according to the Centers for Disease Control and Prevention (CDC), >10% of US adults have chronic kidney disease (CKD), and one in two adults in the 30–64 age group is expected to develop CKD. Yet, development of new therapeutics for treating CKD is sorely lacking, and the disease management in patients primarily relies on the use of antihypertensives and immunomodulators. Glomerular injury and dysfunction are the primary drivers of CKD in patients, including in steroid-resistant nephrotic syndrome (SRNS) that often manifests as focal segmental glomerulosclerosis (FSGS). Yet, there are currently no therapeutics that directly and specifically target the kidney glomerulus.
Podocytes are key cells that form the filtration barrier in the glomerulus, and healthy podocytes are essential for normal kidney function. Podocytes have a large cell body with major cytoskeleton-linked cellular processes, known as foot processes (FPs), that maintain cell shape and function (1, 2). FP effacement is an early finding in glomerular diseases, is common in many patients carrying genetic abnormalities, and results in increased podocyte damage and loss, ultimately leading to glomerulosclerosis and proteinuria (6). FP effacement is a result of increased podocyte FP dynamics measured in vitro by cell motility and recognized as early event in the induction of nephrotic syndrome originally described by Reiser et al. in 2004 (5). Thus agents that directly target podocytes, protect them from FP effacement and injury, and reduce their rate of migration have the potential to be developed into novel, kidney-targeted therapeutics for CKD (4). Yet, efforts for the rational development of podocyte directed therapeutics have been lacking due to lack of quantitative screening assays for drug discovery and development. Conceptually, podocyte-targeting assays in a high-throughput, drug discovery environment can take three different routes (Fig. 1). Cells in multiwell plates are subjected to high content imaging-based assays to quantify changes in key cellular phenotypes, such as cell shape and the organization of its cytoskeleton, as a read-out, as recently described (3). Such an approach identifies agents that affect static cells, but does not provide information on whether key cellular functions, such as motility, will be affected. Another potential approach is to utilize whole organisms, such as zebrafish in a primary screening environment, where validation of direct targeting of podocytes has to be done in subsequent assays.
Fig. 1.
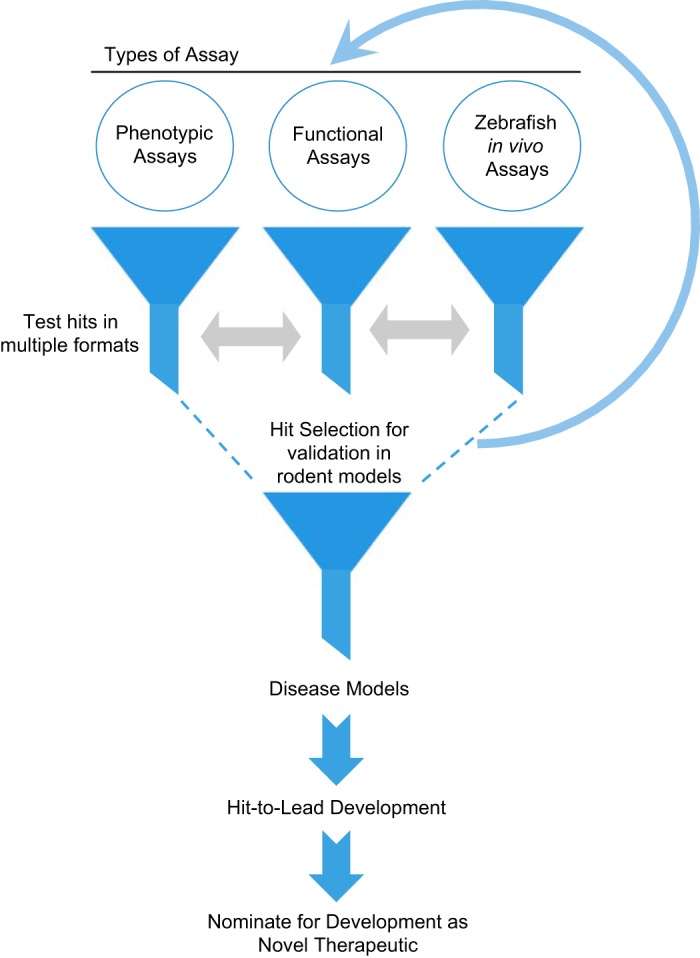
A schematic showing potential drug discovery strategies for identification of novel agents as future therapeutics for chronic kidney disease (CKD). There are 3 assay strategies for an initial (primary) screen. They all involve targeting 1 of the 3 key characteristics: cell shape, motility, and in vivo functionality. A podocyte cell-based phenotypic assay can be used to identify compounds that protect overall cellular cytoskeleton and morphology. A zebrafish-based assay can be used to screen compounds that would protect filtration barrier and kidney function. A 3rd type of assay, as described in the report by Tan et al. (5a), can be used to identify compounds that reduce injury-dependent increase in podocyte motility. Subsequently, the identified hits from each of the 3 types of campaigns could be validated in the other type of assay to select hits that affect these key podocyte qualities before testing in rodent experimental models. Selected compounds can then go through a few rounds of optimization, including target identification, mechanistic studies, and medicinal chemistry-based approaches, before entering a hit-to-lead program and be nominated as a therapeutic lead.
In line with the original concept by Reiser et al. (5), Tan et al. (5a) describe an approach, quantitative changes in the rate of cell migration. Given that FP effacement and increased rate of migration have previously been linked with increased glomerular damage, this approach directly targets podocytes in a highly physiologically relevant functional assay and provides hits that can subsequently be tested in additional phenotypic and in vivo assays for a fuller characterization. In this article, Tan et al. (5a) convincingly show that their new assay system, where they computationally track closure of scratch wounds in monolayers of human podocytes in multiwell plates, can be used in a screening environment, by screen a collection of 725 small molecules to identify, and subsequently validate, 12 compounds that reduced podocyte migration. Not surprisingly, a majority of the hits are known to affect microtubule assembly or block topoisomerases, thus affecting cell migration. By targeting key structural elements of a cell, these compounds likely also help maintain podocyte cellular architecture and shape. Thus the results from this study and previous body of literature suggests that, while there are multiple pathways available for targeting by small molecules in podocytes, maintenance of cell shape and decreasing cell motility are two master phenotypes for development of therapeutically relevant agents. Given these recent developments, we can conclude that podocytes are druggable cells and promising targets for small molecule therapeutic in the fight against kidney disease.
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grants R01-DK-107984 and R01-DK-106512 (to V. Gupta and J. Reiser), by the Nephcure Foundation, and with resources from the Rush University Medical Center.
DISCLOSURES
V. Gupta and J. Reiser are inventors on patent applications relevant to podocyte-based screening assays. They and Rush University Medical Center have the potential for financial gain from their future commercialization.
AUTHOR CONTRIBUTIONS
V.G. and J.R. conception and design of research; V.G. and J.R. prepared figures; V.G. and J.R. drafted manuscript; V.G. and J.R. edited and revised manuscript; V.G. and J.R. approved final version of manuscript.
REFERENCES
- 1.Faul C, Asanuma K, Yanagida-Asanuma E, Kim K, Mundel P. Actin up: regulation of podocyte structure and function by components of the actin cytoskeleton. Trends Cell Biol 17: 428–437, 2007. doi: 10.1016/j.tcb.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Huber TB, Benzing T. The slit diaphragm: a signaling platform to regulate podocyte function. Curr Opin Nephrol Hypertens 14: 211–216, 2005. doi: 10.1097/01.mnh.0000165885.85803.a8. [DOI] [PubMed] [Google Scholar]
- 3.Lee HW, Khan SQ, Faridi MH, Wei C, Tardi NJ, Altintas MM, Elshabrawy HA, Mangos S, Quick KL, Sever S, Reiser J, Gupta V. A podocyte-based automated screening assay identifies protective small molecules. J Am Soc Nephrol 26: 2741–2752, 2015. doi: 10.1681/ASN.2014090859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reiser J, Gupta V, Kistler AD. Toward the development of podocyte-specific drugs. Kidney Int 77: 662–668, 2010. doi: 10.1038/ki.2009.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reiser J, Oh J, Shirato I, Asanuma K, Hug A, Mundel TM, Honey K, Ishidoh K, Kominami E, Kreidberg JA, Tomino Y, Mundel P. Podocyte migration during nephrotic syndrome requires a coordinated interplay between cathepsin L and alpha3 integrin. J Biol Chem 279: 34827–34832, 2004. doi: 10.1074/jbc.M401973200. [DOI] [PubMed] [Google Scholar]
- 5a.Widmeier E, Tan W, Airik M, Hildebrand F. A small molecule screening to detect potential therapeutic 2 targets in (in press) human podocytes. Am J Physiol Renal Physiol. doi: 10.1152/ajprenal.00386.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wiggins RC. The spectrum of podocytopathies: a unifying view of glomerular diseases. Kidney Int 71: 1205–1214, 2007. doi: 10.1038/sj.ki.5002222. [DOI] [PubMed] [Google Scholar]


