Abstract
Krüppel-like factors (KLFs) are a family of zinc-finger transcription factors critical to mammalian embryonic development, regeneration, and human disease. There is emerging evidence that KLFs play a vital role in key physiological processes in the kidney, ranging from maintenance of glomerular filtration barrier to tubulointerstitial inflammation to progression of kidney fibrosis. Seventeen members of the KLF family have been identified, and several have been well characterized in the kidney. Although they may share some overlap in their downstream targets, their structure and function remain distinct. This review highlights our current knowledge of KLFs in the kidney, which includes their pattern of expression and their function in regulating key biological processes. We will also critically examine the currently available literature on KLFs in the kidney and offer some key areas in need of further investigation.
Keywords: Krüppel-like factors, glomerular disease, fibrosis, differentiation, inflammation, endothelial cells
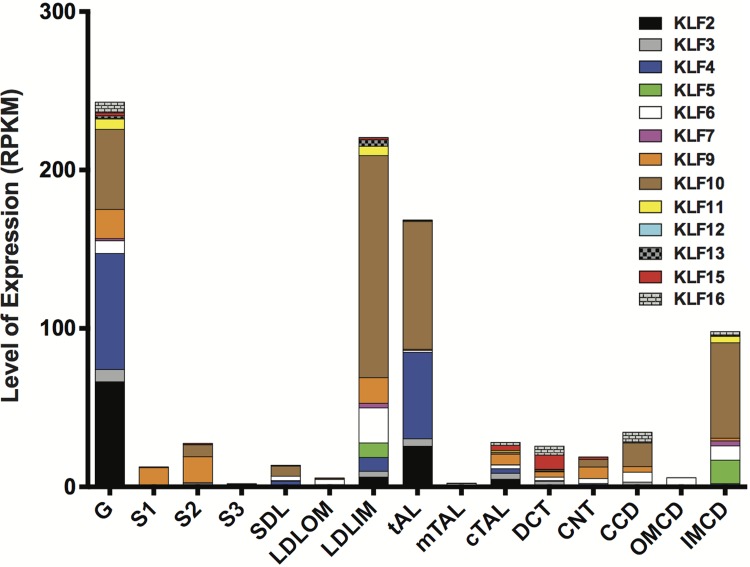
the word Krüppel refers to “cripple” in German. Consequently, when H. U. Gloor identified a critical mutation that led to severe body malformation in Drosophila, he labeled it Krüppel (14). Further work by Christiane Nusslein-Volhard and Eric Weischaus demonstrated that Krüppel mutations in Drosophila contribute to embryonic lethality due to significant defects in body segmentation (51). Krüppel-like factors (KLFs) are a subclass of zinc-finger family of DNA-binding transcriptional regulators that are involved in a broad range of cellular processes (i.e., cell differentiation, apoptosis, cell proliferation) (30). The COOH-terminal region of KLFs is highly conserved with three C2H2 zinc finger domains that mediate transcriptional activity by interacting with GC-rich DNA sequences. In contrast, the specificity of protein-protein and protein-DNA interactions of KLFs are determined by the NH2-terminal region (30). KLFs are typically categorized into the following groups due to similarities in structure and transcriptional activity (1): KLF3, -8, and -12 (2); KLF1, -2, -4, -5, -6, and -7 (3); KLF9, -10, -11, -13, -14, and -16. However, KLF15 and KLF17 have not been classified since their interaction motifs have yet to be determined (30). In the past few years, there has been a dramatic increase in the number of publications on the expression and function of KLFs in the kidney. Furthermore, interrogation of recent gene expression arrays from deep sequencing of microdissected nephron segments of rat renal cortex demonstrate the diverse expression pattern of KLFs in the kidney (25) (Fig. 1). Here, we highlight the critical role of KLFs in kidney disease by reviewing recently reported data. Furthermore, we provide potential areas for further investigations.
Fig. 1.
Expression pattern of Krüppel-like factors in the kidney. Deep sequencing of microdissected nephron segments was performed in rat renal cortex by Lee et al. (25). From these reported findings (25), we extrapolated the Krüppel-like factors’ (KLFs) mRNA expression [reads per kilobase per million reads (RPKM)] from the expression arrays and highlighted its pattern of expression in each nephron segment. Nephron segments are as follows: G (glomeruli), S1 (1st segment of the proximal tubule), S2 (2nd segment of the proximal tubule), S3 (3rd segment of the proximal tubule), SDL (short descending limb of the loop of Henle), LDLOM (long descending limb of the loop of Henle in the outer medulla), LDLIM (long descending limb of the loop of Henle in the inner medulla), tAL (thin ascending limb of the loop of Henle), mTAL (medullary thick ascending limb of the loop of Henle), cTAL (cortical thick ascending limb of the loop of Henle), DCT (distal convoluted tubule), CNT (connecting tubule), CCD (cortical collecting duct), OMCD (outer medullary collecting duct), and IMCD (inner medullary collecting duct). Detailed methods for microdissection and RNA sequencing were previously provided (25).
KLFs in Glomerular Disease
Chronic kidney disease (CKD) is a leading risk factor for cardiovascular disease and stroke (15, 50). Podocytes (visceral glomerular epithelial cells) in normal mature kidneys are regarded as highly differentiated and quiescent cells. In many glomerular diseases such as Focal Segmental Glomerulosclerosis (FSGS) and HIV-associated nephropathy (HIVAN), podocytes are injured (31). In the setting of injury, podocytes undergo a major change in phenotype, resulting in a loss of podocyte cytoskeleton, actin stress fiber formation, and their terminal differentiation markers (3). A recent comparative promoter analysis of podocyte slit diaphragm molecules revealed that many podocyte-specific genes share KLF binding sites in their promoter region (7).
We recently reported that KLF15 or KKLF (kidney-enriched KLF) is required for restoration of podocyte differentiation markers under cell stress (Fig. 2) (29). KLF15 is an early inducible gene and putative binding sites for KLF15 are present in the promoter region of podocyte-specific genes such as Nephrin and Podocin (29). In addition, the overexpression of KLF15 increased the expression of Nephrin in wild-type and HIV-1 infected human podocytes (a model of podocyte dedifferentiation and injury) (29). Furthermore, knockdown of Klf15 exacerbated albuminuria and podocyte effacement with a reduction in podocyte differentiation markers in proteinuric murine models (29). Interestingly, KLF15 is a key regulator of retinoic acid (RA)-induced restoration of podocyte differentiation markers (Nephrin, Synaptopodin, and Podocin) and amelioration of podocyte injury in cultured human podocytes. Furthermore, the loss of Klf15 in mice attenuated RA-mediated restoration of podocyte differentiation markers after lipopolysaccharide treatment (29). KLF15 expression is also reduced in human primary glomerulopathies such as FSGS and HIVAN compared with healthy control subjects (29).
Fig. 2.
Role of Krüppel-like factor 15 in podocyte differentiation. We highlight the pathway by which Krüppel-like factor 15 (KLF15) mediates retinoic acid (RA) and glucocorticoids (GCs)-induced restoration of podocyte differentiation markers [actin cytoskeleton, increased expression of podocyte-specific cytoskeleton and slit diaphragm proteins (Nephrin, Podocin, and Synaptopodin)] under cell stress. GR, glucocorticoid receptor; cAMP, cyclic adenosine monophosphate; PKA, protein kinase A; CREB, cAMP response element-binding protein; GRE, GC response element; CRE, cAMP response element.
Glucocorticoids (GCs) are the initial treatment option for many glomerular diseases, such as Minimal Change Disease (MCD) and FSGS (46). Alternate immunosuppressive therapy is typically not considered until patients have failed GC therapy (35). Other than their immunomodulatory effects, recent studies demonstrate that GCs may exert their therapeutic benefits by direct action on podocytes (16, 35, 48, 49, 54). Similar to RA, we recently reported that the salutary benefits of GCs [stabilization of actin cytoskeleton and expression of podocyte-specific cytoskeleton proteins (Nephrin, Synaptopodin)] in the podocyte are directly mediated by KLF15 in cultured human podocytes and proteinuric murine models (27). In addition, the level of KLF15 expression in the glomeruli directly correlated with responsiveness to GCs in patients with primary FSGS and MCD (27).
Although Klf15−/− mice exhibited increased susceptibility to glomerular injury, no overt injury was observed at baseline (29). Interestingly, screening of other Klfs in glomerular fractions in the Klf15−/− mice revealed a significant increase in Klf4 and Klf6 expression, suggesting a potential compensatory mechanism (data not shown). Based on these findings, we initially investigated the role of KLF6 in glomerular disease. Similar to KLF15, KLF6 is expressed in the tubular as well as the glomerular compartment (10, 28). We also observed that KLF6 is an early injury response gene and podocyte-specific loss of Klf6 increased the susceptibility to FSGS in the resistant C57BL/6 mouse strain (28). Interestingly, KLF6 regulates the expression of cytochrome c oxidase (COX) assembly gene, critical to mitochondrial function in the podocyte. In addition, KLF6 binding sites occupy the promoter region of COX assembly gene. Consequently, the loss of KLF6 in cultured human podocytes exacerbated mitochondrial injury under cell stress, leading to activation of intrinsic apoptotic pathway and eventual cell death (28).
KLF4 was initially described to exhibit differential expression in growth-arrested intestinal epithelial cells (37). Furthermore, induction of KLF4 results in G1/S phase cell cycle arrest in colon cancer cell lines (5). Subsequent studies have clearly demonstrated its role as a negative regulator of cellular proliferation in several types of epithelial cells by inducing cell cycle arrest in the G1/S phase (5, 40). Furthermore, KLF4 is an essential transcription factor in the induction of pluripotency from somatic cells (42). Hayashi et al. initially reported that KLF4 is expressed solely in podocytes based on colocalization studies by immunofluorescence, but subsequent studies from the same laboratory showed that KLF4 is also expressed in endothelial cells (18, 56). Consequently, further studies are required to validate these discrepant findings. Nonetheless, the authors demonstrate that KLF4 expression is reduced in proteinuric murine models and kidney biopsies of nephrotic syndrome, and this correlated with increased methylation of the Nephrin promoter and decreased Nephrin expression (18). Restoration of glomerular Klf4 by either gene transfer of Klf4-containing plasmids, using a podocyte-specific tetracycline-based inducible system, or by treatment with an angiotensin receptor blocker (ARB), attenuated albuminuria and reduced methylation of the Nephrin promoter in proteinuric murine models, suggesting an epigenetic-mediated regulation of podocyte-specific genes (18, 19).
KLF6 and KLF15 have been reported to play a role in mesangial cell function (Table 1). KLF6 expression was induced in cultured glomerular mesangial cells in the setting of sublytic complement-mediated apoptosis (55). In addition, KLF15 expression was reduced in mesangial cells during the proliferative phase of Thy-1 rat model of mesangial glomerulonephritis (21). Interestingly, modulating the expression of KLF15 in cultured mesangial cells inversely regulated the levels of E2F1, cyclin D1, and CDK2 expression. These studies confirm the findings of KLF15 as a key regulator of cell differentiation.
Table 1.
Reported expression and function of KLFs in the kidney
| Name | References | Expression Pattern in the Kidney at Baseline | Experimental Models | Reported Function and Disease Association |
|---|---|---|---|---|
| KLF2 | (1, 39, 43, 56, 57) | Endothelial cells | STZ (mice); Uni-Nx (mice); cultured HUVEC; glomerular endothelial cells; IRI (rats); human kidney biopsies | Diabetic nephropathy; compensatory renal hypertrophy; ischemic AKI |
| KLF4 | (4, 18, 19, 36, 38, 52, 55) | Endothelial cells and podocytes | IRI (mice); Adriamycin (mice); PAN (mice); db/db (mice); UUO (mice); cultured human podocytes; proximal tubular cells; HUVEC; human kidney biopsies | Epigenetic regulation of podocyte gene expression; mediates statin-induced renoprotective effects in ischemic AKI; kidney fibrosis; endothelial injury in antibody-mediated rejection (post-kidney transplantation) |
| KLF5 | (4, 11) | Collecting duct cells | UUO (mice); cultured IMCD cells; proximal tubular cells | Regulates inflammatory response in kidney fibrosis |
| KLF6 | (10, 20, 28, 54) | Podocytes; tubular cells | Adriamycin (mice); STZ (rats); cultured human podocytes; primary mouse podocytes; HK2 cells; human kidney biopsies | Podocyte apoptosis (regulates mitochondrial function); kidney fibrosis (TGF-β pathway) |
| KLF12 | (41) | Inner medullary collecting duct cells | Kidney cortex (mice); cultured MDCK cells | Regulates expression of urea transporter (UT-A1) |
| KLF15 | (12, 13, 21, 27, 29, 44) | Podocytes; mesangial cells; tubular cells and fibroblasts | HIV-1 transgenic mice; Adriamycin (mice); LPS (mice); anti-glomerular antibody (mice); 5/6 Nx + high-protein diet (rats); Thy-1 mesangial GN (rats); cultured human podocytes; primary mouse podocytes; rat renal fibroblasts; human kidney biopsies | Regulates podocyte differentiation markers; attenuates mesangial cell proliferation; kidney fibrosis (inhibit TGF-β-induced CTGF signaling) |
STZ, streptozotocin; Nx, nephrectomy, HUVEC, human umbilical vein endothelial cells; IRI, ischemia-reperfusion injury; PAN, puromycin nephropathy; AKI, acute kidney injury; UUO, unilateral ureteric obstruction; IMCD, inner medullary collecting duct; HK, human kidney; MDCK, Madin-Darby canine kidney; LPS, lipopolysaccharide.
KLFs in Tubular Injury
KLF15 was initially described in the kidney and was subsequently coined as the “Kidney-enriched KLF” (KKLF) (44). Specifically, the authors demonstrated by immunofluorescence that KLF15 was expressed in cells where kidney-specific CLC chloride channels were absent. Interestingly, KLF15 suppresses the expression of CLC-K1 and CLC-K2 channels by transcriptionally regulating myc-associated zinc-finger protein in rat kidney cells (44). In contrast, KLF12 is expressed in the collecting ducts and colocalized to the urea transporter UT-A1 in mouse kidney (41). The authors showed that KLF12 is expressed 15 days postconception and regulates the expression of UT-A1 by increasing its promoter activity in mice (41). However, it is unclear whether KLF12 or KLF15 has a functional role in chloride or urea transport, respectively. Furthermore, gene expression arrays from Lee et al. (25) highlight a substantial level of expression in KLF9 and KLF10 compared with other KLFs in several segments of the nephron. However, little is known about the significance of KLF9 and KLF10 in the kidney. KLF9 was identified as a potential downstream target of mineralocorticoid receptor in mouse distal convoluted tubular epithelial cells in culture (45), but further studies are required to assess whether the expression of these zinc finger proteins in the tubule contributes to channelopathies in the kidney.
KLFs in Interstitial Inflammation
As in endothelial cells, KLF2 and KLF4 are expressed in T cells. Generation of regulatory T cells is critical to preventing autoimmunity in several renal and nonrenal disorders. Specifically, KLF2 was recently shown to regulate the production of T regulatory cells in mice (33), which might serve as a critical target in the treatment of cellular rejection post-kidney transplantation. In addition, KLF4 was reported as a key regulator of macrophage polarization in mice (26). Liao et al. (26) demonstrated that KLF4 expression was markedly upregulated in M2 subtype macrophages compared with M1 macrophages in mice, suggesting an increase in anti-inflammatory and pro-fibrotic phenotype. Furthermore, direct interaction between KLF4 and STAT6 in macrophages inhibited M1 programming by blocking the cofactors for nuclear factor-kappa B (NF-κβ) signaling, while activating signaling for M2 macrophages in mice (26). Since tubulointerstitial inflammation is a critical process in several renal disorders, further investigations in the role of KLFs in mediating inflammation specifically in the kidney are required.
KLFs in Fibrosis
Although the anti-inflammatory effects of KLF4 are clear, the precise role of KLF4 in renal fibrosis remains debatable. KLF4-mediated regulation of the canonical TGF-β pathway appears to be cell-specific. For instance, overexpression of KLF4 in human proximal tubular cells suppressed macrophage migration inhibitory factor (MIF) and monocyte chemotactic protein-1 (MCP-1) levels, key mediators of TGF-β pathway (32). In contrast, other laboratories have reported that KLF4 transcriptionally upregulates the expression of TGF-β in cultured cardiac fibroblasts (23). In turn, TGF-β signaling also regulates KLF4 expression. Specifically, TGF-β1 induces KLF4 phosphorylation via canonical and noncanonical pathways, which interacts with Smad2 to cooperatively activate TGF-β1 receptor in HEK 293T cells (22). In addition, TGF-β signaling through the Cdh1/APC pathway leads to ubiquitination and proteosomal degradation of KLF4 (22). Recent studies demonstrate that KLF4 expression is reduced in mice that underwent unilateral ureteric obstruction (4, 53). However, it remains unclear if this is merely an association or a consequence of fibrosis. Consequently, further studies are required to investigate the role of KLF4 in a cell-specific manner in kidney fibrosis.
KLF5 is reportedly expressed in the collecting duct (Table 1). Fujiu et al. (11) recently demonstrated that mice with haploinsufficiency for Klf5 exhibited markedly less kidney injury after unilateral ureteric obstruction. The authors further showed that the loss of Klf5 contributed to less macrophage M2 subtype accumulation in the kidney. Conversely, cooperative interaction between KLF5 and C/EBPα increased chemotactic proteins that contribute to M1 type macrophage accumulation in mice. The authors also showed that ablation of Klf5 specifically in the collecting duct contributed to progression of kidney fibrosis in mice (11). Recent in vitro studies demonstrate that increasing the matrix stiffness in cultured mouse proximal tubular cells leads to significant upregulation of KLF5 (4). In contrast, increased matrix stiffness leads to suppression of KLF4 expression in these cells. The authors also showed that increasing matrix stiffness activated ERK signaling, which contributed to Yes-associated protein 1 (YAP1) mediated stabilization of KLF5. In addition, reducing matrix stiffness directly lowered ERK/MAPK signaling, YAP1, and KLF5 expression while preserving KLF4 expression in cultured proximal tubular cells (4).
In addition to its critical role in enhancing mitochondrial function in the podocyte, KLF6 is also expressed in the proximal tubule cells in the kidney (Table 1). Previous studies have demonstrated that KLF6 transcriptionally regulates TGF-β expression in liver fibrosis. Similarly, Holian et al. (20) showed that overexpression of KLF6 in cultured proximal tubule cells exposed to high glucose reduced the expression of epithelial markers while increasing mesenchymal markers. However, these findings have yet to be demonstrated in murine models of kidney fibrosis.
Recent studies have also highlighted that KLF15 might play a role in the progression of kidney fibrosis (Table 1). By immunostaining, KLF15 is expressed in the glomerular as well as the tubulointerstitial compartments of the kidney (27, 29). Furthermore, KLF15 expression was reduced in 5/6 nephrectomized rats on a high-protein diet (12). Interestingly, protein restriction increased KLF15 expression with subsequent attenuation in kidney injury in these 5/6 nephrectomized rats (12). Furthermore, global Klf15−/− mice demonstrated increased susceptibility to glomerulosclerosis after uninephrectomy. Interestingly, non-Smad-dependent TGF-β signaling suppressed KLF15 expression in cultured rat renal fibroblasts. Conversely, overexpression of KLF15 directly inhibited canonical TGF-β-induced CTGF signaling in cultured rat renal fibroblasts (13). Collectively, these studies demonstrate that KLFs play an important role in kidney fibrosis. However, redundancy and cooperative interaction between KLFs and TGF-β signaling in kidney fibrosis have yet to be explored.
KLFs in Renovascular Injury
Several members of the KLF family contribute to vascular homeostasis (Table 1). Although some overlap exists between KLFs in their role in endothelial biology, they remain distinct in the mechanisms by which they regulate key downstream targets. Since structural and functional similarities exist between KLF2 and KLF4, it is not surprising that both have been reported to play an active role in maintaining endothelial homeostasis. Furthermore, the specific role of these KLFs in endothelial biology has been highlighted in detail in recent reviews (2, 9).
Although initially characterized in epithelial cells, KLF4 is highly expressed in vascular endothelial cells and modulates their antithrombotic and anti-inflammatory properties. Furthermore, induction of KLF4 in cultured endothelial cells increases the expression of endothelial nitric oxide synthase (eNOS) and thrombomodulin (THBD) (47), whereas knockdown of KLF4 leads to increased Vascular cell adhesion molecule (VCAM-1) expression in TNF-α-treated endothelial cells (17). In response to proinflammatory stimuli, KLF4 is induced early with subsequent activation of anti-inflammatory pathways in cultured endothelial cells (17). Specifically in the kidney, endothelial KLF4 was protective against acute kidney injury (AKI) in the murine model of ischemia-reperfusion injury, where the loss of endothelial Klf4 increased AKI, inflammation, and the expression of adhesion molecules (ICAM-1, VCAM-1) after IRI (56). However, these findings have yet to be validated by other laboratories since the conditional deletion of Klf4 in mice was performed using tyrosine kinase promoter, which is expressed in endothelial cells and hematopoietic cells. Endothelial expression of adhesion molecules is mediated by the NF-κβ pathway (8). Interestingly, KLF4 was demonstrated to regulate NF-κβ pathway by directly inhibiting p65, the subunit required for NF-κβ activation, thereby modulating the expression of endothelial adhesion molecules in cultured endothelial cells (56). The authors also report that the endothelial-specific loss of Klf4 attenuates the pleotropic effects of statins in AKI in mice (56).
Similar to KLF4, KLF2 expression is regulated by laminar shear stress and proinflammatory stimuli and confers an anti-inflammatory and antithrombotic phenotype in the vascular endothelium (2, 17, 34). Klf2 is expressed early during mammalian development, at embryonic day 8.5 (E8.5) (24), and both global and endothelial-specific Klf2 knockout mice die as early as E14 due to a loss in blood vessel integrity, leading to hemorrhage and high-output heart failure (24, 52). In glomerular endothelial cells in culture, the expression of KLF2, and its downstream targets endothelial nitric oxide (eNOS), thrombomodulin (THBD), and endothelin-1, is upregulated upon exposure to chronic laminar flow by activation of the ERK5 signaling pathway (39). In human diabetic kidney biopsy samples and in a streptozotocin (STZ) rat model of diabetes, glomerular KLF2 was reduced compared with controls (57). Endothelial-specific knockdown of Klf2 exacerbated diabetic kidney injury and increased expression of angiogenesis markers (57). More recently, we reported that these mice are also susceptible to increased glomerular injury in the setting of glomerular hyperfiltration after unilateral uninephrectomy (58). The increased shear stress with glomerular hyperfiltration initially contributes to a renoprotective induction in Klf2 expression in cultured endothelial cells. Consequently mice with knockdown of Klf2 have lost this compensatory response, thereby exacerbating glomerular injury. Interestingly, endothelial-specific loss of Klf2 contributes to podocyte injury in these models, suggesting a potential cross-talk that needs to be further explored. Similar to KLF4, statins also induce the expression of KLF2 after ischemia reperfusion injury in mice (43).
After kidney transplantation, endothelial injury secondary to thrombotic microangiopathy (TMA) can occur as a result of chronic antibody-mediated rejection (chronic ABMR) or calcineurin inhibitor toxicity. Although these lesions are well characterized histologically, mechanisms mediating endothelial injury post kidney transplantation remain poorly understood. Interestingly, glomerular KLF2 and KLF4 expression was significantly downregulated in microdissected glomeruli from human kidney biopsies with TMA posttransplantation (1). In contrast, KLF4 expression was the highest increased transcript from gene expression arrays performed on renal cortex with chronic ABMR compared with T cell-mediated rejection (36, 38). Interestingly, the differentially expressed endothelial associated transcripts in chronic ABMR involved in inflammation, angiogenesis, adhesion, and thrombosis were regulated by induction of KLF4 expression in an independent study (47).
Since KLF2 and KLF4 share some redundancy in their function in endothelial cells, it is not surprising that they are both required for vasculogenesis in mouse embryos (6). For instance, single knockouts live longer during embryogenesis than the double knockouts of Klf2 and Klf4 due to severity in vascular injury (6). These data suggest that KLF2 and KLF4 might cooperatively interact to regulate the function of key endothelial transcripts in the setting of vascular injury in the kidney.
Conclusions
In this review, we highlight the diverse and intersecting roles of KLFs in maintaining homeostasis in the kidney. Although a majority of the literature has focused on the role of KLFs in development and regeneration, there is clear evidence from multiple laboratories that they also play a vital role in the progression of human disease. In recent years, there has been a dramatic rise in studies on KLFs in glomerular disease, inflammation, kidney fibrosis, and vascular biology. Nonetheless, significant gaps lie ahead in bridging the link between the expression and function of these zinc-finger transcription factors in renal physiology and disease.
GRANTS
This work was supported by funds from the National Institute of Diabetes and Digestive and Kidney Diseases (DK-102519), the American Heart Association (16-GRNT-31280004), and Dialysis Clinic, Inc., to S. K. Mallipattu.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
S.K.M. performed experiments; S.K.M. analyzed data; S.K.M. interpreted results of experiments; S.K.M. and C.C.E. prepared figures; S.K.M. and C.C.E. drafted manuscript; S.K.M., C.C.E., and J.C.H. edited and revised manuscript; S.K.M., C.C.E., and J.C.H. approved final version of manuscript.
REFERENCES
- 1.Agustian PA, Bockmeyer CL, Modde F, Wittig J, Heinemann FM, Brundiers S, Dämmrich ME, Schwarz A, Birschmann I, Suwelack B, Jindra PT, Ahlenstiel T, Wohlschläger J, Vester U, Ganzenmüller T, Zilian E, Feldkamp T, Spieker T, Immenschuh S, Kreipe HH, Bröcker V, Becker JU. Glomerular mRNA expression of prothrombotic and antithrombotic factors in renal transplants with thrombotic microangiopathy. Transplantation 95: 1242–1248, 2013. doi: 10.1097/TP.0b013e318291a298. [DOI] [PubMed] [Google Scholar]
- 2.Atkins GB, Jain MK. Role of Krüppel-like transcription factors in endothelial biology. Circ Res 100: 1686–1695, 2007. doi: 10.1161/01.RES.0000267856.00713.0a. [DOI] [PubMed] [Google Scholar]
- 3.Barisoni L, Kriz W, Mundel P, D’Agati V. The dysregulated podocyte phenotype: a novel concept in the pathogenesis of collapsing idiopathic focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol 10: 51–61, 1999. [DOI] [PubMed] [Google Scholar]
- 4.Chen WC, Lin HH, Tang MJ. Matrix-stiffness-regulated inverse expression of Krüppel-Like Factor 5 and Krüppel-Like Factor 4 in the pathogenesis of renal fibrosis. Am J Pathol 185: 2468–2481, 2015. doi: 10.1016/j.ajpath.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 5.Chen X, Johns DC, Geiman DE, Marban E, Dang DT, Hamlin G, Sun R, Yang VW. Krüppel-like factor 4 (gut-enriched Krüppel-like factor) inhibits cell proliferation by blocking G1/S progression of the cell cycle. J Biol Chem 276: 30423–30428, 2001. doi: 10.1074/jbc.M101194200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiplunkar AR, Curtis BC, Eades GL, Kane MS, Fox SJ, Haar JL, Lloyd JA. The Krüppel-like factor 2 and Krüppel-like factor 4 genes interact to maintain endothelial integrity in mouse embryonic vasculogenesis. BMC Dev Biol 13: 40, 2013. doi: 10.1186/1471-213X-13-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen CD, Klingenhoff A, Boucherot A, Nitsche A, Henger A, Brunner B, Schmid H, Merkle M, Saleem MA, Koller KP, Werner T, Gröne HJ, Nelson PJ, Kretzler M. Comparative promoter analysis allows de novo identification of specialized cell junction-associated proteins. Proc Natl Acad Sci USA 103: 5682–5687, 2006. doi: 10.1073/pnas.0511257103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collins T, Read MA, Neish AS, Whitley MZ, Thanos D, Maniatis T. Transcriptional regulation of endothelial cell adhesion molecules: NF-kappa B and cytokine-inducible enhancers. FASEB J 9: 899–909, 1995. [PubMed] [Google Scholar]
- 9.Feinberg MW, Lin Z, Fisch S, Jain MK. An emerging role for Krüppel-like factors in vascular biology. Trends Cardiovasc Med 14: 241–246, 2004. doi: 10.1016/j.tcm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Fischer EA, Verpont MC, Garrett-Sinha LA, Ronco PM, Rossert JA. Klf6 is a zinc finger protein expressed in a cell-specific manner during kidney development. J Am Soc Nephrol 12: 726–735, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Fujiu K, Manabe I, Nagai R. Renal collecting duct epithelial cells regulate inflammation in tubulointerstitial damage in mice. J Clin Invest 121: 3425–3441, 2011. doi: 10.1172/JCI57582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao X, Huang L, Grosjean F, Esposito V, Wu J, Fu L, Hu H, Tan J, He C, Gray S, Jain MK, Zheng F, Mei C. Low-protein diet supplemented with ketoacids reduces the severity of renal disease in 5/6 nephrectomized rats: a role for KLF15. Kidney Int 79: 987–996, 2011. doi: 10.1038/ki.2010.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao X, Wu G, Gu X, Fu L, Mei C. Kruppel-like factor 15 modulates renal interstitial fibrosis by ERK/MAPK and JNK/MAPK pathways regulation. Kidney Blood Press Res 37: 631–640, 2013. doi: 10.1159/000355743. [DOI] [PubMed] [Google Scholar]
- 14.Gloor HU. Schädigungsmuster eines Letalfaktors (Kr) von Drosophila melanogaster. Arch Jul Klaus Stiftung 25: 38–44, 1950. [Google Scholar]
- 15.Grundy SM, Garber A, Goldberg R, Havas S, Holman R, Lamendola C, Howard WJ, Savage P, Sowers J, Vega GL. Prevention Conference VI: Diabetes and Cardiovascular Disease: Writing Group IV: lifestyle and medical management of risk factors. Circulation 105: e153–e158, 2002. doi: 10.1161/01.CIR.0000014022.85836.96. [DOI] [PubMed] [Google Scholar]
- 16.Guess A, Agrawal S, Wei CC, Ransom RF, Benndorf R, Smoyer WE. Dose- and time-dependent glucocorticoid receptor signaling in podocytes. Am J Physiol Renal Physiol 299: F845–F853, 2010. doi: 10.1152/ajprenal.00161.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamik A, Lin Z, Kumar A, Balcells M, Sinha S, Katz J, Feinberg MW, Gerzsten RE, Edelman ER, Jain MK. Kruppel-like factor 4 regulates endothelial inflammation. J Biol Chem 282: 13769–13779, 2007. doi: 10.1074/jbc.M700078200. [DOI] [PubMed] [Google Scholar]
- 18.Hayashi K, Sasamura H, Nakamura M, Azegami T, Oguchi H, Sakamaki Y, Itoh H. KLF4-dependent epigenetic remodeling modulates podocyte phenotypes and attenuates proteinuria. J Clin Invest 124: 2523–2537, 2014. doi: 10.1172/JCI69557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayashi K, Sasamura H, Nakamura M, Sakamaki Y, Azegami T, Oguchi H, Tokuyama H, Wakino S, Hayashi K, Itoh H. Renin-angiotensin blockade resets podocyte epigenome through Kruppel-like Factor 4 and attenuates proteinuria. Kidney Int 88: 745–753, 2015. doi: 10.1038/ki.2015.178. [DOI] [PubMed] [Google Scholar]
- 20.Holian J, Qi W, Kelly DJ, Zhang Y, Mreich E, Pollock CA, Chen XM. Role of Kruppel-like factor 6 in transforming growth factor-beta1-induced epithelial-mesenchymal transition of proximal tubule cells. Am J Physiol Renal Physiol 295: F1388–F1396, 2008. doi: 10.1152/ajprenal.00055.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hong Q, Li C, Xie Y, Lv Y, Liu X, Shi S, Ding R, Zhang X, Zhang L, Liu S, Chen X. Kruppel-like factor-15 inhibits the proliferation of mesangial cells. Cell Physiol Biochem 29: 893–904, 2012. doi: 10.1159/000178518. [DOI] [PubMed] [Google Scholar]
- 22.Hu D, Wan Y. Regulation of Krüppel-like factor 4 by the anaphase promoting complex pathway is involved in TGF-beta signaling. J Biol Chem 286: 6890–6901, 2011. doi: 10.1074/jbc.M110.179952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.King KE, Iyemere VP, Weissberg PL, Shanahan CM. Krüppel-like factor 4 (KLF4/GKLF) is a target of bone morphogenetic proteins and transforming growth factor beta 1 in the regulation of vascular smooth muscle cell phenotype. J Biol Chem 278: 11661–11669, 2003. doi: 10.1074/jbc.M211337200. [DOI] [PubMed] [Google Scholar]
- 24.Lee JS, Yu Q, Shin JT, Sebzda E, Bertozzi C, Chen M, Mericko P, Stadtfeld M, Zhou D, Cheng L, Graf T, MacRae CA, Lepore JJ, Lo CW, Kahn ML. Klf2 is an essential regulator of vascular hemodynamic forces in vivo. Dev Cell 11: 845–857, 2006. doi: 10.1016/j.devcel.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 25.Lee JW, Chou CL, Knepper MA. Deep Sequencing in Microdissected Renal Tubules Identifies Nephron Segment-Specific Transcriptomes. J Am Soc Nephrol 26: 2669–2677, 2015. doi: 10.1681/ASN.2014111067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liao X, Sharma N, Kapadia F, Zhou G, Lu Y, Hong H, Paruchuri K, Mahabeleshwar GH, Dalmas E, Venteclef N, Flask CA, Kim J, Doreian BW, Lu KQ, Kaestner KH, Hamik A, Clément K, Jain MK. Krüppel-like factor 4 regulates macrophage polarization. J Clin Invest 121: 2736–2749, 2011. doi: 10.1172/JCI45444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mallipattu SK, Guo Y, Revelo MP, Roa-Peña L, Miller T, Ling J, Shankland SJ, Bialkowska AB, Ly V, Estrada C, Jain MK, Lu Y, Ma’ayan A, Mehrotra A, Yacoub R, Nord EP, Woroniecki RP, Yang VW, He JC. Krüppel-Like Factor 15 mediates glucocorticoid-induced restoration of podocyte differentiation markers. J Am Soc Nephrol 28: 166–184, 2017. doi: 10.1681/ASN.2015060672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mallipattu SK, Horne SJ, D’Agati V, Narla G, Liu R, Frohman MA, Dickman K, Chen EY, Ma’ayan A, Bialkowska AB, Ghaleb AM, Nandan MO, Jain MK, Daehn I, Chuang PY, Yang VW, He JC. Krüppel-like factor 6 regulates mitochondrial function in the kidney. J Clin Invest 125: 1347–1361, 2015. doi: 10.1172/JCI77084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mallipattu SK, Liu R, Zheng F, Narla G, Ma’ayan A, Dikman S, Jain MK, Saleem M, D’Agati V, Klotman P, Chuang PY, He JC. Kruppel-like factor 15 (KLF15) is a key regulator of podocyte differentiation. J Biol Chem 287: 19122–19135, 2012. doi: 10.1074/jbc.M112.345983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McConnell BB, Yang VW. Mammalian Krüppel-like factors in health and diseases. Physiol Rev 90: 1337–1381, 2010. doi: 10.1152/physrev.00058.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meyrier A. Mechanisms of disease: focal segmental glomerulosclerosis. Nat Clin Pract Nephrol 1: 44–54, 2005. doi: 10.1038/ncpneph0025. [DOI] [PubMed] [Google Scholar]
- 32.Mreich E, Chen XM, Zaky A, Pollock CA, Saad S. The role of Krüppel-like factor 4 in transforming growth factor-β-induced inflammatory and fibrotic responses in human proximal tubule cells. Clin Exp Pharmacol Physiol 42: 680–686, 2015. doi: 10.1111/1440-1681.12405. [DOI] [PubMed] [Google Scholar]
- 33.Pabbisetty SK, Rabacal W, Maseda D, Cendron D, Collins PL, Hoek KL, Parekh VV, Aune TM, Sebzda E. KLF2 is a rate-limiting transcription factor that can be targeted to enhance regulatory T-cell production. Proc Natl Acad Sci USA 111: 9579–9584, 2014. doi: 10.1073/pnas.1323493111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parmar KM, Larman HB, Dai G, Zhang Y, Wang ET, Moorthy SN, Kratz JR, Lin Z, Jain MK, Gimbrone MA Jr, García-Cardeña G. Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. J Clin Invest 116: 49–58, 2006. doi: 10.1172/JCI24787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schönenberger E, Ehrich JH, Haller H, Schiffer M. The podocyte as a direct target of immunosuppressive agents. Nephrol Dial Transplant 26: 18–24, 2011. doi: 10.1093/ndt/gfq617. [DOI] [PubMed] [Google Scholar]
- 36.Sellarés J, Reeve J, Loupy A, Mengel M, Sis B, Skene A, de Freitas DG, Kreepala C, Hidalgo LG, Famulski KS, Halloran PF. Molecular diagnosis of antibody-mediated rejection in human kidney transplants. Am J Transplant 13: 971–983, 2013. doi: 10.1111/ajt.12150. [DOI] [PubMed] [Google Scholar]
- 37.Shields JM, Christy RJ, Yang VW. Identification and characterization of a gene encoding a gut-enriched Krüppel-like factor expressed during growth arrest. J Biol Chem 271: 20009–20017, 1996. doi: 10.1074/jbc.271.33.20009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sis B, Jhangri GS, Bunnag S, Allanach K, Kaplan B, Halloran PF. Endothelial gene expression in kidney transplants with alloantibody indicates antibody-mediated damage despite lack of C4d staining. Am J Transplant 9: 2312–2323, 2009. doi: 10.1111/j.1600-6143.2009.02761.x. [DOI] [PubMed] [Google Scholar]
- 39.Slater SC, Ramnath RD, Uttridge K, Saleem MA, Cahill PA, Mathieson PW, Welsh GI, Satchell SC. Chronic exposure to laminar shear stress induces Kruppel-like factor 2 in glomerular endothelial cells and modulates interactions with co-cultured podocytes. Int J Biochem Cell Biol 44: 1482–1490, 2012. doi: 10.1016/j.biocel.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 40.Song B, Smink AM, Jones CV, Callaghan JM, Firth SD, Bernard CA, Laslett AL, Kerr PG, Ricardo SD. The directed differentiation of human iPS cells into kidney podocytes. PLoS One 7: e46453, 2012. doi: 10.1371/journal.pone.0046453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suda S, Rai T, Sohara E, Sasaki S, Uchida S. Postnatal expression of KLF12 in the inner medullary collecting ducts of kidney and its trans-activation of UT-A1 urea transporter promoter. Biochem Biophys Res Commun 344: 246–252, 2006. doi: 10.1016/j.bbrc.2006.03.138. [DOI] [PubMed] [Google Scholar]
- 42.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126: 663–676, 2006. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 43.Tuuminen R, Nykänen AI, Saharinen P, Gautam P, Keränen MA, Arnaudova R, Rouvinen E, Helin H, Tammi R, Rilla K, Krebs R, Lemström KB. Donor simvastatin treatment prevents ischemia-reperfusion and acute kidney injury by preserving microvascular barrier function. Am J Transplant 13: 2019–2034, 2013. doi: 10.1111/ajt.12315. [DOI] [PubMed] [Google Scholar]
- 44.Uchida S, Sasaki S, Marumo F. Isolation of a novel zinc finger repressor that regulates the kidney-specific CLC-K1 promoter. Kidney Int 60: 416–421, 2001. doi: 10.1046/j.1523-1755.2001.060002416.x. [DOI] [PubMed] [Google Scholar]
- 45.Ueda K, Fujiki K, Shirahige K, Gomez-Sanchez CE, Fujita T, Nangaku M, Nagase M. Genome-wide analysis of murine renal distal convoluted tubular cells for the target genes of mineralocorticoid receptor. Biochem Biophys Res Commun 445: 132–137, 2014. doi: 10.1016/j.bbrc.2014.01.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Husen M, Kemper MJ. New therapies in steroid-sensitive and steroid-resistant idiopathic nephrotic syndrome. Pediatr Nephrol 26: 881–892, 2011. doi: 10.1007/s00467-010-1717-5. [DOI] [PubMed] [Google Scholar]
- 47.Villarreal G Jr, Zhang Y, Larman HB, Gracia-Sancho J, Koo A, García-Cardeña G. Defining the regulation of KLF4 expression and its downstream transcriptional targets in vascular endothelial cells. Biochem Biophys Res Commun 391: 984–989, 2010. doi: 10.1016/j.bbrc.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wada T, Pippin JW, Marshall CB, Griffin SV, Shankland SJ. Dexamethasone prevents podocyte apoptosis induced by puromycin aminonucleoside: role of p53 and Bcl-2-related family proteins. J Am Soc Nephrol 16: 2615–2625, 2005. doi: 10.1681/ASN.2005020142. [DOI] [PubMed] [Google Scholar]
- 49.Wada T, Pippin JW, Nangaku M, Shankland SJ. Dexamethasone’s prosurvival benefits in podocytes require extracellular signal-regulated kinase phosphorylation. Nephron Exp Nephrol 109: e8–e19, 2008. doi: 10.1159/000131892. [DOI] [PubMed] [Google Scholar]
- 50.Wannamethee SG, Shaper AG, Perry IJ. Serum creatinine concentration and risk of cardiovascular disease: a possible marker for increased risk of stroke. Stroke 28: 557–563, 1997. doi: 10.1161/01.STR.28.3.557. [DOI] [PubMed] [Google Scholar]
- 51.Wieschaus E, Nusslein-Volhard C, Kluding H. Krüppel, a gene whose activity is required early in the zygotic genome for normal embryonic segmentation. Dev Biol 104: 172–186, 1984. doi: 10.1016/0012-1606(84)90046-0. [DOI] [PubMed] [Google Scholar]
- 52.Wu J, Bohanan CS, Neumann JC, Lingrel JB. KLF2 transcription factor modulates blood vessel maturation through smooth muscle cell migration. J Biol Chem 283: 3942–3950, 2008. doi: 10.1074/jbc.M707882200. [DOI] [PubMed] [Google Scholar]
- 53.Xiao X, Tang W, Yuan Q, Peng L, Yu P. Epigenetic repression of Krüppel-like factor 4 through Dnmt1 contributes to EMT in renal fibrosis. Int J Mol Med 35: 1596–1602, 2015. doi: 10.3892/ijmm.2015.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xing CY, Saleem MA, Coward RJ, Ni L, Witherden IR, Mathieson PW. Direct effects of dexamethasone on human podocytes. Kidney Int 70: 1038–1045, 2006. doi: 10.1038/sj.ki.5001655. [DOI] [PubMed] [Google Scholar]
- 55.Xu K, Zhou Y, Qiu W, Liu X, Xia M, Liu L, Liu X, Zhao D, Wang Y. Activating transcription factor 3 (ATF3) promotes sublytic C5b-9-induced glomerular mesangial cells apoptosis through up-regulation of Gadd45α and KLF6 gene expression. Immunobiology 216: 871–881, 2011. doi: 10.1016/j.imbio.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 56.Yoshida T, Yamashita M, Iwai M, Hayashi M. Endothelial Krüppel-Like Factor 4 mediates the protective effect of statins against ischemic AKI. J Am Soc Nephrol 27: 1379–1388, 2016. doi: 10.1681/ASN.2015040460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhong F, Chen H, Wei C, Zhang W, Li Z, Jain MK, Chuang PY, Chen H, Wang Y, Mallipattu SK, He JC. Reduced Krüppel-like factor 2 expression may aggravate the endothelial injury of diabetic nephropathy. Kidney Int 87: 382–395, 2015. doi: 10.1038/ki.2014.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhong F, Mallipattu SK, Estrada C, Menon M, Salem F, Jain MK, Chen H, Wang Y, Lee K, He JC. Reduced Krüppel-Like Factor 2 aggravates glomerular endothelial cell injury and kidney disease in mice with unilateral nephrectomy. Am J Pathol 186: 2021–2031, 2016. doi: 10.1016/j.ajpath.2016.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]