Abstract
Elevation of hemoglobin concentration, a common adaptive response to high-altitude hypoxia, occurs among Oromo but is dampened among Amhara highlanders of East Africa. We hypothesized that Amhara highlanders offset their smaller hemoglobin response with a vascular response. We tested this by comparing Amhara and Oromo highlanders at 3,700 and 4,000 m to their lowland counterparts at 1,200 and 1,700 m. To evaluate vascular responses, we assessed urinary levels of nitrate (NO3−) as a readout of production of the vasodilator nitric oxide and its downstream signal transducer cyclic guanosine monophosphate (cGMP), along with diastolic blood pressure as an indicator of vasomotor tone. To evaluate hematological responses, we measured hemoglobin and percent oxygen saturation of hemoglobin. Amhara highlanders, but not Oromo, had higher NO3− and cGMP compared with their lowland counterparts. NO3− directly correlated with cGMP (Amhara R2 = 0.25, P < 0.0001; Oromo R2 = 0.30, P < 0.0001). Consistent with higher levels of NO3− and cGMP, diastolic blood pressure was lower in Amhara highlanders. Both highland samples had apparent left shift in oxyhemoglobin saturation characteristics and maintained total oxyhemoglobin content similar to their lowland counterparts. However, deoxyhemoglobin levels were significantly higher, much more so among Oromo than Amhara. In conclusion, the Amhara balance minimally elevated hemoglobin with vasodilatory response to environmental hypoxia, whereas Oromo rely mainly on elevated hemoglobin response. These results point to different combinations of adaptive responses in genetically similar East African highlanders.
Keywords: nitric oxide, cyclic guanosine monophosphate, oxyhemoglobin, deoxyhemoglobin, high altitude, hypoxia, Amhara, Oromo, adaptation
the amount of oxygen delivered to tissues is related to the product of percent oxygen saturation, total hemoglobin, and blood flow. A universal response to acute high-altitude hypoxia and lowered percent of oxygen saturation is elevated hemoglobin. Elevated hemoglobin facilitates higher arterial oxygen content and delivery to tissues. This response occurs to different degrees in genetically closely related Ethiopian highlanders of Amhara and Oromo ethnicity, who have resided above 2,500 m for over 5,000 yr, and for ~500 yr, respectively (1, 18, 25, 28). Percent oxygen saturation is much lower in Oromo compared with Amhara highland natives (18). To compensate for this drop, Oromo highland natives have more hemoglobin than Amhara to maintain oxygen delivery. Population differences in the magnitude of the hemoglobin response suggest the existence of alternative routes of adaptation.
Vasodilation is a potential adaptive mechanism enabling increased oxygen delivery. For example, Tibetan highlanders have a dampened hemoglobin response with high blood flow and nitric oxide levels (5, 8, 11, 29). To determine whether Amhara, like the Tibetans, offset a dampened hemoglobin response with the synthesis of vasodilatory molecules such as nitric oxide (18), we quantified the end product of nitric oxide in the body, nitrate (NO3−), along with the downstream signaling molecule cyclic guanosine monophosphate (cGMP) in urine (2, 3) and evaluated the vasomotor tone by measuring the diastolic blood pressures of Amhara and Oromo lowland and highland natives.
To compare hematological responses, we quantified the amount of oxygen-saturated and -unsaturated hemoglobin, i.e., oxy- and deoxyhemoglobin levels. In this study, we tested the hypothesis that the Amhara adapt to high-altitude hypoxia through the vascular nitric oxide– cGMP pathway to offset a modest hematological response while the Oromo mount a robust hemoglobin response.
MATERIALS AND METHODS
Study participants.
Study participants and sample collection have previously been described (1, 12, 18). All individuals were agropastoralists born and raised at the altitude of residence. High-altitude participants had not traveled to altitudes below 2,500 m in the previous 6 mo while low-altitude participants had not traveled to altitudes above 2,500 m in the same timeframe. They had normal body iron levels and no infection or inflammation, as determined by human malarial plasmodium DNA and C-reactive protein levels (19). At the time of study, environmental conditions were recorded as follows: study group, altitude, average barometric pressure in early morning, temperature, and relative humidity. High-altitude Oromo: 4,000 m, 469 mmHg, 3°C, 40.5%; low-altitude Oromo: 1,700 m, 635 mmHg, 15°C, 32%; high-altitude Amhara: 3,700 m, 498 mmHg, 6°C, 36%; low-altitude Amhara: 1,200 m, 659 mmHg, 22°C, 36%.
Study approval.
Institutional Review Boards at Case Western Reserve University and Cleveland Clinic, Addis Ababa University Faculty of Medicine, and the Ethiopian Science and Technology Commission Ethics Committee approved the study protocols. All participants provided informed consent.
Sample collection.
Venous blood was collected into heparinized tubes with peripheral venipuncture. Hemoglobin level was measured in duplicate with the cyanmethemoglobin method (HemoCue, Ängelholm, Sweden). Spot urine samples were collected, shipped frozen in liquid nitrogen to Cleveland, and stored at −80°C. Blood pressure was the mean of three measurements taken while participants rested in a seated position. Percent oxygen saturation was measured with pulse oximetry (Criticare Model 503 and SpO2; Criticare Systems, Waukesha, WI), and the reported value was the mean of six readings taken 10 s apart. C-reactive protein levels were quantified by ELISA (R&D Systems, Minneapolis, MN).
Equations.
where PaO2 is alveolar partial pressure of oxygen.
Measurements of urinary nitrate and cGMP.
All measurements of urine samples were normalized by urine creatinine concentrations to account for differences in hydration and urine volume. Creatinine concentrations were measured with a Jaffe reaction-based colorimetric assay (Cayman Chemical, Ann Arbor, MI). All samples tested negative for nitrite using the Griess reaction. NO3− was measured by chemiluminescence as previously described (12). cGMP was quantified by diluting urine samples by 10-fold according to the manufacturer’s protocol of the cGMP parameter assay (KGE003, R&D Systems). cGMP/creatinine levels in this study were within the previously reported reference range (0.002 to 1.28 mmol/g) (15).
Statistics.
Data points more than three standard deviations from the mean were considered outliers and excluded from statistical analysis. JMP PRO 10 was used for analysis. Student’s t-tests were used to test the statistical significance for the observed differences between groups. P value less than 0.01 was considered statistically significant to account for multiple testing.
RESULTS
Study participants.
Study participants were 113 men and 44 women of Amhara, and 100 men and 37 women of Oromo (Table 1). The study samples were healthy by self-report and medical examination, nonanemic, nonsmoking, normotensive, and not pregnant. Amhara and Oromo highlanders had similar ages, but Oromo lowlanders were overall younger than the other groups. Study outcomes including NO3−, cGMP, systolic blood pressure, diastolic blood pressure, oxyhemoglobin, and deoxyhemoglobin did not correlate with age.
Table 1.
Participant demographics
| Ethnicity | Altitude, m | N | Age, yr | Weight, kg | Height, m |
|---|---|---|---|---|---|
| Amhara | 1,200 | 60 (m) | 34.7 ± 1.2 | 58.0 ± 0.7 | 1.72 ± 0.01 |
| 14 (f) | 28.1 ± 2.3 | 52.9 ± 2.0 | 1.56 ± 0.01 | ||
| Amhara | 3,700 | 53 (m) | 32.2 ± 1.2 | 52.6 ± 0.7 | 1.66 ± 0.01 |
| 30 (f) | 27.5 ± 1.2 | 48.4 ± 0.9 | 1.59 ± 0.01 | ||
| Oromo | 1,700 | 35 (m) | 24.3 ± 0.9 | 55.2 ± 0.9 | 1.72 ± 0.01 |
| 7 (f) | 28.0 ± 1.8 | 56.4 ± 3.2 | 1.61 ± 0.02 | ||
| Oromo | 4,000 | 65 (m) | 29.3 ± 1.2 | 59.1 ± 0.8 | 1.70 ± 0.01 |
| 30 (f) | 26.3 ± 1.2 | 54.0 ± 1.3 | 1.58 ± 0.01 |
Values are means ± SE. m, Male; f, female.
Elevated NO3− and cGMP levels in Amhara highlanders.
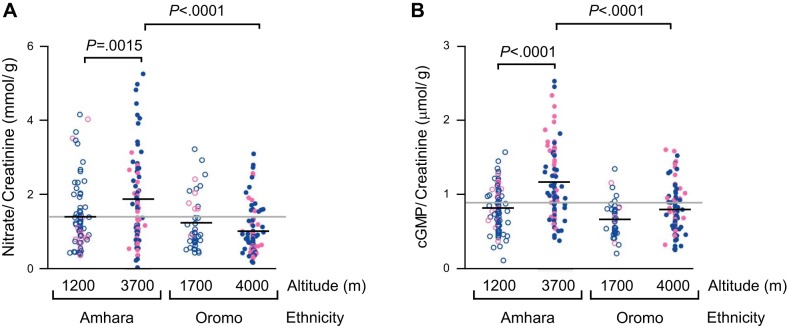
Amhara highland natives had 34% higher in urinary NO3− (P = 0.0015) and 43% higher in cGMP (P < 0.0001) compared with their lowland counterparts; in contrast, Oromo samples did not differ (Fig. 1, A and B). At high altitudes, NO3− and cGMP levels were 86% and 46% higher in Amhara than Oromo (P < 0.0001). These data demonstrate that the nitric oxide/cGMP pathway was activated in Amhara but not Oromo highlanders.
Fig. 1.
Elevated urinary levels of nitrate (NO3−) and cyclic guanosine monophosphate (cGMP) in high-altitude Amhara but not Oromo. Low-altitude (open circles) and high-altitude (close circles) Amhara and Oromo healthy volunteers (men in blue and women in pink) were recruited. Amhara have significantly higher NO3− (A) and cGMP (B) levels at high vs. low altitudes, whereas Oromo do not. Black line indicates sample mean, while gray line represents grand mean of all study groups.
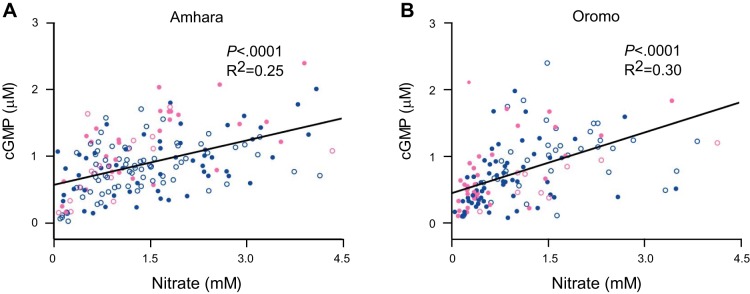
Higher urinary NO3− levels associated directly with higher cGMP (Amhara: R2 = 0.25, P < 0.0001; Oromo: R2 = 0.30, P < 0.0001) (Fig. 2, A and B), consistent with the known NO – cGMP signal transduction pathway (3).
Fig. 2.
Higher NO3− level is associated with higher cGMP in urine samples of Amhara and Oromo. Least square regression analysis shows positive correlation between the vasodilator NO3− and the secondary messenger cGMP. A: 25% variation in cGMP is explained by NO3− in Amhara. B: 30% variation in cGMP is explained by NO3− in Oromo.
Dietary NO3− from liquid samples.
To assess possible dietary contribution to urinary nitrate, we measured the nitrate level of beverage samples collected from the households of study participants, including water, beer, tea, and coffee. The average nitrate levels of Amhara highlanders’ beverages were higher than lowlanders’ beverages [highland Amhara, μM: 464.7 ± 78.4 (n = 9); lowland Amhara, μM: 102.4 ± 40.6 (n = 10); highland Oromo, μM: 86.8 ± 23.0 (n = 3); lowland Oromo, μM: 259 (n = 1)].
Lower diastolic blood pressure in Amhara highlanders.
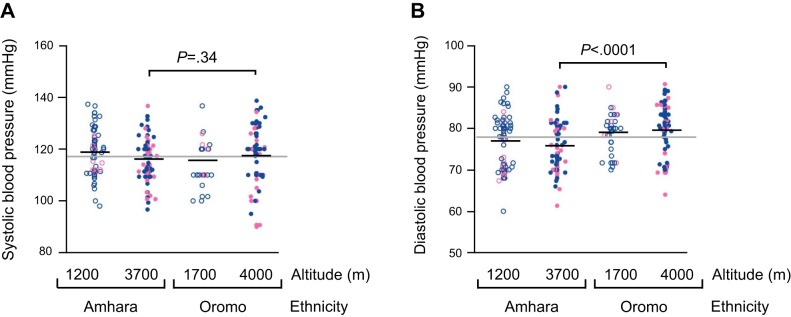
Systolic and diastolic blood pressures are indicators of sympathetic activity and the peripheral vasomotor tone, respectively. Systolic blood pressure was similar across the study groups (Fig. 3A), but at high altitudes, diastolic blood pressure was, on average, 3.7 mmHg lower among Amhara than Oromo (P < 0.0001) (Fig. 3B).
Fig. 3.
Diastolic blood pressures of Amhara were lower than Oromo at high altitudes. A: systolic blood pressures are similar among all groups. B: at high altitudes, Amhara have lower diastolic blood pressures than Oromo. Group mean shown in black line, and grand mean of all study groups in gray.
Oxyhemoglobin concentration was similar at low and high altitudes, while deoxyhemoglobin was higher.
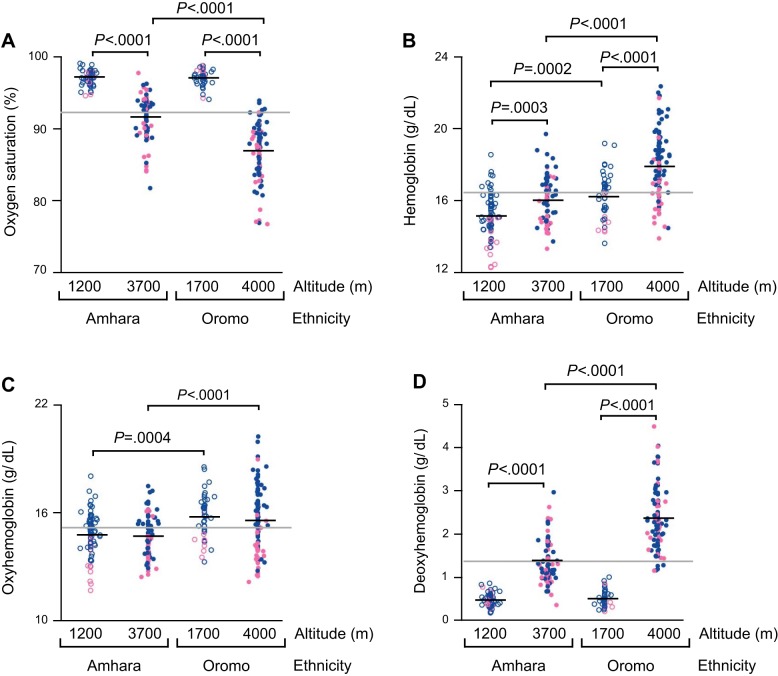
As previously reported (18), percent oxygen saturation of hemoglobin was lower at high altitude in both Amhara and Oromo, but the altitude difference among Oromo was 1.8-fold larger than among Amhara (P < 0.0001) (Fig. 4A). Similarly, the hemoglobin level was higher at high altitude among both ethnicities, but the Oromo hemoglobin response was 1.9-fold greater (P < 0.0001) (Fig. 4B).
Fig. 4.
Oxygen-saturated hemoglobin levels do not change with altitudes in Amhara and Oromo, whereas the increase in deoxyhemoglobin from low to high altitude is much greater in Oromo than Amhara. A: percent oxygen saturation is lower at high altitude in both Amhara and Oromo, but the drop is much greater in Oromo than Amhara. B: hemoglobin levels increase with altitudes in both Amhara and Oromo, but the increase in hemoglobin from low to high altitude is much greater in Oromo than Amhara. C: oxyhemoglobin levels do not change with altitudes, but Oromo have higher oxyhemoglobin levels than Amhara at low and high altitudes. D: deoxyhemoglobin levels increase with altitudes in Amhara and Oromo, but the increase is much greater in Oromo than Amhara.
Neither Amhara nor Oromo showed altitude differences in oxyhemoglobin levels, although Oromo had ~1 g/dl higher oxyhemoglobin levels than Amhara at both altitudes (Amhara-Oromo mean difference at low-altitude: P = 0.0004; at high-altitude: P < 0.0001) (Fig. 4C). These findings indicate that both high-altitude Amhara and Oromo both successfully maintained levels of oxygen-saturated hemoglobin, despite severe hypoxia at high altitudes.
In contrast to oxyhemoglobin, both Amhara and Oromo had higher deoxyhemoglobin with high altitude, but high-altitude Amhara deoxyhemoglobin concentration was triple that of low-altitude Amhara, while Oromo deoxyhemoglobin was quadruple (P < 0.0001). Oromo highlanders had 1.7-fold higher deoxyhemoglobin levels than Amhara highlanders (P < 0.0001) (Fig. 4D).
To consider the potential role of oxygen affinity of hemoglobin in these East African highlanders, we plotted the measured percent oxygen saturation of hemoglobin and estimated oxygen partial pressures of low- and high-altitude samples, alongside the standard oxygen dissociation curve of hypothetical subjects derived from the Hill’s equation (Fig. 5) (6). The average measured oxygen saturations of high-altitude Amhara and Oromo lie on the left side of the standard oxygen dissociation curve. The results suggest a greater affinity of hemoglobin for oxygen in highland natives than predicted from the standard curve at low oxygen partial pressures. Interpolations based on Oromo low- and high-altitude oxygen saturations estimate lower than actual values for the Amhara highlanders at comparable oxygen partial pressures. This suggests a greater affinity for oxygen among high-altitude Amhara than Oromo. Oromo show marked increase in hemoglobin and modest increase in oxygen affinity; Amhara show increased vasodilation and marked increase in oxygen affinity.
Fig. 5.
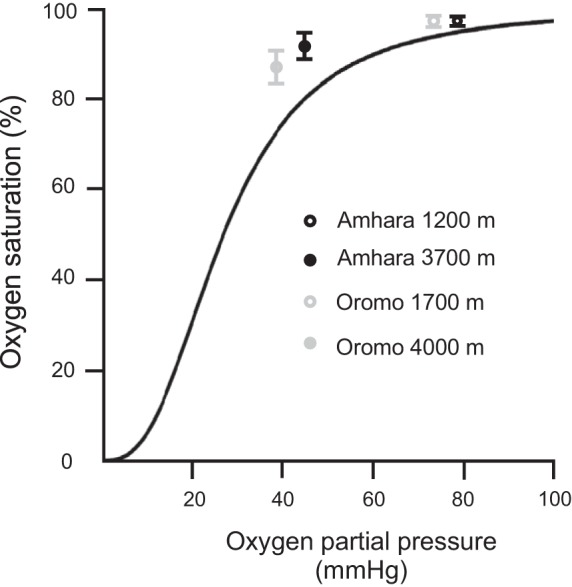
Increased affinity of hemoglobin for oxygen in high-altitude samples, relative to hypothetical subjects derived from the standard oxygen dissociation curve (ODC). The ODC is mathematically derived from the Hill’s equation, with a P50 of 26.8 mmHg and the Hill coefficient of 1.3922 × 10−4 mmHg-2.7 (6). The depicted values of alveolar oxygen partial pressure in Amhara and Oromo are estimated based on the assumption of 47 mmHg water vapor pressure, 40 mmHg arterial partial pressure of carbon dioxide, and 0.8 respiratory exchange ratio (24).
DISCUSSION
Amhara and Oromo at 3,700 and 4,000 m have adapted to survive lifelong hypoxic stress over generations, but the underlying mechanisms remain unclear (1, 12, 18). The major finding of this study is that indigenous Amhara highlanders produce more nitric oxide and its bioactive signal molecule cGMP. The positive correlation between NO3− and cGMP is consistent with previous studies, which showed that nitric oxide increases the accumulation of cGMP (2, 3). In Amhara, elevated nitric oxide-cGMP enable vasodilation, lower diastolic blood pressure and, by extension, increase blood flow, which offset their relatively lower hemoglobin response. Unlike Amhara, Oromo highland natives produce a much greater hemoglobin response to maintain sufficient oxygen-carrying capacity at the cost of lower body iron stores (18) and higher level of circulating deoxyhemoglobin. This evidence suggests different balances of hemoglobin (Oromo) and vascular (Amhara) adaptive responses to hypoxia among East African high-altitude natives.
Plotting percent oxygen saturation and estimated partial pressure of oxygen for study participants, along with the standard oxygen dissociation curve, illustrates that East African high-altitude natives have a higher than predicted affinity of hemoglobin for oxygen. This is consistent with the general pattern of high-altitude native vertebrates but opposite to acutely exposed people (4, 27). Lower body temperature or carbon dioxide levels, increased pH, and decreased 2,3-bisphosphoglycerate are factors that could have caused the leftward shift of the oxygen dissociation curve (14). The oxygen saturation value of high-altitude Amhara is higher than estimated from interpolations based on Oromo low- and high-altitude samples, perhaps related to nitric oxide production (13, 16, 26). High-altitude Amhara rely on nitric oxide-based vasodilation to offset their dampened hemoglobin response, and their hemoglobin picks up oxygen more efficiently in the lung. In contrast, high-altitude Oromo mainly rely on hemoglobin response of much greater magnitude, and their hemoglobin gives up oxygen more readily.
Vasodilation presents an alternative approach to high-altitude adaptation, as evidenced in Tibetans (8, 11), and now among Amhara on a different continent. Urine sampling of NO3− and cGMP allows noninvasive global measurements of these biological molecules produced in the systemic vasculature. Urinary nitrate levels reflect the net result of dietary intake, total body biosynthesis, and metabolic loss of nitric oxide (10). To evaluate whether the ~4.5-fold higher nitrate level in the beverages of the highland Amhara contributed significantly to their relatively high urinary nitrate, we estimated the daily liquid consumption that might affect total body nitrate. Tannenbaum and colleagues (10) showed that ~60% of ingested nitrate is recovered as urinary nitrate. Ingestion of 3,500 μmol nitrate in the Tannenbaum paper led to, on average, 1,865 μmol of urinary nitrate. Working from these data, high-altitude Amhara would need to ingest 2,673 μmol nitrate, corresponding to a daily 6 liters of fluid ingestion. Observations in the field do not support excessive fluid intake. Average fluid intake in U.S. is ~2 liters (23). Furthermore, recorded dietary recalls for the day of the study show that leafy greens (a nitrate rich diet) were rarely consumed in study groups. Thus diet is unlikely to be the major contributor to the urinary nitrate level.
A limitation of the present study is that NO3−, cGMP, and blood pressures were measurements at one time point. Blood flow was not directly measured. However, many studies show that blood flow increases with increasing nitric oxide (7, 9, 22). Thus we infer that blood flow is higher due to the higher NO3− and lower vasomotor tone of high-altitude Amhara.
Another potential limitation is that our lowlander samples were collected at 1,200 and 1,700 m. These sites provided a substantial contrast in partial pressures of oxygen and are similar to previous studies using Denver, Colorado at 1,600 m as a low-altitude reference group (20, 21). An advantage of these lowland groups is the absence of the endemic malaria found at lower altitudes in East Africa, which would impact hemoglobin. Our previous study found no erythropoietin or hepcidin response in the lowlander groups (18), further supporting their use as low-altitude comparison groups.
Furthermore, nitrate levels accounted for only ~30% variation in cGMP in this study. These results suggest other possible upstream signal pathways, other than nitric oxide, that could lead to cGMP production. For example, atrial natriuretic peptides and brain natriuretic peptides, which are released in response to atrial stretch, bind to plasma-membrane guanylate cyclase receptors and in turn generate cGMP (17). Further investigation in the role of these pathways in high-altitude adaptation is warranted.
In conclusion, the discovery of elevated cGMP associated with NO3− among Amhara but not Oromo provides new evidence that chronically exposed populations may adapt differently to the same stress of high-altitude hypoxia. The two closely related ethnic groups have in common elevated oxygen affinity and maintenance of oxyhemoglobin levels. Oromo show substantially higher hematological responses. Amhara activate the NO-cGMP vasodilation pathway. The balance between hematological and vascular adaptation may be essential for human adaptation and survival in extreme hypoxic conditions.
GRANTS
This work was supported by the National Institutes of Health (HL60917) (S. C. Erzurum) and the National Science Foundation (BCS-0452326) (C. M. Beall). H. Cheong is a predoctoral student in the Molecular Medicine Ph.D. Program of the Cleveland Clinic and Case Western Reserve University, funded in part by the Med into Grad initiative of the Howard Hughes Medical Institute. S. C. Erzurum is supported in part by the Alfred Lerner Memorial Chair in Innovative Biomedical Research.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
H.I.C., A.J.J., L.T.M., A.C.G., and C.M.B. analyzed data; H.I.C., A.J.J., S.C.E., and C.M.B. interpreted results of experiments; H.I.C. prepared figures; H.I.C. drafted manuscript; H.I.C., A.J.J., L.T.M., A.C.G., A.G., S.C.E., and C.M.B. edited and revised manuscript; H.I.C. and C.M.B. approved final version of manuscript; A.J.J., L.T.M., A.C.G., and C.M.B. performed experiments.
ACKNOWLEDGMENTS
We are grateful for the permission to conduct the research by the Ethiopian Science and Technology Commission, the Amhara and the Oromia Regional Governments, and the Semien Mountains and Bale Mountains National Parks. We thank the communities for their hospitality and participation in the study. Gezahegn Fentahun, M.D. and the late Daniel Tessema of Addis Ababa University worked in both Ethiopian field sites. Lawrence T. Monocello is now a graduate student in the Department of Anthropology in University of Alabama.
REFERENCES
- 1.Alkorta-Aranburu G, Beall CM, Witonsky DB, Gebremedhin A, Pritchard JK, Di Rienzo A. The genetic architecture of adaptations to high altitude in Ethiopia. PLoS Genet 8: e1003110, 2012. doi: 10.1371/journal.pgen.1003110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Archer SL, Huang JM, Hampl V, Nelson DP, Shultz PJ, Weir EK. Nitric oxide and cGMP cause vasorelaxation by activation of a charybdotoxin-sensitive K channel by cGMP-dependent protein kinase. Proc Natl Acad Sci USA 91: 7583–7587, 1994. doi: 10.1073/pnas.91.16.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnold WP, Mittal CK, Katsuki S, Murad F. Nitric oxide activates guanylate cyclase and increases guanosine 3′:5′-cyclic monophosphate levels in various tissue preparations. Proc Natl Acad Sci USA 74: 3203–3207, 1977. doi: 10.1073/pnas.74.8.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aste-Salazar H, Hurtado A. The affinity of hemoglobin for oxygen at sea level and at high altitudes. Am J Physiol 142: 733–743, 1944. [Google Scholar]
- 5.Beall CM, Brittenham GM, Strohl KP, Blangero J, Williams-Blangero S, Goldstein MC, Decker MJ, Vargas E, Villena M, Soria R, Alarcon AM, Gonzales C. Hemoglobin concentration of high-altitude Tibetans and Bolivian Aymara. Am J Phys Anthropol 106: 385–400, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 6.Dash RK, Bassingthwaighte JB. Erratum to: Blood HbO2 and HbCO2 dissociation curves at varied O2, CO2, pH, 2,3-DPG and temperature levels. Ann Biomed Eng 38: 1683–1701, 2010. doi: 10.1007/s10439-010-9948-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dejam A, Hunter CJ, Tremonti C, Pluta RM, Hon YY, Grimes G, Partovi K, Pelletier MM, Oldfield EH, Cannon RO III, Schechter AN, Gladwin MT. Nitrite infusion in humans and nonhuman primates: endocrine effects, pharmacokinetics, and tolerance formation. Circulation 116: 1821–1831, 2007. doi: 10.1161/CIRCULATIONAHA.107.712133. [DOI] [PubMed] [Google Scholar]
- 8.Erzurum SC, Ghosh S, Janocha AJ, Xu W, Bauer S, Bryan NS, Tejero J, Hemann C, Hille R, Stuehr DJ, Feelisch M, Beall CM. Higher blood flow and circulating NO products offset high-altitude hypoxia among Tibetans. Proc Natl Acad Sci USA 104: 17593–17598, 2007. doi: 10.1073/pnas.0707462104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferguson SK, Glean AA, Holdsworth CT, Wright JL, Fees AJ, Colburn TD, Stabler T, Allen JD, Jones AM, Musch TI, Poole DC. Skeletal muscle vascular control during exercise: impact of nitrite infusion during nitric oxide synthase inhibition in healthy rats. J Cardiovasc Pharmacol Ther 21: 201–208, 2016. doi: 10.1177/1074248415599061. [DOI] [PubMed] [Google Scholar]
- 10.Green LC, Ruiz de Luzuriaga K, Wagner DA, Rand W, Istfan N, Young VR, Tannenbaum SR. Nitrate biosynthesis in man. Proc Natl Acad Sci USA 78: 7764–7768, 1981. doi: 10.1073/pnas.78.12.7764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoit BD, Dalton ND, Erzurum SC, Laskowski D, Strohl KP, Beall CM. Nitric oxide and cardiopulmonary hemodynamics in Tibetan highlanders. J Appl Physiol (1985) 99: 1796–1801, 2005. doi: 10.1152/japplphysiol.00205.2005. [DOI] [PubMed] [Google Scholar]
- 12.Hoit BD, Dalton ND, Gebremedhin A, Janocha A, Zimmerman PA, Zimmerman AM, Strohl KP, Erzurum SC, Beall CM. Elevated pulmonary artery pressure among Amhara highlanders in Ethiopia. Am J Hum Biol 23: 168–176, 2011. doi: 10.1002/ajhb.21130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsia CC. Respiratory function of hemoglobin. N Engl J Med 338: 239–248, 1998. doi: 10.1056/NEJM199801223380407. [DOI] [PubMed] [Google Scholar]
- 14.Jacquez JA. Respiratory Physiology. Washington, DC: Hemisphere, 1979. [Google Scholar]
- 15.Jakob G, Mair J, Vorderwinkler KP, Judmaier G, König P, Zwierzina H, Pichler M, Puschendorf B. Clinical significance of urinary cyclic guanosine monophosphate in diagnosis of heart failure. Clin Chem 40: 96–100, 1994. [PubMed] [Google Scholar]
- 16.Jia L, Bonaventura C, Bonaventura J, Stamler JS. S-nitrosohaemoglobin: a dynamic activity of blood involved in vascular control. Nature 380: 221–226, 1996. doi: 10.1038/380221a0. [DOI] [PubMed] [Google Scholar]
- 17.Kuhn M. Cardiac actions of atrial natriuretic peptide: new visions of an old friend. Circ Res 116: 1278–1280, 2015. doi: 10.1161/CIRCRESAHA.115.306325. [DOI] [PubMed] [Google Scholar]
- 18.Lundgrin EL, Janocha AJ, Koch CD, Gebremedhin A, Di Rienzo A, Alkorta-Aranburu G, Brittenham GM, Erzurum SC, Beall CM. Plasma hepcidin of Ethiopian highlanders with steady-state hypoxia. Blood 122: 1989–1991, 2013. doi: 10.1182/blood-2013-03-491068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McNamara DT, Kasehagen LJ, Grimberg BT, Cole-Tobian J, Collins WE, Zimmerman PA. Diagnosing infection levels of four human malaria parasite species by a polymerase chain reaction/ligase detection reaction fluorescent microsphere-based assay. Am J Trop Med Hyg 74: 413–421, 2006. [PMC free article] [PubMed] [Google Scholar]
- 20.Moore LG, Harrison GL, McCullough RE, McCullough RG, Micco AJ, Tucker A, Weil JV, Reeves JT. Low acute hypoxic ventilatory response and hypoxic depression in acute altitude sickness. J Appl Physiol (1985) 60: 1407–1412, 1986. [DOI] [PubMed] [Google Scholar]
- 21.Palmer SK, Moore LG, Young D, Cregger B, Berman JC, Zamudio S. Altered blood pressure course during normal pregnancy and increased preeclampsia at high altitude (3100 meters) in Colorado. Am J Obstet Gynecol 180: 1161–1168, 1999. doi: 10.1016/S0002-9378(99)70611-3. [DOI] [PubMed] [Google Scholar]
- 22.Petersson J, Phillipson M, Jansson EA, Patzak A, Lundberg JO, Holm L. Dietary nitrate increases gastric mucosal blood flow and mucosal defense. Am J Physiol Gastrointest Liver Physiol 292: G718–G724, 2007. doi: 10.1152/ajpgi.00435.2006. [DOI] [PubMed] [Google Scholar]
- 23.Popkin BM, D’Anci KE, Rosenberg IH. Water, hydration, and health. Nutr Rev 68: 439–458, 2010. doi: 10.1111/j.1753-4887.2010.00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sánchez-Godoy JA. Respiratory physiology. In: Principles of Pulmonary Protection in Heart Surgery, edited by Gabriel EA, Salerno T. London: Springer, 2010, p. 9–26. doi: 10.1007/978-1-84996-308-4_2. [DOI] [Google Scholar]
- 25.Scacchi R, Corbo RM, Rickards O, Destefano GF. Survey of seven plasma-protein polymorphisms in the Amhara and Oromo populations of Ethiopia. Am J Hum Biol 6: 773–781, 1994. doi: 10.1002/ajhb.1310060611. [DOI] [PubMed] [Google Scholar]
- 26.Stamler JS, Jia L, Eu JP, McMahon TJ, Demchenko IT, Bonaventura J, Gernert K, Piantadosi CA. Blood flow regulation by S-nitrosohemoglobin in the physiological oxygen gradient. Science 276: 2034–2037, 1997. doi: 10.1126/science.276.5321.2034. [DOI] [PubMed] [Google Scholar]
- 27.Storz JF. Hemoglobin function and physiological adaptation to hypoxia in high-altitude mammals. J Mammal 88: 24–31, 2007. doi: 10.1644/06-MAMM-S-199R1.1. [DOI] [Google Scholar]
- 28.Tartaglia M, Scano G, DeStefano GF. An anthropogenetic study on the Oromo and Amhara of central Ethiopia. Am J Hum Biol 8: 505–516, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- 29.West JB. High-altitude medicine. Am J Respir Crit Care Med 186: 1229–1237, 2012. doi: 10.1164/rccm.201207-1323CI. [DOI] [PubMed] [Google Scholar]