Abstract
Bronchopulmonary dysplasia (BPD) is a common complication of premature birth. The histopathology of BPD is characterized by an arrest of alveolarization with fibroblast activation. The Wnt/β-catenin signaling pathway is important in early lung development. When Wnt signaling is active, phosphorylation of β-catenin by tyrosine kinases at activating sites, specifically at tyrosine 489 (Y489), correlates with nuclear localization of β-catenin. We examined fetal lung tissue, lung tissue from term newborns, and lung tissue from infants who died with BPD; we found nuclear β-catenin phosphorylation at Y489 in epithelial and mesenchymal cells in fetal tissue and BPD tissue, but not in the lungs of term infants. Using a 3D human organoid model, we found increased nuclear localization of β-catenin phosphorylated at Y489 (p-β-cateninY489) after exposure to alternating hypoxia and hyperoxia compared with organoids cultured in normoxia. Exogenous stimulation of the canonical Wnt pathway in organoids was sufficient to cause nuclear localization of p-β-cateninY489 in normoxia and mimicked the pattern of α-smooth muscle actin (α-SMA) expression seen with fibroblastic activation from oxidative stress. Treatment of organoids with a tyrosine kinase inhibitor prior to cyclic hypoxia-hyperoxia inhibited nuclear localization of p-β-cateninY489 and prevented α-SMA expression by fibroblasts. Posttranslational phosphorylation of β-catenin is a transient feature of normal lung development. Moreover, the persistence of p-β-cateninY489 is a durable marker of fibroblast activation in BPD and may play an important role in BPD disease pathobiology.
Keywords: bronchopulmonary dysplasia, disease modeling, fibrosis, hyperoxia, Wnt/β-catenin
bronchopulmonary dysplasia (BPD) is a leading morbidity in survivors of preterm birth and arises from environmental injuries to the lungs at a vulnerable developmental time point, before the alveolar stage of lung development (5, 15). BPD is characterized by an arrest of development, with a well-described histological pattern of decreased alveolarization and fibroblast activation (5, 21, 32). While the use of surfactant and more gentle mechanical ventilation strategies have reduced the extent of fibrosis in BPD, there remain well-described features of fibroblastic changes, especially the development of bands of fibroblasts that express α smooth muscle actin (α-SMA) within alveolar septa (6). These activated myofibroblasts are believed to contribute to the abnormal developmental trajectory that occurs in BPD through altered mesenchymal-epithelial signaling (6, 20).
BPD is a disease of developmental vulnerability, restricted to prematurely born infants in the saccular stage of lung development. The Wnt/β-catenin signaling pathway has been shown to play a key role in lung development, especially during the saccular stage (13, 31, 33, 37, 38). Enhanced canonical Wnt signaling is associated with elevated β-catenin levels, and β-catenin is more available for phosphorylation at one of several activating sites by tyrosine kinases, including Abl kinase (28). Phosphorylation facilitates nuclear translocation of β-catenin to initiate a program of gene transcription (12, 19). Prior work demonstrated peak Wnt signaling in lung epithelium and mesenchyme toward the end of the canalicular stage of lung development, with a gradual decline until alveolarization begins (12, 24, 38). Thus Wnt signaling is temporally and spatially localized in a pattern that could be important to the pathobiology of BPD.
We hypothesized that posttranslational phosphorylation of β-catenin plays a role in normal lung development and in the abnormal developmental processes that occur in BPD. As there are critical differences between lung development in humans compared to other mammals, we developed a three-dimensional (3D) human organoid to model the fibroblastic changes associated with BPD (34). Organoids cultured in cyclic hypoxia-hyperoxia exhibit fibroblast activation associated with myofibroblast expansion and transformation, a reproducible feature of the fibroblastic changes associated with BPD (34, 35). We now show that posttranslational phosphorylation of β-catenin at activating site tyrosine 489 (p-β-cateninY489) in mesenchymal fibroblasts associates with canalicular stage normal fetal lung and with BPD lung in humans. Moreover, human organoids exposed to alternating hypoxia-hyperoxia demonstrate the myofibroblast transformation and proliferation characteristic of BPD in association with enhanced nuclear p-β-cateninY489, which is modifiable via tyrosine kinase inhibitors.
METHODS
Human fibroblast isolation and cell culture.
Human fetal lung fibroblasts (FLF) were isolated from fetal lungs between 18 and 21 wk gestation. Tissues were minced and dissociated with collagenase/dispase 1 mg/ml (Roche) and DNAse 0.1 mg/ml followed by rotation at 37oC for 45 min. The tissues were then washed with 1% fetal bovine serum (FBS) containing media and a single cell suspension was made with 100-μm and 40-μm cell strainers. Incubation in lysis buffer (BD Pharmingen) for 15 min was used to remove red blood cells and cells were plated in six-well tissue culture plates and cultured in DMEM/F12 with 10% FBS. Cells between passages 5 and 15 were used for experiments.
3D culture on alginate beads and hypoxia-hyperoxia model.
Alginate beads were generated with 3% alginate (Sigma) and functionalized with collagen (Corning) as described previously (34, 35). FLF were cultured onto the alginate beads scaffold using previously described methods of placing FLF and beads into a rotating bioreactor (Synthecon) (34, 35). After 3 h of rotation, the beads were fully coated with cells. A 150-μl volume of cell-coated beads were seeded into each well of a 96-well plate. Organoids were allowed to mature in the wells for 24 h before initiation of the experimental protocol. Organoids were cultured in a 37oC incubator (HeraCell 150i, Thermo Fisher) with 21% oxygen (normoxia), or alternating 10% O2 and 70% O2 (hypoxia-hyperoxia) every 24 h for a total of 4 days as previously described (34, 35). To activate the Wnt/β-catenin signaling pathway independently of oxidative stress, organoids were cultured in media containing 5 μM CHIR-99021 (CHIR; Tocris) during normoxia (38). To assess the role of β-catenin activation under hypoxia-hyperoxia conditions, inhibition of β-catenin phosphorylation was accomplished by supplementing media with 100 nM dasatanib (Santa Cruz Biotechnology) during culture under hypoxia-hyperoxia conditions.
Immunofluorescence.
Immunofluorescence (IF) was performed as described previously (25, 34, 35). The following primary antibodies were used: anti-α-SMA (Sigma, A2547), anti-vimentin (Bioss bs-0756r), anti-p-β-cateninY489 (Developmental Studies Hybridoma Bank, AB_10144551), anti-PDGFRα (platelet-derived growth factor-α) (Cell Signaling Technologies), anti-TTF1 (transcription termination factor 1) (Santa Cruz Biotechnology), anti-Pro-SPB (surfactant protein B) (Abcam). Alexa 594- and Alexa 488-conjugated secondary antibodies (Thermo Fisher) and counterstain DAPI (Vector Laboratories) were also used.
Human lung tissue.
The lungs of human infants who died with BPD from 2007 to 2015 and lungs from term infant stillbirths who died from nonrespiratory causes were obtained from the Translational Pathology Core Laboratory at UCLA. Lungs from five infants from the BPD group and four from the term infant group were examined.
Real-time quantitative PCR.
Total RNA was extracted from organoids using the RNeasy Mini Kit (Qiagen) following the manufacturer’s instructions, including DNase treatment of the column. TaqMan Reverse Transcription Reagents (Thermo Fisher) were used to make cDNA libraries. Real-time quantitative PCR (qPCR) was performed on an Applied Biosystems StepOne-Plus Real Time PCR System using TaqMan PCR Master Mix (Applied Biosystems). The following TaqMan probes were used: ACTA2 (α-SMA) hs00426855, Col1A1 (Collagen I hs00164004), CCND1 (CyclinD1 hs00765553), CTNNB1 (β-catenin hs00355049), PDE5A (Phosphodiesterase 5A hs00153649), TGFB1 (TGFβ1 hs00998133), MMP2 (Matrix Metalloprotease 2) (hs01548727_m1), MMP9 (Matrix Metalloprotease 9) (hs00957562_m1).
Statistics.
Triplicate samples were used in each experiment. Experiments were repeated a minimum of three times. All values are reported as a mean with error bars representing ± SD. Statistical analysis was performed using GraphPad Prism, with the use of two-tailed Student’s t-test for two-group comparisons. The Holm-Sidak method was used to correct for multiple comparisons. P values less than 0.05 were considered to be statistically significant.
Study approval.
Approval for this research was obtained from the UCLA Institutional Review Board.
RESULTS
Nuclear p-β-cateninY489 is temporally extinguished with progressive lung maturation and persistence of p-β-cateninY489 is demonstrated in the lungs of infants with BPD.
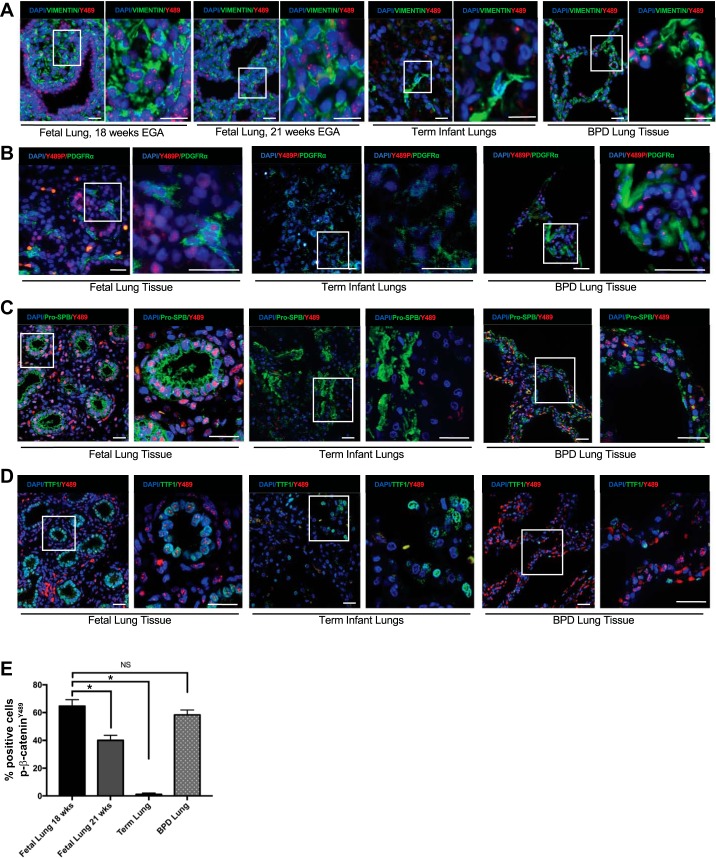
To investigate the expression and localization of p-β-cateninY489, we obtained human lung samples from four midgestation fetuses and term infants who died from nonrespiratory causes. Immunostaining with an antibody specific to p-β-cateninY489 demonstrated the presence of nuclear p-β-cateninY489 at 18 wk gestation in both the epithelial cells and fibroblasts (Fig. 1, A–E), with a 50% reduction in cells expressing nuclear p-β-cateninY489 by 21 wk gestation (Fig. 1, A and D). In the fetal lungs, nuclear p-β-cateninY489 was present in epithelial cells lining the acinar tubules of the lungs, which were coimmunostained with epithelial markers TTF1 and Pro-SPB, as well as in mesenchymal cells, which were identified by coimmunostaining for vimentin and PDGFRα. Immunofluorescence of lungs from four term stillborn infants who died from nonrespiratory causes were nearly devoid of nuclear p-β-cateninY489 (Fig. 1, A–E), though they do show a normal staining pattern for stromal markers vimentin and PDGFRα and epithelial markers Pro-SPB and TTF1 (9, 10, 17, 22, 34). By comparison, autopsy specimens of the lungs from five infants who died with BPD from 2005 to 2014 at postmenstrual ages ranging from 39 to 65 wk exhibited nuclear p-β-cateninY489 in both epithelial and mesenchymal cells of the alveolar septa (Fig. 1, A–D). The percentage of total cells positive for nuclear p-β-cateninY489 in BPD lung was comparable to that seen in fetal lungs at 18 wk gestation and was significantly increased compared to the lungs of term infants (Fig. 1E).
Fig. 1.
Phospho-Y489 β-catenin (p-β-cateninY489) localization in epithelial cells and fibroblasts during lung development and in BPD. A: IF for p-β-cateninY489 in fetal lung sections at 18 wk and 21 wk estimated gestational age, lungs from term infants who died without respiratory pathology, and the lungs with infants with BPD demonstrating nuclear presence of p-β-cateninY489 (red) in the fetal lung cells and BPD lungs. Some cells positive for p-β-cateninY489 also costained positive for vimentin (green), a marker of fibroblasts. B: IF for p-β-cateninY489 (red) and fibroblast marker PDGFRα (green) in fetal lungs (18 wk, term human lungs, and BPD lung tissue. C: IF for p-β-cateninY489 (red) and epithelial marker Pro-SPB (green) in fetal lungs (18 wk, term human lungs, and BPD lung tissue. D: IF for p-β-cateninY489 (red) and epithelial marker TTF1 (green) in fetal lungs (18 wk, term human lungs, and BPD lung tissue. Scale bar = 50 µm. E: quantification of percentage of cells positive for p-β-cateninY489; 10 high-powered fields were counted 3 times for each condition; *P < 0.001; NS, not significant.
3D organoid model of the fibroblastic changes associated with BPD demonstrated a marked increase in nuclear p-β-cateninY489 in response to hypoxia-hyperoxia exposure.
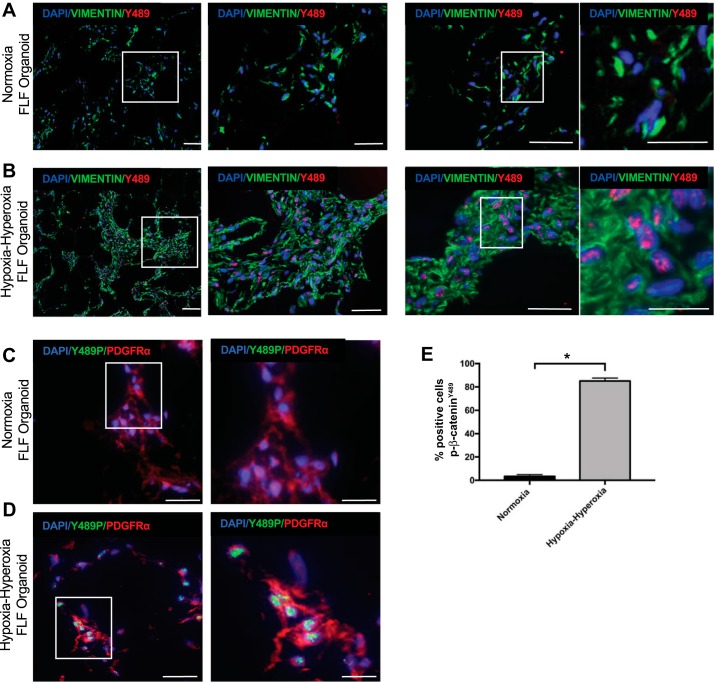
3D mesenchymal organoids cultured in normoxia had very little evidence of nuclear p-β-cateninY489 by immunostaining (Fig. 2, A, C, and E). After exposure to the alternating hypoxia and hyperoxia conditions used to model BPD in mice, the 3D organoids exhibited a significant increase in the percentage of cells with nuclear p-β-cateninY489 (Fig. 2, B, C, and D). As seen in our prior work, all of the fibroblasts in the organoid model expressed vimentin in both normoxia and alternating hypoxia-hyperoxia (34, 35). A subset of the fibroblasts in the organoid were found to express PDGFRα, with PDGFRα-expressing cells being both positive and negative for the nuclear accumulation of p-β-cateninY489.
Fig. 2.
p-β-CateninY489 localization in the 3D mesenchymal organoid model. A: IF for p-β-cateninY489 (red) and vimentin (green) of FLF organoids cultured in normoxic conditions. B: IF for p-β-cateninY489 and vimentin in FLF organoids cultured in alternating hypoxia and hyperoxia. C: IF for p-β-cateninY489 (green) and PDGFRα (red) of FLF organoids cultured in normoxic conditions. D: IF for p-β-cateninY489 (green) and PDGFRα (red) of FLF organoids cultured in alternating hypoxia and hyperoxia. Scale bar = 50 µm. E: quantification of percentage of cells positive for p-β-cateninY489; 10 high-powered fields were counted 3 times for each condition; *P < 1 × 10−4.
Activation of the Wnt/β-catenin signaling pathway in normoxic organoids increased nuclear p-β-cateninY489 and activation of α-SMA.
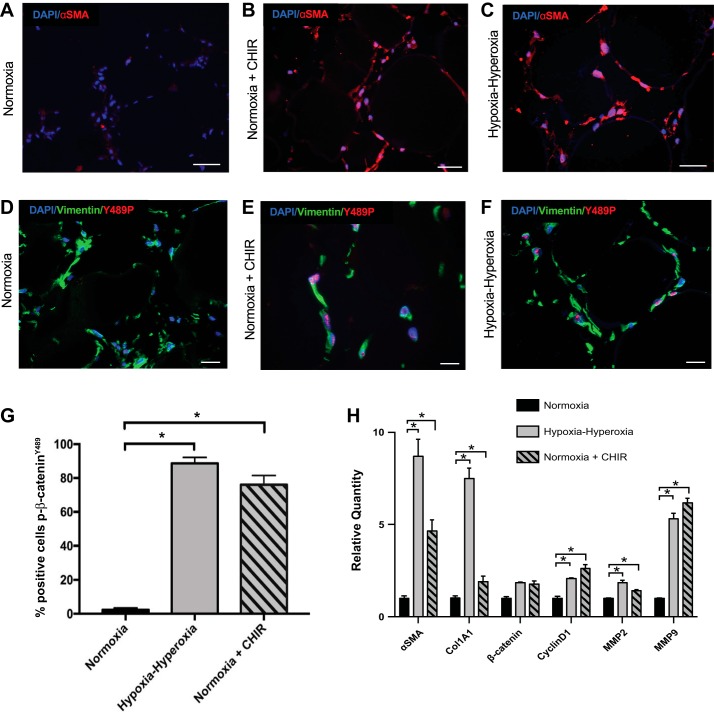
As demonstrated previously, organoids cultured in cyclic hypoxia-hyperoxia demonstrate increased α-SMA expression compared to normoxic organoids (Fig. 3, A–C) (34). CHIR is a small molecule that activates the Wnt/β-catenin pathway via GSK3β inhibition, which allows for the accumulation of β-catenin in the cell. Normoxic organoids treated with CHIR demonstrated a pattern of α-SMA expression that was significantly increased over normoxic organoids without CHIR, and was similar to that observed in organoids after cyclic hypoxia-hyperoxia (Fig. 3, A–C). Moreover, nuclear p-β-cateninY489 was observed in normoxic organoids of CHIR-treated samples that was significantly increased over normoxic organoids without CHIR and was similar to the extent of nuclear p-β-cateninY489 in organoids after cyclic hypoxia-hyperoxia, both qualitatively (Fig. 3, D–F) and quantitatively (Fig. 3G). There was not a significant difference between the CHIR-treated samples and the hypoxia-hyperoxia-exposed organoids in the relative number of cells with nuclear p-β-cateninY489 (P = 0.1). To determine whether CHIR and hypoxia-hyperoxia were acting through the Wnt pathway in our model, we examined the impact of these experimental conditions on downstream targets of Wnt signaling Cyclin D1, MMP2, and MMP9 (26) by qPCR. We found an increase in expression of these genes in both hypoxia-hyperoxia- and CHIR-treated samples (Fig. 3H). In addition, the transcriptional profile of the CHIR-treated organoids was similar to the organoids in cyclic hypoxia-hyperoxia, with significant increases in α-SMA and Col1A1 RNA expression of both samples relative to normoxic controls (Fig. 3H). Expression of Wnt targets was not significantly different when CHIR-treated organoids were compared with hypoxia-hyperoxia treated samples (P = 0.07 for Col1A1, and P values between 0.29 and 0.70 for all other targets). Furthermore, expression of total β-catenin was not significantly different between normoxia-, hypoxia-hyperoxia-, and CHIR-exposed organoids, as would be expected since CHIR enhances β-catenin signaling by inhibiting β-catenin degradation instead of by enhancing transcription and translation.
Fig. 3.
Upregulation of Wnt pathway with CHIR with resulting p-β-cateninY489 localization and fibroblast activation in the 3D mesenchymal organoid model. A–C: IF for α-SMA (red) in FLF organoids grown in normoxia, hypoxia-hyperoxia, and normoxia with CHIR added to culture media. Scale bar = 50 µm. D–F: IF for p-β-cateninY489 (red) and vimentin (green) of FLF organoids grown in normoxia, hypoxia-hyperoxia, and normoxia + 5 µm CHIR. Scale bar = 50 µm. G: quantification of percentage of cells positive for p-β-cateninY489; 10 high-powered fields were counted 3 times for each condition; *P < 1 × 10−4. H: qPCR for markers of fibroblast activation seen in BPD, α-SMA and Col1A1, as well as β-catenin and downstream targets of Wnt signaling Cyclin D1, MMP2, and MMP9. *P < 0.01. Triplicate samples were used in each experiment. Experiments were repeated a minimum of 3 times.
Inhibition of tyrosine kinase activity in hypoxia-hyperoxia exposed organoids using dasatinib prevents both the nuclear localization of p-β-cateninY489 and expression of α-SMA.
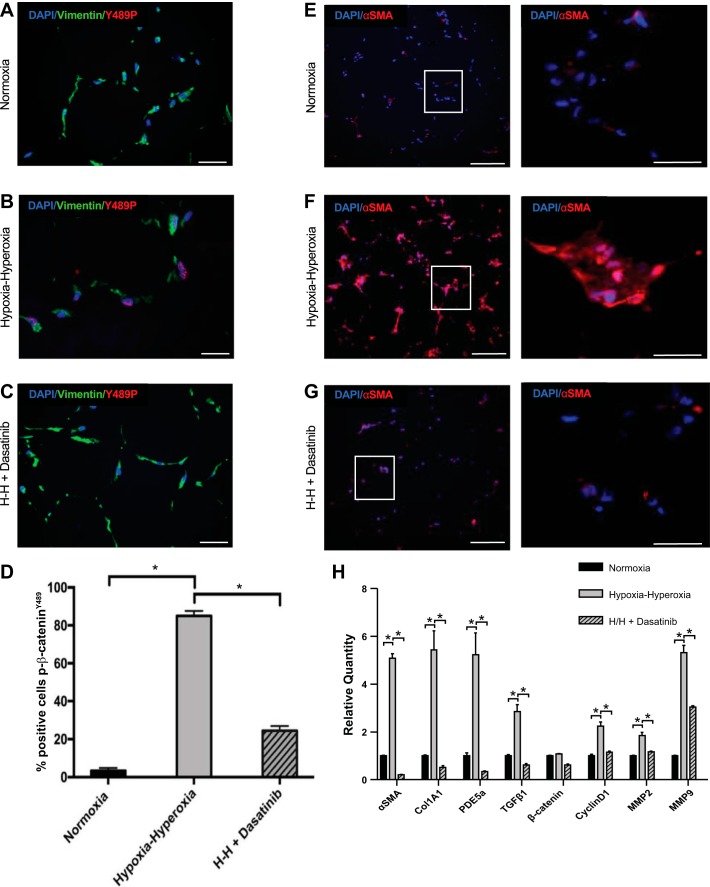
To determine whether we could modulate p-β-cateninY489 in our model system, 3D organoids were treated with the tyrosine kinase inhibitor, dasatinib, prior to and throughout hypoxia-hyperoxia exposure. We used dasatanib, an inhibitor of Abl and Src kinases to inhibit this phosphorylation event as Abl kinase has been shown to phosphorylate β-catenin at the tyrosine 489 site (28). 3D organoids treated with dasatanib and exposed to alternating hypoxia-hyperoxia had significantly decreased nuclear p-β-cateninY489 expression compared with hypoxia-hyperoxia-exposed organoids exposed without dasatinib (Fig. 4, A–D). Moreover, RNA expression of Wnt downstream targets Cyclin D1, MMP2, and MMP9 was decreased to normoxic levels in organoids exposed to both hypoxia-hyperoxia and dasatanib, validating that blocking tyrosine kinase activity with dasatanib decreased Wnt/β-catenin signaling (Fig. 4H). Dasatanib-treated organoids also showed a marked decrease in α-SMA expression compared with hypoxia-hyperoxia cultured organoids that were not treated with dasatinib (Fig. 4, F–H). Transcriptional profiling of organoids cultured in normoxia, or in hypoxia-hyperoxia with or without dasatanib showed that dasatanib prevented the expression of transcripts associated with fibroblastic activation with oxidative stress (Fig. 4H). Specifically, α-SMA, Col1A1, PDE5a, and TGFβ1 RNA were increased in hypoxia-hyperoxia relative to normoxia cultured organoids, and the increase in gene expression was prevented by dasatanib treatment.
Fig. 4.
Inhibition of tyrosine kinase with dasatanib with decreased p-β-cateninY489 localization and fibroblast activation in the mesenchymal organoid model. A–C: IF for p-β-cateninY489 (red) and vimentin (green) of FLF organoids grown in normoxia, hypoxia-hyperoxia (H-H), and hypoxia-hyperoxia + 100 nm dasatanib added to culture media. Scale bar = 50 µm. E–G: IF for α-SMA (red) in FLF organoids grown in normoxia, hypoxia-hyperoxia, and hypoxia-hyperoxia with 100 nm dasatanib added to culture media. Scale bar = 50 µm. D: quantification of percentage of cells positive for p-β-cateninY489; 10 high-powered fields were counted 3 times for each condition; *P < 1 × 10−4. H: qPCR for markers of fibroblast activation seen in BPD, α-SMA, Col1A1, TGFβ1, and PDE5a, as well as β-catenin and downstream targets of Wnt signaling Cyclin D1, MMP2, and MMP9. *P < 0.001. Triplicate samples were used in each experiment. Experiments were repeated a minimum of 3 times.
DISCUSSION
Histologically, BPD is described as an arrest of lung development (5, 8, 15). BPD results from an airway injury sustained during the saccular stage of lung development, just after the peak in Wnt/β-catenin activity during the canalicular stage (38). Thus we hypothesized that aberrations in the temporal pattern of Wnt/β-catenin signaling may play a role in BPD pathobiology. Since Wnt/β-catenin signaling requires the nuclear translocation of phosphorylated β-catenin, we followed p-β-cateninY489 as a marker of Wnt/β-catenin activity. We found that nuclear p-β-cateninY489 is present in a majority of the nuclei of epithelial and mesenchymal cells at 18 wk gestation, with significant stepwise decreases at 21 wk gestation and term (Fig. 1, A and E). This is consistent with prior work that demonstrated a peak of β-catenin in the lungs in the second trimester, with attenuation of nuclear β-catenin by the alveolar stage of lung development (12, 38). While prior work has examined total β-catenin signaling, this is the first time the temporal appearance of nuclear β-catenin phosphorylated at tyrosine 489 has been characterized and described in normal lung development.
Examination of BPD lung tissue demonstrated the persistence of nuclear p-β-cateninY489 in both epithelial and mesenchymal cells of the alveolar septa. All of the infants who died with BPD had a gestational age that corrected to 39 wk or later at the time of their demise and therefore would have been expected to demonstrate no nuclear p-β-cateninY489 in their alveoli had their lungs followed a normal developmental trajectory. The pattern of nuclear p-β-cateninY489 expression in the BPD tissue was comparable to that seen in the 18-wk fetal lungs (Fig. 1, A–D), suggesting that persistence of Wnt signaling in postnatal premature lungs may be an important mechanism for the developmental arrest in BPD. Our data extend earlier reports of increased Wnt/β-catenin expression with BPD by providing direct evidence of phosphorylation and nuclear translocation of p-β-cateninY489 as a mechanism for future therapeutic targeting (24, 27).
These studies were enhanced by the availability of a 3D human organoid model of the fibroblastic changes associated with BPD to manipulate Wnt/β-catenin signaling and to modulate the phosphorylation of β-catenin (34, 35). Unlike the fetal tissue from which they are isolated, the FLF organoids grown in normoxia have minimal nuclear accumulation of p-β-cateninY489. This is likely because the FLF are passaged prior to seeding the organoids, and in the absence of their native microenvironment there is cell turnover and decreased Wnt signaling. Exposure of the 3D organoids to oxidative stress appears to stimulate Wnt signaling by the phosphorylation of β-catenin. CHIR inhibits GSK3β, thereby inhibiting degradation of β-catenin and enhancing total cell β-catenin content, increasing the likelihood of β-catenin phosphorylation, nuclear translocation, and transcription of Wnt target genes, such as Cyclin D1, MMP2, and MMP9 (26, 30). Enhancing p-β-cateninY489 with CHIR in normoxic organoids was sufficient to phenocopy the effects of hypoxia-hyperoxia exposure (Fig. 3), while inhibiting β-catenin phosphorylation with the tyrosine kinase inhibitor dasatinib blocked the hypoxia-hyperoxia phenotype in organoids. The effects of CHIR and dasatinib were consistent within the experiments, not only on activation and inhibition of known downstream targets of p-β-cateninY489, respectively, but on their impact on myofibroblast expansion and transformation. We previously described myofibroblast expansion and transformation in organoids in response to hypoxia-hyperoxia, which was similar to the pattern of fibroblast activation and proliferation in human BPD (34). Our present studies show that modulation of β-catenin signaling with CHIR or dasatinib modulates the BPD-associated fibroblast phenotype in organoids. Our findings are consistent with prior work in animal models of BPD showing that administration of agents that block β-catenin signaling resulted in prevention of the alveolar simplification and vascular pathology typically seen in these models in response to hyperoxia exposure (1, 2, 29).
While animal models may approximate the alveolar simplification of BPD in humans, there exist significant gaps between BPD animal models and BPD in humans (3, 4, 7, 11, 16, 23, 36). Specifically, the trajectory of lung development for rats and mice is different from that in human infants, as these mammals are surfactant sufficient in the saccular stage of lung development (16). Because we are examining the potential role of developmental pathways, we used a 3D human model that would have greater fidelity with the developmental processes at work in human lungs. The 3D human organoid model allowed us to isolate the mesenchymal compartment of the lung from other cell types and to understand the impact of oxidative stress and β-catenin in isolation in one cell type. While BPD in human infants is clearly a disease in which the development of epithelial, endothelial, and mesenchymal cells is affected, we believe that the mesenchyme is one of the key drivers of disease pathogenesis. Our data from the model suggest that activation of the Wnt pathway may be sufficient to initiate the fibroblast activation associated with BPD and that blocking the phosphorylation of β-catenin at Y489 may block the development of myofibroblast differentiation associated with hypoxia-hyperoxia exposure.
There are several limitations in the organoid model used in this study. While BPD tissue clearly shows that p-β-cateninY489 is present in both epithelial and mesenchymal cells (Fig. 1), our 3D organoids utilize only FLF, and are thus unable to model the effects of our experimental exposures on epithelial cells independently or as they interact with fibroblasts. The utilization of a single cell type in our model may lead to a difference between the in vitro signals observed in our model and signaling pathways that are active in vivo, and drugs like dasatanib may have different effects when used with other cell populations. While we believe our model is a good one for exploring the fibroblast activation associated with BPD, future work in more complex models is necessary to examine the role of p-β-cateninY489 in the interaction between multiple cell types. Subtle differences that did not reach statistical significance occurred between the CHIR-treated samples and hypoxia-hyperoxia exposed samples (Fig. 3, A–H) that suggest that additional pathways beyond Wnt are activated with hypoxia-hyperoxia exposure. Our prior work demonstrating evidence for Notch pathway involvement is one such additional pathway (34), and the 3D organoid model provides us with a platform to explore other pathways activated in response to hypoxia-hyperoxia in the search for therapeutic targets.
Because β-catenin is phosphorylated at Y489 by Abl kinase, we used dasatanib, an Abl kinase inhibitor, to interfere with this posttranslational phosphorylation of β-catenin. However, Abl kinase phosphorylates other cellular proteins besides β-catenin, which is likely why dasatanib was shown to have off-target effects in clinical trials on human subjects treated for leukemia (14). It is possible that some of the effects observed in the dasatanib-treated organoids occurred as a result of these off-target effects in the organoids. Notably, dasatanib also inhibits Src kinase, which has been implicated in mouse models of arrested alveolar development (18). However, our data clearly demonstrate a role for dasatinib interference with Wnt signaling, as evidenced by the decreased expression of Wnt downstream targets Cyclin D1, MMP2, and MMP9. While the Wnt-β-catenin pathway is an attractive target for therapy due to the availability of clinically tested inhibitors, as we consider the future therapeutic implications of this work it will be important to identify more specific tyrosine kinase inhibitors of β-catenin phosphorylation in order to minimize off-target side effects.
There is an growing awareness of the role of Wnt/β-catenin signaling in the development of lung disease across the human lifespan, including BPD, idiopathic pulmonary fibrosis, and chronic obstructive pulmonary disease (24). This study provides a developmental context of a specific event in the Wnt/β-catenin signaling pathway, the posttranslational phosphorylation of β-catenin at Y489, with a goal of broadening our understanding of the underlying molecular basis for BPD pathogenesis. This understanding may also open the door toward the development of clinical therapies that target these posttranslational processes. While globally blocking the entire Wnt pathway during development in preterm infants would likely have severe effects on other developmentally sensitive organs, we speculate that blocking tyrosine kinase activity may prevent the fibroblast activation associated with BPD, and furthermore that β-catenin may be a relevant target for BPD-modifying therapies.
GRANTS
This work was supported by a Clinical Fellowship Training Grant from the Eli and Edythe Broad Center of Regenerative Medicine at UCLA and Stem Cell Research and the California Institute of Regenerative Medicine (CIRM), through the Scholars in Translational Medicine Program at the Broad Stem Cell Research Center at UCLA, and NIH K12 HD087023 (J. M. S. Sucre). The work was also supported by R01 GM114259-01 (B. N. Gomperts), CIRM 12-02 (B. N. Gomperts), NSF DGE-1144087 (D. Wilkinson), NIH GM108807 (S. H. Guttentag), and the Julia Carell Stadler Chair in Pediatrics (S. H. Guttentag). Experiments were performed in part through the use of the VU Cell Imaging Shared Resource (supported by NIH grants CA68485, DK20593, DK58404, DK5963).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.M.S.S., B.D., and B.N.G. conceived and designed research; J.M.S.S., P.V., and C.J.A. performed experiments; J.M.S.S., D.W., M.P., and B.N.G. analyzed data; J.M.S.S., P.V., C.J.A., D.W., M.P., S.H.G., and B.N.G. interpreted results of experiments; J.M.S.S. prepared figures; J.M.S.S. drafted manuscript; J.M.S.S., P.V., C.J.A., S.H.G., and B.N.G. edited and revised manuscript; J.M.S.S., C.J.A., S.H.G., and B.N.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to April Pyle, Sherin Devaskar, Josephine Enciso, and Brian Hackett for thoughtful discussions about our model and experiments and to Riet van der Meer for technical assistance. The p-β-catenin-Y489 antibody developed by J. Balsamo and J. Lilien was obtained from the Developmental Studies Hybridoma Bank created by the NICHD of the NIH and maintained at the University of Iowa, Department of Biology.
REFERENCES
- 1.Alapati D, Rong M, Chen S, Hehre D, Hummler SC, Wu S. Inhibition of β-catenin signaling improves alveolarization and reduces pulmonary hypertension in experimental bronchopulmonary dysplasia. Am J Respir Cell Mol Biol 51: 104–113, 2014. doi: 10.1165/rcmb.2013-0346OC. [DOI] [PubMed] [Google Scholar]
- 2.Alapati D, Rong M, Chen S, Lin C, Li Y, Wu S. Inhibition of LRP5/6-mediated Wnt/β-catenin signaling by Mesd attenuates hyperoxia-induced pulmonary hypertension in neonatal rats. Pediatr Res 73: 719–725, 2013. doi: 10.1038/pr.2013.42. [DOI] [PubMed] [Google Scholar]
- 3.Albertine KH. Utility of large-animal models of BPD: chronically ventilated preterm lambs. Am J Physiol Lung Cell Mol Physiol 308: L983–L1001, 2015. doi: 10.1152/ajplung.00178.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ambalavanan N, Morty RE. Searching for better animal models of BPD: a perspective. Am J Physiol Lung Cell Mol Physiol 311: L924–L927, 2016. doi: 10.1152/ajplung.00355.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med 357: 1946–1955, 2007. doi: 10.1056/NEJMra067279. [DOI] [PubMed] [Google Scholar]
- 6.Benjamin JT, Smith RJ, Halloran BA, Day TJ, Kelly DR, Prince LS. FGF-10 is decreased in bronchopulmonary dysplasia and suppressed by Toll-like receptor activation. Am J Physiol Lung Cell Mol Physiol 292: L550–L558, 2007. doi: 10.1152/ajplung.00329.2006. [DOI] [PubMed] [Google Scholar]
- 7.Berger J, Bhandari V. Animal models of bronchopulmonary dysplasia. The term mouse models. Am J Physiol Lung Cell Mol Physiol 307: L936–L947, 2014. doi: 10.1152/ajplung.00159.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bourbon J, Boucherat O, Chailley-Heu B, Delacourt C. Control mechanisms of lung alveolar development and their disorders in bronchopulmonary dysplasia. Pediatr Res 57: 38R–46R, 2005. doi: 10.1203/01.PDR.0000159630.35883.BE. [DOI] [PubMed] [Google Scholar]
- 9.Branchfield K, Li R, Lungova V, Verheyden JM, McCulley D, Sun X. A three-dimensional study of alveologenesis in mouse lung. Dev Biol 409: 429–441, 2016. doi: 10.1016/j.ydbio.2015.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cau F, Pisu E, Gerosa C, Senes G, Ronchi F, Botta C, Di Felice E, Uda F, Marinelli V, Faa G, Fanos V, Moretti C, Fanni D. Interindividual variability in the expression of surfactant protein A and B in the human lung during development. Eur J Histochem 60: 2678, 2016. doi: 10.4081/ejh.2016.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D’Angio CT, Ryan RM. Animal models of bronchopulmonary dysplasia. The preterm and term rabbit models. Am J Physiol Lung Cell Mol Physiol 307: L959–L969, 2014. doi: 10.1152/ajplung.00228.2014. [DOI] [PubMed] [Google Scholar]
- 12.Eberhart CG, Argani P. Wnt signaling in human development: beta-catenin nuclear translocation in fetal lung, kidney, placenta, capillaries, adrenal, and cartilage. Pediatr Dev Pathol 4: 351–357, 2001. doi: 10.1007/s10024001-0037-y. [DOI] [PubMed] [Google Scholar]
- 13.Goss AM, Morrisey EE. Wnt signaling and specification of the respiratory endoderm. Cell Cycle 9: 10–11, 2010. doi: 10.4161/cc.9.1.10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guignabert C, Phan C, Seferian A, Huertas A, Tu L, Thuillet R, Sattler C, Le Hiress M, Tamura Y, Jutant EM, Chaumais MC, Bouchet S, Manéglier B, Molimard M, Rousselot P, Sitbon O, Simonneau G, Montani D, Humbert M. Dasatinib induces lung vascular toxicity and predisposes to pulmonary hypertension. J Clin Invest 126: 3207–3218, 2016. doi: 10.1172/JCI86249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hadchouel A, Franco-Montoya ML, Delacourt C. Altered lung development in bronchopulmonary dysplasia. Birth Defects Res A Clin Mol Teratol 100: 158–167, 2014. doi: 10.1002/bdra.23237. [DOI] [PubMed] [Google Scholar]
- 16.Jobe AH. Animal models, learning lessons to prevent and treat neonatal chronic lung disease. Front Med (Lausanne) 2: 49, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.La Rosa S, Chiaravalli AM, Placidi C, Papanikolaou N, Cerati M, Capella C. TTF1 expression in normal lung neuroendocrine cells and related tumors: immunohistochemical study comparing two different monoclonal antibodies. Virchows Arch 457: 497–507, 2010. doi: 10.1007/s00428-010-0954-0. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Li Y, He H, Liu C, Li W, Xie L, Zhang Y. Csk/Src/EGFR signaling regulates migration of myofibroblasts and alveolarization. Am J Physiol Lung Cell Mol Physiol 310: L562–L571, 2016. doi: 10.1152/ajplung.00162.2015. [DOI] [PubMed] [Google Scholar]
- 19.Lilien J, Balsamo J. The regulation of cadherin-mediated adhesion by tyrosine phosphorylation/dephosphorylation of beta-catenin. Curr Opin Cell Biol 17: 459–465, 2005. doi: 10.1016/j.ceb.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 20.McCulley D, Wienhold M, Sun X. The pulmonary mesenchyme directs lung development. Curr Opin Genet Dev 32: 98–105, 2015. doi: 10.1016/j.gde.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McEvoy CT, Jain L, Schmidt B, Abman S, Bancalari E, Aschner JL. Bronchopulmonary dysplasia: NHLBI Workshop on the Primary Prevention of Chronic Lung Diseases. Ann Am Thorac Soc 11, Suppl 3: S146–S153, 2014. doi: 10.1513/AnnalsATS.201312-424LD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ntokou A, Klein F, Dontireddy D, Becker S, Bellusci S, Richardson WD, Szibor M, Braun T, Morty RE, Seeger W, Voswinckel R, Ahlbrecht K. Characterization of the platelet-derived growth factor receptor-α-positive cell lineage during murine late lung development. Am J Physiol Lung Cell Mol Physiol 309: L942–L958, 2015. doi: 10.1152/ajplung.00272.2014. [DOI] [PubMed] [Google Scholar]
- 23.O’Reilly M, Thébaud B. Animal models of bronchopulmonary dysplasia. The term rat models. Am J Physiol Lung Cell Mol Physiol 307: L948–L958, 2014. doi: 10.1152/ajplung.00160.2014. [DOI] [PubMed] [Google Scholar]
- 24.Ota C, Baarsma HA, Wagner DE, Hilgendorff A, Königshoff M. Linking bronchopulmonary dysplasia to adult chronic lung diseases: role of WNT signaling. Mol Cell Pediatr 3: 34, 2016. doi: 10.1186/s40348-016-0062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paul MK, Bisht B, Darmawan DO, Chiou R, Ha VL, Wallace WD, Chon AT, Hegab AE, Grogan T, Elashoff DA, Alva-Ornelas JA, Gomperts BN. Dynamic changes in intracellular ROS levels regulate airway basal stem cell homeostasis through Nrf2-dependent Notch signaling. Cell Stem Cell 15: 199–214, 2014. doi: 10.1016/j.stem.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pongracz JE, Stockley RA. Wnt signalling in lung development and diseases. Respir Res 7: 15, 2006. doi: 10.1186/1465-9921-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Popova AP, Bentley JK, Anyanwu AC, Richardson MN, Linn MJ, Lei J, Wong EJ, Goldsmith AM, Pryhuber GS, Hershenson MB. Glycogen synthase kinase-3β/β-catenin signaling regulates neonatal lung mesenchymal stromal cell myofibroblastic differentiation. Am J Physiol Lung Cell Mol Physiol 303: L439–L448, 2012. doi: 10.1152/ajplung.00408.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rhee J, Buchan T, Zukerberg L, Lilien J, Balsamo J. Cables links Robo-bound Abl kinase to N-cadherin-bound beta-catenin to mediate Slit-induced modulation of adhesion and transcription. Nat Cell Biol 9: 883–892, 2007. doi: 10.1038/ncb1614. [DOI] [PubMed] [Google Scholar]
- 29.Rong M, Chen S, Zambrano R, Duncan MR, Grotendorst G, Wu S. Inhibition of β-catenin signaling protects against CTGF-induced alveolar and vascular pathology in neonatal mouse lung. Pediatr Res 80: 136–144, 2016. doi: 10.1038/pr.2016.52. [DOI] [PubMed] [Google Scholar]
- 30.Rubinfeld B, Albert I, Porfiri E, Fiol C, Munemitsu S, Polakis P. Binding of GSK3beta to the APC-beta-catenin complex and regulation of complex assembly. Science 272: 1023–1026, 1996. doi: 10.1126/science.272.5264.1023. [DOI] [PubMed] [Google Scholar]
- 31.Shu W, Guttentag S, Wang Z, Andl T, Ballard P, Lu MM, Piccolo S, Birchmeier W, Whitsett JA, Millar SE, Morrisey EE. Wnt/beta-catenin signaling acts upstream of N-myc, BMP4, and FGF signaling to regulate proximal-distal patterning in the lung. Dev Biol 283: 226–239, 2005. doi: 10.1016/j.ydbio.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 32.Silva DM, Nardiello C, Pozarska A, Morty RE. Recent advances in the mechanisms of lung alveolarization and the pathogenesis of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 309: L1239–L1272, 2015. doi: 10.1152/ajplung.00268.2015. [DOI] [PubMed] [Google Scholar]
- 33.Snowball J, Ambalavanan M, Cornett B, Lang R, Whitsett J, Sinner D. Mesenchymal Wnt signaling promotes formation of sternum and thoracic body wall. Dev Biol 401: 264–275, 2015. doi: 10.1016/j.ydbio.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sucre JM, Wilkinson D, Vijayaraj P, Paul M, Dunn B, Alva-Ornelas JA, Gomperts BN. A three-dimensional human model of the fibroblast activation that accompanies bronchopulmonary dysplasia identifies Notch-mediated pathophysiology. Am J Physiol Lung Cell Mol Physiol 310: L889–L898, 2016. doi: 10.1152/ajplung.00446.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilkinson DC, Alva-Ornelas JA, Sucre JM, Vijayaraj P, Durra A, Richardson W, Jonas SJ, Paul MK, Karumbayaram S, Dunn B, Gomperts BN. Development of a three-dimensional bioengineering technology to generate lung tissue for personalized disease modeling. Stem Cells Transl Med sctm.2016-0192, 2016. doi: 10.5966/sctm.2016-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoder BA, Coalson JJ. Animal models of bronchopulmonary dysplasia. The preterm baboon models. Am J Physiol Lung Cell Mol Physiol 307: L970–L977, 2014. doi: 10.1152/ajplung.00171.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang J, Gill AJ, Issacs JD, Atmore B, Johns A, Delbridge LW, Lai R, McMullen TP. The Wnt/β-catenin pathway drives increased cyclin D1 levels in lymph node metastasis in papillary thyroid cancer. Hum Pathol 43: 1044–1050, 2012. doi: 10.1016/j.humpath.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 38.Zhang M, Shi J, Huang Y, Lai L. Expression of canonical WNT/β-CATENIN signaling components in the developing human lung. BMC Dev Biol 12: 21, 2012. doi: 10.1186/1471-213X-12-21. [DOI] [PMC free article] [PubMed] [Google Scholar]