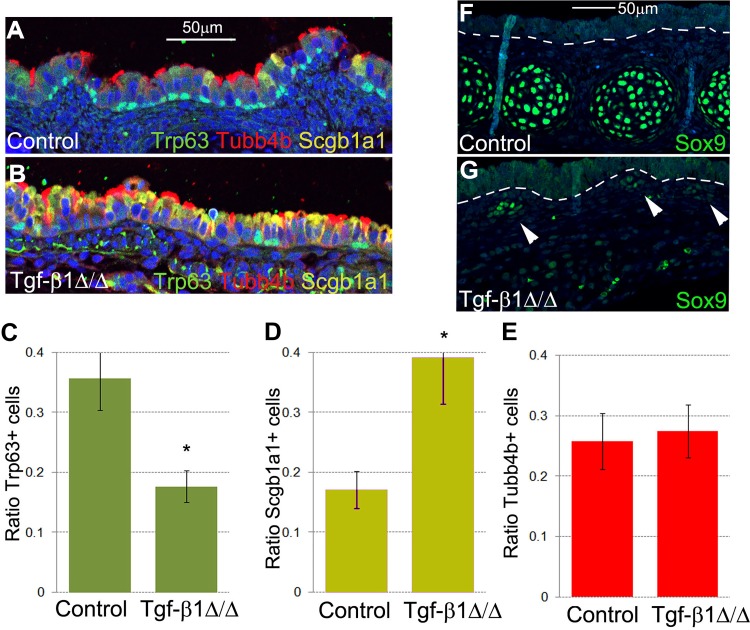
Fig. 6.
Mesenchymal knockout of Tgf-β1 impairs differentiation of tracheal epithelium. A–E: knockout of Tgf-β1 in lung mesenchyme resulted in fewer basal cells and more Clara cells. E: number of ciliated cells was not affected by the lack of mesenchymal expression of Tgf-β1. *P < 0.05. F and G: Sox9 protein expression was downregulated in mutant Tgf-β1Δ/Δ embryonic trachea. White arrowheads indicate focal residual expression of Sox9 by the tracheal mesenchyme. Images are representative of 3 separate embryonic lungs. Values are means ± SD from 3 independent experiments.
Lack of tracheal cartilage alters oxidative and glycogen metabolism in the tracheal epithelium.
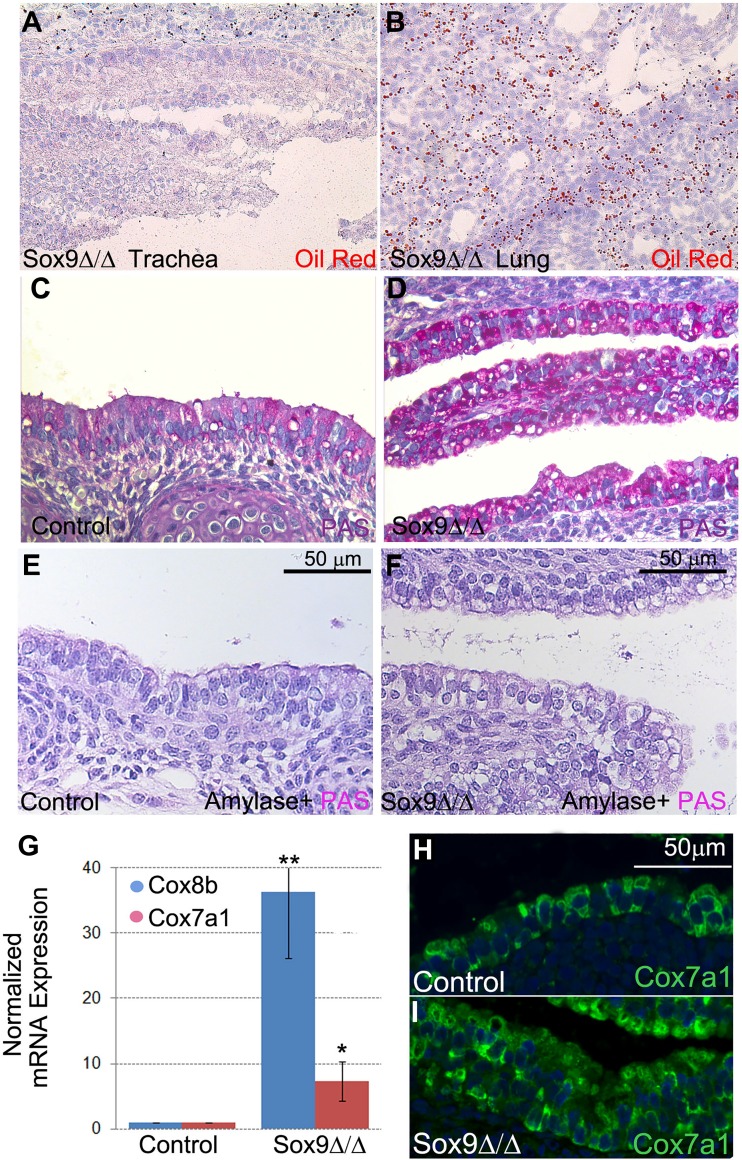
Large cytoplasmic vacuoles with unidentified contents were observed in the epithelium of the mutant Sox9Δ/Δ trachea (Fig. 1, A and B). These vacuoles did not contain surfactant and mucus proteins [Sftpb, Pb1fa1, mucin 5AC (Muc5ac), and Scgb1a1; data not shown] or lipids (Fig. 7, A and B). In contrast, glycogen was found in most of the vacuoles from the mutant trachea, as shown by PAS staining (Fig. 7, C and D). The PAS staining was lost if the slide was pretreated with α-amylase to digest the sugar monomers (Fig. 7, E and F).
Fig. 7.
Lack of cartilage rings increases expression of glycogenic genes and storage of glycogen in tracheal epithelium. A and B: vacuoles in mutant tracheal epithelium do not contain lipids. C and D: marked increase in glycogen storage in periodic acid-Schiff (PAS)-stained Sox9-knockout tracheal epithelium. E and F: pretreatment of samples with α-amylase abolished PAS staining intensity in control and mutant samples. G: normalized real-time quantitative PCR data show increased expression of cytochrome c oxidase subunit VIIa polypeptide 1 (Cox7a1) and cytochrome c oxidase subunit VIIIb (Cox8b), genes involved in mitochondrial oxidative metabolism. Values are means ± SD from 4 independent experiments. H and I: staining for Cox7a1 protein localized in tracheal epithelium, with highest expression in mutant tracheal epithelium. *P < 0.05, **P < 0.01. Images are representative of 4 independent experiments.
To understand the mechanism underlying glycogen accumulation in these airway epithelial cells, gene expression profiles were reanalyzed. We found that Cox7a1 and Cox8b were upregulated in the mutant epithelium (Fig. 7G). Cox7a1 and Cox8b are key components of mitochondrial oxidative metabolism and regulate fat and glucose metabolism in different organs and cell types. Expression of Cox7a1 is associated with increased in vivo glucose uptake (5, 35, 43), and both enzymes are strongly expressed by heart, muscle, and diaphragm. Staining specifically for Cox7a1 protein revealed that its expression is localized in the tracheal epithelium and that it is increased in the mutant Sox9Δ/Δ trachea (Fig. 7, H and I).
DISCUSSION
The proximal airway epithelium is organized in a specific proximal-distal manner: while basal cells are concentrated in the proximal airway, club cell numbers increase with proximity to the distal lung. Recent research has shown that the tracheal epithelium requires adjacent mesenchymal signaling for appropriate differentiation. Volckaert et al. demonstrated a critical role for FGF10 signaling from the tracheal mesenchyme in the differentiation of epithelial basal and club cells (47). Our novel findings and those of others (10, 45) suggest that the tracheal cartilage also has the ability to signal to the adjacent epithelium to induce proper epithelial cell differentiation. Furthermore, the epithelial cells in the mutant trachea presented a markedly altered cytoplasmic morphology with numerous large vacuoles (Fig. 1, A and B) (45).
The lack of cartilage rings affected the mRNA expression of many epithelial differentiation markers (Fig. 1C). For some, such as Pb1fa1 and Sftpb, with increases of >5.5- and >8-fold, respectively, the changes in expression were surprisingly large. Two hypotheses can explain the altered differentiation of the tracheal epithelium in the Sox9 mesenchymal knockout mouse. 1) The tracheal cartilage is a morphogenic entity able to signal inductively to the tracheal epithelium by releasing specific soluble factors, a hypothesis that we have named “cartilage-epithelium cross talk.” 2) The altered differentiation of the tracheal epithelium is caused by changes in mechanical forces (such as compression) (30), acting on the epithelium as a result of the lack of physical support of the cartilage rings.
We have focused on the cartilage-epithelium cross-talk hypothesis. After adding factors secreted by chondrocytes in the form of conditioned medium to tracheal epithelial cells growing in ALI culture, we detected a significant decrease in the club cell population. This decrease in the number of club cells suggests that if conditions are similar in vivo, cartilage indeed regulates the differentiation of club cells in the tracheal epithelium during development (Fig. 2). On the tissue level, excess cartilage leads to tracheal narrowing and can cause airway obstruction and lung disease, while the absence of cartilage poses choking risks and breathing problems, as seen in tracheomalacia (16, 25, 40). On the cellular level, the presence of chondrocytes seems to negatively regulate the abundance of club cells by decreasing the population of mature cells (Fig. 2G). This negative regulation could also be an important factor in determining the relative distribution of club cells in species such as the mouse, where airway cartilage is distributed more proximally, compared with the human and pig, where cartilage and club cells extend more distally.
Our previous research showed that lack of cartilage rings resulted not only in increased numbers of club cells but also a decreased number of basal cells. Our experiments using in vitro ALI cultures with chondrocyte-conditioned medium partially recapitulate the inverse of the phenotype obtained by the lack of cartilage rings in vivo. However, we did not observe a change in the number of basal cells, which indicates that other signaling mechanisms are likely to exist in vivo. We speculate that the complex signaling mechanism may also extend to other elements of the tracheal mesenchyme and vasculature.
Most of the identified genes were downregulated and, as expected, implicated in the differentiation and development of cartilage and connective tissue. We were surprised to find upregulation of several genes related to the inflammatory response in the mutant trachea (see Supplemental Data Set S1). As we expected, the TGF-β signaling pathway was downregulated in the mutant trachea (Fig. 3D; see Supplemental Data Set S1), since chondrocytes express TGF-β ligands during differentiation and condensation. An in vitro experiment showed that both TNF-α and IFN-γ have the ability to alter differentiation of tracheal epithelial cells (Fig. 4) (3, 18).
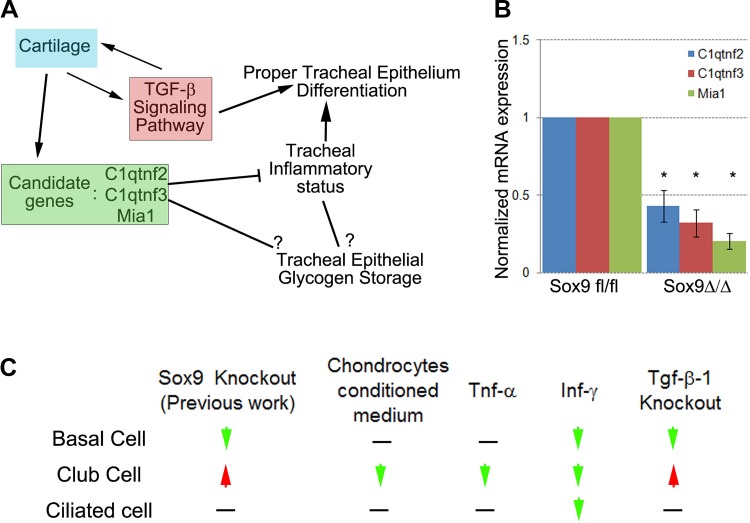
How cartilage affects tracheal inflammation is still under investigation, but we hypothesized that multiple pathways and molecules feed into this phenomenon. 1) Lack of tracheal cartilage resulted in reduced activity of the TGF-β signaling pathway. The role of the TGF-β signaling pathway in the modulation of inflammation is well known (21, 53). 2) Cartilage expresses two important adipokines, C1qtnf2 (Ctrp2) and C1qtnf3 (Ctrp3), which are also members of the TNF family (1, 26, 52). C1qtnf2 and C1qtnf3 are strongly downregulated in mutant trachea (Fig. 8B; see Supplemental Data Set S1). They are key regulators of glycogen and fat metabolism, as well critical mediators of inflammation (7). C1qtnf3 knockout mice develop more severe rheumatoid arthritis than their control littermates (28), suggesting that C1qtnf3 acts as an anti-inflammatory chemokine. 3) Cartilage secretes Mia1, which is also an anti-inflammatory cytokine (54).
Fig. 8.
Concept diagram. A and B: cartilage is necessary for proper tracheal epithelial differentiation and metabolism and regulation of inflammatory-related gene expression in embryonic trachea. Tgf-β ligands, C1qtnf2/3 and melanoma inhibitory activity 1 (Mia1) proteins, are normally secreted by tracheal cartilage and, thus, mediate expression of inflammatory genes by the trachea. TGF-β signaling pathway is pivotal for ontogeny of tracheal basal cells but negatively regulates Clara/club cell differentiation. C1qtnf2/3 peptides are secreted by the cartilage and may contribute to glycogen regulation in tracheal epithelium. Expression of C1qtnf2/3 is reduced in the mutant Sox9Δ/Δ trachea. C: phenotypes across the different experimental systems. Values are means ± SD from 3 independent experiments.
The role of the TGF-β signaling pathway in embryonic tracheal development is unknown. We knocked out Tgf-β1 specifically in the tracheal mesenchyme. In mutant embryos, cartilage was absent, expression of Sox9 protein was reduced, and differentiation of the tracheal epithelium was altered: the number of basal cells was significantly reduced in the mutant Tgf-β1Δ/Δ trachea, while the number of club cells was increased. Interestingly, the compartment-specific knockout of Tgf-β1 in the lung mesenchyme strongly phenocopies the compartment-specific knockout of Sox9 in the lung mesenchyme (45). Our results, therefore, strongly suggest that the TGF-β signaling pathway is pivotal in regulation of the balance between basal cell and Clara/club cell differentiation during embryonic development.
Finally, we identified massive glycogen deposits within the cytoplasmic vacuoles in the mutant tracheal epithelium (Fig. 7, C and D). Expression levels of Cox8b and Cox7a1 were markedly increased in the mutant trachea. Cox7a1 protein is strongly localized in the cytoplasm of the tracheal epithelial cells (Fig. 7, H and I). No antibody for Cox8b was commercially available; therefore, we can only speculate, based on similarity of function, that localization expression and function of Cox8b and Cox7a1 may be similar in the epithelium. The highest expression levels of Cox8b and Cox7a1iare found in heart, muscle, and diaphragm. Cox8b and Cox7a1 are key components in the mitochondrial oxidative chain, playing important roles in fat and glucose metabolism. This is remarkable, since glycogen content is inversely related to the differentiation level of lung epithelial cells (17, 48–51). This finding further supports our hypothesis that the presence of cartilage is essential to the timely progress of final differentiation of tracheal epithelial cells into definitive cell types during upper airway development.
Our research has answered some important questions about tracheal mesenchyme-epithelium cross talk during embryonic development but has provoked many more questions. Future studies are necessary to identify which cartilage-specific molecules signal to the tracheal epithelium and the mechanism of the TGF-β signaling pathway in the compartment-specific regulation of tracheal epithelial differentiation.
GRANTS
This work was supported by National Institutes of Health Grants 5U01 HL-122681-02 to D. Warburton and a grant from the California Institute for Regenerative Medicine to G. Turcatel.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
G.T. and D.W. developed the concept; G.T., K.M., and S.L. performed the experiments and designed the study; M.T., and B.G. performed microarray studies and statistical analysis; G.T., K.M., W.S., and D.W. drafted the manuscript; G.T., K.M, M.T., S.L., B.G., W.S., and D.W. approved the final version of the manuscript.
Supplementary Material
REFERENCES
- 1.Akiyama H, Furukawa S, Wakisaka S, Maeda T. Cartducin stimulates mesenchymal chondroprogenitor cell proliferation through both extracellular signal-regulated kinase and phosphatidylinositol 3-kinase/Akt pathways. FEBS J 273: 2257–2263, 2006. doi: 10.1111/j.1742-4658.2006.05240.x. [DOI] [PubMed] [Google Scholar]
- 1a.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B 57: 289–300, 1995. [Google Scholar]
- 2.El Agha E, Bellusci S. Walking along the fibroblast growth factor 10 route: a key pathway to understand the control and regulation of epithelial and mesenchymal cell-lineage formation during lung development and repair after injury. Scientifica (Cairo) 2014: 538379, 2014. doi: 10.1155/2014/538379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friesel R, Komoriya A, Maciag T. Inhibition of endothelial cell proliferation by gamma-interferon. J Cell Biol 104: 689–696, 1987. doi: 10.1083/jcb.104.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galvis LA, Holik AZ, Short KM, Pasquet J, Lun AT, Blewitt ME, Smyth IM, Ritchie ME, Asselin-Labat ML. Repression of Igf1 expression by Ezh2 prevents basal cell differentiation in the developing lung. Development 142: 1458–1469, 2015. doi: 10.1242/dev.122077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gburcik V, Cleasby ME, Timmons JA. Loss of neuronatin promotes “browning” of primary mouse adipocytes while reducing Glut1-mediated glucose disposal. Am J Physiol Endocrinol Metab 304: E885–E894, 2013. doi: 10.1152/ajpendo.00463.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghafouri B, Kihlström E, Ståhlbom B, Tagesson C, Lindahl M. PLUNC (palate, lung and nasal epithelial clone) proteins in human nasal lavage fluid. Biochem Soc Trans 31: 810–814, 2003. doi: 10.1042/bst0310810. [DOI] [PubMed] [Google Scholar]
- 7.Ghai R, Waters P, Roumenina LT, Gadjeva M, Kojouharova MS, Reid KB, Sim RB, Kishore U. C1q and its growing family. Immunobiology 212: 253–266, 2007. doi: 10.1016/j.imbio.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Gomi K, Arbelaez V, Crystal RG, Walters MS. Activation of NOTCH1 or NOTCH3 signaling skews human airway basal cell differentiation toward a secretory pathway. PLoS One 10: e0116507, 2015. doi: 10.1371/journal.pone.0116507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harvilchuck JA, Zurbrugg RJ, Carlson GP. CC10 mRNA and protein expression in Clara cells of CD-1 mice following exposure to styrene or its metabolites styrene oxide or 4-vinylphenol. Toxicol Lett 183: 28–35, 2008. doi: 10.1016/j.toxlet.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 10.Hines EA, Jones MK, Verheyden JM, Harvey JF, Sun X. Establishment of smooth muscle and cartilage juxtaposition in the developing mouse upper airways. Proc Natl Acad Sci U S A 110: 19444–19449, 2013. doi: 10.1073/pnas.1313223110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hogan BL, Barkauskas CE, Chapman HA, Epstein JA, Jain R, Hsia CC, Niklason L, Calle E, Le A, Randell SH, Rock J, Snitow M, Krummel M, Stripp BR, Vu T, White ES, Whitsett JA, Morrisey EE. Repair and regeneration of the respiratory system: complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell 15: 123–138, 2014. doi: 10.1016/j.stem.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hong KU, Reynolds SD, Giangreco A, Hurley CM, Stripp BR. Clara cell secretory protein-expressing cells of the airway neuroepithelial body microenvironment include a label-retaining subset and are critical for epithelial renewal after progenitor cell depletion. Am J Respir Cell Mol Biol 24: 671–681, 2001. doi: 10.1165/ajrcmb.24.6.4498. [DOI] [PubMed] [Google Scholar]
- 15.Horani A, Nath A, Wasserman MG, Huang T, Brody SL. Rho-associated protein kinase inhibition enhances airway epithelial Basal-cell proliferation and lentivirus transduction. Am J Respir Cell Mol Biol 49: 341–347, 2013. doi: 10.1165/rcmb.2013-0046TE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hysinger EB, Panitch HB. Paediatric Tracheomalacia. Paediatr Respir Rev 17: 9–15, 2016. [DOI] [PubMed] [Google Scholar]
- 17.Ito T, Newkirk C, Strum JM, McDowell EM. Modulation of glycogen stores in epithelial cells during airway development in Syrian golden hamsters: a histochemical study comparing concanavalin A binding with the periodic acid-Schiff reaction. J Histochem Cytochem 38: 691–697, 1990. doi: 10.1177/38.5.2332626. [DOI] [PubMed] [Google Scholar]
- 18.Iwamoto T, Yamada A, Yuasa K, Fukumoto E, Nakamura T, Fujiwara T, Fukumoto S. Influences of interferon-gamma on cell proliferation and interleukin-6 production in Down syndrome derived fibroblasts. Arch Oral Biol 54: 963–969, 2009. doi: 10.1016/j.archoralbio.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 19.Jain D, Atochina-Vasserman EN, Tomer Y, Kadire H, Beers MF. Surfactant protein D protects against acute hyperoxic lung injury. Am J Respir Crit Care Med 178: 805–813, 2008. doi: 10.1164/rccm.200804-582OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knight DA, Holgate ST. The airway epithelium: structural and functional properties in health and disease. Respirology 8: 432–446, 2003. doi: 10.1046/j.1440-1843.2003.00493.x. [DOI] [PubMed] [Google Scholar]
- 21.Kulkarni AB, Huh CG, Becker D, Geiser A, Lyght M, Flanders KC, Roberts AB, Sporn MB, Ward JM, Karlsson S. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci U S A 90: 770–774, 1993. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kulkarni AB, Karlsson S. Transforming growth factor-beta 1 knockout mice. A mutation in one cytokine gene causes a dramatic inflammatory disease. Am J Pathol 143: 3–9, 1993. [PMC free article] [PubMed] [Google Scholar]
- 23.Lin S, Na CL, Akinbi HT, Apsley KS, Whitsett JA, Weaver TE. Surfactant protein B (SP-B)−/− mice are rescued by restoration of SP-B expression in alveolar type II cells but not Clara cells. J Biol Chem 274: 19168–19174, 1999. doi: 10.1074/jbc.274.27.19168. [DOI] [PubMed] [Google Scholar]
- 24.Lin SS, Tzeng BH, Lee KR, Smith RJ, Campbell KP, Chen CC. Cav3.2 T-type calcium channel is required for the NFAT-dependent Sox9 expression in tracheal cartilage. Proc Natl Acad Sci U S A 111: E1990–E1998, 2014. doi: 10.1073/pnas.1323112111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu CH, Huang WS, Wang HH, Wu CP, Chian CF, Perng WC, Tsai CL. Airway obstruction due to tracheomalacia caused by innominate artery compression and a kyphotic cervical spine. Ann Thorac Surg 99: 685–687, 2015. doi: 10.1016/j.athoracsur.2014.04.119. [DOI] [PubMed] [Google Scholar]
- 26.Maeda T, Jikko A, Abe M, Yokohama-Tamaki T, Akiyama H, Furukawa S, Takigawa M, Wakisaka S. Cartducin, a paralog of Acrp30/adiponectin, is induced during chondrogenic differentiation and promotes proliferation of chondrogenic precursors and chondrocytes. J Cell Physiol 206: 537–544, 2006. doi: 10.1002/jcp.20493. [DOI] [PubMed] [Google Scholar]
- 27.Mango GW, Johnston CJ, Reynolds SD, Finkelstein JN, Plopper CG, Stripp BR. Clara cell secretory protein deficiency increases oxidant stress response in conducting airways. Am J Physiol Lung Cell Mol Physiol 275: L348–L356, 1998. [DOI] [PubMed] [Google Scholar]
- 28.Murayama MA, Kakuta S, Maruhashi T, Shimizu K, Seno A, Kubo S, Sato N, Saijo S, Hattori M, Iwakura Y. CTRP3 plays an important role in the development of collagen-induced arthritis in mice. Biochem Biophys Res Commun 443: 42–48, 2014. doi: 10.1016/j.bbrc.2013.11.040. [DOI] [PubMed] [Google Scholar]
- 29.Oh CD, Maity SN, Lu JF, Zhang J, Liang S, Coustry F, de Crombrugghe B, Yasuda H. Identification of SOX9 interaction sites in the genome of chondrocytes. PLoS One 5: e10113, 2010. doi: 10.1371/journal.pone.0010113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piccolo S. Developmental biology: Mechanics in the embryo. Nature 504: 223–225, 2013. doi: 10.1038/504223a. [DOI] [PubMed] [Google Scholar]
- 31.Qu Y, Yang Y, Ma D, Xiao W. Increased trefoil factor 3 levels in the serum of patients with three major histological subtypes of lung cancer. Oncol Rep 27: 1277–1283, 2012. doi: 10.3892/or.2012.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reynolds SD, Malkinson AM. Clara cell: progenitor for the bronchiolar epithelium. Int J Biochem Cell Biol 42: 1–4, 2010. doi: 10.1016/j.biocel.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 43: e47, 2015. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, Randell SH, Hogan BL. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci U S A 106: 12771–12775, 2009. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rönn T, Poulsen P, Hansson O, Holmkvist J, Almgren P, Nilsson P, Tuomi T, Isomaa B, Groop L, Vaag A, Ling C. Age influences DNA methylation and gene expression of COX7A1 in human skeletal muscle. Diabetologia 51: 1159–1168, 2008. doi: 10.1007/s00125-008-1018-8. [DOI] [PubMed] [Google Scholar]
- 36.Shi W, Oshlack A, Smyth GK. Optimizing the noise versus bias trade-off for Illumina whole genome expression BeadChips. Nucleic Acids Res 38: e204, 2010. doi: 10.1093/nar/gkq871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D, Annunziata N, Doetschman T. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature 359: 693–699, 1992. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh G, Katyal SL. Clara cells and Clara cell 10 kD protein (CC10). Am J Respir Cell Mol Biol 17: 141–143, 1997. doi: 10.1165/ajrcmb.17.2.f138. [DOI] [PubMed] [Google Scholar]
- 39.Smyth GK. Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Statistical Applications Genet Mol Biol 3: 1–25, 2004. doi: 10.2202/1544-6115.1027. [DOI] [PubMed] [Google Scholar]
- 40.Snijders D, Barbato A. An update on diagnosis of tracheomalacia in children. Eur J Pediatr Surg 25: 333–335, 2015. doi: 10.1055/s-0035-1559816. [DOI] [PubMed] [Google Scholar]
- 41.Spencer H. Spence's Pathology of the Lung (6th ed.). Cambridge, UK: Cambridge University Press, 2013. [Google Scholar]
- 42.Tekari A, Luginbuehl R, Hofstetter W, Egli RJ. Transforming growth factor beta signaling is essential for the autonomous formation of cartilage-like tissue by expanded chondrocytes. PLoS One 10: e0120857, 2015. doi: 10.1371/journal.pone.0120857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tomilov A, Bettaieb A, Kim K, Sahdeo S, Tomilova N, Lam A, Hagopian K, Connell M, Fong J, Rowland D, Griffey S, Ramsey J, Haj F, Cortopassi G. Shc depletion stimulates brown fat activity in vivo and in vitro. Aging Cell 13: 1049–1058, 2014. doi: 10.1111/acel.12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tompkins DH, Besnard V, Lange AW, Wert SE, Keiser AR, Smith AN, Lang R, Whitsett JA. Sox2 is required for maintenance and differentiation of bronchiolar Clara, ciliated, and goblet cells. PLoS One 4: e8248, 2009. doi: 10.1371/journal.pone.0008248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Turcatel G, Rubin N, Menke DB, Martin G, Shi W, Warburton D. Lung mesenchymal expression of Sox9 plays a critical role in tracheal development. BMC Biol 11: 117, 2013. doi: 10.1186/1741-7007-11-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vladar EK, Brody SL. Analysis of ciliogenesis in primary culture mouse tracheal epithelial cells. Methods Enzymol 525: 285–309, 2013. doi: 10.1016/B978-0-12-397944-5.00014-6. [DOI] [PubMed] [Google Scholar]
- 47.Volckaert T, Campbell A, Dill E, Li C, Minoo P, De Langhe S. Localized Fgf10 expression is not required for lung branching morphogenesis but prevents differentiation of epithelial progenitors. Development 140: 3731–3742, 2013. doi: 10.1242/dev.096560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Warburton D, Parton L, Buckley S, Cosico L, Saluna T. Effects of beta-2 agonist on hepatic glycogen metabolism in the fetal lamb. Pediatr Res 24: 330–332, 1988. doi: 10.1203/00006450-198809000-00011. [DOI] [PubMed] [Google Scholar]
- 49.Warburton D, Parton L, Buckley S, Cosico L, Saluna T. Effects of beta-2 agonist on metabolic regulation in the fetal lamb lung. J Pharmacol Exp Ther 242: 389–393, 1987. [PubMed] [Google Scholar]
- 50.Warburton D, Parton L, Buckley S, Cosico L, Saluna T. Effects of beta-2 agonist on tracheal fluid flow, surfactant and pulmonary mechanics in the fetal lamb. J Pharmacol Exp Ther 242: 394–398, 1987. [PubMed] [Google Scholar]
- 51.Warburton D, Parton L, Buckley S, Cosico L, Saluna T. Effects of glucose infusion on surfactant and glycogen regulation in fetal lamb lung. J Appl Physiol (1985) 63: 1750–1756, 1987. [DOI] [PubMed] [Google Scholar]
- 52.Wong GW, Krawczyk SA, Kitidis-Mitrokostas C, Revett T, Gimeno R, Lodish HF. Molecular, biochemical and functional characterizations of C1q/TNF family members: adipose-tissue-selective expression patterns, regulation by PPAR-gamma agonist, cysteine-mediated oligomerizations, combinatorial associations and metabolic functions. Biochem J 416: 161–177, 2008. doi: 10.1042/BJ20081240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yaswen L, Kulkarni AB, Fredrickson T, Mittleman B, Schiffman R, Payne S, Longenecker G, Mozes E, Karlsson S. Autoimmune manifestations in the transforming growth factor-beta 1 knockout mouse. Blood 87: 1439–1445, 1996. [PubMed] [Google Scholar]
- 54.Yeremenko N, Härle P, Cantaert T, van Tok M, van Duivenvoorde LM, Bosserhoff A, Baeten D. The cartilage protein melanoma inhibitory activity contributes to inflammatory arthritis. Rheumatology (Oxford) 53: 438–447, 2014. doi: 10.1093/rheumatology/ket382. [DOI] [PubMed] [Google Scholar]
- 55.Zhang W, Menke DB, Jiang M, Chen H, Warburton D, Turcatel G, Lu CH, Xu W, Luo Y, Shi W. Spatial-temporal targeting of lung-specific mesenchyme by a Tbx4 enhancer. BMC Biol 11: 111, 2013. doi: 10.1186/1741-7007-11-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.