Abstract
Air-liquid interface (ALI) culture of primary airway epithelial cells enables mucociliary differentiation providing an in vitro model of the human airway, but their proliferative potential is limited. To extend proliferation, these cells were previously transduced with viral oncogenes or mouse Bmi-1 + hTERT, but the resultant cell lines did not undergo mucociliary differentiation. We hypothesized that use of human BMI-1 alone would increase the proliferative potential of bronchial epithelial cells while retaining their mucociliary differentiation potential. Cystic fibrosis (CF) and non-CF bronchial epithelial cells were transduced by lentivirus with BMI-1 and then their morphology, replication kinetics, and karyotype were assessed. When differentiated at ALI, mucin production, ciliary function, and transepithelial electrophysiology were measured. Finally, shRNA knockdown of DNAH5 in BMI-1 cells was used to model primary ciliary dyskinesia (PCD). BMI-1-transduced basal cells showed normal cell morphology, karyotype, and doubling times despite extensive passaging. The cell lines underwent mucociliary differentiation when cultured at ALI with abundant ciliation and production of the gel-forming mucins MUC5AC and MUC5B evident. Cilia displayed a normal beat frequency and 9+2 ultrastructure. Electrophysiological characteristics of BMI-1-transduced cells were similar to those of untransduced cells. shRNA knockdown of DNAH5 in BMI-1 cells produced immotile cilia and absence of DNAH5 in the ciliary axoneme as seen in cells from patients with PCD. BMI-1 delayed senescence in bronchial epithelial cells, increasing their proliferative potential but maintaining mucociliary differentiation at ALI. We have shown these cells are amenable to genetic manipulation and can be used to produce novel disease models for research and dissemination.
Keywords: air-liquid interface, airway model, lung, mucociliary differentiation, primary ciliary dyskinesia
the ciliated epithelium lining the airways provides the first line of defense to inhaled pathogens and particles and plays a crucial role in many respiratory diseases. It is possible to remove respiratory epithelial cells from the nose or upper airways of donors by brushing and to culture them in the laboratory on collagen-coated, semipermeable membranes. The progenitor basal epithelial cells from the brushings cultured at air-liquid interface (ALI) differentiate into a fully ciliated, pseudostratified epithelium closely resembling that found in the airway (3).
If cells are obtained from a donor with a lung disease, e.g., cystic fibrosis, primary ciliary dyskinesia (PCD), asthma, and chronic obstructive pulmonary disease, these ALI cultures provide a surrogate model of the diseased lung for research into pathogenic mechanisms and for the development of new therapeutics (8, 14, 16). However, basal epithelial cells can only be passaged two to three times before they lose their proliferation and differentiation potential (6, 18). Thus, to establish the wider use of basal cells in ALI epithelial culture models, methods are required that enable basal cells to be cultured for longer, genetically engineered, expanded, and stored easily before differentiation on ALI cultures. Such cells would also overcome ethical issues related to repeated brushing of volunteers.
Recent approaches to extend the utility of primary, basal epithelial cells involved culturing them with Rho-associated protein kinase (ROCK) inhibitors on a layer of irradiated feeder cells to provide cell-derived growth factors (18, 27). The requirement for irradiated feeder cells makes the maintenance of basal cell cultures complex, time consuming, and difficult to scale up and may limit the use of this approach to specialist laboratories. Alternatively, induced pluripotent stem cells and embryonic stem cells were differentiated into mature respiratory epithelial cells and used to generate a pseudostratified epithelium expressing CFTR (30). However, the process takes several weeks and often the resulting cultures are not suitable for disease modeling as they are contaminated with endodermal cell types (31) and often present with karyotypic anomalies that may confound drug-screening efforts.
Extended proliferative potential of primary human bronchial epithelial (HBE) cells was described by transduction of basal cells with the mouse polycomb complex protein, Bmi-1, and human telomerase reverse transcriptase (hTERT) (6). Unlike cells transformed with viral oncogenes, Bmi-1+hTERT cell lines had no chromosomal abnormalities and produced a pseudostratified epithelium on ALI but gave only sparse ciliogenesis. This limited differentiation capacity may be explained by reports that hTERT, following long-term growth in culture, upregulates expression of the potent mitogen c-Myc, so promoting entry into the cell cycle (21) and thereby impeding ciliogenesis.
We hypothesized that transduction of BMI-1 alone may overcome these issues observed with Bmi-1+hTERT, to produce basal cells with the potential for extended proliferation that retain their differentiation capacity on ALI. In this study, BMI-1-transduced primary basal epithelial cells from cystic fibrosis (CF) and healthy donors were investigated for their morphology, growth characteristics, and karyotype. We also assessed the cells' mucociliary differentiation potential at ALI along with their Na+ and Cl− transport properties in Ussing chamber studies. We then demonstrate their use for the production of novel engineered disease models by shRNA knockdown of DNAH5, a gene associated with PCD, a ciliopathy with significant lung pathology resulting from abnormal mucociliary clearance. BMI-1 transduction offers a facile method to greatly extend the utility of basal epithelial cells for translational and basic research.
MATERIALS AND METHODS
Materials.
Primary antibodies used in this study can be found in Table 1. Secondary antibodies for immunofluorescence were anti-IgG antibodies conjugated with Alexa Fluor dyes (Invitrogen, Life Technologies). Secondary antibodies for Western blots were horseradish peroxidase (HRP)-conjugated anti-IgG antibodies (Dako, Agilent Technologies).
Table 1.
Primary antibodies used in this study
| Name | Supplier | Dilution WB/IF |
|---|---|---|
| Anti-MUC5AC | Life Technologies | NA/1:100 |
| Anti-acetylated α-tubulin | Sigma-Aldrich | NA/1:500 |
| Anti-BMI-1 | Life Technologies | 1:200/1:100 |
| Anti-GAPDH | Life Technologies | 1:1,000/1:500 |
| Anti-MUC5B | Kind gift from Professor Dallas Swallow (23) | NA/neat |
| Anti-occludin | Invitrogen, Life Technologies | NA/1:100 |
| Anti-p16INK4 | PharMingen, BD Biosciences | 1:200/NA |
| Anti-p63 | Invitrogen, Life Technologies | NA/1:100 |
WB, Western blot; IF, immunofluorescence; NA, not applicable.
Collagen coating.
Tissue culture flasks and Transwells were coated for 1 h at room temperature with 1% (vol/vol) solution of a 3 mg/ml bovine collagen solution (PureCol; Advanced Biomatrix) in phosphate-buffered saline (PBS), then washed with distilled water and air-dried.
Cell culture.
HEK293T cells were cultured in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% (vol/vol) fetal bovine serum. Normal human bronchial epithelial (NHBE) cells, cystic fibrosis human bronchial epithelial cells (CFBE) cells were grown on collagen-coated plastic in bronchial epithelial growth media (BEGM; Lonza). All cells were grown at 37°C and 5% CO2. NHBE and CFBE cells were purchased from Lonza and Epithelix SàRL.
Lentivirus production and transduction.
Full-length human BMI-1 cDNA was PCR cloned from pHR-EF1α-BMI1-IRES-GFP plasmid (20) with XhoI and BamHI sites added and TOPO cloned into pCR4 TOPO vector before being subcloned into pLVX-Puro vector digested with XhoI and BamHI. Lentivirus was produced as previously described (20), concentrated by centrifugation at 4,500 g for 18 h at 4°C, resuspended in OptiMem, and added to cell media to transduce NHBE and CFBE cells (Lonza) at passage 2.
Doubling time analysis.
NHBE and NHBE BMI-1 cells at varying passage numbers were seeded at densities of 30,000 cells per well onto collagen-coated 12-well plates. Cells were detached using trypsin-EDTA following 1–4 days in culture and total cell numbers per well were counted using a hemocytometer. An online calculator was used to calculate the doubling time (Roth V. 2006 Doubling Time Computing, available from: http://www.doubling-time.com/compute.php). Doubling times were calculated using the formula
where cell count values were mean cell counts of three independent wells.
Western blotting.
Cells were lysed with Cell Extraction Buffer (Life Technologies), boiled in the presence of NuPage LDS Sample Buffer (Life Technologies), and loaded onto NuPage Novex 4–12% Bis-Tris gels (Life Technologies). Electrophoresis and protein transfer onto Immobilon-P polyvinylidene fluoride membranes were performed using standard protocols. Antibodies against BMI-1, p16Ink4a, and GAPDH and appropriate HRP-conjugated secondary antibodies were used for probing with bands visualized using Pierce ECL Western Blotting Substrate (Life Technologies, Paisley, UK) and a UVIchemi chemiluminescence imaging system (UVItec).
ALI culture.
Cells grown to ~80% confluence in T75 flasks were trypsinized, seeded at a density of 900,000 cells/cm2 on Transwell inserts (Corning), and grown at an ALI as previously described (8). Cells were maintained at an ALI for 4 wk before analysis was performed.
qRT-PCR.
Unless indicated, all reagents for quantitative RT-PCR (qRT-PCR) were obtained from ThermoFisher. Total RNA was harvested from cells using RNeasy Mini Kit (Qiagen) and potential DNA impurities digested using DNase I enzyme (TURBO DNA-free kit). Purified RNA was reverse transcribed with 2.5 U/µl murine leukemia virus (MuLV) reverse transcriptase at 42°C for 1 h in a reaction containing 1× GeneAmp PCR Gold Buffer, 1 mM each dNTP, 5 µM random hexamers, 5 mM MgCl2, and 1 U/µl RNase inhibitor. The resulting cDNA was used in a qPCR reaction containing 1× Platinum Quantitative PCR SuperMix-UDG with ROX and 1× TaqMan Gene Expression Assay primer/probe set (GAPDH primer/probe set Hs99999905_m1; DNAH5 primer/probe set Hs00292485_m1). The PCR reaction cycles used were 50°C for 2 min, 95°C for 10 min, and 40 cycles of 95°C for 15 s and 60°C for 1 min on an ABI PRISM 7000 Sequence Detection System (Applied Biosystems). Fluorescence data were collected at the end of each 60°C reaction and relative expression levels were calculated by the delta-delta Ct (2−ΔΔCt) method (19).
Immunofluorescence staining and confocal microscopy.
Cells were fixed with 4% paraformaldehyde for 10 min at room temperature, washed with PBS, and permeabilized with PBS-Triton [PBS 0.1% (vol/vol) Triton X-100] for 10 min at room temperature before blocking, immunostaining, and mounting on microscope slides as previously described (26). Images were obtained using an Inverted Zeiss LSM 710 Confocal microscope with the appropriate excitation lasers selected for the dyes used.
Fluorescence microscopy.
Bright-field and fluorescence images were captured with a Nikon Digital Sight DS-QiMC video camera attached to a Nikon Eclipse Ti-U inverted microscope. Videos and images were processed using NIS Elements AR software (Nikon, v4.00.12).
Transmission electron microscopy for cilia ultrastructure.
Ciliated cells cultured at an ALI were scraped and cells washed off with 200 µl warmed BEBM. Cells were fixed by addition of 2 ml of 2.5% glutaraldehyde and stored at 4°C for at least 24 h before further processing as previously described (24). Assessment of cilia ultrastructure was undertaken blinded by Dr. Amelia Shoemark, a member of the PCD diagnostic service team at the Royal Brompton & Harefield NHS Foundation Trust.
High-speed video microscopy.
High-speed video was recorded using a MotionPro X4 high-speed motion camera attached to a Nikon Eclipse Ti-U inverted microscope built with an environmental chamber. Videos were recorded at a frame rate of 500 frames/s using Motion Studio software (IDT Vision, v2.11) with cells maintained at 37°C.
For cilia beat frequency (CBF) assessment, ALI cultures were washed twice with PBS to remove mucus that may have affected CBF. After washing, the cells were allowed to equilibrate at 37°C and 5% CO2 for 20 min before video recording. At least four independent cultures per donor line were videoed with five areas recorded per culture, i.e., at least 20 videos were captured per donor line. To minimize bias videos were recorded from the top, bottom, left, right, and center regions of each culture and CBF was assessed by use of CiliaFA software (25).
Electrophysiology studies.
Cells were grown at ALI for 4 wk on Snapwell membranes (Corning) to enable mucociliary differentiation. Snapwells were then mounted on Ussing chambers and short circuit current (Isc) was measured as previously described (32). Briefly, monolayers were mounted in Ussing chambers in physiological salt solution consisting of 117 mM NaCl, 25 mM NaHCO3, 4.7 mM KCl, 1.2 mM MgSO4, 1.2 mM KH2PO4, 2.5 mM CaCl2, and 11 mM d-glucose. The solution was continuously circulated throughout the course of the experiment and maintained at 37°C while bubbled with 21% O2 + 5% CO2 premixed gas. Monolayers were first maintained under open-circuit conditions until transepithelial potential difference (Vt) and resistance stabilized. The cells were then short-circuited by clamping Vt at 0 mV using a DVC-4000 voltage/current clamp, and Isc was measured and recorded using a PowerLab computer interface. Every 30 s the preparations were returned to open-circuit conditions for 3 s so that the spontaneous Vt could be measured and transepithelial electrical resistance (TEER) calculated. Drugs were circulated in physiological salt solution and added in the order of amiloride (10 μM, apical), forskolin (25 μM, apical and basolateral), and GlyH-101 (10 μM, apical).
RESULTS
Characterization of BMI-1-transduced cells in submerged culture.
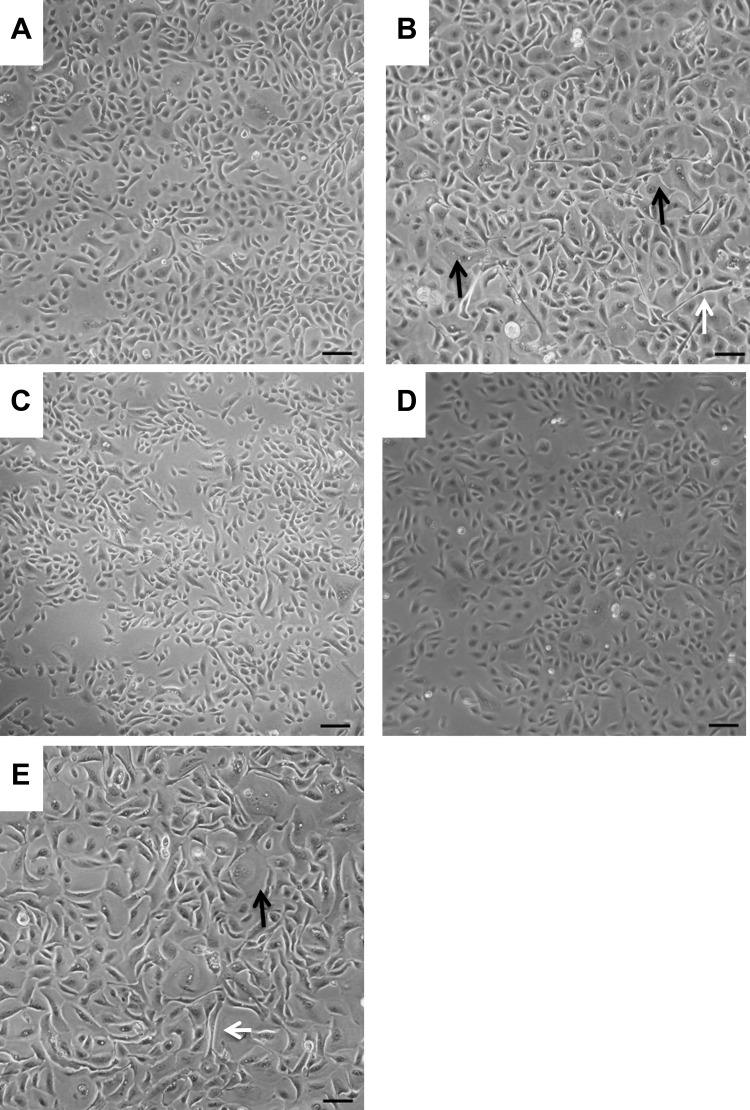
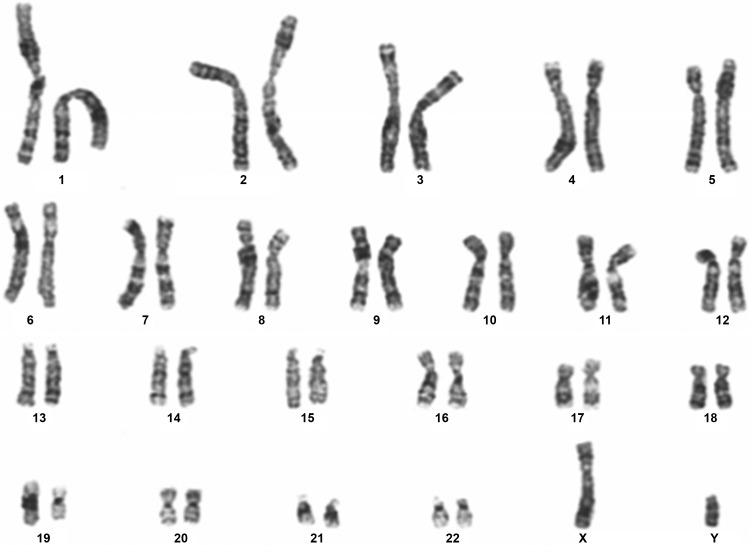
Primary NHBE cells maintained in submerged cultures displayed a characteristic cobblestone appearance (Fig. 1A) but by passage 3 cells became elongated in appearance (white arrow; Fig. 1B) and squamous differentiation was evident (black arrow; Fig. 1B). In contrast, BMI-1-transduced NHBE cells (NHBE-BMI-1) maintained their cobblestone appearance following extensive passaging, for example at passage 11 (Fig. 1C) and passage 17 (Fig. 1D). However, squamous cells became evident following 25 passages (Fig. 1E) after which the cells senesced, with no observable cell division for 10 days. The cells maintained a normal diploid karyotype even at passage 23 (Fig. 2).
Fig. 1.
BMI-1 maintains healthy cell morphology in 2D culture. The morphology of NHBE cells at passage 1 (A) and passage 3 (B) was observed under light microscopy and compared with NHBE BMI-1 cells after passages 11 (C), 17 (D), and 25 (E). White arrows highlight elongated cells and black arrows highlight squamous cells. Scale bars are 100 µm.
Fig. 2.
Karyotype analysis of NHBE-BMI-1 cells. Karyotype of passage 23 NHBE-BMI-1 cells was undertaken by The Doctors Laboratory, London.
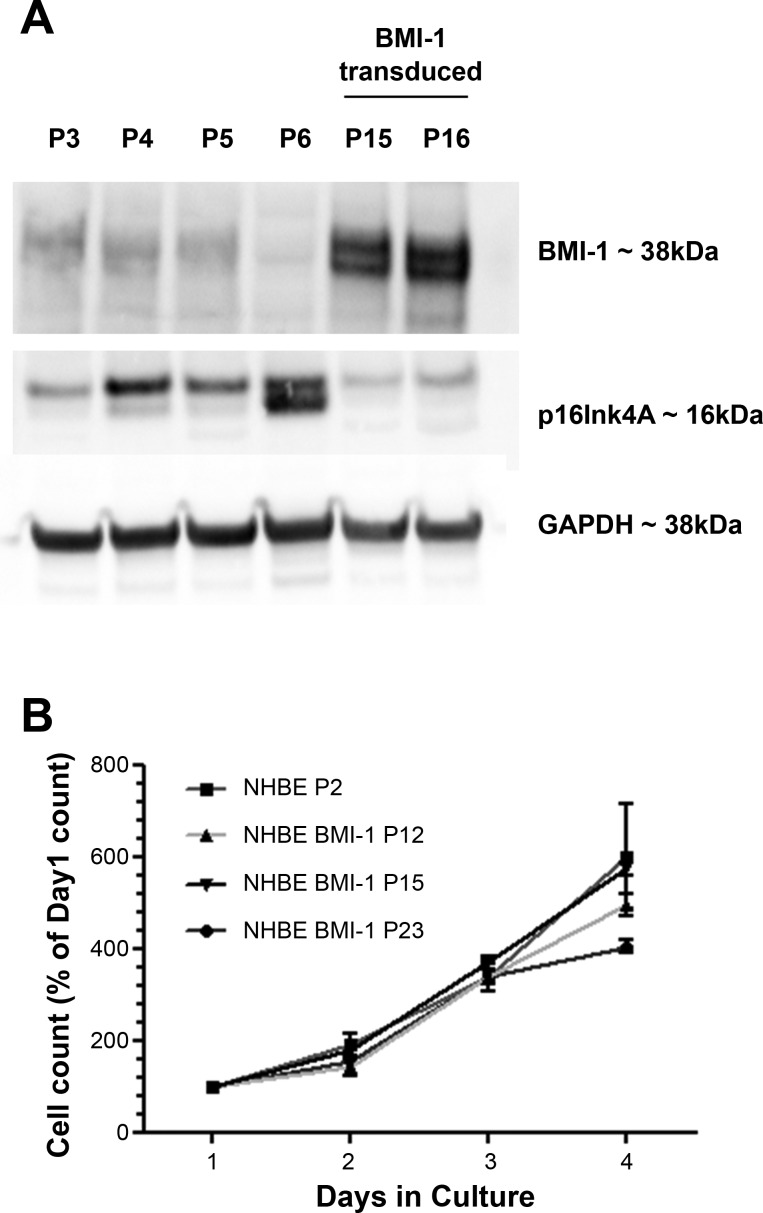
BMI-1 downregulates expression of the prosenescent protein p16Ink4A. NHBE cells transduced with BMI-1 had low levels of p16Ink4A protein and high levels of BMI-1 (Fig. 3A). Levels of BMI-1 in untransduced NHBE cells declined with an increase in passaging while levels of p16Ink4A increased and were higher in senescent, untransduced NHBE cells at passage 6 while BMI-1 expression was not evident by Western blot (Fig. 3A).
Fig. 3.
Elevated p16Ink4a precedes senescence and BMI-1 functions by inhibiting p16Ink4a and retains a normal cell doubling time. Western blot was used to assess levels of BMI-1 and p16Ink4A in serially passaged NHBE cells and BMI-1-transduced cells (A), and cell counting was used to determine the replication kinetics of NHBE and NHBE BMI-1 cells at varying passages (B). Growth curves are presented as percent of mean of day 1 cell count. Data are means ± SE. For each data point n = 3 biological replicates.
SV40 large T-antigen or ROCK inhibition extends the replication potential of basal cells but alters the proliferation rate of the cells (4, 6, 12); therefore we assessed the doubling times of BMI-1-transduced cells at different passages (Fig. 3B). We determined that untransduced cells at passage 2 had a doubling time of 1.18 days, similar to BMI-1-transduced cells at passages 12 and 15 (doubling times of 1.25 and 1.21 days, respectively) although by passage 23 the doubling time had increased to 1.49 days, consistent with observations of senescence at passage 25.
Differentiation of NHBE-BMI-1 cells.
NHBE-BMI-1 basal cells were subsequently analyzed for their differentiation potential when cultured at ALI. After 2 −3 wk culture, both primary NHBE and NHBE-BMI-1 cells produced motile cilia (Supplemental Video S1, A and B, respectively; Supplemental Material for this article is available online at the Journal website). NHBE-BMI-1 cells maintained the ability to differentiate and produce cilia even at passage 15.
To quantify cilia function, we assessed CBF of both primary and BMI-1-transduced NHBE and CFBE cells. Beating cilia from CFBE cells could not be detected, most likely due to the build-up of viscous mucus hindering cilia beating, until cultures were washed. As such, CFBE and NHBE cultures were washed twice before video recording and CBF analysis as detailed in materials and methods.
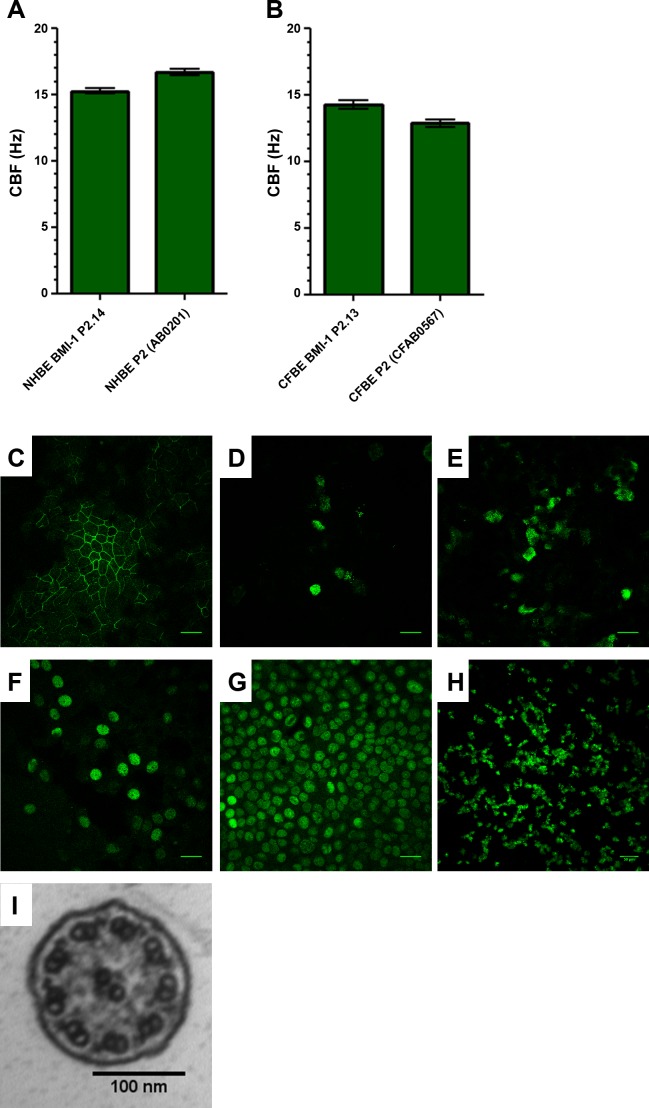
CBF analysis of both primary and BMI-1-transduced NHBE and CFBE cells showed mean values within the normal range for respiratory cilia of 9–17 Hz (25) (Fig. 4, A and B). Primary NHBE and NHBE-BMI-1 cells had a CBF of 16.7 ± 0.2 Hz and 15.3 ± 0.2 Hz, respectively (Fig. 4A) and primary CFBE and CFBE-BMI-1 cells exhibited CBF values of 12.9 ± 0.3 Hz and 14.3 ± 0.3 Hz, respectively.
Fig. 4.
BMI-1 cells retain their mucociliary differentiation capacity. Extensively passaged BMI-1-transduced cells (passage 15) were differentiated on ALI and cilia beat frequency of NHBE (A) and CFBE (B) cells was determined using ciliaFA plugin (25) for ImageJ. Data are means ± SE; n = 4 independent ALI cultures, 5 fields videoed per culture. Immunostaining of NHBE-BMI-1 cells was used to show tight junction formation (occludin; C), mucin production (MUC5AC and MUC5B; D and E, respectively), the presence of basal cells (p63+; F), widespread BMI-1 expression (BMI-1; G), and extensive ciliation (acetylated α-tubulin; H). TEM was used to determine cilia ultrastructure (I). Images are representative of 4 independent ALI cultures per marker. Scale bars for C–H are 50 µm and 100 nm for I.
Further evidence of differentiation was demonstrated by immunodetection, in NHBE-BMI-1 cells, of the tight junction protein occludin (Fig. 3C) and the mucins MUC5AC and MUC5B (Fig. 4, D and E). In addition, basal cells were present and indicated by p63 staining (Fig. 4F) and BMI-1 protein was present in all nuclei (Fig. 4G). The ciliary protein, acetylated α-tubulin, was also detected by immunostaining and highlighted abundant ciliation (Fig. 4H). Further analysis of the cilia in differentiated NHBE-BMI-1 cells by transmission electron microscopy (TEM) showed that they had a normal 9+2 ultrastructure with both inner and outer dynein arms present (Fig. 4I; Tables 2 and 3).
Table 2.
Microtubule organization of motile cilia
| Microtubule Organization | Frequency, % |
|---|---|
| Normal 9+2 | 92.05 |
| Central pair defect | 0.66 |
| Disarranged | 3.31 |
| Other defect | 3.97 |
Table 3.
Dynein arm presence in motile cilia
| Dynein Arms | Frequency, % |
|---|---|
| ODA and IDA present | 100.00 |
| ODA only | 0.00 |
| IDA only | 0.00 |
| ODA and IDA absent | 0.00 |
ODA, outer dynein arms; IDA, inner dynein arms.
Electrophysiology studies.
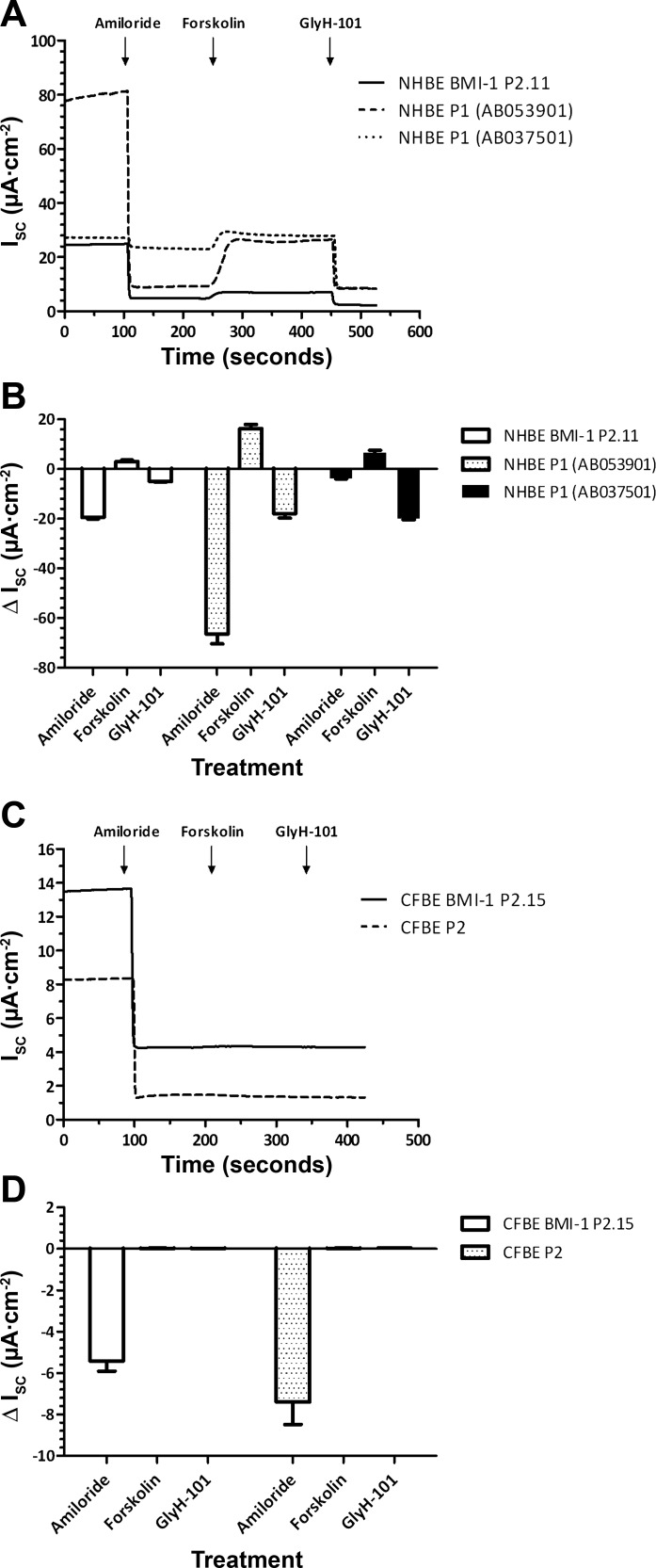
Primary HBE cells grown on ALI develop a TEER with ion transport properties that can be measured by mounting of cultured epithelia on Ussing chambers and addition of drugs that can activate or inhibit specific cell surface ion channels. Cultures of primary NHBE cells from two different donors showed baseline TEER values of 331.1 ± 105.5 and 621.0 ± 33.2 Ω·cm2 (Table 4) and primary CFBE cells developed TEER of 1,307.9 ± 36.6 Ω·cm2. Similarly, BMI-1-transduced NHBE and CFBE cells developed high TEER when grown at an ALI (1268.4 ± 78.4 Ω·cm2 and 917.6 ± 165.3 Ω·cm2, respectively; Table 4), demonstrating the cells retained their ability to form an electrically resistive epithelium.
Table 4.
TEER measurements
| Name | Passage | TEER, Ω·cm2 | n |
|---|---|---|---|
| NHBE (AB053901) | P1 | 621.0 ± 33.2 | 5 |
| NHBE (AB037501) | P1 | 331.1 ± 105.5 | 3 |
| NHBE BMI-1 | P13 | 1,268.4 ± 78.4 | 4 |
| CFBE | P2 | 1,307.9 ± 36.6 | 5 |
| CFBE BMI-1 | P17 | 917.6 ± 165.3 | 6 |
Values are means ± SE. TEER, transepithelial electrical resistance.
Isc analysis in Ussing chambers of NHBE and CFBE cells revealed that both primary NHBE cells and passage 13 NHBE-BMI-1 cells cultured at ALI also had similar electrophysiology. Amiloride (10 μM), an inhibitor of the epithelial Na+ channel ENaC, reduced Isc in all cultures, although the amiloride-sensitive Isc was variable. Subsequent elevation of cellular cAMP with forskolin (25 μM) increased Isc, and this elevation was inhibited by the CFTR inhibitor Gly-H101 (10 μM) (Fig. 5, A and B). Thus ENaC and CFTR-mediated ion transport was retained in NHBE-BMI-1 cells. Primary CFBE cells and passage 17 CFBE BMI-1 cells cultured at ALI also exhibited amiloride-inhibitable Isc but no response to either forskolin or GlyH-101 was observed, as expected due to the lack of CFTR in these cells (Fig. 5, C and D). Thus, CFBE-BMI-1 cells, like NHBE-BMI-1, also maintain the Na+ and Cl− ion transport characteristics of nontransduced primary CF cells.
Fig. 5.
BMI-1 cells form ALI cultures suitable for Ussing chamber studies. Representative Ussing chamber traces and changes in short-circuit current (Isc) in response to administration of amiloride (apical), forskolin (apical and basolateral), and GlyH-101 (apical) in primary and BMI-1-transduced NHBE (A and B) and CFBE (C and D) cells are shown. Data are means ± SE; n = at least 3 independent ALI cultures (see Table 4 for exact values).
Use of BMI-1-transduced cells to generate PCD cell models.
We next explored the potential use of the BMI-1-transduced NHBE cells to generate an in vitro model of PCD. The outer dynein arm protein DNAH5 is the most commonly mutated gene but even so this is a rare disease and cells are often not readily available. Cells with DNAH5 mutations lack the DNAH5 protein in the ciliary axoneme and have missing outer dynein arms (13). NHBE cells transduced with BMI-1 were additionally transduced with a DNAH5 shRNA lentiviral construct that also expresses green fluorescent protein (GFP).
DNAH5 expression in shRNA-transduced cells was silenced by ~75% relative to untransduced cells (Fig. 6A) while scrambled shRNA had no effect on DNAH5 expression indicating silencing specificity.
Fig. 6.
DNAH5 knockdown recapitulates PCD phenotype. A: qRT-PCR was used to assess DNAH5 mRNA expression in NHBE-BMI-1 cells and NHBE BMI-1-transduced with lentivirus expressing either a scrambled or DNAH5-targeting shRNA and grown in submerged 2D culture. **P < 0.01; 1-way ANOVA with Bonferroni’s posttest used to assess significance. Data are means ± SE. B: immunostaining for DNAH5 and acetylated α-tubulin was used to assess the presence or absence of DNAH5 in the ciliary axoneme of shRNA-transduced and untransduced NHBE BMI-1 cells differentiated at ALI. Presence of GFP fluorescence denotes cells transduced with the GFP-shRNA construct and so expressing the shRNA. Scale bars are 20 µm. Images are representative of 4 independent ALI cultures per condition.
NHBE-BMI-1 cells transduced with the two shRNAs were subsequently cultured at ALI to promote differentiation and ciliation. Following mucociliary differentiation, NHBE-BMI-1 GFP-positive cells transduced with scrambled shRNA had motile cilia, (Supplemental Video S2A) whereas GFP-positive DNAH5 shRNA-silenced cells had immotile cilia (Supplemental Video S2B). However, in GFP-negative cells (and by extension also DNAH5 shRNA negative), motile cilia were still observed (Supplemental Video S2C).
In untransduced NHBE-BMI-1 cells and those GFP-positive cells transduced with the scrambled shRNA, DNAH5 was localized to the ciliary axoneme in all ciliated cells assessed as shown by colocalization with acetylated α-tubulin expression. In contrast, in DNAH5 shRNA-transduced GFP-positive cells, only 2.9% (5/173) of ciliated cells had DNAH5 in the ciliary axoneme (Fig. 6B and Table 5).
Table 5.
DNAH5 localization
| Is DNAH5 Located in Ciliary Axoneme? |
||
|---|---|---|
| shRNA Target | Yes | No |
| Untransduced | 157 | 0 |
| Scrambled | 147 | 0 |
| DNAH5 | 5 | 173 |
DISCUSSION
Airway diseases are a significant cause of morbidity and mortality. Mucociliary differentiation of primary airway epithelial cells using ALI culture methods provides an in vitro model that faithfully recapitulates the in vivo airway epithelium for the study of disease pathology and therapies. However, these cells can only be cultured for two to three passages before they lose their ability to differentiate (4). This has important practical, ethical, and cost implications for research in the field. Traditional cell transformation methods, using viral oncogenes that promote entry into the cell cycle, produce immortal cell lines incapable of mucociliary differentiation most likely due to their inability to suspend cell division and allow cilia production and differentiation.
We have shown that prevention of cellular senescence by expression of BMI-1 allows extended passaging of HBE cells from CF and non-CF donors. Western blot analysis highlighted that senescent primary NHBE cells had accumulated high levels of the prosenescent protein p16Ink4a in agreement with other studies (1, 6, 20). BMI-1-transduced cells, however, showed low levels of p16Ink4a, thereby delaying cell senescence as reported previously (15).
In addition to exhibiting delayed senescence, BMI-1-transduced cells retained their cell phenotype, karyotype, ion transport characteristics, and mucociliary differentiation potential with abundant ciliation observed when cultured at ALI. Ussing chamber studies revealed that, like primary HBE cells, BMI-1-transduced NHBE and CFBE cells formed electrically resistive cultures and the direction of change in Isc was as expected upon addition of amiloride, forskolin, and the CFTR inhibitor Gly-H101. We note that baseline TEER values varied between HBE donors as did the magnitude of change in Isc upon addition of amiloride, forskolin, and the CFTR inhibitor Gly-H101. Such variation has also been observed by Tosoni et al. (29), who recently demonstrated baseline TEER values ranged from 309 to 2,963 Ω·cm2 in ALI cultures generated from the cells of 18 healthy donors.
In agreement with our findings, Torr et al. (28) recently demonstrated that transduction of basal cells, from two different donors, with human BMI-1 alone extends the proliferative potential of NHBE cells while retaining their differentiation potential as demonstrated by immunostaining and scanning electron microscopy. Our study extends on these findings demonstrating that passaging capacity of diseased cells (CFBE) can also be extended by this method. Taken together this would suggest BMI-1 transduction of bronchial epithelial cells permits extended passaging and mucociliary differentiation independent of donor and/or disease status although further studies are needed to confirm this.
BMI-1 transduction did not immortalize the HBE cells in contrast to viral antigens such as the SV40 large T-antigen used to produce the 16HBE14o− cell line (4). However, BMI-transduced cells could still be differentiated at 20–25 passages representing a significant advantage of this method over use of viral antigens. Using the ALI culture protocol outlined in the present study one can routinely obtain six to eight functional epithelial Transwells in a 24-well ALI culture format per passage, enabling the generation of a minimum of ~90–100 Transwells from a single donor. This is significantly higher than the 10–15 epithelial Transwells that can be generated with ~1×106 primary bronchial epithelial cells (typical quantity obtained from commercial providers) or brushing of the nasal turbinate of a single donor (29). Furthermore, subculturing of BMI-1 transformed cells, as opposed to seeding ALI cultures, would enable banking of early passage cells and the potential to generate exponentially more functional epithelia at each passage.
Tosoni et al. (29) recently demonstrated that ALI cultures generated from different healthy donors can yield epithelia with vastly different physiological properties and drug responses. The BMI-1 transduction protocol enables the generation of a large number of epithelia generated from donors with similar genetic backgrounds, or indeed from a single donor, allowing the study of disease pathophysiology in a manner that avoids the influence of genetic variability in cells from different donors. This highlights the potential for the development of personalized treatments using BMI-1-transduced cells.
In addition, an extended passaging capacity affords the opportunity for modification of HBE cells to create new models, to better understand disease and find novel treatments. As a proof of concept, we transduced NHBE-BMI-1 cells with shRNA targeted against DNAH5 in an attempt to create a model of PCD. The shRNA construct contained a GFP reporter to allow for selection of cells in which the DNAH5 shRNA was expressed. Focusing on cells expressing GFP, we demonstrated loss of ciliary motility and absence of DNAH5 in the ciliary axoneme of cells transduced with the DNAH5 targeted shRNA so mimicking the phenotype seen in patient cells (13). shRNA-mediated knockdown has been previously used to model PCD in otherwise healthy primary HBE cells (10, 11, 17), but these cells were not long lived so could not be used for further study to assess, for example, protein interactions or novel treatments. Gene addition, shRNA knockdown, or genome editing of BMI-1-transduced HBE cells could therefore provide a more useful tool for the study of a number of airway diseases.
Recently the use of pharmacological Rho-kinase inhibition along with coculture of HBE cells with irradiated feeder-layer fibroblasts has been described to allow indefinite passage of HBE cells while retaining the cells differentiation capacity when placed at ALI (18, 27). However, studies where the mucociliary differentiation potential of CRCs have been assessed have not reported successful mucociliary differentiation beyond passage 11 (2, 22, 27). Furthermore, CRC morphology and doubling times differ significantly to their parent cells with CRC cells being smaller and growing in colonies as well as showing faster proliferation rates (18, 27). Following viral transduction, BMI-1-expressing NHBE and CFBE cells are cultured exactly as nontransformed primary cells, without the need for a feeder layer, a factor that is likely to aid in the rapid uptake of this method of transformation and dissemination of the resulting cell models between laboratories and in the maintenance of cells in biobanks.
In summary, here we have shown that BMI-1 transduction delays senescence in HBE cells from healthy and CF donors while maintaining the cells' mucociliary differentiation potential. We have undertaken extensive characterization of the differentiated cells showing normal ciliary beat frequency and ciliary ultrastructure. Ussing chamber studies with BMI-1 transformed NHBE and CFBE cells showed that these cells exhibit similar Na+ and Cl− ion transport characteristics to their respective primary cells, validating their use as models of CF. Furthermore, we have demonstrated how BMI-1-transduced cells can be engineered by further transduction with DNAH5-targeted shRNA to recapitulate an in vitro disease model of primary ciliary dyskinesia, a valuable feature when studying rare diseases such as PCD where patient samples are difficult to obtain.
GRANTS
This study was funded by the Great Ormond Street Hospital Children’s Charity and the Child Health Research Appeal Trust and was supported by the National Institute for Health Research Biomedical Research Centre at Great Ormond Street Hospital for Children NHS Foundation Trust and University College London.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
M.M.M., A.S., R.A.H., and J.M.D. performed experiments; M.M.M., D.L.B., and S.L.H. analyzed data; M.M.M., A.S., C.O., D.L.B., S.J.H., and S.L.H. interpreted results of experiments; M.M.M. and A.S. prepared figures; M.M.M., D.L.B., and S.L.H. drafted manuscript; M.M.M., A.S., R.A.H., T.V.S., T.R.M., C.O., D.L.B., S.J.H., and S.L.H. edited and revised manuscript; M.M.M., A.S., R.A.H., J.M.D., T.V.S., T.R.M., C.O., D.L.B., S.J.H., and S.L.H. approved final version of manuscript.
Supplementary Material
REFERENCES
- 1.Brookes S, Rowe J, Gutierrez Del Arroyo A, Bond J, Peters G. Contribution of p16(INK4a) to replicative senescence of human fibroblasts. Exp Cell Res 298: 549–559, 2004. doi: 10.1016/j.yexcr.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 2.Butler CR, Hynds RE, Gowers KH, Lee DH, Brown JM, Crowley C, Teixeira VH, Smith CM, Urbani L, Hamilton NJ, Thakrar RM, Booth HL, Birchall MA, De Coppi P, Giangreco A, O’Callaghan C, Janes SM. Rapid expansion of human epithelial stem cells suitable for airway tissue engineering. Am J Respir Crit Care Med 194: 156–168, 2016. doi: 10.1164/rccm.201507-1414OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chu Q, Tousignant JD, Fang S, Jiang C, Eastman SJ, Chen LH, Cheng SH, Scheule RK. Binding and uptake of cationic lipid:pDNA complexes by polarized airway epithelial cells. Hum Gene Ther 10: 25–36, 1999. doi: 10.1089/10430349950019165. [DOI] [PubMed] [Google Scholar]
- 4.Cozens AL, Yezzi MJ, Kunzelmann K, Ohrui T, Chin L, Eng K, Finkbeiner WE, Widdicombe JH, Gruenert DC. CFTR expression and chloride secretion in polarized immortal human bronchial epithelial cells. Am J Respir Cell Mol Biol 10: 38–47, 1994. doi: 10.1165/ajrcmb.10.1.7507342. [DOI] [PubMed] [Google Scholar]
- 6.Fulcher ML, Gabriel SE, Olsen JC, Tatreau JR, Gentzsch M, Livanos E, Saavedra MT, Salmon P, Randell SH. Novel human bronchial epithelial cell lines for cystic fibrosis research. Am J Physiol Lung Cell Mol Physiol 296: L82–L91, 2009. doi: 10.1152/ajplung.90314.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirst RA, Rutman A, Williams G, O’Callaghan C. Ciliated air-liquid cultures as an aid to diagnostic testing of primary ciliary dyskinesia. Chest 138: 1441–1447, 2010. doi: 10.1378/chest.10-0175. [DOI] [PubMed] [Google Scholar]
- 10.Horani A, Brody SL, Ferkol TW, Shoseyov D, Wasserman MG, Ta-shma A, Wilson KS, Bayly PV, Amirav I, Cohen-Cymberknoh M, Dutcher SK, Elpeleg O, Kerem E. CCDC65 mutation causes primary ciliary dyskinesia with normal ultrastructure and hyperkinetic cilia. PLoS One 8: e72299, 2013. doi: 10.1371/journal.pone.0072299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horani A, Ferkol TW, Shoseyov D, Wasserman MG, Oren YS, Kerem B, Amirav I, Cohen-Cymberknoh M, Dutcher SK, Brody SL, Elpeleg O, Kerem E. LRRC6 mutation causes primary ciliary dyskinesia with dynein arm defects. PLoS One 8: e59436, 2013. doi: 10.1371/journal.pone.0059436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horani A, Nath A, Wasserman MG, Huang T, Brody SL. Rho-associated protein kinase inhibition enhances airway epithelial Basal-cell proliferation and lentivirus transduction. Am J Respir Cell Mol Biol 49: 341–347, 2013. doi: 10.1165/rcmb.2013-0046TE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hornef N, Olbrich H, Horvath J, Zariwala MA, Fliegauf M, Loges NT, Wildhaber J, Noone PG, Kennedy M, Antonarakis SE, Blouin JL, Bartoloni L, Nüsslein T, Ahrens P, Griese M, Kuhl H, Sudbrak R, Knowles MR, Reinhardt R, Omran H. DNAH5 mutations are a common cause of primary ciliary dyskinesia with outer dynein arm defects. Am J Respir Crit Care Med 174: 120–126, 2006. doi: 10.1164/rccm.200601-084OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hussain S, Ji Z, Taylor AJ, DeGraff LM, George M, Tucker CJ, Chang CH, Li R, Bonner JC, Garantziotis S. Multiwalled carbon nanotube functionalization with high molecular weight hyaluronan significantly reduces pulmonary injury. ACS Nano 10: 7675–7688, 2016. doi: 10.1021/acsnano.6b03013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobs JJ, Kieboom K, Marino S, DePinho RA, van Lohuizen M. The oncogene and Polycomb-group gene bmi-1 regulates cell proliferation and senescence through the ink4a locus. Nature 397: 164–168, 1999. doi: 10.1038/16476. [DOI] [PubMed] [Google Scholar]
- 16.Kesimer M, Kirkham S, Pickles RJ, Henderson AG, Alexis NE, Demaria G, Knight D, Thornton DJ, Sheehan JK. Tracheobronchial air-liquid interface cell culture: a model for innate mucosal defense of the upper airways? Am J Physiol Lung Cell Mol Physiol 296: L92–L100, 2009. doi: 10.1152/ajplung.90388.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y, Yagi H, Onuoha EO, Damerla RR, Francis R, Furutani Y, Tariq M, King SM, Hendricks G, Cui C, Saydmohammed M, Lee DM, Zahid M, Sami I, Leatherbury L, Pazour GJ, Ware SM, Nakanishi T, Goldmuntz E, Tsang M, Lo CW. DNAH6 and its interactions with PCD genes in heterotaxy and primary ciliary dyskinesia. PLoS Genet 12: e1005821, 2016. doi: 10.1371/journal.pgen.1005821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu X, Ory V, Chapman S, Yuan H, Albanese C, Kallakury B, Timofeeva OA, Nealon C, Dakic A, Simic V, Haddad BR, Rhim JS, Dritschilo A, Riegel A, McBride A, Schlegel R. ROCK inhibitor and feeder cells induce the conditional reprogramming of epithelial cells. Am J Pathol 180: 599–607, 2012. doi: 10.1016/j.ajpath.2011.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25: 402–408, 2001. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.McKay TR, Camarasa MV, Iskender B, Ye J, Bates N, Miller D, Fitzsimmons JC, Foxler D, Mee M, Sharp TV, Aplin J, Brison DR, Kimber SJ. Human feeder cell line for derivation and culture of hESc/hiPSc. Stem Cell Res (Amst) 7: 154–162, 2011. doi: 10.1016/j.scr.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Milyavsky M, Shats I, Erez N, Tang X, Senderovich S, Meerson A, Tabach Y, Goldfinger N, Ginsberg D, Harris CC, Rotter V. Prolonged culture of telomerase-immortalized human fibroblasts leads to a premalignant phenotype. Cancer Res 63: 7147–7157, 2003. [PubMed] [Google Scholar]
- 22.Reynolds SD, Rios C, Wesolowska-Andersen A, Zhuang Y, Pinter M, Happoldt C, Hill CL, Lallier SW, Cosgrove GP, Solomon GM, Nichols DP, Seibold MA. Airway progenitor clone formation is enhanced by Y-27632-dependent changes in the transcriptome. Am J Respir Cell Mol Biol 55: 323–336, 2016. doi: 10.1165/rcmb.2015-0274MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rousseau K, Wickstrom C, Whitehouse DB, Carlstedt I, Swallow DM. New monoclonal antibodies to non-glycosylated domains of the secreted mucins MUC5B and MUC7. Hybrid Hybridomics 22: 293–299, 2003. doi: 10.1089/153685903322538818. [DOI] [PubMed] [Google Scholar]
- 24.Shoemark A, Dixon M, Beales PL, Hogg CL. Bardet Biedl syndrome: motile ciliary phenotype. Chest 147: 764–770, 2015. doi: 10.1378/chest.13-2913. [DOI] [PubMed] [Google Scholar]
- 25.Smith CM, Djakow J, Free RC, Djakow P, Lonnen R, Williams G, Pohunek P, Hirst RA, Easton AJ, Andrew PW, O’Callaghan C. ciliaFA: a research tool for automated, high-throughput measurement of ciliary beat frequency using freely available software. Cilia 1: 14, 2012. doi: 10.1186/2046-2530-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith CM, Kulkarni H, Radhakrishnan P, Rutman A, Bankart MJ, Williams G, Hirst RA, Easton AJ, Andrew PW, O’Callaghan C. Ciliary dyskinesia is an early feature of respiratory syncytial virus infection. Eur Respir J 43: 485–496, 2014. doi: 10.1183/09031936.00205312. [DOI] [PubMed] [Google Scholar]
- 27.Suprynowicz FA, Upadhyay G, Krawczyk E, Kramer SC, Hebert JD, Liu X, Yuan H, Cheluvaraju C, Clapp PW, Boucher RC Jr, Kamonjoh CM, Randell SH, Schlegel R. Conditionally reprogrammed cells represent a stem-like state of adult epithelial cells. Proc Natl Acad Sci USA 109: 20035–20040, 2012. doi: 10.1073/pnas.1213241109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Torr E, Heath M, Mee M, Shaw D, Sharp TV, Sayers I. Expression of polycomb protein BMI-1 maintains the plasticity of basal bronchial epithelial cells. Physiol Rep 4: e12847, 2016. doi: 10.14814/phy2.12847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tosoni K, Cassidy D, Kerr B, Land SC, Mehta A. Using drugs to probe the variability of trans-epithelial airway resistance. PLoS One 11: e0149550, 2016. doi: 10.1371/journal.pone.0149550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong AP, Bear CE, Chin S, Pasceri P, Thompson TO, Huan LJ, Ratjen F, Ellis J, Rossant J. Directed differentiation of human pluripotent stem cells into mature airway epithelia expressing functional CFTR protein. Nat Biotechnol 30: 876–882, 2012. doi: 10.1038/nbt.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong AP, Rossant J. Generation of lung epithelium from pluripotent stem cells. Curr Pathobiol Rep 1: 137–145, 2013. doi: 10.1007/s40139-013-0016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woollhead AM, Sivagnanasundaram J, Kalsi KK, Pucovsky V, Pellatt LJ, Scott JW, Mustard KJ, Hardie DG, Baines DL. Pharmacological activators of AMP-activated protein kinase have different effects on Na+ transport processes across human lung epithelial cells. Br J Pharmacol 151: 1204–1215, 2007. doi: 10.1038/sj.bjp.0707343. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.